Abstract
Background
Flavoring chemicals, or flavorants, have been used in electronic cigarettes (e-cigarettes) since their inception; however, little is known about their toxicological effects. Free radicals present in e-cigarette aerosols have been shown to induce oxidative stress resulting in damage to proliferation, survival, and inflammation pathways in the cell. Aerosols generated from e-liquid solvents alone contain high levels of free radicals but few studies have looked at how these toxins are modulated by flavorants.
Objectives
We investigated the effects of different flavorants on free radical production in e-cigarette aerosols.
Methods
Free radicals generated from 49 commercially available e-liquid flavors were captured and analyzed using electron paramagnetic resonance (EPR). The flavorant composition of each e-liquid was analyzed by gas chromatography mass spectroscopy (GCMS). Radical production was correlated with flavorant abundance. Ten compounds were identified and analyzed for their impact on free radical generation.
Results
Nearly half of the flavors modulated free radical generation. Flavorants with strong correlations included β-damascone, δ-tetradecalactone, γ-decalactone, citral, dipentene, ethyl maltol, ethyl vanillin, ethyl vanillin PG acetal, linalool, and piperonal. Dipentene, ethyl maltol, citral, linalool, and piperonal promoted radical formation in a concentration-dependent manner. Ethyl vanillin inhibited the radical formation in a concentration dependent manner. Free radical production was closely linked with the capacity to oxidize biologically-relevant lipids.
Conclusions
Our results suggest that flavoring agents play an important role in either enhancing or inhibiting the production of free radicals in flavored e-cigarette aerosols. This information is important for developing regulatory strategies aimed at reducing potential harm from e-cigarettes.
Keywords: electronic cigarette, e-cigarettes, e-cig, free radicals, oxidative stress, flavors, flavorants
Graphical Abstract
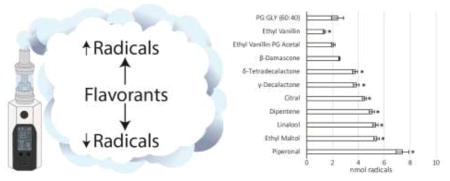
Introduction
Since their inception, electronic cigarettes (e-cigarettes) have been sold and marketed with flavored e-liquids; however, little is known regarding the products formed by these flavoring additives when heated at the high temperatures found in e-cigarettes. Many of the flavoring chemicals, or flavorants, found in these liquids are “generally recognized as safe” (GRAS) when consumed orally (US FDA 21CFR 182.1320); however, the thermal breakdown of these compounds in e-cigarette aerosols has yet to be fully evaluated, particularly in a toxicological context. In fact, the organization responsible for certifying food-safe flavorings for the FDA, the Flavor Extracts Manufacturers Association (FEMA), has specifically stated that they do not evaluate flavor ingredients for use in e-cigarettes or any other exposures other than ingestion.[1]
The development of many tobacco-related diseases, such as cardiovascular disease, chronic obstructive pulmonary disease (COPD), and cancer are all thought to be influenced or induced by oxidative stress and oxidative damage.[2–5] Oxidative stress can be induced by reactive oxygen species (ROS) and reactive nitrogen species (RNS), of which free radicals are a major constituent.[6] In 2010, the Surgeon General released a report in which it identified free radical induced oxidative stress from tobacco smoke as being a contributor to the development of smoking-related diseases.[7] Free radicals are found in high concentrations in cigarette smoke (>1016 molecules/puff).[8–10] Similarly, previous studies done by our lab and others have shown relatively high levels of reactive free radicals in e-cigarette aerosols (>1013 molecules/puff) by electron paramagnetic resonance (EPR).[11–14] We found that free radical generation was highly dependent on the propylene glycol content of the e-cigarette liquid. [14] In addition to free radicals, a number of other studies have also found an assortment of other toxic agents in e-cigarette aerosols including nitrosamines, heavy metals, diethylene glycol, and reactive organic compounds such as formaldehyde and acetaldehyde.[15–21]
Flavoring additives represent an important component of tobacco products as they have been shown to directly influence tobacco product preference and use, and have historically been used to attract younger consumers.[22–26] Despite banning characterizing flavors (fruits, candy, etc.) in cigarettes in the 2009 Family Smoking Prevention and Tobacco Control Act, flavorings are still utilized in virtually all other tobacco products, including e-cigarettes.[23, 27] Recent studies, including the Population Assessment of Tobacco and Health (PATH) Study, reported that flavor was the primary reason for using a particular tobacco product among youth and young adults.[28, 29] Nationwide, a survey of young adults reported that their first and usual e-cigarette flavor was something other than tobacco flavored.[29] Preferences for non-tobacco flavored e-cigarettes have also been seen in adults as a recent study found that over 75% of adult users of e-cigarettes preferred flavors other than tobacco for their e-liquids.[30] While the popularity of flavored e-cigarette products continues to grow, the potential harms from these flavoring additives remains largely unknown.
To date, only a handful of studies have examined the toxicity of specific flavorants. Specifically, exposure to cinnamaldehyde, 2-methoxycinnamaldehyde, and diacetyl have been shown to cause cytotoxicity at concentrations typically found in e-cigarette liquids.[31–34] The flavorants acetoin and maltol also appear to be potent inducers of inflammation.[35] A wide variety of volatile organic compounds have been identified in both in flavored e-cigarette liquids and their aerosols.[36] More recently, benzene has been shown to form as a result of the thermal decomposition of benzaldehyde, a natural fruit flavorant common in many e-liquid flavors.[37] Benzaldehyde has also been shown to cause respiratory airways irritation in animal exposure studies.[34] Another study found that toxic aldehydes are produced primarily from the decomposition of flavor compounds during vaping. Altogether, these studies suggest that flavor compounds may play an important role in the potential toxicity of e-cigarettes.[38]
While the effects that e-cigarette operating parameters and e-cigarette solvents have only recently been investigated with respect to the delivery of toxins, the effect that e-cigarette flavoring additives have on the generation of these toxic compounds remains largely unknown. Of the studies performed that specifically address this topic of flavorants, only a few compounds have been identified as being harmful.[31, 34, 37] While many of these studies demonstrated cytotoxic effects of various flavorants, there have been no studies that have looked at the effects of flavorants on the generation of free radicals in e-cigarettes. Thus, in this study, we systematically evaluated the free radical generation of forty-nine commercially available, nicotine-free e-liquid flavor concentrates in e-cigarette aerosols. We also identified the individual flavorants found in the e-liquids and evaluated the effects of ten specific flavorants on free radical generation.
Materials and Methods
E-cigarette, coil, and atomizer tank
The e-cigarette used for this study was a Wismec Reuleaux RX200S Mod (MyVaporStore.com) in temperature control mode. Three high amperage Samsung INR18650-25R, 2500mAh, 3.7v batteries were used to power the device. The batteries were recharged after 250 puffs were performed using the device. The heating element used was a commercially available 0.5 Ω stainless steel coil (SS316) Uwell Crown Coil (MyVaporStore.com). The atomizer tank used had a capacity of 4 mL and was composed of stainless steel and glass (Uwell Crown Tank; MyVaporStore.com).
Reagents
Arachidonic acid (AA), cis-4,7,10,13,16,19-docosahexaenoic acid (DHA), cis-5,8,11,14,17-eicosapentaenoic acid (EPA), glycerol (GLY), hexane, phenyl-N-tert-butylnitrone (PBN), propylene glycol (PG), 2,2,6,6-tetramethyl-1-piperidinyloxyl (TEMPO), and tert-benzene, and tris(hydroxymethyl)aminomethane hydrochloride (TRIS-HCl) were purchased from Sigma-Aldrich (St. Louis, MO) and used as received.
E-cigarette flavor concentrates and flavorants
A commercially available kit containing forty-nine popular nicotine free e-liquid flavor concentrates was purchased from NicVape.com (Spartanburg, SC). Food grade certified flavorants (β-damascone, δ-tetradecalactone, γ-decalactone, citral, dipentene, ethyl maltol, ethyl vanillin, linalool, and piperonal) were purchased from Sigma-Aldrich. Food grade certified ethyl vanillin propylene glycol acetal was obtained from Vigon (East Stroudsburg, PA).
Profiling of Flavorants
E-liquid compositions were analyzed as described previously by gas chromatography-mass spectrometry (GCMS).[39] Using a Gerstel MPS2 multipurpose autosampler (Gerstel GmbH & Co. KG. Mülheim an der Ruhr, Germany), samples (1 μL) were introduced and split 50:1 into an Agilent Technologies 7890A gas chromatograph (Agilent Technologies, Inc., Santa Clara, CA) with an Agilent 5975C mass selective detector. The GC inlet was a silanized glass straight design inlet liner (78.5 mm long × 6.5 mm o.d. × 0.75 mm i.d.) (Supelco, Bellefonte, PA) and the column was an Agilent J&W VF-35ms capillary column (60 m × 0.25 mm × 0.25 μm) with helium (Airgas) as the carrier gas. The inlet and MS source were both maintained at 280°C. The temperature profile consisted of: injection at 50°C with a 2 min hold, a linear increase of 10°C/min to 240°C, and an isothermal hold at 240°C for 10 min. The MS was set to a scan of 30–300 amu. Chromatogram peaks were analyzed using Mass Hunter Qualitative Analysis B.06.00, software and chemical identities were found by library searching against the NIST11 EI mass spectral database. Quantitation of specific chemicals was done using external standards dissolved in propylene glycol and run using the same method.
E-cigarette apparatus
The e-cigarette setup used here was similar to that used in our previous study.[14] In brief, the e-cigarette’s fire button was activated by a 12 V relay timer switch (SainSmart; Amazon.com) and a second relay switch was connected to a 12 VDC solenoid valve (RioRand; Amazon.com). Upstream and downstream ends of the solenoid valve were connected to of the solenoid valve were connected an impinger and a flow meter respectively. The flow meter was connected to the house vacuum and adjusted to a flow rate of 500 mL/min. A diagram of this setup is shown in Supplemental Figure 1.
Solvent components, temperature, and wattage factors
To investigate the thermal degradation of flavors, e-liquid flavor concentrates were diluted, per the manufacturer’s instructions, to 20% in a mixture of PG and GLY for a final PG:GLY ratio of 60:40 (v:v). To investigate the thermal degradation of individual flavorants, each flavorant was dissolved in a PG:GLY mixture (60:40, PG:GLY) at similar concentrations to those found in the e-liquid flavors. All experiments were conducted using the e-cigarette device in constant temperature mode set at 225°C and 50W.
Generation of e-cigarettes aerosols
Aerosols were generated under normal laboratory conditions using puffing parameters previously used to resemble typical usage.[11, 40] Puffs were simulated using the following puffing topography: puff duration, 5 seconds; interpuff interval, 30 seconds; flow rate, 500 mL/min; and number of puffs, 40.
Spin trapping of free radicals in e-cigarette aerosols
Free radicals in e-cigarette aerosols were trapped and quantified as previously reported.[14] In brief, e-cigarette aerosols were passed through a 25 mL impinger containing 6 mL of 0.05 M PBN in hexane. The nitrone spin trap, PBN, has been used extensively for the detection of radical species in cigarette smoke.[10, 11, 41] Hexane was evaporated and the remaining residue was reconstituted in 500 μL of tert-benzene. High purity quartz EPR tubes were filled with 200 μL of the reconstituted tert-benzene solution and deoxygenated using a freeze-pump-thaw technique with a Schlenk line.[42] Samples were deoxygenated as described previously.[11] In brief, the samples received three freeze-pump-thaw argon cycles before being blanketed with gaseous argon.
EPR measurements
The spectra derived from PBN radical adducts was measured using a Bruker eScan R spectrometer (Bruker-Biospin, Billerica, MA) operating in X-band. The EPR parameters were as follows: microwave frequency, 9.7 GHz; modulation frequency, 86.0 kHz; microwave power, 6.00 mW; scan range, 60G; modulation amplitude, 2.04 G; sweep time, 5.24 s; time constant, 10.24 ms; and conversion time, 10.24 ms. All measurements were carried out at room temperature (22 ± 1°C). The spin quantification of the radical signals obtained was performed in MatLab. Each spectrum was processed automatically to produce a double integral. In the process, point-based-spline baseline correction was applied to the absorption data (first integral) prior calculation of the second integral. Conversion factors from double integral values to spin concentrations we obtained from the known concentrations of a stable radical standard, TEMPO.[43]
Lipid peroxidation analysis
Lipid peroxidation studies were performed as previously reported.[14] In brief, 6 mL of 50 μg/mL AA, DHA, and EPA in 0.1 M TRIS-HCl (pH 7.4) was added to an impinger. Flavorants were then vaped as they had been done for the EPR measurements with the aerosol passing through the impinger containing the AA, DHA, and EPA. The impinger solution was then analyzed for secondary lipid oxidation products using a thiobarbituric acid reactive substances (TBARS) assay kit (Cayman, Ann Arbor, MI). The samples were also analyzed for 8-isoprostane formation using an 8-isoprostane enzyme-linked immunosorbent assay (ELISA) (Eagle Biosciences, Inc., Nashua, NH). Both TBARS and 8-isoprostane levels were compared to controls that received the same puffing treatment but in the absence of the e-cigarette to remove the effect of bubbling as a variable.
Data analysis
All measurements were done in triplicate and free radical concentrations were compared to the flavor-free PG:GLY (60:40) base using a one-way ANOVA and Dunnett’s multiple comparison for the flavor samples via GraphPad Prism (San Diego, CA). Comparison of the free radical concentrations for the individual flavorants were done using a one-way ANOVA and Tukey’s multiple comparison to compare the effects of different concentrations and the base (60:40). Comparisons for the TBARS and 8-isoprostane measurements were done using a one-way ANOVA and Dunnett’s multiple comparison to compare all samples to the base (60:40). Significant differences were identified at the p<0.05 level. Pearson’s correlation values for each chemical’s peak area and the flavor’s free radical content were determined using the SciPy statistical packages for Python.[44, 45]
Results
Flavor effects on radical production
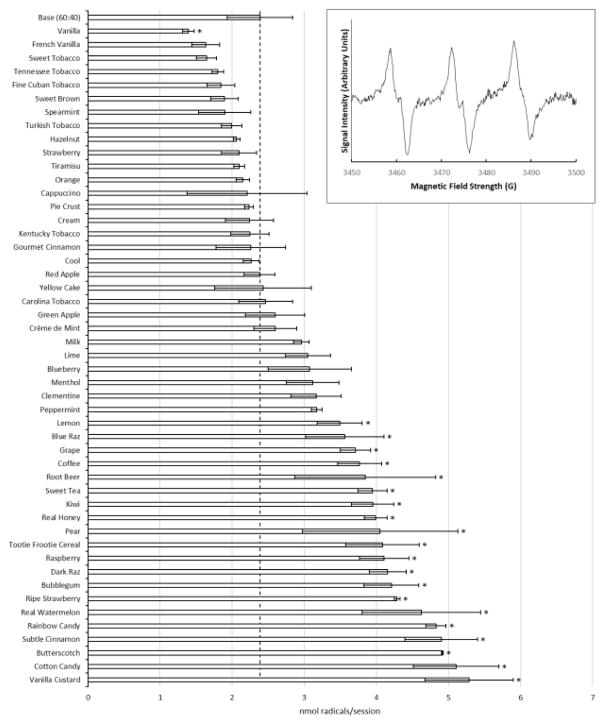
The impact of adding flavoring agents to PG:GLY base on radical production was examined with 49 different flavors. Overall, radical production varied widely across the different flavored e-liquids tested (Figure 1). Nearly 43% of the flavors resulted in significant increases in radical production as compared to the base PG:GLY (60:40) mixture. Significant increases (p<0.05) in radical production were observed for Lemon (46%), Blue Raz (49%), Grape (56%), Coffee (58%), Root Beer (61%), Sweet Tea (65%), Kiwi (66%), Real Honey (67%), Pear (70%), Tootie Frootie Cereal (71%), Raspberry (72%), Dark Raz (74%), Bubblegum (76%), Ripe Strawberry (80%), Real Watermelon (94%), Rainbow Candy (102%), Subtle Cinnamon (105%), Butterscotch (106%), Cotton Candy (114%), Vanilla Custard (122%). Interestingly, significant reductions in radical production below baseline were observed as a result of adding Vanilla flavoring. Direct interactions with PBN, or autoxidation, with the flavors were measured and accounted for less than 7% of the radicals seen in the vaped samples. (Supplemental Figure 2)
Figure 1.
The effects of different commercially available e-liquid flavor concentrates on radical production. Asterisks (*) represent a statistical difference (p<0.05) from the base PG:GLY (60:40) mixture. Inset graph is a representative spectra showing the base PG:GLY (60:40).
A six-line spectrum is typically associated with PBN adducts, the overmodulation of our spectra results in a broader three-line spectra. (Figure 1) Overmodulation can cause peak broadening which distorts the PBN’s characteristic six-line spectra. This overmodulation was done intentionally in order to reliably quantitate and detect low level concentrations of radicals. The six-line spectrum can be seen with more concentrated samples at lower modulation amplitudes. (Supplemental Figure 6)
Flavor concentrate chemical composition, correlation, and concentration
Among the forty-nine flavor concentrates analyzed by GCMS, nearly 300 unique chemicals were identified. Of these 10% were found in 5 or more flavor concentrates and 3% in 10 or more concentrates. Ethyl vanillin PG acetal and ethyl maltol were the two most common flavoring additives occurring in more than 45% of flavors. In order to identify specific flavorants that might impact free radical production, relative abundance of the different flavorants in each e-liquid concentrate was correlated with radical production. The 10 most highly correlated flavor compounds (positively or negatively) were chosen for further analysis. (Table 1) Ethyl vanillin PG acetal, ethyl vanillin, β-damascone, and δ-tetradecalactone were chosen as they exhibited negative correlations suggesting they may inhibit radical production. Conversely, γ-decalactone, neral, ethyl maltol, piperonal, d-limonene, and linalool were chosen as they exhibited the strongest positive correlations suggesting they contribute to increased radical production. The concentrations of these flavorants in their e-liquid flavor concentrates were then determined using GCMS from standards and used as a range for further testing. (Table 1)
Table 1.
Flavorants identified in the e-liquids.
| Chemical | n | Pearson’s a Correlation | p Value | Flavors Containing | Concentration b Ranges (mg/mL) |
|---|---|---|---|---|---|
| Ethyl vanillin PG acetal | 50 | −0.2243 | 0.1173 | Blue Raz, Bubblegum, Butterscotch, Cream, French Vanilla, Hazelnut, Raspberry, Root Beer, Sweet Tobacco, Vanilla, Vanilla Custard | 0.42 – 113.12 |
| Ethyl vanillin | 50 | −0.0943 | 0.5146 | Blue Raz, Bubblegum, Cream, Hazelnut, Sweet Tea | 0.62 – 3.98 |
| β-Damascone | 50 | −0.1781 | 0.2159 | Kentucky Tobacco, Sweet Tea, Sweet Tobacco | 0.02 – 1.50 |
| δ-Tetradecalactone | 50 | −0.1795 | 0.2123 | Cream, Milk, Tootie Frootie Cereal, Vanilla, Vanilla Custard | 0.99 – 9.30 |
| Linalool | 50 | 0.1769 | 0.2190 | Blue Raz, Bubblegum, Clementine, Lime, Orange, Pear, Rainbow Candy, Raspberry, Subtle Cinnamon, Sweet Tea, Tootie Frootie Cereal | 4.78 – 22.80 |
| D-Limonene | 50 | 0.2359 | 0.0990 | Bubblegum, Clementine, Kiwi, Lemon, Lime, Orange, Rainbow Candy, Real Watermelon, Root Beer, Spearmint, Tootie Frootie Cereal | 0.16 – 76.52 |
| Piperonal | 50 | 0.3336 | 0.0179 | Butterscotch, Cream, French Vanilla, Raspberry, Real Honey, Sweet Tea, Vanilla, Vanilla Custard, Yellow Cake | 0.01 – 7.52 |
| Ethyl maltol | 50 | 0.4235 | 0.0022 | Blue Raz, Cotton Candy, French Vanilla, Grape, Kiwi, Raspberry, Real Honey, Real Watermelon, Ripe Strawberry, Root Beer, Strawberry, Sweet Tobacco, Tennessee Tobacco, Vanilla Custard, Yellow Cake | 1.19 – 61.23 |
| Neral | 50 | 0.2857 | 0.0443 | Bubblegum, Lime, Rainbow Candy, Tootie Frootie Cereal | 3.89 – 21.83 |
| γ-Decalactone | 50 | 0.3284 | 0.0199 | Blue Raz, Butterscotch, Kiwi, Pear, Rainbow Candy, Raspberry, Ripe Strawberry, Tootie Frootie Cereal | 0.44 – 8.16 |
The Pearson’s correlation values compare the area under the curve for each peak to the free radical concentration for that flavor.
The concentration ranges are those found in the undiluted e-liquid flavor concentrates.
Flavorant effects on radical production
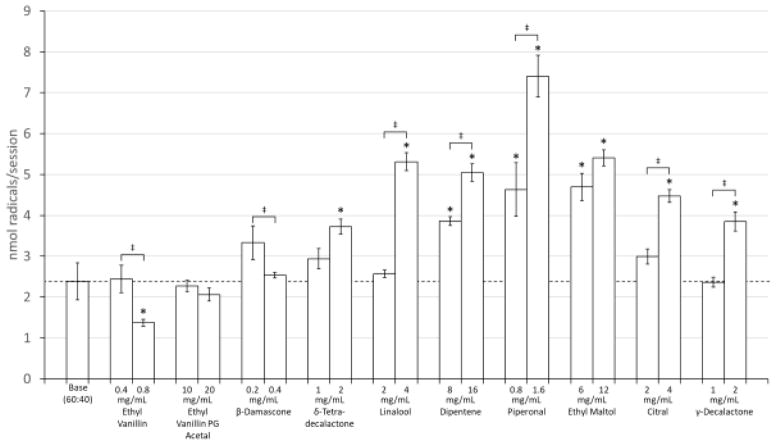
The flavorants selected above were tested individually by adding to PG:GLY (60:40) mixtures within the concentration ranges found in the commercially obtained flavors and then diluted to 20% with additional PG:GLY (60:40) in order to match the final concentration ranges of the commercial e-liquids. The results show that free radical generation can either be increased or decreased as a result of flavorant additions. (Figure 2) The addition of ethyl vanillin significantly decreased radical production by 42% from baseline at the 0.8 mg/mL concentration. While ethyl vanillin PG acetal showed a slight decrease (14%) in radical production at 20 mg/mL, the effect was not significant. β-Damascone also showed a small but not significant decrease. δ-tetradecalactone at 2 mg/mL showed a significant increase (56%) in radical production. Linalool, at a concentration 4 mg/mL, significantly increased radical production (122%) as compared to the baseline and was significantly higher than the 2 mg/mL concentration. Dipentene, a mixture of isomers including d-limonene, also produced significant 62–112% increases in radical production at both the 8 mg/mL and 16 mg/mL concentrations and both values were significantly higher than baseline and significantly different from one another. The addition of 0.8 mg/mL and 1.6 mg/mL piperonal resulted in significant 94% and 210% increases in radical production, respectively, as compared to the baseline and were significantly different from each other. Radical production also increased significantly with increasing concentrations of ethyl maltol (97% at 6 mg/mL and 127% at 12 mg/mL and citral, a mixture of both neral and geranial, in a concentration dependent fashion (26% at 2 mg/mL and 88% at 4 mg/mL). Higher concentrations of γ-decalactone (2 mg/mL) significantly increased radical production by 61% above baseline. Direct interactions with PBN, or autoxidation, with the flavorants were measured and accounted for less than 6% of the radicals seen in the vaped samples. (Supplemental Figure 3)
Figure 2.
Concentration dependent flavorant effects on radical production. Asterisks (*) represent a statistical difference (p<0.05) from the base PG:GLY (60:40) mixture. ‡ represents a statistical difference (p<0.05) between the chemical’s concentrations.
Flavorant effects on lipid peroxidation
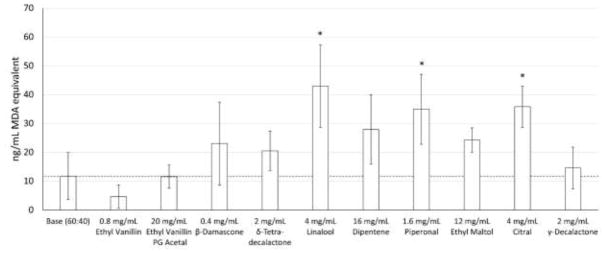
The flavorants were then used to examine their potential for modulating lipid peroxidation products using malondialdehyde (MDA) formation as a marker. (Figure 3) Linalool (4 mg/mL), piperonal (1.6 mg/mL), and citral (4 mg/mL) all resulted in significant increases (257%, 197%, and 205% respectively) of lipid peroxidation products. The addition of ethyl vanillin (0.8 mg/mL) reduced lipid peroxidation by 60% from baseline; however, the decrease was not significant. Ethyl vanillin PG acetal (20 mg/mL) showed a mild decrease (2%) but this change was not significant. β-Damascone (0.4 mg/mL), dipentene (16 mg/mL), ethyl maltol (12 mg/mL), γ-decalactone (2 mg/mL), and δ-tetradecalactone (2 mg/mL) all showed mild increases in lipid peroxidation (96%, 139%, 106%, 24%, and 75% respectively); however, the changes were not significant. The effects of direct interactions with the un-vaped flavorants on TBARS formation were measured and accounted for less than 7% of the TBARS formation seen in the vaped samples. (Supplemental Figure 4)
Figure 3.
Flavor chemical effects on lipid (AA, DHA, and EPA) peroxidation. Asterisks (*) represent a statistical difference (p<0.05) from the base PG:GLY (60:40) mixture.
Flavorant effects on 8-isoprostane formation
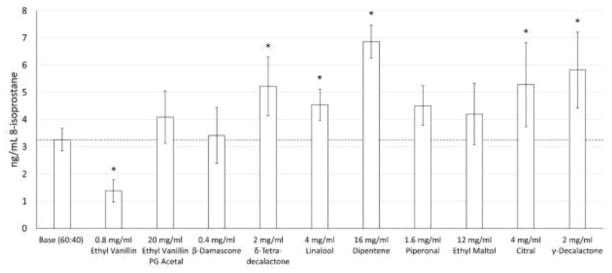
The flavorants’ biological impact on the oxidation of AA and subsequent formation of its oxidation product, 8-isoprostane, were then examined. (Figure 4) δ-Tetradecalactone (2 mg/mL), linalool (4 mg/mL), dipentene (16 mg/mL), citral (4 mg/mL) and γ-decalactone (2 mg/mL) resulted in significant increases in 8-isoprostane formation (60%, 39%, 111%, 62% and 78%, respectively). Ethyl vanillin PG acetal (20 mg/mL), piperonal (1.6 mg/mL), ethyl maltol (12 mg/mL), and β-damascone (0.4 mg/mL) showed a mild increases in 8-isprostatane formation (25%, 38%, 29%, and 5%, respectively), but these changes were not significant. The effects of direct interactions with the un-vaped flavorants on 8-isoprostane formation were measured and accounted for less than 12% of the 8-isoprostane formation seen in the vaped samples. (Supplemental Figure 5)
Figure 4.
Flavor chemical effects on AA oxidation to 8-isoprostane. Asterisks (*) represent a statistical difference (p<0.05) from the base PG:GLY (60:40) mixture.
Discussion
In our study, we found that flavorants in e-liquids can have a direct impact on the formation of highly reactive free radicals. Overall, aerosols from 49 different flavored e-liquids showed nearly an 8-fold range in radical production. Since conditions such as temperature and PG:GLY ratios were held constant, the differences in radical production could be directly attributed to the flavorants. As flavor descriptors are largely subjective, and the chemical composition of e-liquids is proprietary, we took the approach of identifying the specific flavorants in each flavored e-liquid by GCMS. Using these data, we performed correlational analyses between the relative abundance of each flavorant in and their corresponding free radical output. Using this approach, we selected 10 specific flavorants that had the strongest correlation (positive or negative) in terms of radical production for further investigation. It should be noted that future studies should address whether other flavorants also contribute to radical production.
Of the 10 flavorants examined, 7 (ethyl vanillin, δ-tetradecalactone, linalool, dipentene, piperonal, ethyl maltol, citral, and γ-decalactone) were found to significantly modulate the generation of radicals in e-cigarette aerosols. Three of the highest radical producing flavorants (linalool, dipentene, and citral) are non-phenolic terpenes which are found in a number of different plants. The addition of each of these compounds yielded a concentration-dependent increase in radical production, suggesting that they contribute to radical formation. Many of these non-phenolic terpenes are used heavily in the fragrance, food, and beverage industries to add floral and citrus aromas and flavors.[46] Several studies have found that some of these compounds can undergo autoxidation to form hydroperoxides, which have been linked to allergic contact dermatitis in some people.[47–49] Focusing specifically at the hydroperoxides formed from linalool and d-limonene, one study found that that these hydroperoxides can rapidly degrade into a number of different allyloxyl and carbon-centered radicals all of which reacted readily with histidine amino acids in vitro.[48] These studies seem to explain a possible route for the increase in radical generation observed in our studies and suggest that the addition of non-phenolic terpene flavor compounds should be investigated further.
Ethyl maltol, a common flavoring ingredient used to impart sweet and caramel-like aromas to fragrances, foods, and beverages, also produced a concentration dependent increase in radicals.[46] Ethyl maltol and maltol have both been shown to interact with iron or copper to produce a number of different hydroxypyranone complexes that can promote the generation of radicals.[50, 51] These complexes by themselves have also been shown to be toxic in animal models.[52] Trace amounts of iron or copper may be present in the e-liquid solvent, wick, or coil and in turn be responsible for the formation of these complexes and their subsequent radical generation. This is also of concern as these compounds may react with biologically available iron or copper. A recent study looking at inflammatory responses in lung cells found that maltol increased inflammatory cytokines iterleukin-8 (IL-8) and tumor necrosis factor alpha (TNF-α) production and decreased barrier function in human bronchial epithelial (Beas2B) and human lung fibroblasts (HFL-1) cell lines.[35]
Lactones, such as δ-tetradecalactone and γ-decalactone, are a group of flavorants composed of intramolecular hydroxy fatty acid esters and used to impart fruity and creamy notes to fragrances, foods and beverages.[46] Radical production from some lactones has been reported and specific radical products have been identified.[53] Unfortunately, radicals formed by γ-decalactone or δ-tetradecalactone have still yet to be investigated.
The addition of piperonal also increased radical generation in the e-cigarette aerosols in a concentration dependent manner. Piperonal is an aromatic aldehyde that is used to add cherry and vanilla like properties to fragrances, foods, and beverages.[46] Despite the increase in radical generation in the aerosols we observed, there has been little research done looking at the oxidative properties of piperonal.
An interesting finding from our study was that certain flavorants may actually inhibit the formation of radicals. Ethyl vanillin PG acetal and ethyl vanillin, both used in fragrances, foods, and beverages to impart a vanilla characteristic, showed similar inhibitions of radicals. While the decrease in radicals by ethyl vanillin PG acetal was not significant, decreases observed with higher concentrations of the unacetalated ethyl vanillin were significant. This differential effect may indicate that the aldehyde group present in ethyl vanillin but not in ethyl vanillin PG acetal may play a role in its antioxidant potential. A recent study found that ethyl vanillin and vanillin both can act as a strong antioxidants in vitro and in vivo further suggesting a role in radical inhibition.[54] This study also suggests the importance of the aldehyde group found on both ethyl vanillin and vanillin as the antioxidant properties were not observed with vanillyl alcohol or vanillic acid, both of which lack the aldehyde group.[54] The radical inhibition effects of ethyl vanillin suggest its possible use an additive in e-liquids reduce free radical production during aerosol formation. Further tests will be needed to determine if there are any toxic compounds formed during the aerosolizing process of e-liquids.
While no significant differences were seen compared to the base for β-damascone, a flavorant used to add floral aromas to fragrances, foods, and beverages, the trend suggests a decrease in radical formation. β-damascone is known to be a nucleophilic Michael addition acceptor capable of scavenging radicals by reacting with nucleophiles and has been found to decrease inflammatory cytokine gene expressions of interleukin-10 (IL-10) and TNF-α in human colon epithelial cells (T84).[55]
In order to assess the potential biological impact of free radicals produced from e-cigarettes, we assessed their reactivity with biologically relevant lipids in vitro. Free radicals are highly reactive with polyunsaturated fatty acids resulting in the initiation of lipid peroxidation.[56] Thiobarbituric acid-reactive substances (TBARS) are a well-established byproduct of the reaction of free radicals with biologically-relevant unsaturated lipids such as AA, DHA, and EPA. Hence, TBARS have long been used as a marker of oxidative stress in blood and tissues and, in a recent study, elevated levels of TBARS were found in the lung homogenate of mice exposed to e-cigarette aerosols[12] Free radicals are also responsible for promoting the oxidation of AA to form 8-isoprostane, a well-characterized marker of oxidative stress.[57] The finding of increased levels of lung TBARS and plasma 8-isoprostane in vivo provide evidence that the e-cigarette-derived radicals may have a biological impact.[58] Here we show that direct exposure of e-cigarette aerosols to biologically relevant lipids resulted in the production of both TBARS and 8-isoprostane. Further, we observed TBARS and 8-isoprostane were elevated with addition of flavorants which enhance free radical production including linalool, piperonal, and citral for TBARS and δ-tetradecalactone, linalool, dipentene, citral, and γ-decalactone for 8-isoprostane. Conversely, addition of ethyl vanillin, which decreased free radical production, also decreased both TBARS and 8-isoprostane formation. These findings suggest a tight linkage between free radical exposure and the oxidation of AA, DHA, and EPA, highlighting the potential toxicological importance of e-cigarette-derived free radicals
Overall, our results demonstrate that flavorants can modulate radical production in e-cigarette aerosols. While our study did not look into all of the over 300 chemicals found within the 49 flavors tested or in the other flavored e-liquids on the market, we did find significant correlations between certain common flavorants and their effects on radical generation. Specifically, we found that linalool, dipentene, piperonal, ethyl maltol and citral, all appear to promote radical production in a concentration dependent manner suggesting these flavorant additions may pose more oxidative harm to the e-cigarette user. Conversely, we also found that ethyl vanillin and, to a lesser extent, β-damascone appear to block radical production in a concentration dependent manner suggesting that adding these flavorants may in fact reduce oxidative harm for the consumer. More research will be needed to further investigate these flavorants and the products they form when heated and aerosolized.
Supplementary Material
Highlights.
Flavoring chemicals can modulate the production of free radicals in e-cigarettes
Citral, dipentene, ethyl maltol, linalool, and piperonal all promote radical formation
Ethyl vanillin dose-dependently inhibits radical delivery
Flavorants can modulate lipid peroxidation induced by e-cigarette aerosols
Acknowledgments
Funding Sources
This work was supported in part by the National Institute on Drug Abuse of the National Institutes of Health and the Center for Tobacco Products of the U.S. Food and Drug Administration (under Award Number P50-DA-036107). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Food and Drug Administration.
Abbreviations
- AA
arachidonic acid
- COPD
chronic obstructive pulmonary disease
- DHA
cis-4,7,10,13,16,19-docosahexaenoic acid
- ELISA
enzyme-linked immunosorbent assay
- EPA
cis-5,8,11,14,17-eicosapentaenoic acid
- EPR
electron paramagnetic resonance
- GLY
glycerol
- PBN
phenyl-N-tert-butylnitrone
- PG
propylene glycol
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- TBARS
thiobarbituric acid reactive substances
- TEMPO
2,2,6,6-tetramethyl-1-piperidinyloxyl
- TRIS-HCl
tris(hydroxymethyl)aminomethane hydrochloride
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Flavor and Extract Manufacturers Association. [Accessed April 25 2017];Safety assessment and regulatory authority to use flavors—focus on e-cigarettes. 2013 https://www.femaflavor.org/safety-assessment-and-regulatory-authority-use-flavors-focus-electronic-nicotine-delivery-systems.
- 2.Pryor WA. Cigarette smoke radicals and the role of free radicals in chemical carcinogenicity. Environ Health Perspect. 1997;105(Suppl 4):875. doi: 10.1289/ehp.97105s4875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Messner B, Bernhard D. Smoking and cardiovascular disease mechanisms of endothelial dysfunction and early atherogenesis. Arterioscler Thromb Vasc Biol. 2014;34(3):509–515. doi: 10.1161/ATVBAHA.113.300156. [DOI] [PubMed] [Google Scholar]
- 4.Dekhuijzen P. Antioxidant properties of N-acetylcysteine: their relevance in relation to chronic obstructive pulmonary disease. Eur Respir J. 2004;23(4):629–636. doi: 10.1183/09031936.04.00016804. [DOI] [PubMed] [Google Scholar]
- 5.MacNee W, Rahman I. Is oxidative stress central to the pathogenesis of chronic obstructive pulmonary disease? Trends Mol Med. 2001;7(2):55–62. doi: 10.1016/s1471-4914(01)01912-8. [DOI] [PubMed] [Google Scholar]
- 6.Faux SP, Tai T, Thorne D, Xu Y, Breheny D, Gaca M. The role of oxidative stress in the biological responses of lung epithelial cells to cigarette smoke. Biomarkers. 2009;14(Suppl 1):90–6. doi: 10.1080/13547500902965047. [DOI] [PubMed] [Google Scholar]
- 7.U.S. Department of Health and Human Services. How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; Atlanta, GA: U.S: 2010. [PubMed] [Google Scholar]
- 8.Bartalis J, Chan WG, Wooten JB. A new look at radicals in cigarette smoke. Anal Chem. 2007;79(13):5103–5106. doi: 10.1021/ac070561+. [DOI] [PubMed] [Google Scholar]
- 9.Dellinger B, Khachatryan L, Masko S, Lomnicki S. Free radicals in tobacco smoke. Mini Rev Org Chem. 2011;8(4):427–433. [Google Scholar]
- 10.Goel R, Bitzer Z, Reilly SM, Trushin N, Foulds J, Muscat J, Liao J, Elias RJ, Richie JP., Jr Variation in Free Radical Yields from U.S. Marketed Cigarettes. Chem Res Toxicol. 2017;30(4):1038–1045. doi: 10.1021/acs.chemrestox.6b00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goel R, Durand E, Trushin N, Prokopczyk B, Foulds J, Elias RJ, Richie JP., Jr Highly reactive free radicals in electronic cigarette aerosols. Chem Res Toxicol. 2015;28(9):1675–7. doi: 10.1021/acs.chemrestox.5b00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sussan TE, Gajghate S, Thimmulappa RK, Ma J, Kim JH, Sudini K, Consolini N, Cormier SA, Lomnicki S, Hasan F, Pekosz A, Biswal S. Exposure to electronic cigarettes impairs pulmonary anti-bacterial and anti-viral defenses in a mouse model. PLoS One. 2015;10(2):e0116861. doi: 10.1371/journal.pone.0116861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Canistro D, Vivarelli F, Cirillo S, Babot Marquillas C, Buschini A, Lazzaretti M, Marchi L, Cardenia V, Rodriguez-Estrada MT, Lodovici M, Cipriani C, Lorenzini A, Croco E, Marchionni S, Franchi P, Lucarini M, Longo V, Della Croce CM, Vornoli A, Colacci A, Vaccari M, Sapone A, Paolini M. E-cigarettes induce toxicological effects that can raise the cancer risk. Sci Rep. 2017;7(1):2028. doi: 10.1038/s41598-017-02317-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bitzer ZT, Goel R, Reilly SM, Foulds J, Muscat J, Elias RJ, Richie JP., Jr Effects of Solvent and Temperature on Free Radical Formation in Electronic Cigarette Aerosols. Chem Res Toxicol. 2018;31(1):4–12. doi: 10.1021/acs.chemrestox.7b00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Long GA. Comparison of select analytes in exhaled aerosol from e-cigarettes with exhaled smoke from a conventional cigarette and exhaled breaths. Int J Env Res Public Health. 2014;11(11):11177–91. doi: 10.3390/ijerph111111177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jensen RP, Luo W, Pankow JF, Strongin RM, Peyton DH. Hidden Formaldehyde in E-Cigarette Aerosols. New Engl J Med. 2015;372(4):392–394. doi: 10.1056/NEJMc1413069. [DOI] [PubMed] [Google Scholar]
- 17.Kosmider L, Sobczak A, Fik M, Knysak J, Zaciera M, Kurek J, Goniewicz ML. Carbonyl compounds in electronic cigarette vapors: effects of nicotine solvent and battery output voltage. Nicotine Tob Res. 2014;16(10):1319–26. doi: 10.1093/ntr/ntu078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams M, Villarreal A, Bozhilov K, Lin S, Talbot P. Metal and silicate particles including nanoparticles are present in electronic cigarette cartomizer fluid and aerosol. PLoS One. 2013;8(3):e57987. doi: 10.1371/journal.pone.0057987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uchiyama S, Ohta K, Inaba Y, Kunugita N. Determination of carbonyl compounds generated from the E-cigarette using coupled silica cartridges impregnated with hydroquinone and 2,4-dinitrophenylhydrazine, followed by high-performance liquid chromatography. Anal Sci. 2013;29(12):1219–22. doi: 10.2116/analsci.29.1219. [DOI] [PubMed] [Google Scholar]
- 20.Wang P, Chen W, Liao J, Matsuo T, Ito K, Fowles J, Shusterman D, Mendell M, Kumagai K. A Device-Independent Evaluation of Carbonyl Emissions from Heated Electronic Cigarette Solvents. PLoS One. 2017;12(1):e0169811. doi: 10.1371/journal.pone.0169811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng T. Chemical evaluation of electronic cigarettes. Tob Control. 2014;23(suppl 2):ii11–ii17. doi: 10.1136/tobaccocontrol-2013-051482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cummings KM, Morley CP, Horan JK, Steger C, Leavell N-R. Marketing to America’s youth: evidence from corporate documents. Tob Control. 2002;11(suppl 1):i5–i17. doi: 10.1136/tc.11.suppl_1.i5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown JE, Luo W, Isabelle LM, Pankow JF. Candy Flavorings in Tobacco. New Engl J Med. 2014;370(23):2250–2252. doi: 10.1056/NEJMc1403015. [DOI] [PubMed] [Google Scholar]
- 24.Wayne GF, Connolly GN. How cigarette design can affect youth initiation into smoking: Camel cigarettes 1983–93. Tob Control. 2002;11(suppl 1):i32–i39. doi: 10.1136/tc.11.suppl_1.i32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kostygina G, Glantz SA, Ling PM. Tobacco industry use of flavours to recruit new users of little cigars and cigarillos. Tob Control. 2016;25(1):66–74. doi: 10.1136/tobaccocontrol-2014-051830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Connolly GN. Sweet and spicy flavours: new brands for minorities and youth. Tob Control. 2004;13(3):211–212. doi: 10.1136/tc.2004.009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.FDA. [Accessed April 25 2017];Family Smoking Prevention And Tobacco Control Act. 2009 https://www.fda.gov/tobaccoproducts/labeling/rulesregulationsguidance/ucm237092.htm.
- 28.Villanti AC, Johnson AL, Ambrose BK, Cummings KM, Stanton CA, Rose SW, Feirman SP, Tworek C, Glasser AM, Pearson JL, Cohn AM, Conway KP, Niaura RS, Bansal-Travers M, Hyland A. Flavored Tobacco Product Use in Youth and Adults: Findings From the First Wave of the PATH Study (2013–2014) Am J Prev Med. 2017;53(2):139–151. doi: 10.1016/j.amepre.2017.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harrell MB, Weaver SR, Loukas A, Creamer M, Marti CN, Jackson CD, Heath JW, Nayak P, Perry CL, Pechacek TF, Eriksen MP. Flavored e-cigarette use: Characterizing youth, young adult, and adult users. Prev Med Rep. 2017;5:33–40. doi: 10.1016/j.pmedr.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yingst JM, Veldheer S, Hammett E, Hrabovsky S, Foulds J. A method for classifying user-reported electronic cigarette liquid flavors. Nicotine Tob Res. 2017;19(11):1381–1385. doi: 10.1093/ntr/ntw383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grana R, Benowitz N, Glantz SA. E-cigarettes: a scientific review. Circulation. 2014;129(19):1972–86. doi: 10.1161/CIRCULATIONAHA.114.007667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barrington-Trimis JL, Samet JM, McConnell R. Flavorings in electronic cigarettes: an unrecognized respiratory health hazard? JAMA. 2014;312(23):2493–4. doi: 10.1001/jama.2014.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Behar RZ, Davis B, Wang Y, Bahl V, Lin S, Talbot P. Identification of toxicants in cinnamon-flavored electronic cigarette refill fluids. Toxicol In Vitro. 2014;28(2):198–208. doi: 10.1016/j.tiv.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 34.Kosmider L, Sobczak A, Prokopowicz A, Kurek J, Zaciera M, Knysak J, Smith D, Goniewicz ML. Cherry-flavoured electronic cigarettes expose users to the inhalation irritant, benzaldehyde. Thorax. 2016;71(4):376–7. doi: 10.1136/thoraxjnl-2015-207895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gerloff J, Sundar IK, Freter R, Sekera ER, Friedman AE, Robinson R, Pagano T, Rahman I. Inflammatory Response and Barrier Dysfunction by Different e-Cigarette Flavoring Chemicals Identified by Gas Chromatography-Mass Spectrometry in e-Liquids and e-Vapors on Human Lung Epithelial Cells and Fibroblasts. Appl In Vitro Toxicol. 2017;3(1):28–40. doi: 10.1089/aivt.2016.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lim HH, Shin HS. Determination of volatile organic compounds including alcohols in refill fluids and cartridges of electronic cigarettes by headspace solid-phase micro extraction and gas chromatography-mass spectrometry. Anal Bioanal Chem. 2017;409(5):1247–1256. doi: 10.1007/s00216-016-0049-0. [DOI] [PubMed] [Google Scholar]
- 37.Pankow JF, Kim K, McWhirter KJ, Luo W, Escobedo JO, Strongin RM, Duell AK, Peyton DH. Benzene formation in electronic cigarettes. PLoS One. 2017;12(3):e0173055. doi: 10.1371/journal.pone.0173055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khlystov A, Samburova V. Flavoring Compounds Dominate Toxic Aldehyde Production during E-Cigarette Vaping. Environ Sci Technol. 2016;50(23):13080–13085. doi: 10.1021/acs.est.6b05145. [DOI] [PubMed] [Google Scholar]
- 39.Sandy C, Winsor H, Armitage R, Garside A. [Accessed 12 July 2016];Qualitative Analysis of E-Cigarette Liquids Using Gas Chromotagraphy/Mass Spectrometry. 2015 https://www.agilent.com/cs/library/applications/ecigarette_analysis_v5.pdf.
- 40.Farsalinos K, Romagna G, Tsiapras D, Kyrzopoulos S, Voudris V. Evaluation of Electronic Cigarette Use (Vaping) Topography and Estimation of Liquid Consumption: Implications for Research Protocol Standards Definition and for Public Health Authorities’ Regulation. Int J Env Res Public Health. 2013;10(6):2500. doi: 10.3390/ijerph10062500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Church DF. Spin trapping organic radicals. Anal Chem. 1994;66(7):418A–427A. [Google Scholar]
- 42.Yu L-X, Dzikovski BG, Freed JH. A protocol for detecting and scavenging gas-phase free radicals in mainstream cigarette smoke. J Vis Exp. 2012;(59):e3406–e3406. doi: 10.3791/3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hagen WR. Biomolecular EPR spectroscopy. CRC Press; Boca Raton: 2009. [Google Scholar]
- 44.Walt Svd, Colbert SC, Varoquaux G. The NumPy Array: A Structure for Efficient Numerical Computation. Comput Sci Eng. 2011;13(2):22–30. [Google Scholar]
- 45.Millman KJ, Aivazis M. Python for Scientists and Engineers. Comput Sci Eng. 2011;13(2):9–12. [Google Scholar]
- 46.De Rovira DA. Dictionary of flavors. 3. John Wiley & Sons, Inc; Chichester, West Sussex; Hoboken, NJ: 2017. [Google Scholar]
- 47.Baschieri A, Ajvazi MD, Tonfack JLF, Valgimigli L, Amorati R. Explaining the antioxidant activity of some common non-phenolic components of essential oils. Food Chem. 2017;232:656–663. doi: 10.1016/j.foodchem.2017.04.036. [DOI] [PubMed] [Google Scholar]
- 48.Kao D, Chaintreau A, Lepoittevin JP, Gimenez-Arnau E. Synthesis of allylic hydroperoxides and EPR spin-trapping studies on the formation of radicals in iron systems as potential initiators of the sensitizing pathway. J Org Chem. 2011;76(15):6188–200. doi: 10.1021/jo200948x. [DOI] [PubMed] [Google Scholar]
- 49.Bezard M, Gimenez-Arnau E, Meurer B, Grossi L, Lepoittevin JP. Identification of carbon-centred radicals derived from linalyl hydroperoxide, a strong skin sensitizer: a possible route for protein modifications. Bioorg Med Chem. 2005;13(12):3977–86. doi: 10.1016/j.bmc.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 50.Maxton DG, Thompson RP, Hider RC. Absorption of iron from ferric hydroxypyranone complexes. Br J Nutr. 1994;71(2):203–7. doi: 10.1079/bjn19940127. [DOI] [PubMed] [Google Scholar]
- 51.Murakami K, Ishida K, Watakabe K, Tsubouchi R, Haneda M, Yoshino M. Prooxidant action of maltol: role of transition metals in the generation of reactive oxygen species and enhanced formation of 8-hydroxy-2′-deoxyguanosine formation in DNA. BioMetals. 2006;19(3):253–7. doi: 10.1007/s10534-005-6998-y. [DOI] [PubMed] [Google Scholar]
- 52.Li Z, Lu J, Wu C, Pang Q, Zhu Z, Nan R, Du R, Chen J. Toxicity Studies of Ethyl Maltol and Iron Complexes in Mice. Biomed Res Int. 2017;2017:2640619. doi: 10.1155/2017/2640619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Naumov S, Janovský I, Knolle W, Mehnert R, Turin DA. Low-temperature EPR and quantum chemical study of lactone radical cations and their transformations. Radiat Phys Chem. 2005;73(4):206–212. [Google Scholar]
- 54.Tai A, Sawano T, Yazama F. Antioxidant properties of ethyl vanillin in vitro and in vivo. Biosci, Biotechnol, Biochem. 2011;75(12):2346–50. doi: 10.1271/bbb.110524. [DOI] [PubMed] [Google Scholar]
- 55.Mueller D, Triebel S, Rudakovski O, Richling E. Influence of triterpenoids present in apple peel on inflammatory gene expression associated with inflammatory bowel disease (IBD) Food Chem. 2013;139(1–4):339–46. doi: 10.1016/j.foodchem.2013.01.101. [DOI] [PubMed] [Google Scholar]
- 56.Tsikas D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal Biochem. 2017;524:13–30. doi: 10.1016/j.ab.2016.10.021. [DOI] [PubMed] [Google Scholar]
- 57.Milne GL, Yin H, Morrow JD. Human biochemistry of the isoprostane pathway. J Biol Chem. 2008;283(23):15533–7. doi: 10.1074/jbc.R700047200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Carnevale R, Sciarretta S, Violi F, Nocella C, Loffredo L, Perri L, Peruzzi M, Marullo AG, De Falco E, Chimenti I, Valenti V, Biondi-Zoccai G, Frati G. Acute Impact of Tobacco vs Electronic Cigarette Smoking on Oxidative Stress and Vascular Function. Chest. 2016;150(3):606–12. doi: 10.1016/j.chest.2016.04.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.