Abstract
PEGylated asparaginase (pegaspargase) can be administered via intramuscular (IM) injection or intravenous (IV) infusion with a hypersensitivity reaction (HSR) incidence ranging 3–41%. We evaluated grade ≥3 HSRs when given IM vs. IV on six Children’s Oncology Group (COG) leukemia trials (2003–2015) to determine differences in HSR rates. 54,280 doses were administered to 16,534 patients. Considering all doses of pegaspargase during induction, consolidation, and delayed intensification, grade ≥3 HSR rate with IM injection was 5.4% (n = 482/8981) compared to 3.2% for IV (n = 245/7553) (p < .0001). If only the second and third doses of pegaspargase were analyzed, where the majority of grade ≥3 HSRs occur, the rate following IM injection was 10.1% (n = 459/4534) compared to 5.0% (n = 222/4443) for IV (p < .0001). On standardized treatment protocols conducted by the COG during 2003–2015, grade ≥3 HSR rates to pegaspargase occurred less frequently with IV infusion than IM injection.
Keywords: Pegaspargase, asparaginase, hypersensitivity, intravenous, intramuscular, ALL
Introduction
Acute lymphoblastic leukemia (ALL) is the most common pediatric malignancy, accounting for 25% of all cancers in children <15 years of age [1]. Outcomes have steadily improved over time with overall mean survival now >85%, [2] in part through the incorporation of asparaginase with multi-agent chemotherapy. The predominant asparaginase preparation used in contemporary ALL therapy in North America is PEGylated Escherichia coli asparaginase (pegaspargase) which can be administered either via intravenous infusion (IV) or intramuscular (IM) injection. The mechanism of action of asparaginase is the enzymatic breakdown of asparagine and glutamine into aspartic acid, glutamic acid, and ammonia. As lymphoblasts cannot synthesize asparagine, a necessary amino acid for cell growth and differentiation, the absence of asparagine from surrounding sources leads to diminished lymphoblast survival [3]. Through its prolonged depletion of asparagine, pegaspargase results in reduced DNA, RNA, and protein synthesis leading to cell death [3].
As all asparaginases are foreign proteins with strong immunogenicity, allergic, and/or anaphylactic reactions are reported to occur in 3–41% of patients receiving asparaginase therapy [4–8]. When IV infusion of pegaspargase began to replace the IM injection as the predominant route of administration in North America there were reports of a greater rate of hypersensitivity reactions (HSR) following IV infusion [9,10]. Although these reports were primarily single institution case reports with a limited number of patients, concern over the rate of HSR with IV administered pegaspargase was growing amongst pediatric oncology providers. We therefore evaluated the reported HSR rate following pegaspargase therapy on six Children’s Oncology Group (COG) ALL clinical trials conducted between 2003 and March 2015 to determine if there was a difference in the rate of grade ≥3 HSR between IV and IM administration.
Methods
Toxicity data from six COG ALL trials (AALL0232/NCT00075725, AALL0331/NCT00103285, AALL0434/NCT00408005, AALL07P4/NCT00671034, AALL0932/NCT01190930, and AALL1131/NCT01406756) (Supplementary Figure 1) were analyzed for HSR adverse events attributed to pegaspargase. Investigators performed all human investigations after approval by their local Human Investigations Committee and in accord with an assurance filed with and approved by the Department of Health and Human Services, where appropriate. In addition, such data was anonymized to protect the identities of subjects involved in the research. All investigators obtained informed consent from each participant or each participant's guardian. See Table 1 for a description of each of these trials that includes the dates of patient accrual; number of evaluable/evaluated patients; number of protocol-specified pegaspargase doses and the treatment phase it was to be administered; the phase of therapy evaluated for HSR to pegaspargase and the number of doses reviewed; and whether concomitant protocol specified steroid therapy was to be administered during the treatment phase. Patients with Down syndrome were not included in this analysis as these patients were not consistently included across all six COG studies in this analysis. Patients with NCI Standard-Risk (SR) ALL received a standard COG BFM backbone which did not include pegaspargase during Consolidation or Interim Maintenance while patients with T-ALL or High-Risk (HR) B-ALL received a COG augmented BFM backbone where pegaspargase was given twice during Consolidation and twice during Interim Maintenance, when escalating methotrexate with pegaspargase was used. Trials AALL0331 [11] and AALL0932 enrolled 5164 and 5195 patients with SR B-ALL respectively, in which for the purpose of this analysis, we included only those patients that received a total of two doses of pegaspargase (n = 1380) for a more homogeneous comparison, excluding patients on AALL0331 randomized to augmented Interim Maintenance and Delayed Intensification (DI) where two extra doses of pegaspargase were given. Patients enrolled on AALL0232 [12] (HR B-ALL) or AALL0434 [13] (T-ALL or T-lymphoblastic lymphoma (LL)) randomized to escalating methotrexate with pegaspargase for Interim Maintenance I were excluded from the analysis when comparing total rates of grade ≥3 HSR. However, these patients were included for comparisons for pegaspargase grade ≥3 HSR occurring after dose #2 or #3 (during Consolidation) to provide a more uniform group of HR B-ALL and T-ALL/LL patients receiving similar therapy. These analyses did not include HR and Very High-Risk (VHR) patients with B-ALL who enrolled on AALL1131 and were randomized to experimental arms 1 or 2, or patients who were enrolled on AALL07P4 [14] and randomized to receive EZN-2285 (calaspargase pegol; SC-PEG). The dose of pegaspargase across all six trials was 2500 IU/m2 with no dose capping allowed on protocol with an infusion time of 1–2 h recommended for intravenous administration.
Table 1.
COG trials evaluated for IM vs. IV grade ≥3 HSR reactions.
| COG AALL0331 | B-precursor ALL-NCI standard risk (ages >1 year and <10 years) | |
| Route of pegaspargase administration evaluated: | IM Amendment to ‘May give IV’ on 5/30/11 occurred after accrual closed | |
| Accrual interval: [open |closed] | 4/15/2005 | 5/28/2010 | |
| Number of patients entered | 5305 | |
| Excluded intensive/experimental arms of the post Induction randomizations for LR and SR, on which additional doses of asparaginase were administered | ||
| Number doses of pegaspargase administered: | 2 (1 during induction, 1 during delayed intensification (DI) | |
| Treatment phase evaluated for HSR and number of doses evaluated: | DI (1899 pegaspargase doses) | |
| Concomitant steroid (therapy component): | Yes (dexamethasone days 1–7 & 15–21); pegaspargase given day 4 OR 5 OR 6 | |
| COG AALL0932 | B-precursor ALL-NCI standard risk (ages >1 year and <10 years) | |
| Route of pegaspargase administration evaluated: | IV IM not permitted | |
| Accrual interval: [open | closed] | 8/9/2010 | still accruing | |
| Number of patients entered | 6614 | |
| Number doses of pegaspargase administered: | 2 (1 during induction, 1 during DI) | |
| Excluded P9904 based regimen for LR and regimen for HR, on which additional doses of asparaginase were administered | ||
| Treatment phase evaluated for HSR and number of doses evaluated: | DI (2300 pegaspargase doses) | |
| Concomitant steroid (therapy component): | Yes (dexamethasone days 1–7 & 15–21); pegaspargase given day 4 OR 5 OR 6 | |
| COG AALL0232 | B-precursor ALL-NCI high risk (ages >1 year and <31 years) | |
| Route of pegaspargase administration evaluated: | IM Small number of patients who received IV toward end of study were excluded | |
| Accrual interval: [open | closed] | 4/15/2005 | 1/21/2011 | |
| Number of patients entered | 3083 | |
| Excluded patients who received extended induction on AALL0232. Patients assigned to receive escalating methotrexate with pegaspargase in Interim Maintenance phase, were excluded from the comparison of hypersensitivity rates in DI. The study was amended (04/04/2011) after accrual was completed to change from IM to allow IV administration of PEG. Patients who were in DI after 04/04/2011 were excluded from the above comparison. | ||
| Number doses of pegaspargase administered: | 5 (1 during induction, 2 during consolidation, and 2 during DI) | |
| Treatment phase evaluated for HSR and number of doses evaluated: | Consolidation (5006 pegaspargase doses) | |
| Concomitant steroid (therapy component): | No | |
| COG AALL1131 | B-precursor ALL-NCI high risk (Ages >1 year and <31 years) | |
| Route of pegaspargase administration evaluated: | IV IM not permitted | |
| Accrual interval: [open | closed] | 2/27/2012 | Still accruing | |
| Number of patients entered | 1768 | |
| Excluded patients assigned to experimental arms 1 or 2 of the VHR randomization on AALL1131. | ||
| Number doses of pegaspargase administered: | 5 (1 during induction, 2 during consolidation, and 2 during DI) | |
| Treatment phase evaluated for HSR and number of doses evaluated: | Consolidation (870 pegaspargase doses) | |
| Concomitant steroid (therapy component): | No | |
| COG AALL07P4 | B-precursor ALL-NCI High-Risk (ages >1 year and <31 years) | |
| Route of pegaspargase administration evaluated: | IV IM not permitted | |
| Accrual interval: [open | closed] | 7/21/2008 | 9/4/2012 | |
| Number of patients entered | 54 | |
| Patients assigned to receive escalating methotrexate with pegaspargase in Interim Maintenance phase were excluded from the comparison of HSR rates in DI. Only patients who were in induction, consolidation or DI before 8/13/2012 were included in the HSR analysis. Patients randomized to calaspargase pegol were excluded. | ||
| Number doses of pegaspargase administered: | 5 (1 during induction, 2 during consolidation, and 2 during DI) | |
| Treatment phase evaluated for HSR and number of doses evaluated: | Consolidation (86 pegaspargase doses) | |
| Concomitant steroid (therapy component): | No | |
| COG AALL0434 | T-cell ALL & T-cell non-Hodgkin lymphoma (lymphoblastic lymphoma) (ages >1 year and <31 years) | |
| Route of pegaspargase administration evaluated: | IM → IV Amendment on 8/13/2012 permitted IV administration hence both IM and IV could be evaluated on this trial | |
| Accrual interval: [open | closed] | 1/22/2007 | 7/25/2014 | |
| Number Patients Entered | 1649 | |
| Patients who were in induction, consolidation, or DI before or after 8/13/2012 were considered to have had IM or IV routes, respectively. | ||
| Patients assigned to receive escalating methotrexate with pegaspargase in Interim Maintenance phase, were excluded from the comparison of hypersensitivity rates in DI. | ||
| Number doses of pegaspargase administered: | 3 or more, depending on randomization | |
| Treatment phase evaluated for HSR and number of doses evaluated: | Consolidation (2300 pegaspargase doses) | |
| Concomitant Steroid (Therapy Component): | No | |
| Number of pegaspargase doses evaluated for HSR: | 15,893 of which 2300 were evaluated during consolidation (763 patients for IM administration and 397 patients for IV administration | |
NCI: National Cancer Institute; IM: intramuscular; IV: intravenous; LR: low-risk; SR: standard-risk; DI: delayed intensification; HSR: hypersensitivity reaction.
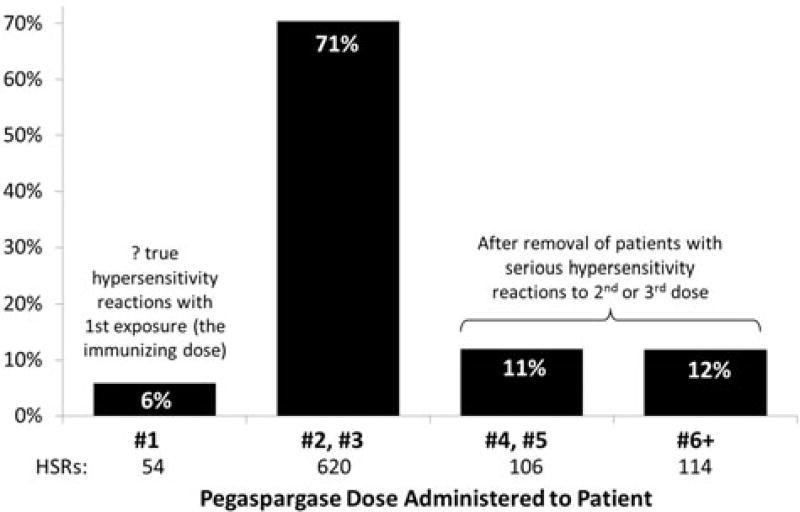
For each trial, sites were required to provide information regarding specific toxicities using the NCI common toxicity criteria adverse event (CTCAE) versions 3.0 or 4.0 (National Institutes of Health, Rockville, MD). Grade ≥3 HSR were defined as grade ≥3 allergic reaction or anaphylaxis according to the CTCAE version 4.0 or allergic reaction/hypersensitivity in version 3.0 (Table 2). In May 2009, CTCAE version 4.0 replaced version 3.0 for adverse event (AE) reporting, therefore trials AALL0932 and AALL1131 were prospectively graded using version 4.0 as they opened to accrual after this date and the remaining trials in this analysis (AALL0331, AALL0232, AALL0434, and AALL07P4) using version 3.0 had to be retrospectively graded to meet AE reporting for version 4.0. All data collected in CTCAE version 3.0 were therefore mapped to version 4.0 in the COG database using NCI’s mapping software. In all of these trials except AALL07P4, only grade ≥3 toxicity data were collected by the COG and therefore only grade ≥3 HSR are reported in this analysis. AALL07P4 assessed all grades (including grade 1 and 2) of toxicity because the trial randomized patients to receive either pegaspargase or a new PEGylated formulation of asparaginase (calaspargase pegol; SC-PEG) that used a succinimidyl carbamate (SC) linker rather than the succinimidyl succinate linker used in pegaspargase [14]. The second and third doses of pegaspargase accounted for 71% of all HSRs and thus were primarily used to assess grade ≥3 HSR rates (Figure 1). All six COG trials included protocol language strongly discouraging use of premedication with antihistamines to decrease the risk of overt allergy symptoms to pegaspargase.
Table 2.
Modification of the CTCAE HSR criteria.
| Version | CTCAE term | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|---|
| 3.0 | Allergic reaction/hypersensitivity (including drug fever) | Allergic reaction transient flushing or rash; drug fever <38°C (<100.4 °F) | Rash; flushing; urticaria; dyspnea; drug fever ≥38 °C (≥100.4 °F) | Symptomatic bronchospasm, with or without urticaria; parenteral medication(s) indicated; allergy-related edema/angioedema; hypotension | Anaphylaxis |
| 4.0 Immune system | Allergic reaction | Transient flushing or rash, drug fever <38 °C (<100.4 °F); intervention not indicated | Intervention or infusion interruption indicated; responds promptly to symptomatic treatment (e.g. antihistamines, NSAIDS, narcotics); prophylactic medications indicated for ≤24 h | Prolonged (e.g. not rapidly responsive to symptomatic medication and/or brief interruption of infusion); recurrence of symptoms following initial improvement; hospitalization indicated for clinical sequelae (e.g. renal impairment, pulmonary infiltrates) | Life-threatening consequence; urgent intervention indicated |
| Anaphylaxis | – | – | Symptomatic bronchospasm, with or without urticaria; parenteral intervention indicated; allergy-related edema/angioedema; hypotension | Life-threatening consequence; urgent intervention indicated |
NSAIDS: non-steroidal anti-inflammatory drugs.
Figure 1.
Percentage by protocol dose of 892 Grade ≥3 hypersensitivity reactions to 54,280 doses of pegaspargase in 16,534 patients on six COG ALL trials, as of 03/2015.
Statistical methods
The six COG trials analyzed in this report include two studies for SR B-ALL (AALL0331 and AALL0932), two for HR B-ALL (AALL0232 and AALL1131), one for T-ALL, and T-LL (AALL0434) and a pilot study investigating calaspargase pegol in HR B-ALL (AALL07P4). Data current as of 31 March 2015 for these studies were included in this report. Rates of toxicities were compared using a Chi square test with p values .05 being considered significant.
Results
Tables 1 and 3 summarize the protocols and patient characteristics across all six trials. In all, 54,280 doses of pegaspargase were evaluated in 16,534 patients, of which 33,179 doses were specifically compared for grade ≥3 HSRs after IM (21,797 doses) and IV (11,382 doses) administration. The studies varied in the number of pegaspargase doses administered; time interval between pegaspargase administration; whether steroid therapy was part of the phase of treatment when the pegaspargase was administered; type of ALL treated; and the age of the patients enrolled on study (Table 1). Patients with SR B-ALL enrolled on AALL0331 or AALL0932 received only two doses of pegaspargase while the HR patients with B-ALL and patients with T-ALL/LL received five or more doses. Most patients included in this analysis received pegaspargase via IM administration compared to IV (8981 vs. 7664; p <.0001). Patients with SR B-ALL had a similar distribution of IM vs. IV pegaspargase (5164 (IM) vs. 5195 (IV); p = NS) compared to patients with NCI HR B-ALL and T-ALL/LL who received a greater number of IM doses (2771 vs. 1962; p = .02 and 1046 vs. 507; p = .72, respectively). When including all regimens, patients who received IM pegaspargase were more likely to have a grade ≥3 HSR after dose 2 or 3 during Consolidation/Delayed Intensification compared to patients receiving IV pegaspargase (10.1% vs. 5.0%; p <.0001). Similarly, grade ≥3 HSR were more common after doses 1 through 5 during all treatment phases for IM compared to IV (5.4 vs. 3.2%; p < .0001) (Table 4).
Table 3.
Patient Characteristics across six COG trials.
| B-cell NCI HR | B-cell NCI SR | T-cell | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||
| Variable | AALL0232 | AALL07P4 | AALL1131 | AALL0331 | AALL0932 | AALL0434 | AALL0434 | |
| Age | <10 | 939 (33.9%) | 18 (33.3%) | 674 (37.5%) | 5164 (100%) | 5195 (100%) | 565 (54.0%) | 269 (53.1%) |
| ≥10 | 1832 (66.1%) | 36 (66.7%) | 1123 (62.5%) | 0 (0%) | 0 (0%) | 481 (46.0%) | 238 (46.9%) | |
| Gender | Male | 1524 (55.0%) | 31 (57.4%) | 1013 (56.4%) | 2775 (53.7%) | 2755 (53.0%) | 776 (74.2%) | 377 (74.4%) |
| Female | 1247 (45.0%) | 23 (42.6%) | 784 (43.6%) | 2389 (46.3%) | 2440 (47.0%) | 270 (25.8%) | 130 (25.6%) | |
| WBC | <50 | 1587 (57.3%) | 32 (59.3%) | 983 (54.7%) | 5164 (100%) | 5195 (100%) | 434 (41.5%) | 221 (43.6%) |
| ≥50 | 1184 (42.7%) | 22 (40.7%) | 814 (45.3%) | 0 (0%) | 0 (0%) | 612 (58.5%) | 286 (56.4%) | |
| Race | American Indian or Alaska native | 12 (0.4%) | 0 (0%) | 16 (0.9%) | 34 (0.7%) | 44 (0.8%) | 3 (0.3%) | 3 (0.6%) |
| Asian | 91 (3.3%) | 1 (1.8%) | 76 (4.2%) | 250 (4.8%) | 245 (4.7%) | 51 (4.9%) | 32 (6.3%) | |
| Native Hawaiian or other Pacific Islander | 13 (0.5%) | 0 (0%) | 8 (0.4%) | 21 (0.4%) | 19 (0.4%) | 9 (0.9%) | 3 (0.6%) | |
| Black or African American | 200 (7.2%) | 6 (11.1%) | 125 (7.0%) | 317 (6.1%) | 294 (5.7%) | 139 (13.3%) | 56 (11.0%) | |
| White | 2102 (75.9%) | 42 (77.8%) | 1322 (73.6%) | 3869 (75.0%) | 3888 (74.8%) | 733 (70.0%) | 364 (71.8%) | |
| Unknown | 353 (12.7%) | 5 (9.3%) | 250 (13.9%) | 673 (13.0%) | 705 (13.6%) | 111 (10.6%) | 49 (9.7%) | |
| Ethnicity | Hispanic or Latino | 637 (23.0%) | 18 (33.3%) | 509 (28.3%) | 1059 (20.5%) | 1203 (23.2%) | 141 (13.5%) | 81 (16.0%) |
| Not Hispanic or Latino | 2033 (73.4%) | 34 (63.0%) | 1243 (69.2%) | 3874 (75.0%) | 3864 (74.4%) | 858 (82.0%) | 413 (81.5%) | |
| Unknown | 101 (3.6%) | 2 (3.7%) | 45 (2.5%) | 231 (4.5%) | 128 (2.4%) | 47 (4.5%) | 13 (2.5%) | |
| Comparison of demographics between IM and IV groups | IM | IV | p Value (Chi square test) | ||
|
| |||||
| Age | <10 | 6668 (74.2%) | 6156 (81.5%) | <.0001 | |
| ≥10 | 2313 (25.8%) | 1397 (18.5%) | |||
| Gender | Male | 5075 (56.5%) | 4176 (55.3%) | .1158 | |
| Female | 3906 (43.5%) | 3377 (44.7%) | |||
| WBC | <50 | 7185 (80.0%) | 6431 (85.1%) | <.0001 | |
| ≥50 | 1796 (20.0%) | 1122 (14.9%) | |||
| Race | White | 6704 (74.6%) | 5616 (74.3%) | .3440 | |
| Othera | 1140 (12.7%) | 928 (12.3%) | |||
| Unknown | 1137 (12.7%) | 1009 (13.4%) | |||
| Ethnicity | Hispanic or Latino | 1837 (20.5%) | 1811 (24.0%) | <.0001 | |
| Not Hispanic or Latino | 6765 (75.3%) | 5554 (73.5%) | |||
| Unknown | 379 (4.2%) | 188 (2.5%) | |||
| Comparison of serious HSR rates between IM and IV by demographics | IM | IV | p Value (Chi square test) | ||
|
| |||||
| Age | <10 | No HSR | 6486 (97.3%) | 5986 (97.2%) | .9116 |
| HSR | 182 (2.7%) | 170 (2.8%) | |||
| ≥10 | No HSR | 2007 (86.8%) | 1328 (95.1%) | <.0001 | |
| HSR | 306 (13.2%) | 69 (4.9%) | |||
| WBC | <50 | No HSR | 6898 (96.0%) | 6251 (97.2%) | .0001 |
| HSR | 287 (4.0%) | 180 (2.8%) | |||
| ≥50 | No HSR | 1595 (88.8%) | 1063 (94.7%) | <.0001 | |
| HSR | 201 (11.2%) | 59 (5.3%) | |||
| Ethnicity | Hispanic or Latino | No HSR | 1727 (94.0%) | 1756 (97.0%) | <.0001 |
| HSR | 110 (6.0%) | 55 (3.0%) | |||
| Not Hispanic or Latino | No HSR | 6408 (94.7%) | 5373 (96.7%) | <.0001 | |
| HSR | 357 (5.3%) | 181 (3.3%) | |||
| Unknown | No HSR | 358 (94.5%) | 185 (98.4%) | .0280 | |
| HSR | 21 (5.5%) | 3 (1.6%) | |||
Other: American Indian or Alaska native, Asian, Native Hawaiian or other Pacific Islander, Black, or African American
SR: standard-risk; HR: high-risk.
Table 4.
Rates of grade ≥3 HSR across all six COG studies.
| IM | IV | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| COG regimen | Dose and phase of pegaspargase evaluated |
Number of patients |
Grade ≥3 HSRs |
Rate (%) |
Number of patients |
Grade ≥3 HSRs |
Rate (%) |
p Value (Chi square test) |
| Regimen with IM then IV | Dose 2 and 3 during consolidation | 763a | 104 | 13.6 | 397a | 42 | 10.6 | .13 |
| Regimens with 2 doses | Dose 2 during delayed Intensification | 1380b | 7 | 0.5 | 2851c | 53 | 1.9 | .0005 |
| Regimens with 3+ doses | Dose 2 and 3 during consolidation | 3132d | 442 | 14.1 | 1342e | 163 | 12.1 | .08 |
| Regimens with 3+ doses | Doses 2 and 3 during CONS or DI | 4534f | 459 | 10.1 | 4443g | 222 | 5.0 | <.0001 |
| Regimens with 2 doses | Doses 1 and 2 during induction and DI | 5164 | 20 | 0.4 | 4848 | 70 | 1.4 | <.0001 |
| Regimens with 3+ doses | Doses 1–5 during all phasesh | 3817 | 462 | 12.1 | 2705 | 175 | 6.5 | <.0001 |
| All regimens | Doses 1–5 during all phasesh | 8981 | 482 | 5.4 | 7553 | 245 | 3.2 | <.0001 |
AALL0434
AALL0331
AALL0932
AALL0232, AALL0434
AALL1131, AALL0434, AALL07P4
AALL0331, AALL0232, AALL0434
AALL0932, AALL1131, AALL0434, AALL07P4
Induction, consolidation, and delayed intensification.
CONS: consolidation; DI: delayed intensification.
When comparing rates of grade ≥3 HSR to pegaspargase in patients with NCI SR B-ALL based-on phase of therapy (Induction or DI), there was no significant difference comparing route of administration (IM vs. IV) during Induction (0.23 vs. 0.33%; p = .35), however, there was less ≥3 grade allergic reactions/anaphylaxis during DI with IM pegaspargase compared to IV administration (0.51 vs. 1.86%; p = .0005) (Supplementary Table 1). Additionally, the overall rate of grade ≥3 HSR to pegaspargase across all treatment phases for SR patients enrolled on AALL0331 was significantly lower following IM administration compared to patients on AALL0932 with IV administration (0.4 vs. 1.3%, p < .0001).
When comparing rates of grade ≥3 HSR to pegaspargase in patients with NCI HR B-ALL or T-ALL/LL based on phase of therapy (Induction, Consolidation, or Delayed Intensification), there was no significant difference between IM and IV pegaspargase (Supplementary Table 1). In addition, grouping the HR and T-ALL/LL patients across all studies (AALL0232, AALL0434, AALL1131, and AALL07P4) who were to receive a greater number of pegaspargase doses, there was no significant difference in grade ≥3 HSR comparing IM vs. IV (14.1 vs. 12.1%, respectively; p = .08) (Table 4).
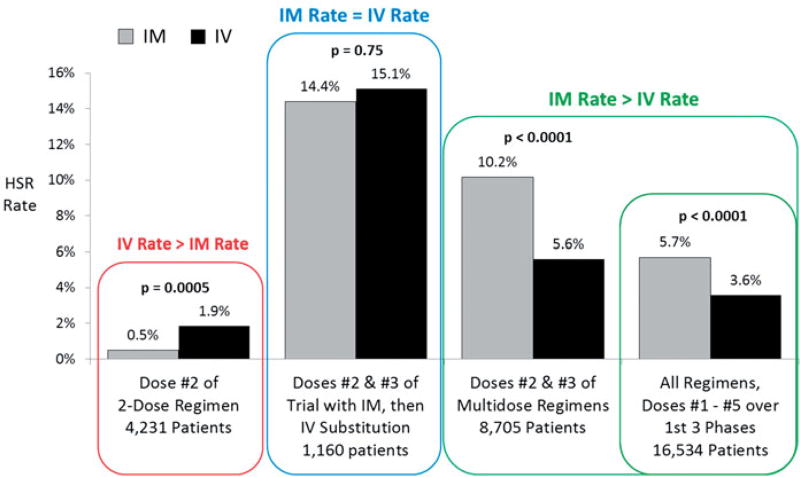
The grade ≥3 HSR rate was higher with IM compared to IV administration when all multi-dose (>2 doses) regimens evaluated were aggregated, whether only the second and third doses were assessed (the HSR prone doses) or all doses during Induction, Consolidation and DI (Figure 2). Overall, when all regimens and all doses of pegaspargase were evaluated up to the end of DI, the HSR rate in 8981 patients treated with IM pegaspargase was 5.4%, statistically more frequent than the 3.2% rate reported in 7553 patients receiving IV injection (p < .0001) (Table 4).
Figure 2.
Rate of grade ≥3 hypersensitivity reactions to pegaspargase in patients with newly diagnosed ALL and Lymphoblastic Lymphoma on six COG trials from December 2003 to March 2015: IM and IV administration.
Of the six COG trials evaluated in this report, AALL0434 and AALL0232 are the most informative regarding grade ≥3 HSR based on their similar chemotherapy backbone for the control arm, having the same number of pegaspargase doses and timing of therapy, the same Interim Maintenance 1 randomization (high-dose methotrexate vs. escalating methotrexate with pegaspargase), and same overlap in study period (2007–2011) (Table 1). Additionally, AALL0434 was the only COG trial of the six without significant differences in patient age, WBC, race and/or ethnicity between patients receiving IM vs. IV pegaspargase. The rate of grade ≥3 HSR for the second and third doses of pegaspargase during Consolidation, without concomitant steroid therapy, in the 3529 patients treated on these two studies ranged from 14.3 to 13.6% for IM pegaspargase compared to 10.6% for IV (p = .048 and 0.14, respectively) (Supplementary Figure 2).
Discussion
This analysis of children, adolescents and young adults with ALL treated on six COG clinical trials provides the largest collection of data regarding grade ≥3 HSR to pegaspargase based on route of administration. This report covers a 13-year period during which two important changes occurred. First, IM pegaspargase was replaced with IV administration and second, there was a significant change in how HSR events were reported from CTCAE version 3.0 to 4.0. Overall, we found the rate of grade ≥3 HSR to pegaspargase based on route of administration occurred less frequently with IV infusion than IM injection. There are varieties of potential confounding variables that may have contributed to these differences, which include different reporting practices for HSR that occurred with IV infusions vs. IM injection, variations in corticosteroid administration prior to the dose of pegaspargase as a component of protocol specified therapy, and/or patient demographics. Regarding age, we found that serious HSR occurred less frequently in patients less than 10 years of age regardless of route of administration compared to those older than 10, where when serious HSR were to occur, they were reported more often after IM pegaspargase vs. IV (13.2 vs. 4.9%; p < .0001; Table 3). Similar findings were seen based on presenting WBC and patient ethnicity where serious HSR were more common after IM pegaspargase compared to IV administration (Table 3). After adjusting for ethnicity, WBC, age and the number of pegaspargase doses given (2 doses vs. >3) in multiple logistic regression analysis, serious HSR rates were higher after IM administration compared to IV (Table 5).
Table 5.
Multiple logistic regression.
| Multiple logistic regression | ||||
|---|---|---|---|---|
|
|
||||
| Effect | Odds ratio | 95% Wald Confidence limits |
p Value | |
| Group: IM vs. IV* | 1.59 | 1.35 | 1.87 | <.0001 |
| Ethnic: Hispanic or Latino vs. Not Hispanic/Latino (ref) | 1.04 | 0.87 | 1.25 | .65 |
| Ethnic: Unknown vs. Not Hispanic/Latino (ref) | 0.96 | 0.62 | 1.47 | .77 |
| WBC: ≥50 K vs. <50 K (ref) | 0.79 | 0.65 | 0.95 | .01 |
| Age: ≥10 yrs vs. <10 yrs (ref) | 0.93 | 0.77 | 1.13 | .47 |
| Dose: dose 3+ vs. dose 2 (ref) | 13.60 | 10.29 | 17.96 | <.0001 |
Reference for logistic regression.
In evaluating the pegaspargase doses during Delayed Intensification (dose 4 and 5), the grade ≥3 HSR rate for either IV or IM routes were much lower than during the Consolidation phase; likely the result of the patients who were significantly allergic being removed from the group at risk. In addition, patients likely became more immunosuppressed as they progressed further into their treatment regimens and therefore may have become immunotolerant to subsequent doses of asparaginase, particularly when considering the higher doses of dexamethasone delivered during Delayed Intensification.
AALL0434 is likely the most definitive trial to compare the difference in grade ≥3 HSR rate for IM vs. IV pegaspargase. This trial is of greater significance for this assessment because the route of pegaspargase administration was changed from IM to allow for IV administration after about two-thirds of the patients were accrued (13 August 2012), and is the only study in which the comparison of IV vs. IM administration can be performed within the same clinical trial. AALL0434 had a total of 1046 IM-treated patients compared to 507 IV-treated patients. The results of this study identified no significant difference in grade ≥3 HSR for the 2nd and 3rd pegaspargase doses during Consolidation between IM (13.6%) and IV (10.6%). In addition, a previous comparison of HR B-ALL patients enrolled on AALL0232 and AALL1131 where IM and IV pegaspargase was administered respectively, identified similar rates of grade ≥3 HSR to pegaspargase during Consolidation (14.4 vs. 12.6%; p = .18) [15].
The different version of CTCAE reporting across studies that may have influenced the grade of HSR reported. In 2009, the CTCAE HSR definitions and grading were changed to include more signs and symptoms of HSR and specific grading criteria. The net effects of this change translated to increase reporting of HSR due to providing two separate categories; allergic reactions and anaphylaxis. Previously in CTCAE version 3.0 a single category existed with allergic reaction/hypersensitivity. As well, version 4.0 added ‘any’ intervention or infusion interruption as an AE when previously only ‘parenteral medication’ was specified. This change stated that any medication intervention or prophylaxis (antihistamines, nonsteroidal anti-inflammatory drugs, and narcotics) were criteria for HSR reporting. The enhanced AE definition also resulted in increased reporting of HSR specifically due to IV infusion since the new criteria added ‘infusion interruption’, including ‘brief interruption of infusion’ which previously did not exist. As IM injections cannot be interrupted (other than by withholding a subsequent injection if multiple injections are required to administer one dose), this AE could only be reported with IV administration. Thus, in fact a higher rate of HSR could be expected with IV compared to IM administration. COG studies with patients on treatment at the time of the CTCAE modification (AALL0331, AALL0232, AALL0434, and AALL07P4) had their HSR retroactively adjusted to fit the new criteria which may have unintentionally introduced error into the reporting.
Concomitant steroid therapy may have influenced whether a grade ≥3 HSR occurred following pegaspargase administration. None of the doses of pegaspargase administered during the Consolidation phase of therapy was given with a steroid and the first (or only) dose of pegaspargase administered during Delayed Intensification was given after three to four days of dexamethasone. Thus, the regimens varied as to whether dose #2 of pegaspargase was administered with dexamethasone. Taking this into consideration, the higher overall rate of grade ≥3 HSR that occurred in the multi-dose regimens of pegaspargase compared with the two dose only regimen, where co-administration of steroids occurred, may be explained in part by concomitant steroid therapy.
We identified significantly lower rates of grade ≥3 HSR when pegaspargase was given IV compared to IM when including all regimens for doses 2 and 3 during Consolidation and Delayed Intensification and doses 1 through 5 across all treatment phases. A possible explanation for the difference could be the inclusion of infusion interruption as a grade 2 allergic reaction in CTCAE version 4. As a HSR to pegaspargase will often occur immediately (within seconds to minutes of the start of the infusion) when administered IV, possibly before the reaction can escalate to serious (grade ≥3 allergy/anaphylaxis), the infusion could be stopped and medication given which would keep the AE at a grade 2 and therefore not reported as a grade ≥3 HSR. In contrast, IM administration of pegaspargase provides the patient with the entire dose of asparaginase which could result in more severe or prolonged HSR graded as grade ≥3 and might be in part the reason more patients were observed to have grade ≥3 HSR when given IM compared to IV across all regimens.
In subgroup analysis, only patients with SR B-ALL who received two doses of pegaspargase treated on AALL0331 receiving IM pegaspargase had a significantly lower rate of grade ≥3 HSR compared to similar patients enrolled on AALL0932 where pegaspargase was given IV. However, the clinical significance of this difference is limited, as both groups reported extremely low rates of grade ≥3 HSR below 2%, and thus IV administration tends to remain the preferred route of administration in these patients given the relative ease of delivery over IM injection. The interval between the initial sensitizing dose of pegaspargase and the second and third doses may explain some of the variability we observed between SR and HR patients. The lower rate of grade ≥3 HSR for SR patients contrasts to those who received ≥3 doses of pegaspargase and reported considerably higher-grade ≥3 HSR rates (ranging 9–15%). Likely, the second antigenic exposure elicited a hyper-amnestic response resulting in a higher HSR rate with the third dose. It is not clear, however, why the two dose less-intensive regimens had a higher-grade ≥3 HSR rate with IV infusion compared to IM injection. It is possible the infusion of pegaspargase may be associated with a greater rise and peak of serum ammonia levels compared to IM injection [16–20] which can result in a variety of symptoms mistaken as HSR including nausea, vomiting, headache, and rash [16,21]. As hyperammonemia secondary to asparagine depletion is more likely to occur following IV infusion than IM injection, more AEs may have been reported as HSR and attributed to IV pegaspargase. Whether the AEs observed with IV administration of pegaspargase-included non-immune mediated infusion reactions rather than true hypersensitivity is unknown. However, as greater than 98% of SR two dose-treated patients did not have a grade ≥3 HSR renders the higher rate with IV infusion reported clinically insignificant. Additionally, likely the most direct comparison in patients with HR ALL during Consolidation therapy where doses 2 and 3 of pegaspargase are given and the majority of ≥3 HSR are to occur, similar rates of ≥3 HSR were reported between IM and IV administration (14.1 vs. 12.1%, p = .08). In conclusion, grade ≥3 HSR rates to pegaspargase occurred less frequently with IV infusion than IM injection on standardized protocols from >200 medical centers across the US, Canada, Australia, and New Zealand conducted by the COG during 2003–2015, and importantly no deaths (Grade 5 HSR) were reported among the 16,645 patients treated with 54,280 doses of either IV or IM pegaspargase.
Supplementary Material
Acknowledgments
Funding
This work was funded by COG Chair’s Operations grants U10 CA98543 and U10 CA180886, COG Statistics and Data Center grants U10 CA098413, and U10 CA180899.
Footnotes
Supplemental data for this article can be accessed here.
Potential conflict of interest: Disclosure forms provided by the authors are available with the full text of this article online at https://doi.org/10.1080/10428194.2017.1397658.
References
- 1.Linet MS, Ries LA, Smith MA, et al. Cancer surveillance series: recent trends in childhood cancer incidence and mortality in the United States. J Nat Cancer Inst. 1999;91:1051–1058. doi: 10.1093/jnci/91.12.1051. [DOI] [PubMed] [Google Scholar]
- 2.Hunger SP, Loh ML, Whitlock JA, et al. Children’s Oncology Group’s 2013 blueprint for research: acute lymphoblastic leukemia. Pediatr Blood Cancer. 2013;60:957–963. doi: 10.1002/pbc.24420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kawedia JD, Rytting ME. Asparaginase in acute lymphoblastic leukemia. Clin Lymphoma Myeloma Leuk. 2014;14(Suppl):S14–S17. doi: 10.1016/j.clml.2014.06.017. [DOI] [PubMed] [Google Scholar]
- 4.Woo MH, Hak LJ, Storm MC, et al. Hypersensitivity or development of antibodies to asparaginase does not impact treatment outcome of childhood acute lymphoblastic leukemia. J Clin Oncol. 2000;18:1525–1532. doi: 10.1200/JCO.2000.18.7.1525. [DOI] [PubMed] [Google Scholar]
- 5.Zalewska-Szewczyk B, Andrzejewski W, Mlynarski W, et al. The anti-asparagines antibodies correlate with L-asparagines activity and may affect clinical outcome of childhood acute lymphoblastic leukemia. Leuk Lymphoma. 2007;48:931–936. doi: 10.1080/10428190701292049. [DOI] [PubMed] [Google Scholar]
- 6.Raetz EA, Salzer WL. Tolerability and efficacy of L-asparaginase therapy in pediatric patients with acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2010;32:554–563. doi: 10.1097/MPH.0b013e3181e6f003. [DOI] [PubMed] [Google Scholar]
- 7.Liu C, Kawedia JD, Cheng C, et al. Clinical utility and implications of asparaginase antibodies in acute lymphoblastic leukemia. Leukemia. 2012;26:2303–2309. doi: 10.1038/leu.2012.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nachman JB, Sather HN, Sensel MG, et al. Augmented post-induction therapy for children with high-risk acute lymphoblastic leukemia and a slow response to initial therapy. N Engl J Med. 1998;338:1663–1671. doi: 10.1056/NEJM199806043382304. [DOI] [PubMed] [Google Scholar]
- 9.Pidaparti M, Bostrom B. Comparison of allergic reactions to pegasparaginase given intravenously versus intramuscularly. Pediatr Blood Cancer. 2012;59:436–439. doi: 10.1002/pbc.23380. [DOI] [PubMed] [Google Scholar]
- 10.Petersen WC, Jr, Clark D, Senn SL, et al. Comparison of allergic reactions to intravenous and intramuscular pegaspargase in children with acute lymphoblastic leukemia. Pediatr Hematol Oncol. 2014;31:311–317. doi: 10.3109/08880018.2013.876134. [DOI] [PubMed] [Google Scholar]
- 11.Mattano LA, Devidas M, Friedmann AM, et al. Outstanding outcome for children with standard risk-low (SR-Low) acute lymphoblastic leukemia (ALL) and no benefit to intensified peg-asparaginase (PEGASNase) therapy: results of children’s oncology group (COG) study AALL0331. Blood. 2014;124:793. [Google Scholar]
- 12.Larsen EC, Devidas M, Chen S, et al. Dexamethasone and high-dose methotrexate improve outcome for children and young adults with high-risk B-acute lymphoblastic leukemia: a report from children’s oncology group study AALL0232. J Clini Oncol. 2016;34:2380–2388. doi: 10.1200/JCO.2015.62.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Winter SS, Dunsmore KP, Devidas M, et al. Safe integration of nelarabine into intensive chemotherapy in newly diagnosed T-cell acute lymphoblastic leukemia: children’s oncology group study AALL0434. Pediatr Blood Cancer. 2015;62:1176–1183. doi: 10.1002/pbc.25470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Angiolillo AL, Schore RJ, Devidas M, et al. Pharmacokinetic and pharmacodynamic properties of calaspargase pegol Escherichia coli L-asparaginase in the treatment of patients with acute lymphoblastic leukemia: results from Children’s Oncology Group Study AALL07P4. J Clin Oncol. 2014;32:3874–3882. doi: 10.1200/JCO.2014.55.5763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salzer W, Burke MJ, Larsen EC, et al. Incidence of allergic reactions to pegaspargase (PEG) administered intramuscularly versus intravenously (IM vs. IV) in children and young adults with high risk B-lymphoblastic leukemia (HR B-ALL): results of Children’s Oncology Group (COG) Studies AALL0232/AALL1131. Blood. 2015;126:1303. [Google Scholar]
- 16.Heitink-Polle KM, Prinsen BH, de Koning TJ, et al. High incidence of symptomatic hyperammonemia in children with acute lymphoblastic leukemia receiving pegylated asparaginase. JIMD Rep. 2013;7:103–108. doi: 10.1007/8904_2012_156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jaing TH, Lin JL, Lin YP, et al. Hyperammonemic encephalopathy after induction chemotherapy for acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2009;31:955–956. doi: 10.1097/MPH.0b013e3181b8beb1. [DOI] [PubMed] [Google Scholar]
- 18.Nussbaum V, Lubcke N, Findlay R. Hyperammonemia secondary to asparaginase: a case series. J Oncol Pharm Practice. 2014;22:161–164. doi: 10.1177/1078155214551590. [DOI] [PubMed] [Google Scholar]
- 19.Tong WH, Pieters R, de Groot-Kruseman HA, et al. The toxicity of very prolonged courses of PEGasparaginase or Erwinia asparaginase in relation to asparaginase activity, with a special focus on dyslipidemia. Haematologica. 2014;99:1716–1721. doi: 10.3324/haematol.2014.109413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steiner M, Attarbaschi A, Kastner U, et al. Distinct fluctuations of ammonia levels during asparaginase therapy for childhood acute leukemia. Pediatr Blood Cancer. 2007;49:640–642. doi: 10.1002/pbc.21022. [DOI] [PubMed] [Google Scholar]
- 21.Jorck C, Kiess W, Weigel JF, et al. Transient hyperammonemia due to L-asparaginase therapy in children with acute lymphoblastic leukemia or non-Hodgkin lymphoma. Pediatr Hematol Oncol. 2011;28:3–9. doi: 10.3109/08880018.2010.484852. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.