Abstract
Objective
Expiratory functions that clear aspiration from the airway are compromised in patients with neurogenic dysphagia for whom cough and expiratory force may also be impaired by the primary disease process. The relationship between expiratory function, cough, and aspiration is less clear in head and neck cancer (HNC) survivors for whom the disease process does not directly impact the lower respiratory system. Our objective was to compare mechanisms of airway clearance (expiratory force and cough) with aspiration status in post-radiated HNC survivors.
Study Design
Cross-sectional study.
Methods
103 disease-free HNC survivors ≥3-months post-radiotherapy referred for modified barium swallow studies were prospectively enrolled regardless of dysphagia status. Maximum expiratory pressures (MEPs) and peak cough flow (PCF) measures were taken at enrollment and examined as a function of aspiration status using generalized linear regression methods.
Results
34 (33%) patients aspirated. MEP and PCF demonstrated a moderate positive correlation (Pearson’s r =0.35). Adjusting for sex and age, MEPs were on average 19.2% lower (21.1 cmH2O, 95% CI 5.3, 36.8) among aspirators. PCF was also 14.9% lower (59.6 L/min, 95% CI 15.8, 103.3) among aspirators after adjusting for age and sex.
Conclusions
Expiratory functions were depressed in post-radiated HNC aspirators relative to non-aspirators, suggesting that airway protection impairments may extend beyond disrupted laryngopharyngeal mechanisms in the local treatment field. Exercises to strengthen subglottic expiratory force generating capacity may offer an adjunctive therapeutic target to improve airway protection in chronic aspirators after head and neck radiotherapy.
Keywords: head and neck cancer, radiotherapy, aspiration, cough
INTRODUCTION
Dysphagia therapies increasingly incorporate targets in the respiratory system to aid in airway protection among populations at risk for aspiration. Therapies in this manner include use of novel biofeedback paradigms used to train patients to swallow at the optimal point in the respiratory cycle (i.e., respiratory swallow training, RST),1 and well as resistive exercise in the expiratory muscle strength training (EMST) program.2 Respiratory exercise, most commonly EMST, was initially applied for this indication in neurogenic populations at risk for aspiration and is proposed to aide in airway protection by strengthening subglottal expiratory force generating capacity for a more effective cough for airway clearance or by exercising airway closure muscles such submental suprahyoid elevators.3–6
Expiratory functions that clear aspiration from the airway are compromised in patients with neurogenic dysphagia for whom motor planning and neuromodulation of cough and expiratory force is also impaired by the primary disease process. The relationship between expiratory strength, cough, and aspiration, however, is less clear in head and neck cancer (HNC) survivors for whom the disease process does not directly impact the lower respiratory system. Our objective was to compare mechanisms of airway clearance (expiratory force and cough) with aspiration status in post-radiated HNC survivors. We hypothesized that: 1) maximum expiratory pressures (MEP, the direct therapeutic target of EMST) would be lower among aspirating HNC survivors relative to non-aspirating HNC survivors, and 2) cough strength would be lower among aspirating survivors relative to non-aspirating HNC survivors.
METHODS
A cross-sectional sample (n=103) was prospectively enrolled from the swallowing clinics in the Head and Neck Center of the University of Texas MD Anderson Cancer Center (Houston, TX, USA) between March, 2016 and February, 2017. Eligible patients included adults with history of curative-intent radiotherapy (in the past 15 years) at MD Anderson for a new primary head and neck cancer who were referred to the Section of Speech Pathology and Audiology for swallowing evaluation. Patients with a history of 1) recurrent disease, 2) second primary of the head and neck, central nervous system, or thoracic cavity, or 3) prior head and neck surgery (excluding diagnostic procedures, transoral surgery, or non-radical neck dissection) at the time of enrollment were excluded. Modified barium swallow (MBS) study schedules were screened. Of 166 potentially eligible participants identified during screening, 103 (62%) consented and were included in this analysis (n=20 declined participation, n=29 did not show for MBS appointments, and n=14 were missed by research personnel). This analysis was approved by the Institutional Review Board, and all subjects provided informed consent.
Expiratory testing
All patients completed two expiratory testing measures at the time of enrollment: 1) maximum expiratory pressures (MEPs), and 2) peak cough flow (PCF). MEPs and PCF are complementary measures of respiratory muscle strength supported by international thoracic and respiratory medical societies.7 MEPs were measured using a digital manometer (Micro Respiratory Pressure Meter, CareFusion, Yorba Linda, California). For each trial, the participant was instructed to inhale to total lung capacity (“fill your lungs as much as possible”), seal the lips fully around a flanged mouthpiece and exhale forcefully (“blow out as fast and as hard as you can”). Voluntary PCF was measured using the digital Mini Wright Peak Flow Meter (KW-Med, Inc., Antioch, Illinois). Patients were instructed to inhale to total lung capacity (TLC) and to “cough hard like there is something stuck in the throat” while standing. Both MEP and PCF are obtained from TLC after full inspiration, making their results in part conditional on patient effort and adequate coaching. Thus, MEP and PCF were calculated as the best of three trials within 10% variance.
Swallowing Evaluation
Swallowing evaluations included a standardized MBS study using a protocol previously described.8 MBS were acquired and recorded at 30 frames/second. Patients were administered 2 trials each of 5-mL, 10-mL, and cup sips of Varibar thin liquid barium, teaspoon presentation of Varibar pudding, and a ¼ cracker coated in Varibar pudding in a mid-sagittal lateral plane, and single presentation of barium in AP coronal plane. Penetration-Aspiration Scale (PAS) scores and the Dynamic Imaging Grade of Swallowing Toxicity (DIGEST) were assigned by blinded post hoc analysis by a trained laboratory rater.
Penetration-Aspiration Scale
The Penetration-Aspiration Scale is a commonly reported 8-point ordinal scale that ranks the safety of the swallow by the depth of bolus entry into the airway and the patient’s response (0=no airway entry, 8=silent aspiration).9 PAS scores were first assigned for each bolus trial based on all attempts to swallow the bolus. In this manner, bolus-level PAS scores represented the highest grade of penetration and aspiration events at any point in a single bolus trial (i.e., before, during, or after swallowing). The maximum PAS score across all bolus trials was used for analysis. Maximum PAS was selected as this parameter is associated with elevated risk of aspiration pneumonia in post-radiated HNC survivors.10 Patients with maximum PAS scores of 1 –5 were designated as non-aspirators, and those with PAS scores of 6 – 8 were designated as aspirators.
DIGEST
DIGEST is a validated tool to grade the severity of pharyngeal dysphagia based on results of an MBS study.8 DIGEST first assigns two component scores: 1) safety classification and 2) efficiency classification. To derive the safety classification, the rater assigns the maximum PAS score observed across a series of standard bolus trials with a modifier applied to account for the frequency and amount of penetration/aspiration events. To derive the efficiency classification, the rater assigns an estimation of the maximum percentage of pharyngeal residue on an ordinal scale (<10%, 10–49%, 50–90%, and >90%) with modifiers to assign a pattern of residue across bolus types. The summary DIGEST rating aligns with NCI’s Common Terminology Criteria for Adverse Events framework for toxicity reporting in oncology trials, and is based on the interaction of the safety and efficiency classification (grade 0=no pharyngeal dysphagia, 1=mild, 2=moderate, 3=severe, 4=life threatening). In a validation study of 100 HNC patients, intra-rater reliability was excellent (weighted Kappa=0.82–0.84) with substantial to almost perfect agreement between raters (weighted Kappa=0.67–0.81). DIGEST significantly discriminated levels of pharyngeal pathophysiology (MBSImP™©11 r=0.77, p<0.0001), swallow efficiency (Oropharyngeal Swallow Efficiency:12 r=−0.56, p<0.0001), perceived dysphagia (MDADI:13 r=−0.41, p<0.0001), and oral intake (PSS-HN Normalcy of Diet:14 r=−0.49, p<0.0001) in the validation study.
Statistical Plan
Two measures of expiratory function, MEP (primary outcome measure) and PCF (secondary outcome measure), were examined separately as a function of aspiration status. The distribution MEP and PCF approximated a normal distribution. MEP and PCF between aspirators and non-aspirators were initially compared using two-group t-tests. Other bivariate associations were examined using a Fisher’s exact test or a two-group t-test. To describe the bivariate relationship between MEP and PCF, we used a Pearson’s correlation coefficient along with 95% confidence limits constructed using Fisher’s z transformation. Correlation coefficients were interpreted per Cohen as small, medium, and large effect sizes corresponding to a Pearson’s r of .1, .3, and .5, respectively or an η2 of .02, .13, and .2615
Multiple linear regression models were then fit for the purpose of predicting mean MEP and PCF for aspirators and non-aspirators after adjusting for clinically important covariates. Age and sex were set a priori as known confounders to retain in final models. Additional candidates covariates considered for modeling included BMI, smoking status and smoking pack years, tumor T-stage, months since last radiation, radiation dose, and chemotherapy. Tumor site, COPD diagnosis, and chemotherapy use (yes or no) lacked variability; therefore, these variables were excluded from modeling procedures. Months since last radiation treatment was log transformed to better approximate a normal distribution. Because very few patients were current smokers, smoking pack-years rather than current, former, or never smoking status was used to quantify smoking exposure. Radiation dose had little variability with approximately 77% of patients receiving a cumulative dose of 70 Gy. Therefore, we dichotomized radiation dose into two categories (<70 vs. ≥70 Gy). Variables were entered into the model using a backward stepwise selection procedure that retained variables significant at p < .05. Age and sex, known confounders, were retained in the models regardless of statistical significance level. Preliminary screening procedures included inspection of variable distributions using scatter plots and box plots to detect univariate and bivariate outliers. Examination of model residuals confirmed the assumption of linearity. Model diagnostics were performed to assess multicollinearity, overly influential observations, and interactions. Least squares means and 95% confidence limits for MEP and PCF by aspiration status are reported.
Sensitivity analyses considered secondary dysphagia classification methods: 1) maximum PAS as an ordinal variable, and 2) DIGEST classification of pharyngeal dysphagia. To describe the relationship of MEP or PCF with an ordinal variable (PAS or DIGEST), we used a polyserial correlation coefficient with p-values based on the likelihood ratio test. All statistical procedures were conducted in SAS for Windows version 9.4 TS1M0 (Cary, NC).
RESULTS
Patient characteristics
103 disease-free HNC survivors were included. Mean age was 61 (SD: 9) and 91% were male. The majority of patients had a history of multimodality cancer treatment, and more than half were treated for oropharyngeal primary tumors. Among 90 patients with oropharyngeal or unknown primary tumors, HPV and p16 status was unknown in 14 (16%), negative in 2 (2%), and positive in 74 (82%). Median time post-cancer treatment was 11.6 months (range 3.0 to 159.5), and 89% of patients had completed treatment within 30 months before enrollment. Table I summarizes clinical and demographic characteristics of the study population. One-third (n=34, 33%) of patients aspirated (Figure 1), and 17% (n=18) had severe to profound (grade ≥3) MBS-detected pharyngeal dysphagia per DIGEST (Table II, Figure 2).
Table I.
Sample characteristics by aspiration status among 103 participants
| Characteristic | All patients | Aspirators | Non-Aspirators | P-value |
|---|---|---|---|---|
| Sex | .473 | |||
| Male | 94 (91%) | 30 (88%) | 64 (93%) | |
| Female | 9 (9%) | 4 (12%) | 5 (7%) | |
|
| ||||
| Age | ||||
| Mean±SD | 61.34±9.19 | 65.4±7.6 | 59.3±9.3 | .001 |
|
| ||||
| T Staging | .084 | |||
| T0/T1 | 22 (21%) | 4 (12%) | 18 (26%) | |
| T2 | 33 (32%) | 9 (26%) | 24 (35%) | |
| T3 | 23 (22%) | 8 (24%) | 15 (22%) | |
| T4 | 25 (24%) | 13 (38%) | 12 (17%) | |
|
| ||||
| N Staging | .507 | |||
| N0 | 11 (11%) | 5 (15%) | 6 (9%) | |
| N1 | 11 (11%) | 4 (12%) | 7 (10%) | |
| N2 | 75 (73%) | 22 (65%) | 53 (77%) | |
| N3 | 6 (6%) | 3 (9%) | 3 (4%) | |
|
| ||||
| Site | .267 | |||
| Oropharynx | 87 (84%) | 26 (76%) | 61 (88%) | |
| Nasopharynx | 1 (1%) | 1 (3%) | 0 (0%) | |
| Hypopharynx | 3 (3%) | 1 (3%) | 2 (3%) | |
| Larynx | 9 (9%) | 5 (15%) | 4 (6%) | |
| Unknown | 3 (3%) | 1 (3%) | 2 (3%) | |
| Primary | ||||
|
| ||||
| Treatment | .002 | |||
| CRT | 69 (67%) | 18 (53%) | 51 (74%) | |
| Induction + CRT | 21 (20%) | 12 (35%) | 9 (13%) | |
| Induction + RT | 3 (3%) | 3 (9%) | 0 (0%) | |
| RT alone | 2 (2%) | 1 (3%) | 1 (1%) | |
| Surgery + | 6 (6%) | 0 (0%) | 6 (9%) | |
| POCRT | 2 (2%) | 0 (0%) | 2 (3%) | |
| Surgery + PORT | ||||
|
| ||||
| Smoking Status | .305 | |||
| Never | 68 (66%) | 19 (56%) | 49 (71%) | |
| Former | 29 (28%) | 13 (38%) | 16 (23%) | |
| Current | 6 (6%) | 2 (6%) | 4 (6%) | |
|
| ||||
| Smoking Pack-Years for Former or Current Smokers | ||||
| Mean±SD | 35.7±19.6 | 36.1±20.8 | 35.5±19.2 | .938 |
|
| ||||
| BMI | .007 | |||
| Underweight | 2 (2%) | 2 (6%) | 0 (0%) | |
| Healthy weight | 34 (33%) | 17 (50%) | 17 (25%) | |
| Overweight | 47 (46%) | 11 (32%) | 36 (52%) | |
| Obese | 20 (19%) | 4 (12%) | 16 (23%) | |
|
| ||||
| MEP | ||||
| Mean±SD | 119.2±39.3 | 100.8±35.2 | 128.2±38.3 | <0.001 |
|
| ||||
| PCF | ||||
| Mean±SD | 438.3±114.9 | 380.3±112.9 | 466.8±105.4 | <0.001 |
Figure 1.
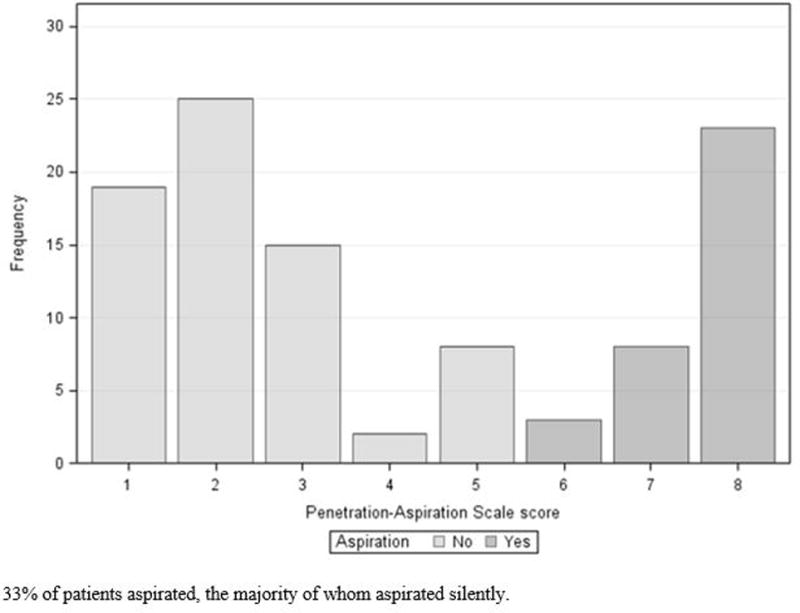
Distribution of maximum penetration-aspiration scale scores among 103 patients
Table II.
Pharyngeal dysphagia severity according to DIGEST classification
| No. (%) of Patients | |
|---|---|
| DIGEST | |
| 0, no dysphagia | 20 (19%) |
| 1, mild | 37 (36%) |
| 2, moderate | 28 (27%) |
| 3, severe | 16 (16%) |
| 4, profound (life-threatening) | 2 (2%) |
Abbreviation: DIGEST, Dynamic Imaging Grade of Swallowing Toxicity
Figure 2.
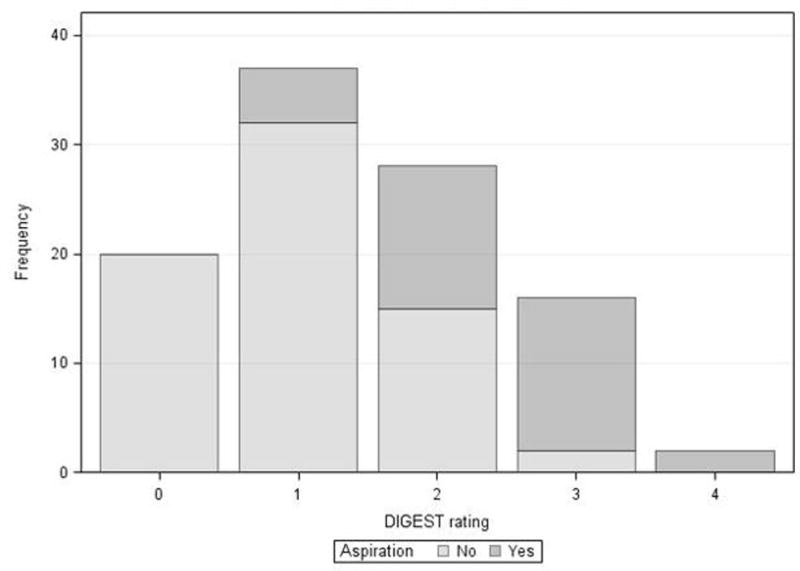
DIGEST by aspiration status among 103 patients
Expiratory function in HNC patients
MEP and PCF demonstrated a moderate, positive correlation (Pearson r=0.35, 95% CI: 0.17–0.51, Figure 3).
Figure 3.
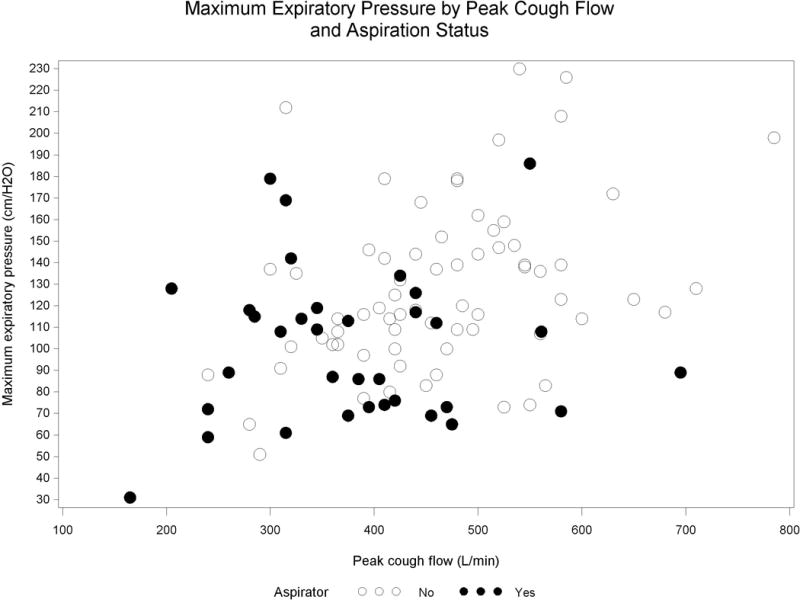
Maximum expiratory pressures by peak cough flow and aspiration status among 103 patients
MEPs and Aspiration Status
MEPs were 21% lower (mean difference 27.4 cmH2O, 95% CI 11.9, 42.9) among aspirators relative to non-aspirators (mean ± SD: 100.8 ± 35.2 aspirators v. 128.2 ± 38.3 non-aspirators, p<0.001), corresponding to a medium effect size (η2 = .11, 95% CI .02, .23). Generalized linear modeling indicated that aspirator status was significantly associated with MEP (F(1,101) = 12.30, p<0.001), accounting for approximately 11% of the variance in MEP. On multivariate analysis controlling for sex and age (Table III), MEPs remained significantly associated with aspiration status. After multivariate adjustment, estimated MEPS remained 19.2% lower among aspirators relative to non-aspirators (mean difference 21.1 cmH2O, 95% CI 5.3, 36.8). Sensitivity analysis considering alternate dysphagia severity classifiers included analysis of maximum PAS as an ordinal variable and summary pharyngeal dysphagia grade per DIGEST. Maximum PAS scores, showed a moderate negative correlation with MEP (r = -.38, SE = .09, p <.001). There was also a moderate negative correlation between MEP and DIGEST pharyngeal dysphagia grade (r = −.40, SE = .09, p < .001).
Table III.
Multiple regression model for maximum MEP (n = 103)
| Variable | Parameter Estimate |
Standard Error |
95% Confidence Interval | Standardized Estimate |
p | |
|---|---|---|---|---|---|---|
| Intercept | 175.43 | 25.00 | 125.83 | 225.04 | <.0001 | |
| Age (year) | −0.75 | 0.41 | −1.56 | 0.07 | −0.17 | .0728 |
| Patient sex (female) | −39.93 | 12.71 | −65.14 | −14.71 | −0.29 | .0022 |
| Aspiration (yes) | −21.05 | 7.95 | −36.82 | −5.29 | −0.25 | .0094 |
| Aspirator | Adjusted Mean | 95% Confidence Limit | |
|---|---|---|---|
| Yes | 88.59 | 73.10 | 104.07 |
| No | 109.64 | 95.56 | 123.72 |
| Difference | −21.05 | −36.82 | −5.29 |
F(3,99) = 8.30, p < .0001 R2 = .20 R2adj = .18
PCF and Aspiration Status
PCF was 18.8% lower (mean difference 86.5 L/min, 95% CI 41.6, 131.4) among aspirators relative to non-aspirators (mean ± SD: 380.3±112.9 aspirators v. 466.8±105.4 non-aspirators, p<0.001), corresponding to a medium effect size (η2 = .13, 95% CI .03, 0.25). On multivariate analysis controlling for age and sex (Table IV), PCFs remained significantly associated with aspiration status. After multivariate adjustment, estimated PCF was 14.9% lower among aspirators (mean difference 59.6 L/min, 95% CI 15.8, 103.3). Sensitivity analysis considering maximum PAS scores as an ordinal variable, exhibited a moderate negative correlation with PCF (r = -.33, SE = .09, p<0.001). There was also a moderate negative correlation between PCF and DIGEST pharyngeal dysphagia grade (r = −.28, SE = .10, p = .003).
Table IV.
Multiple regression model for maximum PCF (n = 103)
| Variable | Parameter Estimate |
Standard Error |
95% Confidence Interval | Standardized Estimate |
p | |
|---|---|---|---|---|---|---|
| Intercept | 676.79 | 69.39 | 539.11 | 814.48 | <.0001 | |
| Age (year) | −3.37 | 1.14 | −5.64 | −1.10 | −0.27 | .0040 |
| Patient sex (female) | −140.48 | 35.27 | −210.46 | −70.49 | −0.35 | .0001 |
| Aspiration (yes) | −59.56 | 22.05 | −103.32 | −15.80 | −0.24 | .0081 |
| Aspirator | Adjusted Mean | 95% Confidence Limit | |
|---|---|---|---|
| Yes | 340.40 | 297.43 | 383.37 |
| No | 399.96 | 360.88 | 439.03 |
| Difference | −59.56 | −103.32 | −15.80 |
F(3,99) = 12.76, p < .0001 R2 = .28 R2adj = .26
DISCUSSION
Aspiration is a potentially life-threatening effect of head and neck radiotherapy. In the post-radiated head and neck cancer population, aspiration is thought to result from sensorimotor disruption of laryngopharyngeal functions that prevent the airway from fully closing during a swallow or cause incomplete bolus clearance prompting aspiration of post-swallow bolus residue. For this reason, much attention is given to radiation dose-distributions to dysphagia-aspiration related structures and resultant biomechanical aberrations in laryngopharyngeal functioning during swallowing after radiotherapy.16,17 Airway protection, however, is not only dependent on airway closure during a swallow. Rather, airway protection represents a continuum of integrated behaviors from airway closure to effective cough to clear the airway if foreign body entry occurs. A framework for this continuum of behaviors has been proposed by Troche et al, emphasizing shared central and peripheral neural substrates of swallowing and cough.18 Considering both sides of the continuum of airway protection, we hypothesized that expiratory functions would be reduced in aspirating HNC survivors. Herein, we identified significant associations between aspiration status and measures of respiratory muscle strength in a cross-sectional sample of post-radiated head and neck cancer survivors. Both MEPs (expiratory force generating capacity) and PCF (cough strength) were significantly lower in aspirators relative to non-aspirators, and these (medium) effect sizes were stable in multivariate models adjusting for confounding effects of age and sex.
As hypothesized, MEP was significantly lower in aspirating HNC survivors relative to non-aspirating survivors. This is a novel finding that supports the premise that strengthening subglottic expiratory force generating capacity (i.e., MEP) might be an appropriate adjunctive therapy target to improve airway protection in the HNC population. The relationship of MEP and aspiration is largely unknown, to our knowledge, not previously reported in published work. We chose to examine the relationship of MEP and aspiration because MEP is the direct therapeutic target of EMST, which is increasingly used for therapy for airway protection in dysphagia or dysphagia at risk populations. The reason for the strong relationship between MEP and aspiration status in HNC survivors is not entirely clear. We speculate that pulmonary capacity and reserve may provide a physiologic foundation that leads to greater ability to protect the airway. For example, greater respiratory capacity could associate with longer laryngeal vestibule closure during swallowing. Future studies could examine which biomechanical or temporal parameters of swallowing physiology drive this association between aspiration and MEP. Longitudinal studies are also needed to understand whether depressed MEPs were present at baseline (before cancer therapy) representing less respiratory reserve or whether MEPs diminish over time in HNC patients who develop aspiration as a chronic toxicity of cancer therapy. No matter the reason, depressed MEPS in aspirating HNC survivors might be improved by strengthening exercise with EMST.19
We also found that PCF was significantly lower in aspirating HNC survivors relative to non-aspirating survivors. The relationship between PCF and aspiration, as well as related outcomes such as dysphagia and pneumonia, is far more commonly studied than MEP, but to our knowledge not previously published in HNC populations. PCF is often studied as it relates to dysphagia broadly or pneumonia rather than specifically as it relates to aspiration status, as was the focus of this study. PCF is depressed in populations with dysphagia related to neurogenic pathology. For instance, PCF is depressed in stroke patients with dysphagia relative to matched healthy controls (Kimura 201320) as well as in PD participants with penetration or aspiration on videofluoroscopy relative to PD participants with normal airway closure during swallow.21,22 Perhaps most notable in reference to the relationship of PCF and airway protection is the significant association between voluntary PCF and pneumonia, previously reported in acute stroke populations with regression models estimating approximately 2% reduction in pneumonia risk for every 10 L/min increase in PCF.22 MEP and PCF are directly related.23 For instance, in patients with neurodegenerative disease (i.e., Duchenne Muscular Dystrophy and ALS), MEP and PCF were strongly inversely correlated (r= −0.75, p<0.001) and the determination correlation suggested that approximately 58% of PCF changes could be predicted by MEP changes.20 We likewise found a moderate correlation between PCF and MEP in this HNC population. Given the correlation between MEP and PCF, we might also propose the hypothesis that pneumonia risk may be decreased by strengthening MEP and PCF with exercise therapies. Pneumonia was not examined in this study, and this hypothesis requires testing in future studies.
Herein, we report novel associations between measures of expiratory function and aspiration status using prospective, cross-sectional sampling of post-radiated HNC survivors. The significant correlations between aspiration status and expiratory muscle strength parameters maintained in multivariate analysis. These associations were derived from observations in a sample of 103 subjects with 34 aspirators, limiting precision of the estimates and requiring validation in a sample that was not used to derive the model. Longitudinal data collection is underway in effort to validate these observations in a new sample, and also to understand the evolution of these associations from cancer diagnosis through post-radiation survivorship. Limited expiratory testing was performed including MEPs and spirometry PCF to ensure brief testing of volunteer participants recruited during busy clinical visits. It is likely that more detailed analysis of cough airflow parameters on pneumotachograph or the additional testing of inspiratory pressures might help to better describe the nature of the observed respiratory and swallowing relationship. This study used a cross-sectional design recruiting patients referred for modified barium swallow studies. This sampling method inherently introduces threat of referral bias with likely over-sampling of survivors with dysphagia. It is noteworthy that 58% of MBS were scheduled for routine dysphagia surveillance per institutional post-radiation swallowing pathways rather than purely by symptomatic referral. Accordingly, more than 55% of the sample had no or mild pharyngeal dysphagia (DIGEST grade ≤1). Finally, expansions of this work might include dose-response analysis considering non-target bystander dose to shared respiratory/swallowing regions during cancer treatment.
CONCLUSION
Expiratory functions were depressed in post-radiated HNC aspirators relative to non-aspirators, suggesting that airway protection impairments may extend beyond disrupted laryngopharyngeal mechanisms in the local treatment field. Exercises to strengthen subglottic expiratory force generating capacity may offer a novel therapeutic target to improve airway protection in chronic aspirators after head and neck radiotherapy.
Acknowledgments
Financial Disclosures and Conflicts of Interest: This work with accomplished with support of the MD Anderson Institutional Research Grant Program Survivorship Seed Monies Award. Dr. Hutcheson receives funding support from the National Cancer Institute (R03CA188162-01). Drs. Hutcheson and Lai, receive funding support from the National Institutes of Health (NIH)/National Institute for Dental and Craniofacial Research (1R01DE025248-01/R56DE025248-01. The authors also acknowledge infrastructure support from NCI Cancer Center Support Grant (P30CA016672).
Footnotes
Portions of this work were presented at the American Laryngological Association at the 2017 Combined Otolaryngology Spring Meeting in San Diego, California, April 26-28, 2017.
Level of Evidence: 2b
Bibliography
- 1.Martin-Harris B, McFarland D, Hill EG, et al. Respiratory-swallow training in patients with head and neck cancer. Arch Phys Med Rehabil. 2015;96:885–893. doi: 10.1016/j.apmr.2014.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sapienza C, Troche M, Pitts T, Davenport P. Respiratory strength training: concept and intervention outcomes. Semin Speech Lang. 2011;32:21–30. doi: 10.1055/s-0031-1271972. [DOI] [PubMed] [Google Scholar]
- 3.Hegland KW, Davenport PW, Brandimore AE, Singletary FF, Troche MS. Rehabilitation of swallowing and cough functions following stroke: an expiratory muscle strength training trial. Arch Phys Med Rehabil. 2016;97:1345–1351. doi: 10.1016/j.apmr.2016.03.027. [DOI] [PubMed] [Google Scholar]
- 4.Pitts T, Bolser D, Rosenbek J, Troche M, Okun MS, Sapienza C. Impact of expiratory muscle strength training on voluntary cough and swallow function in Parkinson disease. Chest. 2009;135:1301–1308. doi: 10.1378/chest.08-1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Troche MS, Okun MS, Rosenbek JC, et al. Aspiration and swallowing in Parkinson disease and rehabilitation with EMST: a randomized trial. Neurology. 2010;75:1912–1919. doi: 10.1212/WNL.0b013e3181fef115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Plowman EK, Watts SA, Tabor L, et al. Impact of expiratory strength training in amyotrophic lateral sclerosis. Muscle Nerve. 2016;54:48–53. doi: 10.1002/mus.24990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.American Thoracic Society/European Respiratory Society. ATS/ERS Statement on respiratory muscle testing. Am J Respir Crit Care Med. 2002;166:518–624. doi: 10.1164/rccm.166.4.518. [DOI] [PubMed] [Google Scholar]
- 8.Hutcheson KA, Barrow MP, Barringer DA, et al. Dynamic Imaging Grade of Swallowing Toxicity (DIGEST): Scale development and validation. Cancer. 2016;123:62–70. doi: 10.1002/cncr.30283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosenbek JC, Robbins JA, Roecker EB, Coyle JL, Wood JL. A penetration-aspiration scale. Dysphagia. 1996;11:93–98. doi: 10.1007/BF00417897. [DOI] [PubMed] [Google Scholar]
- 10.Hunter KU, Lee OE, Lyden TH, et al. Aspiration pneumonia after chemo-intensity-modulated radiation therapy of oropharyngeal carcinoma and its clinical and dysphagia-related predictors. Head Neck. 2014;36:120–125. doi: 10.1002/hed.23275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin-Harris B, Brodsky MB, Michel Y, et al. MBS measurement tool for swallow impairment-MBSImp: establishing a standard. Dysphagia. 2008;23:392–405. doi: 10.1007/s00455-008-9185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rademaker AW, Pauloski BR, Logemann JA, Shanahan TK. Oropharyngeal swallow efficiency as a representative measure of swallowing function. J Speech Hear Res. 1994;37:314–325. doi: 10.1044/jshr.3702.314. [DOI] [PubMed] [Google Scholar]
- 13.Chen AY, Frankowski R, Bishop-Leone J, et al. The development and validation of a dysphagia-specific quality-of-life questionnaire for patients with head and neck cancer: the M. D. Anderson dysphagia inventory. Arch Otolaryngol Head Neck Surg. 2001;127:870–876. [PubMed] [Google Scholar]
- 14.List MA, Ritter-Sterr C, Lansky SB. A performance status scale for head and neck cancer patients. Cancer. 1990;66:564–569. doi: 10.1002/1097-0142(19900801)66:3<564::aid-cncr2820660326>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 15.Cohen J. Statistical power analysis for the behavioral sciences. Hilsdale NJ: Lawrence Earlbaum Associates; 1988. p. 2. [Google Scholar]
- 16.M. D. Anderson Head Neck Cancer Symptom Working Group. Beyond mean pharyngeal constrictor dose for beam path toxicity in non-target swallowing muscles: Dose-volume correlates of chronic radiation-associated dysphagia (RAD) after oropharyngeal intensity modulated radiotherapy. Radiother Oncol. 2016;118:304–314. doi: 10.1016/j.radonc.2016.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eisbruch A, Kim HM, Feng FY, et al. Chemo-IMRT of oropharyngeal cancer aiming to reduce dysphagia: swallowing organs late complication probabilities and dosimetric correlates. Int J Radiat Oncol Biol Phys. 2011;81:e93–99. doi: 10.1016/j.ijrobp.2010.12.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Troche MS, Brandimore AE, Godoy J, Hegland KW. A framework for understanding shared substrates of airway protection. J Appl Oral Sci. 2014;22:251–260. doi: 10.1590/1678-775720140132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baker S, Davenport P, Sapienza C. Examination of strength training and detraining effects in expiratory muscles. J Speech Lang Hear Res. 2005;48:1325–1333. doi: 10.1044/1092-4388(2005/092). [DOI] [PubMed] [Google Scholar]
- 20.Kimura Y, Takahashi M, Wada F, Hachisuka K. Differences in the peak cough flow among stroke patients with and without dysphagia. J UOEH. 2013;35:9–16. doi: 10.7888/juoeh.35.9. [DOI] [PubMed] [Google Scholar]
- 21.Hegland KW, Okun MS, Troche MS. Sequential voluntary cough and aspiration or aspiration risk in Parkinson’s disease. Lung. 2014;192:601–608. doi: 10.1007/s00408-014-9584-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kulnik ST, Birring SS, Moxham J, Rafferty GF, Kalra L. Does respiratory muscle training improve cough flow in acute stroke? Pilot randomized controlled trial. Stroke. 2015;46:447–453. doi: 10.1161/STROKEAHA.114.007110. [DOI] [PubMed] [Google Scholar]
- 23.Suarez AA, Pessolano FA, Monteiro SG, et al. Peak flow and peak cough flow in the evaluation of expiratory muscle weakness and bulbar impairment in patients with neuromuscular disease. Am J Phys Med Rehabil. 2002;81:506–511. doi: 10.1097/00002060-200207000-00007. [DOI] [PubMed] [Google Scholar]


