Abstract
Despite remarkable progresses in vaccinology, therapeutic cancer vaccines have not achieved their full potential. We previously showed that an excessively long duration of antigen presentation critically reduced the quantity and quality of vaccination-induced T cell responses and subsequent anti-tumor efficacy. Here, using murine model and tumor cell lines, we studied L-Tyrosine amino acid-based microparticles as a peptide vaccine adjuvant with a short-term antigen depot function for the induction of tumor-specific T cells. L-Tyrosine microparticles did not induce dendritic cell maturation, and their adjuvant activity was not mediated by inflammasome activation. Instead, prolonged antigen presentation in vivo translated into increased numbers and anti-tumor activity of vaccination-induced CD8+ T cells. Indeed, prolonging antigen presentation by repeated injection of peptide in saline resulted in an increase in T cell numbers similar to that observed after vaccination with peptide/L-Tyrosine microparticles. Our results show that the duration of antigen presentation is critical for optimal induction of anti-tumor T cells, and can be manipulated through vaccine formulation.
Introduction
Immunotherapy is a potent modality in the treatment of several cancers, thanks to the major success of immune checkpoint blockade therapy with anti-CTLA4 and anti-PD1/PD-L1 monoclonal antibodies. Immune checkpoint blockade potentiates pre-existing tumor-specific T cell responses to mediate tumor destruction (1). However, many tumors induce insufficient spontaneous T cell responses, a limitation that can potentially be overcome by anti-cancer vaccination. Unfortunately this approach has yet to deliver robust therapeutic efficacy (2, 3). With recent advances in the personalized identification of tumor antigens (Ag) (i.e. neoepitopes derived from mutated gene products) (4) and better understandings of vaccine adjuvants (i.e. delivery systems and immunopotentiators), new avenues are open for more potent therapeutic cancer vaccines (5). For example, Gubin et al. showed in a murine model that immunization using tumor neoantigens (peptides) was as effective as checkpoint blockade (6). Recently, personal neoantigen vaccines were demonstrated to be safe and effective in treating patients with high risk melanoma (7, 8). Exciting results from these studies provide a strong rationale for cancer vaccine as a standalone treatment or in combination with checkpoint blockade or other immunotherapies.
We have previously shown that for peptide-based cancer vaccines, the choice of antigen delivery system can affect the ensuing anti-tumor immune response (9). Long-lived water-in-oil emulsions of Incomplete Freund's Adjuvant (IFA), which greatly prolong Ag presentation time through a long-lived Ag depot function, diminished therapeutic efficacy when used as adjuvant for short antigenic peptides. Specifically, tumor-specific CD8 T cells became sequestered at the persisting, antigen-rich vaccination site, where they underwent apoptosis without reaching the tumor. While a very short-lived, water-based formulation showed no T cell sequestration and consequently improved anti-tumor activity, T cell responses were weaker, possibly because Ag was cleared too rapidly to allow maximal T cell priming. We therefore hypothesized that Ag delivery systems can be created to extend Ag presentation time sufficiently long to allow induction of an optimal T cell response, but not so long as to induce T cell sequestration at the vaccination site. Here, we report that microparticles consisting of the poorly soluble amino acid L-Tyrosine are a promising peptide Ag delivery system for the induction of potent anti-tumor immune responses. Mechanistically, L-Tyrosine functioned as a short-lived depot, extending the Ag presentation time during a critical window for optimal T cell priming. Interestingly, this effect could be largely mimicked by repeated injections of peptide in saline, thus suggesting a simple strategy for increasing the potency of peptide-based anti-cancer vaccines. Overall, our results point to duration of antigen presentation as a critical factor in vaccine-induced T cell priming, which can be controlled by proper choice of vaccine adjuvant.
Materials and Methods
Reagents and Chemicals
The synthetic, high-affinity H-2Db-restricted heteroclitic mouse gp10025–33 peptide (KVPRNQDWL) and H-2Kb–restricted chicken OVA-I 257–264 peptide (SIINFEKL) were purchased from Peptides International (Louisville, KY) at a purity >95%. Optima-Grade acetonitrile, methanol, and water were purchased from Thermo-Fisher Scientific (Waltham, MA). Mass Spectrometry-grade formic acid (Fluka; 98%) was purchased from Sigma Aldrich (St. Louis, MO). 1× Phosphate Buffered Saline (PBS) was purchased from Mediatech Inc. (Manassas, VA). Sodium hydroxide (molecular biology grade) and hydrochloric acid (36.5% v/v) were purchased from Fisher Scientific (Fair Lawn, NJ). Sodium chloride, sodium phosphate monobasic, sodium phosphate dibasic, and L-Tyrosine (cell culture grade) were purchased from Sigma (St. Louis, MO). Mouse cytokine/chemokine milliplex kit (catalog # MXMCY70KPX25MGBK) was purchased from EMD Millipore (Massachusetts, USA). OVA-I dextramer H2-Kb was purchased from Immudex (Fairfax, VA).
Mice
All mouse protocols were reviewed and approved by the Institutional Animal Care and Use Committee at The University of Texas - M.D. Anderson Cancer Center. Pmel-1 TCR transgenic mice on C57BL/6 background (the Jackson Laboratory, ME) were crossed with CD90.1 congenic mice to yield pmel-1+/+CD90.1+/+ mice (hereafter referred as pmel-1 mice). C57BL/6 (B6) mice were purchased from Charles River (Wilmington, MA). B16-F10 melanoma bearing mice were established by injecting 300,000 B16 cells in a volume of 0.1 ml subcutaneously. Tumor bearing mice received treatments on day 6 after tumor injection when the average tumor size was approximately 30 mm2. ASC knock-out mice were a kind gift from Dr. Thirumala-Devi Kanneganti at St. Jude Children's Research Hospital.
Cell culture
B16-F10 melanoma cell line, from ATCC (Manassas, VA), was cultured in complete medium including RPMI 1640 with 10% FBS, 100 μg/ml streptomycin and 100 μg/ml penicillin (Life Technologies, Carlsbad, CA.)
Vaccination
Peptides in saline and IFA were prepared as previously described (9). Preparation of peptide with L-Tyrosine was adapted from a protocol for grass pollen/L-Tyrosine described by Wheeler et al. (10). A peptide solution was prepared by dissolving 4 mg of peptide in 2 mL of 1×-PBS. After dissolution, 0.667 mL of strong PBS (0.83 M Na2HPO4, 0.25 M NaH2PO4, 0.137 M NaCl) was added to the solution. Next, 0.667 mL each of 3.2 M sodium hydroxide and 1.3 M L-Tyrosine in 3.9 M hydrochloric acid were added simultaneously, mixed, resulting in a final solution volume of 4 ml. The suspension was centrifuged and supernatant was discarded. Remaining peptide/L-Tyrosine pellet was dissolved in PBS to make up a volume of 4 ml and ready for injection. Final peptide concentration was approximately 0.25 mg/ml. Each mouse received 50 μg peptide in 200 μl vaccine (100 μl ×2 vaccination sites). For the quantification of tumor specific CD8+ T cells, pmel-1 splenocytes were intravenously transferred to B6 mice in the same day with vaccination. Covax, including anti-CD40 antibody (clone FGK4.5/FGK45, BioXcell, New Hampshire), IL-2 (TECIN, Hoffman LaRoche) and Imiquimod cream 5% (Fougera, Melville, NY) was given on the same day with peptide vaccination. Anti-CD40 dose: 50 μg subcutaneously; IL-2: 100,000 IU, once on day 0 and twice on day 1 and 2 intraperitoneally; imiquimod cream: 50 mg, applied topically on vaccine site.
Adoptive transfer of pmel-1 T cells
Unless specified, each mouse received approximately 80,000 naïve pmel-1 CD8 T cells from pmel-1 donor mouse via i.v. tail vein injection. For in vivo antigen detection experiments, pmel-1 CD8 T cells were purified using CD8 T cell enrichment kit (StemCell Technologies, Vancouver, BC, Canada) then labeled with CFSE as described elsewhere (11). Each mouse received 2×106 CFSE labeled pmel-1 CD8 T cells i.v.
Quantification of gp100 and OVA-I specific T cells
gp100 specific CD8 T cell responses of mice receiving pmel-1 T cells were detected basing on congenic Thy1.1 (CD90.1). Endogenous gp100 and OVA-I specific CD8 T cell responses were detected by IFN-g and OVA-I dextramer using flow cytometry, respectively.
FACS analysis
Mice were tail-bled on the indicated days. Extracellular staining was performed using FACS buffer containing 2% FBS. Intracellular cytokine staining was performed using the cytofix/cytoperm kit from BD Biosciences (San Jose, CA) basing on the manufacturer's recommendation. Granzyme B staining was done without stimulation while IFN-γ staining was done after 4 hours of stimulation with 1 μM gp10025-33 peptide. Antibodies were either purchased from eBioscience or BD Biosciences: CD8a (clone 56-6.7), CD4 (GK1.5), CD90.1 (HIS51), IFN-γ (XMG1.2), TNF-α (MP6-XT22), Granzyme B (NGZB), CD19 (eBio1D3), CD3e (145-2C11), NK1.1 (PK136), CD44 (IM7), B220 (RA3-6B2), CD11b (M1/70), CD11c (N418), F4/80 (BM8), CD62L (MEL-14), CD27 (A7R34) MHCII (M5/114.15.2), CD40 (HM40-3), CD86 (GL-1), Ly6G (1A8), Ly6C (AL-21).
Cytokine/chemokine assay
On day 1, 2, 3 and 7 post vaccination, skins at vaccine site were depilated, weighted, mechanically disrupted in ice cold PBS (1 ml/sample) and centrifuged for supernatant collection. The cytokines/chemokines in the supernatant were measured using Milliplex mouse cytokine/chemokine panel (Millipore) according to the manufacturer's instructions. Fluorescence signal was measured on Luminex 100/200 system and data were analyzed using Excel software. Final cytokine/chemokine readouts were normalized by sample weight.
Quantification of peptides (gp100 and OVA-I) in L-Tyrosine formulation
After the peptide/L-Tyrosine co-precipitation (as described in vaccination section), the final volumes of the supernatant and crystal fractions were determined to be 2.85 mL and 1.15 mL, respectively. The individual fractions were stored at 4 °C until analysis. Peptide stock (2.49 mg/mL) and intermediate (100 μg/mL) solutions were prepared in water, and were stored at 4 °C until analysis. The intermediate solution was used to prepare calibration standards at 50.0, 25.0, 10.0, 2.00, and 1.00 μg/mL concentrations in water. Prior to sample processing, the peptide loaded particle and supernatant fractions were warmed to room temperature. The peptide-loaded L-Tyrosine particles contained in the crystal fraction were dissolved by an addition of 4 mL of formic acid followed by gentle vortex-mixing. Once the particles were completely dissolved, an additional 1.88 mL aliquot of water was added to the sample to increase the final sample volume to 7.00 ml. In prior to analysis, three individual sample dilutions were prepared at 10×, 50×, and 100× in water.
LC-MS/MS System Conditions
Sample analysis was performed on an Agilent 1290 Infinity Binary UHPLC coupled to an Agilent 6460 tandem-mass spectrometer. Mobile phase A (MPA) and mobile phase B (MPB) used for this study were 0.1% formic acid in water and 0.1% formic acid in acetonitrile, respectively. The chromatographic column used was an Agilent Zorbax RRHD Eclipse Plus C18 (2.1 × 50 mm, 1.8 micrometer; dead volume: ∼0.12 mL; dead time: ∼0.60 min at 0.200 mL/min). The column was heated to 40 °C, and the chromatographic flow rate was 0.200 mL/min. The gradient elution program was set as follows: dwell at initial conditions of 90:10 MPA:MPB for 1.5 minutes post-injection; ramp to 20:80 MPA:MPB at 4.0 minutes post-injection; ramp to 0:100 MPA:MPB at 5.0 minutes post-injection; and ramp back to initial conditions (90:10 MPA:MPB) at 5.5 minutes post-injection until the gradient stops at 6.5 minutes post-injection. The overall cycle-time for a single injection was approximately 7.0 minutes. The mass spectrometer acquisition source parameters were as follows: source: Agilent Jet Stream ESI source; gas temperature: 275°C; gas flow: 6 L/min; nebulizer: 40 psi; sheath gas temperature: 325 °C; sheath gas flow: 9 L/min; capillary voltage: +3,750 V; nozzle voltage: 0 V. The molecule specific acquisition parameters were as follows: precursor to product transition: m/z 490.n2 to m/z 848.4; MS1 and MS2 were set to unit resolution; dwell time: 250 ms; fragmentor voltage: 100 V; collision energy voltage: 10 V; cell acceleration voltage: 7 V; and the source polarity was set to positive mode.
Statistical analysis
All results are expressed as mean ± s.e.m (standard error of the mean. Group size was at least 3 mice per group. Tumor challenge experiments had group size of 10 mice/group. Data were analyzed using unpaired t-test where p value < 0.05 is considered as significant. Survival experiments used log-rank (Mantel–Cox) test for survival analysis. All experiments were repeated at least once with comparable results.
Results
L-Tyrosine formulation potentiates peptide vaccine-induced CD8 T cell responses
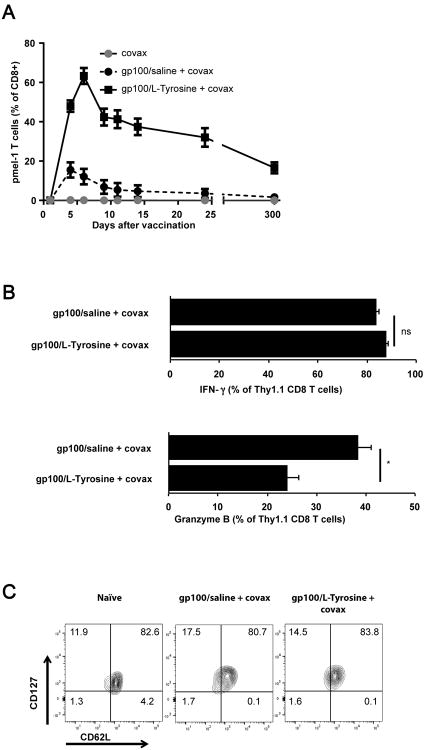
L-Tyrosine is an amino acid with poor water-solubility and the capacity to adsorb macromolecular grass and tree allergens during pH change-induced flash-precipitation (figure S1A and S1C) (10). In vivo, injected L-Tyrosine microparticles dissolve over a period of several days and L-Tyrosine-formulated macromolecular grass and tree pollen allergens have been used in human allergen desensitization vaccines (10, 12). Here, we tested whether L-Tyrosine could also be used as a short-term slow release formulation for short (9 amino acid) gp10025–33 (hereafter referred to as gp100) antigenic peptide. After co-precipitation of L-Tyrosine and gp100 peptide (hereafter referred to as gp100/L-Tyrosine), we determined the gp100 peptide content in the resulting microparticles (Table SI). Approximately 25 percent of initial gp100 peptide input was reproducibly retained in the gp100/L-Tyrosine formulation. We previously showed that covax (a molecularly defined adjuvant consisting of agonistic anti-CD40 antibody, the Toll-Like Receptor (TLR)7 agonist, imiquimod, and IL-2) could remarkably improve vaccination-induced CD8+ T cell responses (9). We therefore vaccinated mice with gp100/L-Tyrosine and covax and measured the resulting gp100-specific pmel-1 T cell response. gp100/L-Tyrosine induced superior T cell numbers in peripheral blood compared to the equivalent dose (50 μg/mouse) of gp100 in saline formulation (figure 1A). Functionally, L-Tyrosine induced similar IFN-γ but significantly less granzyme B production by pmel-1 T cells (figure 1B and 1C). Three hundred days later, gp100/L-Tyrosine-vaccinated mice contained significantly more memory T cells than mice vaccinated with gp100/saline. T cells in both groups displayed a central memory (CD62LhiCD127hi) phenotype (figure 1D). Noticeably, peptide formulated in either the D- or L- optical isomer of Tyrosine induced similar T cell responses, suggesting the ability of L-Tyrosine's to form microparticles was more important for its vaccine adjuvant activity than its possible pharmacological activity (figure S2A). In the absence of covax, gp100/L-Tyrosine induced a very modest pmel-1 T cell response (figure S2B), confirming the importance of including specific APC-activating (TLR7 agonist and agonistic anti-CD40 mAb) and T cell survival signals (IL-2) for inducing a robust T cell response after vaccination with synthetic peptides.
Figure 1.
L-Tyrosine is a potent vaccine adjuvant for the induction of CD8+ T cell responses. A) Pmel-1 T cell level as a percentage of CD8 + T cells in the blood of mice at different time point. B) IFN-γ and C) granzyme B production by pmel-1 T cells. D) Memory phenotype of pmel-1 T cells on day 300 post vaccination. Mice received 8×105 pmel-1 T cells and indicated treatments on day 0. Peptide dose was 50 μg per mouse. Data are shown as the mean ± s.e.m. Statistical differences between the two groups were determined by student t-test. n = 5 mice per group per experiment. Data are representative of 3 independent experiments.*: P< 0.05, **: P< 0.01, ns: not significant.
L-Tyrosine-based vaccination induces chemokine production and immune cell recruitment without direct activation of dendritic cells or inflammasome
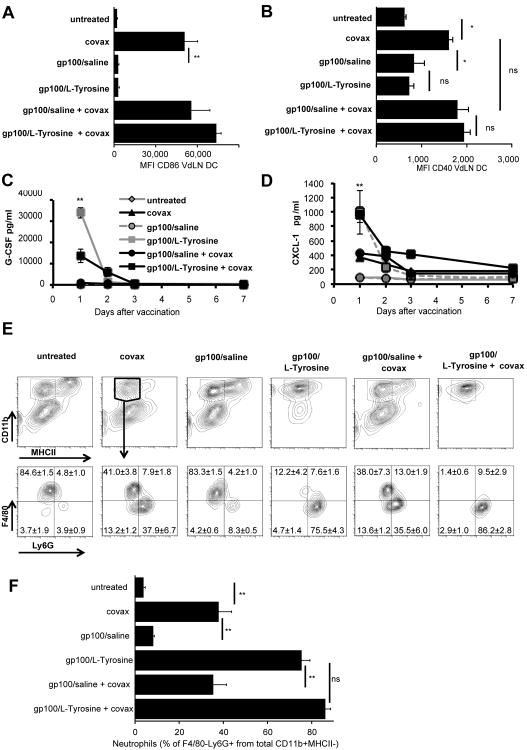
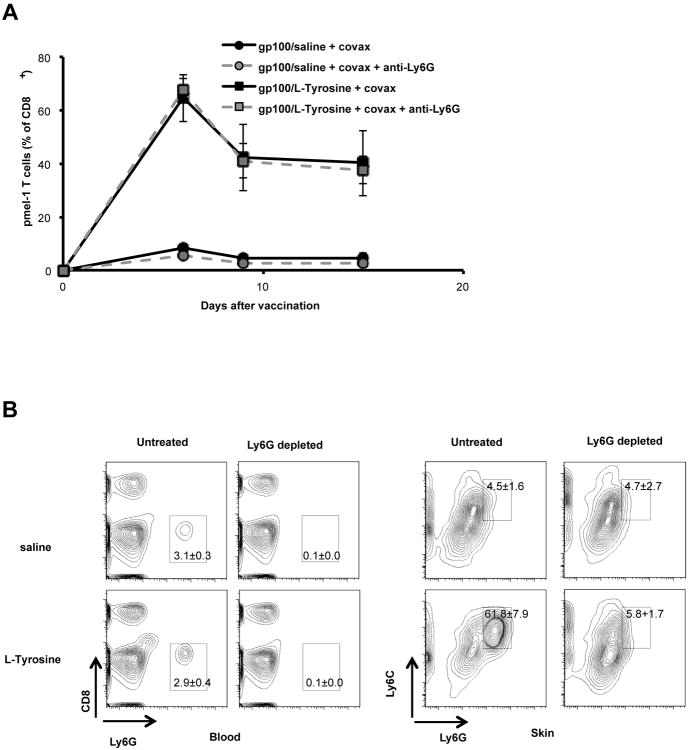
Successful vaccination requires the induction of local inflammation, resulting in recruitment and activation of antigen presenting cells (APC) such as dendritic cells (DC). To understand the contribution of these processes in gp100/L-Tyrosine-induced T cell immunity, we characterized chemokine production and leukocyte recruitment at the cutaneous vaccination site. L-Tyrosine microparticles did not induce upregulation of co-stimulatory molecules such as CD86 and CD40 on DCs (figure 2A and 2B). Instead, L-Tyrosine induced particularly high expression of the granulocyte/neutrophil attractant, CXCL-1, as well as G-CSF, which was accompanied by increased recruitment of neutrophils to the vaccination site (figure 2C, 2D, 2E and 2F). To determine whether this neutrophil recruitment contributed to superior CD8 T cell priming by L-Tyrosine microparticles, we depleted neutrophils with a Ly6G-specific antibody. We found no difference in gp100-specific CD8 T cell responses between Ly6G-depleted and control groups (figure 3), suggesting that while the particulate nature of L-Tyrosine vaccine triggered the influx of neutrophils, these did not contribute to the enhanced T cell priming.
Figure 2.
Local inflammatory response to L-Tyrosine-based vaccination. L-Tyrosine did not induced CD86 and CD40 upregulation on dendritic cells at VdLN (A and B) but induced chemo-attractant CXCL-1 and G-CSF at vaccine site (C and D). L-Tyrosine induced massive neutrophil (CD11b+, MHCII-, F4/80-, Ly6G+) infiltration to vaccine site (E and F). Statistical differences between the two groups were determined by the unpaired two-tailed t-test. n = 3-4 mice per group per experiment. Data are representative of 2 independent experiments (mean ± s.e.m.). *: P< 0.05, **: P< 0.01, ns: not significant. VdLN: vaccine draining lymph node, DC: dendritic cell.
Figure 3.
Neutrophils do not contribute to T cell priming after L-Tyrosine vaccine. Anti-Ly6G Ab was given 2 days prior to vaccination and every 3 days afterward. A) Pmel-1 T cells level in the blood after indicated treatments were shown. The depletion of Ly6G population was confirmed in peripheral blood (B) and the skin at vaccine sites (C) on day 4 after vaccination. Mice received 8×105 pmel-1 T cells and indicated treatments on day 0.n = 5 mice per group. Data are representative of 2 independent experiments.
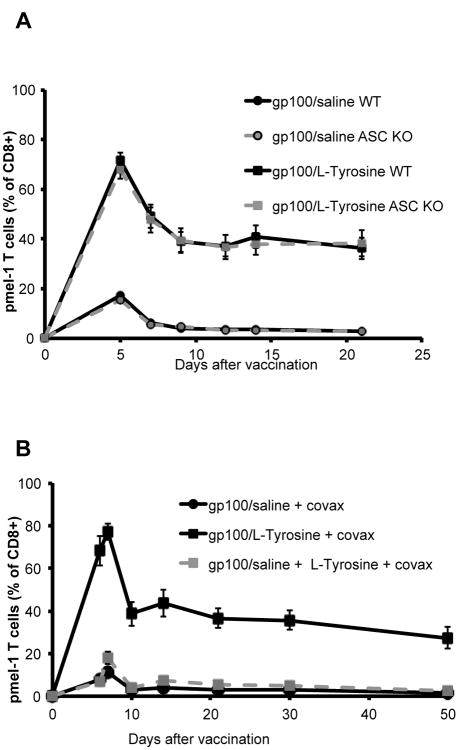
Because particulate materials have previously been shown to exert vaccine adjuvant activity through inflammation mediated by activation of the inflammasome (13, 14), and the recruitment of neutrophils is a strong indicator of inflammation (15), we determined whether the adjuvant activity of L-Tyrosine was mediated by the inflammasome. We measured gp100 specific pmel-1 T cell responses after L-Tyrosine vaccination in wild-type and genetically inflammasome-deficient ASC knock-out mice. We found no difference in T cell response among wild-type and genetically inflammasome-deficient ASC-KO mice (figure 4A). In addition, vaccination with gp100 peptide mixed with “empty” L-Tyrosine microparticles (preparation shown in figure S1B) did not improve T cell response, as opposed to vaccination with co-precipitated gp100 peptide and L-Tyrosine (figure 4B). Thus the vaccine adjuvant activity of L-Tyrosine required co-precipitation of peptide and L-Tyrosine into mixed microparticles, and “empty” L-Tyrosine microparticles did not demonstrate any vaccine adjuvant activity even when co-injected with free peptide antigen, as would be expected if the microparticles induced local inflammation. Taken together, the activity of L-Tyrosine did not appear to be mediated by inflammasome activation or direct activation of APCs.
Figure 4.
L-Tyrosine adjuvant activity does not require inflammasome activation. A) No difference in T cell responses between wild-type and ASC KO groups was found, regardless of vaccine formulations. B) L-Tyrosine adjuvant activity requires peptide co-precipitation. Mice received 8×105 pmel-1 T cells and indicated treatments on day 0. n= 5 mice per group. Data are representative of 3 independent experiments. WT: wild type, ASC KO: ASC knockout.
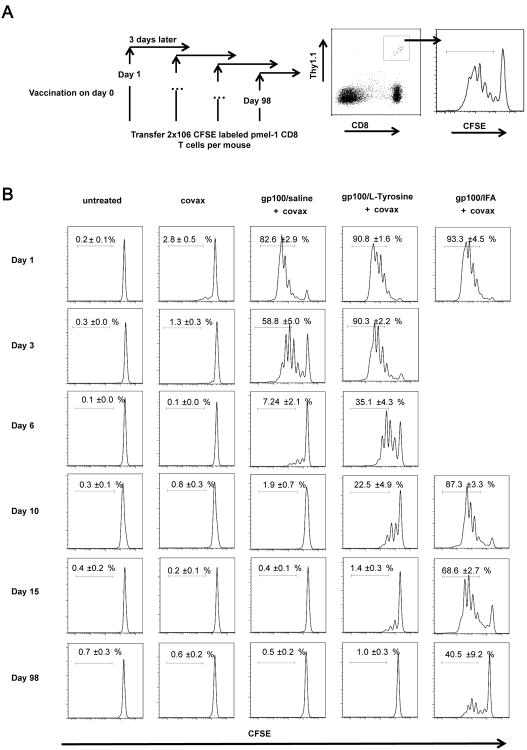
L-Tyrosine formulation extends the duration of antigen presentation
Since L-Tyrosine was only active when co-precipitated with peptide, we hypothesized that L-Tyrosine extends the duration of peptide presentation to naïve CD8+ T cells compared to free peptide delivered in saline. To test this hypothesis, we vaccinated mice with gp100/L-Tyrosine, gp100/saline or gp100/IFA (all with covax) and then transferred CFSE-labeled, naïve gp100-specific pmel-1 CD8 T cells at different time points after vaccination. IFA formulation with peptide was included because it has been well documented to extend Ag presentation time over a long period of time and served as a positive control (9, 16, 17). The productive presentation of gp100 peptide antigen in vivo was detected by measuring the proliferation of naïve gp100 specific pmel-1 T cells, as indicated by CFSE dilution, at 72 hours after their adoptive transfer. We found that gp100/L-Tyrosine extended the duration of gp100 peptide presentation beyond gp100/saline by approximately 3-4 days, but not to the extent caused by gp100/IFA, which was still potently presented after 98 days, in line with our previous observations (figure 5 and figure S3 and (9)). Thus, L-Tyrosine microparticles functioned as a peptide vaccine formulation that caused an intermediate duration of antigen presentation to T cells in vivo.
Figure 5.
L-Tyrosine formulation extends the duration of antigen presentation. A) A schematic of the experiment design. All mice were treated with indicated treatments on day 0. At indicated time points, 2×106 CFSE labeled pmel-1 CD8+ T cells were transferred to hosts. 72 hours post transfer, vaccine draining lymph nodes were harvested and CFSE dilution of pmel-1 T cells was measured by flow cytometry. n = 3 - 5 mice per group. Data are representative of 2 independent experiments.
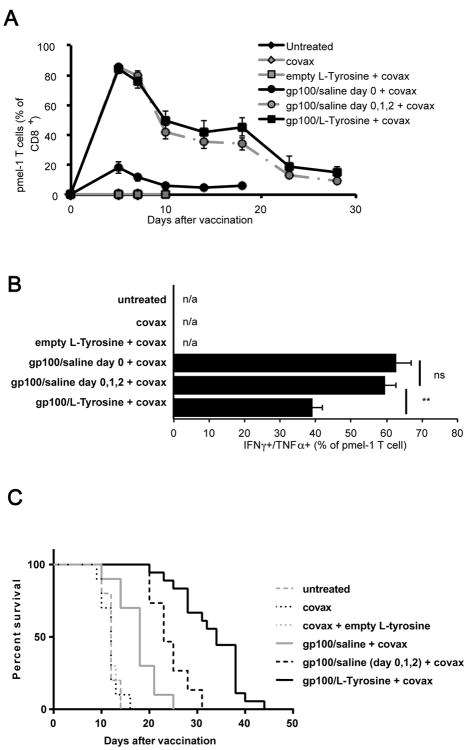
Repeated dosing of free peptides recapitulates the vaccine adjuvant effect of single-dose L-Tyrosine-formulated peptides
Since the extended duration of Ag presentation by L-Tyrosine formulation correlated with its ability to induce a superior T cell response, we determined whether a similar result could be attained by extending Ag presentation through repeated injections of unformulated, soluble peptide. Indeed, T cell levels were very comparable between mice receiving 1 dose of gp100/L-Tyrosine and 3 doses of gp100/saline on 3 consecutive days (figure 6A), while cytokine (IFNγ/TNFα) production was reduced (figure 6B). This may explain why tumor rejection after repeated peptide injection was improved compared to single gp100/saline injection, but still not efficient as gp100/L-Tyrosine (figure 6C).
Figure 6.
Anti-tumor efficacy of different vaccine formulations regimens. Mice received 8×105 pmel-1 T cells and indicated treatments on day 0. A) Pmel-1 T cell response after different vaccine formulations were followed and B) their function at day 7 post vaccination was shown (mean ± s.e.m.). C) Anti-tumor efficacy of corresponding groups of the same experiment. n = 10 – 20 mice/group. Peptide dose was 50ug per mice. Data pooled from 2 independent experiments. Differences in survival among groups were compared using log-rank test. *: P< 0.05, **: P< 0.01, ns: not significant, n/a: not available.
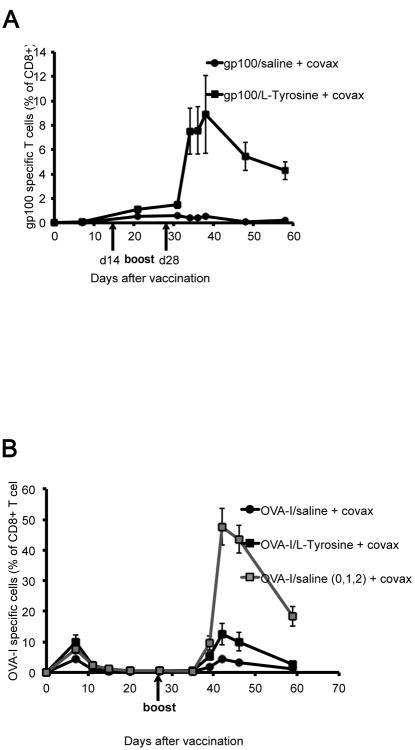
To rule out the possibility that our findings applied uniquely to our specific model system based on gp100 antigen and gp100-specific, transgenic T cells, we examined endogenous T cell responses to gp100 and OVA-I257–264 (SIINFEKL) peptides. When testing endogenous T cell responses to gp100, we observed a very low response after 1 vaccination, likely due to the reported very low T cell precursor frequency to this self antigen (18). However, after 2 booster vaccination, mice receiving gp100/L-Tyrosine showed a dramatically stronger gp100-specific CD8+ T cell response than mice receiving gp100/saline (figure 7A). We then tested endogenous T cell responses to the unrelated non-self antigen, OVA-I, and observed a strong CD8+ T cell response after two vaccinations, with OVA-I/L-Tyrosine and especially three successive daily OVA-I/saline vaccinations giving a clearly enhanced T cell response. Overall, these results demonstrate that approaches that prolong antigen presentation in vivo deserve further investigation in the development of human cancer vaccines.
Figure 7.
T cell responses from the endogenous repertoire. A) Endogenous gp100 specific T cell responses after saline and L-Tyrosine vaccines, detected by IFN-γ positive CD8 T cells. B) Endogenous OVA-I specific T cell responses after saline and L-Tyrosine vaccines, detected by OVA-I dextramer H2-Kb positive CD8 T cells. Boosters were given as indicated times, with same dose and formulations as for priming. n = 3 - 5 mice per group.
Discussion
Cancer vaccines are widely explored as a mean to induce tumor-specific T cells, but thus far clinical success has been limited. One reason is a profound lack of sufficiently potent vaccine adjuvants available for the potentiation of cancer vaccines aimed at inducing robust tumor-specific T cell responses. Vaccine adjuvants can be broadly categorized into 2 groups: Ag delivery systems and immunopotentiators (19–21).
Most Ag delivery systems influence the availability of Ag in vivo, either by protecting Ag from rapid clearance (e.g. by proteases) and/or delivering the Ag to LN, either directly or indirectly (by targeting tissue APC that traffic to LN). Importantly, Ag delivery systems can also serve as carriers for immunopotentiators) as well as function as innate immune activators themselves. For example, IFA and other water-in-oil emulsion adjuvants (Montanide™ oil series, SEPPIC Corp.) can both retain and slowly release the Ag at the vaccination site and cause local inflammation(16). Alum, which has been used for over 80 years, is the most widely used vaccine adjuvant for human and veterinary vaccines(20). The Ag depot effect has generally been accepted as a major mechanism of action of alum until recently. Hutchinson et al. showed that Ab production induced by alum remained intact even when the injection site was surgically removed shortly (2 hours) after vaccination(22). This observation suggested adjuvant activity of alum is likely due to its immunopotentiating activity. Indeed, alum was shown to activate the NALP3 inflammasome in DC (23). Inflammasome activation results in production of pro-inflammatory cytokines such as IL-1β and IL-18 (24) (25). In contrast, the adjuvant activity of L-Tyrosine microparticles did not require inflammasome activation, but could be largely mimicked by extending Ag presentation through repeated Ag injection, suggesting the depot function of L-Tyrosine was a major determinant of its adjuvant activity. Micro- and nanoparticles that can be generated in well-defined structure, size, and shape, offer several advantages in vaccine design. Particles can be loaded with Ag and different molecular immunopotentiators (e.g. STING agonist cdGMP, TLR9 agonist CpG etc.), and target desired cell types (such as APC) or tissues (such as lymph nodes or tumors)(26–30). Size, shape, half-life, surface charge, hydrophobicity, material choice as well as the type of physicochemical interaction with peptide all contribute to adjuvanticity of particles. In terms of size, particles with the size of 200 nm or larger are trapped by local APC which eventually migrate to draining LN. Particles of 20-200 nm drain passively through LN where they will be taken up by LN resident DC (17, 24). Particles smaller than 10 nm drain through blood capillaries (30). 20-200 nm size range is ideal for DC while 500 nm or larger preferentially target macrophage (32). Micro-/nanoparticles can activate innate immune components, depending on their materials. Poly(D,L-lactic-co-glycolide) (PLG) and polystyrene particles are Inflammasome activators enhancing IL-1β production by DC in a phagocytosis-dependent manner (31) while carbon nanotubes activate the complement system and subsequent inflammatory responses via binding to the complement factor, C1q(33). The lipid layer of liposomes can be positively charged (cationic liposome) to promote interaction with cell membranes (which is negatively charged)(34). The persistence of particle liposomes in vivo can be extended when they are coated with polyethylene glycol (PEG) or other biocompatible polymers(35). Beside the half-life of Ag carriers, Ag – carrier interaction is another factor controlling Ag persistence. Generally, encapsulation and chemical conjugation provide stronger interaction between Ag and carrier than adsorption which is basically a charge or hydrophobic interaction(30). Virus-like particles (VLP), formed by viral structural proteins, are distinct from other antigen delivery systems due to their unique formation. Repetitive antigenic epitopes displayed on VLP cross-link B cell receptors, leading to humoral responses while their ability to target to DC (and be cross-presented on MHC Class I) is responsible for cellular response(36–38). Target antigens can be genetically fused with structural proteins in non-enveloped VLP or integrated in the outer surface (derived from host cell membrane) in enveloped VLP (39).
Immunopotentiators induce co-stimulatory signals on APC to increase immune responses. In the current study we used a combination of 3 defined immunopotentiators: IL-2, CD40 agonist mAb and imiquimod, collectively called covax. This combination of multiple specific immunological signals, similar to those induced by a viral infection, induces a potent and functional T cell response to suppress established tumors. Versions of covax that include 1 or 2 of these 3 components are dramatically less powerful (data not shown). Indeed, experimental single-agent vaccine adjuvants such as IFA, GM-CSF or poly I:C have met with limited success in clinical trials of vaccines against established tumors.
L-tyrosine microparticle adjuvant has been used clinically in pollen allergy vaccines and its safety has been extensively documented(12). Formulated with pollen allergens it promotes Th1 (particularly IgG2a) antibody responses and therefore desensitizes allergic reactions(40). In comparison, same allergens formulated with alum induce Th2 responses. Although the exact nature of the interaction between L-tyrosine particles and peptide antigens remains to be thoroughly investigated, we speculate that peptide antigen is adsorbed to L-tyrosine by hydrophobic interaction. Our preliminary observation suggests that peptides with higher grand average hydropathy (GRAVY) scores may adsorb more efficiently to L-tyrosine particles (unpublished data). As previously mentioned, the interaction between Ag and other delivery systems can be manipulated through chemical conjugation, encapsulation or charge interaction. It would be interesting to compare the activity of such other Ag delivery platforms with L-tyrosine. In particular, it would be interesting to examine the relationship between the duration of Ag presentation of different Ag formulations with the magnitude and quality of the T cell responses they induce. However, beside a certain duration of Ag presentation, each Ag delivery system will have additional, unique attributes, including the ability to activate the inflammasome and complement systems, to attract influx of specific immune cells, particle size, stability and propensity for in vivo dispersal, surface charge and resulting uptake by specific (immune) cell types. This would make it impossible to isolate the impact of duration of Ag presentation on T cell immunity from a study of multiple different vaccine adjuvants. To more directly probe the impact of extended duration of Ag presentation on T cell response, we therefore administered peptide in saline over a varying number of days, keeping all other variables constant, and found that duration of Ag presentation was a powerful driver of T cell immunity. This demonstrated that prolonging the availability of Ag by repeated administration directly induced a more powerful T cell response. It is important to recognize that different vaccine adjuvants and formulations have many important effects on T cell immunity beyond controlling the duration of antigen presentation. It is also quite possible that individual peptides may benefit most from different vaccine adjuvants, depending on the unique physicochemical attributes of the peptide. In our studies, gp100-specificic immunity was strongly enhanced by formulating the gp100 peptide in L-Tyrosine as well as by repeated peptide administration in saline, while OVA-specific immunity was only modestly enhanced by formulation in L-Tyrosine, and benefited from more repeated administration in saline. It will therefore be interesting to determine, for individual peptides, the relative potency and safety of the wide variety of currently approved and experimental vaccine formulations and adjuvants, including L-Tyrosine microparticles and repeated peptide administration in saline, in order to gain a deeper understanding of their relative utility in clinical vaccine applications.
The contribution of Ag exposure time to the expansion and differentiation of T cells has been previously recognized. Initial studies suggested that a very brief Ag stimulation (∼2 hours) was enough for CD8 T cells to enter autonomous clonal expansion (41, 42). Subsequent studies showed that longer antigenic stimulation (20-64 hours) was required for CD8 T cell to acquire full effector function and memory differentiation after expansion (43–45). In vitro settings used in these studies, however, did not truly mimic conditions of T cell priming. To overcome such shortcomings, independent groups used Listeria monocytogenes (LM) infection followed by antibiotic treatment as an in vivo model to study the role of Ag presentation time to CD8 T cell response. Mercado et al. showed that the magnitude and kinetics of Ag specific T cell response was only determined within the first 24 hour of LM infection(45)(44). Williams et al. found that reducing infection time diminished memory differentiation but not expansion of CD8 T cells (47). Importantly, when LM was increased to high dose (10 folds), reduction of infection time had minimal effect on memory CD8 T cell differentiation. The authors proposed that the initial dose of Ag and co-stimulatory signals/cytokines dictated the differentiation of effector to memory T cells. However, another possibility was that high dose LM infection resulted in more Ag that required longer time for complete clearance after antibiotic administration. In fact, manipulating bacterial clearance through antibiotics was a caveat of these studies. Although antibiotics block the production of new protein Ag, previously produced Ag requires time to be completely cleared and meanwhile can still prime T cells. Also, antibiotics curtail pathogeninduced innate immune activation by nucleic acids and TLR ligands. To circumvent these issues, Prlic et al. developed a model using peptide loaded-diphtheria receptor (DTR) expressing DC to finely tune Ag presentation time(48). The authors found that duration of TCR stimulus controlled the magnitude but not functionality of CD8 T cells response. Blair et al. altered the Ag availability to T cells by employing monoclonal Ab that blocks Ag/MHC from engaging TCR of Ag specific T cells(49). With this elegant model, they reached similar conclusions as Prlic and colleagues. However, in all these cases, the natural duration of Ag presentation was shortened, reducing the resulting T cell response level. In a setting of anti-cancer vaccination, the goal would be to induce a T cell response of the greatest magnitude and anti-tumor function. We therefore employed an opposite tactic where Ag presentation was not limited but extended beyond its “natural” duration (i.e. the ubiquitously employed single injection of peptide Ag), either by formulation with L-tyrosine or through repeated injection of peptide in saline. Using this approach, we confirmed the critical contribution of Ag exposure time for the activation and differentiation of CD8 T cells, and demonstrate that artificially extending the duration of Ag presentation results in superior T cell number and consequent anti-tumor activity. Importantly, repeated injection of free peptide model circumvents shortcomings of using different formulations to study contribution of Ag presentation to T cell responses for reasons mentioned above. Theoretically, the magnitude of T cell response by prolonged Ag stimulation may be controlled by number of Ag specific T cell recruited to clonal expansion (50). Nonetheless, Ag specific T cells were recruited to clonal expansion very efficiently in our vaccine settings, with virtually no naïve T cells remaining after 5 days, regardless of vaccine formulation (data not shown). This finding is consistent with previous reports, where naïve T cell precursor recruitment was also highly efficient and near-complete (41, 48, 51). Therefore, we conclude that increased magnitude of CD8 T cell response either after L-Tyrosine or by repeated injection is caused by increased Ag driven clonal expansion rather than enhanced recruitment of Ag-specific naïve T cells into proliferation.
Intriguingly, although repeated injection of peptide and L-Tyrosine formulation induced similar tumor specific T cell levels, the resulting anti-tumor efficacy of L-Tyrosine was more potent. This discrepancy could imply that L-Tyrosine has other, undiscovered impacts on T cell responses besides the extended Ag presentation effect we described here. In fact, meta- and ortho-Tyrosine have been shown to mediate a form of concomitant tumor resistance, a phenomenon where primary tumor suppresses the growth of distant secondary tumors (52). L-Tyrosine used in our vaccine, however, was para-L-Tyrosine, which was described as not mediating concomitant immunity. It appears more likely that 3 daily injections of peptide in saline and 1 injection of peptide in L-Tyrosine microparticles do not produce identical kinetics of Ag release and presentation by APCs, resulting in similar quantity but not quality of the ensuing T cells response, as we observed. This indicates that there may be more effective regimens of repeated Ag injection, for example a gradual increase of Ag dose followed by a gradual decrease over time, more closely mimicking the kinetics of Ag production during a viral infection; these permutations remain to be explored. From a clinical perspective, repeated Ag injection in saline is attractive because it simplifies the preparation process but it will complicate the administration since patients will have to remain hospitalized for multiple days. In contrast, formulating Ag in an appropriate depot adjuvant will complicate vaccine preparation but reduce the patients' hospital stays. The dose and frequency of Ag administration may depend on the kinetics of Ag presentation and are likely to be unique for each peptide, based on its solubility, length, susceptibility to proteases and peptidases, and affinity for the MHC Class I binding groove, which protects bound peptides from degradation. T cells with a low affinity TCR are less likely to undergo prolonged expansion than high affinity T cell clones (53, 54). A recent report described a mathematical model that helps predict T cell response basing on antigen affinity and dose (55). If broadly applicable, this model could be very helpful in determining optimal peptide dose and possibly schedule of administration. Still, in formulation with L-Tyrosine, the kinetics of Ag presentation of an individual peptide will depend on the efficiency to (dis)associate with L-Tyrosine microparticles, and how long the peptide will persist once released in vivo. Future refinements in peptide vaccine adjuvants may include formulations that are relatively insensitive to the physicochemical nature of the antigenic peptide, allowing for standardized peptide incorporation efficiency. This will facilitate the development of multi-peptide vaccines, reducing the chance of tumor escape through Ag loss (54, 55, 56). Given recent progress in the design of therapeutic vaccines, including tumor Ag selection, Ag delivery platform, and immunopotentiators, the development of effective therapeutic vaccine for cancer is accelerating. The duration of antigen presentation is an important parameter that can be controlled through vaccine formulation to drive more effective anti-cancer T cell responses for the therapy of patients with cancer.
Supplementary Material
Acknowledgments
We thank Dr. Thirumala-Devi Kanneganti from St. Jude Children's Research Hospital for generously providing the ASC knockout mice, Dr. Louis M. Vence and Yi Xiaohui for expertise and technical support on cytokine/chemokine assays.
This work was supported by an Individual Investigator Research Award RP140522 from Cancer Prevention Research Institute of Texas (CPRIT) (to WWO), Vietnam Education Foundation Fellowship (to HK), National Cancer Institute T32 training grant CA009599 (to DM), CPRIT training grant RP140106 (to CSI), Cancer Center Support Grant (CCSG) by the National Cancer Institute (P30CA16672) to the Flow Cytometry Core, CPRIT grant RP130397, NIH high-end instrument grant number 1S10OD012304-01 to the Proteomics and Metabolomics Core Facility of the University of Texas – MD Anderson Cancer Center.
Footnotes
Disclosures: The authors have no competing interests.
References
- 1.Topalian SL, Taube JM, Anders RA, Pardoll DM. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer. 2016;16:275–287. doi: 10.1038/nrc.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klebanoff CA, Acquavella N, Yu Z, Restifo NP. Therapeutic cancer vaccines: are we there yet? Immunol Rev. 2011;239:27–44. doi: 10.1111/j.1600-065X.2010.00979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348:69–74. doi: 10.1126/science.aaa4971. [DOI] [PubMed] [Google Scholar]
- 5.van der Burg SH, Arens R, Ossendorp F, van Hall T, Melief CJM. Vaccines for established cancer: overcoming the challenges posed by immune evasion. Nat Rev Cancer. 2016;16:219–233. doi: 10.1038/nrc.2016.16. [DOI] [PubMed] [Google Scholar]
- 6.Gubin MM, Zhang X, Schuster H, Caron E, Ward JP, Noguchi T, Ivanova Y, Hundal J, Arthur CD, Krebber WJ, Mulder GE, Toebes M, Vesely MD, Lam SSK, Korman AJ, Allison JP, Freeman GJ, Sharpe AH, Pearce EL, Schumacher TN, Aebersold R, Rammensee HG, Melief CJM, Mardis ER, Gillanders WE, Artyomov MN, Schreiber RD. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature. 2014;515:577–581. doi: 10.1038/nature13988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ott PA, Hu Z, Keskin DB, Shukla SA, Sun J, Bozym DJ, Zhang W, Luoma A, Giobbie-Hurder A, Peter L, Chen C, Olive O, Carter TA, Li S, Lieb DJ, Eisenhaure T, Gjini E, Stevens J, Lane WJ, Javeri I, Nellaiappan K, Salazar AM, Daley H, Seaman M, Buchbinder EI, Yoon CH, Harden M, Lennon N, Gabriel S, Rodig SJ, Barouch DH, Aster JC, Getz G, Wucherpfennig K, Neuberg D, Ritz J, Lander ES, Fritsch EF, Hacohen N, Wu CJ. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature. 2017;547:217–221. doi: 10.1038/nature22991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sahin U, Derhovanessian E, Miller M, Kloke BP, Simon P, Löwer M, Bukur V, Tadmor AD, Luxemburger U, Schrörs B, Omokoko T, Vormehr M, Albrecht C, Paruzynski A, Kuhn AN, Buck J, Heesch S, Schreeb KH, Müller F, Ortseifer I, Vogler I, Godehardt E, Attig S, Rae R, Breitkreuz A, Tolliver C, Suchan M, Martic G, Hohberger A, Sorn P, Diekmann J, Ciesla J, Waksmann O, Brück AK, Witt M, Zillgen M, Rothermel A, Kasemann B, Langer D, Bolte S, Diken M, Kreiter S, Nemecek R, Gebhardt C, Grabbe S, Höller C, Utikal J, Huber C, Loquai C, Türeci Ö. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature. 2017;547:222. doi: 10.1038/nature23003. [DOI] [PubMed] [Google Scholar]
- 9.Hailemichael Y, Dai Z, Jaffarzad N, Ye Y, Medina MA, Huang XF, Dorta-Estremera SM, Greeley NR, Nitti G, Peng W, Liu C, Lou Y, Wang Z, Ma W, Rabinovich B, Sowell Ryan T, Schluns KS, Davis RE, Hwu P, Overwijk WW. Persistent antigen at vaccination sites induces tumor-specific CD8+ T cell sequestration, dysfunction and deletion. Nat Med. 2013;19:465–472. doi: 10.1038/nm.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wheeler AW, Moran DM, Robins BE, Driscoll A. l-Tyrosine as an immunological adjuvant. Int Arch Allergy Appl Immunol. 1982;69:113–119. doi: 10.1159/000233157. [DOI] [PubMed] [Google Scholar]
- 11.Lyons AB. Analysing cell division in vivo and in vitro using flow cytometric measurement of CFSE dye dilution. J Immunol Methods. 2000;243:147–154. doi: 10.1016/s0022-1759(00)00231-3. [DOI] [PubMed] [Google Scholar]
- 12.Baldrick P, Richardson D, Wheeler AW. Review of L-tyrosine confirming its safe human use as an adjuvant. J Appl Toxicol. 2002;22:333–344. doi: 10.1002/jat.869. [DOI] [PubMed] [Google Scholar]
- 13.Ben-Sasson SZ, Hogg A, Hu-Li J, Wingfield P, Chen X, Crank M, Caucheteux S, Ratner-Hurevich M, Berzofsky JA, Nir-Paz R, Paul WE. IL-1 enhances expansion, effector function, tissue localization, and memory response of antigen-specific CD8 T cells. J Exp Med. 2013;210:491–502. doi: 10.1084/jem.20122006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, Fitzgerald KA, Latz E. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol. 2008;9:847–856. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dinarello CA, Simon A, van der Meer JWM. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov. 2012;11:633–652. doi: 10.1038/nrd3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stills HF. Adjuvants and Antibody Production: Dispelling the Myths Associated with Freund's Complete and Other Adjuvants. ILAR J. 2005;46:280–293. doi: 10.1093/ilar.46.3.280. [DOI] [PubMed] [Google Scholar]
- 17.Bijker MS, van den Eeden SJF, Franken KL, Melief CJM, van der Burg SH, Offringa R. Superior induction of anti-tumor CTL immunity by extended peptide vaccines involves prolonged, DC-focused antigen presentation. Eur J Immunol. 2008;38:1033–1042. doi: 10.1002/eji.200737995. [DOI] [PubMed] [Google Scholar]
- 18.Rizzuto GA, Merghoub T, Hirschhorn-Cymerman D, Liu C, Lesokhin AM, Sahawneh D, Zhong H, Panageas KS, Perales MA, Altan-Bonnet G, Wolchok JD, Houghton AN. Self-antigen-specific CD8+ T cell precursor frequency determines the quality of the antitumor immune response. J Exp Med. 2009;206:849–866. doi: 10.1084/jem.20081382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khong H, Overwijk WW. Adjuvants for peptide-based cancer vaccines. J Immunother Cancer. 2016;4:56. doi: 10.1186/s40425-016-0160-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cox JC, Coulter AR. Adjuvants—a classification and review of their modes of action. Vaccine. 1997;15:248–256. doi: 10.1016/s0264-410x(96)00183-1. [DOI] [PubMed] [Google Scholar]
- 21.Lindblad EB. Aluminium adjuvants—in retrospect and prospect. Vaccine. 2004;22:3658–3668. doi: 10.1016/j.vaccine.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 22.Hutchison S, Benson RA, Gibson VB, Pollock AH, Garside P, Brewer JM. Antigen depot is not required for alum adjuvanticity. FASEB J Off Publ Fed Am Soc Exp Biol. 2012;26:1272–1279. doi: 10.1096/fj.11-184556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eisenbarth SC, Colegio OR, O'Connor W, Sutterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453:112–1126. doi: 10.1038/nature06939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lamkanfi M, Dixit VM. Mechanisms and functions of inflammasomes. Cell. 2014;157:1013–1022. doi: 10.1016/j.cell.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 25.Seubert A, Monaci E, Pizza M, O'Hagan DT, Wack A. The adjuvants aluminum hydroxide and MF59 induce monocyte and granulocyte chemoattractants and enhance monocyte differentiation toward dendritic cells. J Immunol. 2008;180:5402–5412. doi: 10.4049/jimmunol.180.8.5402. [DOI] [PubMed] [Google Scholar]
- 26.Liu H, Moynihan KD, Zheng Y, Szeto GL, Li AV, Huang B, Van Egeren DS, Park C, Irvine DJ. Structure-based programming of lymph-node targeting in molecular vaccines. Nature. 2014;507:519–522. doi: 10.1038/nature12978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hanson MC, Crespo MP, Abraham W, Moynihan KD, Szeto GL, Chen SH, Melo MB, Mueller S, Irvine DJ. Nanoparticulate STING agonists are potent lymph node-targeted vaccine adjuvants. J Clin Invest. 2015;125:2532–2546. doi: 10.1172/JCI79915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mehta NK, Moynihan KD, Irvine DJ. Engineering New Approaches to Cancer Vaccines. Cancer Immunol Res. 2015;3:836–843. doi: 10.1158/2326-6066.CIR-15-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang AZ, Langer R, Farokhzad OC. Nanoparticle delivery of cancer drugs. Annu Rev Med. 2012;63:185–198. doi: 10.1146/annurev-med-040210-162544. [DOI] [PubMed] [Google Scholar]
- 30.Zhao L, Seth A, Wibowo N, Zhao CX, Mitter N, Yu C, Middelberg APJ. Nanoparticle vaccines. Vaccine. 2014;32:327–337. doi: 10.1016/j.vaccine.2013.11.069. [DOI] [PubMed] [Google Scholar]
- 31.Sharp FA, Ruane D, Claass B, Creagh E, Harris J, Malyala P, Singh M, O'Hagan DT, Pétrilli V, Tschopp J, O'Neill LAJ, Lavelle EC. Uptake of particulate vaccine adjuvants by dendritic cells activates the NALP3 inflammasome. Proc Natl Acad Sci U S A. 2009;106:870–875. doi: 10.1073/pnas.0804897106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xiang SD, Scholzen A, Minigo G, David C, Apostolopoulos V, Mottram PL, Plebanski M. Pathogen recognition and development of particulate vaccines: Does size matter? Methods. 2006;40:1–9. doi: 10.1016/j.ymeth.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 33.Salvador-Morales C, Flahaut E, Sim E, Sloan J, Green MLH, Sim RB. Complement activation and protein adsorption by carbon nanotubes. Mol Immunol. 2006;43:193–201. doi: 10.1016/j.molimm.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 34.Schwendener RA. Liposomes as vaccine delivery systems: a review of the recent advances. Ther Adv Vaccines. 2014;2:159–182. doi: 10.1177/2051013614541440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Milla P, Dosio F, Cattel L. PEGylation of proteins and liposomes: a powerful and flexible strategy to improve the drug delivery. Curr Drug Metab. 2012;13:105–119. doi: 10.2174/138920012798356934. [DOI] [PubMed] [Google Scholar]
- 36.Li K, Peers-Adams A, Win SJ, Scullion S, Wilson M, Young VL, Jennings P, Ward VK, Baird MA, Young SL. Antigen incorporated in virus-like particles is delivered to specific dendritic cell subsets that induce an effective antitumor immune response in vivo. J Immunother Hagerstown Md 1997. 2013;36:11–19. doi: 10.1097/CJI.0b013e3182787f5e. [DOI] [PubMed] [Google Scholar]
- 37.Win SJ, Ward VK, Dunbar PR, Young SL, Baird MA. Cross-presentation of epitopes on virus-like particles via the MHC I receptor recycling pathway. Immunol Cell Biol. 2011;89:681–688. doi: 10.1038/icb.2010.161. [DOI] [PubMed] [Google Scholar]
- 38.Chackerian B. Virus-like particles: flexible platforms for vaccine development. Expert Rev Vaccines. 2007;6:381–390. doi: 10.1586/14760584.6.3.381. [DOI] [PubMed] [Google Scholar]
- 39.Kushnir N, Streatfield SJ, Yusibov V. Virus-like particles as a highly efficient vaccine platform: diversity of targets and production systems and advances in clinical development. Vaccine. 2012;31:58–83. doi: 10.1016/j.vaccine.2012.10.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wheeler AW, Woroniecki SR. Immunological adjuvants in allergy vaccines: Past, present future. Allergol Int. 2001;50:295–301. [Google Scholar]
- 41.Kaech SM, Ahmed R. Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naïve cells. Nat Immunol. 2001;2:415–422. doi: 10.1038/87720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Stipdonk MJ, Lemmens EE, Schoenberger SP. Naïve CTLs require a single brief period of antigenic stimulation for clonal expansion and differentiation. Nat Immunol. 2001;2:423–429. doi: 10.1038/87730. [DOI] [PubMed] [Google Scholar]
- 43.Gett AV, Sallusto F, Lanzavecchia A, Geginat J. T cell fitness determined by signal strength. Nat Immunol. 2003;4:355–360. doi: 10.1038/ni908. [DOI] [PubMed] [Google Scholar]
- 44.van Stipdonk MJB, Hardenberg G, Bijker MS, Lemmens EE, Droin NM, Green DR, Schoenberger SP. Dynamic programming of CD8+ T lymphocyte responses. Nat Immunol. 2003;4:361–365. doi: 10.1038/ni912. [DOI] [PubMed] [Google Scholar]
- 45.Curtsinger JM, Johnson CM, Mescher MF. CD8 T Cell Clonal Expansion and Development of Effector Function Require Prolonged Exposure to Antigen, Costimulation, and Signal 3 Cytokine. J Immunol. 2003;171:5165–5171. doi: 10.4049/jimmunol.171.10.5165. [DOI] [PubMed] [Google Scholar]
- 46.Mercado R, Vijh S, Allen SE, Kerksiek K, Pilip IM, Pamer EG. Early programming of T cell populations responding to bacterial infection. J Immunol. 2000;165:6833–6839. doi: 10.4049/jimmunol.165.12.6833. [DOI] [PubMed] [Google Scholar]
- 47.Williams MA, Bevan MJ. Shortening the Infectious Period Does Not Alter Expansion of CD8 T Cells but Diminishes Their Capacity to Differentiate into Memory Cells. J Immunol. 2004;173:6694–6702. doi: 10.4049/jimmunol.173.11.6694. [DOI] [PubMed] [Google Scholar]
- 48.Prlic M, Hernandez-Hoyos G, Bevan MJ. Duration of the initial TCR stimulus controls the magnitude but not functionality of the CD8+ T cell response. J Exp Med. 2006;203:2135–2143. doi: 10.1084/jem.20060928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Blair DA, Turner DL, Bose TO, Pham QM, Bouchard KR, Williams KJ, McAleer JP, Cauley LS, Vella AT, Lefrançois L. Duration of Antigen Availability Influences the Expansion and Memory Differentiation of T Cells. J Immunol. 2011;187:2310–2321. doi: 10.4049/jimmunol.1100363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Au-Yeung BB, Zikherman J, Mueller JL, Ashouri JF, Matloubian M, Cheng DA, Chen Y, Shokat KM, Weiss A. A sharp T-cell antigen receptor signaling threshold for T-cell proliferation. Proc Natl Acad Sci U S A. 2014;111:E3679–3688. doi: 10.1073/pnas.1413726111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van Heijst JWJ, Gerlach C, Swart E, Sie D, Nunes-Alves C, Kerkhoven RM, Arens R, Correia-Neves M, Schepers K, Schumacher TNM. Recruitment of antigen-specific CD8+ T cells in response to infection is markedly efficient. Science. 2009;325:1265–1269. doi: 10.1126/science.1175455. [DOI] [PubMed] [Google Scholar]
- 52.Ruggiero RA, Bruzzo J, Chiarella P, di Gianni P, Isturiz MA, Linskens S, Speziale N, Meiss RP, Bustuoabad OD, Pasqualini CD. Tyrosine Isomers Mediate the Classical Phenomenon of Concomitant Tumor Resistance. Cancer Res. 2011;71:7113–7124. doi: 10.1158/0008-5472.CAN-11-0581. [DOI] [PubMed] [Google Scholar]
- 53.Zehn D, Lee SY, Bevan MJ. Complete but curtailed T-cell response to very low-affinity antigen. Nature. 2009;458:211–214. doi: 10.1038/nature07657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gronski MA, Boulter JM, Moskophidis D, Nguyen LT, Holmberg K, Elford AR, Deenick EK, Kim HO, Penninger JM, Odermatt B, Gallimore A, Gascoigne NRJ, Ohashi PS. TCR affinity and negative regulation limit autoimmunity. Nat Med. 2004;10:1234–1239. doi: 10.1038/nm1114. [DOI] [PubMed] [Google Scholar]
- 55.Lever M, Lim HS, Kruger P, Nguyen J, Trendel N, Abu-Shah E, Maini PK, van der Merwe PA, Dushek O. Architecture of a minimal signaling pathway explains the T-cell response to a 1 million-fold variation in antigen affinity and dose. Proc Natl Acad Sci. 2016;113:E6630–E6638. doi: 10.1073/pnas.1608820113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Walter S, Weinschenk T, Stenzl A, Zdrojowy R, Pluzanska A, Szczylik C, Staehler M, Brugger W, Dietrich PY, Mendrzyk R, Hilf N, Schoor O, Fritsche J, Mahr A, Maurer D, Vass V, Trautwein C, Lewandrowski P, Flohr C, Pohla H, Stanczak JJ, Bronte V, Mandruzzato S, Biedermann T, Pawelec G, Derhovanessian E, Yamagishi H, Miki T, Hongo F, Takaha N, Hirakawa K, Tanaka H, Stevanovic S, Frisch J, Mayer-Mokler A, Kirner A, Rammensee HG, Reinhardt C, Singh-Jasuja H. Multipeptide immune response to cancer vaccine IMA901 after single-dose cyclophosphamide associates with longer patient survival. Nat Med. 2012;18:1254–1261. doi: 10.1038/nm.2883. [DOI] [PubMed] [Google Scholar]
- 57.Bai XF, Liu J, Li O, Zheng P, Liu Y. Antigenic drift as a mechanism for tumor evasion of destruction by cytolytic T lymphocytes. J Clin Invest. 2003;111:1487–1496. doi: 10.1172/JCI17656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Melero I, Gaudernack G, Gerritsen W, Huber C, Parmiani G, Scholl S, Thatcher N, Wagstaff J, Zielinski C, Faulkner I, Mellstedt H. Therapeutic vaccines for cancer: an overview of clinical trials. Nat Rev Clin Oncol. 2014;11:509–524. doi: 10.1038/nrclinonc.2014.111. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.