Abstract
Background
Falls lead to a disproportionate burden of death and disability among older adults despite evidence-based recommendations to screen regularly for fall risk and clinical trials demonstrating the effectiveness of multifactorial interventions to reduce falls. The Centers for Disease Control and Prevention developed STEADI (Stopping Elderly Accidents, Deaths, and Injuries) to assist primary care teams to screen for fall risk and reduce risk of falling in older adults.
Purpose of the Study
This paper describes a practical application of STEADI in a large academic internal medicine clinic utilizing the Kotter framework, a tool used to guide clinical practice change.
Design and Methods
We describe key steps and decision points in the implementation of STEADI as they relate to the recommended strategies of the Kotter framework. Strategies include: creating a sense of urgency, building a guiding coalition, forming a strategic vision and initiative, enlisting volunteers, enabling success by removing barriers, generating short-term wins, sustaining change, and instituting change.
Results
Fifty-six patients were screened during pilot testing; 360 patients were screened during the first 3 months of implementation. Key to successful implementation was (a) the development of electronic health record (EHR) tools and workflow to guide clinical practice and (b) the proactive leadership of clinical champions within the practice to identify and respond to barriers.
Implications
Implementing falls prevention in a clinical setting required support and effort across multiple stakeholders. We highlight challenges, successes, and lessons learned that offer guidance for other clinical practices in their falls prevention efforts.
Keywords: Falls screening, Falls risk evaluation, Clinical decision support, Implementation science, Electronic health record
Introduction
Falls are the leading cause of injury-related death and one of the leading causes of overall mortality in older adults (Centers for Disease Control and Prevention [CDC], 2013). Injury death rates from falls nearly doubled between 2000 and 2013, from 29 to 56 per 100,000 population (CDC, 2013). The increasing death rate combined with a growing older adult population means the direct medical costs of falls in the United States are projected to increase from about $35 billion in 2012 to over $100 billion in 2030 (Houry, Florence, Baldwin, Stevens, & McClure, 2016). Multiple individual and multifactorial interventions have been shown to reduce fall risk. Effective interventions include fall prevention classes, Tai Chi, environmental modification, vitamin D supplementation, medication optimization, and targeted clinical assessments and referrals (Chang et al., 2004; Hanley, Silke, & Murphy, 2010). Despite these strategies, provider uptake of falls screening and interventions has been low, in part due to unclear roles across the primary care team, discrepant recommendations of various falls prevention interventions (frequency and duration of Tai Chi, target vitamin D levels; Bischoff-Ferrari et al., 2009; Kalyani et al., 2010; Tinetti, Gordon, Sogolow, Lapin, & Bradley, 2006), and the complexity of employing financial and quality incentives (Jones, Ghosh, Horn, Smith, & Vogt, 2011; Landis & Galvin, 2014; Shubert, Smith, Prizer, & Ory, 2014).
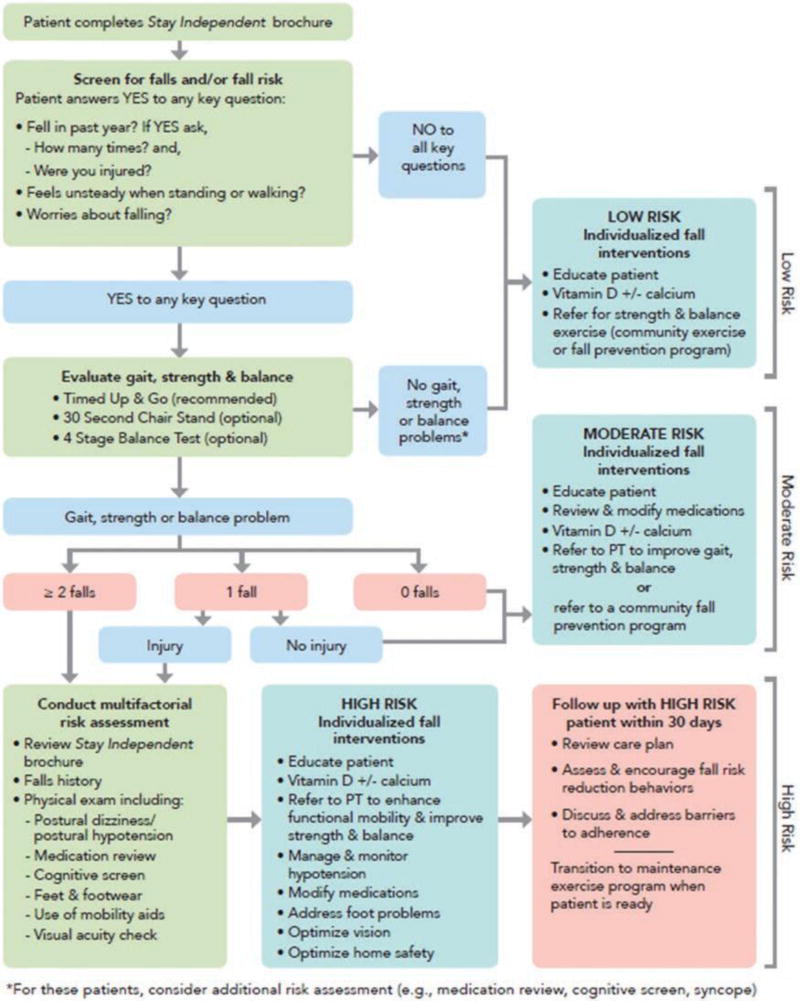
In response to this growing public health problem, the CDC developed STEADI (Stopping Elderly Accidents, Deaths, and Injuries) to encourage and facilitate falls screening and management in primary care (Stevens & Phelan, 2013; http://www.cdc.gov/steadi/). The foundation of STEADI is the STEADI algorithm (see Figure 1), which is adapted from the 2010 American Geriatrics Society and British Geriatrics Society (AGS/BGS) clinical practice guidelines for prevention of falls in older adults (AGS/BGS Panel, 2011). The algorithm begins with a 12-question patient self-assessment “Stay Independent” questionnaire (http://www.cdc.gov/steadi/pdf/stay_independent_brochure-a.pdf). The Stay Independent self-assessment is used as a screening tool, with a score of 4 or more or an affirmative response to any of three key questions (falling in the last year, being worried about falling, or feeling unsteady) prompting additional assessments. These assessments—including gait, strength, and balance testing; vision exam; orthostatic blood pressure measurement; medication review; physical exam; cognitive screen; and falls history—identify fall risk factors amenable to targeted interventions. Interventions include community Tai Chi or other evidence-based falls prevention classes to improve strength and balance, physical therapy for gait training or mobility-aid evaluation, occupational therapy for home safety evaluation, vision correction, management of orthostasis, vitamin D supplementation, and medication optimization to eliminate or reduce medications associated with falls.
Figure 1.
CDC STEADI falls screening and management algorithm. CDC = Centers for Disease Control and Prevention; STEADI = Stopping Elderly Accidents, Deaths, and Injuries.
Despite the simplicity of these individual interventions and the accessibility of the education materials, evaluating and mitigating falls risk still represents a significant investment of time and energy for busy providers (Shubert et al., 2014). To assist providers in implementing the STEADI algorithm, STEADI includes additional information for the care team, such as basic information about falls, case studies, conversation starters, and standardized gait and balance assessments (Timed Up and Go [TUG] test, 30 second chair stand, and 4-stage balance test) with instructional videos and online trainings (www.cdc.train.org). STEADI also includes educational handouts and brochures about fall prevention specifically designed for older adults and their families.
In 2011, the CDC funded three health departments— Oregon, New York, and Colorado—to create sustainable strategies over a 5-year period to reduce falls risk in older adults at the community, health system, and practice levels. Our state’s Oregon Health Authority (OHA), part of the state health department, approached authors C. M. Casey and E. Eckstrom to consider implementing STEADI within their clinical practice at the Oregon Health & Science University (OHSU) Internal Medicine and Geriatrics Clinic. This paper reports on the integration of STEADI into this clinic, focusing on strategies, successes, challenges, and lessons learned in the implementation process.
Methods
Practice Change Model
Various models have been used to facilitate practice change, including the plan-do-study-act (PDSA) framework, the Systems Engineering Initiative for Patient Safety (SEIPS) model (Carayon et al., 2006; Perry, Bell, Shaw, Fitzpatrick, & Sampson, 2014), and the RE-AIM (Reach, Effectiveness, Adoption, Implementation, and Maintenance) framework (Gaglio, Shoup, & Glasgow, 2013; Glasgow, Vogt, & Boles, 1999; Shubert, Altpeter, & Busby-Whitehead, 2011). These models are effective at analyzing the overall impact of a practice change. SEIPS, for example, allows the investigation of the interaction of system components such as how organizational culture affects the environment into which tools are introduced (Carayon et al., 2006). RE-AIM promotes quantitative evaluation of study areas such as adoption percentage and long-term behavior change (Glasgow et al., 1999). A PDSA, by its very nature, is oriented around small tests of change—plan, do, and then evaluate and revise, in a cyclical pattern.
We felt that introducing falls intervention protocols into a busy clinic environment would require a more holistic approach with a focus on the process of practice change. We wanted to develop, with providers, a shared priority to reduce falls, and thereby achieve a greater chance of creating lasting change. We learned of the Kotter framework during our project implementation and felt it aligned well with our approach to implement STEADI. The Kotter framework, developed in 1995, is an eight-step process for organizational change used in various health care change initiatives (Campbell, 2008; Kotter, 2012; Kotter & Schlesinger, 2008; Stoller, 2010). Essential elements of the Kotter framework include creating a sense of urgency, building a guiding coalition, forming a strategic vision and initiative, enlisting volunteers, enabling success by removing barriers, generating short-term wins, sustaining change, and instituting change. We use the Kotter framework here to highlight and organize our description of the project’s implementation. This framework was selected early in the implementation process and guided the implementation, with a particular focus on the importance of enabling success by removing barriers.
Implementation Process
We implemented STEADI through four main strategies: (a) developing a workflow that aligned with (and did not disrupt) the usual clinic flow; (b) integrating the STEADI algorithm within Epic, our electronic health record (EHR) system; (c) pilot testing the workflow and EHR tools by the STEADI clinic champions for several months before clinic-wide implementation; and (d) using 1-hr in-person trainings to educate providers and medical assistants on fall burden, fall risk assessments, and how to use the workflow and EHR tools. All participating providers and medical assistants signed informed consent, and the project was approved by the Institutional Review Board. Clinic staff refers to medical assistants and front office staff unless otherwise specified.
Data Collection and Analysis
The STEADI EHR tool included the following falls-related Current Procedural Terminology Category II codes (CPT II codes): (a) documentation of falls in past year (no falls/ single fall without injury [CPT II 1101] or an injurious fall/ multiple falls [CPT II 1100]); (b) assessment of falls risk (CPT II 3288); and (c) development of a falls care plan (CPT II 0518). Our clinical data analyst used these codes to generate weekly and monthly data reports on the numbers of screened patients. Regular reporting of clinical performance provided to providers and staff allowed for prompt performance feedback, identification of data collection issues, and the need for changes to the workflow and EHR tools. At 6 months, the state health department facilitated the data analysis required for providers to receive Maintenance of Certification credit through the American Boards of Internal Medicine (ABIM) and Family Medicine (ABFM).
Retrospective chart review was also conducted to evaluate STEADI implementation. STEADI visits were identified through CPT II codes 3288F and 0518F. Data collection included variables related to STEADI workflow, STEADI EHR tool utilization, and provider and medical assistant completion of falls risk factor assessments and interventions.
A Facilitators and Barriers survey was distributed to all STEADI-trained providers and medical assistants after 3 months; it was available electronically and as hard copy. This 21-question survey included both closed and open ended questions, and solicited feedback that guided additional process improvements.
Descriptive statistics were used to evaluate adoption of the STEADI workflow and EHR tool. Data were stored and protected per institutional policy and were analyzed using Microsoft Excel.
Results
Results of implementation of our project are reported using the Kotter practice change framework.
Create a Sense of Urgency
Clinic champions created a sense of urgency for the STEADI project by highlighting the growing falls epidemic, building on prior successes from other PatientCentered Medical Home (PCMH) projects in the clinic, and ensuring recognition and external credit for project completion (such as Maintenance of Certification credits through the ABIM and ABFM). An additional organizational incentive for change included a benefit of some health plans to pay practices a per-member-per-month payment for falls screening. Taken together, the data, organizational support, and complimentary institutional initiatives highlighted the need for falls prevention and provided a window of opportunity to launch fall prevention efforts.
Build a Guiding Coalition
Our guiding coalition consisted of the STEADI clinic champions (geriatrics-trained members of the clinic who served as project leaders), state health leaders (the state’s geriat ric education center and state falls prevention program); computer analysts (both an EHR developer and clinical data analyst); and clinic leaders. This group met at least monthly either in-person or by telephone. Each guiding coalition member had incentives to participate. For example, the state health department was satisfying grant objectives, whereas clinic leadership wanted to satisfy required reporting elements for our PCMH and Comprehensive Primary Care (CPC) initiatives. Other key stakeholders in this initiative were the institution’s rehabilitation department (physical and occupational therapy) and the health care team (providers, medical assistants, and front office staff).
Form a Strategic Vision and Initiative
Our project’s vision built on the vision of the CDC in creating STEADI—to optimize a clinical setting to proactively identify and address fall risk in older adults (i.e., to make fall prevention a routine part of clinical care). Because falls risks are multifactorial and require multifaceted interventions, our STEADI initiative combined state-level efforts, community-based referrals, and clinic-, provider-, and patient-based components to identify and address fall risk (see Supplementary Figure 1). Diverse partners found common ground because STEADI encouraged a multisectoral approach to reducing falls. Each partner was able to use its own existing strengths and resources to meaningfully contribute to the project’s vision.
Enlist a Volunteer Army
We asked for volunteers to participate in the project and were pleased that 16 of 24 providers and 10 of 12 medical assistants across 4 of the clinic’s 5 teams agreed to implement STEADI. All four front office staff, who work across clinic teams, participated. All providers were physicians, except for one nurse practitioner and one physician assistant. The only team that did not participate was the HIV team that had a number of separate clinical initiatives under way.
To train volunteers, participating providers attended a 1-hr lunchtime discussion designed to increase knowledge of falls prevention strategies and introduce them to the STEADI workflow and Epic tools. Physicians were incentivized to participate because STEADI qualified as an eligible Maintenance of Certification project through the ABIM and ABFM. The project was presented to front office and medical assistant staff at a required 1-hr monthly staff meeting. Identification of existing national and institutional efforts with which STEADI could align, including the clinic’s PCMH and CPC initiatives, helped to increase buy-in for the project.
Enable Action by Removing Barriers
A critical component of implementation was to proactively identify and mitigate barriers throughout the STEADI implementation process. By systematically designing the workflow and Epic EHR tools before pilot testing, refining both as a result of pilot testing, and then continuously evaluating the project as it rolled out, we quickly identified barriers as they arose.
Part 1: The STEADI Workflow
We matched the STEADI workflow to the clinic’s existing approach for other screening efforts, which included using a “health maintenance modifier.” A health maintenance modifier is a clinical alert that can be added to a patient’s appointment notes to help identify and implement recommended screenings. The clinic routinely uses this approach to ensure patients receive colonoscopy, mammography, flu vaccines, and other evidence-based screening recommendations when due. The STEADI project created an “annual falls screening health maintenance modifier” to alert staff and providers that a patient was due for falls screening. The health maintenance modifier, applied to patients’ charts automatically based on age 65 and older, helped front office staff identify eligible patients due for falls screening when they checked in for their appointment.
Pilot testing revealed it was extremely time-consuming to perform a full STEADI evaluation on patients with dementia because they could not easily complete the self-assessment form nor follow directions, so these patients were excluded from the project. Frequent fallers were also excluded, as they did not need “screening” for fall risk. Hospice and nonambulatory patients were also excluded. Each of these exclusions helped to enhance the feasibility of the project. The project team adapted the EHR tools to incorporate these criteria, and each provider reviewed their panel of patients 65 and older to remove ineligible patients. Eligible patients, therefore, included patients not excluded due to dementia, being a frequent faller, nonambulatory, or on hospice; patients not previously screened in the prior year; and patients of participating providers.
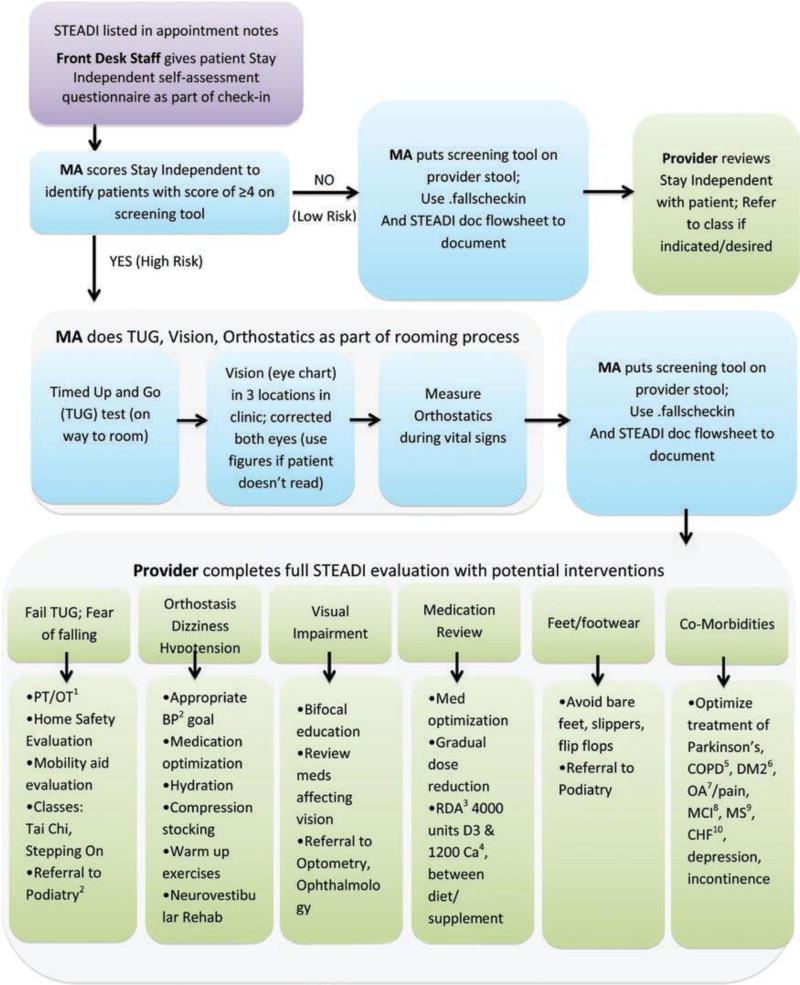
It was important that the STEADI workflow matched how providers, staff, and patients normally experienced a clinic appointment, dividing roles among front desk staff, medical assistants, and providers (see Figure 2). Because some of the assessments were most efficiently completed by medical assistants as part of the rooming process (TUG, vision testing, orthostatics), the clinic workflow simplified the STEADI algorithm from three levels of risk (high, moderate, low) to two levels of risk based solely on results of the patient self-assessment, with a score of ≤3 indicating low risk and ≥4 indicating high risk. This simplification allowed medical assistants to work within their scope of practice and helped them quickly identify high-risk patients needing additional assessments during the rooming process.
Figure 2.
STEADI workflow for front desk staff, medical assistants (MA), providers. STEADI = Stopping Elderly Accidents, Deaths, and Injuries. 1Physical therapy/occupational therapy; 2Blood pressure; 3Recommended daily allowance; 4Calcium; 5Chronic obstructive pulmonary disease; 6Diabetes mellitus type 2; 7Osteoarthritis; 8Mild cognitive impairment; 9Multiple sclerosis; 10Congestive heart failure.
STEADI includes three options for assessing gait, strength, and balance. We implemented only the TUG test because it yielded the most valuable clinical information on gait in the least amount of time. Medical assistants could complete the TUG during the rooming process. Because of the Health Insurance Portability and Accountability Act (HIPAA), we could not perform the TUG in a public area, so we created two TUG “stations” in private hallways within the clinic, equipped with stopwatches, a chair with arms, and markings on the floor 10 feet from the chair. Vision charts were hung in locations adjacent to the TUG stations, so medical assistants could do vision screening immediately after performing the TUG. During implementation, we discovered variations in how medical assistants were measuring orthostatic blood pressure. We provided additional training to take blood pressure first lying for 5 min and then standing at 1 min, based on evidence that this approach has the highest sensitivity to detect orthostasis (Mader, Hornick, & Winger, 1987). Completing the standard medication reconciliation while the patient was lying down during their blood pressure measurements helped save time.
Part 2: The STEADI EHR Tool
To make STEADI implementation feasible, key STEADI elements needed to be built into an Epic clinical decision support tool. To simplify integration, we created tools based on the EHR technology that providers and medical assistants already used regularly—dot phrases, doc flowsheets, and Smartsets. Providers and medical assistants were familiar with using “dot phrases,” structured note templates that include pertinent history, exam, assessment, and plan components. Medical assistants were familiar with entering data into “doc flowsheets,” which are data entry tables for various scored measures, like depression and alcohol use screenings. Data from doc flowsheets can be pulled into a visit note to streamline documentation. Lastly, providers were accustomed to using all-in-one order sets called “Smartsets,” which include access to note templates, relevant orders (in this case physical therapy and other consults, labs, etc.), associated diagnoses, and patient education materials in a single location within the EHR. STEADI EHR elements included: (a) provider note templates for low and high-risk patients; (b) doc flowsheets for the Stay Independent questionnaire responses, TUG, vision screen, and orthostatic measurements; and (c) a STEADI Smartset including access to the note templates, all pertinent orders, associated falls-related diagnoses and CPT II codes, and patient education materials (see Supplementary Figure 2).
The falls-related CPT II codes, built into the STEADI Smartset, were an important mechanism to collect data for the institution to report on falls-related national quality measures and for ABIM/ABFM Maintenance of Certification reporting. A Smartset is signed by a provider like an order, and the STEADI Smartset was built so that it could not be signed without selecting the appropriate CPT II codes. Selection of the CPT II codes also satisfied the health maintenance modifier, so patients would be flagged for a STEADI evaluation only once per year.
Educational materials were also part of the Smartset, including the CDC STEADI patient brochures and additional information developed by the project team with regard to bifocal lenses, adequate hydration, and local Tai Chi classes. Building educational materials into the Smartset avoided maintaining an inventory of brochures across multiple clinic work areas.
Part 3: The Roll Out
During the first months of clinic-wide implementation, the STEADI clinic champions conducted weekly walking rounds to identify workflow and EHR issues, encourage adoption of the workflow, and to shadow medical assistants to observe their technique in conducting the STEADI assessments. Regular e-mails updated providers and staff on changes made as a result of feedback. Additional trainings were held as necessary for new providers and staff or if more education was needed, such as how to correctly perform orthostatic blood pressure measurements.
STEADI “brown bag” lunch sessions encouraged additional sharing of successes and barriers to refine the EHR tools and workflow. As a result of feedback from providers at these sessions, we adapted the falls screen doc flowsheet from recording a dichotomous falls risk score (low risk vs high risk) to documenting each of the 12 responses from the Stay Independent questionnaire into the doc flowsheet. This change saved providers time because they could view each response within Epic instead of needing to look at the paper form; it also provided better data to evaluate risk factors across the clinic population.
Because of the multifaceted nature of STEADI and to encourage practice using the EHR tools, we built a ramp-up period into the implementation plan. In the first 2 months of clinic-wide roll out, we asked providers to preview their clinic schedules and preselect only one patient per day for STEADI evaluation, so they would not be overwhelmed.
We encouraged providers to start with patients whom they thought would be low risk, to allow them to focus on mastering the overall STEADI workflow first, before moving on to more complex, higher-risk patients. The weekly STEADI walking rounds offered immediate solutions to technical issues and on-demand refresher training on the Epic tools.
Generate Short-term Wins
The clinic champions (a geriatrician and geriatric nurse practitioner) pilot-tested the STEADI workflow and Epic tools with 56 patients aged 75 and older for 3–4 months before broader implementation. Of the 45 patients (80%) with documented fall risk scores, 25 (56%) scored high risk for falls. Two patients had their falls evaluations deferred to future visits, but the other 23 patients received a complete STEADI falls evaluation. Documentation of various falls risk assessments ranged from 78% for history of falls to 91% for documenting vision assessment and a vitamin D level. A falls-related care plan was documented for more than 90% of patients. We viewed these results as short term wins, especially because they came from a sample of older and higher-risk patients than we expected as part of clinic-wide implementation. This success reinforced the feasibility of implementing STEADI across the clinic among patients aged 65 and older. The STEADI workflow and EHR tools were iteratively modified before clinic-wide implementation.
Timely data reporting was critical to the project’s development. As part of clinic-wide implementation, we created weekly STEADI data reports so that providers and staff could see their number of screenings conducted and see how well they were doing. During weekly walking rounds, we also identified major issues that required immediate attention such as ineligible patients appearing as “due” for STEADI, missing stopwatches, or difficulty accessing the various EHR tools. Providers and staff embraced these on demand tutorials and saw the quick resolution as short term wins.
Sustain Acceleration
In the first 3 months after clinic-wide implementation across four clinic teams, the 16 STEADI providers completed 360 falls screenings. Other than the 56 patients screened as part of the STEADI pilot, there were no other documented falls screenings at our clinic prior to implementation. These 360 screenings represented about 19% of the providers’ eligible patients (in this early phase, providers were encouraged to pick just one patient per clinic for STEADI so as not to be overwhelmed). Providers and staff embraced this early success. Of the high-risk patients, providers used the dot phrase 77% of the time to access the provider note template for evaluation; medical assistants recorded the patient’s fall risk scores 98% of the time.
A Facilitators and Barriers survey was completed by eight providers and six medical assistants after 3 months of implementation. Respondents acknowledged the value of falls screening and the usefulness of the STEADI tools (Table 1). Providers expressed concern regarding whether falls screening could easily fit into a regular office visit and whether STEADI was truly feasible in a busy primary care practice—both issues related to limited time and competing medical priorities. Providers and medical assistants universally expressed appreciation for the project team’s responsiveness to challenges.
Table 1.
Results of Facilitators and Barriers Survey
| Survey question | Strongly agree |
Somewhat agree |
Somewhat disagree |
Strongly disagree |
|---|---|---|---|---|
| 1. The culture at my clinic is supportive for implementing new protocols such as the STEADI project. | 35.7% | 64.3% | 0.0% | 0.0% |
| 2. The opportunity to receive Maintenance of | Certification (MOC) credits for ABIM encourages me to participate (provider only responses).a | 87.5% | 12.5% | 0.0% | 0.0% |
| 3. The project champions are readily available to help me implement STEADI in my practice. | 71.5% | 14.3% | 7.1% | 7.1% |
| 4. There is strong evidence to support falls screening among older adults. | 78.6% | 14.3% | 0.0% | 7.1% |
| 5. Fall screening is not as important as other preventive screenings, such as cancer screening and osteoporosis screening. | 7.1% | 7.1% | 28.6% | 57.2% |
| 6. There is strong evidence that the STEADI interventions can help to prevent falls. | 61.5% | 30.8% | 0.0% | 7.7% |
| 7. The STEADI protocol is effective at streamlining the falls screening process. | 28.6% | 57.2% | 7.1% | 7.1% |
| 8. Time constraints and competing medical priorities are significant barriers to implementing STEADI in my practice. | 50.0% | 28.6% | 21.4% | 0.0% |
| 9. The STEADI training did not prepare me to effectively screen and prevent falls among older adults. | 7.1% | 7.1% | 42.9% | 42.9% |
| 10. The clinic staff members are well-prepared to administer the STEADI protocol. | 50.0% | 35.7% | 14.3% | 0.0% |
| 11. The complexity of falls screening and management makes it difficult to adopt falls screening into my practice. | 14.3% | 35.7% | 35.7% | 14.3% |
| 12. Patients are generally receptive to being screened for falls. | 35.7% | 50.0% | 14.3% | 0.0% |
| 13. My patients generally do not follow my recommendations to reduce their risk of falls. | 0.0% | 23.1% | 76.9% | 0.0% |
| 14. The STEADI Smartset and dot phrases make it easier to administer falls screening and provide patients with good information to improve their fall risk. | 57.1% | 28.6% | 14.3% | 0.0% |
| 15. The extent of necessary falls prevention documentation discourages falls screening. | 21.4% | 35.7% | 28.6% | 14.3% |
| 16. Poor reimbursement for falls screening and management discourages me from incorporating STEADI into my practice. | 0.0% | 15.4% | 53.8% | 30.8% |
| 17. Receiving data on my STEADI performance helps to improve my falls screening practices. | 21.4% | 64.3% | 14.3% | 0.0% |
| 18. Falls screening (STEADI) does not fit easily within a Medicare Wellness Visit. | 21.4% | 50.0% | 14.3% | 14.3% |
Notes: Number of respondents: 14. ABIM = American Board of Internal Medicine; STEADI = Stopping Elderly Accidents, Deaths, and Injuries.
Denominator for question 2 includes only the eight providers.
Part of sustaining the project’s momentum required proactively addressing the issue of the time required to complete STEADI (as much as 15–20 min), as was reported in the Facilitators and Barriers survey. Many patients had multiple other issues on their clinic visit agenda; sometimes, there was not sufficient time to also complete a full “high-risk evaluation” for a patient who scored high risk. We recognized the need for an alternative workflow that allowed a provider to defer a full falls risk evaluation to a future visit. Thus, adding a “deferred visit” option acknowledged the initial screening but allowed the provider to schedule the full high-risk evaluation for a different day. In such cases, the provider could recommend that the patient be scheduled for a Medicare Wellness Visit, because falls risk screening is required as part of these visits, and they are typically longer visits. Medicare Wellness Visits are a covered annual visit for Medicare beneficiaries. EHR developers modified the STEADI Epic tools to support this workflow by adding a dot phrase and note template for a “deferred visit” (see Supplementary Figure 2).
Institute Change
The short-term successes of STEADI fueled gradual culture change throughout the clinic so that STEADI became a recommended part of all faculty and resident practices. All of the clinic’s 45 internal medicine residents, as well as 4 more faculty providers, were trained as a second cohort of STEADI providers. Within 18 months, the clinic had screened over 870 patients, 45% of its eligible patients 65 years and older, with roughly 35% scoring as high risk. Falls screenings continued throughout all quarters of the project’s first year, representing ongoing uptake of the project. On a weekly basis, STEADI screenings increased from the 30% range to almost 50% of eligible patients. Due to this success, clinic leadership identified STEADI falls screening rates as one of its top quality metrics moving forward.
Success of the STEADI project also encouraged uptake beyond the internal medicine clinic. Because of the project’s early successes and an overlap in leadership between the STEADI project and the institution’s Medicare Wellness Visit project, the institution used the STEADI EHR tools for Medicare Wellness Visits across all of the institution’s primary care clinics. A separate workgroup convened monthly to operationalize these visits and was co-led by one of the STEADI champions. Individual departments modified the STEADI workflow as needed to fit their own needs. For example, one Family Medicine clinic conducts the initial falls screening questionnaire by phone as part of the pre-visit screen. If a patient is high risk, the clinic increases the appointment length by 15 min to accommodate a high-risk STEADI evaluation.
Implications
We adapted and integrated the CDC STEADI algorithm for falls screening and management into a large academic primary care practice; successfully training all faculty, residents, and staff in the protocols; screening 45% of eligible patients during our study period; and gaining leadership support to continue screening and broaden implementation across the institution. Keys to the project’s success were early buy-in from clinic leadership, faculty, and staff; careful attention to building a clinic workflow that was feasible and responsive to provider and staff needs; and development of Epic EHR tools that allowed staff and providers to confidently and efficiently complete all components of the STEADI algorithm. The project has also had national impact. Based largely on OHSU’s STEADI implementation and EHR tools, in December 2015, the Epic organization released a new Clinical Program, Preventing Falls in Primary Care Using STEADI, which provides instructions and tools for integrating STEADI into any health system using Epic.
One of the biggest lessons learned was the importance of attending to the details of the workflow and Epic tool development. We recognized early in the process that the EHR and workflow must be woven together in an efficient, flexible way that built on existing tools and processes. The countless small revisions made to these tools helped ensure they were relevant. In-person feedback at every step of implementation ensured medical assistants, front office staff, and faculty providers could give input that led to improvements in the workflow and EHR tools.
Moving forward, we will work with stakeholders to sustain momentum and institute long-term change. Ongoing attention to the workflow and EHR tools continues to be a priority, and our electronic patient portal is now being used to increase screening rates and identify high-risk patients proactively to bring them in to clinic for a full falls evaluation. We are working to involve more team members (such as nurse care managers and pharmacists) in high-risk evaluations to reduce burden on providers. Because STEADI includes community-based resources, our linkages to local and state resources—such as the state health department— are critical. We were not able to study patient adherence to recommendations to access to community-based services, such as falls prevention or Tai Chi classes, but do hope to improve the clinic’s referral links to these resources. In this era of the Triple Aim to improve patient care and population health while decreasing cost, falls reduction offers a logical intersection between population health and primary care (Berwick, Nolan, & Whittington, 2008; IHI, 2015).
Conclusion
The STEADI project at OHSU successfully adopted the CDC STEADI algorithm in a large academic primary care setting by having clinical champions embedded within the practice, customizing a clinical workflow that functions within existing processes, developing EHR tools specific for falls screening and management in older adults, and partnering with the OHA (the state public health department) to link patients to community resources. Ongoing, frequent communication to keep partners informed, provide data, solicit feedback, and engage collaborators has been instrumental to the project’s success. With continued support at all levels of the organization, we have the potential to sustain falls screening as a standard of care within the clinic, across the institution, and beyond.
Supplementary Material
Acknowledgments
Matt Grant, BS, OHSU Epic support and clinical reporting; Megan Morgove, MS, of the Oregon Geriatric Education Center; Lisa Shields, BA, of the Oregon Public Health Division; Katie Bensching, MD, and Clea Cadham, MPH, of OHSU Division of General Internal Medicine and Geriatrics; Eric Wiser, MD, of OHSU Family Medicine.
Funding
This work was supported by the Health Resources and Services Administration (HRSA) of the US Department of Health and Human Services (HHS) (grant number UB4HP19057) titled “Oregon Geriatric Education Center” (total award amount of $2,138,357, 0% financed with nongovernmental sources). This information or content and conclusions are those of the author and should not be construed as the official position or policy of, nor should any endorsements be inferred by HRSA, HHS, or the US Government. Portions of the work were also conducted under an Intergovernmental Personnel Act (IPA) agreement with Centers for Disease Control and Prevention (CDC). The findings and conclusions in this manuscript are those of the authors and do not necessarily represent the official position of the CDC.
References
- Berwick DM, Nolan TW, Whittington J. The triple aim: Care, health, and cost. Health Affairs (Project Hope) 2008;27:759–769. doi: 10.1377/hlthaff.27.3.759. [DOI] [PubMed] [Google Scholar]
- Bischoff-Ferrari HA, Dawson-Hughes B, Staehelin HB, Orav JE, Stuck AE, Theiler R, Henschkowski J. Fall prevention with supplemental and active forms of vitamin D: A meta-analysis of randomised controlled trials. British Medical Journal. 2009;339:b3692. doi: 10.1136/bmj.b3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell RJ. Change management in health care. Health Care Manager. 2008;27:23–39. doi: 10.1097/01.hcm.0000285028.79762.a1. [DOI] [PubMed] [Google Scholar]
- Carayon P, Schoofs Hundt A, Karsh BT, Gurses AP, Alvarado CJ, Smith M, Flatley BP. Work system design for patient safety: The SEIPS model. Quality & Safety in Health Care. 2006;15:i50–i58. doi: 10.1136/qshc.2005.015842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Web-based Injury Statistics Query and Reporting System (WISQARS) [online] Atlanta, GA: National Center for Injury Prevention and Control; 2013. Retrieved July 13, 2015, from www.cdc.gov/injury/wisqars. [Google Scholar]
- Chang JT, Morton SC, Rubenstein LZ, Mojica WA, Maglione M, Suttorp MJ, Shekelle PG. Interventions for the prevention of falls in older adults: Systematic review and metaanalysis of randomised clinical trials. British Medical Journal. 2004;328:1–7. doi: 10.1136/bmj.328.7441.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaglio B, Shoup JA, Glasgow RE. The RE-AIM framework: A systematic review of use over time. American Journal of Public Health. 2013;103:e38–e46. doi: 10.2105/AJPH.2013.301299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasgow RE, Vogt TM, Boles SM. Evaluating the public health impact of health promotion interventions: The RE-AIM framework. American Journal of Public Health. 1999;89:1322–1327. doi: 10.2105/AJPH.89.9.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley A, Silke C, Murphy J. Community-based health efforts for the prevention of falls in the elderly. Clinical Interventions in Aging. 2010;2011:19–25. doi: 10.2147/CIA.S9489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houry D, Florence C, Baldwin G, Stevens J, McClure R. The CDC injury center’s response to the growing public health problem of falls among older adults. American Journal of Lifestyle Medicine. 2016;10:74–77. doi: 10.1177/1559827615600137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute for Healthcare Improvement. Triple aim for populations. 2015. Retrieved July 13, 2015, from http://www.ihi.org/Topics/TripleAim/Pages/default.aspx.
- Jones TS, Ghosh TS, Horn K, Smith J, Vogt RL. Primary care physicians perceptions and practices regarding fall prevention in adult’s 65 years and over. Accident; Analysis and Prevention. 2011;43:1605–1609. doi: 10.1016/j.aap.2011.03.013. [DOI] [PubMed] [Google Scholar]
- Kalyani RR, Stein B, Valiyil R, Manno R, Maynard JW, Crews DC. Vitamin D treatment for the prevention of falls in older adults: Systematic review and meta-analysis. Journal of the American Geriatrics Society. 2010;58:1299–1310. doi: 10.1111/j.1532-5415.2010.02949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotter JP. Accelerate! Harvard Business Review. 2012;90:44–52. [PubMed] [Google Scholar]
- Kotter JP, Schlesinger LA. Choosing strategies for change. Harvard Business Review. 2008;86 130+ [PubMed] [Google Scholar]
- Landis SE, Galvin SL. Implementation and assessment of a fall screening program in primary care practices. Journal of the American Geriatrics Society. 2014;62:2408–2414. doi: 10.1111/jgs.13137. [DOI] [PubMed] [Google Scholar]
- Mader SL, Hornick T, Winger J. Effect of initial recumbent or sitting positions on postural blood pressure measurements [abstract] . Gerontologist. 1987;27:206A. [Google Scholar]
- Panel on Prevention of Falls in Older Persons, American Geriatrics Society, & British Geriatrics Society. Summary of the updated American Geriatrics Society/British Geriatrics Society clinical practice guideline for prevention of falls in older persons. Journal of the American Geriatrics Society. 2011;59:148–157. doi: 10.1111/j.1532-5415.2010.03234.x. [DOI] [PubMed] [Google Scholar]
- Perry J, Bell F, Shaw T, Fitzpatrick B, Sampson EL. The use of PDSA methodology to evaluate and optimise an inner city memory clinic: A quality improvement project. BMC Geriatrics. 2014;14:4–4. doi: 10.1186/1471-2318-14-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shubert TE, Altpeter M, Busby-Whitehead J. Using the RE-AIM framework to translate a research-based falls prevention intervention into a community-based program: Lessons learned. Journal of Safety Research. 2011;42:509–516. doi: 10.1016/j.jsr/2011.09.003. [DOI] [PubMed] [Google Scholar]
- Shubert TE, Smith ML, Prizer LP, Ory MG. Complexities of fall prevention in clinical settings: A commentary. Gerontologist. 2014;54:550–558. doi: 10.1093/geront/gnt079. [DOI] [PubMed] [Google Scholar]
- Stevens JA, Phelan EA. Development of STEADI: A fall prevention resource for health care providers. Health Promotion Practice. 2013;14:706–714. doi: 10.1177/1524839912463576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoller JK. Implementing change in respiratory care. Respiratory Care. 2010;55:749–757. [PubMed] [Google Scholar]
- Tinetti ME, Gordon C, Sogolow E, Lapin P, Bradley EH. Fall-risk evaluation and management: Challenges in adopting geriatric care practices. Gerontologist. 2006;46:717–725. doi: 10.1093/geront/46.6.717. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.