Abstract
Objective
Callous-unemotional (CU) traits are characterized by a lack of guilt and empathy, and low responsiveness to distress and fear in others. Children with CU traits are at-risk for engaging in early and persistent conduct problems. Individuals showing CU traits have been shown to have reduced neural responses to others’ distress (e.g., fear). However, the neural components of distress responses in children with CU traits have not been investigated in early childhood. In the current study, we examined neural responses that underlie the processing of emotionally-valenced vocal stimuli using the event-related potential technique in a group of preschoolers.
Method
Participants between 2 and 5 years old took part in an auditory oddball task containing English-based pseudowords spoken with either a fearful, happy, or a neutral prosody while electroencephalography data were collected. The mismatch negativity (MMN) component, an index of the automatic detection of deviant stimuli within a series of stimuli, was examined in association with two dimensions of CU traits (i.e., callousness-uncaring and unemotional dimensions) reported by primary caregivers.
Results
Findings suggest that the callousness-uncaring dimension of CU traits in early childhood is associated with reduced responses to fearful vocal stimuli.
Conclusions
Reduced neural responses to vocal fear could be a biomarker for callous-uncaring traits in early childhood. These findings are relevant for clinicians and researchers attempting to identify risk factors for early callous-uncaring traits.
Keywords: Callous-uncaring traits, event-related potential, early childhood
Callous-unemotional (CU) traits are typically characterized by deficits in guilt and empathy and low responsiveness to distress and fear in others. Children with CU traits are at increased risk for developing early-onset, persistent and severe conduct problems (e.g., aggressive behavior; Fontaine et al., 2011; Frick, Ray, Thornton, & Kahn, 2014). In addition, the presence of CU traits heightens risk for the development of psychopathy in adulthood, a disorder that encompasses a constellation of affective (e.g., callousness), interpersonal (e.g., manipulativeness), and behavioral (e.g., impulsiveness) features (Lynam et al., 2007). The evidence for a distinctive group of children displaying CU traits within the wider group of children with conduct problems led to the inclusion in the Diagnostic and Statistical Manual of Mental Disorders – 5th edition of CU traits as a specifier of Conduct Disorder, labeled “limited prosocial emotions” (American Psychiatric Association, 2013).
To date, most of the developmental research on CU traits has focused on school-age children and adolescents. Recently, there has been a growing interest in studying CU traits in early childhood (Hyde et al., 2013; Kimonis et al., 2016), notably for its value in helping to identify children at risk for persistent and severe behavior problems and for prevention efforts (Frick, Ray, Thornton, & Kahn, 2014). Recent studies have identified CU traits in children as young as two (Hyde et al., 2013), and meta-analytic findings indicate that there is a large, positive association between CU traits and conduct problems in early childhood (Longman, Hawes, & Kohlhoff, 2015). These findings suggest that CU traits in very young children are a meaningful risk factor for concurrent and future behavior problems.
Emotion Processing and CU Traits
Research suggests that difficulty identifying, processing, and responding to negative emotional stimuli is a core feature of CU traits (Frick et al., 2014). Theories about the developmental origins of CU traits suggest that difficulties processing and responding to negative emotional stimuli, often encompassed within descriptions of temperamental fearlessness (Frick & Viding, 2009), may be one possible mechanism in the development of CU traits (Blair, 2005; Frick & Morris, 2004; van Goozen, 2015; Waller et al., 2016). Blair (2013) suggests that children who do not readily detect distress in others (e.g., fear) are less likely to experience the aversion and subsequent withdrawal that are typically experienced when committing behaviors that cause distress to others. Without experiencing this aversion, children may have low internal motivation to discontinue their behaviors that cause distress to others. Because this aversion is thought of as a possible precursor to empathy (Kochanska, 1993; 1997), this could ultimately lead to disrupted conscience development in childhood. Recent research confirms that temperamental fearlessness in infancy is positively associated with parent-reported CU traits in toddlerhood, suggesting that, fearlessness is a precursor to CU traits in early childhood (Waller et al., 2016). Given this developmental model, examining early emotion processing capacities may provide crucial information about the mechanisms through which CU traits develop in early childhood.
Much of the research on emotion processing in children and adults displaying CU traits has focused on the perception of facial emotional stimuli. Recent meta-analytic evidence identified that adults with CU/psychopathic traits show deficits in recognizing fearful, sad, and surprised facial expressions, as compared to control participants (Marsh & Blair, 2008). Findings also suggest that individuals with CU traits show diminished neural activation in response to distressed faces when compared to controls (Jones, Laurens, Herba, Barker, & Viding, 2009; Marsh et al., 2008). Substantially less research has focused on auditory stimuli (e.g., processing and identifying linguistic stimuli spoken with varying emotional prosodies). However, it is important to determine whether difficulties processing distressed stimuli in early childhood occur across sensory modalities, or if such difficulties are specific to visually presented emotional stimuli. Identifying what sensory modalities are affected by emotion processing difficulties may provide important information about the neural mechanisms of the emotion processing patterns that characterize children and adults with CU traits. The handful of studies that have examined auditory stimuli suggest that individuals displaying CU traits may have deficits in recognizing the linguistic stimuli spoken in a fearful tone (Blair et al., 2002; Dawel et al., 2012; Stevens et al., 2001). In addition, the studies that have examined auditory processing were mainly based on adolescents and adults – to our knowledge, there has been no study published with a sample of preschoolers.
Little is known about emotion recognition processing difficulties in children with CU traits in early childhood. Given that early childhood is a sensitive period for empathy development (Eisenberg, Spinrad, & Sadovsky, 2006), emotion processing difficulties present at this age may be particularly detrimental. Recent findings suggest that difficulties in emotion recognition are present in preschool-age children with CU traits (Kimonis et al., 2016). These difficulties may be particularly associated with the recognition of fear, as preschoolers with low concern for others (e.g., act like he/she did not care when someone felt bad or sad) were found to have deficits in recognizing fearful facial stimuli (White et al., 2016).
Neural Correlates of Vocal Emotion Processing and CU Traits
Despite the progress in studying CU traits in early childhood, the neural aspects of emotion processing in young children, including the processing of vocal emotions and how they relate to CU traits are not well understood. Prior research examining the neural correlates of vocal emotion processing has notably focused on the mismatch negativity (MMN), an event-related potential (ERP) component thought to be sensitive to changes in emotional expression (Schirmer & Escoffier, 2010; Schirmer, Striano, & Friederici, 2005). The MMN is a negative-going component that typically occurs from 150 – 250 milliseconds after the onset of an auditory stimulus, with longer latencies observed in childhood. The MMN is typically elicited during auditory oddball tasks, in which one stimulus (the frequent stimulus) is presented more frequently than an infrequent one (the oddball; Näätänen, Paavilainen, Rinne, & Alho, 2007). The novelty of the oddball stimulus should elicit increased attentional processing that is not elicited by the frequent stimulus. To reflect these processing differences, the MMN is computed as a difference waveform in which the waveform in the frequent condition is subtracted from the waveform in the oddball condition. As such, a larger, more negative MMN is indicative of increased processing of the oddball stimulus relative to the frequent stimulus, and is thought to reflect the enhanced processing of the differences between these stimuli.
The MMN component has been utilized to examine the auditory emotion processing capacities of a sample of adolescents with severe conduct problems. Hung et al. (2013) reported that the impulsive-antisocial features of psychopathy (which are often referred to as factor 2 of psychopathy) were associated with an enhanced neural processing to fearful vocal stimuli, whereas the affective-interpersonal features of psychopathy (which include CU traits and are often referred to as factor 1 of psychopathy) were not associated with a reduced neural processing to fearful vocal stimuli. This latter finding contradicted past research showing a reduced neural processing of facial fearful emotions in youth with CU traits (e.g., Jones et al., 2009; Marsh et al., 2008). However, Hung et al. (2013) did not specifically assess CU traits in their sample, therefore additional research is warranted. To our knowledge, researchers have yet to examine the association between CU traits and the neural correlates of auditory emotion processing in early childhood. Exploring this association has the potential to contribute to our understanding of the etiology of CU traits.
Current study
In the current study, we used the ERP technique to assess neurophysiological activity during an emotionally-valenced passive auditory oddball task in a sample of preschoolers displaying varying levels of CU traits. The passive oddball task contained vocal stimuli, English-based pseudowords, spoken in either a fearful, happy, or neutral prosody. The fear condition, which included stimuli spoken with a fearful tone (target) or a neutral tone (frequent), was the primary condition of interest given past studies that suggest that emotion processing difficulties are more specific to fear/distress emotions in individuals with CU traits. The happy condition, which included stimuli spoken with a happy tone (target) or a neutral tone (frequent), was included as a control condition, as difficulties processing positive emotions have not been widely noted in the CU literature (Marsh & Blair, 2008). Within the emotion processing task, the MMN was used to investigate the neural correlates of vocal emotion processing. Based on previous research, we expected that CU traits would be associated with a pattern of neurophysiological activity indicative of atypical processing of vocal fear (as measured by the morphology of the MMN component). In particular, we expected that CU traits would be associated with a reduced processing of vocal fear stimuli. However, we expected this effect to be specific to the fear stimuli, and did not expect to see a similar effect in the happy condition.
Method
Participants
Analyses were conducted on 27 participants, taken from an initial pool of 44 children (23 female) between the age of 2 and 5 years whose primary caregivers completed a screening measure assessing the child’s eligibility to participate in the laboratory portion of the study. Children were not eligible to participate if they 1) spoke a language other than English (because the auditory task was based on the pronunciation rules of English), 2) had significant developmental delays, or 3) had ever been referred to social services, as this may indicate maltreatment or severe family adversity, suggesting the possibility that environmental adversity may have contributed to the development of conduct problems and CU traits. For instance, children exposed to environmental adversity may show key differences in the mechanisms and expressions of their CU traits as well as in their neural responses to emotional stimuli. These children may show increased neural activation during threatening situations, instead of decreased activation as expected in children with CU traits not exposed to adverse environmental experiences (Fanti, Demetriou, & Kimonis, 2013; Khan et al., 2013; McCrory, De Brito, & Viding, 2011). Primary caregivers of eligible children were then contacted to schedule a follow-up laboratory visit in which the child participated in two tasks while electroencephalography (EEG) data were collected. To include children displaying a broad range of CU traits, a convenience sample of children was recruited from both a community setting (n = 39) and a University-based clinic for children with disruptive behavior disorders (n = 5).
Of those who completed the initial screening measures (n = 44), 32 children (18 female, M age = 46.85 months, SD = 9.9 months) participated in the EEG portion of the study. The 12 children who did not complete the lab portion of the experiment were excluded because 1) they met one of the exclusion criteria (n = 3), 2) caregivers were no longer interested in participating (n = 7), or 3) caregivers were interested in participating, but were unable to schedule a lab visit (n = 2). The children who were and were not included in the final sample did not differ significantly from one another in terms of CU traits, conduct problems, or demographic variables. Five of these children were unable to be included in the final sample because their EEG data contained excessive movement artifacts. For the final sample with usable EEG data included in analysis (n = 27, 14 female), the family’s socioeconomic status, as calculated using the Hollingshead Four Factor Index (Hollingshead, 1975), ranged from 20 to 66 (M = 49.80, SD = 13.86). The children’s ethnicity, as identified by primary caregivers, was 73% Caucasian, 21% Mixed Race, 3% Black, and 3% other. The children’s verbal abilities, as measured by a standardized measure of receptive vocabulary (Peabody Picture Vocabulary Test, Fourth Edition; PPVT-4; Dunn & Dunn, 2007), were within the normal range for preschool-age children (standardized score: M = 113.88, SD = 12.04, range = 89–131). According to primary caregiver report, no child had visual or hearing impairments.
Procedures
After completing the screening measure, the child and his or her primary caregiver participated in a laboratory visit in which EEG data were collected. During the lab visit, the children participated in a passive auditory oddball task and an emotional face-viewing paradigm. The current report focuses exclusively on results from the auditory oddball paradigm. After the EEG tasks, the children completed the PPVT-4. During the visit, the child’s primary caregiver completed a battery of questionnaires, including measures of child behavior problems. The current study complied with all the ethical standards of the American Psychological Association, and the institutional review board at Indiana University approved all procedures. All families received monetary compensation and a small toy for the child to thank them for their participation.
Measures
ERP measures of vocal emotions
A passive auditory oddball task served as an index of the child’s capacity to process emotional vocal affect. Due to the variability in behavioral response capacities of young children, we opted for a passive paradigm in order to retain as many trials as possible. During this task, which lasted for approximately 6 minutes, each child was instructed to sit as still as possible and watch a silent video of an aquarium. While the child watched the video, English-based disyllabic pseudowords spoken with either a fearful, happy, or neutral prosody by a female speaker recruited from a university theater department were played at 75 decibels from an 8-ohm speaker located one meter above the child’s head. The stimuli, selected from a larger corpus of vocal emotions (Darcy, Fontaine, Cepeda, & Krueger, under review), consisted of four pseudowords spoken with a fearful and a neutral prosody and four pseudowords spoken with a happy and a neutral prosody (the control condition). Emotion accuracy of the pseudowords was verified by a sample of 30 listeners (100% native English speakers; 83% female; M age = 22.80, SD = 6.02). Duration and peak intensity were similar across all stimuli. The task was divided into two blocks, one block in which fearful stimuli and neutral stimuli were played (the experimental condition of interest), and another block in which happy stimuli and neutral stimuli were played (the control condition). Both the fearful and happy stimuli were presented infrequently (24 times per block, in which each emotional stimulus was repeated 6 times) and the neutral stimuli were presented frequently (56 times per block, in which each neutral stimulus was repeated 14 times), amounting to 160 trials in total. Each pseudoword stimulus lasted for approximately 600 milliseconds. Each trial had a duration of 1500 milliseconds, and trials were presented continuously. During the task, a research assistant sat with the child to redirect the child if he or she was moving or not attending to the aquarium video. Block order alternated across participants, and resulting ERP waveforms were time locked to the presentation of either the fear, neutral (fear baseline), happy, or neutral (happy baseline) stimuli.
CU traits
The primary caregivers completed the Inventory of Callous-Unemotional Traits (ICU) preschool-version, a widely used measure of CU traits (Frick, 2004). The items of the ICU were assessed using a four-point scale ranging from not at all true (0) to definitely true (3). We used the two-factor structure proposed by Henry et al. (2016): (1) callousness-uncaring dimension (17 items, e.g., “seems very cold and uncaring), and (2) unemotional dimension (5 items, e.g., “does not show his/her emotions to others”). Cronbach α values for the callousness-uncaring and the unemotional dimensions were .90 and .78, respectively. We also examined a higher order factor that included all of the items on the ICU – referred to as the ICU total score (Kimonis et al., 2008). The Cronbach’s α value for the ICU total score was .89.
Control variables
Conduct problems were assessed using the externalizing problems scale (24 items, e.g., “hits others”) of the Child Behavior Checklist for ages 1 ½ to 5 (CBCL; Achenbach & Rescorla, 2000), a widely used instrument of behavior problems in early childhood. Caregivers indicated if each behavior problem was not true (0), somewhat or sometimes true (1), or very or often true (2). One item included in the externalizing problems scale, “doesn’t seem to feel guilty after misbehaving”, conceptually overlaps with two ICU items, “feels bad or guilty when he/she has done something wrong” (reverse scored) and “shows no remorse when he/she has done something wrong”. To avoid this overlap, this CBCL item was not included in our conduct problems index. The Cronbach’s α value for the conduct problems scale was .96. Child sex and age in months, variables shown to be associated with emotion processing capacities (Herba & Phillips, 2004), were also included as control variables in analysis.
Recordings and data processing
Netstation Acquisition Software 4.4.2 (Electrical Geodesic, Inc., Eugene, OR) was used to collect and process the continuous EEG data recorded during the oddball task. Data were collected using a 128-electrode Hydrocel Geodesic Sensor Net set at a sampling rate of 250 Hz. Throughout the recording session, all electrode impedances were adjusted to be at or below 50 kΩ. After collection, the continuous waveforms were band-pass filtered from 0.3 to 30 Hz, and then segmented into 1200 milliseconds epochs beginning 200 milliseconds prior to the presentation of each stimulus. Each epoch was manually inspected for artifacts, and then automatically examined for artifacts. Automatic artifact rejection included the identification and removal of channels that contained a voltage shift greater than 150 μV during a given segment of length 80 milliseconds and the removal of epochs that contained 20 or more bad channels. For a child’s ERP data to be included in analysis, the child had to have at least 9 artifact-free trials in each stimulus condition. Of the 32 children who participated in the auditory oddball task, 27 children met the criterion and were able to be included in the final sample. There were no apparent significant differences between children who did and did not provide usable EEG data on any of the variables examined in the analysis, suggesting that data were missing at random or completely at random. The average number of trials retained in each condition was 18.59 trials in the Happy condition (SD = 3.35), 42.15 trials in the Happy baseline condition (SD = 7.46), 19.41 trials in the Fear condition (SD = 3.59), and 43.63 trials in the Fear baseline condition (SD = 7.22). Each individual’s epoched data were then re-referenced to their average reference (the average of all scalp electrodes), and baseline corrected by subtracting the average activity from the epoch’s 200 milliseconds baseline.
After processing, the primary components of the ERP waveform were statistically decomposed using a sequential temporospatial principal components analysis (PCA), which objectively and empirically determines the regions of electrodes and time frames that parsimoniously account for the majority of the variance in the waveforms (corresponding to ERP components; Dien & Frishkoff, 2005). Sequential temporospatial PCA is more likely to isolate the underlying neural signal when compared to examining individual electrodes (Dien & Frishkoff, 2005), and this technique is especially useful when analyzing data from young children that are marred with movement artifacts (Dien, 2012). Sequential temporospatial PCA was conducted using ERP PCA toolkit (Dien, 2010). The initial temporal PCA identified 15 temporal factors accounting for 95% of the variance, and the temporospatial PCA identified 6 temporospatial factors accounting for 83% of the variance in the entire waveform. The temporospatial factor corresponding to the MMN was selected based on a priori expectations about the latency and topography of the component. This factor peaked from 300 – 400 milliseconds post-stimulus, and included a frontocentral positivity with a corresponding posterior negativity. The MMN difference waveform was computed by subtracting the amplitude in the baseline (neutral) condition from the corresponding amplitude in the emotion condition. Although the MMN is typically observed to be a frontocentral negativity in adults, this may not be the case for children. In early childhood, evidence suggests that the MMN appears as a frontocentral positivity with a corresponding posterior negativity, especially if the differences between stimuli are subtle (Maurer, Bucher, Brem, & Brandeis, 2003). Additionally, Maurer et al. (2003) determined that similar neural generators in the superior temporal plane underlie both the frontocentral MMN negativity observed in adults and the frontocentral MMN positivity observed in young children. These findings suggest that the frontocentral positive MMN, and its corresponding posterior negativity, that were present in the current study likely reflect activation in the same neural generators as the traditional frontocentral negative MMN component observed in adults (see the Results section).
Data analysis
The analyses proceeded in two steps. First, we examined the association between CU traits (examining the callous-uncaring and unemotional dimensions separately, as well as the ICU total score) and the MMN difference waveform amplitude in both the fear and happy conditions using Pearson correlations. Next, we used multiple regression to examine any significant associations between CU traits and the amplitude of the MMN waveform while controlling for covariates (i.e., conduct problems, age in months, and sex) to rule out the possibility that these variables accounted for any observed associations. All models were fitted using the R statistical software package (R Core Team, 2012).
Results
Grand-averaged waveform
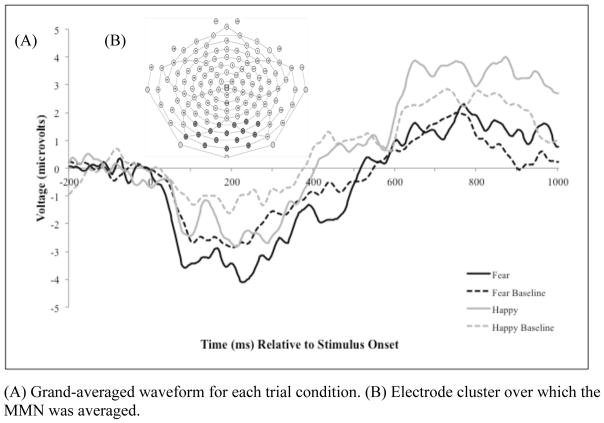
The temporospatial factor representing the MMN consisted of a frontocentral positivity with a corresponding dipole of a posterior negativity. Because the posterior negativity was larger than the frontocentral positivity, thereby driving the factor, the values from the negative, posterior portion of the factor were used in analysis (Dien, 2012; results from the frontocentral positivity are available upon request). See Figure 1 for the grand averaged waveform in each condition across the electrode cluster associated with the chosen temporospatial factor. The mean amplitude of the MMN difference waveform in the fear condition was more negative than the MMN difference waveform in the happy condition, suggesting that the fear tones were perceived to differ more substantially from the neutral condition than the happy condition. Descriptive values for all the analyzed variables are provided in Table 1.
Figure 1.
Grand-averaged Waveform Across a Posterior Electrode Group Determined via Temporospatial PCA and Corresponding to the MMN Component
Table 1.
Descriptive Statistics for MMN Difference Waveform Amplitude, CU Traits, and Control Variables
| Variable | n | Mean | SD | Range |
|---|---|---|---|---|
| Amplitude MMN difference waveform | ||||
| Fear condition (μV) | 27 | −1.22 | 6.88 | −11.34 – 19.17 |
| Happy condition (μV) | 27 | −0.37 | 5.37 | −11.27 – 12.44 |
|
| ||||
| CU traits | ||||
| Callous-uncaring traits | 26a | 13.42 | 4.70 | 7 – 25 |
| Unemotional traits | 26a | 2.38 | 2.04 | 0 – 6 |
| ICU total score | 26a | 16.46 | 5.48 | 7 – 33 |
|
| ||||
| Control variables | ||||
| Conduct problems | 27 | 7.52 | 5.19 | 0 – 19 |
| Age (months) | 27 | 46.74 | 10.48 | 27 – 66 |
Note.
Data on CU traits were missing for one child.
Fear MMN Amplitudes
Correlations between the Fear MMN amplitudes and CU traits are presented in Table 2. There were significant associations between callous-uncaring traits and the Fear MMN (r = 0.56, p < .01) and between the ICU total score and the Fear MMN (r = 0.56, p < .01). More positive Fear MMN amplitudes, indicating a smaller difference between the child’s neural response to the fear stimuli (oddball) and to the neutral stimuli (frequent), were associated with higher levels of callous-uncaring traits and overall higher scores on the ICU. This finding suggests that children with higher levels of callous-uncaring traits were less likely to differentiate between pseudowords spoken in a fearful tone and those spoken in a neutral tone than children with lower levels of callous-uncaring traits. However, because much of the variance in the ICU total score was attributable to variance in the items that loaded onto the callousness-uncaring factor, further models focused specifically on the callousness-uncaring subscale. Additionally, because there was a small, non-significant association between the unemotional subscale and Fear MMN amplitudes (r = 0.17, p < .10), the unemotional subscale was not examined in further models.
Table 2.
Correlations between the MMN Difference Waveform Amplitude, CU Traits and Control Variables
| 1. | 2. | 3. | 4. | 5. | 6. | 7. | |
|---|---|---|---|---|---|---|---|
| 1. Fear MMN amplitudes | - | ||||||
| 2. Happy MMN amplitudes | −0.03 | - | |||||
| 3. Callous-uncaring traitsa | 0.56** | −0.38† | - | ||||
| 4. Unemotional traitsa | 0.17 | −0.13 | 0.10 | - | |||
| 5. ICU total scorea | 0.56** | −0.31 | 0.92*** | 0.50** | - | ||
| 6. Conduct problems | 0.01 | −0.06 | 0.42* | −0.11 | 0.31 | - | |
| 7. Age (months) | −0.03 | 0.11 | 0.00 | 0.28 | 0.15 | 0.03 | - |
Note:
Data on CU traits were missing for one child.
p ≤ .05
p ≤ .01
p ≤ .001
p = .054
Control variables
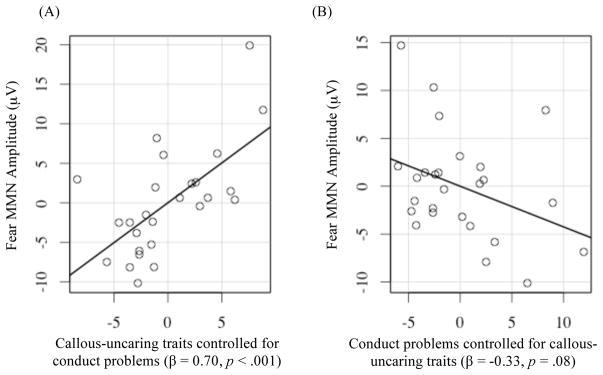
Using multiple regression, we examined whether the significant association between callous-uncaring traits and the Fear MMN held after including conduct problems (Part A) as well as child age and sex (Part B; see Table 3). When the child’s conduct problems score was included as a covariate, the association between callous-uncaring traits and the Fear MMN became slightly stonger (see Figure 2). In addition, the association between conduct problems and the Fear MMN became stronger when accounting for CU traits. Findings suggest that children with high levels of conduct problems showed larger (more negative) Fear MMN amplitudes (although this correlation did not surpass the traditional threshold for statistical significance; β = −0.33, p = .08; see Figure 2). The inclusion of child age and sex in the model did not change the overall pattern of associations between callous-uncaring traits and the Fear MMN (see Table 3, Part B).
Table 3.
Multiple Regression Analyses Predicting Fear MMN Amplitudes with Control Variables
| β | p-value | |
|---|---|---|
| Part A | ||
| Callous-uncaring traits | 0.70 | < .001 |
| Control variable | ||
| Conduct problems | −0.33 | .08 |
| F(2,23) = 10.24, p < .05, R2 = .41 | ||
|
| ||
| Part B | ||
| Callous-uncaring traits | 0.71 | < .001 |
| Control variables | ||
| Conduct problems | −0.33 | .09 |
| Age (months) | 0.02 | .92 |
| Sex* | −0.07 | .71 |
| F(4,21) = 3.66, p < .05, R2 = .41 | ||
Note:
Female = 1
Figure 2.
Partial Regression Plots Showing Unique and Contrasting Associations Between Fear MMN Amplitudes and (A) Callous-uncaring Traits and (B) Conduct Problems
Happy MMN Amplitudes
The happy condition was included as a control condition, so we did not anticipate finding an association between MMN amplitudes in the happy condition and CU traits. Contrary to expectations, there was a non-significant but trending association between the Happy MMN amplitudes and callous-uncaring traits (r = −0.38, p = .054), such that higher levels of callous-uncaring traits were associated with more negative Happy MMN amplitudes (see Table 2). We provisionally take this as early evidence that more negative Happy MMN amplitudes (which indicate a larger difference between the child’s neural response to the happy and neutral stimuli) were associated with higher levels of callous-uncaring traits. This finding may indicate that children showing higher levels of callous-uncaring traits are more adept at differentiating between happy pseudowords and neutral pseudowords. The correlation between the amplitude of the Happy MMN difference waveform and unemotional traits was not statistically significant (see Table 2).
Discussion
The current study examined the association between primary caregiver-reported CU traits and the MMN component, an ERP component that is considered an index of the extent to which differences in emotional valence are perceived and processed. We found an association between neural responses to vocal fear and caregiver reports of callous-uncaring traits: poorer abilities to detect emotional valence, inferred from smaller (less negative) Fear MMN amplitudes, were associated with higher levels of callous-uncaring traits. These results support the interpretation that preschoolers displaying higher levels of CU traits, and more specifically callous-uncaring traits, processed vocal fear less sensitively than preschoolers with fewer CU traits. A second possible interpretation is that the processing deficits seen in children with higher levels of callous-uncaring traits may have resulted from inattention to the differences between neutral and fearful vocal tones.
This finding lends support to theories that suggest that one potential process in which CU traits develop is through difficulties in perceiving distress in others. Such difficulties in emotion processing may disrupt normal empathy development, which could result in the cluster of cold and low-empathy traits that typify children displaying CU traits (Blair, 2013). Our findings, which demonstrate an association between the neural processing of fear stimuli and callous-uncaring traits, provide evidence that this process may be active in early childhood. Given that early childhood is a crucial window in which socialization occurs, emotion processing difficulties at this age, and subsequent disrupted empathy development, could have a cascade of ramifications on later development, especially if the child fails to learn from key socialization experiences and develops a negative interaction pattern with caregivers, teachers, and peers.
The current study demonstrates, for the first time, that young children displaying callous-uncaring traits show the same type of emotion processing deficits to emotionally-valenced vocal stimuli as older children and adults (Dawel et al., 2012). Additionally, the findings from the current study extend our understanding of the association between CU traits and emotion processing in early childhood in several other key ways. First, the association between CU traits and the Fear MMN emerged with the callous-uncaring scale, but not with the unemotional scale. The lack of association with the unemotional scale was expected, as unemotionality, as assessed by the ICU, may lack the precision to capture the atypical emotional responses that are characteristic of children displaying elevated levels of CU traits (Henry et al., 2016). In addition, several researchers have raised concerns about the characteristically low correlations between unemotional traits, as assessed by the ICU, and indexes of antisociality (Hawes et al., 2014; Henry et al., 2016).
Next, our findings suggest that preschoolers displaying callous-uncaring traits show reduced neural responses to fearful vocal stimuli, while also showing enhanced neural responses to happy vocal stimuli (although this latter association was only trending). These findings support a growing literature that suggests that emotion processing difficulties in individuals with CU traits may be specific to emotions associated with distress (i.e., especially fear), and not simply a general deficit in emotion processing (Marsh et al., 2008; White et al., 2012). Additionally, our findings suggest that children who are displaying higher levels of callous-uncaring traits may be more adept at differentiating and processing happy vocal stimuli. One potential interpretation of this finding depends on the general hypothesis that children with CU traits show higher levels of reward sensitivity (O’Brien & Frick, 1996). A bias towards reward may increase the child’s tendency to recognize and respond to vocal stimuli spoken with a happy prosody, as words spoken with a happy intonation often indicate that some sort of reward (either social or formal) will follow. Although we are interested in this finding, we are mindful that the association between callous-uncaring traits and the MMN in the happy condition did not reach the .05 level of significance. Replications will be crucial.
Finally, although it did not surpass the traditional level of statistical significance, a contrasting association with the Fear MMN between callous-uncaring traits and conduct problems emerged. This finding suggests that callous-uncaring traits and conduct problems may be associated differently with the perception of vocal fear, such that children who show more callous-uncaring traits may be less responsive to vocal fear tones, whereas children with more conduct problems, that are not associated with callousness-uncaring traits, may be more responsive to vocal fear tones. This latter association emerged only when accounting for the shared variance between callous-uncaring traits and conduct problems, indicating the presence of a two-way suppressor effect. A suppressor effect occurs when the inclusion of an additional predictor in a multiple regression model increases the predictive power of another predictor through accounting for outcome-irrelevant variance (Tzelgov & Henik, 1991; Wiggins, 1973). By accounting for the shared variance between the predictor and the suppressor variable, a suppressor variable makes visible the association between the non-overlapping portion of the predictor of interest and the criterion (Tzelgov & Henik, 1991). In our findings, both callous-uncaring traits and conduct problems act as suppressor variables, increasing the ability of the other to predict Fear MMN scores by accounting for the shared variance between callous-uncaring traits and conduct problems. Interestingly, accounting for callous-uncaring traits increased the association between conduct problems and enhanced Fear MMN responses, supporting the conceptualization of children with conduct problems, without callous-uncaring traits, as being hyper-sensitive to threat cues (in behavioral studies – Frick et al., 2003 and in fMRI studies – Lozier et al., 2014; Viding et al., 2012). Notably, this type of a suppressor effect has been found in previous studies assessing CU traits and conduct problems in older samples (Frick et al., 1999; Lozier et al., 2014; Sebastian et al., 2012), suggesting this suppressor effect may be a robust effect across ages. However, in the current study, even when accounting for the shared variance with callous-uncaring traits, the association between conduct problems and the Fear MMN failed to surpass the traditional level of statistical significance. This could be indicative of a lack of statistical power, therefore replication of this finding using a larger sample of children in this age range will be crucial. Such data may also allow for analyses relying on a person-centered approach, in which subgroups of children (e.g., children high on conduct problems and CU traits vs. children high on conduct problems only) could be compared. Such analyses could help to further clarify the patterns of neural responses to emotional stimuli in preschoolers showing conduct problems with CU traits (e.g., reduced responsiveness to vocal fear) compared to preschoolers showing conduct problems without CU traits (e.g., heightened responsiveness to vocal fear).
Strengths and limitations
This study has several important strengths. To our knowledge, this is the first study to explore the association between auditory emotion processing and CU traits in preschool-age children. The findings of the current study suggest that early-appearing callous-uncaring traits are associated with fear processing difficulties. This finding contributes to an emerging literature focusing on examining such traits in early childhood. Next, the focus on the neural correlates of auditory emotional processing, rather than behavioral indexes of emotional recognition and processing, makes an important contribution to this literature. Relatively few studies examining CU traits in children use cognitive neuroscience approaches to examine the neural correlates of callous-unemotionality, but there are several important benefits to using these methods, especially when studying young children. Focusing on EEG/ERPs, rather than explicit behavior, better accommodates passive tasks, which are useful in early childhood when behavioral response capacities are still developing and thus less stable, potentially complicating the interpretation of behavioral task performance. In addition, neural processing indexes, such as the MMN, have the potential to serve as biomarkers, biologically-based indexes that are detectable prior to robust manifestations of the behavior or psychopathology that convey heightened risk for the later dysfunction (Ritsner & Gottesman, 2009).
This study also has limitations. First, the range of CU traits displayed in our sample was relatively narrow, consistent with a primarily community sample. We did include several children recruited from a clinic for young children with disruptive behavior disorders (not CU traits specifically), however, we could not compare clinical and non-clinical samples of children due to the sample size differences. While our dimensional approach to defining CU traits makes our findings more generalizable to wider community, it does not establish the generalizability to preschoolers displaying clinically significant levels of CU traits. Nevertheless, the association between MMN amplitudes and callous-uncaring traits had a large effect size, suggesting that it may be clinically meaningful.
The auditory emotion processing task used in the current study included only one speaker’s voice, therefore it is possible that our findings could have been influenced by the specific vocal characteristics of this speaker. While there are various reasons only one speaker was used in the current task (i.e., efforts to hold the speaker-specific properties [indexical properties] of the task constant and keep the task as short as possible), future research based on auditory emotional stimuli may benefit from including multiple speakers to rule out the possibility that any effects could be due to some facet of the speaker’s voice. Our sample in the current study is smaller than in most behavioral studies focusing on children with CU traits. However, the number of children with usable EEG data (n = 27) was within the range of typical sample sizes of comparable, developmental ERP studies (average of 15 – 27 children per study; Hoyniak, 2017). However, because this study, like many developmental neuroscience studies, may have modest power, we may have been unable to detect subtle effects present in our data. Future studies that replicate and extend our findings with a larger sample will be crucial to increase confidence in these findings.
Clinical implications
The findings of the current study have several potential clinical implications. First, if the MMN to vocal fear is a biomarker for callous-uncaring traits, then this suggests the hypothesis that targeting and improving emotion processing capacities in early childhood could improve social development outcomes, with more normative empathy. Intervention efforts geared towards young children may be crucial for disrupting and altering the course of development of CU traits, and preventing severe, persistent conduct problems. Such interventions could be especially effective given the malleability of children’s behaviors in early childhood (Brennan & Shaw, 2015; Stormont, 2002). Additionally, the introduction of the limited prosocial emotions specifier to the DSM-5 demonstrates the growing emphasis on understanding CU traits that occur in the context of conduct problems, making it more crucial than ever to understand the early manifestations of CU traits and their neural correlates.
Research Highlights.
We examined the neural responses that underlie the processing of vocal stimuli spoken in fearful and happy tones using the event-related potential (ERP) technique in a group of preschoolers.
The mismatch negativity (MMN) ERP component was examined in association with two dimensions of callous-unemotional traits (i.e., callousness-uncaring and unemotional dimensions).
Callous-uncaring traits were associated with reduced neural responses to fearful vocal stimuli.
Reduced neural responses to vocal fear could be a potential biomarker for callous-uncaring traits in early childhood.
Acknowledgments
This project was supported by a Project Development Team within the ICTSI NIH/NCRR Grant Number TR000006 and the Department of Criminal Justice, Indiana University Bloomington. Caroline Hoyniak is supported by a Graduate Research Fellowship from the National Science Foundation Grant Number 1342962. Dr. Nathalie Fontaine is a Research Scholar, Junior 1, Fonds de recherche du Québec–Santé. We thank Professors Dennis Molfese, Aina Puce and Bennett Bertenthal for their support, and gratefully acknowledge the contribution of the research assistants, families, and children.
Footnotes
Conflicts of interest: None.
References
- Achenbach TM, Rescorla L. ASEBA Preschool Forms & Profiles: An Integrated System of Multi-informant Assessment. Aseba; 2000. [Google Scholar]
- American Psychological Association (APA) Diagnostic and statistical manual of mental disorders. 5th edn. Washington, DC: APA; 2013. [Google Scholar]
- Blair RJR. Responding to the emotions of others: Dissociating forms of empathy through the study of typical and psychiatric populations. Consciousness and Cognition. 2005;14:698–718. doi: 10.1016/j.concog.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Blair RJR. The neurobiology of psychopathic traits in youths. Nature Reviews Neuroscience. 2013;14:786–799. doi: 10.1038/nrn3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RJR, Mitchell DG, Richell RA, Kelly S, Leonard A, Newman C, Scott SK. Turning a deaf ear to fear: Impaired recognition of vocal affect in psychopathic individuals. Journal of Abnormal Psychology. 2002;111:682–686. doi: 10.1037/0021-843X.111.4.682. [DOI] [PubMed] [Google Scholar]
- Brennan LM, Shaw DS. Prenatal and early childhood prevention of antisocial behavior. In: Krohn MD, Lane J, editors. The Handbook of Juvenile Delinquency and Juvenile Justice. Chichester, West Sussex: Wiley Blackwell; 2015. pp. 351–370. [Google Scholar]
- Darcy I, Fontaine NMG, Cepeda G, Krueger F. The Hoosier Prosody Corpus: A data collection of recorded pseudo-words for evaluating the prosodic processing of emotions. Behavior Research Methods (under review) [Google Scholar]
- Dawel A, O’Kearney R, McKone E, Palermo R. Not just fear and sadness: Meta-analytic evidence of pervasive emotion recognition deficits for facial and vocal expressions in psychopathy. Neuroscience & Biobehavioral Reviews. 2012;36:2288–2304. doi: 10.1016/j.neubiorev.2012.08.006. [DOI] [PubMed] [Google Scholar]
- Dien J. The ERP PCA Toolkit: An open source program for advanced statistical analysis of event-related potential data. Journal of Neuroscience Methods. 2010;187:138–145. doi: 10.1016/j.jneumeth.2009.12.009. [DOI] [PubMed] [Google Scholar]
- Dien J. Applying principal components analysis to event-related potentials: A tutorial. Developmental Neuropsychology. 2012;37:497–517. doi: 10.1080/87565641.2012.697503. [DOI] [PubMed] [Google Scholar]
- Dien J, Frishkoff GA. Introduction to principal components analysis of event-related potentials. In: Handy TC, editor. Event related potentials: A methods handbook. Cambridge, Massachussetts: MIT Press; 2005. pp. 189–207. [Google Scholar]
- Dunn LM, Dunn DM. PPVT-4: Peabody picture vocabulary test. Minneapolis, MN: Pearson Assessments; 2007. [Google Scholar]
- Eisenberg N, Spinrad TL, Sadovsky A. Empathy-related responding in children. In: Killen M, Smetana JG, editors. Handbook of Moral Development. Mahwah, NJ: Lawrence Erlbaum Associates Publishers; 2006. pp. 517–549. [Google Scholar]
- Fanti KA, Demetriou CA, Kimonis ER. Variants of callous-unemotional conduct problems in a community sample of adolescents. Journal of Youth and Adolescence. 2013;42:964–979. doi: 10.1007/s10964-013-9958-9. [DOI] [PubMed] [Google Scholar]
- Fontaine NM, McCrory EJ, Boivin M, Moffitt TE, Viding E. Predictors and outcomes of joint trajectories of callous–unemotional traits and conduct problems in childhood. Journal of Abnormal Psychology. 2011;120:730–742. doi: 10.1037/a0022620. [DOI] [PubMed] [Google Scholar]
- Frick PJ. Unpublished rating scale. University of New Orleans; 2004. The inventory of callous-unemotional traits. [Google Scholar]
- Frick PJ, Cornell AH, Bodin SD, Dane HE, Barry CT, Loney BR. Callous-unemotional traits and developmental pathways to severe conduct problems. Developmental Psychology. 2003;39:246–260. doi: 10.1037/0012-1649.39.2.246. [DOI] [PubMed] [Google Scholar]
- Frick PJ, Lilienfeld SO, Ellis M, Loney B, Silverthorn P. The association between anxiety and psychopathy dimensions in children. Journal of Abnormal Child Psychology. 1999;27:383–392. doi: 10.1023/A:1021928018403. [DOI] [PubMed] [Google Scholar]
- Frick PJ, Morris AS. Temperament and developmental pathways to conduct problems. Journal of Clinical Child and Adolescent Psychology. 2004;33:54–68. doi: 10.1207/S15374424JCCP3301_6. [DOI] [PubMed] [Google Scholar]
- Frick PJ, Ray JV, Thornton LC, Kahn RE. Can callous-unemotional traits enhance the understanding, diagnosis, and treatment of serious conduct problems in children and adolescents? A comprehensive review. Psychological Bulletin. 2014;140:1–57. doi: 10.1037/a0033076. [DOI] [PubMed] [Google Scholar]
- Frick PJ, Viding E. Antisocial behavior from a developmental psychopathology perspective. Development and Psychopathology. 2009;21:1111–1131. doi: 10.1017/S0954579409990071. [DOI] [PubMed] [Google Scholar]
- Hawes SW, Byrd AL, Henderson CE, Gazda RL, Burke JD, Loeber R, Pardini DA. Refining the parent-reported Inventory of Callous-Unemotional Traits in boys with conduct problems. Psychological Assessment. 2014;26:256–266. doi: 10.1037/a0034718. [DOI] [PubMed] [Google Scholar]
- Henry J, Pingault JB, Boivin M, Rijsdijk F, Viding E. Genetic and environmental aetiology of the dimensions of Callous-Unemotional traits. Psychological Medicine. 2016;46:405–414. doi: 10.1017/S0033291715001919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herba C, Phillips M. Annotation: Development of facial expression recognition from childhood to adolescence: Behavioural and neurological perspectives. Journal of Child Psychology and Psychiatry. 2004;45:1185–1198. doi: 10.1111/j.1469-7610.2004.00316.x. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Unpublished manuscript. Yale University; 1975. Four factor index of social status. [Google Scholar]
- Hoyniak C. Changes in the NoGo N2 Event-Related Potential component across childhood: A systematic review and meta-analysis. Developmental Neuropsychology. 2017;42:1–24. doi: 10.1080/87565641.2016.1247162. [DOI] [PubMed] [Google Scholar]
- Hung AY, Ahveninen J, Cheng Y. Atypical mismatch negativity to distressful voices associated with conduct disorder symptoms. Journal of Child Psychology and Psychiatry. 2013;54(9):1016–1027. doi: 10.1111/jcpp.12076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde LW, Shaw DS, Gardner F, Cheong J, Dishion TJ, Wilson M. Dimensions of callousness in early childhood: Links to problem behavior and family intervention effectiveness. Development and Psychopathology. 2013;25:347–363. doi: 10.1017/S0954579412001101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones A, Laurens K, Herba C, Barker G, Viding E. Amygdala hypoactivity to fearful faces in boys with conduct problems and callous-unemotional traits. American Journal of Psychiatry. 2009;166:95–102. doi: 10.1176/appi.ajp.2008.07071050. [DOI] [PubMed] [Google Scholar]
- Kahn RE, Frick PJ, Youngstrom EA, Kogos Youngstrom J, Feeny NC, Findling RL. Distinguishing primary and secondary variants of callous-unemotional traits among adolescents in a clinic-referred sample. Psychological Assessment. 2013;25:966–978. doi: 10.1037/a0032880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimonis ER, Fanti KA, Anastassiou-Hadjicharalambous X, Mertan B, Goulter N, Katsimicha E. Can callous-unemotional traits be reliably measured in preschoolers? Journal of Abnormal Child Psychology. 2016;44:625–638. doi: 10.1007/s10802-015-0075-y. [DOI] [PubMed] [Google Scholar]
- Kimonis ER, Frick PJ, Skeem JL, Marsee MA, Cruise K, Munoz LC, … Morris AS. Assessing callous–unemotional traits in adolescent offenders: Validation of the Inventory of Callous–Unemotional traits. International Journal of Law and Psychiatry. 2008;31:241–252. doi: 10.1016/j.ijlp.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Kochanska G. Toward a synthesis of parental socialization and child temperament in early development of conscience. Child Development. 1993;64:325–347. doi: 10.2307/1131254. [DOI] [Google Scholar]
- Kochanska G. Multiple pathways to conscience for children with different temperaments: From toddlerhood to age 5. Developmental Psychology. 1997;33:228–240. doi: 10.1037/0012-1649.33.2.228. [DOI] [PubMed] [Google Scholar]
- Longman T, Hawes DJ, Kohlhoff J. Callous–unemotional traits as markers for conduct problem severity in early childhood: A meta-analysis. Child Psychiatry & Human Development. 2015:1–9. doi: 10.1007/s10578-015-0564-9. doi:0.1007/s10578-015-0564-9. [DOI] [PubMed] [Google Scholar]
- Lozier LM, Cardinale EM, VanMeter JW, Marsh AA. Mediation of the relationship between callous-unemotional traits and proactive aggression by amygdala response to fear among children with conduct problems. JAMA Psychiatry. 2014;71:627–636. doi: 10.1001/jamapsychiatry.2013.4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynam DR, Caspi A, Moffitt TE, Loeber R, Stouthamer-Loeber M. Longitudinal evidence that psychopathy scores in early adolescence predict adult psychopathy. Journal of Abnormal Psychology. 2007;116:155–165. doi: 10.1037/0021-843X.116.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh AA, Blair R. Deficits in facial affect recognition among antisocial populations: A meta-analysis. Neuroscience & Biobehavioral Reviews. 2008;32:454–465. doi: 10.1016/j.neubiorev.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh AA, Finger EC, Mitchell DG, Reid ME, Sims C, Kosson DS, … Blair R. Reduced amygdala response to fearful expressions in children and adolescents with callous-unemotional traits and disruptive behavior disorders. The American Journal of Psychiatry. 2008;165:712–720. doi: 10.1176/appi.ajp.2007.07071145. [DOI] [PubMed] [Google Scholar]
- Maurer U, Bucher K, Brem S, Brandeis D. Development of the automatic mismatch response: From frontal positivity in kindergarten children to the mismatch negativity. Clinical Neurophysiology. 2003;114:808–817. doi: 10.1016/S1388-2457(03)00032-4. [DOI] [PubMed] [Google Scholar]
- McCrory E, De Brito SA, Viding E. The impact of childhood maltreatment: A review of neurobiological and genetic factors. Frontiers in Psychiatry. 2011:48. doi: 10.3389/fpsyt.2011.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Näätänen R, Paavilainen P, Rinne T, Alho K. The mismatch negativity (MMN) in basic research of central auditory processing: A review. Clinical Neurophysiology. 2007;118:2544–2590. doi: 10.1016/0304-3940(89)90513-2. [DOI] [PubMed] [Google Scholar]
- O’Brien BS, Frick PJ. Reward dominance: Associations with anxiety, conduct problems, and psychopathy in children. Journal of Abnormal Child Psychology. 1996;24:223–240. doi: 10.1007/BF01441486. [DOI] [PubMed] [Google Scholar]
- Team, R. C. R: A language and environment for statistical computing. 2012. [Google Scholar]
- Ritsner MS, Gottesman II. Where do we stand in the quest for neuropsychiatric biomarkers and endophenotypes and what next? In: Ritsner MS, editor. The handbook of neuropsychiatric biomarkers, endophenotypes and genes. Springer; 2009. pp. 3–21. [Google Scholar]
- Schirmer A, Escoffier N. Emotional MMN: Anxiety and heart rate correlate with the ERP signature for auditory change detection. Clinical Neurophysiology. 2010;121:53–59. doi: 10.1016/j.clinph.2009.09.029. [DOI] [PubMed] [Google Scholar]
- Schirmer A, Striano T, Friederici AD. Sex differences in the preattentive processing of vocal emotional expressions. Neuroreport. 2005;16:635–639. doi: 10.1097/00001756-200504250-00024. [DOI] [PubMed] [Google Scholar]
- Sebastian CL, McCrory EJP, Cecil CAM, Lockwood PL, De Brito SA, Fontaine NMG, Viding E. Neural responses to affective and cognitive Theory of Mind in children with conduct problems and varying levels of callous-unemotional traits. Archives of General Psychiatry. 2012;69:814–822. doi: 10.1001/archgenpsychiatry.2011.2070. [DOI] [PubMed] [Google Scholar]
- Stevens D, Charman T, Blair R. Recognition of emotion in facial expressions and vocal tones in children with psychopathic tendencies. The Journal of Genetic Psychology. 2001;162:201–211. doi: 10.1080/00221320109597961. [DOI] [PubMed] [Google Scholar]
- Stormont M. Externalizing behavior problems in young children: Contributing factors and early intervention. Psychology in the Schools. 2002;39:127–138. doi: 10.1002/pits.10025. [DOI] [Google Scholar]
- Tzelgov J, Henik A. Suppression situations in psychological research: Definitions, implications, and applications. Psychological Bulletin. 1991;109(3):524–536. doi: 10.1037/0033-2909.109.3.524. [DOI] [Google Scholar]
- van Goozen SH. The role of early emotion impairments in the development of persistent antisocial behavior. Child Development Perspectives. 2015;9:206–210. doi: 10.1111/cdep.12134. [DOI] [Google Scholar]
- Viding E, Sebastian CL, Dadds MR, Lockwood PL, Cecil CA, De Brito SA, McCrory EJ. Amygdala response to preattentive masked fear in children with conduct problems: The role of callous-unemotional traits. American Journal of Psychiatry. 2012;169:1109–1116. doi: 10.1176/appi.ajp.2012.12020191. [DOI] [PubMed] [Google Scholar]
- Waller R, Trentacosta CJ, Shaw DS, Neiderhiser JM, Ganiban JM, Reiss D, Hyde LW. Heritable temperament pathways to early callous-unemotional behaviour. British Journal of Psychiatry. 2016;209:475–482. doi: 10.1192/bjp.bp.116.181503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SF, Briggs-Gowan MJ, Voss JL, Petitclerc A, McCarthy KR, Blair RJ, Wakschlag LS. Can the fear recognition deficits associated with callous-unemotional traits be identified in early childhood? Journal of Clinical and Experimental Neuropsychology. 2016;38(6):672–684. doi: 10.1080/13803395.2016.1149154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SF, Williams WC, Brislin SJ, Sinclair S, Blair KS, Fowler KA, Blair RJ. Reduced activity within the dorsal endogenous orienting of attention network to fearful expressions in youth with disruptive behavior disorders and psychopathic traits. Development and Psychopathology. 2012;24:1105–1116. doi: 10.1017/S0954579412000569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins JS. Personality and prediction: Principles of personality assessment. Addison-Wesley Pub. Co; 1973. [Google Scholar]