Abstract
p38 mitogen-activated protein kinase (MAPK) consists of two major isoforms: p38α and p38β; however, it remains unclear which isoform is more important for chronic pain development. Recently, we developed potent, long-lasting, and p38 MAPK subtype-specific antisense oligonucleotides (ASOs). We examined the therapeutic effects of isoform-specific ASOs in several chronic pain models following single intrathecal injection (300 µg/10 µl) in CD1 mice. In the chronic constriction injury (CCI) model, p38α MAPK ASO, given on post-operative day 5, reduced CCI-induced mechanical allodynia in male but not female mice. In contrast, mechanical allodynia after CCI in both sexes was not affected by p38β MAPK ASO. Intrathecal injection of p38α or p38β ASO resulted in a partial reduction (≈50%) of spinal p38α or p38β mRNA level, respectively, in both sexes at two weeks. In contrast, intrathecal injection of the ASOs did not affect p38α and p38β MAPK mRNA levels in dorsal root ganglia. Intrathecal p38α ASO also reduced postoperative pain (mechanical and cold allodynia) in male mice after tibia fracture. However, intrathecal p38α ASO had no effect on mechanical allodynia in male mice after paclitaxel treatment. Intrathecal p38α MAPK ASO pre-treatment also prevented TLR4-mediated mechanical allodynia and downregulated levels of p38α MAPK and phosphorylated p38 MAPK following intrathecal treatment of lipopolysaccharide. In summary, our findings suggest that p38α MAPK is the major p38 MAPK isoform in the spinal cord and regulates chronic pain in a sex and model-dependent manner. Intrathecal p38α MAPK ASO may offer a new treatment for some chronic pain conditions.
Keywords: antisense oligonucleotides (ASOs), p38 mitogen-activated protein kinase (MAPK), microglia, neuropathic pain, sex, spinal cord
Introduction
Chronic pain, such as neuropathic pain, inflammatory pain and cancer pain, is a disease caused by peripheral inflammation and abnormal processing in the sensory nervous system (Ji et al., 2014; Kuner, 2010). Recent progress has indicated that non-neuronal cells, including glial cells and immune cells, regulate the development and maintenance of chronic pain through multiple mechanisms, such as neuroinflammation, neural-glial crosstalk, and secretion of pain molecules (Grace et al., 2014; Ji et al., 2016; Milligan and Watkins, 2009; Ren and Dubner, 2010). Recently, glial activation was implicated in patients suffering chronic pain (Loggia et al., 2015). Microglial cells are considered as resident macrophages in the central nerve system (CNS). Accumulating literatures indicate that spinal microglial activation is an essential step for the generation of pathological pain (Coull et al., 2005; Ji et al., 2013; Tsuda et al., 2005). In particular, Toll-like receptor 4 (TLR4) has been strongly implicated in microglial activation and pathogenesis of chronic pain (Christianson et al., 2011; Sorge et al., 2011; Tanga et al., 2005). TLR4 also contributes to opioid induced antinociceptive tolerance and opioid induced hyperalgesia (Grace et al., 2016; Watkins et al., 2009). Mounting evidence suggests that the role of microglial cells in chronic pain is sex dependent. For example, spinal TLR4 modulates inflammatory and neuropathic pain only in male mice (Sorge et al., 2011). Intrathecal injection of a microglial inhibitor (such as minocycline) attenuated mechanical allodynia in male but not female mice following peripheral nerve injury (Chen et al., 2017; Sorge et al., 2015). However, morphological activation of microglia (microgliosis) is not evident in male rodent models of chronic post-ischemia pain and chemotherapy-induced neuropathy (Robinson et al., 2014; Tian et al., 2017).
Numerous studies indicate that p38 MAPK in spinal cord microglia contributes to the development of neuropathic, inflammatory, and cancer pain (Ji et al., 2009; Jin et al., 2003b; Kobayashi et al., 2008; Malon and Cao, 2016; Milligan et al., 2003; Tsuda et al., 2004; Zhuang et al., 2007). GRK2 determines duration of hyperalgesia via possible signaling of CX3CR1/p38/IL-1 in spinal microglia (Willemen et al., 2010). Recently, we found that chronic constriction injury (CCI) induced identical increases of microglial markers CXC3CR1 and IBA-1 in both sexes. However, we also found that p38 MAPK activation (phosphorylation) was primarily increased in spinal microglia of male mice, and importantly, a specific p38 MAPK inhibitor skepinone reduced CCI-evoked mechanical allodynia in male but not female mice (Taves et al., 2016). These findings imply that some microglial signaling (e.g., p38 activation, P2X4 upregulation, and caspase-6 response) but not overall morphological activation in microglia is male dependent (Berta et al., 2016; Sorge et al., 2015; Taves et al., 2016).
There are four isoforms of p38 MAPK, p38α, p38β, p38γ and p38δ, which differ in the expression patterns and activation mechanisms (Cuadrado and Nebreda, 2010). Previous studies showed that p38α and p38β MAPK are expressed in rodent spinal cord; and furthermore, pharmacological intervention using antisense oligonucleotides (ASOs) indicated that distinct role p38α and p38β MAPK in persistent pain is model and condition dependent (Dong et al., 2014; Svensson et al., 2005). Svensson et al. reported that spinal p38β but not p38α MAPK isoform mediates tissue injury-induced hyperalgesia and spinal sensitization [35]. Dong et al. showed that intrathecal p38β MAPK ASOs reduced bone cancer pain in rats [8]. However, it remains unclear which p38 isoform such as p38α or p38β regulates neuropathic pain and postoperative pain in a sex-dependent manner.
In this study, we examined whether p38α and p38β MAPK play a distinct role in several pathological pain models using p38 MAPK subtype-specific ASOs. The ASOs used here were optimized for CNS delivery, and are potent and long-lasting due to the use of second generation ASOs with 2’-O-methoxyethyl (MOE) modifications and backbones containing phosphothioate linkages (Bennett and Swayze, 2010). We found that ASOs are well tolerated in mice after intrathecal injection (300 µg). We also report the following interesting findings. (1) Intrathecal injection of p38α but not p38β MAPK ASO attenuated nerve injury-induced mechanical allodynia in male mice. (2) Intrathecal p38α and p38β ASO had no effects on nerve injury-induced mechanical allodynia in female mice. (3) A single intrathecal p38α or p38β ASO treatment produced a partial but sustained reduction of p38α or p38β mRNA levels in spinal cord but not dorsal root ganglion (DRG) tissues of both sexes. (4) Intrathecal p38α but not p38β ASO reduced mechanical and cold allodynia in male animals after bone fracture. (5) Neither p38α nor p38β ASO reduced chemotherapy-induced mechanical allodynia in males following intrathecal injection. (6) Intrathecal p38α MAPK ASO pre-treatment prevented LPS-induced mechanical allodynia and decreased the levels of p38α MAPK and phosphorylated p38 MAPK in spinal cord tissues. These findings support a role of spinal p38α in some chronic pain conditions of male mice.
Materials and Methods
2.1. Animals
Wild-type CD1 (male and female, 8–10 weeks old) were purchased from Charles River Laboratories and housed at the vivarium animal facility of Duke University Medical Center. The protocol of animal experiments was approved by the Animal Care Committee of Duke University Medical Center.
2.2. Drugs and administration
Antisense oligonucleotides (ASO) targeting mouse p38α and p38β were provided by Ionis Pharmaceuticals and diluted in sterile PBS (for sequences, see Table 1). Paclitaxel and lipopolysaccharide (LPS) were purchased from Sigma. Intrathecal injection was performed as described previously (Taves et al., 2016), mice were anesthetized with isoflurane and a spinal cord puncture was performed between the L5 and L6 level to deliver drugs (10 µl) using a 30G needle.
Table 1.
ASO sequences and targets
| ASO | Sequence | Target | Species |
|---|---|---|---|
| ION 792142 | 5’-CGTCCAACACTGGAATTGGC-3’ | p38α | Mouse, rat |
| ION 725040 | 5’-TGCTCAATTTCATGGGTGCC-3’ | p38β | Mouse, rat |
2.2. Surgery
Chronic constriction injury (CCI) was performed as described previously (Chen et al., 2015; Taves et al., 2016). Briefly, the left sciatic nerve was exposed at mid-thigh level under isoflurane anesthesia, and three loose silk ligatures (6-0 suture) approximately 1 mm apart were made around the sciatic nerve and the incision was closed with non-absorbable silk suture (5-0). It was reported that CCI with gut but not silk suture caused hyperalgesia in rats (Maves et al., 1993). However, in our hands, CCI with silk suture consistently produces marked and sustained mechanical allodynia and heat hyperalgesia in mice (Chen et al., 2015; Han et al., 2016b; Taves et al., 2016). We also observed robust neuroinflammation in the DRG and spinal cord after CCI with silk suture (Chen et al., 2015). It is possible that the onset of neuropathic pain could be slower with silk suture. Tibial facture (TF) was performed under isoflurane anesthesia (Zhang et al., 2016b). Muscles were disassociated following an incision on the left hindpaw. A 0.38-mm stainless steel pin was inserted into the tibia intramedullary canal, followed by the osteotomy, and the incision was sutured with 6-0 Prolene. To produce chemotherapy-associated neuropathic pain, paclitaxel (2 mg/kg, i.p.) was injected at day 0, 2, 4, and 6 (Xu et al., 2015).
2.3. Behavioral analysis
All behavioral tests were performed in boxes on an elevated metal mesh floor under stable room temperature and humidity. Mice were habituated to the environment for at least 2 days before the experiments. To assess mechanical allodynia, the plantar surface of left hind-paw was stimulated using a series of von Frey fibers with logarithmically increasing stiffness (0.02–2.56 gram, Stoelting), presented perpendicularly to the central plantar surface. 50% paw withdrawal threshold was determined following Dixon's up-down method. The frequency response was measured by stimulating the hind-paw with a 0.4 gram von Frey hair for ten times and the percentage withdrawal response was calculated as frequency (Taves et al., 2016). To assess cold allodynia, two acetone applications (20 µl each) were gently applied to the hindpaw bottom using a pipette and the responses to acetone were scored: 0, no response; 1, quick withdrawal, paw stamping or flicking; 2, prolonged withdrawal or repeated flicking of the paw; 3, repeated paw flicking and licking (Chen et al., 2016; Han et al., 2016a). All the behavioral tests were performed in a blinded manner.
2.4. Real-time PCR
Tissues sent frozen at −80°C to Ionis Pharmaceuticals for PCR analysis. Upon arrival, tissues were homogenized in guanidine isothiocyanate solution (Invitrogen, Carlsbad, CA) containing 8% 2-mercaptoethanol. Total RNA was then isolated using the RNeasy 96 Kit (Qiagen, Germantown, MD) that included in-column DNA digestion with 50U of DNase I (Invitrogen, Carlsbad, CA). Single step real-time reverse-transcription polymerase chain reaction (RT-PCR) was performed with gene specific primers (Table 2) (IDT technologies, Coralville, IA) using the conditions 50°C for 15 minutes, 95°C for 2 minutes followed by 40 cycles of 95°C for 15 seconds, 60°C for 1 minute. Relative RNA quantities analyzed based on standard curves made from RNA extracted from tissues of vehicle treated animals.
Table 2.
RT-PCR primers and probes
| Target | Mouse p38α | Mouse p38β |
|---|---|---|
| Forward | 5’-TGTGAACGAAGACTGTGAGC-3’ | 5’-CGCCAGAAGGTGGCTGTAAA-3’ |
| Reverse | 5’-GCATCCAATTCAGCATGATCTC-3’ | 5’-TGTCCTCCTCGCGTGGAT-3’ |
| Probe | 5’CCGAGCCAGCCCAAAATCCAGAAT-3’ | 5’AAGCTGTCTCGCCCTTTCCAATCGC-3’ |
2.5 Western blotting
L3–L5 spinal cord tissues were homogenized in a RIPA lysis buffer (10×, Millipore) containing protease and phosphatase inhibitors. Protein samples were quantified using BCA Protein Assay (Pierce) and separated on an SDS-PAGE gel (Bio-rad), transferred, and probed with antibodies against p38α MAPK (1:1000, Cell signaling), phosphorylated p38 MAPK (1:1000, Cell signaling), GAPDH (1:10000, Cell signaling). Then, these blots were incubated with corresponding horseradish peroxidase-conjugated secondary antibodies (GE Healthcare). Specific bands were visualized with enhanced chemiluminescence (Thermo scientific) and quantified with ImageJ software (NIH).
2.6 Statistics
The data were expressed as mean ± S.E.M., as indicated in figure legends. Statistical analyses were made using Prism GraphPad 6.0. For behavioral tests, differences between groups were analyzed using One-Way or Two-Way ANOVA followed by Bonferroni’s post-hoc test. For PCR analysis, differences between groups were compared by One-way ANOVA with Bonferroni’s post-hoc test. For western blotting, differences between groups were analyzed using t test. p<0.05 was taken as the criterion for statistically significance.
Results
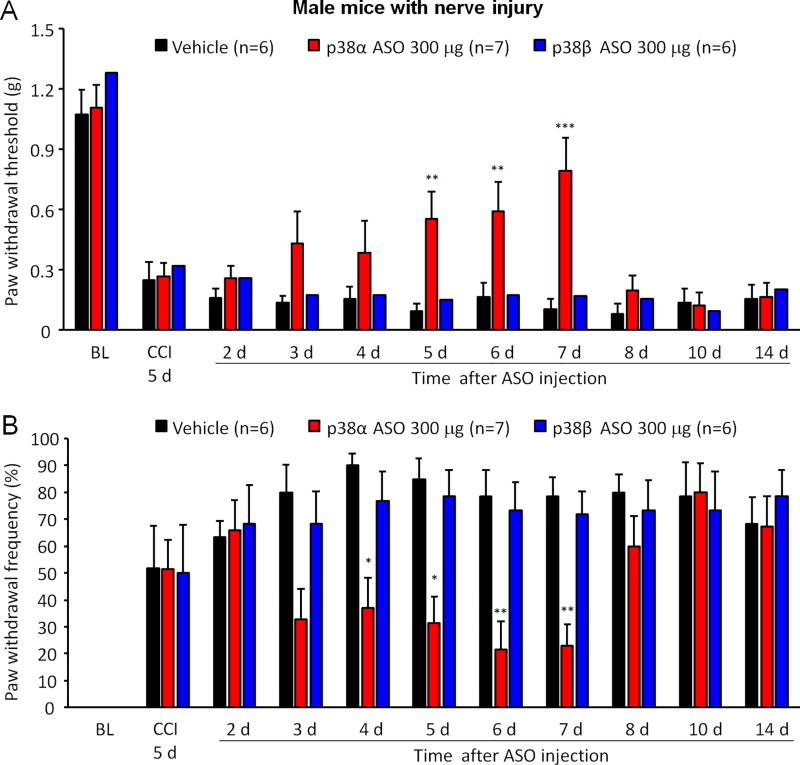
3.1. Intrathecal injection of p38α but not p38β MAPK ASO alleviated mechanical allodynia in male mice with CCI injury
In order to define specific contribution of p38α and p38β MAPK isoform to neuropathic pain, we performed the chronic constriction injury (CCI) of the sciatic nerve and administrated 300 µg p38α or p38β MAPK ASO intrathecally to CCI-injured male mice on post-operative day (POD) 5. We used two different methods to determine mechanical allodynia in response to von Frey hair stimuli: paw withdrawal threshold (PWT) and paw withdrawal frequency (PWF). All male animals developed mechanical allodynia on POD 5, as indicated by decreased PWT (F(10, 160) = 49.11, p < 0.001) and increased PWF (F(10, 160) = 19.13, p < 0.001) (Figure 1A and 1B). Intrathecal injection of p38α MAPK ASO produced significant inhibition of mechanical allodynia by increasing PWT (F (1, 121) = 23.79, p < 0.001) and decreasing PWF (F(1, 121) = 20.60, p < 0.001), as compared to vehicle group, and such analgesic effects lasted several days, from post-injection day 4 to day 7 (Figure 1A and 1B). Surprisingly, intrathecal delivery of p38β MAPK ASO failed to attenuate CCI-induced mechanical allodynia (PWF: F(1, 110) = 3.092, p > 0.05; PWT: F(1, 110) = 0.07662, p > 0.05) (Figure 1A and 1B). Previously, we reported that intrathecal injection of skepinone, a highly selective inhibitor for p38α MAPK, reduced CCI-induced neuropathic pain in male rodents (Taves et al., 2016). These behavioral data imply that p38α may be the major subtype regulating spinal p38 signaling in neuropathic pain in male rodents.
Figure 1. Intrathecal injection of p38α but not p38β MAPK ASO alleviates mechanical allodynia in male mice with CCI injury.
(A, B) Intrathecal injection of 300 µg p38α but not p38β MAPK ASO 5 days after CCI increased paw withdrawal threshold (A) and decreased paw withdrawal frequency (B) in male mice. **p<0.01 and *p<0.05 in comparison to vehicle (PBS). Two-Way ANOVA with Bonferroni’s post-hoc test, n=6–7 mice/per group. Paw withdrawal frequency was induced by a 0.4 g von Frey hair.
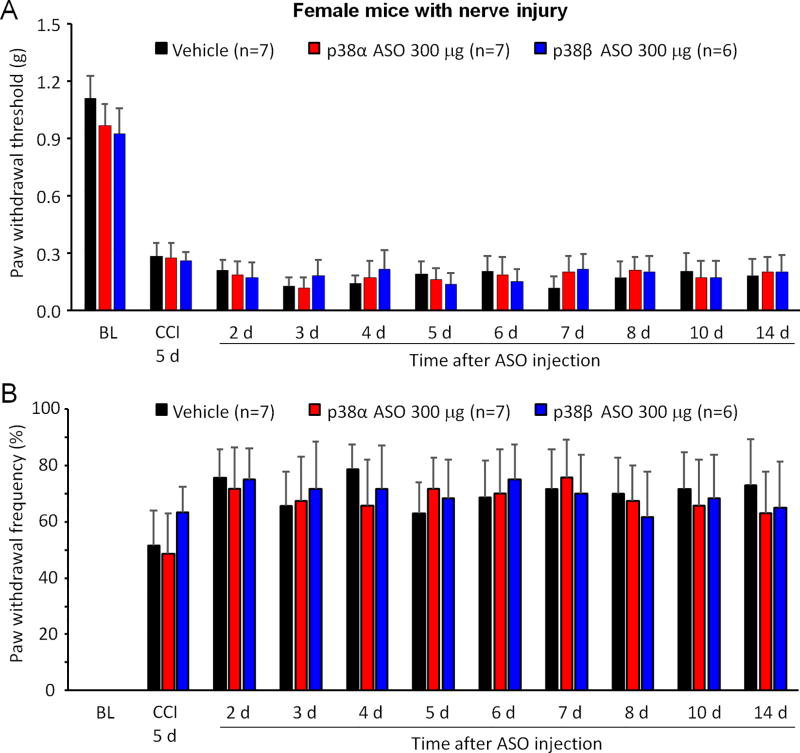
3.2. Intrathecal injection of p38α or p38β MAPK ASO failed to affect mechanical allodynia in female mice with CCI
CCI increased the number of IBA-1 labeled microglia in both male and female rodents, but only induced p38 MAPK phosphorylation in male animals at the early stage of neuropathy (Taves et al., 2016). Therefore, p38 MAPK subtypes may not be involved in CCI-induced neuropathic pain. To confirm it, p38α or p38β MAPK ASO (300 µg) was also administrated intrathecally to CCI-injured female mice on POD 5. Our results showed that CCI induced mechanical allodynia in all female animals on POD 5, as indicated by decreased PWT (F(10, 170) = 50.01, p < 0.001) and increased PWF (F(10, 170) = 24.66 p < 0.001) (Figure 2A and B). Intrathecal injection of p38α or p38β MAPK ASO on POD 5 failed to reduce mechanical allodynia in female mice with CCI injury. These results further confirm a critical role of p38α MAPK signaling in neuropathic pain of males.
Figure 2. Intrathecal injection of p38α or p38β MAPK ASO fails to affect mechanical allodynia in CCI-injured female mice.
(A, B) Intrathecal injection of 300 µg p38α or p38β MAPK ASO 5 days after CCI did not alter paw withdrawal threshold (A) or paw withdrawal frequency (B) in CCI-injured female mice. Two-Way ANOVA with Bonferroni’s post-hoc test, n=6–7 mice per group. Paw withdrawal frequency was induced by a 0.4 g von Frey hair.
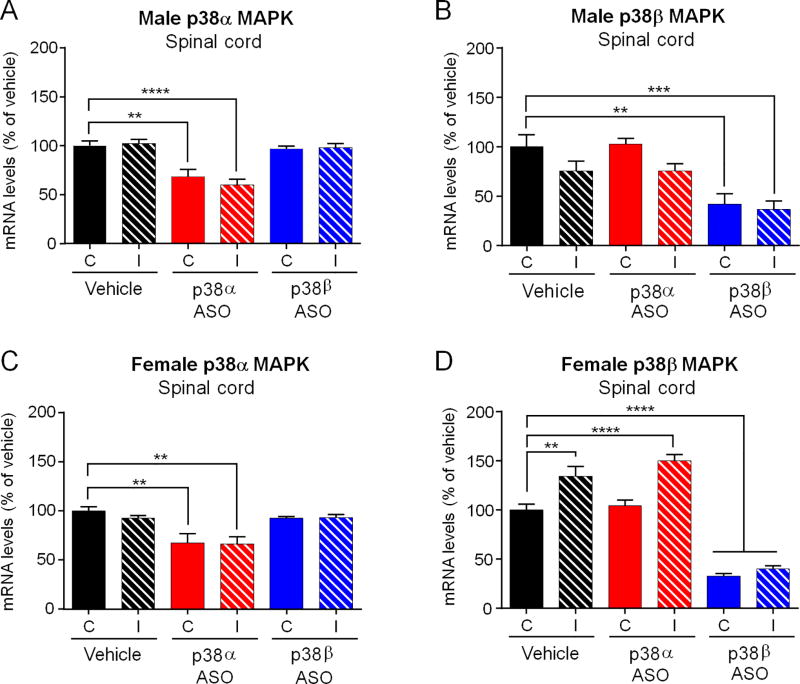
3.3. Single intrathecal injection of p38α or p38β MAPK ASO reduced the expression of p38α or p38β MAPK mRNA in the spinal cord tissues in CCI mice of both sexes
To ascertain whether intrathecal ASO treatment targets the respective expression of p38α or p38β MAPK in the spinal cord, we harvested spinal cord tissues 2 weeks after the treatment (ASO, 300 µg, 19 days post CCI surgery) and analyzed mRNA levels of p38 MAPK isoforms on both ipsilateral and contralateral spinal cords using real-time PCR. In control male mice, there was no difference in p38α or p38β MAPK mRNA levels in the ipsilateral and contralateral spinal cord (Figure 3A and B). Intrathecal p38α MAPK ASO only lowered the spinal mRNA levels of p38α MAPK, but not p38β MAPK, in both sexes and on both sides (Figure 3A, F(5,32) = 1.013, p < 0.001; and C, F(5, 34) = 4.036, p < 0.001). Moreover, intrathecal p38β MAPK ASO only decreased the spinal mRNA levels of p38β but not p38α MAPK, in both sexes and on both sides (Figure 3B, F(5,32) = 0.3317, p < 0.001), and D, F(5,34) = 1.019, p < 0.001). Notably, the knockdown effects were partial (around 35% reduction of p38α MAPK mRNA and 60% reduction of p38β MAPK mRNA in both sexes) but sustained when examined 2 weeks after a single ASO injection. It is also noteworthy that p38β MAPK ASO was more effective than p38α MAPK ASO in suppressing p38β MAPK mRNA expression (60% reduction) than p38α expression in the spinal cord, respectively.
Figure 3. Intrathecal injection of p38α or p38β MAPK ASO reduces the respective expression of p38α or p38β mRNA in spinal cord of mice with CCI injury.
(A–D) Intrathecal injection of p38α MAPK ASO only reduced spinal mRNA levels of p38α MAPK, but not p38β MAPK, on both sides and in both males (A,B) and females (C,D). ****p<0.0001, ***p<0.001, **p<0.01. C, contralateral; I, ipsilateral to CCI. One-way ANOVA with Bonferroni’s post-hoc test, n=6–7 mice/group. Spinal cord tissues were collected two weeks after the ASO treatment.
In spinal cord from vehicle-treated female animals, there was no difference in p38α MAPK mRNA level between both sides (Figure 3C), whereas higher levels of p38β MAPK mRNA was found on the ipsilateral side, suggesting an upregulation of p38β but not p38α MAPK mRNA levels in females after nerve injury (Figure 3D). Similar to the data from male mice, intrathecal p38α or p38β MAPK ASO only reduced the spinal levels of p38α or p38β MAPK mRNA in female mice, respectively (Figure 3C and D). Together, these results indicated that spinal p38α or p38β MAPK ASO treatment produced isoform-specific knockdown of p38α or p38β MAPK expression in the spinal cords of both sexes.
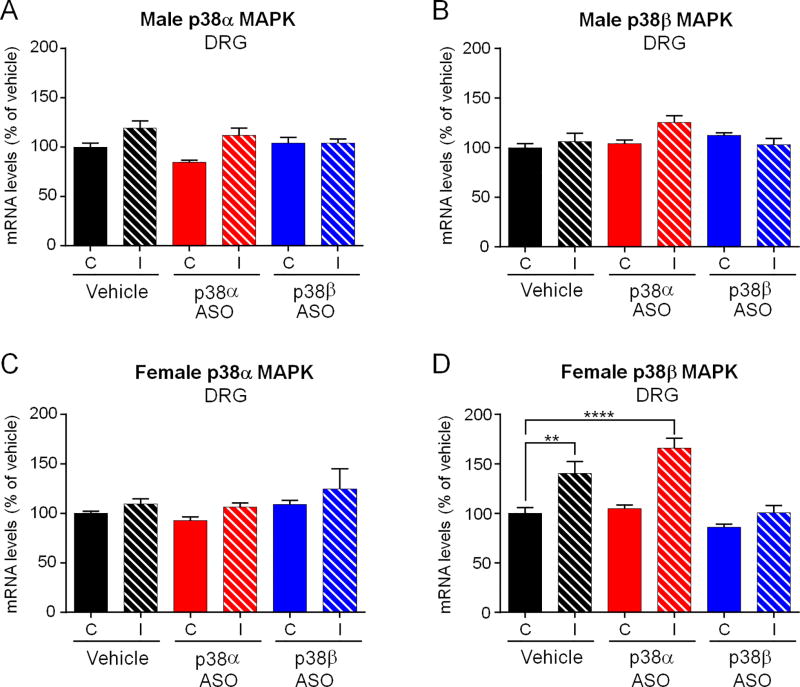
3.4. Effects of single intrathecal injection of p38α or p38β MAPK ASO on the expression of p38α or p38β mRNA in DRG tissues in CCI mice of both sexes
Previous studies have shown that intrathecal injections of ASOs reduced target gene expression (e.g., TRPA1 after intrathecal infusion) in DRG tissue (Matsuda et al., 2017; Obata et al., 2005). Next, we investigated whether a single intrathecal bolus of ASO would affect p38α or p38β MAPK mRNA levels in lumbar DRG tissues. In vehicle-treated male mice, p38α or p38β MAPK was not expressed in an inducible pattern in ipsilateral or contralateral DRG (Figure 4A and B). In female control animals, we found that there was no difference of p38α MAPK expression between both sides, whereas p38β MAPK was expressed in a higher mRNA level at ipsilateral side, comparing to that in contralateral DRG (Figure 4C and D, F(5,34) = 2.195, p < 0.0001). Intrathecal p38α or p38β MAPK ASO treatment failed to affect target mRNA levels on neither side or in neither sex, when compared to the contralateral side of vehicle control. However, nerve injury-induced upregulation of p38β MAPK mRNA in female DRGs was suppressed by p38β MAPK ASO treatment (Figure 4D, F(5, 34) = 2.195, p < 0.001). These results indicate that 1) p38α in DRG may not be involved in ASO-evoked analgesic effects in neuropathic pain in male mice and 2) p38β MAPK regulation in DRG by nerve injury and p38β MAPK ASO in female mice does not contribute to neuropathic pain, given the fact that p38β MAPK ASO failed to evoke analgesia in females.
Figure 4. Intrathecal injection of p38α or p38β MAPK ASO does not alter p38α or p38β MAPK mRNA expression in DRG tissues after CCI.
(A–D) Intrathecal injection of p38α or p38β MAPK ASO did not affect p38α or p38β MAPK mRNA levels in lumbar (L3–L5) DRG tissues on both sides and in male mice (A, B) and female mice (C, D). ****p<0.0001 and **p<0.01. C, contralateral; I, ipsilateral to CCI. One-way ANOVA with Bonferroni’s post-hoc test, n=6–7 mice/group. Note that nerve injury increased p38β mRNA in the ipsilateral DRGs of vehicle- and p38α ASO-treated females, compared to contralateral DRGs (D). However, p38β MAPK ASO-treated females, p38β MAPK mRNA levels did not change, compared to vehicle-treated females (D). DRG tissues were collected two weeks after the ASO treatment.
3.5. Intrathecal injection of p38α but not p38β MAPK ASO alleviated postoperative pain in male mice after tibia fracture but not neuropathic pain chemotherapy
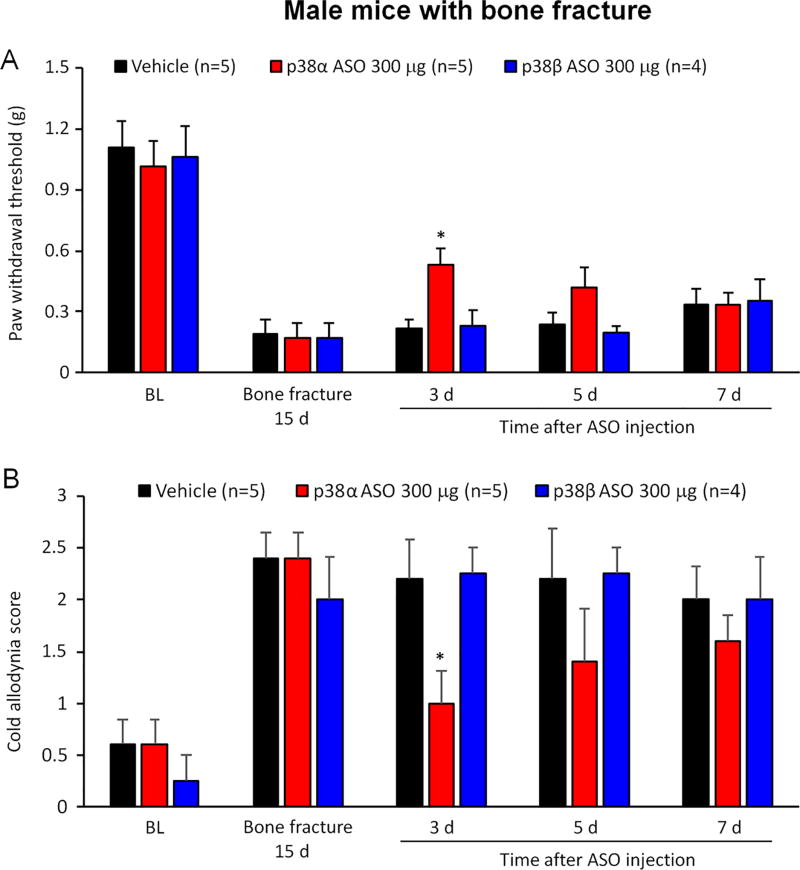
Bone fracture such as tibia facture results in persistent postoperative pain as well as microglial activation in the spinal cord (Wei et al., 2016; Zhang et al., 2016a). We evaluated the effects of p38α and p38β MAPK ASO (300 µg, intrathecal) on male mice in a tibia fracture model. Following tibia fracture, all male mice developed mechanical allodynia (F(4, 44) = 43.55, p < 0.001) and cold allodynia (F(4, 44) = 14.03, p < 0.001) on POD 15 (Figure 5). Intrathecal injection of p38α MAPK, but not p38β MAPK, ASO produced analgesic effects on post-injection day 3 (Figure 5).
Figure 5. Intrathecal injection of p38α but not p38β MAPK ASO reduces postoperative pain in male mice with tibia fracture.
(A, B) Tibia fracture induced mechanical allodynia in male mice. Intrathecal injection of 300 µg p38α but not p38β MAPK ASO, given 15 days after surgery, increased paw withdrawal threshold (A) and reduced cold allodynia score (B) in male mice with bone fracture. *p<0.05 in comparison to respective vehicle (PBS). Two-Way ANOVA with Bonferroni’s post-hoc test, n = 4–5 mice per group.
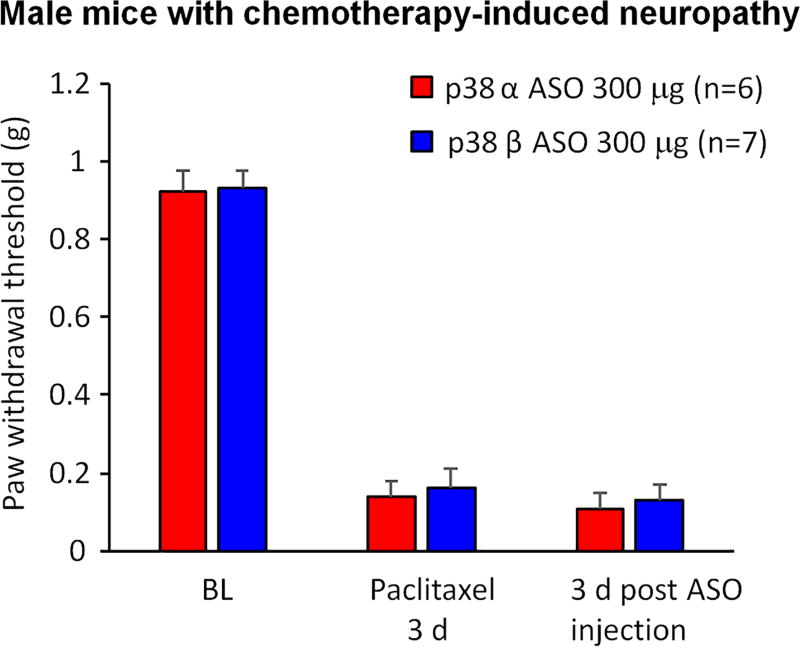
Chemotherapy agents such as paclitaxel produce peripheral neuropathy and neuropathic pain (Jaggi and Singh, 2012). After paclitaxel treatment, male mice developed robust mechanical allodynia on day 3 after the first paclitaxel treatment (F(2, 22) = 174.9, p < 0.001, Figure 6). We found that p38α or p38β MAPK ASO, given 3 days after the after the first paclitaxel treatment, failed to affect mechanical allodynia in paclitaxel-treated mice, 3 days after the ASO treatment (Figure 6).
Figure 6. Intrathecal injection of p38α or p38β MAPK ASO fails to affect neuropathic pain in male mice after chemotherapy-induced neuropathy.
Paclitaxel (2 mg/kg, i.p., 4 injections on day 0, 2, 4, and 6) induced robust mechanical allodynia in male mice. Intrathecal injection of 300 µg p38α or p38β MAPK ASO 3 days after the first paclitaxel treatment had no effects on paw withdrawal threshold in these animals. Two-Way ANOVA with Bonferroni’s post-hoc comparison, n = 6–7 mice per group.
3.5. Intrathecal p38α MAPK ASO prevented TLR4-induced mechanical allodynia in male mice and decreased protein levels of p38α MAPK and phosphorylated p38 MAPK in the spinal cord
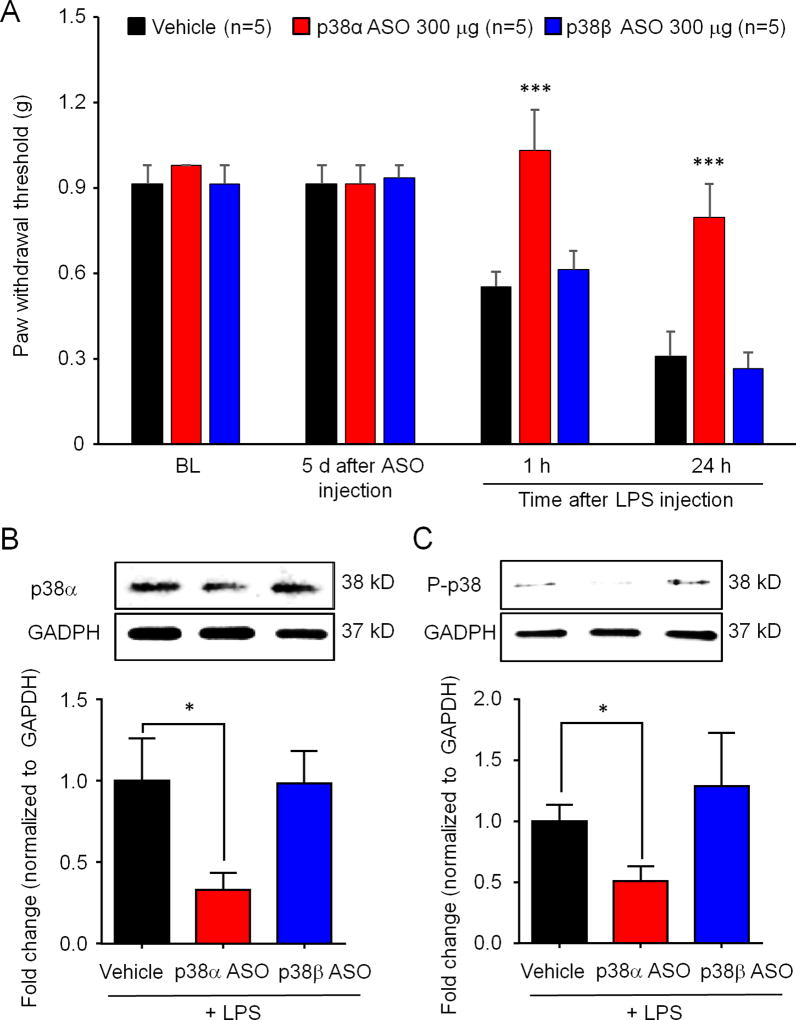
TLR4 has been strongly implicated in spinal microglial activation and generation of pathological pain, and intrathecal TLR4 agonist, lipopolysaccharide (LPS), caused mechanical allodynia only in male animals (Sorge et al., 2011). We evaluate the effects p38 MAPK ASOs (300 µg, intrathecal) on intrathecal LPS (10 µg) induced pain in male mice. Intrathecal injection of p38α or p38β MAPK ASO did not affect pain baseline mechanical sensitivity 5 days post-ASO injection (Figure 7A). However, p38α MAPK, but not p38β MAPK, ASO blocked intrathecal LPS-induced mechanical allodynia (Figure 7A, F(3, 48) = 24.34, P < 0.0001). We also collected spinal cord tissues at 1 h post-LPS injection and examined the protein levels of p38α MAPK and phosphorylated p38 MAPK (P-p38) using Western blotting. We found that p38α MAPK ASO downregulated the levels of p38α and P-p38 (Figure 7B and C, t test, p<0.05), suggesting that p38α MAPK signaling is required by intrathecal LPS/TLR4-evoked allodynia.
Figure 7. Intrathecal p38α MAPK ASO prevents the TLR4-induced mechanical allodynia and decreases protein levels of p38α MAPK and phosphorylated p38 MAPK in spinal cord following intrathecal LPS treatment.
(A–C) Mice received intrathecal 300 µg p38α or p38β MAPK ASO 5 days before intrathecal injection of LPS (10 µg). (A) LPS-induced mechanical allodynia was blocked by p38α but not p38β MAPK ASO. Two-Way ANOVA with Bonferroni’s pro-test, n=5 per group. ***p<0.001 in comparison to vehicle (PBS). (B) p38α MAPK ASO downregulated the levels p38α MAPK and phosphorylated p38 MAPK. Student’s t- test, n=3 per group, *p<0.05.
Discussion
One of the major findings of this study is that spinal p38α MAPK plays a predominant role in the development of pathological pain. Despite extensive preclinical studies on p38 MAPK, conducted in various animal models of pain in last 15 years, it is still unclear which p38 MAPK isoform plays a key role in regulating persistent pathological pain. p38 MAPK consists of four isoforms (α, β, γ and δ) with distinct expression in different tissues (Cuadrado and Nebreda, 2010). It appears that α and β are two major p38 isoforms in the rodent spinal cord (Svensson et al., 2005). However, our knowledge about p38 isoforms in pain regulation is still limited, in part because commercial antibodies for phosphorylated p38 (p-p38) MAPK and inhibitors of p38 MAPK are not isoform-specific (Ji and Suter, 2007). Of note, the oral administration of p38 inhibitor (losmapimod), targeting p38α and p38β MAPK, failed to produce analgesic effects in patients suffering neuropathic pain (Ostenfeld et al., 2013). In another trial, p38α inhibitor dilmapimod alleviated pain via oral route in patients with neuropathic pain (Anand et al., 2011). These results implicated that p38α MAPK would be a better target for chronic pain therapy, comparing to non-specific inhibitors of p38 MAPK. In this study, we test the effects of p38α and p38β MAPK ASOs in three mouse models of pathological pain after CCI, tibial fracture, chemotherapy and intrathecal LPS following a single intrathecal injection. Our results demonstrated that mechanical allodynia, a cardinal feature of chronic pain, was not affected by intrathecal p38β MAPK ASO is all four models of persistent pain, despite the fact that p38β MAPK ASO produced more inhibition of p38β MAPK (60%) than the inhibition (35%) of p38α MAPK by p38α MAPK ASO. This result argues against a role of p38β in MAPK chronic pain. Our data also showed that mechanical allodynia after CCI, bone fracture and intrathecal LPS was attenuated by p38α MAPK ASO, suggesting that p38α is the major isoform for regulating pathological pain in the spinal cord. However, chemotherapy-evoked mechanical allodynia was not reduced by p38α MAPK ASO, in agreement with a previous report that p38 MAPK inhibitor prevented but did not reverse paclitaxel-induced behavioral hypersensitivity via DRG neuronal mechanisms (Li et al., 2015). Also, p38 MAPK inhibitor, SCIO-469, provided in the chow, did not attenuate bone cancer pain in rodents (Svensson et al., 2008). Thus, it is likely that the primary action of spinal and microglial p38α may be found during the development of chronic pain when there is remarkable inflammation. This notion is also supported by our recent study. Skepinone-L is a new p38α MAPK inhibitor with high potency and excellent selectivity in vitro and in vivo (Koeberle et al., 2012). Intrathecal administration of skepinone-L, one week post CCI, effectively blocked nerve injury-induced mechanical allodynia (Taves et al., 2016). Previous studies used ASOs to assess distinct roles of p38 MAPK informs in rat models of persistent pain. It was shown that intrathecal p38β (but not p38α) MAPK ASO attenuated formalin- and neurokinin-1-induced spontaneous pain (Svensson et al., 2005) and carrageenan-induced thermal hyperalgesia (Fitzsimmons et al., 2010). Possibly, p38β MAPK may contribute to the generation of some persistent pain, or rat p38β MAPK may equal to mouse p38α functionally in pain processing.
Another major finding of this study is that despite p38α MAPK knockdown in both male and female mice, only male mice responded to intrathecal p38α MAPK ASO by showing increased PWT and decreased PWF in the CCI model. By contrast, neither p38α ASO nor p38β ASO changed PWT and PWF in female mice with CCI, despite the fact that CCI caused an upregulation of p38β but not p38α mRNA levels in the spinal cord of female mice (Figure 3D). Furthermore, p38α MAPK ASO pre-treatment downregulated protein levels of p38α MAPK and phosphorylated p38 MAPK in male mice treated with intrathecal LPS, suggesting that decreased p38α MAPK expression may affect the phosphorylation (function) of p38 MAPK in TLR4-mediated chronic pain. In agreement, we recently found that intrathecal skepinone-L, given one week post CCI, attenuated nerve injury-induced mechanical allodynia exclusively in male mice, whereas peri-neural injection of this inhibitor reduced mechanical allodynia in both sexes (Taves et al., 2016). We postulate this male-specific effect of skepinone and p38α ASO is a result of microglial modulation in the spinal cord. In agreement, our recent work found that (1) CCI increased p38 phosphorylation (P-p38) levels in the spinal cord dorsal horn of male but not female mice and (2) CCI increased P-p38 immunostaining primarily in CX3CR1-postive microglia in male mice (Taves et al., 2016). In addition to p38 MAPK, other microglial signaling molecules such as TLR4, P2X4, and BDNF also regulate pathological pain, especially neuropathic pain, in male rodents (Sorge et al., 2011; Sorge et al., 2015). Furthermore, caspase-6, a microglial activator produced by primary afferents in the spinal cord, regulates persistent pain in male mice (Berta et al., 2016). However, microglial inhibitor minocycline was also shown to reduce neuropathic pain in female rats in the late phase of spinal cord injury (Chen et al., 2012) and attenuate bone cancer pain in female animals inoculated with breast cancer cells (Yang et al., 2015), suggesting that microglia in female animals may also have an active role in some pain conditions.
In addition to a central and microglial regulation of pain by p38 MAPK in the spinal cord, we should not exclude a peripheral regulation of pain by p38 MAPK in DRG neurons and peripheral immune cells. Inflammation and nerve injury cause p38 MAPK activation in DRG neurons (Ji et al., 2002b; Jin et al., 2003a; Obata and Noguchi, 2004; Schafers et al., 2003). Intrathecal injections of ASOs, siRNA, or MAPK inhibitors have been shown to reduce gene expression in DRGs of rats and mice (Ji et al., 2002b; Jin et al., 2003a; Matsuda et al., 2017; Obata and Noguchi, 2004; Schafers et al., 2003). p38 MAPK activation in DRG neurons was implicated in inflammatory pain and neuropathic pain (Ji et al., 2002a; Jin et al., 2003a; Obata et al., 2004). Interestingly, we did not find significant knockdown of p38α and p38β MAPK expression in mouse DRG of both sexes after the intrathecal treatment of ASOs in the CCI model, further supporting a dominant role of p38 signaling in spinal microglia in neuropathic pain.
The lack of target reduction in DRG following ASOs treatment was surprising, as ASOs can suppress targets in DRG in pain models (Obata et al., 2005). The primary differences between previous studies and ours are the targets and the dosing paradigm. It is feasible that differential cell-type expression of the target may alter the apparent response when comparing whole tissues with different cellular milieu (i.e microglial targets vs. neuronal targets). Alternatively, a single bolus injection, as we've done here to mimic a likely clinical paradigm, allows for broad ASO distribution throughout the CNS, where alternative dosing methods, like slow infusion, can lead to more focal ASO accumulation (Rigo et al., 2014). It is plausible, that the previous dosing paradigms led to more focal accumulation of ASO at the lumbar site of administration, and thus more accumulation in lumbar DRG. It is likely that a different dosing paradigm, or a single bolus of a more potent ASO will achieve target reductions in DRG. Furthermore, as we collected spinal cord and DRG tissue for PCR test on day 14 post-ASO injection (day 19 post-CCI), mRNA levels of these p38 MAPK subtypes, it is not excluded that DRG levels of p38 MAPK subtypes may be affected at early time point following ASOs treatment.
In conclusion, our results show that p38α MAPK is the major p38 MAPK isoform for the development of pathological pain via possible regulation of spinal cord microglial signaling. Importantly, this regulation of pathological pain by p38α MAPK is also sex-dependent and model-dependent. As an emerging therapeutic platform, ASOs can readily regulate target gene expression and have been implied in neurodegenerative disorders (Evers et al., 2015). Intrathecal delivery of ASOs has proven successful in treating children with spinal muscular atrophy (Finkel et al., 2016), and are currently being developed for treatment of various neurodegenerative disorders. Thus, targeting p38α MAPK with specific ASOs may offer a new treatment for some clinical pain conditions with marked inflammation. Several pain-related clinical trials were reported with p38 inhibitors. In one trial, p38α MAPK inhibitor dilmapimod, administered via oral route, alleviated pain in patients with neuropathic pain (Anand et al., 2011). In another trial, oral administration of p38 MAPK inhibitor losmapimod that targets both p38α and p38β MAPK did not produce significant analgesic effects in patients suffering neuropathic pain (Ostenfeld et al., 2013). The lack of response could reflect insufficient losmapimod levels in the spinal cord (Ostenfeld et al., 2013). It may also result from different pain models/conditions between lumbosacral radiculopathy in patients and animal models of neuropathic pain (Ostenfeld et al., 2013), as we showed in this study. It appears that p38 inhibition is not very effective in reversing pathological pain in the late-phase (Chen et al., 2014). Another limitation is that an intrathecal dose of p38α MAPK ASO only caused a partial reduction of p38α MAPK expression in the spinal cord, associated with a transient inhibition of neuropathic pain for a few days. Further improvement of p38α MAPK ASO efficacy or knocking down additional MAPK isoforms such as JNK1 MAPK (Zhuang et al., 2006) should be considered in future studies.
Acknowledgments
This study is supported by NIH R01 grants DE17794, DE22743, and NS87988 to R.R.J. We thank Mark Andrade and the Oligo Synthesis Group for providing ASOs and Andy Watt and the Rapid Throughput Screening Group for in vitro lead identification.
Footnotes
Conflict of interest statement
BF, AM, HK2 are employees and shareholders of Ionis Pharmaceuticals. However, XL, LZ, NT, RRJ are employees of Duke University and did not receive research fund and compensations from Ionics.
References
- Anand P, Shenoy R, Palmer JE, Baines AJ, Lai RYK, Robertson J, Bird N, Ostenfeld T, Chizh BA. Clinical trial of the p38 MAP kinase inhibitor dilmapimod in neuropathic pain following nerve injury. European Journal of Pain. 2011;15:1040–1048. doi: 10.1016/j.ejpain.2011.04.005. [DOI] [PubMed] [Google Scholar]
- Bennett CF, Swayze EE. RNA Targeting Therapeutics: Molecular Mechanisms of Antisense Oligonucleotides as a Therapeutic Platform. Annu. Rev. Pharmacol. Toxicol. 2010;50:259–293. doi: 10.1146/annurev.pharmtox.010909.105654. [DOI] [PubMed] [Google Scholar]
- Berta T, Qadri YJ, Chen G, Ji RR. Microglial Signaling in Chronic Pain with a Special Focus on Caspase 6, p38 MAP Kinase, and Sex Dependence. Journal of dental research. 2016 doi: 10.1177/0022034516653604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Luo X, Qadri MY, Berta T, Ji RR. Sex-Dependent Glial Signaling in Pathological Pain: Distinct Roles of Spinal Microglia and Astrocytes. Neuroscience bulletin. 2017 doi: 10.1007/s12264-017-0145-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Park CK, Xie RG, Berta T, Nedergaard M, Ji RR. Connexin-43 induces chemokine release from spinal cord astrocytes to maintain late-phase neuropathic pain in mice. Brain. 2014;137:2193–2209. doi: 10.1093/brain/awu140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Park CK, Xie RG, Ji RR. Intrathecal bone marrow stromal cells inhibit neuropathic pain via TGF-beta secretion. Journal of Clinical Investigation. 2015;125:3226–3240. doi: 10.1172/JCI80883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Xie RG, Gao YJ, Xu ZZ, Zhao LX, Bang S, Berta T, Park CK, Lay M, Chen W, Ji RR. beta-arrestin-2 regulates NMDA receptor function in spinal lamina II neurons and duration of persistent pain. Nat Commun. 2016;7:12531. doi: 10.1038/ncomms12531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MJ, Kress B, Han X, Moll K, Peng W, Ji RR, Nedergaard M. Astrocytic CX43 hemichannels and gap junctions play a crucial role in development of chronic neuropathic pain following spinal cord injury. Glia. 2012;60:1660–1670. doi: 10.1002/glia.22384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson CA, Dumlao DS, Stokes JA, Dennis EA, Svensson CI, Corr M, Yaksh TL. Spinal TLR4 mediates the transition to a persistent mechanical hypersensitivity after the resolution of inflammation in serum-transferred arthritis. Pain. 2011;152:2881–2891. doi: 10.1016/j.pain.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, Gravel C, Salter MW, De Koninck Y. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438:1017–1021. doi: 10.1038/nature04223. [DOI] [PubMed] [Google Scholar]
- Cuadrado A, Nebreda AR. Mechanisms and functions of p38 MAPK signalling. The Biochemical journal. 2010;429:403–417. doi: 10.1042/BJ20100323. [DOI] [PubMed] [Google Scholar]
- Dong H, Xiang HB, Ye DW, Tian XB. Inhibitory effects of intrathecal p38 beta antisense oligonucleotide on bone cancer pain in rats. Int. J. Clin. Exp. Pathol. 2014;7:7690–7698. [PMC free article] [PubMed] [Google Scholar]
- Evers MM, Toonen LJA, van Roon-Mom WMC. Antisense oligonucleotides in therapy for neurodegenerative disorders. Adv. Drug Deliv. Rev. 2015;87:90–103. doi: 10.1016/j.addr.2015.03.008. [DOI] [PubMed] [Google Scholar]
- Finkel RS, Chiriboga CA, Vajsar J, Day JW, Montes J, De Vivo DC, Yamashita M, Rigo F, Hung G, Schneider E, Norris DA, Xia S, Bennett CF, Bishop KM. Treatment of infantile-onset spinal muscular atrophy with nusinersen: a phase 2, open-label, dose-escalation study. Lancet. 2016;388:3017–3026. doi: 10.1016/S0140-6736(16)31408-8. [DOI] [PubMed] [Google Scholar]
- Fitzsimmons BL, Zattoni M, Svensson CI, Steinauer J, Hua X-Y, Yaksh TL. Role of spinal p38α and β MAPK in inflammatory hyperalgesia and spinal COX-2 expression. Neuroreport. 2010;21:313–317. doi: 10.1097/WNR.0b013e32833774bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace PM, Hutchinson MR, Maier SF, Watkins LR. Pathological pain and the neuroimmune interface. Nat. Rev. Immunol. 2014 doi: 10.1038/nri3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace PM, Strand KA, Galer EL, Urban DJ, Wang X, Baratta MV, Fabisiak TJ, Anderson ND, Cheng K, Greene LI, Berkelhammer D, Zhang Y, Ellis AL, Yin HH, Campeau S, Rice KC, Roth BL, Maier SF, Watkins LR. Morphine paradoxically prolongs neuropathic pain in rats by amplifying spinal NLRP3 inflammasome activation. Proceedings of the National Academy of Sciences of the United States of America. 2016 doi: 10.1073/pnas.1602070113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Q, Kim YH, Wang X, Liu D, Zhang Z-J, Bey AL, Lay M, Chang W, Berta T, Zhang Y, Jiang Y-H, Ji R-R. SHANK3 Deficiency Impairs Heat Hyperalgesia and TRPV1 Signaling in Primary Sensory Neurons. Neuron. 2016a;92:1279–1293. doi: 10.1016/j.neuron.2016.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Q, Kim YH, Wang X, Liu D, Zhang ZJ, Bey AL, Lay M, Chang W, Berta T, Zhang Y, Jiang YH, Ji RR. SHANK3 Deficiency Impairs Heat Hyperalgesia and TRPV1 Signaling in Primary Sensory Neurons. Neuron. 2016b;92:1279–1293. doi: 10.1016/j.neuron.2016.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaggi AS, Singh N. Mechanisms in cancer-chemotherapeutic drugs-induced peripheral neuropathy. Toxicology. 2012;291:1–9. doi: 10.1016/j.tox.2011.10.019. [DOI] [PubMed] [Google Scholar]
- Ji R-R, Chamessian A, Zhang Y-Q. Pain regulation by non-neuronal cells and inflammation. Science. 2016;354:572–577. doi: 10.1126/science.aaf8924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji R-R, Samad TA, Jin S-X, Schmoll R, Woolf CJ. p38 MAPK Activation by NGF in Primary Sensory Neurons after Inflammation Increases TRPV1 Levels and Maintains Heat Hyperalgesia. Neuron. 2002a;36:57–68. doi: 10.1016/s0896-6273(02)00908-x. [DOI] [PubMed] [Google Scholar]
- Ji RR, Berta T, Nedergaard M. Glia and pain: Is chronic pain a gliopathy? Pain. 2013 doi: 10.1016/j.pain.2013.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji RR, Gereau RW, Malcangio M, Strichartz GR. MAP kinase and pain. Brain Res. Rev. 2009;60:135–148. doi: 10.1016/j.brainresrev.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji RR, Samad TA, Jin SX, Schmoll R, Woolf CJ. p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron. 2002b;36:57–68. doi: 10.1016/s0896-6273(02)00908-x. [DOI] [PubMed] [Google Scholar]
- Ji RR, Suter MR. p38 MAPK, microglial signaling, and neuropathic pain. Mol Pain. 2007;3:33. doi: 10.1186/1744-8069-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji RR, Xu ZZ, Gao YJ. Emerging targets in neuroinflammation-driven chronic pain. Nat. Rev. Drug Discov. 2014;13:533–548. doi: 10.1038/nrd4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin SX, Zhuang ZY, Woolf CJ, Ji RR. p38 mitogen-activated protein kinase is activated after a spinal nerve ligation in spinal cord microglia and dorsal root ganglion neurons and contributes to the generation of neuropathic pain. J. Neurosci. 2003a;23:4017–4022. doi: 10.1523/JNEUROSCI.23-10-04017.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin SX, Zhuang ZY, Woolf CJ, Ji RR. P38 mitogen-activated protein kinase is activated after a spinal nerve ligation in spinal cord microglia and dorsal root ganglion neurons and contributes to the generation of neuropathic pain. J. Neurosci. 2003b;23:4017–4022. doi: 10.1523/JNEUROSCI.23-10-04017.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Yamanaka H, Fukuoka T, Dai Y, Obata K, Noguchi K. P2Y12 receptor upregulation in activated microglia is a gateway of p38 signaling and neuropathic pain. J. Neurosci. 2008;28:2892–2902. doi: 10.1523/JNEUROSCI.5589-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeberle SC, Romir J, Fischer S, Koeberle A, Schattel V, Albrecht W, Grütter C, Werz O, Rauh D, Stehle T, Laufer SA. Skepinone-L is a selective p38 mitogen-activated protein kinase inhibitor. Nat Chem Biol. 2012;8:141–143. doi: 10.1038/nchembio.761. [DOI] [PubMed] [Google Scholar]
- Kuner R. Central mechanisms of pathological pain. Nat. Med. 2010;16:1258–1266. doi: 10.1038/nm.2231. [DOI] [PubMed] [Google Scholar]
- Li Y, Zhang H, Kosturakis AK, Cassidy RM, Zhang H, Kennamer-Chapman RM, Jawad AB, Colomand CM, Harrison DS, Dougherty PM. MAPK signaling downstream to TLR4 contributes to paclitaxel-induced peripheral neuropathy. Brain, behavior, and immunity. 2015;49:255–266. doi: 10.1016/j.bbi.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loggia ML, Chonde DB, Akeju O, Arabasz G, Catana C, Edwards RR, Hill E, Hsu S, Izquierdo-Garcia D, Ji RR, Riley M, Wasan AD, Zurcher NR, Albrecht DS, Vangel MG, Rosen BR, Napadow V, Hooker JM. Evidence for brain glial activation in chronic pain patients. Brain. 2015;138:604–615. doi: 10.1093/brain/awu377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malon JT, Cao L. Calcitonin gene-related peptide contributes to peripheral nerve injury-induced mechanical hypersensitivity through CCL5 and p38 pathways. J Neuroimmunol. 2016;297:68–75. doi: 10.1016/j.jneuroim.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda M, Oh-Hashi K, Yokota I, Sawa T, Amaya F. Acquired Exchange Protein Directly Activated by Cyclic Adenosine Monophosphate Activity Induced by p38 Mitogen-activated Protein Kinase in Primary Afferent Neurons Contributes to Sustaining Postincisional Nociception. Anesthesiology. 2017;126:150–162. doi: 10.1097/ALN.0000000000001401. [DOI] [PubMed] [Google Scholar]
- Maves TJ, Pechman PS, Gebhart GF, Meller ST. Possible chemical contribution from chromic gut sutures produces disorders of pain sensation like those seen in man. Pain. 1993;54:57–69. doi: 10.1016/0304-3959(93)90100-4. [DOI] [PubMed] [Google Scholar]
- Milligan ED, Twining C, Chacur M, Biedenkapp J, O'Connor K, Poole S, Tracey K, Martin D, Maier SF, Watkins LR. Spinal glia and proinflammatory cytokines mediate mirror-image neuropathic pain in rats. J. Neurosci. 2003;23:1026–1040. doi: 10.1523/JNEUROSCI.23-03-01026.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan ED, Watkins LR. Pathological and protective roles of glia in chronic pain. Nat. Rev. Neurosci. 2009;10:23–36. doi: 10.1038/nrn2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obata K, Katsura H, Mizushima T, Yamanaka H, Kobayashi K, Dai Y, Fukuoka T, Tokunaga A, Tominaga M, Noguchi K. TRPA1 induced in sensory neurons contributes to cold hyperalgesia after inflammation and nerve injury. J. Clin. Invest. 2005;115:2393–2401. doi: 10.1172/JCI25437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obata K, Noguchi K. MAPK activation in nociceptive neurons and pain hypersensitivity. Life Sci. 2004;74:2643–2653. doi: 10.1016/j.lfs.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Obata K, Yamanaka H, Kobayashi K, Dai Y, Mizushima T, Katsura H, Fukuoka T, Tokunaga A, Noguchi K. Role of mitogen-activated protein kinase activation in injured and intact primary afferent neurons for mechanical and heat hypersensitivity after spinal nerve ligation. J. Neurosci. 2004;24:10211–10222. doi: 10.1523/JNEUROSCI.3388-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostenfeld T, Krishen A, Lai RY, Bullman J, Baines AJ, Green J, Anand P, Kelly M. Analgesic efficacy and safety of the novel p38 MAP kinase inhibitor, losmapimod, in patients with neuropathic pain following peripheral nerve injury: a double-blind, placebo-controlled study. European Journal of Pain. 2013;17:844–857. doi: 10.1002/j.1532-2149.2012.00256.x. [DOI] [PubMed] [Google Scholar]
- Ren K, Dubner R. Interactions between the immune and nervous systems in pain. Nat. Med. 2010;16:1267–1276. doi: 10.1038/nm.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigo F, Chun SJ, Norris DA, Hung G, Lee S, Matson J, Fey RA, Gaus H, Hua Y, Grundy JS, Krainer AR, Henry SP, Bennett CF. Pharmacology of a central nervous system delivered 2'-O-methoxyethyl-modified survival of motor neuron splicing oligonucleotide in mice and nonhuman primates. J Pharmacol Exp Ther. 2014;350:46–55. doi: 10.1124/jpet.113.212407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson CR, Zhang H, Dougherty PM. Astrocytes, but not microglia, are activated in oxaliplatin and bortezomib-induced peripheral neuropathy in the rat. Neuroscience. 2014;274:308–317. doi: 10.1016/j.neuroscience.2014.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafers M, Svensson CI, Sommer C, Sorkin LS. Tumor necrosis factor-alpha induces mechanical allodynia after spinal nerve ligation by activation of p38 MAPK in primary sensory neurons. J. Neurosci. 2003;23:2517–2521. doi: 10.1523/JNEUROSCI.23-07-02517.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorge RE, Lacroix-Fralish ML, Tuttle AH, Sotocinal SG, Austin JS, Ritchie J, Chanda ML, Graham AC, Topham L, Beggs S, Salter MW, Mogil JS. Spinal cord Toll-like receptor 4 mediates inflammatory and neuropathic hypersensitivity in male but not female mice. J. Neurosci. 2011;31:15450–15454. doi: 10.1523/JNEUROSCI.3859-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorge RE, Mapplebeck JC, Rosen S, Beggs S, Taves S, Alexander JK, Martin LJ, Austin JS, Sotocinal SG, Chen D, Yang M, Shi XQ, Huang H, Pillon NJ, Bilan PJ, Tu Y, Klip A, Ji RR, Zhang J, Salter MW, Mogil JS. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat. Neurosci. 2015;18:1081–1083. doi: 10.1038/nn.4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson CI, Fitzsimmons B, Azizi S, Powell HC, Hua X-Y, Yaksh TL. Spinal p38β isoform mediates tissue injury-induced hyperalgesia and spinal sensitization. J. Neurochem. 2005;92:1508–1520. doi: 10.1111/j.1471-4159.2004.02996.x. [DOI] [PubMed] [Google Scholar]
- Svensson CI, Medicherla S, Malkmus S, Jiang Y, Ma JY, Kerr I, Brainin-Mattos J, Powell HC, Luo ZD, Chakravarty S, Dugar S, Higgins LS, Protter AA, Yaksh TL. Role of p38 mitogen activated protein kinase in a model of osteosarcoma-induced pain. Pharmacol Biochem Behav. 2008;90:664–675. doi: 10.1016/j.pbb.2008.05.016. [DOI] [PubMed] [Google Scholar]
- Tanga FY, Nutile-McMenemy N, DeLeo JA. The CNS role of Toll-like receptor 4 in innate neuroimmunity and painful neuropathy. Proc. Natl. Acad. Sci. U. S. A. 2005 doi: 10.1073/pnas.0501634102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taves S, Berta T, Liu D-L, Gan S, Chen G, Kim YH, Van de Ven T, Laufer S, Ji R-R. Spinal inhibition of p38 MAP kinase reduces inflammatory and neuropathic pain in male but not female mice: Sex-dependent microglial signaling in the spinal cord. Brain, Behavior, and Immunity. 2016;55:70–81. doi: 10.1016/j.bbi.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian G, Luo X, Tang C, Cheng X, Chung SK, Xia Z, Cheung CW, Guo Q. Astrocyte contributes to pain development via MMP2-JNK1/2 signaling in a mouse model of complex regional pain syndrome. Life Sci. 2017;170:64–71. doi: 10.1016/j.lfs.2016.11.030. [DOI] [PubMed] [Google Scholar]
- Tsuda M, Inoue K, Salter MW. Neuropathic pain and spinal microglia: a big problem from molecules in "small" glia. Trends Neurosci. 2005;28:101–107. doi: 10.1016/j.tins.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Tsuda M, Mizokoshi A, Shigemoto-Mogami Y, Koizumi S, Inoue K. Activation of p38 mitogen-activated protein kinase in spinal hyperactive microglia contributes to pain hypersensitivity following peripheral nerve injury. Glia. 2004;45:89–95. doi: 10.1002/glia.10308. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Hutchinson MR, Rice KC, Maier SF. The "toll" of opioid-induced glial activation: improving the clinical efficacy of opioids by targeting glia. Trends Pharmacol. Sci. 2009;30:581–591. doi: 10.1016/j.tips.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei T, Guo TZ, Li WW, Kingery WS, Clark JD. Acute versus chronic phase mechanisms in a rat model of CRPS. Journal of neuroinflammation. 2016;13:14. doi: 10.1186/s12974-015-0472-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willemen HL, Eijkelkamp N, Wang H, Dantzer R, Dorn GW, Kelley KW, Heijnen CJ, Kavelaars A. Microglial/macrophage GRK2 determines duration of peripheral IL-1beta-induced hyperalgesia: contribution of spinal cord CX3CR1, p38 and IL-1 signaling. Pain. 2010;150:550–560. doi: 10.1016/j.pain.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu ZZ, Kim YH, Bang SS, Zhang Y, Berta T, Wang F, Oh SB, Ji RR. Inhibition of mechanical allodynia in neuropathic pain by TLR5-mediated A-fiber blockade. Nat. Med. 2015;21:1326–1331. doi: 10.1038/nm.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Li H, Li TT, Luo H, Gu XY, Lu N, Ji RR, Zhang YQ. Delayed Activation of Spinal Microglia Contributes to the Maintenance of Bone Cancer Pain in Female Wistar Rats via P2X7 Receptor and IL-18. J. Neurosci. 2015;35:7950–7963. doi: 10.1523/JNEUROSCI.5250-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M-D, Barde S, Yang T, Lei B, Eriksson LI, Mathew JP, Andreska T, Akassoglou K, Harkany T, Hökfelt TGM, Terrando N. Orthopedic surgery modulates neuropeptides and BDNF expression at the spinal and hippocampal levels. Proc. Natl. Acad. Sci. U. S. A. 2016a;113:E6686–E6695. doi: 10.1073/pnas.1614017113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang MD, Barde S, Yang T, Lei B, Eriksson LI, Mathew JP, Andreska T, Akassoglou K, Harkany T, Hokfelt TG, Terrando N. Orthopedic surgery modulates neuropeptides and BDNF expression at the spinal and hippocampal levels. Proceedings of the National Academy of Sciences of the United States of America. 2016b;113:E6686–E6695. doi: 10.1073/pnas.1614017113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang ZY, Kawasaki Y, Tan PH, Wen YR, Huang J, Ji RR. Role of the CX3CR1/p38 MAPK pathway in spinal microglia for the development of neuropathic pain following nerve injury-induced cleavage of fractalkine. Brain Behav. Immun. 2007;21:642–651. doi: 10.1016/j.bbi.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang ZY, Wen YR, Zhang DR, Borsello T, Bonny C, Strichartz GR, Decosterd I, Ji RR. A peptide c-Jun N-terminal kinase (JNK) inhibitor blocks mechanical allodynia after spinal nerve ligation: respective roles of JNK activation in primary sensory neurons and spinal astrocytes for neuropathic pain development and maintenance. J. Neurosci. 2006;26:3551–3560. doi: 10.1523/JNEUROSCI.5290-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]