Abstract
In Arabidopsis, seedless silique development or parthenocarpy can be induced by the application of various plant growth regulators (PGRs) to unfertilized pistils. Ecotype-specific responses were observed in the Arabidopsis ecotypes Columbia and Landsberg relative to the type of PGR and level applied. The parthenocarpic response was greatest in ecotype Landsberg, and comparisons of fruit growth and morphology were studied primarily in this ecotype. Gibberellic acid application (10 μmol pistil−1) caused development similar to that in pollinated pistils, while benzyladenine (1 μmol pistil−1) and naphthylacetic acid (10 μmol pistil−1) treatment produced shorter siliques. Naphthylacetic acid primarily modified mesocarp cell expansion. Arabidopsis mutants were employed to examine potential dependencies on gibberellin biosynthesis (ga1-3, ga4-1, and ga5-1) and perception (spy-4 and gai) during parthenocarpic silique development. Emasculated spy-4 pistils were neither obviously parthenocarpic nor deficient in PGR perception. By contrast, emasculated gai mutants did not produce parthenocarpic siliques following gibberellic acid application, but silique development occurred following pollination or application of auxin and cytokinin. Pollinated gai siliques had decreased cell numbers and morphologically resembled auxin-induced parthenocarpic siliques. This shows that a number of independent and possibly redundant pathways can direct hormone-induced parthenocarpy, and that endogenous gibberellins play a role in regulating cell expansion and promoting cell division in carpels.
Fruit and seed development are initiated following fertilization and are coordinated processes (Gillaspy et al., 1993). The absence of fertilization results in either senescence of the entire flower or a cessation of carpel development following the abscission of other floral organs (Vercher et al., 1984, 1989; Vercher and Carbonell, 1991; Granell et al., 1992; O'Neill and Nadeau, 1997). The limiting factor for the growth of unpollinated carpels appears to be the reduced endogenous growth hormone level prior to the onset of senescence (Pharis and King, 1985; Gillaspy et al., 1993). Developing seeds are usually considered to be essential determinants of fruit growth (Nitsch, 1950; Archbold and Dennis, 1985) because they synthesize high levels of plant growth hormones (Eeuwens and Schwabe, 1975; Sponsel, 1983; Talon et al., 1990a; García-Martínez et al., 1991a, 1991b; Ben-Cheikh et al., 1997; Rodrigo et al., 1997).
In some species, parthenocarpic fruit develops in the absence of fertilization and is seedless, indicating that it is possible to uncouple fruit formation from seed development. Parthenocarpy has a genetic basis (Pike and Peterson, 1969; Lin et al., 1984; Nuez et al., 1986; Vardy et al., 1989a, 1989b) and is selected for in seedless fruit-breeding programs (Sykes and Lewis, 1996). Parthenocarpy can also be induced in a diverse range of agricultural species with the exogenous application of GAs, auxins, and cytokinins (Schwabe and Mills, 1981). It has been assumed that exogenous plant growth regulators (PGRs) substitute for hormones synthesized by developing seeds. Furthermore, elevated levels of endogenous auxins and GAs have been observed in the fruit of plants exhibiting naturally occurring parthenocarpy (George et al., 1984; Talon et al., 1990d, 1992), suggesting that elevated hormone levels in fruit tissue other than seeds may be sufficient to induce fruit development. This was directly demonstrated by Rotino et al. (1997), who obtained seedless transgenic eggplant and tomato plants by specifically elevating auxin levels in ovules by means of chimeric auxin biosynthesis genes. Although parthenocarpic fruit development can be induced following exogenous PGR application, and elevated endogenous hormone levels have been observed during parthenocarpic fruit set in some species, the molecular events controlling the initiation of fruit development and their link to plant hormone signal transduction processes remain unknown.
Arabidopsis can be used to identify the genes controlling carpel morphogenesis (Gu et al., 1998) and hormone signal transduction (Jacobsen and Olzewski, 1993; Hobbie et al., 1994; Kieber et al., 1997; Hobbie, 1998; Phillips, 1998). The functional fruit and seed dispersal units of Arabidopsis are siliques, and their development in Arabidopsis is dependent on fertilization and seed set (Ohad et al., 1996; Chaudhury et al., 1997; Meinke and Sussex, 1979). Barendse et al. (1986) previously demonstrated that GA is an essential component for silique development in Arabidopsis, because both seed and fruit development in the ga1-biosynthetic mutant were dependent on the application of exogenous GA3 following pollination. Although reciprocal crosses between ga1 mutants and wild-type plants showed that silique development was primarily determined by maternal endogenous GAs, Barendse et al. (1986) also showed that determinants other than GAs were also involved in silique development in the GA-deficient genotypes.
The available biosynthetic and hormone perception mutants in Arabidopsis make it an ideal species with which to investigate how fruit growth is initiated at the molecular level and to understand the role of plant hormones during fruit development. Parthenocarpic silique development can occur in Arabidopsis following the application of GA3 (Jacobsen and Olzewski, 1993; Chaudhury et al., 1994). Jacobsen and Olzewski (1993) reported that mutants at the SPINDLY locus have altered GA perception and that parthenocarpic silique elongation occurs independent of fertilization in these plants. Apart from this genetic research, seedless fruit formation has not been studied to any great extent in Arabidopsis.
To further understand the molecular basis for parthenocarpy, we have analyzed the ability of various plant growth regulators to elicit silique development following their application to the pistils of emasculated flowers. We then genetically analyzed how the process was mediated by comparing silique growth and morphology of PGR-induced parthenocarpic siliques with those of Arabidopsis mutants blocked in GA biosynthesis and perception. In this paper we demonstrate the relationships between growth-regulator-induced parthenocarpy, hormone signal transduction, and silique development.
MATERIALS AND METHODS
Plant Growth
Arabidopsis plants were grown at 20°C in a walk-in growth chamber (Phoenix Biosystems, Adelaide, Australia) with a 16-h daylength and a light intensity of 150 μmol m−2 s−1. Eight plants were grown in 13- × 7- × 4-cm deep containers in a 1:1:1 peat:sand:perlite mix containing 1 g L−1 FeSO4, 3 g L−1 fertilizer (Osmocote Plus, Scotts-Sierra, Maysville, OH), 2 g L−1 dolomite, 0.5 g L−1 gypsum, and 0.5 g L−1 lime. Plants were watered daily.
Seeds of ga1-3, which require GA for germination, were surface-sterilized and plated onto Murashige and Skoog medium (Murashige and Skoog, 1962) containing 1% (w/v) Suc and 1% (w/v) agarose, pH 5.7, to which sterile GA3 had been added after autoclaving to a final concentration of 0.1 mm. Petri dishes were kept at 20°C in a 16-h daylength at 35 μmol m−2 s−1. Seedlings were transferred to soil after 7 d.
Silique Emasculation, Controlled Pollination, and Application of Growth Regulators
For each experiment, buds of ecotype Landsberg (Ler and LER) and ecotype Columbia (Col-1 and Col-1 er2), were emasculated at stage 11 to 12 (Bowman, 1993) approximately 1 to 2 d pre-anthesis. To avoid damage to the inflorescence meristem from emasculation, extra-fine scissors (Castro-Viejo, ProSciTech, Thuringowa, Australia) were used instead of fine forceps to remove sepals, petals, and anthers, leaving an exposed pistil. Controlled pollination was performed on anthesis stage pistils (stage 13; Bowman, 1993) by dusting a freshly dehisced anther over the extended stigmatic papillae until pollen was seen adhering to the stigmatic surface. Alternatively, pistils were left unpollinated as controls or treated with PGR.
Growth regulators were applied to emasculated pistils at stage 13 (Bowman, 1993) unless specified otherwise. Each pistil was uniformly coated from the tip of the stigmatic papillae to the pedicel with a 1-μL droplet containing 0.01, 0.1, 1.0, or 10 μmol of GA3, BA, IAA, or NAA, with 0.04% (v/v) Triton X-100 as a surfactant. Each solution was buffered to pH 7.0. Pistils treated with a control solution of 0.04% (v/v) Triton X-100 were identical in length to unpollinated pistils at 7 DPA.
Final silique length following pollination or PGR treatment was measured at 7 DPA. The growth rates of individual Arabidopsis siliques were measured over a 10- to 12-d period by taking repeated digital images of treated pistils at 12-h intervals (RD-175 digital camera, Minolta, Osaka). Growth data for each treatment were established by the examination of a minimum of five individual pistils, and each pistil was measured from a separate plant. Growth curves were fitted to data with an exponential growth function using graphing software (Sigmaplot version 4.0, Jandel Scientific, San Rafael, CA).
Pistil Receptivity to Pollen and to GA3
Emasculated flowers (n = 10 pistils) were pollinated or treated with a single application of 10 μmol GA3 pistil−1 (n = 7 pistils) at daily intervals post anthesis to determine the period of receptivity to pollen or GA3. Each GA3-treated pistil from separate plants was assessed. Final silique length was determined 12 DPA. Pistil receptivity was the period during which siliques elongated or set seed in response to pollination with respect to the days post anthesis.
Morphological Analysis of Carpel and Silique Development
Pistils at anthesis and developing siliques following various treatments were fixed in 4% (v/v) glutaraldehyde and 10 mm sodium cacodylate (pH 6.9). Tissue was rinsed once in 10 mm sodium cacodylate (pH 6.9), and then dehydrated through a graded-ethanol series to 100% and embedded in Spurr's resin (Spurr, 1969). Sections (0.6 μm) were cut from the embedded tissue using a microtome (Ultracut E, Reichert-Jung, Wien, Austria) and stained with 0.1% (w/v) toluidine blue. Stained sections were photographed using the digital camera attached to an Axioplan microscope (Carl Zeiss, Jena, Germany). Morphometric analysis was performed on captured images of each section by downloading to Photoshop (version 4.0, Adobe Systems, San Jose, CA) and by measuring cells using imaging software (Image Tool version 1.27, The University of Texas Health Science Center, San Antonio, http://ddsdx.uthscsa.edu/).
To determine how the pattern of cell division and expansion occurred during silique development, the average number of cells in the exocarp, mesocarp, and endocarp tissues was counted from cross-sections of pistils at anthesis and siliques at 7 DPA (n = 3–10). Mean cell length normal to the plane of silique elongation was also ascertained from lateral carpel longitudinal sections (n = 8–64 cells per section each from 3–10 sections). From these data we determined the magnitude of extension of individual cell types and calculated the total number of cells in the longitudinal sectional area of a pistil or silique. The latter measurement was calculated directly by dividing the mean silique length by the cell length normal to the plane of silique growth.
Analysis of Various Arabidopsis Mutants for Silique Elongation following Emasculation
A selection of existing Arabidopsis mutants was investigated for their ability to form fruit following emasculation: amp1-1; ctr1-1; etr1-3; ein2-1; ein3-1; ein4; ein5-1; ein6, ein7; spy-1; spy-3; spy-4; gar2-1; gai; ga1-3; ga4-1; ga5-1; abi4; axr1-3; axr2; axr4-2; and aux1-7. Pistils were emasculated and assessed at 12 DPA. All mutants were obtained from the Arabidopsis Biological Resource Center (Ohio State University, Columbus) except spy-4, which was a gift from Dr. Steve Swain, and amp1-1, which was a gift from Dr. Abed Chaudhury.
RESULTS
Silique Growth and Elongation in Arabidopsis
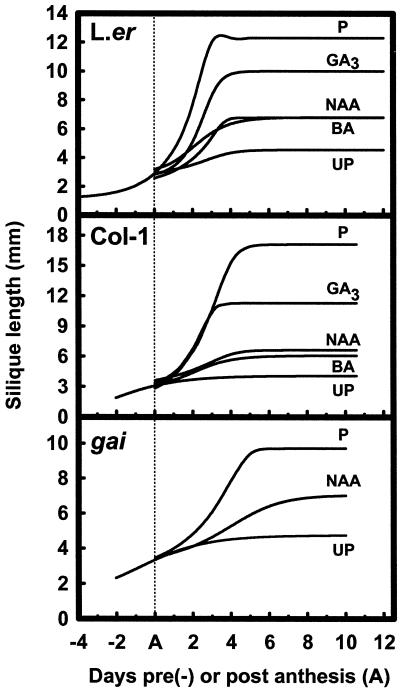
Arabidopsis pistils from ecotypes Ler, LER, Col-1, and Col-1 er2 pollinated at stage 13 (anthesis; Bowman, 1993) increased in fresh weight until 6 to 7 DPA (not shown), and siliques were 4- to 5-fold longer than their initial anthesis length (Table I). Other floral organs, excluding the developing silique, senesced soon after pollination and abscised during silique development. In pollinated pistils of Ler and Col-1, the siliques elongated exponentially until 3 and 5 DPA, respectively (Fig. 1, top and middle). The fruit matured and carpel valves became yellow from around 12 DPA until the siliques shattered and shed matured seed several days later. We observed that the Col-1 and LER ecotypes were comparable with respect to final pollinated silique length, but significantly longer than those of Col-1 er2 and Ler (Table I), indicating that regardless of the ecotype background, the erecta mutation significantly reduced the ability of pollinated pistils to elongate. The final post-pollination silique length obtained in these backgrounds was comparable to that observed by Torii et al. (1996).
Table I.
Elongation of Arabidopsis siliques in response to pollination and PGR treatments
| Stage and Treatment | Ecotype/Mutant
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Ler | LER | Col-1 er2 | Col-1 | Ws-O | spy-4 | gaia | ga5-1 | |
| mm | ||||||||
| Anthesis pistils | 2.8 ± 0.2 | 3.2 ± 0.2 | 2.9 ± 0.3 | 3.2 ± 0.4 | 3.2 ± 0.2 | 2.8 ± 0.3 | 2.9 ± 0.3 | 3.2 ± 0.3 |
| Emasculated without pollination | 4.1 ± 0.4b | 4.8 ± 0.4a | 3.7 ± 0.2c | 4.1 ± 0.3b | 4.5 ± 0.1ab | 4.9 ± 0.4a | 4.5 ± 0.4a | 5.0 ± 0.2a |
| Emasculated with pollination | 11.5 ± 1.0c (6.7) | 16.7 ± 1.5a (8.3) | 11.3 ± 0.6c (10.6) | 16.0 ± 1.7a (15.3) | 17.3 ± 1.1a (10.6) | 14.6 ± 0.8b (5.7) | 9.5 ± 1.2d (4.1) | 10.5 ± 1.0cd (4.2) |
| IAA, 10 μmol pistil−1 | 7.5 ± 0.9 (3.7) | 7.9 ± 2.4 (2.9) | 6.9 ± 0.7 (5.0) | 5.2 ± 0.4 (2.3) | ||||
| NAA, 10 μmol pistil−1 | 6.8 ± 0.8 (3.1) | 8.9 ± 3.5 (3.6) | 4.0 ± 1.7ns (1.4)b | 5.9 ± 1.4 (3.2) | 8.7 ± 1.4 (2.8) | 7.6 ± 1.2 (2.9) | ||
| BA, 0.1 μmol pistil−1 | 5.9 ± 0.8 (2.4) | 7.9 ± 0.5 (2.9) | 5.3 ± 0.6 (3.0) | 4.5 ± 1.3ns (1.5)b | ||||
| BA, 1 μmol pistil−1 | 5.7 ± 0.4 (2.2) | 6.2 ± 1.1 (1.9)b | 2.9 ± 0.3ns (0)b | 5.1 ± 0.7 (2.2) | 5.4 ± 1.1ns (1.3)b | 6.1 ± 0.2 (2.0) | ||
| GA3, 0.1 μmol pistil−1 | 8.2 ± 0.8 (4.2) | 11.0 ± 3.2 (4.8) | 5.9 ± 0.9 (3.8) | 7.4 ± 0.4 (4.9) | ||||
| GA3, 1 μmol pistil−1 | 9.5 ± 1.5 (5.2) | 12.2 ± 1.6 (5.6) | 7.6 ± 0.2 (6.0) | 9.2 ± 1.1 (7.2) | ||||
| GA3, 10 μmol pistil−1 | 10.0 ± 1.1 (5.6) | 14.5 ± 1.9 (7.0) | 9.2 ± 0.5 (7.9) | 10.6 ± 1.4 (8.9) | 9.8 ± 0.8 (4.9) | 8.4 ± 1.1 (2.7) | 5.3 ± 0.7 (1.5)b | 10.0 ± 1.1 (3.9) |
Results are means ± sd. Numbers in parentheses represent the fold increase in silique length as described in “Results.” ns indicates that the mean silique lengths are not significantly different from unpollinated pistils harvested at 7 DPA.
Mean silique length of cross-pollinated gai siliques (gai self-pollinated siliques attained 8.0 ± 1.7 mm).
Siliques less than 2-fold the length difference between anthesis and unpollinated 7-d pistils.
Figure 1.
Top and middle, Silique elongation of emasculated anthesis pistils after pollination (P), without pollination (UP), or treated with GA3 (10 μmol pistil−1), NAA (10 μmol pistil−1), or BA (1 μmol pistil−1) in the Ler (top) and Col-1 (middle) ecotypes. Bottom, Silique elongation in the gai background after emasculation of anthesis-stage pistils left unpollinated (UP) or after cross-pollination (P) or NAA treatment (10 μmol pistil−1). For estimates of error (±sd) refer to Table I.
Unpollinated pistils also shed their floral organs, yet they continued to elongate slightly from their normal anthesis length at a considerably reduced rate of growth compared with pollinated siliques (Fig. 1, top and middle). Unpollinated pistil elongation continued for 3 and 4 DPA in Col-1 and Ler ecotypes, respectively (Fig. 1, top and middle), and after 7 DPA the unfertilized pistils senesced yet failed to dehisce. These pistils had increased in length approximately another one-third to one-half of their original anthesis length (Table I). Therefore, an assessment of unpollinated pistil length at 7 DPA was used as the baseline to evaluate and compare post-anthesis silique development induced by pollination or PGR application in the different ecotypes. It was also noted that the final length attained by unpollinated pistils was ecotype dependent and determined by the presence or absence of the erecta mutation (Table I).
Arabidopsis Silique Growth Responses to PGRs
We compared the parthenocarpic responses of pistils following PGR application in different ecotype backgrounds that either contained or lacked the erecta mutation. We used a modified measurement of elongation to discriminate between parthenocarpy and the ability of an unpollinated pistil to slightly elongate. We subtracted the mean anthesis length from the final length attained at 7 DPA, and then divided this difference by the mean difference between the anthesis length and the unpollinated pistil length. Those pistils that exceeded a 2-fold increase in this measure were considered to be parthenocarpic siliques (Table I). Dehiscence of carpel valves was also used as an indicator of silique maturation.
Auxins (NAA or IAA), cytokinin (BA), and GA3 applied at anthesis to emasculated pistils stimulated fertilization-independent silique growth in the Ler, LER, Col-1 er2, and Col-1 ecotypes (Table I). The type and amount of PGR applied differentially influenced the magnitude of elongation and the external morphological appearance of the silique as examined at 7 DPA (Fig. 2). In all cases, however, the extent of PGR-induced silique elongation was always significantly lower than that observed in fertilized pistils (P < 0.05). Nevertheless, the elongated siliques that formed following auxin and GA3 treatment matured at 10 DPA and shattered open several days later, indicating that carpel valve dehiscence zones were functional. Compared with auxin- and GA3-induced siliques, pollinated siliques matured from 12 DPA. Cytokinin treatment often delayed silique maturation and carpel valve dehiscence compared with pollinated siliques.
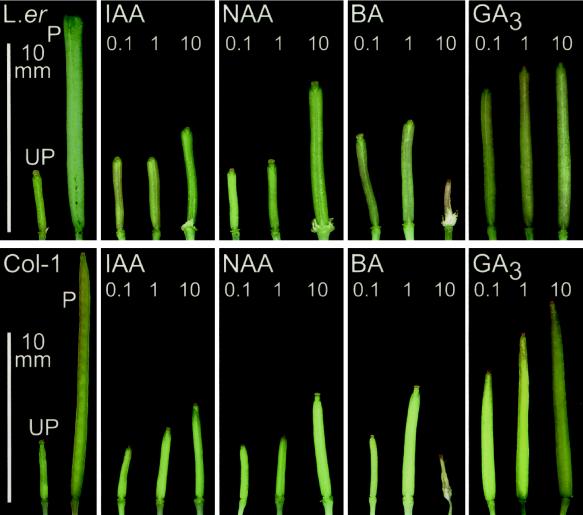
Figure 2.
Siliques 7 DPA after treatment with IAA, NAA, GA3, or BA in the Ler background (top) or Columbia (bottom), with respective application levels in micromoles per pistil indicated in each panel. UP, Unpollinated; P, pollinated.
Auxin treatments of 10 μmol NAA pistil−1 or 10 μmol IAA pistil−1 produced parthenocarpic siliques in all of the ecotypes tested except Col-1 er2, in which there was no apparent elongation observed following NAA application at 10 μmol pistil−1 (Fig. 2; Table I). In general, the application of auxin at levels below 10 μmol pistil−1 did not result in significant elongation (Fig. 2), while auxin levels above 50 μmol pistil−1 caused pistils to degenerate (not shown). Application of BA at 0.1 μmol pistil−1 was only effective in inducing parthenocarpic silique elongation in the Ler, LER, and Col-1 er2 ecotypes. However, a higher level of BA (1 μmol pistil−1) was able to induce parthenocarpy in the Col-1 ecotype. In all ecotypes, BA application below 0.1 μmol pistil−1 failed to yield a parthenocarpic response, while treatments above 10 μmol pistil−1 frequently damaged the pistils (Fig. 2). These results indicated that the Col-1 ecotype was the least sensitive to auxin and generally less sensitive to BA than the Landsberg ecotype (Table I; Fig. 2).
Treatment of Arabidopsis pistils with GA3 at 10 μmol pistil−1 was most effective at inducing silique elongation and resulted in the longest siliques at 10 DPA compared with those induced after auxin or cytokinin treatment (Table I; Fig. 2). GA3 was also effective at inducing silique elongation in the presence of the erecta mutation, and elongation was evident in both the Col-1 and Ler ecotypes from levels as low as 10 nmol pistil−1 (not shown). Elongation of Ler and Col-1 pistils following 10 μmol GA3 pistil−1 treatment resulted in a growth rate comparable to that observed in pollinated pistils (Fig. 1, top and middle). Ecotype-specific differences in elongation and outward appearance following GA3 application were also apparent (Table I; Fig. 2). GA3-treated Col-1 pistils were the most similar to pollinated siliques, even considering that pistil elongation was dosage dependent (Table I). In both ecotypes, silique growth following NAA and BA treatment progressed at a slower rate than in GA3-treated or fertilized pistils (Fig. 1, top and middle).
Pistil Receptivity to Pollen and GA3 in Col-1 and Ler Ecotypes
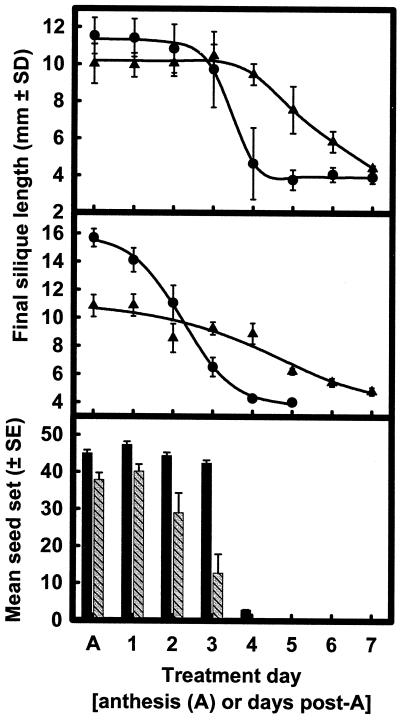
We tested the receptivity period of emasculated pistils to pollination- and GA3-induced growth by applying pollen or GA3 on sequential DPA and then assessing the final silique length. Figure 3 shows that pistil receptivity to pollen extends from anthesis (stage 13; Bowman, 1993) to 3 to 4 DPA in Ler and 3 DPA in Col-1 (Fig. 3, top and middle). Silique length and seed set declined beyond 4 DPA, and once the pistils reached 5 DPA seeds were not set following pollination (Fig. 3). Pistils were responsive to 10 μmol GA3 pistil−1 treatment up to 6 DPA (Fig. 3), indicating that they were receptive to GA3 for a significantly longer period of time than they were to pollination.
Figure 3.
Receptivity period for pollination- and GA3-induced silique elongation was determined by a single treatment of either GA3 (▴; 10 μmol pistil−1) or pollination (●) to emasculated pistils of Arabidopsis at various DPA in the Ler (top) and Col-1 (middle) ecotypes. Seed set (bottom) was also determined with respect to the DPA following pollination in Ler (black bars) and Col-1 (hatched bars).
Analysis of Hormone Biosynthesis and Perception Mutants for Silique Elongation following Emasculation
Given that different plant growth regulators stimulate fertilization-independent silique development, various Arabidopsis mutants altered in their biosynthesis and perception of hormones were surveyed for silique elongation independent of fertilization. Emasculation of these mutants revealed that they were unable to significantly elongate their siliques over the slight elongation normally observed in unpollinated pistils. These non-parthenocarpic mutants included: amp1-1, a mutant exhibiting elevated endogenous cytokinin levels; abi4, an ABA-insensitive mutant; ctr1-1, etr1-3, ein2-1, ein3-1, ein4, ein5-1, ein6, and ein7, ethylene perception mutants; gar2–1 and gai, GA perception mutants; ga1-3, ga4-1, and ga5-1, GA biosynthesis mutants; and axr1-3, axr2, axr4-2, and aux1-7, auxin perception mutants. Auxin application (NAA, 10 μmol pistil−1) to emasculated auxin-resistant mutants axr1-3, axr2, and aux1-7 induced silique elongation (data not shown), indicating that these lesions were independent of auxin-induced parthenocarpy. Additional experiments were carried out with the GA perception mutants spy-1, spy-3, spy-4, and gai; and also the GA biosynthesis mutants ga1-3, ga4-1, and ga5-1. These are described in the sections below.
Spy-4 Silique Development following Emasculation and Response to PGR Application
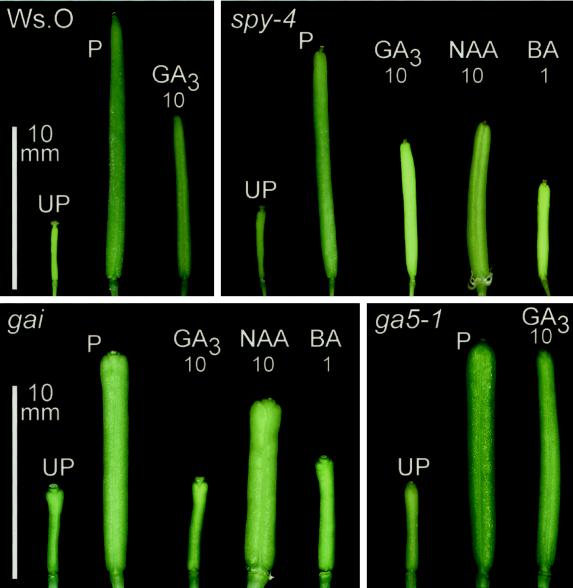
Jacobsen and Oleszewski (1993) reported that several alleles of the SPINDLY locus exhibit parthenocarpic silique development. We assessed the involvement of SPINDLY during growth-regulator-induced parthenocarpy by comparing the elongation response of emasculated spy-4 pistils with and without PGR application (Fig. 4; Table I). Initially, the length attained by emasculated spy-4 pistils was compared with emasculated pistils of Wassilewskija-O (Ws-O), the parental background of the spy-4 mutation, and we found that emasculated spy-4 pistils did not significantly elongate further than emasculated Ws-O (Table I; Fig. 4). Even though numerous spy-4 plants were assessed (n = 87), parthenocarpic silique elongation was not observed (Fig. 4, top; Table I). Similar results were obtained when spy-1 and spy-3 plants were emasculated and examined (n = 24 and 18 plants, respectively).
Figure 4.
Ws-O siliques pollinated (P), unpollinated (UP), and GA3 treated (10 μmol pistil−1) compared with spy-4 unpollinated (UP), pollinated (P), and GA3-, NAA-, or BA-treated (10, 10, and 1 μmol pistil−1, respectively; top) siliques. gai and ga5-1 unpollinated pistils and pollinated siliques (Ler; bottom) compared with GA3, NAA, and BA treatment (10, 10, and 1 μmol pistil−1, respectively), as described in “Materials and Methods.”
To determine whether there was a difference in the response of emasculated spy-4 pistils to the perception of various PGRs, we applied GA3 and NAA at 10 μmol pistil−1 and BA at 1 μmol pistil−1. GA3 and NAA application resulted in the elongation of siliques (Table I; Fig. 4), indicating that spy-4 pistils were able to perceive these PGRs in pistil tissues at anthesis. Although spy-4 pistils responded to these PGRs, emasculated pistils treated with BA did not significantly exceed the length of unpollinated Ws-O pistils at 7 DPA (Table I). Nonetheless, when BA was applied to emasculated spy-4 pistils, they expanded (Fig. 4). Comparison of the silique length attained from GA3-treated Ws-O pistils with that of GA3-treated spy-4 pistils revealed a slight, insignificant reduction in the capacity to elongate in response to exogenously applied GA3. Taken together, these results show that under our growth conditions, spy alleles do not exhibit parthenocarpy following emasculation and that the spy-4 allele is responsive to GA3-induced parthenocarpic silique elongation.
PGR-Induced Silique Elongation in the gai Background
Considering that GA application to Ler and Col-1 pistils produced the longest siliques with similar outward morphology to pollinated siliques, GA perception during fruit set was further examined by assessing the responses of emasculated pistils to PGRs in the GA-insensitive mutant background gai. The gai mutant displays repressed growth and reduced GA responsiveness caused by a semi-dominant mutation that confers dysfunctional activity to the GAI protein (Peng et al., 1997) but does not confer GA deficiency (Talon et al., 1990b).
Initially we found that self-pollinated gai pistils frequently produced shorter siliques with a higher degree of variability in silique length than that observed in Ler, the ecotype background of the gai mutant allele (Table I). Seed set was also low and variable in self-pollinated gai. By contrast, gai siliques following cross-pollination with Ler pollen were not significantly different in length and were less variable than gai siliques following self-pollination (Table I). We interpreted this to mean that gai had defective pollen that decreased seed set and silique elongation. In subsequent experiments, silique growth in gai pistils was therefore determined following cross-pollination with Ler pollen.
Following pollination, siliques of gai mutants elongated until 5 to 6 DPA, which was 2 to 3 d longer than that observed for Ler (Fig. 1, bottom). Comparisons between the top and bottom panels of Figure 1 show that the difference in elongation period was because pollinated gai siliques had a reduced growth rate and a significantly reduced mean silique length (Table I). Given that the mean length of anthesis-stage pistils of gai and Ler were similar (Table I), differences in silique length must have arisen during postanthesis development. Following pollination, gai siliques were dehiscent, indicating the development of functional dehiscence zones.
Emasculated gai pistils did not elongate following application of 10 μmol GA3 pistil−1. Unpollinated and GA3-treated gai pistils were morphologically alike (Fig. 4; Table I) and indehiscent, indicating that GA3-induced parthenocarpy was blocked in the mutant gai background. However, silique elongation was still observed following exogenous application of NAA and, to a lesser degree, BA (Fig. 4; Table I). NAA treatment also substantially increased silique expansion over that observed following pollination of gai pistils (Fig. 4, bottom left). Comparison of silique elongation in auxin-induced gai pistils to pollinated gai pistils also revealed that the initiation, rate, and cessation of silique development differed (Fig. 1, bottom). Therefore, while it appears that normal functional activity of GAI is necessary for transducing GA signals, the analysis of the gai mutant allele indicated that this pathway does not appear to be critical for alternative PGR-induced parthenocarpic responses.
Structural Comparisons of Unpollinated, Pollinated, and Induced Siliques
Up to this point, parthenocarpic induction had been measured as the ability of a silique to elongate and dehisce. In the present study, we also compared the structure of anthesis pistils, unpollinated pistils, and elongated siliques following pollination or PGR treatment in several mutant backgrounds by histological sectioning.
Arabidopsis gynoecium structure and post-pollination development has been described previously (Gasser and Robinson-Beers, 1993; Sessions and Zambryski, 1995; Gu et al., 1998), but without specific reference to exocarp, mesocarp, supportive sclerenchyma, and endocarp development. Figures 5 and 6 show that there are six to seven cell layers associated with the carpel wall, of which four form distinct cell types. The single outer epidermal layer of the carpel differentiates into the exocarp layer of mature siliques. Three to four chlorenchyma or parenchymal cell-type layers develop as the mesocarp layer. A supportive sclerenchymal layer adjoins the innermost mesocarp layer, and an adjacent endodermal layer forms the endocarp that faces into the locule. Cell counts from semi-thin carpel cross-sections were used to specifically determine how carpel valves expand in width (Table II). We also determined the cell length normal to the plane of elongation from longitudinal sections, as shown in Table III. Using the mean cell length for a given cell type, the total cell number was calculated for the length of a silique for a single longitudinal lateral section (Table IV).
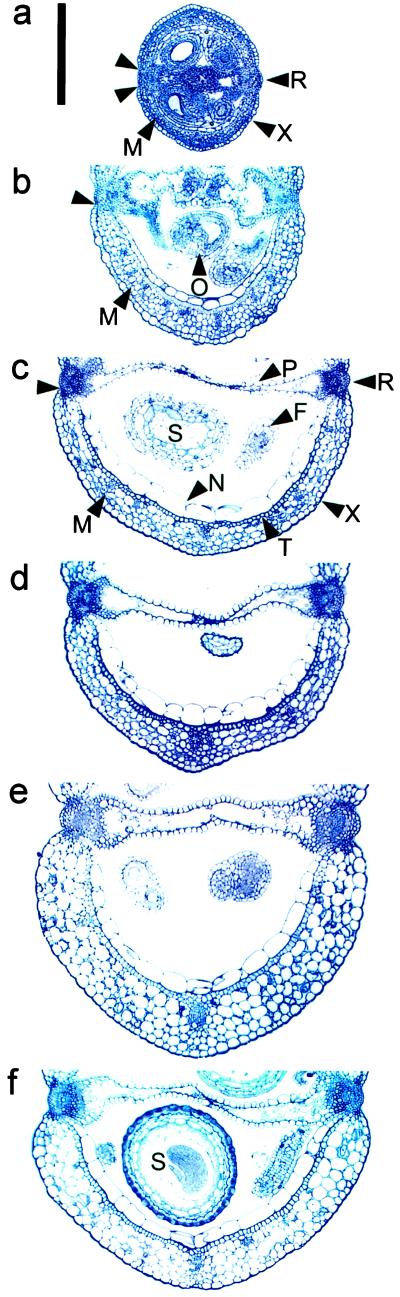
Figure 5.
Carpel wall cross-sections illustrating the degree of carpel expansion and development from an anthesis-stage pistil compared with 7-DPA unpollinated, pollinated, and PGR-treated siliques from the gai and Ler backgrounds. a, Anthesis-stage Ler pistil; b, unpollinated Ler pistil; c, pollinated Ler silique; d, Ler silique induced with 10 μmol GA3 pistil−1; e, Ler silique induced with 10 μmol NAA pistil−1; f, pollinated gai silique. Unmarked arrowheads indicate dehiscence zones; X, exocarp; M, mesocarp; T, supportive sclerenchyma; N, endocarp; S, seed; O, ovule; P, septum; F, funiculus; R, replum. Scale bar = 250 μm.
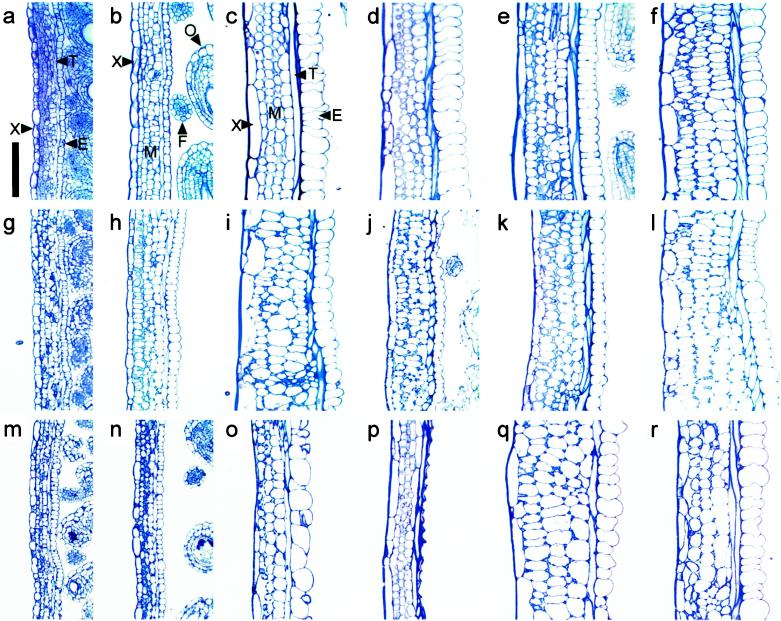
Figure 6.
Longitudinal carpel wall sections of Ler at anthesis (a) and at 7 DPA for an unpollinated silique (b), a pollinated silique (c), and parthenocarpic siliques induced by 10 μmol GA3 pistil−1 (d), 1 μmol BA pistil−1 (e), 10 μmol NAA pistil−1 (f). g to l, Silique wall sections of the respective Ler treatments in the gai background. Carpel wall sections of spy-4 anthesis pistil (m) and 7 at DPA for an unpollinated pistil (n), pollinated silique (o), and emasculated spy-4 pistil induced to grow with 10 μmol GA3 pistil−1 (p). Rescue of carpel wall structure in the ga5-1 biosynthetic mutant, 7-d pollinated ga5-1 silique (q), ga5-1 parthenocarpic silique induced with 10 μmol GA3 pistil−1 (r). X, Exocarp; M, mesocarp; T, supportive sclerenchyma; E, endocarp; O, ovule; F, funiculus. Scale bar = 100 μm.
Table II.
Comparison of cell number in cross-sections of different Arabidopsis carpels at anthesis and 7 DPA
| Treatment | DPA | Carpel Cell No. per Tissue Type
|
||
|---|---|---|---|---|
| Exocarp | Mesocarp | Endocarp | ||
| Anthesis | 0 | 81 ± 18.5 | 177 ± 5.0 | 25.3 ± 5.0 |
| Ler unpollinated | 7 | 82 ± 10.6 | 171 ± 33 | 28.0 ± 8.7 |
| Ler + pollination | 7 | 79 ± 10.6 | 167 ± 7.0 | 32.7 ± 12.2 |
| Ler + 10 μmol−1 pistil GA3 | 7 | 69 ± 2.3 | 154 ± 6.9 | 23.3 ± 4.2 |
| Ler + 10 μmol−1 pistil NAA | 7 | 67 ± 8.1 | 180 ± 17.8 | 24.0 ± 6.9 |
| gai + pollination | 7 | 67 ± 11.7 | 149 ± 7.6 | 20.7 ± 1.2 |
| ga5-1 + pollination | 7 | 67 ± 10.0 | 131 ± 18.1 | 20.0 ± 4.3 |
Results are means ± sd.
Table III.
Comparison of the mean cell length, normal to the silique elongation axis, in Arabidopsis carpel tissue layers from anthesis and 7-DPA pollinated or PGR-treated pistils
| Stage of Silique Development and Treatment Type
| ||||||||
|---|---|---|---|---|---|---|---|---|
| Tissue | Anthesis (2.8 ± 0.2) | Ler UPa (4.1 ± 0.4) | Ler +Pb (11.5 ± 1.0) | Ler +GA3c (10.0 ± 1.1) | Ler +NAAd (6.8 ± 0.8) | gai +Pb (9.5 ± 1.2) | ga5-1 +Pb (10.5 ± 1.0) | ga5-1 +GA3c (10.0 ± 1.1) |
| μm | ||||||||
| Exocarp | 15 ± 8 | 28 ± 15 | 49 ± 32 | 82 ± 48 | 59 ± 24 | 46 ± 41 | 67 ± 42 | 60 ± 33 |
| Mesocarp 1 | 10 ± 4 | 11 ± 4 | 13 ± 5 | 13 ± 3 | 16 ± 6 | 20 ± 6 | 19 ± 5 | 20 ± 6 |
| Mesocarp 2 | 11 ± 3 | 11 ± 3 | 12 ± 3 | 17 ± 4 | 13 ± 5 | 20 ± 6 | 23 ± 6 | 18 ± 7 |
| Mesocarp 3 | 11 ± 4 | 14 ± 5 | 21 ± 8 | 17 ± 7 | 17 ± 6 | 29 ± 10 | 26 ± 8 | 30 ± 10 |
| Endocarp | 7 ± 2 | 13 ± 3 | 22 ± 6 | 20 ± 7 | 20 ± 7 | 28 ± 7 | 28 ± 8 | 24 ± 8 |
Results are means ± sd silique length for each treatment.
UP, Unpollinated.
P, Pollinated.
10 μmol GA3 pistil−1.
10 μmol NAA pistil−1.
Table IV.
Comparison of the mean cell number, in longitudinal sections of Arabidopsis carpel tissues from anthesis and 7-DPA pollinated or PGR-treated pistils
| Stage of Silique Development and Treatment Type
| ||||||||
|---|---|---|---|---|---|---|---|---|
| Tissue | Anthesis (2.8 ± 0.2) | Ler UPa (4.1 ± 0.4) | Ler +Pb (11.5 ± 1.0) | Ler +GA3c (10.0 ± 1.1) | Ler +NAAd (6.8 ± 0.8) | gai +Pb (9.5 ± 1.2) | ga5-1 +Pb (10.5 ± 1.0) | ga5-1 +GA3c (10.0 ± 1.1) |
| Exocarp | 227 ± 20 | 196 ± 23 | 277 ± 26 | 176 ± 38 | 133 ± 13 | 241 ± 33 | 223 ± 26 | 232 ± 29 |
| Mesocarp 1 | 315 ± 17 | 396 ± 15 | 951 ± 41 | 788 ± 28 | 494 ± 25 | 440 ± 28 | 609 ± 18 | 567 ± 35 |
| Mesocarp 2 | 287 ± 12 | 389 ± 11 | 983 ± 34 | 642 ± 24 | 601 ± 28 | 437 ± 23 | 489 ± 14 | 623 ± 21 |
| Mesocarp 3 | 289 ± 19 | 326 ± 17 | 617 ± 30 | 664 ± 34 | 493 ± 22 | 306 ± 13 | 446 ± 16 | 440 ± 20 |
| Endocarp | 420 ± 19 | 351 ± 14 | 556 ± 28 | 560 ± 25 | 420 ± 35 | 303 ± 16 | 415 ± 18 | 450 ± 20 |
Results are means ± sds silique lengths for each treatment.
UP, Unpollinated.
P, Pollinated.
10 μmol GA3 pistil−1.
10 μmol NAA pistil−1.
Our observations of cross-sections showed that the increase in the carpel width of unpollinated pistils and mature, pollination-induced siliques was entirely due to cellular expansion in all of the component tissues (Table II; Fig. 5). This was because cell numbers in each tissue post- anthesis were not significantly greater than the mean number present in cross-sections of anthesis pistils (Table II). This indicates that the number of cells in a given cross-section of a mature silique is determined prior to anthesis and remains static during silique formation. Therefore, pollination and fertilization directly influence the degree of cell expansion in this plane, as pollinated pistils expanded far more than 7-d unpollinated pistils (Fig. 5).
The length of unpollinated pistils increased slightly post anthesis because cell length normal to the plane of elongation increased in the exocarp and endocarp layers (Table III). However, the cell number per longitudinal section remained similar to anthesis pistils (Table IV). Endocarp and exocarp were composed of relatively uniform cells (Figs. 5b and 6b). Mesocarp cells remained similar in length to anthesis pistils, but their numbers were greater than those observed in anthesis stage pistils (Tables III and IV). This indicated that some cellular division occurred in the mesocarp cell layer. In contrast to mature siliques (Figs. 5c and 6c), the secondary wall thickening of sclerenchyma cells and the development of mature senescence zones were characteristically absent in unpollinated pistils (Figs. 5b and 6b).
Pollination induced a significant increase in cell division and length normal to the plane of elongation in specific tissue layers (Table III). Unlike unpollinated pistils, the exocarp of pollinated siliques was composed of cells varying in length (Fig. 6c). The number of exocarp cells was increased slightly compared with that observed at anthesis (Table IV). We inferred that exocarp cells predominantly expand post pollination. Mesocarp cells were separated into three layers to analyze cell length and number normal to the plane of elongation (Table IV). Cells adjacent to the exocarp were designated mesocarp 1, cells bounded by other mesocarp cells mesocarp 2, and cells adjacent to the sclerenchyma layer mesocarp 3. Mesocarp 1 and mesocarp 2 cells from mature siliques post pollination were wider (Figs. 5c and 6c) and longer than those observed in both unpollinated and anthesis stage pistils (Table III). Mesocarp 3 cells in mature siliques were twice their original anthesis length (Table III). Cell number for all mesocarp layers in this longitudinal section increased approximately 3-fold over the number present at anthesis (Table IV).
The number of endocarp cells normal to the plane of elongation increased almost 2-fold after pollination (Table IV). These cells increased in length (Table III) and width by expanding into the carpel locule space (Figs. 5c and 6c). During silique development following pollination, vascular differentiation also occurred in the replum and in the medial and lateral bundles of the carpel valve. Fully developed carpel valves were demarcated by mature dehiscence zones subadjacent to the replum (Fig. 5c, unmarked arrowheads).
We found that GA3 treatment of unfertilized pistils induced differentiation analogous to mature pollinated siliques (Figs. 5d and 6d). This was illustrated by similar cell numbers (Tables II and IV) and the degree of cellular elongation in each layer (Table III) except the mesocarp, where cell number was slightly reduced compared with pollinated siliques following GA3 treatment (Table IV). Structural comparison of auxin-induced siliques and pollinated pistils indicated gross cellular expansion primarily in the exocarp and mesocarp cell layers (Figs. 5e and 6f). This resulted in increased silique wall width and only a small increase in cell length perpendicular to the plane of elongation (Table III). Generally, cell numbers normal to the plane of elongation in the mesocarp and endocarp of auxin-treated siliques were more similar to unpollinated siliques than the GA3-treated or pollinated pistils (Figs. 5e and 6f; Tables III and IV). NAA-induced siliques also displayed less secondary wall deposition in sclerenchyma cells, and the endocarp cells had reduced expansion into the carpel locule. Siliques forming following auxin and cytokinin treatment frequently contained four mesocarp cell layers (Figs. 5e and 6, e and f).
Silique Structure in GA Perception Mutants
Carpel valve structure was examined in gai and spy-4 mutants following pollination and PGR application. Gross cellular expansion was observed in the exocarp and mesocarp cell layers of pollinated gai siliques (Figs. 5f and 6i). This was similar to that observed following auxin treatment in unfertilized Ler pistils (Fig. 6f). Closer inspection indicated that the mesocarp and endocarp cells of pollinated gai (Fig. 6i) were longer than those of pollinated Ler or NAA-treated pistils (Table III). Furthermore, their cell number normal to the plane of elongation was essentially the same as unpollinated Ler siliques (Table III). Therefore, gai silique elongation following pollination occurred principally by cellular expansion with minimal cell division. This was not a result of cross-pollination with Ler pollen, because self-pollinated gai silique structure was comparable (data not shown).
Treatment of gai pistils with GA3 failed to stimulate elongation, and the pistils resembled unfertilized pistils (Fig. 4). Following sectioning, we also observed that a small amount of mesocarp expansion and increased secondary thickening in sclerenchyma occurred in GA-treated gai pistils (Fig. 6j), indicating minimal differentiation. Treatment of emasculated gai pistils with BA and NAA (Fig. 6, k and l, respectively) produced carpel valves with structures similar to that observed following application of these hormones to emasculated Ler pistils.
We also examined spy-4 silique structure after pollination or treatment with GA3 (Fig. 6, m–p). Both unpollinated pistils and pollinated spy-4 siliques displayed a slender carpel valve phenotype and small mesocarp cells (Fig. 6, n and o) compared with the respective treatment in Ler and gai siliques. Like unpollinated Ler pistils, unpollinated spy-4 pistils remained undifferentiated (Fig. 6n). Furthermore, parthenocarpic spy-4 siliques induced by GA3 treatment had extreme reduction in mesocarp cell size and the endocarp cells failed to form normally (Fig. 6p). These results may be a function of the Ws-O background in spy-4 plants and require further investigation to exclude ecotype-specific responses.
Analysis of Silique Structure in GA Biosynthetic Mutants after Pollination or PGR Treatment
The dependency of silique elongation on endogenous levels of GA was examined by comparing unpollinated and post-pollination silique structures in the GA biosynthesis mutants ga4-1 and ga5-1. Both mutants examined were in the Ler background. ga4-1 is blocked in the 3β-hydroxylation step of GA20 to GA1 and also GA9 to GA4 (Chiang et al., 1995; Fig. 7). The ga5-1 mutant is impaired at the 20-oxidation step but is still responsive to exogenous GA3, as is ga4-1 (Talon et al., 1990c; Fig. 7). Both ga4-1 and ga5-1 mutants have reduced levels of endogenous active GA4 and GA1 in shoots and rosettes (Talon et al., 1990c). The 20-oxidase encoded by GA5 has also been shown to be regulated by both gai and spy (Xu et al., 1995; Peng et al., 1997; Fig. 7).
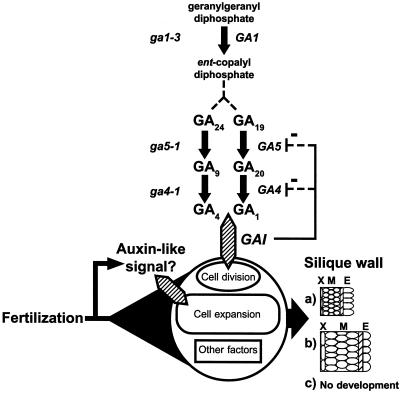
Figure 7.
A model suggesting how GA biosynthesis and perception may determine silique structure in Arabidopsis. Signals from fertilization allow cell division, cell expansion, and differentiation during development. This may include activation of certain steps within the GA signal transduction cascade required for normal differentiation. Levels of active GAs (GA1, GA3, or GA4) would specifically limit cell division and the biosynthetic mutants (italics) would block or alter this process. One exception occurs at GA4, where other known 3β-hydrolyases may allow synthesis of active GAs. Based on mutant analysis described here, GAI may participate in GA perception by transducing signals that regulate cell division. a, At high GA levels cell differentiation occurs as for normal pollinated siliques; b, at low levels of active GA an auxin-like effect dominates with limited cellular division; and c, at very low levels of GA pistils cannot differentiate into siliques. The steps in GA biosynthesis between ent-copalyl diphosphate and GA19 or GA24 are abbreviated for simplicity. Other steps are detailed in Hedden and Kamiya (1997) and Sponsel et al. (1997). X, Exocarp; M, mesocarp; E, endocarp.
The structure of unpollinated and pollinated ga4-1 siliques (data not shown) resembled unpollinated pistils and pollinated siliques of Ler (Fig. 6, b and c, respectively). By contrast, pollinated ga5-1 siliques (Figs. 4, bottom right, and 6q) exhibited a carpel valve phenotype similar to pollinated gai siliques and also to parthenocarpic siliques obtained from NAA treatment of Ler pistils. Cell length in the mesocarp and endocarp was similar in both ga5-1 and gai siliques post pollination (Table III). However, unlike gai, parthenocarpic silique elongation was induced in ga5-1 following GA3 application (Fig. 4, bottom right), and carpel valve structure following this treatment was similar to that observed in Ler siliques following pollination (Fig. 6r; Table III). This indicates that appropriate levels of endogenously active GAs are required for normal carpel development post pollination in Ler, and that the GA response in gai was blocked.
We have shown that parthenocarpic silique development was induced in both spy-4 and gai following the application of auxin. However, ga1-3 mutants that lack normal functional ent-copalyl diphosphate synthase (Fig. 7), an enzyme that catalyzes the first committed step in the GA biosynthesis pathway (Sun and Kamiya, 1994), did not produce parthenocarpic siliques after auxin application (1 and 10 μmol NAA pistil−1; data not shown). This indicates that the alternate parthenocarpy pathway induced by NAA is only active if the early steps in endogenous GA biosynthesis are correctly maintained.
DISCUSSION
Pollination and subsequent double-fertilization events in the carpel induce a coordinated sequence of cell division, cell expansion, and cell differentiation events that result in mature fruit and seed structures. The nature and sequence of the signals that stimulate or limit these processes are unknown. In the absence of fertilization, Arabidopsis pistils treated with GA, auxin, and cytokinin produced parthenocarpic siliques that varied in length and morphology depending on the ecotype and the growth regulator applied. The ability to induce silique growth with different classes of PGR indicates that Arabidopsis pistils are receptive to a variety of hormonal signals that can subsequently promote parthenocarpic growth and thus uncouple silique growth from the normally linked process of seed development.
Structural differences between fertilization-induced fruit and various PGR-induced parthenocarpic fruit have also been observed in tomato (Bünger-Kibler and Bangerth, 1982), blueberry (Cano-Medrano and Darnell 1997), rape (Srinivasan and Morgan, 1996), citrus (Guardiola et al., 1993), watermelon (Sedgley et al., 1977), and pea (Vercher et al., 1987; Vercher and Carbonell, 1991). In Arabidopsis the application of a single PGR could not reproduce the exact silique length, shape, and growth rate observed following pollination. However, application of GA3 at 10 μm pistil−1 gave the greatest silique elongation and the most similar structural development to pollination-induced siliques.
The shorter silique length following GA application in Arabidopsis may reflect the lack of seeds that may physically add to the gross structural arrangement of the silique, as was observed in rape (Srinivasan and Morgan, 1996). It is conceivable that the Arabidopsis seeds also contribute morphogenic molecules that influence maternal carpel tissue development, as has been suggested for other species (Denny, 1992). Comparable post-pollination silique length in Arabidopsis might be obtained following repeated PGR or combinations of PGR application, as observed in rape (Srinivasan and Morgan, 1996) and pea (van Huizen et al., 1997).
GA3 primarily influenced mesocarp cell division in a manner similar to that observed following pollination in Arabidopsis. By contrast, gross mesocarp and exocarp cell enlargement occurred following auxin treatment in Arabidopsis. In tomato, GA3 treatment has been observed to induce mesocarp cell expansion with restricted cellular division, while auxin treatment stimulated cell division (Bünger-Kibler and Bangerth, 1982). PGR-induced parthenocarpy in Arabidopsis was also different from that observed in the related crucifer rape, where GA3 induced cellular expansion in mesocarp tissues (Srinivasan and Morgan, 1996).
Taken together, these observations demonstrate that while pollination-independent fruit development can occur following PGR application in some species, a particular PGR can induce cell division in the fruit tissue of one species but produce cell enlargement during fruit development in another. The potential to successfully induce parthenocarpic fruit development with PGRs may reflect the status of the carpel with respect to the developmental potential for cell division, expansion, and cell differentiation processes at the time of growth regulator application. Therefore, endogenous hormone synthesis and the perception of an endogenous hormone or exogenously applied PGR in a particular tissue are likely to be key factors in hormone-induced parthenocarpy.
GA Biosynthesis and Parthenocarpic Silique Development in Arabidopsis
Barendse et al. (1986) determined that GAs and other maternally derived factors were essential for post-pollination silique development in Arabidopsis. Here we have shown that exogenous GA application stimulates parthenocarpic silique development, producing siliques with a structure most similar to siliques derived post pollination. How does the biosynthesis of GAs determine parthenocarpic fruit growth and what are the genes involved in the GA biosynthesis pathway that affect parthenocarpic silique development?
To address these questions, we examined parthenocarpic silique development in various GA biosynthesis mutants showing reduced levels of endogenous GAs in shoots (Talon et al., 1990c). Figure 7 shows the mutants examined in relation to their role in the known GA biosynthetic pathway in Arabidopsis. ga1-3 mutants lack functional ent-copalyl diphosphate synthase (Sun and Kamiya 1994; Fig. 7) and produce very low levels of active GAs (Hedden and Kamiya, 1997). Barendse et al. (1986) showed that these mutants have an absolute requirement for exogenous GA to produce siliques following fertilization, and we have found that ga1-3 mutants do not produce parthenocarpic siliques following NAA treatment, indicating that a threshold of endogenous biosynthesis of GAs is an essential component for both parthenocarpic and pollination-induced silique development (Fig. 7). This is supported by the observation that spy mutants partially rescue the ga1-3 phenotype (Jacobsen and Olszewski, 1993; Silverstone et al., 1997), possibly by elevating the basal response level that would normally be suppressed in wild-type plants (Jacobsen and Olszewski, 1993), thus avoiding sub-threshold levels of GA biosynthesis that would prevent a variety of essential processes.
The ga4-1 mutant is blocked in the 3-β-hydroxylation of GA20 to GA1 or GA9 to GA4, a final step in yielding active GAs (Talon et al., 1990c; Chiang et al., 1995; Fig. 7). Levels of GA1 and GA4 observed in this mutant are 3-fold lower than Ler (Talon et al., 1990c). Following pollination, the silique walls of the ga4-1 mutant were similar in structure to that of pollinated Ler. This could mean that the lower level of active GAs in the ga4-1 mutant can sustain structural development of the pollinated silique (Fig. 7), which would agree with our observations that parthenocarpy can be induced in Arabidopsis with low levels of GA3. Products from the recently identified GA4H gene (Yamaguchi et al., 1998), which has similarity to the GA4 gene, can catalyze the 3β-hydroxylation of GA20 to GA1 and may contribute to the active GA levels in ga4-1. Alternatively, ga4-1 mutants may not block the synthesis of all biologically active GAs (Sponsel et al., 1997). Thus, GA4-1 may not be a critical determinant of silique development in Arabidopsis because of its functional redundancy.
GA5-1 is one of three 20-oxidase cDNAs thought to encode a stem-specific isoform that can catalyze several steps in GA biosynthesis, including the conversion of GA24 to GA9, and their 13-hydroxylated counterparts, GA19 to GA20 (Phillips et al., 1995; Sponsel et al., 1997; Fig. 7). Xu et al. (1995) and Talon et al. (1990d) have confirmed that the mutant has low levels of active GA1 and GA4 compared with Ler. Sponsel et al. (1997) observed that the ga5-1 mutant had slightly smaller siliques following fertilization (8.3 ± 0.3 mm) and suggested that low levels of GA 20-oxidase activity were responsible for the short-silique phenotype. In the present study, there was no significant difference in length between pollinated ga5-1 and pollinated Ler siliques or GA3-induced siliques in the ga5-1 mutant (Table I), but there were visible differences in carpel valve structure. Pollination of ga5-1 pistils resulted in reduced mesocarp cell division and increased mesocarp cell expansion, resembling aspects of NAA-induced parthenocarpy. By contrast, parthenocarpic silique structure following emasculation and GA3 treatment in ga5-1 was identical to the normal structure of pollinated Ler. The act of pollination coupled with lower active GA levels in ga5-1 resulted in altered mesocarp structure, indicating that endogenous GAs limit cell division and structural differentiation of specific silique tissues (Fig. 7).
It is possible that fertilization induces an auxin-like signal in the pistil (O'Neill and Nadeau, 1997) and that this signal is in turn regulated in the mesocarp by an appropriate level of GA, resulting in the observed silique development in wild-type plants (Fig. 7). Regulation of GA 20-oxidase activity and mRNA levels during pea pod growth by both auxin and GA3 has been previously reported (van Huizen et al., 1995, 1997; García-Martínez et al., 1997). Recent double-mutant analysis of ga5-2 ga6-2 by Sponsel et al. (1997) supports the idea that sub-threshold levels of biologically active GAs severely limit silique development. Therefore, development may proceed if there are suboptimal levels of active GA in the Arabidopsis mesocarp, as in the ga5-1 mutant, but the auxin-like effect would dominate, resulting in greater cellular expansion and an alteration in mesocarp structure (Fig. 7).
The GA biosynthesis mutants show that a basal level of endogenous GA activity is essential for parthenocarpic silique development, that subtle alterations in GA biosynthesis can lead to changes in differentiation of specific silique tissues, and that while GAs are critical for silique development, they do not appear to be the sole endogenous developmental cue.
GA Perception and Parthenocarpic Silique Development
Endogenous synthesis of GA is important in maintaining silique development post pollination. We have also demonstrated that exogenous GA3 application can stimulate parthenocarpic silique development, indicating that exogenous GA3 is perceived in addition to endogenously synthesized GA. GAI is important for the perception of GA (Kornneef et al., 1985; Fig. 7). Active GA1 levels in the gai mutant are 27 times greater than the normally observed levels in Ler (Talon et al., 1990b). Therefore, in contrast to the GA biosynthesis mutants, active GAs are overabundant in gai. This is perhaps because transcript levels of GA4 and GA5 genes are up-regulated and allow higher levels of GA biosynthesis (Peng et al., 1997; Cowling et al., 1998). Experimental evidence indicates that GAI appears to regulate transcription of the 20-oxidase encoded by GA5 in a manner that represses transcript levels when endogenously active GAs are sufficient (Peng et al., 1997). Thus, GAI functions as a GA-derepressable modulator of plant growth (Peng et al., 1997). GAI encodes a protein that appears to be a nuclear-encoded transcriptional coactivator that is similar to the Arabidopsis SCARECROW and RGA genes (Silverstone et al., 1998). We utilized the gai mutant allele that encodes an altered product lacking 17 amino acids, a domain critical for GA response and repression (Peng et al., 1997).
Contrary to previous findings (Kornneef et al., 1985), we found that the gai mutation does extend to the floral unit, because parthenocarpic silique growth was blocked when gai mutants were treated with GA3. However, silique development was not blocked following pollination, and parthenocarpy was also triggered in this mutant following auxin and cytokinin treatment. Silique growth in pollinated gai plants proceeded primarily by cell expansion and resembled auxin-induced parthenocarpic siliques. A post-pollination auxin-like signal that acts independently of GA perception may explain the cellular expansion phenotype (Fig. 7), because the gai mutant may be blocked in one or more downstream processes required for mediating the correct structural development post pollination.
spy mutants display a phenotype similar to plants repeatedly exposed to GA (Jacobsen and Olszewski, 1993), and the spy-4 allele is completely epistatic to gai (Jacobsen et al., 1996). There is some debate about whether SPY activates or deactivates GAI and RGA in Arabidopsis (Silverstone et al., 1998), which could affect GA4 and GA5 transcription levels. spy mutants were reported as parthenocarpic (Jacobsen and Olszewski, 1993), but we found that spy-4 did not exhibit parthenocarpic silique elongation or differentiation when left unpollinated. Emasculated spy-4 pistils did, however, respond to GA3 treatment in that they had even smaller mesocarp cells than pollination-induced siliques. Control points other than SPY may facilitate exogenously induced GA3 parthenocarpy in Arabidopsis.
Arabidopsis Can Be Used to Elucidate the Molecular Basis of Parthenocarpy
In this study we have described the roles for GA biosynthesis and perception during Arabidopsis silique development. We observed that the application of a range of PGRs can induce parthenocarpic silique development with various structural differentiation. Analysis of GA mutants indicated that active GAs and their perception can limit or stimulate cell division and differentiation in Arabidopsis carpels (Fig. 7). However, gai mutants appear to be dependent on alternative endogenous signals that allow pollination- and auxin-induced silique development to occur. Perhaps in one of several steps activated post fertilization, GAI functions to balance signals from GA and other sources (Fig. 7). The possibility of fertilization-stimulated auxin-like cues for silique development and cellular expansion could be investigated using the appropriate perception mutants or hormone-induced genetic elements.
Experimental evidence indicates that ethylene and auxin do play a significant part during pollinated ovary development and senescence of unpollinated pistils (O'Neill et al., 1993; Komori et al., 1997; O'Neill and Nadeau, 1997), but empirical evidence is required for understanding the interaction of ethylene perception. Appropriate mutagenesis screens in Arabidopsis should identify mutants that are parthenocarpic and can elongate siliques in the absence of fertilization. A basis for such a screen would be similar to those conducted to identify mutants exhibiting components of apomixis (Ohad et al., 1996; Chaudhury et al., 1997) except that plants producing seedless siliques would be examined. This approach could clarify the roles of endogenous hormones during the initiation of fruit development and may also aid in the elucidation of the molecular basis of parthenocarpy.
ACKNOWLEDGMENTS
The authors thank Arabidopsis Biological Resource Center and Drs. Steve Swain and Abed Chaudhury for seeds. Special thanks to Drs. Susan Barker, Paul Boss, and Steve Swain for critically reading the manuscript, and to Dr. Abed Chaudhury and the Koltunow Lab team for reviews and support.
Footnotes
This work was supported by the Horticultural Research and Development Corporation, Australia (to A.V.-S. and A.M.K.), by the Commonwealth Scientific and Industrial Research Organization (Australia), and by an Australian Postgraduate Award (to A.V.-S.).
LITERATURE CITED
- Archbold DD, Dennis FG. Strawberry receptacle growth and endogenous IAA content as affected by growth regulator application and achene removal. J Am Soc Hortic Sci. 1985;110:816–820. [Google Scholar]
- Barendse GWM, Kepczynski J, Karssen CM, Koornneef M. The role of endogenous gibberellins during fruit and seed development: studies on gibberellin-deficient genotypes of Arabidopsis thaliana. Physiol Plant. 1986;67:315–319. [Google Scholar]
- Ben-Cheikh W, Perez-Botella J, Tadeo FR, Talon M, Primo-Millo E. Pollination increases gibberellin levels in developing ovaries of seeded varieties of citrus. Plant Physiol. 1997;114:557–564. doi: 10.1104/pp.114.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman J. Arabidopsis: An Atlas of Morphology and Development. New York: Springer-Verlag; 1993. [Google Scholar]
- Bünger-Kibler S, Bangerth F. Relationship between cell number, cell size and fruit size of seeded fruits of tomato (Lycopersicon esculentum Mill.), and those induced parthenocarpically by the application of plant growth regulators. Plant Growth Regul. 1982;1:143–154. [Google Scholar]
- Cano-Medrano R, Darnell RL. Cell number and cell size in parthenocarpic vs. pollinated blueberry (Vaccinium ashei) fruits. Ann Bot. 1997;80:419–425. [Google Scholar]
- Chaudhury AM, Lavithis M, Taylor PE, Craig S, Singh MB, Singer ER, Knox RB, Dennis ES. Genetic control of male fertility in Arabidopsis thaliana: structural analysis of premeiotic developmental mutants. Sex Plant Reprod. 1994;7:17–28. doi: 10.1007/s004250050348. [DOI] [PubMed] [Google Scholar]
- Chaudhury AM, Ming L, Miller C, Craig S, Dennis ES, Peacock WJ. Fertilization-independent seed development in Arabidopsis thaliana. Proc Natl Acad Sci USA. 1997;94:4223–4228. doi: 10.1073/pnas.94.8.4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang H-H, Hwang I, Goodman HM. Isolation of the Arabidopsis GA4 locus. Plant Cell. 1995;7:195–201. doi: 10.1105/tpc.7.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowling RJ, Kamiya Y, Seto H, Harberd NP. Gibberellin dose-response regulation of GA4 gene transcript levels in Arabidopsis. Plant Physiol. 1998;117:1195–1203. doi: 10.1104/pp.117.4.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny OJ. Xenia includes metaxenia. HortScience. 1992;27:722–728. [Google Scholar]
- Eeuwens CJ, Schwabe WW. Seed and pod wall developments in Pisum sativum L. in relation to extracted and applied hormones. J Exp Bot. 1975;26:1–14. [Google Scholar]
- García-Martínez JL, López-Diaz I, Sánchez-Beltrán MJ, Phillips AL, Ward DA, Gaskin P, Hedden P. Isolation and transcript analysis of gibberellin 20-oxidase genes in pea and bean in relation to fruit development. Plant Mol Biol. 1997;33:1073–1084. doi: 10.1023/a:1005715722193. [DOI] [PubMed] [Google Scholar]
- García-Martínez JL, Martí M, Sabater T, Maldonado A, Vercher Y. Development of fertilized ovules and their role in the growth of the pea pod. Physiol Plant. 1991a;83:411–416. [Google Scholar]
- García-Martínez JL, Santes C, Croker SJ, Hedden P. Identification, quantification and distribution of gibberellins in fruits of Pisum sativum L. cv. Alaska during pod development. Planta. 1991b;184:53–60. doi: 10.1007/BF00208236. [DOI] [PubMed] [Google Scholar]
- Gasser CS, Robinson-Beers K. Pistil development. Plant Cell. 1993;5:1231–1239. doi: 10.1105/tpc.5.10.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George WL, Scott JW, Splittstoesser WE. Parthenocarpy in tomato. Hortic Rev. 1984;6:65–84. [Google Scholar]
- Gillaspy G, Ben-David H, Gruissem W. Fruits: a developmental perspective. Plant Cell. 1993;5:1439–1451. doi: 10.1105/tpc.5.10.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granell A, Harris N, Pisabarro AG, Carbonell J. Temporal and spatial expression of a thiolprotease gene during pea ovary senescence, and its regulation by gibberellin. Plant J. 1992;2:907–915. doi: 10.1046/j.1365-313x.1992.t01-5-00999.x. [DOI] [PubMed] [Google Scholar]
- Gu Q, Ferrandiz C, Yanofsky MF, Martienssen R. The FRUITFULL MADS-box gene mediates cell differentiation during Arabidopsis fruit development. Development. 1998;125:1509–1517. doi: 10.1242/dev.125.8.1509. [DOI] [PubMed] [Google Scholar]
- Guardiola JL, Barrés MT, Albert C, García-Luis A. Effects of exogenous growth regulators on fruit development in Citrus unshiu. Ann Bot. 1993;71:169–176. [Google Scholar]
- Hedden P, Kamiya Y. Gibberellin biosynthesis: enzymes, genes and their regulation. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:431–460. doi: 10.1146/annurev.arplant.48.1.431. [DOI] [PubMed] [Google Scholar]
- Hobbie L, Timpte C, Estelle M. Molecular genetics of auxin and cytokinin. Plant Mol Biol. 1994;26:1499–1519. doi: 10.1007/BF00016487. [DOI] [PubMed] [Google Scholar]
- Hobbie LJ. Auxin: molecular genetic approaches in Arabidopsis. Plant Physiol Biochem. 1998;36:91–102. [Google Scholar]
- Jacobsen SE, Binkowski KA, Olszewski NE. SPINDLY, a tetratricopeptide repeat protein involved in gibberellin signal transduction in Arabidopsis. Proc Natl Acad Sci USA. 1996;93:9292–9296. doi: 10.1073/pnas.93.17.9292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen SE, Oleszewski NE. Mutations at the SPINDLY locus of Arabidopsis alter signal transduction. Plant Cell. 1993;5:887–896. doi: 10.1105/tpc.5.8.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieber JJ. The ethylene response pathway in Arabidopsis. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:277–296. doi: 10.1146/annurev.arplant.48.1.277. [DOI] [PubMed] [Google Scholar]
- Komori S, Soejima J, Tsuchiya S, Masuda T, Bessho H, Ito Y. Two types of unfruitfulness found in artificial pollination experiments of apple. J Jpn Soc Hortic Sci. 1997;66:289–295. [Google Scholar]
- Koornneef M, Elgersma A, Hanhart CJ, van Loenen-Martinet EP, van Rijn L, Zeevart JAD. A gibberellin insensitive mutant of Arabidopsis thaliana. Plant Physiol. 1985;65:33–39. [Google Scholar]
- Lin S, George WL, Splittstoesser WE. Expression and inheritance of parthenocarpy in “Severianin” tomato. J Hered. 1984;75:62–66. [Google Scholar]
- Meinke DW, Sussex IM. Embryo lethal mutants of Arabidopsis thaliana: a model system for genetic analysis of plant embryo development. Dev Biol. 1979;12:50–61. doi: 10.1016/0012-1606(79)90097-6. [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F. Medium for growth and bioassays with tobacco tissue cultures. Physiol Plant. 1962;15:473–497. [Google Scholar]
- Nitsch JP. Growth and morphogenesis of the strawberry as related to auxin. Am J Bot. 1950;37:211–215. [Google Scholar]
- Nuez F, Costa J, Cuartero J. Genetics of the parthenocarpy for tomato varieties “Sub-Arctic Plenty”, “75/59” and “Severianin”. Z Pflanzenzuecht. 1986;96:200–206. [Google Scholar]
- Ohad N, Margossian L, Hsu Y-C, Williams C, Repetti P, Fischer RL. A mutation that allows endosperm development without fertilization. Proc Natl Acad Sci USA. 1996;93:5319–5324. doi: 10.1073/pnas.93.11.5319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill SD, Nadeau JA. Post-pollination flower development. Hortic Rev. 1997;19:1–58. [Google Scholar]
- O'Neill SJ, Nadeau JA, Zhang XS, Bui AQ, Halevy AH. Interorgan regulation of ethylene biosynthetic genes by pollination. Plant Cell. 1993;5:419–432. doi: 10.1105/tpc.5.4.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, Carol P, Richards DE, King KE, Cowling RJ, Murphy GP, Harberd NP. The Arabidopsis GAI gene defines a signaling pathway that negatively regulates gibberellin responses. Genes Dev. 1997;11:3194–3205. doi: 10.1101/gad.11.23.3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pharis RP, King R. Gibberellins and reproductive development in seed plants. Annu Rev Plant Physiol. 1985;36:517–568. [Google Scholar]
- Phillips AL. Gibberellins in Arabidopsis. Plant Physiol Biochem. 1998;36:115–124. [Google Scholar]
- Phillips AL, Ward DA, Uknes S, Appleford NE, Lange T, Huttly AK, Gaskin P, Graebe JE, Hedden P. Isolation and expression of three gibberellin 20-oxidase cDNA clones from Arabidopsis. Plant Physiol. 1995;108:1049–1057. doi: 10.1104/pp.108.3.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike LM, Peterson CR. Inheritance of parthenocarpy in the cucumber (Cucumis sativus L.) Euphytica. 1969;18:101–105. [Google Scholar]
- Rodrigo MJ, García-Martínez JL, Santes C, Gaskin P, Hedden P. The role of gibberellins A1 and A3 in fruit growth of Pisum sativum L. and the identification of gibberellins A4 and A7 in young seeds. Planta. 1997;201:446–455. [Google Scholar]
- Rotino GL, Perri E, Zottini M, Sommer H, Spena A. Genetic engineering of parthenocarpic plants. Nat Biotechol. 1997;15:1398–1401. doi: 10.1038/nbt1297-1398. [DOI] [PubMed] [Google Scholar]
- Schwabe WW, Mills JJ. Hormones and parthenocarpic fruit set: a literature survey. Hortic Abstr. 1981;51:661–699. [Google Scholar]
- Sedgley M, Newbury HJ, Possingham JV. Early fruit development in the watermelon: anatomical comparison of pollinated, auxin-induced parthenocarpic and unpollinated fruits. Ann Bot. 1977;41:1345–1355. [Google Scholar]
- Sessions RA, Zambryski PC. Arabidopsis gynoecium structure in the wild type and in ettin mutants. Development. 1995;121:1519–1532. doi: 10.1242/dev.121.5.1519. [DOI] [PubMed] [Google Scholar]
- Silverstone AL, Ciampaglio CN, Sun TP. The Arabidopsis RGA gene encodes a transcriptional regulator repressing the gibberellin signal transduction pathway. Plant Cell. 1998;10:155–169. doi: 10.1105/tpc.10.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstone AL, Mak PYA, Martínez EC, Sun T-P. The new RGA locus encodes a negative regulator of gibberellin response in Arabidopsis thaliana. Genetics. 1997;146:1087–1099. doi: 10.1093/genetics/146.3.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sponsel VM. The localization, metabolism and biological activity of gibberellins in maturing and germinating seeds of Pisum sativum cv. Progess No. 9. Planta. 1983;159:454–468. doi: 10.1007/BF00392082. [DOI] [PubMed] [Google Scholar]
- Sponsel VM, Schmidt FW, Porter SG, Nakayama M, Kohlstruk S, Estelle M. Characterization of new gibberellin-responsive semidwarf mutants of Arabidopsis. Plant Physiol. 1997;115:1009–1020. doi: 10.1104/pp.115.3.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurrs AP. A low viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969;26:31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Srinivasan A, Morgan DG. Growth and development of the pod wall in spring rape (Brassica napus) as related to the presence of seeds and exogenous phytohormones. J Agric Sci. 1996;127:487–500. [Google Scholar]
- Sun T-P, Kamiya Y. The Arabidopsis GA1 locus encodes the cyclase ent-kaurene synthetase A of gibberellin biosynthesis. Plant Cell. 1994;6:1509–1518. doi: 10.1105/tpc.6.10.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes SR, Lewis S. Comparing Imperial mandarin and Silverhill satsuma mandarin as seed parents in a breeding program aimed at developing new seedless citrus cultivars for Australia. Aust J Exp Agric. 1996;36:731–738. [Google Scholar]
- Talon M, Hedden P, Primo-Millo E. Gibberellins in Citrus sinensis: a comparison between seeded and seedless varieties. J Plant Growth Regul. 1990a;9:201–206. [Google Scholar]
- Talon M, Koornneef M, Zeevart JAD. Accumulation of C19-gibberellins in the gibberellin-insensitive dwarf mutant gai of Arabidopsis thaliana. Planta. 1990b;182:501–505. doi: 10.1007/BF02341024. [DOI] [PubMed] [Google Scholar]
- Talon M, Koornneef M, Zeevaart JAD. Endogenous gibberellins in Arabidopsis thaliana and possible steps blocked in the biosynthetic pathways of the semi-dwarf ga4 and ga5 mutants. Proc Natl Acad Sci USA. 1990c;87:7983–7987. doi: 10.1073/pnas.87.20.7983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talon M, Zacarias L, Primo-Millo E. Hormonal changes associated with fruit set and development in mandarins differing in their parthenocarpic ability. Physiol Plant. 1990d;79:400–406. [Google Scholar]
- Talon M, Zacarias L, Primo-Millo E. Gibberellins and parthenocarpic ability in developing ovaries of seedless mandarins. Plant Physiol. 1992;99:1575–1581. doi: 10.1104/pp.99.4.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torii KU, Mitsukawa N, Oosumi T, Matsuura Y, Yokoyama R, Whittier RF, Komeda Y. The Arabidopsis ERECTA gene encodes a putative receptor protein kinase with extracellular leucine-rich repeats. Plant Cell. 1996;8:735–746. doi: 10.1105/tpc.8.4.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Huizen R, Ozga JA, Reinecke DM. Seed and hormonal regulation of gibberellin 20-oxdiase expression in pea pericarp. Plant Physiol. 1997;115:123–128. doi: 10.1104/pp.115.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Huizen R, Ozga JA, Reinecke DM, Twitchin B, Mander LN. Seed and 4-chloroindole-3-acetic acid regulation of gibberellin metabolism in pea pericarp. Plant Physiol. 1995;109:1213–1217. doi: 10.1104/pp.109.4.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardy E, Lapushner D, Genizi A, Hewitt J. Genetics of parthenocarpy in tomato under a low temperature regime I. Line RP75/79. Euphytica. 1989a;41:1–8. [Google Scholar]
- Vardy E, Lapushner D, Genizi A, Hewitt J. Genetics of parthenocarpy in tomato under a low temperature regime II. cultivar “Severianin.”. Euphytica. 1989b;41:9–15. [Google Scholar]
- Vercher Y, Carbonell J. Changes in the structure of ovary tissues and in the ultrastructure of mesocarp cells during ovary senescence or fruit development induced by plant growth substances in Pisum sativum. Physiol Plant. 1991;81:518–526. [Google Scholar]
- Vercher Y, Carrasco P, Carbonell J. Biochemical and histochemical detection of endoproteolytic activities involved in ovary senescence or fruit development in Pisum sativum. Physiol Plant. 1989;76:405–411. [Google Scholar]
- Vercher Y, Molowny A, Carbonell J. Gibberellic acid effects on the ultrastructure of the endocarp cells of unpollinated ovaries of Pisum sativum. Physiol Plant. 1987;71:302–308. [Google Scholar]
- Vercher Y, Molowny A, Lopez C, García-Martínez JL, Carbonell J. Structural changes in the ovary of Pisum sativum L. induced by pollination and gibberellic acid. Plant Sci Lett. 1984;36:87–91. [Google Scholar]
- Xu YL, Li L, Wu KQ, Peeters AJM, Gage DA, Zeevaart JAD. The GA5 locus of Arabidopsis thaliana encodes a multifunctional gibberellin 20-oxidase: molecular cloning and functional expression. Proc Natl Acad Sci USA. 1995;92:6640–6644. doi: 10.1073/pnas.92.14.6640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S, Smith MW, Brown RGS, Kamiya Y, Sun T-P. Phytochrome regulation and differential expression of gibberellin 3β-hydroxylase genes in germinating Arabidopsis seeds. Plant Cell. 1998;10:2115–2126. doi: 10.1105/tpc.10.12.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]