Abstract
Extracellular ATP interacts with purinergic type 2 (P2) receptors and elicits many crucial biological functions. Extracellular ATP is sequentially hydrolyzed to ADP and AMP by the actions of defined nucleotidases, such as CD39, and AMP is converted to adenosine, largely by CD73, an ecto-5′-nucleotidase. Extracellular adenosine interacts with P1 receptors and often opposes the effects of P2 receptor activation. The balance between extracellular ATP and adenosine in the blood and extracellular fluid is regulated chiefly by the activities of CD39 and CD73, which constitute the CD39-adenosinergic axis. In recent years, several studies have shown this axis to play critical roles in transport of water/sodium, tubuloglomerular feedback, renin secretion, ischemia reperfusion injury, renal fibrosis, hypertension, diabetic nephropathy, transplantation, inflammation, and macrophage transformation. Important developments include global and targeted gene knockout and/or transgenic mouse models of CD39 or CD73, biological or small molecule inhibitors, and soluble engineered ectonucleotidases to directly impact the CD39-adenosinergic axis. This review presents a comprehensive picture of the multiple roles of CD39-adenosinergic axis in renal physiology, pathophysiology, and therapeutics. Scientific advances and greater understanding of the role of this axis in the kidney, in both health and illness, will direct development of innovative therapies for renal diseases.
Keywords: Purinergic signaling, Ectonucleotidases, Extracellular nucleotides, P2 receptors, P1 receptors, Kidney, Transplantation
Prologue
Ever since Dr. Geoffrey Burnstock first coined the term in 1970s, the field of “purinergic signaling” has transformed into a distinct and complex system [1–6], registering an exponential growth since early 1990s. Purinergic signaling has implications for virtually in every mammalian system, playing one or more critical roles in physiology and/or pathophysiology thereby offering novel drug targets for the treatment of a variety of diseases [7–13]. The situation is both exciting and complex with respect to the kidney, where purinergic signaling plays a variety of roles in health and disease, many of which offer therapeutic potential. In recent years, excellent reviews have been published on the physiology, pathophysiology, and experimental therapeutics of purinergic signaling in the kidney [14–36]. This review is not intended to update the scope of the purinergic receptors in the kidney, but it specifically focuses on CD39-adenosinergic axis, which functions as a dynamic pathway that regulates the availability of ligands for P2 and P1 receptors in the extracellular milieu, and thus has profound implications in renal physiology, pathophysiology, and therapeutics. As the readers will soon realize, at this stage, our knowledge of CD39-adenosinergic axis signaling remains rudimentary.
Organization of CD39-adenosinergic axis
Despite its high intracellular concentrations (3–5 mM), adenosine triphosphate (ATP) cannot freely diffuse out of cells due to its negative charge. However, ATP can be released from healthy cells by regulated exocytosis or through specific transport processes, such as nucleotide transporters (NT) or pannexin (PNX) or connexin (CNX) hemichannels or through multi-drug resistance (MDR) gene products [3, 37, 38]. Intracellular ATP can also be released in an unregulated fashion during hypoxia or following cell death. Extracellular concentrations of ATP even in low micromolar range (5 to 20 μM), which is an order lower than its intracellular concentrations, can elicit a variety of biological functions in mammalian systems by interacting with P2Y or P2X receptor subtypes. P2X receptors (subtypes 1–7) are ATP-gated channels that open up, allowing ions (Na+, K+, Ca2+) to either influx or efflux. P2Y receptors (subtypes 1, 2, 4, 6, and 11–14) are G protein-coupled with downstream effector signaling pathways that cause an increase in either intracellular free Ca2+ or cyclic adenosine monophosphate (cAMP). Both P2Y and P2X receptor subtypes are widely expressed along the mammalian nephron and collecting duct system [20, 24, 27, 31, 39]. However, as shown in Fig. 1, the released ATP is sequentially hydrolyzed to ADP and AMP by CD39 or NTPDase1 (nucleoside triphosphate diphosphohydrolase-1), which is expressed in vascular and tubular structures of the kidney [40–42]. ATP can also be directly hydrolyzed to AMP by nucleotide pyrophosphatases (NPPs), expressed in the kidney [41]. AMP is converted to adenosine by the action of CD73 (5′-ectonucleotidase), which is also expressed in the kidney. While ATP binds to P2Y and P2X receptors, adenosine is a potent agonist of P1 receptors (subtypes A1, A2A, A2B, and A3). Similar to P2Y receptors, P1 receptors are G protein-coupled, with complex downstream signaling pathways. The activation P1 receptor subtypes result in increased or decreased activity of adenylyl cyclase (AC), thus altering cellular cAMP levels. This pathway leading from ATP to the generation of adenosine, known as the CD39-adenosinergic axis (enclosed in the gray box in Fig. 1), is active locally, and it apparently regulates the balance between P2 and P1 receptor activity in cells. In many organs or cells, the activation of P1 receptors opposes the biological effects initiated by the P2 receptors, thus acting as a feedback loop, which is apparently regulated by the activities of CD39 and CD73 [43–47]. While the expression of purinergic receptors and the ectonucleotidases and their functions are documented in many organs, how the expression and activities of the latter are regulated is not understood at this stage. This is a major gap in our knowledge of the operation of CD39-adenosinergic axis. However, the availability of CD39 or CD73 gene knockout mice or mice overexpressing human CD39 (hCD39), and reagents such as potato apyrase, soluble engineered ectonucleotidases, and small molecules that interact with P1 or P2 receptors or CD39 (polyoxymetalates) have enabled us to gain insights into the roles of CD39-adenosinergic axis in renal physiology and pathophysiology. In the following, we will provide an overview of the role of this axis in several pathophysiological conditions that are relevant to the kidney and direct the readers for review articles that provide more details and in-depth presentation.
Fig. 1.
Schematic representation of the components of the complex purinergic signaling pathways mediated by extracellular nucleotides. The horizontal gray colored box shows the components of CD39-adenosinergic axis. The vertical box encloses the components of extracellular cAMP-adenosine pathway. On the extreme right, the formation of extracellular adenine and its interaction with adenine receptor are shown. NT nucleotide transporter; PNX pannexin; CNX connexin; cAMP cyclic AMP; CD39 nucleoside triphosphate diphosphohydrolase 1 (NTPDase1); NPP nucleotide pyrophosphatase; CD73 5′-ectonucleotidase; AC adenylyl cyclase; ePDE ecto-phosphodiesterase; ns-AP tissue-nonspecific alkaline phosphatases; AdeR adenine receptor; ADA adenosine deaminase. For further details, refer to the text (with permission from Peti-Peterdi et al. [30])
Extracellular cAMP-adenosine pathway
Although the CD39-adenosinergic axis represents the major pathway for the generation of extracellular adenosine, there is evidence that under certain circumstances, extracellular cAMP can also be a source of adenosine, which is referred to as the extracellular cAMP-adenosine pathway (shown in the vertical box in Fig. 1). Extracellular cAMP has two sources: the blood and tissues. Unlike ATP, which is unstable in extracellular milieu, cAMP is relatively stable and can be transported to distant organs through the blood circulation, where it can be converted to adenosine. cAMP released from organs such as the liver in the context of high glucagon levels in the blood (e.g., diabetes mellitus, pancreatitis, cirrhosis of liver) can reach the kidney through blood circulation. cAMP can also be released locally within the kidney during heightened receptor-mediated activation of adenylyl cyclase (AC). Intracellular cAMP reaches the extracellular milieu through the same transport system used by ATP and other nucleotides. Extracellular cAMP is converted to AMP by the action of ecto-phosphodiesterase (ePDE) or tissue-nonspecific alkaline phosphatase (ns-AP). The AMP thus formed is converted to adenosine by CD73.
The above two pathways have their specific advantages and disadvantages. Generation of adenosine locally by the activity of the CD39-adenosinergic axis has the advantage of tissue-specific tight local regulation. While the extracellular cAMP-adenosine pathway works more like a hormonal system, but without feedback regulation, the CD39-adenosinergic axis functions as an autocrine/paracrine purinergic signaling with tight feedback regulation [30].
Adenine (P0) receptor
In recent years, another purinergic receptor that selectively binds adenine base, but not adenosine (adenine base + sugar), has been reported. Extracellular adenosine is not converted to adenine, but is broken down to inosine by the action of adenosine deaminase. Adenine is generated in the cells during purine salvage pathway, and it can be transported out of the cells by the same nucleotide transporters (Fig. 1). Similar to P2Y and P1 receptors, the adenine receptor (also known as P0 receptor) is G protein-coupled that selectively binds adenine, but not adenosine. Although not the focus of this review, we have localized the adenine (P0) receptor in the rat kidney and showed that adenine is a signaling molecule in the kidney [48, 49]. In this context, it is interesting to note that blood levels of adenine are markedly increased in patients with chronic renal failure, and positively correlate with severity of the disease [50].
CD39-adenosinergic axis in renal physiology
Tubular transport of water and sodium
One of the main functions of the kidney is maintenance of water and sodium homeostasis of the body by regulating tubular transport. The roles of the neurohypophyseal peptide hormones arginine vasopressin (AVP) or anti-diuretic hormone (ADH) in regulating the water homeostasis, and the mineralocorticoid hormone aldosterone, in regulating sodium homeostasis are well known [51, 52]. In recent years, it has been established that extracellular nucleotides, acting through P2Y2 and P2Y12 receptors, also play significant roles in the transport of water and sodium in the kidney, mainly by opposing the actions of one or both hormones on the kidney [13, 16, 18, 20, 21, 23]. We used a transgenic (TG) mouse model globally overexpressing hCD39 including in the kidney, which had elevated tissue and blood levels of adenosine [53] and showed that these mice manifest defective water and sodium handling [42, 54]. Under basal conditions, TG mice exhibited impaired urinary concentrating ability despite normal AVP levels and had impaired AVP release in response to water deprivation. However, TG mice kidneys were responsive to exogenous desmopressin (dDAVP), a selective vasopressin V2 receptor agonist [54]. Thus, ectonucleotidases modulated purinergic signaling impacting urinary concentration. Furthermore, high-salt diet and aldosterone clamping experiments in TG mice conducted by us supported the concept that nucleotides facilitate natriuresis by countering aldosterone effect and also revealed aldosterone-independent down-regulation of major sodium transporters and channel subunits by purinergic signaling [42]. Thus, scavenging of extracellular ATP by overexpression of hCD39 resulted in some unexpected observations.
Tubuloglomerular feedback and renin secretion
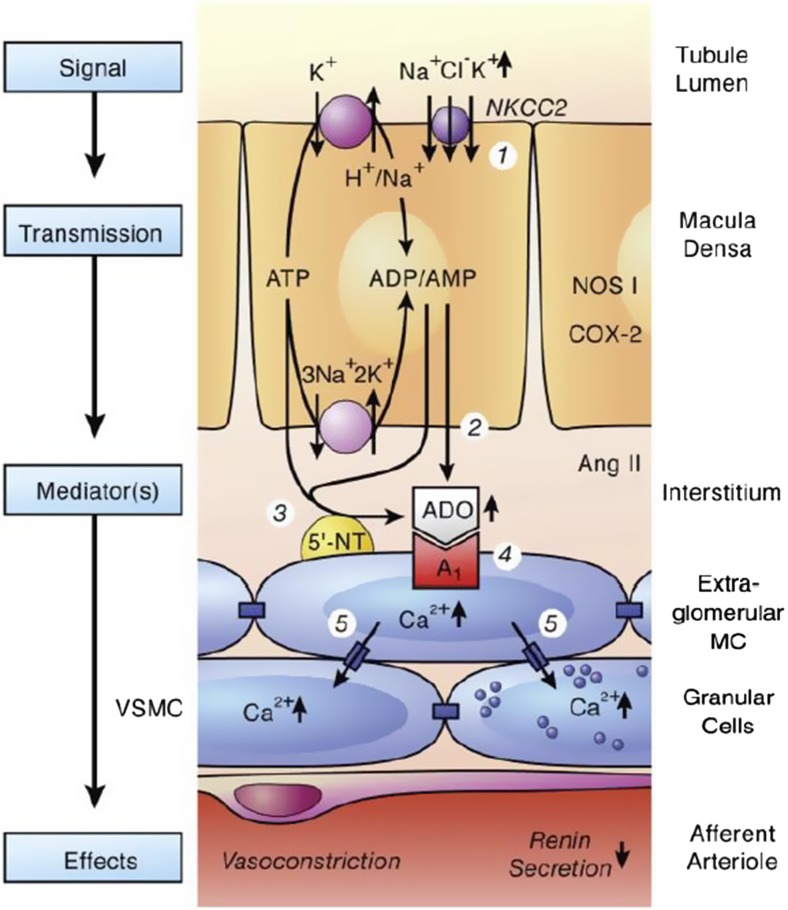
The renal blood flow (RBF) and glomerular filtration rate (GFR) are maintained independent of renal perfusion pressure (RPP) over a defined range (80–180 mmHg). This autoregulation is possible due to two intrarenal mechanisms, namely the myogenic mechanism and the tubuloglomerular feedback (TGF) [55, 56]. TGF operates by sensing of salt concentration of the distal nephron by the macula densa cells of the juxtaglomerular apparatus (JGA), leading to signal transduction to the afferent arteriole thus regulating the GFR. Both ATP and adenosine have been shown to play mediator roles in TGF (Fig. 2). While ATP is the initial signaling molecule [57, 58], adenosine released from the hydrolysis of ATP appears to be the ultimate signaling molecule for TGF [59, 60]. Accordingly, TGF responses were blunted in mice lacking either adenosine A1 receptor [61, 62] or 5′-ectonucleotidase/CD73 [63]. However, it appears that synchronous release of renin from macula dense cells is dependent on ATP-mediated propagation of intra- and intercellular Ca2+ waves through juxtaglomerular cells [64]. Accordingly, it has been shown that maximum TGF responses were reduced in Cd39 null mice, whereas macula densa- and pressure-dependent inhibition of renin secretion remained intact as compared to wild-type mice [66].
Fig. 2.
Suggested role of CD39-adenosinergic axis in the regulation of tubuloglomerular feedback mechanism. Numbers in circles refer to the following sequence of events. 1, Increase in concentration-dependent uptake of Na+, K+, and Cl− via the bumetanide-sensitive Na+-K+-2Cl− co-transporter (NKCC2); 2 and 3, transport-dependent, intra- and/or extracellular generation of adenosine (ADO); the extracellular generation involves ecto-5′-nucleotidase (5′-NT); 4, extracellular ADO activates adenosine A1 receptors triggering an increase in cytosolic Ca2+ in extraglomerular mesangial cells (MC); 5, the intensive coupling between extraglomerular MC, granular cells containing renin, and smooth muscle cells of the afferent arteriole (VSMC) by gap junctions allows propagation of the increased Ca2+ signal resulting in afferent arteriolar vasoconstriction and inhibition of renin release. Factors such as nitric oxide, arachidonic acid breakdown products, or angiotensin (ANG) II modulate the described cascade. NOS 1, neuronal nitric oxide synthase; COX-2, cyclooxygenase-2 (with permission from Vallon et al. [65])
CD39-adenosinergic axis in acute kidney injury
Ischemia reperfusion injury
Ischemia reperfusion injury (IRI) continues to be the major form of acute kidney injury (AKI) in the hospital setting and is associated with significant morbidity and mortality [67]. IRI occurs when there is interruption of blood flow to the kidneys after which blood flow is re-established. Although essential to prevent ongoing ischemic damage, reperfusion triggers a robust inflammation and oxidative stress response, resulting in organ dysfunction [68]. Although the pathophysiology of IRI is not completely understood [69], several studies have documented the role of CD39-adenosinergic axis in IRI [17, 70–74]. As shown in Fig. 3, during IRI, ATP is released from the inflammatory, apoptotic, or necrotic cells into the extracellular space. In IRI occurring in the kidney, liver, bowel, heart, lung, brain, and islet cells, CD39 is the major generator of adenosine [26], which is an innate anti-inflammatory metabolite and tissue protectant, especially during hypoxic conditions, that serves to limit tissue injury. Hypoxia induces the expression of both Cd39 and Cd73, through hypoxia-inducible specificity protein 1 (Sp1) and hypoxia-inducible factor (HIF), respectively. Cd39 is also upregulated within the kidney following ischemic preconditioning, which results in higher pericellular adenosine concentration and less renal IRI [70]. Finally, adenosine itself increases the expression of CD73. As shown in Fig. 3, adenosine mediates its anti-inflammatory effects via A1 receptors on proximal tubular cells (PTC), A2A receptors on T regulatory cells (Treg), and A2B receptor on endothelial cells (EC) and circulating neutrophils. During ischemia, HIF inhibits transcription of equilibrative nucleoside transporter-1 (ENT1) enabling adenosine to remain in the extracellular space (Fig. 3). HIF also increases the expression of A1 and A2B receptors. Sphingosine kinase-1 (SK1) and sphingosine-1-phosphate receptor (S1P1R) in the PTC augment the adenosine-A1R interactions. In the Treg, A2AR activation increases the protein expression of programmed death-1 (PD-1), which suppresses innate immune responses (Fig. 3). Consistent with these mechanisms, IRI in Cd39 null mice in which hydrolysis of ATP is severely diminished [17], or mice treated with POM-1 (polyoxymetalate-1) an inhibitor of Cd39 [70], is severe.
Fig. 3.
CD39-adenosinergic axis in the pathophysiology of ischemia-reperfusion injury. ADO adenosine; Sp1 hypoxia-inducible specificity protein 1; HIF hypoxia-inducible factor; PTC proximal tubule cells; Treg T regulatory cells; EC endothelial cells; ENT1 equilibrative nucleoside transporter; SK1 sphingosine kinase-1; S1P1R sphingosine-1-phosphate receptor; PD-1 programmed-death 1. For further details, refer to the text (modified from Roberts et al. [26] and reproduced with permission)
We have previously shown that transgenic mice overexpressing hCD39 are protected from both warm and cold IRI of the kidneys [71] and IRI of the heart [75]. Furthermore, less severe hepatic injury was observed in donor livers from transgenic mice overexpressing hCD39 transplanted into wild-type recipients after prolonged (18 h) cold storage [76]. Similarly, the administration of apyrase, a soluble form of CD39, which increases tissue levels of adenosine abolished IRI in the kidney [70], heart [77], liver [78, 79], and intestines [80].
In contrast to CD39, the impact of CD73 in renal IRI is variable. Some studies have reported a protective role for CD73 [70, 81], whereas in mild renal IRI, CD73 deficiency or inhibition appears protective [82]. This latter observation may be due to accumulation of AMP, although direct evidence supporting this hypothesis is currently lacking. Interestingly, the volatile anesthetic isoflurane induces CD73 and protects against renal IRI [83].
Adenosine receptors are widely expressed on circulating leukocytes, immune cells, and vascular cells, which all play an important role in IRI. Notably, the A2B receptor has a hypoxia responsive element (hypoxia-inducible factor (HIF)) within the promoter region modifying the expression of the receptor [84]. Indeed, the A2B receptor is upregulated within the kidney as early as 24 h following IRI [85] and it is the expression on the renovasculature which is essential for mitigating IRI [86].
Role of Treg in AKI vis-à-vis CD39-adenosinergic axis
Regulatory T cells (Treg) have an intrinsic reno-protective function. By multiple mechanisms, Tregs suppress inflammation and prevent AKI. Through a series of experiments, Kinsey et al. have shown that depletion of Treg exacerbates renal IRI [87] whereas following ischemic preconditioning, which augments pericellular adenosine concentrations [70, 88], Treg numbers are increased and renal IRI is constrained [89, 90]. The authors went on to show the mechanism underpinning the observed protective effect: adenosine, generated by CD73 on Treg, increased the expression of PD-1 through the A2A receptor, both of which are expressed on Treg [91, 92]. With the advent of Treg therapy to control inflammation, these data may provide a novel therapeutic approach to ameliorate acute kidney injury (AKI) following IRI [93].
Macrophage transformation and inflammation in AKI
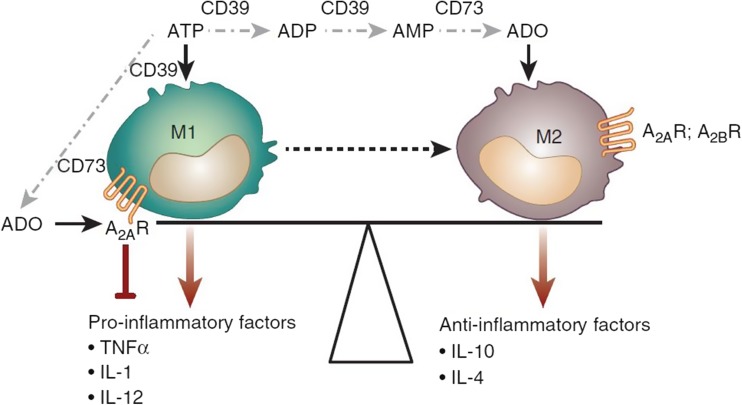
Macrophages are critical mediators and regulators of inflammation in various pathophysiological conditions, including AKI [94, 95]. Macrophage phenotype also controls long-term AKI outcomes—kidney regeneration versus progression to chronic kidney disease (CKD) characterized by fibrosis (see below) [93, 96]. Recent studies revealed that CD39 and CD73 aid in macrophage transformation. M1 macrophages (classically activated macrophages) develop early after renal injury and propagate inflammation by elaborating pro-inflammatory factors. M2 macrophages (alternatively activated macrophages), which appear later, produce anti-inflammatory factors and support renal repair. As shown in Fig. 4, the CD39-adenosinergic axis influences the balance between the M1 and M2 macrophages and thereby inflammation and repair processes in the kidney following injury [26].
Fig. 4.
Role of CD39-adenosinergic axis in macrophage transformation. Adenosine inhibits the expression of pro-inflammatory cytokines by M1 macrophages via A2AR signaling and promotes a shift to the anti-inflammatory M2 phenotype via A2BR signaling. For further details, refer to the text (with permission from Roberts et al. [99])
Renal fibrosis
Fibrosis is a characteristic feature of all forms of chronic kidney disease, culminating in renal failure [97]. Despite significant advances in deciphering the pathophysiological mechanisms of renal fibrosis, mostly derived from animal models [98], there are very few options to prevent or slow the progression of fibrosis in clinical settings. Fibrosis is preceded by inflammation, and although short-term activation of A2A and A2B adenosine receptors decreases inflammation, chronic exposure to adenosine promotes inflammation and fibrosis through A2B receptor [85, 99, 100]. T cells precede the influx of macrophages and can independently promote renal fibrosis. Signaling through A2A receptors inhibits T cell proliferation (Fig. 5). Accordingly, fibrosis is exacerbated in A2A receptor knockout mice [26, 85, 99, 100]. Conversely, chronic signaling through A2B receptor, predominantly expressed on fibroblasts, promotes renal fibrosis [101]. Furthermore, increased signaling through A2B also appears to play a role in the development of tubulointerstitial fibrosis in angiotensin II-treated mice [102]. Indeed, in kidney biopsies from patients with CKD, elevated levels of CD73 and A2B receptor mRNA expression have been demonstrated as compared to patients without CKD [102]. Intriguingly, impaired ability to generate adenosine in CD73 knockout mice results in renal fibrosis by 6 months of age [103].
Fig. 5.
Adenosine signaling via A2A and A2B receptors reduces inflammation, resulting in reduced fibrosis. Left: renal injury promotes macrophage and T-effector cell infiltration that are associated with increased inflammation and fibrosis. Right: adenosine signaling via A2AR and A2BR reduces infiltration of T-effector and M1 macrophages and promotes generation of Treg and M2 macrophages, which are associated with reduced inflammation and less fibrosis. For further details, refer to the text (with permission from Roberts et al. [99])
Adenosine signaling plays a complex role in the development of renal fibrosis following IRI. A2B adenosine receptor activation offers protection acutely, but may contribute to the progression of fibrosis following an episode of ischemia. The A2B receptor is upregulated 24 h following renal IRI which persists for 4 weeks following IRI [85, 99]. Intriguingly, whereas the administration of apyrase or the overexpression of hCD39 confers similar acute protection, the effect on chronic IRI is contrasting. Mice treated with a single dose of apyrase do not develop chronic kidney disease and the upregulation of A2B receptor is blunted. In contrast, hCD39-TG mice develop chronic kidney disease following IRI despite acute protection coincident with increased renal adenosine content [100]. Notably, the whole animal overexpression of human CD39 does not attenuate the development of renal fibrosis in unilateral ureteral obstruction (UUO) model [104] presumably an effect of the underpinning mechanism of fibrosis in this model.
CD39-adenosinergic axis in renal transplantation
Renal transplantation remains the optimal form of renal replacement therapy for patients with end stage renal disease. Despite exceptional short-term survival, long-term survival is limited by the development of chronic allograft dysfunction which in the kidney manifests with interstitial fibrosis and tubular atrophy [105]. Recently, novel approaches have been proposed to improve allograft outcomes [106]. IRI (see above) is an obligatory insult in transplantation, which may lead to delayed graft function which is a risk for acute rejection and long-term graft loss. Intracellular ATP is released in the donor kidney at the time of harvesting due to ischemia and again at the time of vascular anastomosis following reperfusion. Unique to transplantation is a period of cold ischemia occurring between the time of procurement and engraftment. We have shown the expression of CD39 on endothelial cells, monocytes, dendritic cells, Langerhans cells, NK (natural killer) cells, and natural killer T cells [107]. Organs that overexpress CD39 have improved graft function in both kidney [71] and liver [76] mouse transplant models encompassing extended cold preservation. Moreover, several studies during the past two decades indicated that the activity of CD39 influences severity of inflammation and autoimmune response [108–113].
Treg are essential to transplantation tolerance and their therapeutic efficacy is well documented in animal models. CD39 has been identified as a marker of both murine [114, 115] and human Treg [116]. Furthermore, in mice, the generation of adenosine by the concerted actions of CD39 and CD73 is integral to their function and the transfer of Treg from CD39 deficient mice resulted in more rapid rejection of skin grafts [114]. Using the markers of CD4, CD25, and CD39, human Treg can be monitored in patients with end stage renal failure and following renal transplantation with a reduction in Treg number noted during acute transplant rejection [116, 117]. Ex vivo Treg expansion and delivery into renal transplant recipients as a therapy are currently undergoing rigorous study [118]. Figure 6 summarizes our current knowledge about the purinergic protective mechanisms in solid organ transplantation.
Fig. 6.
Protective mechanisms in solid organ transplantation. Extracellular adenosine is generated from the enzymatic hydrolysis of nucleotides by CD39 and CD73 expressed on endothelial cells (EC) and B cells. Adenosine signals via A2AR on circulating cells including regulatory T cells (Treg) and via A2BR expressed both on the vasculature and inflammatory cells. Experimental strategies which improve graft outcome for each solid organ transplant are listed in boxes (with permission from Roberts et al. [125])
CD39-adenosinergic axis in hypertension and diabetic nephropathy
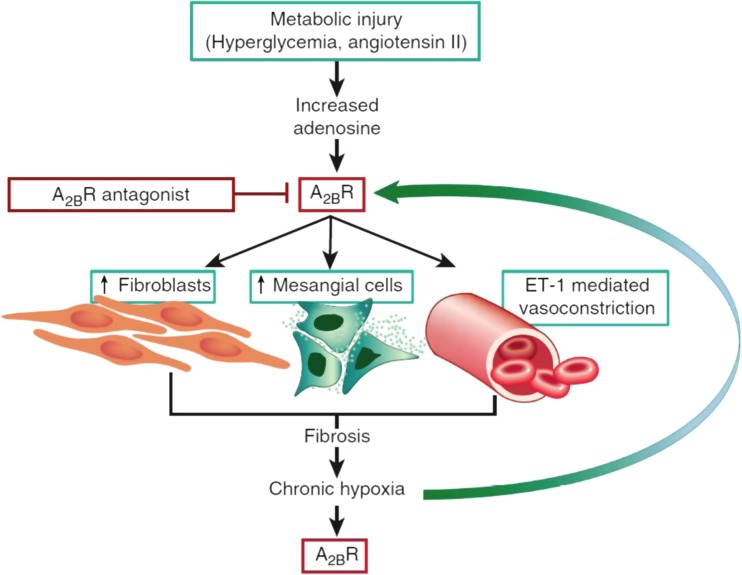
Hypertension and diabetic nephropathy continue to be the major causes of chronic kidney disease (CKD), leading to end-stage renal disease (ESRD) that requires maintenance hemodialysis or renal transplantation. The hydrolysis of adenine nucleotides has been reported to be enhanced in platelets of patients with diabetes and hypertension and was associated with increased expression of CD39. Increasing glucose concentration apparently had a direct effect on ATP hydrolysis [119, 120]. Functional CD39 polymorphism influences the susceptibility to type 2 diabetes mellitus (T2DM) and diabetic nephropathy in African-Americans [121]. Hyperglycemia induced formation of extracellular adenosine in glomeruli and podocytes, apparently due to increased expression of CD73 [122, 123]. Hyperglycemia upregulated expression of HIF-1α [124], which in turn impacted and increased A2B receptor expression in mesangial cells and podocytes [84, 123]. This observation, coupled with increased expression of TGF-β and vascular endothelial growth factor (VEGF) in glomeruli, resulted in the development of glomerulosclerosis. These alterations were reduced by the pharmacological inhibition of A2B receptor (Fig. 7) [99, 125].
Fig. 7.
Experimental models of hypertension and diabetes reveal increased adenosine generation and A2B receptor expression. A2BR activation on fibroblasts and mesangial cells promotes extracellular matrix deposition driving the development of renal fibrosis. Vasoconstriction mediated by endothelin-1 promotes hypoxia which is perpetuated by renal fibrosis. Chronic hypoxia further drives adenosine generation and A2BR activity, creating a vicious cycle of chronic hypoxia and renal fibrosis (with permission from Roberts et al. [99])
Therapeutic implications
The CD39-adenosinergic axis is a potential platform with multiple anchors for the development of novel therapeutic modalities for the treatment or management of renal conditions described above. One potential strategy involves modulation or tilting the axis more toward formation of adenosine relative to the extracellular ATP/ADP concentrations, so that the activity of adenosine or P1 receptors is higher with beneficial effects. This can be achieved by the administration of enzymes that hydrolyze ATP to ADP and AMP, such as soluble engineered ectonucleotidases. Initial studies using a commercially available (APT102) engineered human nucleoside triphosphate diphosphhydrolase-3 (CD39L3) in a canine model of arterial thrombosis were very encouraging [126]. Conversely, various inhibitors of ectonucleotidases patented during the past few years [127], and the availability of therapeutic antibodies that selectively inhibit CD73 [128], widened the scope for modulating the CD39-adenosinergic axis in experimental therapeutics.
The other strategy is to modulate the activity of adenosine or P1 receptor subtypes. In this context, the field of adenosine receptors as drug targets is further advanced with the availability of agonists and/or antagonists for various subtypes [12, 129, 130]. Comparatively, the availability of selective agonists or antagonists of P2 receptors is limited.
Summary points
The CD39-adenosinergic axis is central to the regulation of purinergic signaling in the kidney
The regulated generation of adenosine by CD39 and CD73 has major impacts on renal physiology with respect to water/salt excretion, tubuloglomerular feedback/renin secretion with blood pressure control.
Alterations in the balance of extracellular nucleotides to nucleosides in disease states have major effects on inflammation and impact outcomes of ischemic reperfusion and metabolic stress.
Differential expression of CD39 and CD73 by the vascular, T regulatory cells, and other immunity cells modulates inflammatory and immune reactions in experimental models of renal inflammation, fibrosis, and transplantation.
Strategies for developing therapeutic modalities that can treat or manage kidney diseases by modulating the CD39-adenosinergic axis are available, and they need to be tested in animal models of various kidney diseases.
Acknowledgements
Authors’ work cited in this review has been supported by grants from the US Department of Veterans Affairs (I01BX000596); US Department of Defense Peer Reviewed Medical Research Program (W81XWH-16-1-0464); the National Institutes of Health (R21 DK-081041, P01-HL107152, R21 CA-164970); the National Kidney Foundation of Utah and Idaho, and the resources and facilities at the Veterans Affairs Salt Lake City Health Care System (Salt Lake City, UT).
Compliance with ethical standards
Financial disclosures
BKK received research funding from AstraZeneca, AB; SCR has research funding from Tizona and Boston Biomedical.
Conflict of interest
Bellamkonda K. Kishore declares that he has no conflict of interest.
Simon C. Robson declares that he has no conflict of interest.
Karen M. Dwyer declares that she has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Contributor Information
Bellamkonda K. Kishore, Email: BK.Kishore@hsc.utah.edu
Simon C. Robson, Email: srobson@bidmc.harvard.edu
Karen M. Dwyer, Email: karen.dwyer@deakin.edu.au
References
- 1.Burnstock G. Purinergic signaling: past, present and future. Braz J Med Biol Res. 2009;42(1):3–8. doi: 10.1590/s0100-879x2008005000037. [DOI] [PubMed] [Google Scholar]
- 2.Burnstock G. Introductory overview of purinergic signalling. Front Biosci. 2011;3:896–900. doi: 10.2741/e298. [DOI] [PubMed] [Google Scholar]
- 3.Burnstock G. Purinergic signaling: its unpopular beginning, its acceptance and its exciting future. BioEssays. 2012;34(3):218–225. doi: 10.1002/bies.201100130. [DOI] [PubMed] [Google Scholar]
- 4.Burnstock G. Introduction and perspective, historical note. Front Cell Neurosci. 2013;7:227. doi: 10.3389/fncel.2013.00227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burnstock G. The Paton lecture: purinergic signalling: from discovery to current developments. Exp Physiol. 2014;99(1):16–34. doi: 10.1113/expphysiol.2013.071951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burnstock G. Short- and long-term (trophic) purinergic signalling. Philos Trans R Soc B. 2016;371(1700):20150422. doi: 10.1098/rstb.2015.0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacobson KA. Introduction to adenosine receptors as therapeutic targets. Handb Exp Pharmacol. 2009;193:1–24. doi: 10.1007/978-3-540-89615-9_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacobson KA, Boeynaems JM. P2Y nucleotide receptors: promise of therapeutic applications. Drug Discov Today. 2010;15(13-14):570–578. doi: 10.1016/j.drudis.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fredholm BB. Adenosine receptors as drug targets. Exp Cell Res. 2010;316(4):1284–1288. doi: 10.1016/j.yexcr.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Samsel M, Dzierzbicka K. Therapeutic potential of adenosine analogues and conjugates. Pharmacol Rep. 2011;63(3):601–617. doi: 10.1016/s1734-1140(11)70573-4. [DOI] [PubMed] [Google Scholar]
- 11.Riksen NP, Rongen GA. Targeting adenosine receptors in the development of cardiovascular therapeutics. Expert Rev Clin Pharmacol. 2012;5(2):199–218. doi: 10.1586/ecp.12.8. [DOI] [PubMed] [Google Scholar]
- 12.Chen JF, Eltzschig HK, Fredholm BB. Adenosine receptors as drug targets – what are the challenges? Nat Rev Drug Discov. 2013;12(4):265–286. doi: 10.1038/nrd3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kishore BK, Carlson NG, Ecelbarger CM, Kohan DE, Müller CE, Nelson RD, Peti-Peterdi J, Zhang Y. Targeting renal purinergic signalling for the treatment of lithium-induced nephrogenic diabetes insipidus. Acta Physiol (Oxf) 2015;214(2):176–188. doi: 10.1111/apha.12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jankowski M. Purinergic regulation of glomerular microvasculature and tubular function. J Physiol Pharmacol. 2008;59:121–135. [PubMed] [Google Scholar]
- 15.Wildmann SS, King BF. P2X receptors: epithelial ion channels and regulators of salt and water transport. Nephrol Physiol. 2008;108(3):60–67. doi: 10.1159/000122028. [DOI] [PubMed] [Google Scholar]
- 16.Vallon V. P2 receptors in the regulation of renal transport mechanisms. Am J Physiol Ren Physiol. 2008;294(1):F10–F27. doi: 10.1152/ajprenal.00432.2007. [DOI] [PubMed] [Google Scholar]
- 17.Lu B, Rajakumar SV, Robson SC, Lee EK, Crikis S, d’Apice AJ, Cowan PJ, Dwyer KM. The impact of purinergic signaling on renal ischemia-reperfusion injury. Transplantation. 2008;86(12):1707–1712. doi: 10.1097/TP.0b013e31819022bc. [DOI] [PubMed] [Google Scholar]
- 18.Kishore BK, Nelson RD, Miller RL, Carlson NG, Kohan DE. P2Y2 receptor and water transport in the kidney. Purinergic Signal. 2009;5(4):491–499. doi: 10.1007/s11302-009-9151-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vallon V, Osswald H. Adenosine receptors and the kidney. Handb Exp Pharmacol. 2009;193:443–470. doi: 10.1007/978-3-540-89615-9_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prætorius H, Leipziger J. Intrarenal purinergic signaling in the control of renal tubular transport. Annu Rev Physiol. 2010;72(1):377–393. doi: 10.1146/annurev-physiol-021909-135825. [DOI] [PubMed] [Google Scholar]
- 21.Leipziger J. Luminal nucleotides are tonic inhibitors of renal tubular transport. Curr Opin Nephrol Hypertens. 2011;20(5):518–522. doi: 10.1097/MNH.0b013e3283487393. [DOI] [PubMed] [Google Scholar]
- 22.Laubach VE, French BA, Okusa MD. Targeting of adenosine receptors in ischemia-reperfusion injury. Expert Opin Ther Targets. 2011;15(1):103–118. doi: 10.1517/14728222.2011.541441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vallon V, Rieg T. Regulation of renal NaCl and water transport by the ATP/UTP/P2Y2 receptor system. Am J Physiol Ren Physiol. 2011;301(3):F463–F475. doi: 10.1152/ajprenal.00236.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guan Z, Inscho EW. Role of adenosine 5′-triphosphate in regulating renal microvascular function and in hypertension. Hypertension. 2011;58(3):333–340. doi: 10.1161/HYPERTENSIONAHA.110.155952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Booth JW, Tam FW, Unwin RJ. P2 purinoceptors: renal pathophysiology and therapeutic potential. Clin Nephrol. 2012;78(08):154–163. doi: 10.5414/cn107325. [DOI] [PubMed] [Google Scholar]
- 26.Roberts V, Lu B, Rajakumar S, Cowan PJ, Dwyer KM. The CD39-adenosinergic axis in the pathogenesis of renal ischemia-reperfusion injury. Purinergic Signal. 2013;9(2):135–143. doi: 10.1007/s11302-012-9342-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burnstock G, Evans LC, Bailey MA. Purinergic signalling in the kidney in health and disease. Purinergic Signal. 2014;10(1):71–101. doi: 10.1007/s11302-013-9400-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guan Z, Fellner RC, Van Beusecum J, Inscho EW. P2 receptors in renal autoregulation. Curr Vasc Pharmacol. 2014;12(6):818–828. doi: 10.2174/15701611113116660152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Howarth AR, Conway BR, Bailey MA. Vascular inflammatory actions of P2X receptors in renal injury. Auton Neurosci. 2015;191:135–140. doi: 10.1016/j.autneu.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 30.Peti-Peterdi J, Kishore BK, Pluznick JL. Regulation of vascular and renal function by metabolite receptors. Annu Rev Physiol. 2015;78(1):391–414. doi: 10.1146/annurev-physiol-021115-105403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Beusecum J, Inscho EW. Regulation of renal function and blood pressure control by P2 purinoceptrs in the kidney. Curr Opin Pharmacol. 2015;21:82–88. doi: 10.1016/j.coph.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Franco M, Bautista-Pérez R, Pérez-Méndez O. Purinergic receptors in tubulointerstitial inflammatory cells: a pathophysiological mechanism of salt-sensitive hypertension. Acta Physiol (Oxf) 2015;214(1):75–87. doi: 10.1111/apha.12471. [DOI] [PubMed] [Google Scholar]
- 33.Solini A, Usuelli V, Florina P. The dark side of extracellular ATP in kidney disease. J Am Soc Nephrol. 2015;26(5):1007–1016. doi: 10.1681/ASN.2014070721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Menzies RI, Unwin RJ, Bailey MA. Renal P2 receptors and hypertension. Acta Physiol (Oxf) 2015;213(1):232–241. doi: 10.1111/apha.12412. [DOI] [PubMed] [Google Scholar]
- 35.Menzies RI, Tam FW, Unwin RJ, Bailey MA. Purinergic signaling in kidney disease. Kidney Int. 2017;91(2):315–325. doi: 10.1016/j.kint.2016.08.029. [DOI] [PubMed] [Google Scholar]
- 36.Ilatovskaya DV, Palygin O, Staruschenko A. Functional and therapeutic importance of purinergic signaling in polycystic kidney disease. Am J Physiol Ren Physiol. 2016;311(6):F1135–F1139. doi: 10.1152/ajprenal.00406.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lazarowski ER, Sesma JI, Seminario-Vidal L, Kreda SM. Molecular mechanisms of purine and pyrimidine nucleotide release. Adv Pharmacol. 2011;61:221–261. doi: 10.1016/B978-0-12-385526-8.00008-4. [DOI] [PubMed] [Google Scholar]
- 38.Lohman AW, Isakson BE. Differentiating connexin hemichannels and pannexin channels in cellular ATP release. FEBS Lett. 2014;588(8):1379–1388. doi: 10.1016/j.febslet.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Unwin RJ, Bailey MA, Burnstock G. Purinergic signaling along the renal tubule: the current state of play. News Physiol Sci. 2003;18(6):237–241. doi: 10.1152/nips.01436.2003. [DOI] [PubMed] [Google Scholar]
- 40.Kishore BK, Isaac J, Fausther M, Tripp SR, Shi H, Gill PS, Braun N, Zimmerman H, Sévigny J, Robson SC. Expression of NTPDase1 and NTPDase2 in murine kidney: relevance to regulation of P2 receptor signaling. Am J Physiol Ren Physiol. 2005;288(5):F1032–F1043. doi: 10.1152/ajprenal.00108.2004. [DOI] [PubMed] [Google Scholar]
- 41.Vekaria RM, Shirley DG, Sévigny J, Unwin RJ. Immunolocalization of ectonucleotidases along the rat nephron. Am J Physiol Ren Physiol. 2006;290(2):F550–F560. doi: 10.1152/ajprenal.00151.2005. [DOI] [PubMed] [Google Scholar]
- 42.Zhang Y, Robson SC, Morris KL, Heiney KM, Dwyer KM, Kishore BK, Ecelbarger CM. Impaired natriuretic response to high-NaCl diet plus aldosterone infusion in mice overexpressing human CD39, an ectonucleotidase (NTPDase1) Am J Physiol Ren Physiol. 2015;308(12):F1398–F1408. doi: 10.1152/ajprenal.00125.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Robson SC, Sévigny J, Zimmermann H (2006) The E-NTPDase family of ectonucleotidases: structure function relationship and pathophysiological significance. Purinergic Signal 2(2):409–430. 10.1007/s11302-006-9003-5 [DOI] [PMC free article] [PubMed]
- 44.Schetinger MR, Morsch VM, Bonan CD, Wyse AT. NTPDase and 5′-nucleotidase activities in physiological and disease conditions: new perspectives for human health. Biofactors. 2007;31(2):77–98. doi: 10.1002/biof.5520310205. [DOI] [PubMed] [Google Scholar]
- 45.Yegtkin GG. Nucleotide- and nucleoside-converting ectoenzymes: important modulators of purinergic signaling cascade. Biochim Biophys Acta. 2008;1783(5):673–694. doi: 10.1016/j.bbamcr.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 46.Kukulski F, Levesque SA, Sévigny J. Impact of ectoenzymes on P2 and P1 receptor signaling. Adv Pharmacol. 2011;61(5):263–299. doi: 10.1016/B978-0-12-385526-8.00009-6. [DOI] [PubMed] [Google Scholar]
- 47.Zimmermann H, Zebisch M, Sträter N. Cellular function and molecular structure of ecto-nucleotidases. Purinergic Signal. 2012;8(3):437–502. doi: 10.1007/s11302-012-9309-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kishore BK, Zhang Y, Gevorgyan K, Kohan DE, Schiedel AC, Müller CE, Peti-Peterdi J. Cellular localization of adenine receptors in the rat kidney and their functional significance in the inner medullary collecting duct. Am J Physiol Ren Physiol. 2013;305(9):F1298–F1305. doi: 10.1152/ajprenal.00254.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thimm D, Schiedel AC, Peti-Peterdi J, Kishore BK, Müller CE. The nucleobase adenine as a signalling molecule in the kidney. Acta Physiol (Oxf) 2015;213(4):808–818. doi: 10.1111/apha.12452. [DOI] [PubMed] [Google Scholar]
- 50.Slominska E, Szolkiewicz M, Smolenski RT, Rutkowski B, Swierczynski J. High plasma adenine concentration in chronic renal failure and its relation to erythrocyte ATP. Nephron. 2002;91:286–291. doi: 10.1159/000058406. [DOI] [PubMed] [Google Scholar]
- 51.Knepper MA, Kwon TH, Nielsen S. Molecular physiology of water balance. N Engl J Med. 2015;372(14):1349–1358. doi: 10.1056/NEJMra1404726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shibata S. 30 years of mineralocorticoid receptor: mineralocorticoid receptor and NaCl transport mechanisms in the renal distal nephrol. J Endocrinol. 2017;234(1):T35–T47. doi: 10.1530/JOE-16-0669. [DOI] [PubMed] [Google Scholar]
- 53.Dwyer KM, Robson SC, Nandurkar HH, Campbell DJ, Gock H, Murray-Segal LJ, Fisicaro N, Mysore TB, Kaczmarek E, Cowan PJ, d’Apice AJ. Thromboregulatory manifestations in human CD transgenic mice and the implications for thrombotic disease and transplantation. J Clin Invest. 2004;113(10):1440–1446. doi: 10.1172/JCI19560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang Y, Morris KL, Sparrow SK, Dwyer KM, Enjyoji K, Robson SC, Kishore BK. Defective renal water handling in transgenic mice over-expressing human CD39/NTPDase1. Am J Physiol Ren Physiol. 2012;303(3):F420–F430. doi: 10.1152/ajprenal.00060.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Calström M, Wilcox CS, Arendshors WJ. Renal autoregulation in health and disease. Physiol Rev. 2015;95(2):405–511. doi: 10.1152/physrev.00042.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ferenbach DA, Bonventre JV. Kidney tubules: intertubular, vascular, and glomerular cross-talk. Curr Opin Nephrol Hypertens. 2016;25(3):194–202. doi: 10.1097/MNH.0000000000000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nishiyama A, Navar LG. ATP mediates tubuloglomerular feedback. Am J Phys Regul Integr Comp Phys. 2002;283(1):R273–R275. doi: 10.1152/ajpregu.00071.2002. [DOI] [PubMed] [Google Scholar]
- 58.Bell PD, Komlos P, Zhang ZR. ATP as a mediator of macula densa cell signaling. Purinergic Signal. 2009;5(4):461–471. doi: 10.1007/s11302-009-9148-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Franco M, Bell PD, Navar LG. Effect of adenosine A1 analogue on tubuloglomerular feedback mechanism. Am J Physiol Ren Physiol. 1989;257:F231–F236. doi: 10.1152/ajprenal.1989.257.2.F231. [DOI] [PubMed] [Google Scholar]
- 60.Brown R, Ollerstam A, Johansson B, Skøtt O, Gebre-Medhin S, Fredholm B, Persson AE. Abolished tubuloglomerular feedback and increased plasma renin in adenosine A1 receptor-deficient mice. Am J Phys Regul Integr Comp Phys. 2001;281(5):R1362–R1327. doi: 10.1152/ajpregu.2001.281.5.R1362. [DOI] [PubMed] [Google Scholar]
- 61.Sun D, Samuelson LC, Yang T, Huang Y, Paliege A, Saunders T, Briggs J, Schnermann J. Mediation of tubuloglomerular feedback by adenosine: evidence from mice lacking adenosine 1 receptors. Proc Natl Acad Sci U S A. 2001;98(17):9983–9988. doi: 10.1073/pnas.171317998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li L, Lai EY, Huang Y, Eisner C, Mizel D, Wilcox AC, Schnermann J. Renal afferent arteriolar and tubuloglomerular feedback reactivity in mice with conditional deletions of adenosine 1 receptors. Am J Physiol Ren Physiol. 2012;303(8):F1166–F1176. doi: 10.1152/ajprenal.00222.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Castrop H, Huang Y, Hashimoto S, Mizel D, Hansen P, Theilig F, Bachmann S, Deng C, Briggs J, Schnermann J. Impairment of tubuloglomerular feedback regulation of GFR in ecto-5′-nucleotidase/CD73-deficient mice. J Clin Invest. 2004;114(5):634–642. doi: 10.1172/JCI21851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yao J, Suwa M, Li B, Kawamura K, Morioka T, Oite T. ATP-dependent mechanism for coordination of intercellular Ca2+ signaling and renin secretion in rat juxtaglomerular cells. Circ Res. 2003;93(4):338–345. doi: 10.1161/01.RES.0000086802.21850.5D. [DOI] [PubMed] [Google Scholar]
- 65.Vallon V, Mühlbauer B, Osswald H (2006) Adenosine and kidney function. Physiol Rev 86(3):901-40. 10.1152/physrev.00031.2005 [DOI] [PubMed]
- 66.Oppermann M, Friedman DJ, Faulhaber-Walter R, Mizel D, Castrop H, Enjyoji KE, Robson SC, Schnermann J. Tubuloglomerular feedback and renin secretion in NTPDase1/CD39-deficient mice. Am J Physiol Ren Physiol. 2008;294(4):F965–F970. doi: 10.1152/ajprenal.00603.2007. [DOI] [PubMed] [Google Scholar]
- 67.Koyner JL, Ceda J, Goldstein SL, Jaber BL, Liu KD, Shea JA, Faubel S, Acute Kidney Injury Advisory Group of the American Society of Nephrology The daily burden of acute kidney injury: a survey of U.S. nephrologists on world kidney day. Am J Kidney Dis. 2014;64:394–401. doi: 10.1053/j.ajkd.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 68.Malek M, Nematbacksh M. Renal ischemia/reperfusion injury: from pathophysiology to treatment. J Renal Inj Prev. 2015;4(2):20–27. doi: 10.12861/jrip.2015.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zuk A, Bonventre JV. Acute kidney injury. Annu Rev Med. 2016;67(1):293–307. doi: 10.1146/annurev-med-050214-013407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Grenz A, Zhang H, Hermes M, Eckle T, Klingel K, Huang DY, Müller CE, Robson SC, Osswald H, Eltzschig HK. Contribution of E-NTPDase1 (CD39) to renal protection from ischemia-reperfusion injury. FASEB J. 2007;21(11):2863–2873. doi: 10.1096/fj.06-7947com. [DOI] [PubMed] [Google Scholar]
- 71.Crikis S, Ly B, Murray-Segal LM, Selan C, Robson SC, D’Apice AJ, Nandurkar HH, Cowan PJ, Dwyer KM. Transgenic overexpression of CD39 protects against renal ischemia-reperfusion and transplant vascular injury. Am J Transplant. 2010;10(12):2586–2595. doi: 10.1111/j.1600-6143.2010.03257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vincent IS, Okusa MD. Adenosine 2A receptors in acute kidney injury. Acta Physiol (Oxf) 2015;214(3):303–310. doi: 10.1111/apha.12508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rabadi MM, Lee HT. Adenosine receptors and renal ischemia reperfusion injury. Acta Physiol (Oxf) 2015;213(1):222–231. doi: 10.1111/apha.12402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sung SJ, Li L, Huang L, Lawler J, Ye H, Ronin DL, Vincent IS, Le TH, Yu J, Göridt N, Schrader J, Okusa MD. Proximal tubule CD73 is critical in renal ischemia-reperfusion injury protection. J Am Soc Nephrol. 2016;28(3):888–902. doi: 10.1681/ASN.2016020229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cai M, Huttinger ZM, He H, Zhang W, Li F, Goodman LA, Wheeler DG, Druhan LJ, Zweler JL, Dwyer KM, He G, d’Apice AJF, Robson SC, Cowan PJ, Gumina RJ. Transgenic over expression of ectonucleotide triphosphate diphosphohydroase-1 protects against murine myocardial ischemic injury. J Mol Cell Cardiol. 2011;51(6):927–935. doi: 10.1016/j.yjmcc.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pommey S, Lu B, McRae J, Stagg J, Hill P, Salvaris E, Robson SC, d’Apice AJF, Cowan PJ, Dwyer KM. Liver grafts from CD39-overexpressing rodents are protected from ischemia reperfusion injury due to reduced numbers of resident CD4+ T cells. Hepatology. 2013;57(4):1597–1606. doi: 10.1002/hep.25985. [DOI] [PubMed] [Google Scholar]
- 77.Eltizschig HK, Köhler D, Eckle T, Kong T, Robson SC, Colgan SP. Central role of Sp1-regulated CD39 in hypoxia/ischemia protection. Blood. 2009;113(1):224–232. doi: 10.1182/blood-2008-06-165746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sun X, Imai M, Nowak-Machen M, Guckelberger O, Enjyoji K, Wu Y, Khalpey Z, Berberat P, Munasinghe J, Robson SC. Liver damage and systemic inflammatory responses are exacerbated by the genetic deletion of CD39 in total hepatic ischemia. Purinergic Signal. 2011;7(4):427–434. doi: 10.1007/s11302-011-9239-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hart ML, Gorzolla IC, Schittenhelm J, Robson SC, Eltschig HK. SP1-dependent induction of CD39 facilitates hepatic ischemic preconditions. J Immunol. 2010;184(7):4017–4024. doi: 10.4049/jimmunol.0901851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Guckelberger O, Sun XF, Sévigny J, Imai M, Kaczmarek E, Enjyoji K, Kruskal JB, Robson SC. Beneficial effects of CD39/ecto-nucleoside triphosphate diphosphohydrolase-1 in murine intestinal ischemia-reperfusion injury. Thromb Haemost. 2004;91:576–586. doi: 10.1160/TH03-06-0373. [DOI] [PubMed] [Google Scholar]
- 81.Jian R, Sun Y, Wang Y, Yu J, Zhong L, Zhou P. CD73 protects kidney from ischemia-reperfusion injury through reduction of free radicals. Acta Pathol Microbiol Immunol Scand. 2011;120(2):130–138. doi: 10.1111/j.1600-0463.2011.02827.x. [DOI] [PubMed] [Google Scholar]
- 82.Rajakumar SV, Lu B, Crikis S, Robson SC, d’Apice AJ, Cowan PJ, Dwyer KM. Deficiency or inhibition of CD73 protects in mild kidney ischemia-reperfusion injury. Transplantation. 2010;90(12):1260–1264. doi: 10.1097/TP.0b013e3182003d9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kim M, Ham A, Kim JY, Brown KM, D’Agati V, Lee HT. The volatile anesthetic isoflurane induces ecto-5′-nucleotidases (CD73) to protect against renal ischemia and reperfusion injury. Kidney Int. 2013;84(6):90–103. doi: 10.1038/ki.2013.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kong T, Westerman KA, Faigle M, Eltzschig HK, Colgan SP. HIF-dependent induction of adenosine A2B receptor in hypoxia. FASEB J. 2006;20(13):2242–2250. doi: 10.1096/fj.06-6419com. [DOI] [PubMed] [Google Scholar]
- 85.Roberts V, Lu B, Dwyer KM, Cowan PJ. Adenosine receptor expression in the development of renal fibrosis following ischemic injury. Transplant Proc. 2014;46(10):3257–3261. doi: 10.1016/j.transproceed.2014.09.151. [DOI] [PubMed] [Google Scholar]
- 86.Grenz A, Osswald H, Eckle T, Yang D, Zhang H, Tran ZV, Klingel K, Ravid K, Eltzschig HK. The reno-vascular A2B adenosine receptor protects the kidney from ischemia. PLoS Med. 2008;5(6):e137. doi: 10.1371/journal.pmed.0050137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kinsey GR, Sharma R, Huang L, Li L, Vergis AL, Ye H, ST J, Okusa MD. Regulatory T cells suppress innate immunity in kidney ischemia-reperfusion injury. J Am Soc Nephrol. 2009;20(8):1744–1753. doi: 10.1681/ASN.2008111160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zimmerman MA, Kam I, Eltzschig H, Grenz A. Biological implications of extracellular adenosine in hepatic ischemia and reperfusion injury. Am J Transplant. 2013;13(10):2524–2529. doi: 10.1111/ajt.12398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kinsey GR, Huang L, Vergis AL, Li L, Okusa MD. Regulatory T cells contribute to the protective effect of ischemic preconditioning in the kidney. Kidney Int. 2010;77(9):771–780. doi: 10.1038/ki.2010.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kinsey GR, Okusa MD. Expanding role of T cells in acute kidney injury. Curr Opin Nephrol Hypertens. 2013;23(1):9–16. doi: 10.1097/01.mnh.0000436695.29173.de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kinsey GR, Huang L, Jaworska K, Khutsishivili K, Becker DA, Ye H, Lobo PI, Okusa MD. Autocrine adenosine signaling promotes regulatory T cell-mediated renal protection. J Am Soc Nephol. 2012;23(9):1528–1537. doi: 10.1681/ASN.2012010070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kinsey GR, Sharma R, Okusa MD. Regulatory T cells in AKI. J Am Soc Nephrol. 2013;24(11):1720–1726. doi: 10.1681/ASN.2013050502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kinsey GR. Macrophage dynamics in AKI to CKD progression. J Am Soc Nephrol. 2014;25:207–215. doi: 10.1681/ASN.2013101110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liu YC, Zou XB, Chai YF, Yao YM. Macrophage polarization in inflammatory diseases. Int J Biol Sci. 2014;10(5):520–529. doi: 10.7150/ijbs.8879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cao Q, Harris DC, Wang Y. Macrophages in kidney injury, inflammation, and fibrosis. Physiology (Bethesda) 2015;30(3):183–194. doi: 10.1152/physiol.00046.2014. [DOI] [PubMed] [Google Scholar]
- 96.Lech M, Gröbmayr R, Ryu M, Lorenz G, Hartter I, Mulay SR, Susanti HE, Kobayashi KS, Flavell RA, Anders HJ (2014) Macrophage phenotype controls long-term AKI outcomes – kidney regeneration versus atrophy. J Am Soc Nephrol 25(2):292–304. 10.1681/ASN/2013020152 [DOI] [PMC free article] [PubMed]
- 97.Duffiled JS. Cellular and molecular mechanisms of kidney fibrosis. J Clin Invest. 2014;124(6):2299–2306. doi: 10.1172/JCI72267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nogueira A, Pires MJ, Oliveira PA. In vivo. 2017. Pathophysiological mechanisms of renal fibrosis: a review of animal models and therapeutic strategies; pp. 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Roberts VS, Cowan PJ, Alexander SI, Robson SC, Dwyer KM. The role of adenosine receptors A2A and A2B signaling in renal fibrosis. Kidney Int. 2014;86(4):685–692. doi: 10.1038/ki.2014.244. [DOI] [PubMed] [Google Scholar]
- 100.Roberts V, Campbell DJ, Lu B, Chia J, Cowan PJ, Dwyer KM. The differential effect of apyrase treatment and hCD39 overexpression on chronic renal fibrosis following ischemia reperfusion injury. Transplantation. 2017;101(2017 Feb 14):e194–e204. doi: 10.1097/TP.0000000000001679. [DOI] [PubMed] [Google Scholar]
- 101.Dai Y, Zhang W, Wen J, Zhang Y, Kellems RE, Xia Y. A2B adenosine receptor-mediated induction of IL-6 promotes CKD. J Am Soc Nephrol. 2011;22(5):890–901. doi: 10.1681/ASN.2010080890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang W, Zhang Y, Wang W, Dai Y, Ning C, Luo R, Sun K, Glover L, Grenz A, Sun H, Tao L, Zhang W, Colgan SP, Blackburn MR, Eltzschig HK, Kellems RE, Xia Y. Elevated ecto-5′-nucleotidase-mediated increased renal adenosine signaling via A2B adenosine receptor contributes to chronic hypertension. Circ Res. 2013;112(11):1466–1478. doi: 10.1161/CIRCRESAHA.111.300166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ozüyaman B, Ding Z, Buchheiser A, Koszaika P, Braun N, Godecke A, Decking UK, Zimmermann H, Schrader J. Adenosine produced via the CD73/ecto-5′-nucleotidase pathway has no impact on erythropoietin production but is associated with reduced kidney weight. Pflugers Arch. 2006;452(3):324–331. doi: 10.1007/s00424-006-0045-x. [DOI] [PubMed] [Google Scholar]
- 104.Roberts V, Lu B, Chia J, Cowan PJ, Dwyer KM. CD39 overexpression does not attenuate renal fibrosis in the unilateral ureteric obstructive model of chronic kidney disease. Purinergic Signal. 2016;12(4):653–660. doi: 10.1007/s11302-016-9528-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lea-Henry T, Chacko B (2017) Management considerations in the failing renal allograft. Nephrology (Carlton). 10.1111/nep.13165 [DOI] [PubMed]
- 106.Malvezzi P, Rostaing L. Renal transplantation in 2016. Novel approaches to improve recipient and allograft outcomes. Nat Rev Nephrol. 2017;13(2):73–74. doi: 10.1038/nrneph.2016.190. [DOI] [PubMed] [Google Scholar]
- 107.Koziak K, Sévigny J, Robson SC, Siegel JB, Kaczmarek E. Analysis of CD39/ATP disphosphohydrolase (ATPDase) expression in endothelial cells, platelets and leukocytes. Thromb Haemost. 1999;82(5):1538–1544. [PubMed] [Google Scholar]
- 108.Robson SC, Wu Y, Sun X, Knosalla C, Dwyer K, Enjyoji K. Ectonucleotidases of CD39 family modulate vascular inflammation and thrombosis in transplantation. Semin Thromb Hemost. 2005;31(02):217–233. doi: 10.1055/s-2005-869527. [DOI] [PubMed] [Google Scholar]
- 109.Atkins B, Dwyer K, Enjyoji K, Robson SC. Ecto-nucleotidases of the CD39/NTPDase family modulate platelet activation and thrombus formation: potential therapeutic targets. Blood Cells Mol Dis. 2006;36(2):217–222. doi: 10.1016/j.bcmd.2005.12.025. [DOI] [PubMed] [Google Scholar]
- 110.Beldi G, Enjyoji K, Wu Y, Miller L, Banz Y, Sun X, Robson SC. The role of purinergic signaling in the liver and in transplantation: effects of extracellular nucleotides on hepatic graft vascular injury, rejection and metabolism. Front Biosci. 2008;13:2588–2603. doi: 10.2741/2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Salcido-Ochoa F, Tsang J, Tam P, Falk K, Rotzschke O. Regulatory T cells in transplantation: does extracellular adenosine triphosphate metabolism through CD39 play a crucial role? Transplant Rev. 2010;24(2):52–66. doi: 10.1016/j.trre.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 112.Scalea J, Hanecamp I, Robson SC, Yamada K. T-cell-mediated immunological barriers to xenotransplantation. Xenotransplantation. 2010;19:23–30. doi: 10.1111/j.1399-3089.2011.00687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chernogorova P, Zeiser R. Ectonucleotidases in solid organ and allogenic hematopoietic cell transplantation. J Biomed Biotechnol. 2012;2012:208204. doi: 10.1155/2012/208204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, Chen JF, Enjyoji K, Linden J, Oukka M, Kuchroo VK, Strom TB, Robson SC. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;11(6):1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Borsellino G, Kleinewietfield M, Di Mitri D, Sternjak A, Diamantini A, Giometto R, Höpner S, Centronze D, Bernard D, Dell’Acqua ML, Rossini PM, Battistini L, Rötzschke O, Falk S. Expression of ectonucleotidase CD39 by Fox3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood. 2007;110(4):1225–1232. doi: 10.1182/blood-2006-12-064527. [DOI] [PubMed] [Google Scholar]
- 116.Dwyer KM, Hanidziar D, Putheti P, Hill PA, Pommey S, McRae JI, Wingerhalter A, Doherty G, Deaglio S, Koulmanda M, Gao W, Robson SC, Strom TB. Expression of CD39 by human peripheral blood CD+ CD25+ T cells denotes a regulatory memory phenotype. Am J Transplant. 2010;10(4):2410–2420. doi: 10.1111/j.1600-6143.2010.03291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.McRae JL, Chia JS, Pommey SA, Dwyer KM (2016, 2016) Evaluation of CD4+ T cell populations in peripheral blood of patients following kidney transplantation and during acute allograft rejection. Nephrology. 10.1111/nep12894 [DOI] [PubMed]
- 118.Tang Q, Bleustone JA (2013) Regulatory T-cell therapy in transplantation: moving to the clinic. Cold Spring Harb Perpspect Med 3(11). 10.1101/cshperpect.1015552 [DOI] [PMC free article] [PubMed]
- 119.Lunkes GI, Lunkes D, Stefanello F, Morsch A, Morsch VM, Mazzanti CM, Schetinger MR. Enzymes that hydrolyze adenine nucleotides in diabetes and associated pathologies. Thromb Res. 2003;109:189–194. doi: 10.1016/s0049-3848(03)00178-6. [DOI] [PubMed] [Google Scholar]
- 120.Lunkes GI, Lunkes DS, Leal D, Araujo Mdo C, Correa M, Becker L, Rosa CS, Morsch VM, Schetinger MR. Effect of high glucose levels in human platelet NTPDase and 5′-nucleotidase activities. Diabetes Res Clin Pract. 2008;81(3):351–357. doi: 10.1016/j.diabres.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 121.Friedman DJ, Talbert ME, Bowden DW, Freedman BI, Mukanya Y, Enjyoji K, Robson SC. Functional ENTPD1 polymorphisms in African Americans with diabetes and end-stage renal disease. Diabetes. 2009;58(4):999–1006. doi: 10.2337/db08-1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Karczewska J, Piwkowska A, Rogacka D, Stepinski J, Angielski S, Jankowsko M. Purinergic modulation of glucose uptake into cultured rat podocytes: effect of diabetic milieu. Biochem Biophys Res Commun. 2011;404(2):723–727. doi: 10.1016/j.bbrc.2010.12.051. [DOI] [PubMed] [Google Scholar]
- 123.Roa H, Gaiardo C, Troncoso E, Fuentealba V, Escudero C, Yañez A, Sobrevia L, Pastor-Anglada M, Quezada C, San Martin R. Adenosine mediates transforming growth factor-beta 1 release in kidney glomeruli of diabetic rats. FEBS Lett. 2009;583(19):3192–3198. doi: 10.1016/j.febslet.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 124.Makino H, Miyamoto Y, Sawai K, Mori K, Mukoyama M, Nakao K, Yoshimasa Y, Suga S. Altered gene expression related to glomerulogenesis and podocyte structure in early diabetic nephropathy of db/db mice and its restoration by pioglitazone. Diabetes. 2006;55(10):2747–2756. doi: 10.2337/db05-1683. [DOI] [PubMed] [Google Scholar]
- 125.Roberts V, Stagg J, Dwyer KM (2014) The role of ectonucleotidases CD39 and CD73 and adenosine signaling in solid organ transplantation. Front Immunol 5. 10.3389/fimmun.2014.00064 [DOI] [PMC free article] [PubMed]
- 126.Moeckel D, Jeong SS, Sun X, Broekman M, Nguyen A, Dorospouls JHF, Marcus AJ, Robson SC, Chen R, Abendschein D. Optimizing human apyrase to treat arterial thrombosis and limit reperfusion injury without increasing bleeding risk. Sci Tranl Med. 2014;6:248ra105. doi: 10.1126/scitranslmed.3009246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Al-Rashida M, Qazi SU, Batool N, Hameed A, Iqbal J (2017) Ectonucleotidase inhibitors: a patent review (2011-2016). Expert Opin Ther Pat:1–14. 10.1080/13543776.1369958 [DOI] [PubMed]
- 128.Geoghegan JC, Diedrich G, Lu X, Rosenthal K, Sachsenmeier KF, Wu H, Dall’Acqua WF, Damschroder MM. Inhibition of CD73 AMP hydrolysis by a therapeutic antibody with a dual, non-competitive mechanism of action. mAbs. 2016;8:454–467. doi: 10.1080/19420862.2016.1143182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Jacobson KA, Goa ZG. Adenosine receptors as therapeutic targets. Nat Rev Drug Dis. 2006;5(3):247–264. doi: 10.1038/nrd1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sachdeva S, Gupta M. Adenosine and its receptors as therapeutic targets: an overview. Saudi Pharma J. 2013;21:245–253. doi: 10.1016/j.jsps.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]