SUMMARY
Cyclospora cayetanensis is a coccidian parasite associated with diarrheal illness. In the United States, foodborne outbreaks of cyclosporiasis have been documented almost every year since the mid-1990s. The typical approach used to identify this parasite in human stools is examination of acid-fast-stained smears under bright-field microscopy. UV fluorescence microscopy of wet mounts is more sensitive and specific than acid-fast staining but requires a fluorescence microscope with a special filter not commonly available in diagnostic laboratories. In this study, we evaluated a new DNA extraction method based on the Universal Nucleic Acid Extraction (UNEX) buffer and compared the performances of four published real-time PCR assays for the specific detection of C. cayetanensis in stool. The UNEX-based method had an improved capability to recover DNA from oocysts compared with the FastDNA stool extraction method. The best-performing real-time PCR assay was a C. cayetanensis-specific TaqMan PCR that targets the 18S ribosomal RNA gene. This new testing algorithm should be useful for detection of C. cayetanensis in human stool samples.
Keywords: Cyclospora cayetanensis, stool DNA extraction, coccidia, molecular diagnostics
INTRODUCTION
Cyclospora cayetanensis is a coccidian parasite associated with the diarrheal illness cyclosporiasis. The majority of reported U.S. cases of cyclosporiasis have been associated with foodborne outbreaks or with international travel to tropical or subtropical areas (Centers for Disease Control and Prevention, 2004; Centers for Disease Control and Prevention, 2013; Hall et al., 2011; Hall et al., 2012; Herwaldt, 2000; Ho et al., 2002). Symptoms can persist from weeks to months if the infection is not diagnosed and treated (Herwaldt, 2000).
Laboratory diagnosis of Cyclospora infections relies on detecting the oocysts shed in the stool of infected patients. Cyclospora oocysts have environmentally resistant outer cell walls that make them acid-fast and autofluorescent under UV light (Erickson & Ortega, 2006). Examination of acid-fast-stained stool smears under bright-field microscopy is the typical approach used by clinical laboratories to identify Cyclospora in stools, but this method has suboptimal sensitivity and specificity. UV fluorescence microscopy of wet mounts is an alternative, more accurate approach for diagnosing Cyclospora infection (Berlin et al., 1998). However, a UV excitation filter set that is not commonly available in clinical laboratories is needed for this procedure; with the preferred UV filter set (330 to 365 nm), intense blue fluorescence of Cyclospora oocysts is obtained.(Berlin et al., 1998; Relman et al., 1996). A molecular method such as PCR can provide sensitive and specific detection, as well as species-level identification (Eberhard et al., 1999; Li et al., 2015). Relman and colleagues developed the first-described PCR assay for C. cayetanensis (Relman et al., 1996). This nested PCR assay has since been used for various applications, including confirmatory diagnostic testing (Pieniazek et al., 1996). However, the nested format and the requirement for DNA sequencing analysis of the PCR product for species-level identification limit the usefulness of this method.
To date, four real-time PCR assays for detection of C. cayetanensis have been described: two utilize a species-specific TaqMan probe to detect unique regions in the small subunit ribosomal RNA (18S rRNA) gene (Varma et al., 2003; Verweij et al., 2003), and two rely on DNA binding dyes and amplicon melt curve analysis for specificity (Lalonde & Gajadhar, 2011; Marangi et al., 2015). Each of these four assays has the potential to identify the parasite to the species level in a one-step reaction. However, for any PCR-based assay to be successful in detecting C. cayetanensis in stool, the preceding DNA extraction step must be able to disrupt the tough outer oocyst wall to recover the DNA. The aims of this study were to evaluate a new DNA extraction method, previously used to detect coccidia in experimentally contaminated food (Shields et al., 2013); and to compare the analytical performances of the four published real-time PCR assays for the detection of C. cayetanensis in stool.
MATERIALS AND METHODS
Human specimens
We used 137 human stool specimens to evaluate the DNA extraction and PCR methods. The human stool specimens had been sent to the Centers for Disease Control and Prevention (CDC) for diagnostic confirmation or as part of outbreak investigations during 2004–2015 and were used in accordance with the CDC Human Subjects Research protocol entitled “Use of residual diagnostic specimens from humans for laboratory methods research.” The stool samples were unpreserved or had been collected in commonly used fixatives for parasitology, including Zn-PVA, EcoFix, or TotalFix.
Thirty-two of the 137 human stools were classified as positive for C. cayetanensis, based on positive UV fluorescence microscopy (n = 30) or DNA sequencing confirmation of PCR products obtained by the nested PCR assay (n = 2) (Berlin et al., 1998; Relman et al., 1996). One of the microscopy-positive specimen, selected because of a large volume (about 100 ml) and sufficiently high concentration of oocysts (an oocyst count of 1.3 × 104 oocysts per ml, as determined using a hemocytometer), was used to assess the analytical sensitivities (detection limits) of the four PCR assays: four aliquots of this stool were serially diluted in parasite-free stool to obtain four separate dilution series down to 1.3 oocysts per ml. The definition of the detection limit was the lowest number of oocysts that an assay detected in all four aliquots.
For specificity analysis, we used 105 of the 137 human stool specimens, 15 stool samples from non-human primates, 3 stool samples from rats, and 2 DNA samples. The human stool specimens were positive by microscopy for either Entamoeba histolytica/dispar (n = 24 specimens); Enterocytozoon/Encephalitozoon spp. (n = 13); Giardia duodenalis (n = 6); Cryptosporidium spp. (n = 3); Blastocystis hominis, Dientamoeba fragilis, or Iodamoeba butschlii (each n = 2); or for Balantidium coli, Chilomastix mesnili, Entamoeba coli, Entamoeba hartmani, hookworm, or Trichomonas hominis (each n = 1); or were negative by microscopy for parasites and microsporidia (n = 47). The non-human primate samples, positive for simian Cyclospora spp. as described elsewhere (Eberhard et al., 1999; Eberhard et al., 2001), included eight Cyclospora papionis-positive samples from Papio anubis in Ethiopia, two C. papionis-positive samples from P. anubis in Kenya, two Cyclospora colobi-positive samples from Colobus angolensis in Kenya, and three Cyclospora cercopitheci-positive samples from Cercopithecus aethiops in Kenya. The rat stools (collected during an environmental investigation unrelated to this study) were positive by microscopy for Eimeria spp. (n = 2 samples) and Hymenolepis nana (n = 1). DNA samples extracted from Eimeria tenella and Eimeria acervulina were also included.
DNA extraction
Two methods for extraction of total genomic DNA from stool were compared using fifty human stool samples (25 C. cayetanensis-positive and 25 specificity controls). Method 1 used a modification of the FastDNA® method (da Silva et al., 1999). Aliquots of ~0.3–0.5 ml of each stool were subjected to bead beating in a FastPrep-24 cell disruptor instrument (MP Biomedicals, Santa Ana, CA, USA). An internal DNA quality control plasmid (Duffy et al., 2013), here called pIC, was added immediately after the bead-beating step to allow for detection of DNA extraction failure (see below). Potential inhibitors remaining after the FastDNA extraction were removed by further purification with the QIAquick PCR purification kit, according to the manufacturer’s instructions (QIAGEN Inc., Valencia, CA, USA).
Method 2 was based on the commercially available UNEX (Universal Nucleic Acid Extraction) buffer. An aliquot of ~0.5 ml of stool was added to a matrix E bead-beating tube (MP Biomedicals), along with 60 µl of proteinase K (QIAGEN) and 600 µl of UNEX buffer (Phthisis Diagnostics, Charlottesville, VA, USA). The tube was incubated for 15 min at 56°C for protein digestion, followed by disruption in the FastPrep-24 cell disruptor instrument at a speed of 6.0 m/s for 1 min. One pg of the internal control plasmid pIC was added, and the tube was then centrifuged at maximum speed (>13,000 × g) for 1 min to pellet the debris. The supernatant was passed through a DNA-binding column (DNeasy mini spin column from QIAGEN). After two wash cycles with ethanol-containing wash buffers, the DNA was eluted from the column in 80 µl of AE buffer (QIAGEN). To purify the eluate further, it was passed through a Zymo-Spin IV-HRC column (Zymo Research Corp., Irvine, CA, USA).
The remaining 87 human stool specimens and the 18 animal stool samples were extracted with the UNEX-based method only, since that method performed better than the Fast DNA method for extraction of C. cayetanensis DNA from stool (see results).
Conventional nested PCR for Cyclospora
The nested PCR assay contained 0.3 µM of each primer (Relman et al., 1996) and the AmpliTaq Gold PCR Master Mix (Life Technologies, Grand Island, NY, USA). The total volume was 50 µl, with 5 µl of extracted DNA added to the first reaction and 3 µl of the undiluted product from the first step added to the nested reaction. The cycling parameters were 95°C for 5 min to activate the polymerase, followed by 30 cycles of 95°C for 15 s, 57°C for 15 s, and 72°C for 1 min for the first step; and 35 cycles of 95°C for 15 s, 65°C for 15 s, and 72°C for 1 min for the nested step. The PCR products were visualized on 1.5% agarose gels stained with ethidium bromide.
Real-time PCR for C. cayetanensis
We compared four real-time PCR assays: two TaqMan assays we refer to as the Verweij (Verweij et al., 2003) and the Varma (Varma et al., 2003) assays, which target different parts of the 18S rRNA gene; a SYBR Green assay (Lalonde & Gajadhar, 2011) that targets the 18S rRNA gene; and an EvaGreen assay (Marangi et al., 2015) that targets the internal transcribed region 2 (ITS2). The TaqMan real-time PCR assays were performed in a Mx3000P™ thermocycler, whereas the DNA binding dye assays were performed in an AriaMx thermocycler to facilitate high-resolution melt (HRM) analysis. A 2-µl aliquot of template DNA was added to each reaction.
We used previously described reaction conditions for the EvaGreen assay (Marangi et al., 2015). For the SYBR Green assay, we only used the Cyclospora-specific primers (CycloF and CycloR) (Lalonde & Gajadhar, 2011). We performed the Varma assay with the conditions described in the original publication (Varma et al., 2003) and with various modifications in attempts to improve amplification performance. The results presented here were obtained using QuantiTect Probe PCR Master Mix (QIAGEN) instead of AmpliTaq Master Mix with BSA. We improved the specificity of the Verweij assay by making two modifications: 1) increasing the annealing/extension temperature from 60°C to 67°C, and 2) correcting the reverse primer sequence to 5’-AAT GCC ACG GTA GGC CAA TA-3’ (the reverse primer sequence in the original publication by Verweij et al. was missing a base, in comparison with the target gene). We also replaced Platinum QPCR Supermix (Life Technologies) for the reagents from Eurogentec and decreased the primer and probe concentrations to 0.5 µM and 0.1 µM, respectively.
Detection of the internal DNA quality control plasmid (pIC)
The pIC is a recombinant plasmid with the cDNA of an Arabidopsis thaliana gene (GenBank accession number NM_114612.3) inserted into vector pZErO-2. The pIC plasmid was linearized by PstI digestion and diluted to 0.1 pg/µl before adding it to stool during DNA extraction. DNA samples that contained the pIC were analyzed in a real-time PCR assay containing 0.1 µM each of the primers IAC Fw and IAC Rv (Duffy et al., 2013) and 0.05 µM of the TaqMan probe ICP2 (5’-HEX-CCACTGCTAAAGGTAGCCCACGTC-BHQ1-3’), using standard TaqMan cycling structure.
Statistical analysis
The proportions of samples that were correctly identified were tabulated and confidence intervals were calculated using the Clopper-Pearson method (Clopper & Pearson, 1934). We compared the performances of the FastDNA and UNEX-based extraction methods by calculating the mean Ct value for each method. We assumed that the lower the mean Ct value the better the performance of the DNA extraction method. To evaluate operator-associated variability, two laboratorians processed the same specimens independently. In the statistical analyses, the negative samples, which, by definition, were not assigned Ct values, were considered as right-censored outcomes, in an adaptation of a previously described model for left-censored data (Jin et al., 2011). Thus, a model was fit to the data in SAS software version 9.3 (SAS Institute, Inc., Cary, NC, USA) to estimate the mean difference in the Ct values between the two methods, for each laboratorian; an interaction term between the method and laboratorian was included in the model, and a 5% level of significance was used.
The diagnostic performances of the assays were evaluated as described elsewhere (Griner et al., 1981). In brief, sensitivity was calculated as the probability that the result was positive when C. cayetanensis-positive specimens were tested, and specificity as the probability that the result was negative when C. cayetanensis-negative specimens/samples were tested.
RESULTS
Evaluation of the UNEX-based method
To compare the FastDNA and UNEX-based methods for DNA extraction from stool, two different laboratorians processed 25 C. cayetanensis-positive and 25 specificity-control specimens. Both laboratorians performed both methods in parallel (i.e., for a total of 200 DNA extractions) without knowing which specimens were positive or negative. The results are summarized in Table 1. All of the results obtained with the UNEX-based method agreed with the microscopy results (i.e., no false-positive or false-negative results). In contrast, three C. cayetanensis-positive samples tested negative for C. cayetanensis after DNA extraction with the FastDNA method, even though the pIC was amplified from all three samples. The statistical modeling supported the conclusion that UNEX was better than FastDNA at recovering Cyclospora DNA from stool: the estimated mean difference in the Ct values between the two methods was significantly different from zero for both laboratorians, indicating that the UNEX-based method had a lower estimated Ct mean (Table 2). Furthermore, the FastDNA method had more manual pipetting steps and was therefore more labor-intensive than the UNEX-based method.
Table 1.
Comparison of the FastDNA and UNEX-based DNA extraction methods for PCR detection of Cyclospora cayetanensis.
| No. of stool samples positive (percentage, and 95% confidence interval) in the Verweij assay after DNA extraction using: |
||||
|---|---|---|---|---|
|
|
||||
| The FastDNA method | The UNEX-based method | |||
|
|
||||
| Laboratorian A | Laboratorian B | Laboratorian A | Laboratorian B | |
| Microscopy-positive samples (n = 25) | 19 (76, 55–91%) | 22 (88, 69–97%) | 25 (100, 86–100%) | 25 (100, 86–100%) |
| Microscopy-negative samples (n = 25) | 1 (4, 0.1–20%) | 2 (8, 1–26%) | 0 (0, 0–14%) | 0 (0, 0–14%) |
Table 2.
Estimated mean differences in the Verweij assay Ct values from a right-censored regression model of the performances of the FastDNA and UNEX-based DNA extraction methods, stratified by laboratorian
| Laboratorian | Difference in mean Ct value between FastDNA and UNEX (95% confidence interval) |
P value |
|---|---|---|
| A | 4.6 (3.1–6.1) | <0.001 |
| B | 2.3 (0.8–3.8) | 0.003 |
Comparison of real-time PCR methods
We compared the performances of the four real-time PCR assays with that of the conventional nested PCR assay using 32 Cyclospora-positive human stool specimens and 125 specificity controls (Table 3). The main difference among the assays was in their analytical sensitivities (i.e., detection limits). The EvaGreen assay was the most sensitive, with a detection limit of two oocysts per DNA extraction (i.e., seven oocysts per ml of stool). The Verweij and SYBR Green assays had the same detection limit as the conventional nested assay (i.e., 15 oocysts per DNA extraction, or 50 per ml), whereas the detection limit for the Varma assay was 200 oocysts per sample (~700 oocysts/ml). The Varma assay also failed to detect C. cayetanensis DNA in three of the Cyclospora-positive human stools, such that its diagnostic sensitivity was 93%.
Table 3.
Analytical and diagnostic performance of the conventional and real-time PCR assays for detection of Cyclospora cayetanensis following DNA extraction by the UNEX method.
| Assay | Target gene | Detection mode |
Analytical sensitivity (oocysts per 0.3 ml) |
Analytical specificitya (95% CI) |
Diagnostic specificityb (95% CI) |
Diagnostic sensitivityb (95% CI) |
Reference |
|---|---|---|---|---|---|---|---|
| Nested | 18S rRNA | Agarose gel | 15 | 100% (83–100%) | 100% (96–100%)c | 97% (84–99%) | (Relman et al., 1996) |
| Varma | 18S rRNA | TaqMan probe | 200 | 100% (83–100%) | 100% (96–100%) | 93% (81–98%) | (Varma et al., 2003) |
| Verweij | 18S rRNA | TaqMan probe | 15 | 100% (83–100%) | 100% (96–100%) | 100% (87–100%) | (Verweij et al., 2003) |
| SYBR Green | 18S rRNA | DNA binding dye | 15 | 85% (62–96%) | 100% (96–100%) | 100% (87–100%) | (Lalonde & Gajadhar, 2011) |
| EvaGreen | ITS2 | DNA binding dye | 2 | 100% (83–100%) | 98% (93–99%) | 100% (87–100%) | (Marangi et al., 2015) |
As determined using 20 samples positive for Eimeria and simian Cyclospora spp.
As determined using 137 human stool specimens (32 C. cayetanensis-positive specimens and 105 negative controls)
Requires DNA sequencing analysis of the amplicon
The specificities were 100% for both the conventional nested PCR and the Varma assays, as neither assay amplified any of the specificity controls. The specificity of the Verweij assay was dependent on the annealing temperature; the assay amplified DNA from several of the simian Cyclospora samples when the previously published annealing temperature of 60°C was used (data not shown) but was specific for C. cayetanensis when the annealing temperature was increased to 67°C.
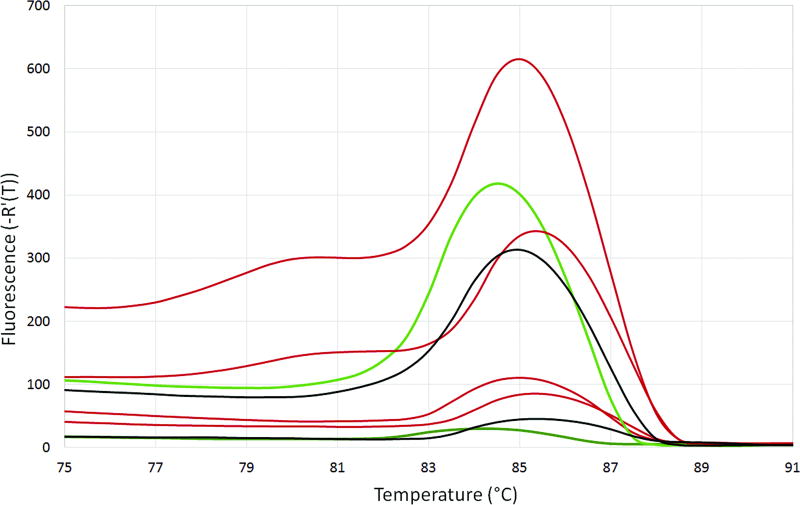
The PCR primers used in the SYBR Green assay were originally designed to detect and distinguish multiple coccidian parasites using HRM analysis. In this study, these primers amplified C. cayetanensis, simian Cyclospora spp., and Eimeria spp. DNA. Fig. 1 depicts the results of the HRM analyses for representative study samples. Eimeria spp. displayed a distinct melt curve profile with a main peak at ~86°C. Cyclospora spp. displayed a melt curve pattern with three peaks. All of the C. cayetanensis specimens had one melt peak in the range of 79.2–79.8°C. Whereas most (14 of 16) of the simian samples had a peak in the range of 80.2–80.8°C, the melt peaks were 79.4°C for one C. papionis sample and 79.6°C for one C. cercopitheci sample. The other two peaks in the three-peak pattern (at ~85.5°C and 88.5°C) overlapped between C. cayetanensis and the simian Cyclospora samples. Therefore, the SYBR Green assay could not reliably distinguish C. cayetanensis from the simian Cyclospora spp., resulting in an analytical specificity of 85%.
Fig. 1.
High-resolution melt analysis curves using the SYBR Green assay. The first derivative of the fluorescence multiplied by −1 [−R’(T)] is plotted against the temperature (°C). Blue = simian Cyclospora spp. (C. cercopitheci, C. papionis, and C. colobi); red = C. cayetanensis; green = Eimeria acervulina.
The EvaGreen assay was designed to specifically amplify C. cayetanensis DNA. All of the C. cayetanensis specimens in this study displayed a single melt peak at 85.0–85.5°C. Unfortunately, two of the human specificity controls (one stool positive for microsporidia and one parasite/microsporidia-free stool) also produced PCR products with similar melt curves as those of the C. cayetanensis-positive specimens (Fig. 2). Thus, the EvaGreen assay had a 98% diagnostic specificity in this study.
Fig. 2.
High-resolution melt analysis curves using the EvaGreen assay. The first derivative of the fluorescence multiplied by −1 [−R’(T)] is plotted against the temperature (°C). Red = C. cayetanensis-positive stools (Tm = 85.0–85.5); green = C. cayetanensis-negative stools with Tm = 84.0–84.5; black = C. cayetanensis-negative stools with Tm = 85.0–85.5 (i.e., false positives).
DISCUSSION
We have developed a new molecular testing algorithm for the specific detection of C. cayetanensis in human stool specimens. The molecular techniques we used previously—the FastDNA extraction method followed by amplification with the nested PCR assay—had several limitations, including occasional failure to detect Cyclospora DNA in microscopy-positive stool, vulnerability to amplicon contamination, and the need to conduct DNA sequencing analysis for species-level identification. The new algorithm consists of an improved DNA extraction method (UNEX), followed by a real-time PCR assay (the Verweij assay) that detects C. cayetanensis to the species level. In our comparison of four published real-time PCR assays, the Verweij assay had the overall best performance. The Varma assay had suboptimal sensitivity, and the two DNA binding dye-based assays were less specific than the Verweij assay.
There are few options available for the laboratory diagnosis of Cyclospora infection. There is no in vitro culture system or animal model to isolate C. cayetanensis in the laboratory (Eberhard et al., 2000). No antigen-based or serologic method for human diagnosis is available. PCR-based molecular detection of C. cayetanensis in stool can be helpful to confirm the findings obtained by microscopy and to diagnose the infection in persons with low-level shedding of the parasite. To our knowledge, there is only one FDA-cleared test for diagnosis of C. cayetanensis infection: the FilmArray Gastrointestinal Panel, a multiplex PCR-based test that simultaneously detects DNA from 22 different pathogens. However, this test requires stool in Cary Blair transport medium and cannot be applied to stools collected in fixatives commonly used in parasitology.
It can be challenging to extract PCR-ready C. cayetanensis DNA from stool. Substances in stool can co-purify with the DNA and inhibit amplification in the subsequent PCR reaction (Monteiro et al., 1997). In addition, C. cayetanensis oocysts have tough cell walls, with a thick, carbohydrate-rich outer layer, that can make DNA extraction difficult (Erickson & Ortega, 2006). In a published comparison of four DNA extraction methods for coccidian parasites in experimentally contaminated food items, another method that used the UNEX buffer produced DNA extracts with less inhibitory effect on PCR (Shields et al., 2013). In our comparison using stool specimens, the UNEX-based method yielded better results than the FastDNA method, which likely reflects enhanced capability to break open the oocysts.
In conclusion, we evaluated methods for DNA extraction and real-time PCR detection of C. cayetanensis in human stool specimens. The UNEX-based DNA extraction followed by the Verweij TaqMan assay is suitable for molecular detection of C. cayetanensis in stool and can be used as an complement to microscopy, for example in cases of inconclusive microscopy findings.
KEY FINDINGS.
We developed an efficient method for the molecular detection of Cyclospora cayetanensis in human stool.
We compared two DNA extraction methods and four real-time PCR methods.
The UNEX-based method was better than FastDNA for extraction of DNA from Cyclospora-positive stool.
A TaqMan assay that targets the 18S rRNA gene was the best-performing PCR assay.
Acknowledgments
We thank colleagues at the U.S. State Public Health Laboratories and Dawn Roellig in the Waterborne Disease Prevention Branch (WDPB) of the CDC for sharing stool specimens with our laboratory. We also thank Michael J. Arrowood in the WDPB for quantifying Cyclospora oocysts in the stool specimen used for assessing the detection limits; Palmer Orlandi, Jr., from the U.S. Food and Drug Administration (FDA) for providing the Eimeria DNA controls; and Francisca Abanyie for critical reviews of earlier versions of this manuscript. The findings and conclusions in this manuscript are those of the authors and do not necessarily represent the official position of the CDC or the FDA.
References
- Berlin OG, Peter JB, Gagne C, Conteas CN, Ash LR. Autofluorescence and the detection of Cyclospora oocysts. Emerg Infect Dis. 1998;4:127–128. doi: 10.3201/eid0401.980121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Outbreak of cyclosporiasis associated with snow peas--Pennsylvania, 2004. MMWR Morb Mortal Wkly Rep. 2004;53:876–878. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Outbreaks of cyclosporiasis--United States, June–August 2013. MMWR Morb Mortal Wkly Rep. 2013;62:862. [PMC free article] [PubMed] [Google Scholar]
- Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika. 1934;26:404–413. [Google Scholar]
- da Silva AJ, Bornay-Llinares FJ, Moura IN, Slemenda SB, Tuttle JL, Pieniazek NJ. Fast and reliable extraction of protozoan parasite DNA from fecal specimens. Mol Diagn. 1999;4:57–64. doi: 10.1016/s1084-8592(99)80050-2. [DOI] [PubMed] [Google Scholar]
- Duffy T, Cura CI, Ramirez JC, Abate T, Cayo NM, Parrado R, Bello ZD, Velazquez E, Munoz-Calderon A, Juiz NA, Basile J, Garcia L, Riarte A, Nasser JR, Ocampo SB, Yadon ZE, Torrico F, de Noya BA, Ribeiro I, Schijman AG. Analytical performance of a multiplex real-time PCR assay using TaqMan probes for quantification of Trypanosoma cruzi satellite DNA in blood samples. PLoS Negl Trop Dis. 2013;7:e2000. doi: 10.1371/journal.pntd.0002000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhard ML, da Silva AJ, Lilley BG, Pieniazek NJ. Morphologic and molecular characterization of new Cyclospora species from Ethiopian monkeys: C. cercopitheci sp.n., C. colobi sp.n., and C. papionis sp.n. Emerg Infect Dis. 1999;5:651–658. doi: 10.3201/eid0505.990506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhard ML, Njenga MN, DaSilva AJ, Owino D, Nace EK, Won KY, Mwenda JM. A survey for Cyclospora spp. in Kenyan primates, with some notes on its biology. J Parasitol. 2001;87:1394–1397. doi: 10.1645/0022-3395(2001)087[1394:ASFCSI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Eberhard ML, Ortega YR, Hanes DE, Nace EK, Do RQ, Robl MG, Won KY, Gavidia C, Sass NL, Mansfield K, Gozalo A, Griffiths J, Gilman R, Sterling CR, Arrowood MJ. Attempts to establish experimental Cyclospora cayetanensis infection in laboratory animals. J Parasitol. 2000;86:577–582. doi: 10.1645/0022-3395(2000)086[0577:ATEECC]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Erickson MC, Ortega YR. Inactivation of protozoan parasites in food, water, and environmental systems. J Food Prot. 2006;69:2786–2808. doi: 10.4315/0362-028x-69.11.2786. [DOI] [PubMed] [Google Scholar]
- Griner PF, Mayewski RJ, Mushlin AI, Greenland P. Principles of test selection and use. Ann Intern Med. 1981;94:559–563. [PubMed] [Google Scholar]
- Hall RL, Jones JL, Herwaldt BL. Surveillance for laboratory-confirmed sporadic cases of cyclosporiasis--United States, 1997–2008. MMWR Surveill Summ. 2011;60:1–11. [PubMed] [Google Scholar]
- Hall RL, Jones JL, Hurd S, Smith G, Mahon BE, Herwaldt BL. Population-based active surveillance for Cyclospora infection--United States, Foodborne Diseases Active Surveillance Network (FoodNet), 1997–2009. Clin Infect Dis. 2012;54(Suppl 5):S411–417. doi: 10.1093/cid/cis049. [DOI] [PubMed] [Google Scholar]
- Herwaldt BL. Cyclospora cayetanensis: a review, focusing on the outbreaks of cyclosporiasis in the 1990s. Clin Infect Dis. 2000;31:1040–1057. doi: 10.1086/314051. [DOI] [PubMed] [Google Scholar]
- Ho AY, Lopez AS, Eberhart MG, Levenson R, Finkel BS, da Silva AJ, Roberts JM, Orlandi PA, Johnson CC, Herwaldt BL. Outbreak of cyclosporiasis associated with imported raspberries, Philadelphia, Pennsylvania, 2000. Emerg Infect Dis. 2002;8:783–788. doi: 10.3201/eid0808.020012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y, Hein MJ, Deddens JA, Hines CJ. Analysis of lognormally distributed exposure data with repeated measures and values below the limit of detection using SAS. Ann Occup Hyg. 2011;55:97–112. doi: 10.1093/annhyg/meq061. [DOI] [PubMed] [Google Scholar]
- Lalonde LF, Gajadhar AA. Detection and differentiation of coccidian oocysts by real-time PCR and melting curve analysis. J Parasitol. 2011;97:725–730. doi: 10.1645/GE-2706.1. [DOI] [PubMed] [Google Scholar]
- Li N, Ye J, Arrowood MJ, Ma J, Wang L, Xu H, Feng Y, Xiao L. Identification and morphologic and molecular characterization of Cyclospora macacae n. sp. from rhesus monkeys in China. Parasitol Res. 2015;114:1811–1816. doi: 10.1007/s00436-015-4367-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marangi M, Koehler AV, Zanzani SA, Manfredi MT, Brianti E, Giangaspero A, Gasser RB. Detection of Cyclospora in captive chimpanzees and macaques by a quantitative PCR-based mutation scanning approach. Parasit Vectors. 2015;8:274. doi: 10.1186/s13071-015-0872-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro L, Bonnemaison D, Vekris A, Petry KG, Bonnet J, Vidal R, Cabrita J, Megraud F. Complex polysaccharides as PCR inhibitors in feces: Helicobacter pylori model. J Clin Microbiol. 1997;35:995–998. doi: 10.1128/jcm.35.4.995-998.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieniazek NJ, Slemenda SB, da Silva AJ, Alfano EM, Arrowood MJ. PCR confirmation of infection with Cyclospora cayetanensis. Emerg Infect Dis. 1996;2:357–359. doi: 10.3201/eid0204.960415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relman DA, Schmidt TM, Gajadhar A, Sogin M, Cross J, Yoder K, Sethabutr O, Echeverria P. Molecular phylogenetic analysis of Cyclospora, the human intestinal pathogen, suggests that it is closely related to Eimeria species. J Infect Dis. 1996;173:440–445. doi: 10.1093/infdis/173.2.440. [DOI] [PubMed] [Google Scholar]
- Shields JM, Joo J, Kim R, Murphy HR. Assessment of three commercial DNA extraction kits and a laboratory-developed method for detecting Cryptosporidium and Cyclospora in raspberry wash, basil wash and pesto. J Microbiol Methods. 2013;92:51–58. doi: 10.1016/j.mimet.2012.11.001. [DOI] [PubMed] [Google Scholar]
- Varma M, Hester JD, Schaefer FW, 3rd, Ware MW, Lindquist HD. Detection of Cyclospora cayetanensis using a quantitative real-time PCR assay. J Microbiol Methods. 2003;53:27–36. doi: 10.1016/s0167-7012(02)00209-9. [DOI] [PubMed] [Google Scholar]
- Verweij JJ, Laeijendecker D, Brienen EA, van Lieshout L, Polderman AM. Detection of Cyclospora cayetanensis in travellers returning from the tropics and subtropics using microscopy and real-time PCR. Int J Med Microbiol. 2003;293:199–202. doi: 10.1078/1438-4221-00252. [DOI] [PubMed] [Google Scholar]