Abstract
Diabetes mellitus is characterized by increased levels of reactive oxygen species (ROS), leading to high levels of adenosine triphosphate (ATP) and the activation of purinergic receptors (P2X7), which results in cell death. Klotho was recently described as a modulator of oxidative stress and as having anti-apoptotic properties, among others. However, the roles of P2X7 and klotho in the progression of diabetic nephropathy are still unclear. In this context, the aim of the present study was to characterize P2X7 and klotho in several stages of diabetes in rats. Diabetes was induced in Wistar rats by streptozotocin, while the control group rats received the drug vehicle. From the 1st to 8th weeks after the diabetes induction, the animals were placed in metabolic cages on the 1st day of each week for 24 h to analyze metabolic parameters and for the urine collection. Then, blood samples and the kidneys were collected for biochemical analysis, including Western blotting and qPCR for P2X7 and klotho. Diabetic rats presented a progressive loss of renal function, with reduced nitric oxide and increased lipid peroxidation. The P2X7 and klotho expressions were similar up to the 4th week; then, P2X7 expression increased in diabetes mellitus (DM), but klotho expression presented an opposite behavior, until the 8th week. Our data show an inverse correlation between P2X7 and klotho expressions through the development of DM, which suggests that the management of these molecules could be useful for controlling the progression of this disease and diabetic nephropathy.
Keywords: Diabetes mellitus, Oxidative stress, Purinergic receptor, Klotho, Kidneys
Introduction
In 1978, Geoffrey Burnstock [1] proposed the terms P1 for adenosine-active receptors and P2 for ATP receptors. Later, in 1986, his studies with Kennedy [2] showed evidence of a subclassification of the P2 receptors into the P2X and P2Y subtypes: P2X receptors are trimeric ion channels that are formed by seven members, and P2X7 highlights the P2X family due to the long C terminal, which was attributed to most of the biological functions of this receptor. P2X7 when rapidly activated by ATP is permeable to cations, and when it is exposed to prolonged concentrations of ATP, many non-selective pores open in the cell membrane, allowing for the passage of hydrophilic molecules of 0.9 kDa in size. For these reasons, P2X7 can lead to cell death by apoptosis or the necrosis pathway [3, 4].
Although ATP was initially identified as an energy molecule, it can exert other biologic roles: ATP is physiologically synthesized by mitochondria and exits the cell by exocytosis into vesicles [5, 6]. However, it is known that in pathologic conditions, such as those in diabetes mellitus (DM), extracellular ATP increases significantly due to oxidative stress [7]. Rucker and Cols [8] showed that macrovascular and microvascular complications from diabetes reduce the enzyme activity of ectonucleotidases that catalyze the conversion of ATP to adenosine, maintaining increased levels of extracellular ATP.
All of these characteristics make DM a key disease that, in addition to activating purinergic receptors through high levels of ATP, also anticipates aging, which in turn is believed to be caused by molecular damage due to the accumulation of reactive species of oxygen (ROS) that originate from mitochondrial dysfunction [9]. Not all diabetics develop diabetic nephropathy, and approximately 30–40% of diabetic patients progress to nephropathy with sustained microalbuminuria [10]. This condition, known as an early marker of diabetic nephropathy, leads to glomerular hyperfiltration [11], and many studies have shown the contribution of the purinergic system to these changes.
Adenosine may produce vasoconstriction in afferent arterioles by the A1 receptor or vasodilation in efferent arterioles by the A2 receptor [12]. The study by Sällström et al. [13] showed that the absence of the A1 adenosine receptor leads to the development of diabetes-induced glomerular hyperfiltration, suggesting the protective effects of adenosine on the kidney and, in turn, adenosine signaling also mediates the fibrotic phenotype in epithelial tubules. Thus, in this case, adenosine receptor blockers may be useful against fibrosis [14]. Tubulointerstitial inflammation plays a key role in the development of diabetic nephropathy, and the P2X4 receptor was related to the initiation of this procedure with the activation of the NOD 3 (non-obese diabetic 3) receptor inflammasome [15]. Menzie et al. [16] demonstrate that a P2X7 inhibitor can decrease the diabetic damages renal in the nephropathy progression.
Renal disease presents novel effects. Among them is the suppression of the klotho transcription highlight itself because the renal tissue is the main expression center of this protein. Klotho is a single-pass transmembrane protein, and klotho may be cleaved and released into the blood to become a soluble form [17]. The two forms of the klotho protein, the membrane klotho and secreted klotho are reduced in renal tissue in early diabetic nephropathy [18, 19]. Klotho was initially related with longevity, but currently, many studies attribute it to its anti-inflammatory action [20], phosphaturia modeling [21], stabilization of the ion channel of the cell surface, and anti-apoptotic properties [22], reduction in oxidative stress [23], and production of nitric oxide [24].
Studies by Vergani et al. [25, 26] showed that P2X members, mainly P2X7, should be related to the inflammatory procedure in cytokine production. Additionally, a previous study in our laboratory showed that this P2X7 receptor was related to nitric oxide and oxidative stress, as this receptor was upregulated at the 8th week of diabetes when renal tissue presented characteristics of damage and nephropathy [27]. The time at which P2X7 begins to significantly express and suppress klotho is unknown. In this context, the present study aimed to characterize the P2X7 and klotho expressions in the progression of diabetic nephropathy.
Materials and methods
Animals
Male Wistar rats (n = 100) that were 7 weeks of age and weighed an average of 210 g were obtained from the Central Animal Housing of Escola Paulista de Medicina. The rats were kept at a controlled temperature of 22 ± 2 °C in an environment with a regular period of light and dark cycle of 12:12 h and with standard chow and water ad libitum. The protocol was approved by the Ethics Committee in Research of Universidade Federal de Sao Paulo under protocol #2056100314.
Surgical procedure
When the animals were 8 weeks of age, they were anesthetized with ketamine chloridrate (67 mg/kg, i.m.; Dopalen®, Sesp, Sao Paulo, Brazil) and xylazine chloridrate (9 mg/kg, i.m.; Xilazina®, Rhobiofarma, Sao Paulo, Brazil), and the left kidney of each animal was removed. The unilateral nephrectomy had the aim of accelerating the diabetic nephropathy. All of the procedures were performed under sterile conditions, and the animals were left resting afterwards. In the recovery period, non-steroidal opioid meloxicam (2 mg/kg, s.c.) (Maxicam®, Sao Paulo, Brazil) was administered soon after the procedure once per day for 2 days [28].
Induction of DM
When the animals were 9 weeks of age, half of the animals received a simple administration of streptozotocin (Sigma-Aldrich, Sao Paulo, Brazil) dissolved in 0.1 M citrate buffer at pH 4.5 in the tail vein (60 mg/kg i.v.), while the other half (control animals) received the drug vehicle. Diabetes was confirmed 48 h after induction and was defined as a fasting glycemia level above 200 mg/dL. Animals that failed this criterion were excluded.
Metabolic profile
All of the animals were placed in metabolic cages (Tecniplast, Italy) for 24 h, receiving water and chow ad libitum, after the confirmation of diabetes and from the 1st to 8th weeks of the protocol; diuresis, water, and food intake were recorded on the 1st day of each week. At the end of each week, we collected 24 h urine samples and a small aliquot of blood from the retro-orbital plexus under anesthesia, after 3 h of fasting (on the same day). The urine and plasma samples were stored at − 20 °C for further analysis.
Euthanasia
At the end of each week of the protocol, the animals were euthanized with a high dose of anesthetic (90 mg/kg ketamine chloridrate at and 18 mg/kg xylazine chloridrate, both i.m.), and an incision was made in the diaphragm. The cut was done in the abdominal region, and the kidney was removed and stored in the freezer at a temperature of − 80 °C for preparation for the nitric oxide (NO), thiobarbituric acid reactive substances (TBARS), and klotho and P2X7 receptor protein analyses and expression determinations.
Renal function
The plasma and urinary levels of creatinine were measured by colorimetric assay using a Labtest Creatinine kit (Centerlab Ltda, Sao Paulo, Brazil). The plasma levels of urea were measured using a Labtest Urea CE kit (Centerlab Ltda, Sao Paulo, Brazil). The proteinuria was measured by a Sensiprot Labtest kit (Centerlab Ltda, Sao Paulo, Brazil).
NO measurement
The NO was measured in the plasma, urine, and renal cortex samples by chemiluminescence using the Nitric Oxide Analyzer (NOA™280, Sievers Instruments Inc., CO, USA), a high-sensitivity detector for measuring NO, which is based on the gas-phase chemiluminescent reaction between NO and ozone. The emission of a photon from electrically excited nitrogen dioxide is in the red and near-infrared region of the spectrum, and it is detected by a thermoelectrically cooled red-sensitive photomultiplier tube. The sensitivity for the measurement of NO and its reaction products in liquid samples is ~ 1 picomole [29].
Estimation of lipid peroxidation
Lipid peroxidation was estimated in the plasma, urine, and kidney using the TBARS method, with a molar extinction coefficient of 1.56 × 105 cm/mol [30, 31].
Quantitative PCR
The extraction was performed using TRIzol reagent (Life Technologies, Sao Paulo, Brazil), and the RNA layer was purified and its concentrations determined using a spectrophotometer (Synergy HT Biotek, VT, USA). The cDNA strands were synthesized from the RNA samples using the SuperScriptVILO MasterMix reagent (Life Technologies, CA, USA) at a ratio of 4 μL of the reagent to 2.5 μg of RNA. The reaction was done by TaqMan (Applied Biosystem, USA) with klotho (Rn00580123_m1) and P2X7 (Rn00570451_m1) probes, using plectin as the housekeeping gene (Rn 00673737_m1). All of the qPCR reactions were conducted in duplicate using LineGene 9620 thermocycler (Bioer, USA). Negative controls were made for each probe, and the cycling conditions were as follows: 2 min at 50 °C followed by 10 min at 95 °C; after this, the samples were submitted to 40 cycles (15 s at 95 °C followed by 1 min at 60 °C with reading). The threshold cycle (Ct) values of the target gene and endogenous control were normalized by the Ct values of the housekeeping gene (ΔCt). The difference, obtained between the ΔCt value of the target gene and the ΔCt of the endogenous control, resulted in the values of ΔΔCt. Thus, the relative gene expression was calculated by applying the equation 2−ΔΔCt [32].
Western blotting
The kidney was homogenized; the total proteins were measured by the Bradford method and soon afterwards, 40 μg protein of each sample was prepared and run on 10% polyacrylamide gel. The blots were then incubated with anti-klotho (E-21): sc-22220 (1:500) and anti-P2X7 antibodies (H-265): sc-25698 (1:100 dilution, both acquired from Santa Cruz Biotechnology, CA, USA). As a loading control, blots were incubated with an anti-actin antibody (C-11): sc-1615 (1:1000 dilution, Santa Cruz Biotechnology, CA, USA). The bands were visualized by chemiluminescent substrate (#34080) (Thermo Fisher Scientific Inc., IL, USA), and the nitrocellulose membranes were analyzed by chemiluminescence imaging in gel documentation (Alience4.7 Uvitec, Cambridge, UK). The bands of proteins were quantified using ImageJ software (National Institutes of Health, MD, USA).
Statistical analysis
The results were expressed as the mean ± SEM or as box plot graphics with the medians and means. After confirmation of a normal distribution by the Kolmogorov-Smirnov test, the values were submitted to repeated measures ANOVA with a Newman-Keuls post hoc test for the parametric data or to Friedman with Dunn’s test for the non-parametric data. For comparison between two groups of rats at the same week, an unpaired Student’s t test was used. Significance was defined as p < 0.05. Correlation was done with Pearson’s r for the parametric data or with Spearman’s r for the non-parametric data.
Results
In the present study, the diabetic rats presented with all of the characteristics of DM, i.e., polyuria, polyphagia, and polydipsia, from the 1st week to the 8th week of the protocol when compared to the control animals (Table 1).
Table 1.
Analysis of metabolic profile and renal function from 1st to 8th weeks of diabetes in rats
| Weeks | 1st | 2nd | 3rd | 4th | ||||
| Diabetes | − | + | − | + | − | + | − | + |
| Metabolic parameters | ||||||||
| Water intake (mL/24 h) | 35.6 ± 3.8 | 136.8 ± 18.0e | 36.6 ± 2.7 | 162.6 ± 21.1e | 36.6 ± 3.1 | 163.0 ± 19.4e | 40.4 ± 4.1 | 159.4 ± 18.2e |
| Food intake (mg/24 h) | 21.2 ± 1.2 | 31.2 ± 2.4e | 21.7 ± 1.0 | 31.6 ± 3.4e | 20.8 ± 1.0 | 34.4 ± 2.4e | 20.8 ± 0.9 | 31.4 ± 1.0e |
| Diuresis (mL/24 h) | 15.4 ± 1.2 | 106.2 ± 14.8e | 16.8 ± 1.4 | 128.6 ± 17.9e | 16.0 ± 1.3 | 132.0 ± 14.5e | 17.4 ± 3.1 | 120.9 ± 14.6e |
| Body weight (g) | 262.2 ± 10.1 | 247.6 ± 8.4 | 261.9 ± 14.5 | 230.7 ± 18.7 | 278.3 ± 12.3 | 194.8 ± 26.4e | 305.5 ± 11.0 | 248.3 ± 6.3e |
| Glycemia (mg/dL) | 102.2 ± 2.2 | 370.6 ± 46.9e | 100.6 ± 3.2 | 363.4 ± 53.4e | 101.6 ± 2.9 | 446.2 ± 30.0e | 103.2 ± 1.5 | 447.8 ± 32.9e |
| Renal function | ||||||||
| Plasmatic creatinine (mg/dL) | 1.05 ± 0.1 | 0.99 ± 0.1 | 0.95 ± 0.1 | 1.13 ± 0.1 | 0.86 ± 0.2 | 1.13 ± 0.1 | 1.03 ± 0.05 | 1.14 ± 0.1 |
| Clearance of creatinine (mg/min) | 0.79 ± 0.1 | 1.13 ± 0.2 | 0.95 ± 0.2 | 1.05 ± 0.1 | 1.00 ± 0.2 | 1.00 ± 0.2 | 0.85 ± 0.3 | 1.25 ± 0.2 |
| Plasmatic urea (mg/dL) | 33.60 ± 1.3 | 46.50 ± 1.4e | 33.60 ± 1.5 | 47.10 ± 0.6e | 34.70 ± 1.4 | 53.70 ± 3.1e | 35.30 ± 0.7 | 55.40 ± 4.2e |
| Proteinuria (mg/24 h) | 20.97 ± 0.8 | 30.73 ± 3.9e | 22.79 ± 1.7 | 43.81 ± 5.4e | 22.17 ± 1.8 | 33.63 ± 4.2e | 23.08 ± 3.1 | 45.29 ± 4.3e |
| Weeks | 5th | 6th | 7th | 8th | ||||
| Diabetes | − | + | − | + | − | + | − | + |
| Metabolic parameters | ||||||||
| Water intake (mL/24 h) | 30.0 ± 1.9 | 154.4 ± 17.6e | 31.8 ± 0.7 | 156.6 ± 5.8e | 30.7 ± 0.4 | 160.0 ± 11.6e | 31.40 ± 1.1 | 175.8 ± 7.4e |
| Food intake (mg/24 h) | 17.3 ± 0.9 a | 27.9 ± 1.6e | 17.7 ± 0.3 a | 27.9 ± 1.1e | 17.1 ± 0.6 a | 33.72 ± 1.0e | 13.82 ± 0.5 d | 33.5 ± 0.3e |
| Diuresis (mL/24 h) | 16.3 ± 1.8 | 119.2 ± 6.2e | 14.7 ± 0.7 | 121.7 ± 8.1e | 14.8 ± 0.6 | 139.8 ± 8.1e | 13.80 ± 0.5 | 151.5 ± 12.5e |
| Body weight (g) | 353.6 ± 10.5 a | 169.8 ± 17.2be | 347.7 ± 7.2 a | 198.5 ± 17.1e | 361.5 ± 10.6 a | 203.8 ± 10.5e | 414.3 ± 9.2 d | 168.6 ± 11.1be |
| Glycemia (mg/dL) | 102.8 ± 2.3 | 492.6 ± 44.3e | 102.4 ± 2.4 | 555.4 ± 20.2ce | 106.4 ± 3.4 | 552.2 ± 13.5 ce | 96.40 ± 1.6 | 594.8 ± 6.2ae |
| Renal function | ||||||||
| Plasmatic creatinine (mg/dL) | 0.95 ± 0.1 | 1.21 ± 0.1 | 0.89 ± 0.1 | 1.27 ± 0.1e | 0.78 ± 0.2 | 1.44 ± 0.1e | 1.0 ± 0.1 | 1.55 ± 0.1e |
| Clearance of creatinine (mg/min) | 1.11 ± 0.1 | 0.72 ± 0.1e | 1.01 ± 0.1 | 0.73 ± 0.08e | 1.06 ± 0.1 | 0.63 ± 0.05e | 0.91 ± 0.04 | 0.65 ± 0.07e |
| Plasmatic urea (mg/dL) | 29.10 ± 0.7 | 51.40 ± 2.0e | 30.80 ± 1.9 | 48.20 ± 3.9e | 27.40 ± 1.4 | 54.90 ± 3.9e | 30.80 ± 0.9 | 58.20 ± 2.4e |
| Proteinuria (mg/24 h) | 20.94 ± 1.8 | 43.91 ± 12.5e | 19.27 ± 2.8 | 32.27 ± 3.2e | 21.28 ± 2.3 | 40.00 ± 2.8e | 20.29 ± 2.9 | 47.75 ± 5.7e |
Values expressed as mean ± SEM. ANOVA of repeated measures with Newman-Keuls post test, n = 6 for both groups, significant when p < 0.05: (a) vs 1st to 4th weeks of the same group, (b) vs 1st week of the same group, (c) vs 1st and 2nd weeks of the same group, and (d) vs all previous of the same group. Unpaired Student’s t test, significant when p < 0.05: (e) vs control of same week
The diabetic animals had a progressive reduction in body weight, which decreased significantly from the 3rd to the 8th weeks of the protocol when compared to the controls of the same week (Table 1). This is also in agreement with the weight loss due to high metabolism observed in DM. The control rats presented a food intake that was unchanged until the 4th week. After this time, they reduced their feeding, and this could be related to their growth. The highest food intake was observed in the diabetic animals, and when we compared these samples to the control group of the same week, we observed that increases were significant (Table 1).
As expected, the diabetic rats presented with a progressive increase of blood glucose, compared to the respective weekly controls from the 1st to 8th weeks (Table 1). The renal function, which was assessed by plasma creatinine levels, in the diabetic animals progressively increased when compared to that of respective weekly controls. The creatinine clearance from the diabetic rats showed a significant reduction after the 5th week compared to that of the respective weekly controls (Table 1). The plasmatic urea of the diabetic rats was significantly increased compared to that of the respective weekly controls from the 1st to 8th weeks of the protocol (Table 1).
The proteinuria of the diabetic animals showed a high and crescent protein excretion among them, and when compared to the control groups, the diabetics were increased during all of the weeks (Table 1).
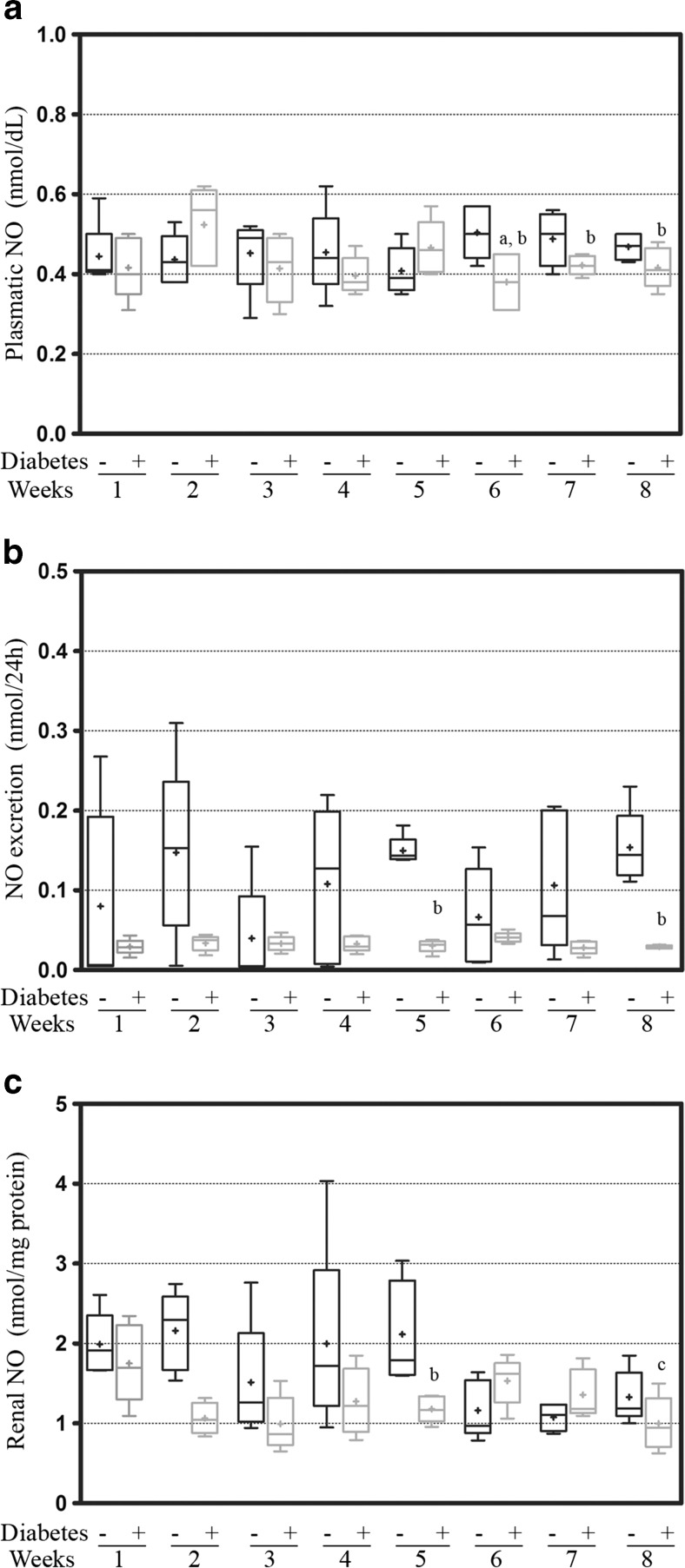
Nitric oxide samples in the plasma from the diabetic rats were observed and showed that the levels from the 6th week were reduced compared to the levels of the 2nd week in the diabetic rats and to the levels of the controls of the same week (0.38 ± 0.03 nmol/dL vs 0.52 ± 0.4 nmol/dL, p < 0.05 and 0.50 ± 0.3 nmol/dL, p = 0.0208; respectively). We also observed that these levels in the diabetic animals at the 7th was reduced compared to the control of the same week (0.42 ± 0.01 nmol/dL vs 0.51 ± 0.2 nmol/dL, p = 0.0052) (Fig. 1a).
Fig. 1.
The measurements of NO from the a plasma, b urine, and c renal cortex tissues from the first to eighth weeks of diabetes. The values are expressed in the box-plot containing the median (line) and average (plus sign); significance is set at p < 0.05; n = 5. One-way ANOVA with repeated measures and Newman-Keuls post-hoc test: a vs second week of diabetes; c vs first week of diabetes. Unpaired Student’s t test: b vs control of the same week. NO nitric oxide
The values of urinary excretion of nitric oxide of the diabetic animals were reduced compared to the respective controls at 8 weeks, showing significance at the 5th and 8th weeks (0.03 ± 0.01 vs 0.14 ± 0.01 nmol/24 h, p = 0.0001 and 0.03 ± 0.01 vs 0.15 ± 0.02 nmol/24 h, p = 0.0003; respectively) (Fig. 1b). Similar to the excretion values were the renal nitric oxide values, for which the diabetic kidneys showed a reduction along all of the weeks compared to the control group of the same week, and they were significantly reduced at the 5th week (1.18 ± 0.07 vs 1.99 ± 0.17 nmol/mg protein, p = 0.0134) (Fig. 1c). Among the diabetic rats, this reduction became significant at the 8th week when compared to the 1st week (0.9 ± 0.1 vs 1.75 ± 0.2 nmol/mg protein, p = 0.0226) (Fig. 1c).
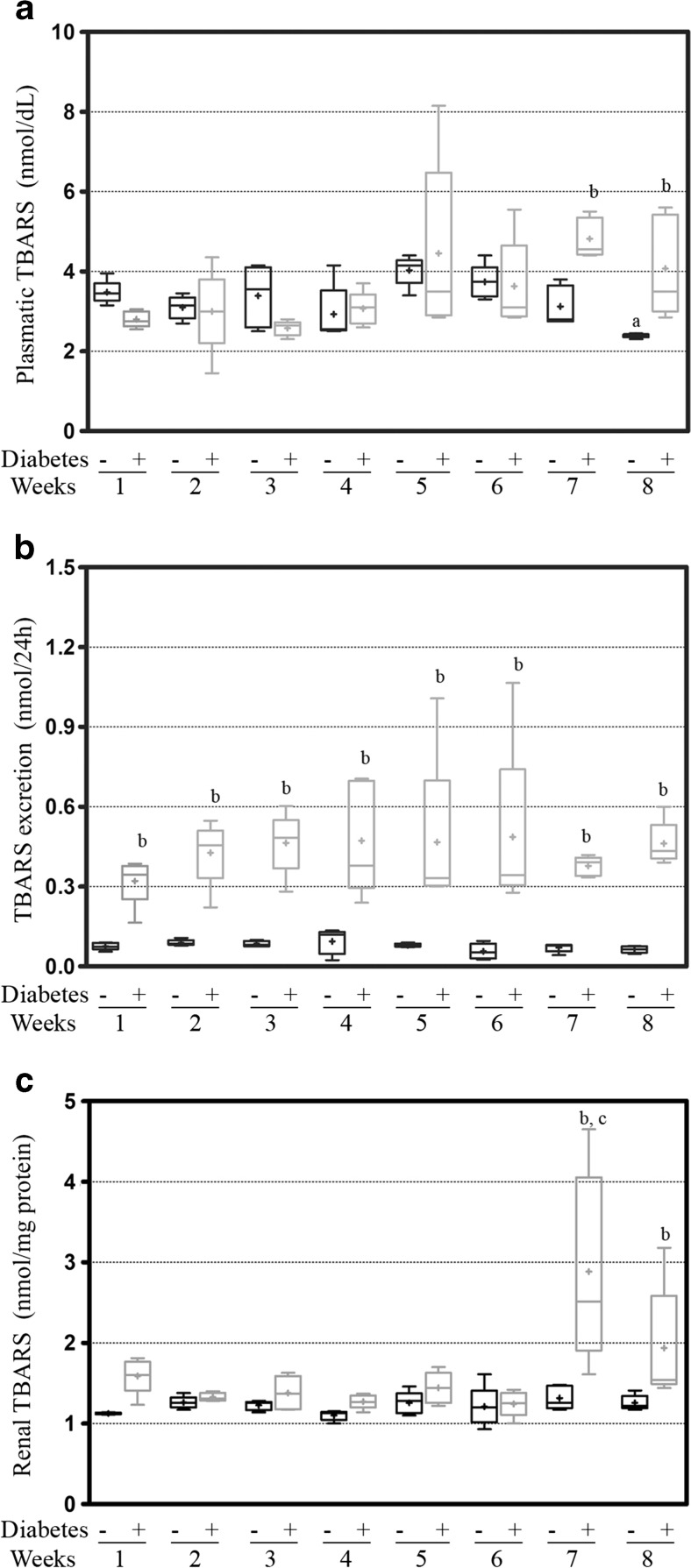
The estimation of plasmatic lipid peroxidation in the diabetic animals showed first differences at the 5th week (4.5 ± 0.9 vs 4.0 ± 0.17, NS) and remained elevated until the end of the protocol, which was a significant compared to that of the respective control groups at the 7th and 8th weeks (4.8 ± 0.2 vs 3.1 ± 0.2, p = 0.0006, and 4.1 ± 0.6 vs 2.4 ± 0.02, p = 0.0179; respectively). Among the control animals were observed variations in the TBARS concentrations, and the animals of the 5th week had a significant increase in the oxidative stress compared with the previous weeks (4.9 ± 0.6 vs 3.5 ± 0.1 nmol/dL, p = 0.0332; 3.1 ± 0.1 nmol/dL, p = 0.0024; 3.2 ± 0.3 nmol/dL, p = 0.0343; and 2.9 ± 0.3 nmol/dL, p = 0.0151; respectively of the 1st, 2nd, 3rd, and 4th weeks) and afterwards, this peak was significantly reduced at the 8th week (2.4 ± 0.02 nmol/dL, p = 0.0001) (Fig. 2a). The urinary excretion of TBARS analysis showed that the diabetic animals had a progressive increase, which was significant compared to the respective controls for all of the weeks (0.32 ± 0.04 vs 0.076 ± 0.01 nmol/24 h, p = 0.0003; 0.43 ± 0.05 vs 0.088 ± 0.01 nmol/24 h, p = 0.0003; 0.46 ± 0.05 vs 0.085 ± 0.01 nmol/24 h, p = 0.0001; 0.47 ± 0.09 vs 0.094 ± 0.02 nmol/24 h, p = 0.0046; 0.47 ± 0.13 vs 0.079 ± 0.01 nmol/24 h, p = 0.0216; 0.48 ± 0.15 vs 0.057 ± 0.01 nmol/24 h, p = 0.0191; 0.38 ± 0.02 vs 0.071 ± 0.01 nmol/24 h, p = 0.0001; 0.46 ± 0.04 vs 0.064 ± 0.01 nmol/24 h, p = 0.0001; respectively for the 1st, 2nd, 3rd, 4th, 5th, 6th, 7th, and 8th weeks) (Fig. 2b). The renal levels of oxidative stress in the diabetic kidneys presented a peak of TBARS production in the 7th week that was significantly higher than that of the previous weeks (2.89 ± 0.05 vs 1.6 ± 0.1 nmol/mg protein, p = 0.0438; 1.3 ± 0.2 nmol/mg protein, p = 0.0193; 1.4 ± 0.9 nmol/mg protein, p = 0.0238; 1.3 ± 0.4 nmol/mg protein, p = 0.0166; 1.4 ± 0.9 nmol/mg protein, p = 0.0282; 1.3 ± 0.7 nmol/mg protein, p = 0.0157; respectively for the 1st, 2nd, 3rd, 4th, 5th, and 6th). This value kept increasing in the 8th week and was significant compared to that of the control of the same week (2.89 ± 0.05 vs 1.3 ± 0.6 nmol/mg protein, p = 0.0192, and 1.9 ± 3.2 vs 1.3 ± 0.4 nmol/mg protein, p = 0.0715; respectively for the 7th and 8th weeks) (Fig. 2c).
Fig. 2.
The measurements of TBARS from the (a) plasma, (b) urine, and (c) renal cortex tissue from the first to eighth weeks of the diabetes protocol. The values are expressed in the box-plot containing the median (line) and average (plus sign); significance is set at p < 0.05; n = 5. One-way ANOVA with repeated measures and Newman-Keuls post-hoc test: a vs the second week to sixth week of diabetes; c vs all previous weeks of diabetes. Unpaired t test of Student: b vs control of same week. TBARS thiobarbituric acid reactive substances
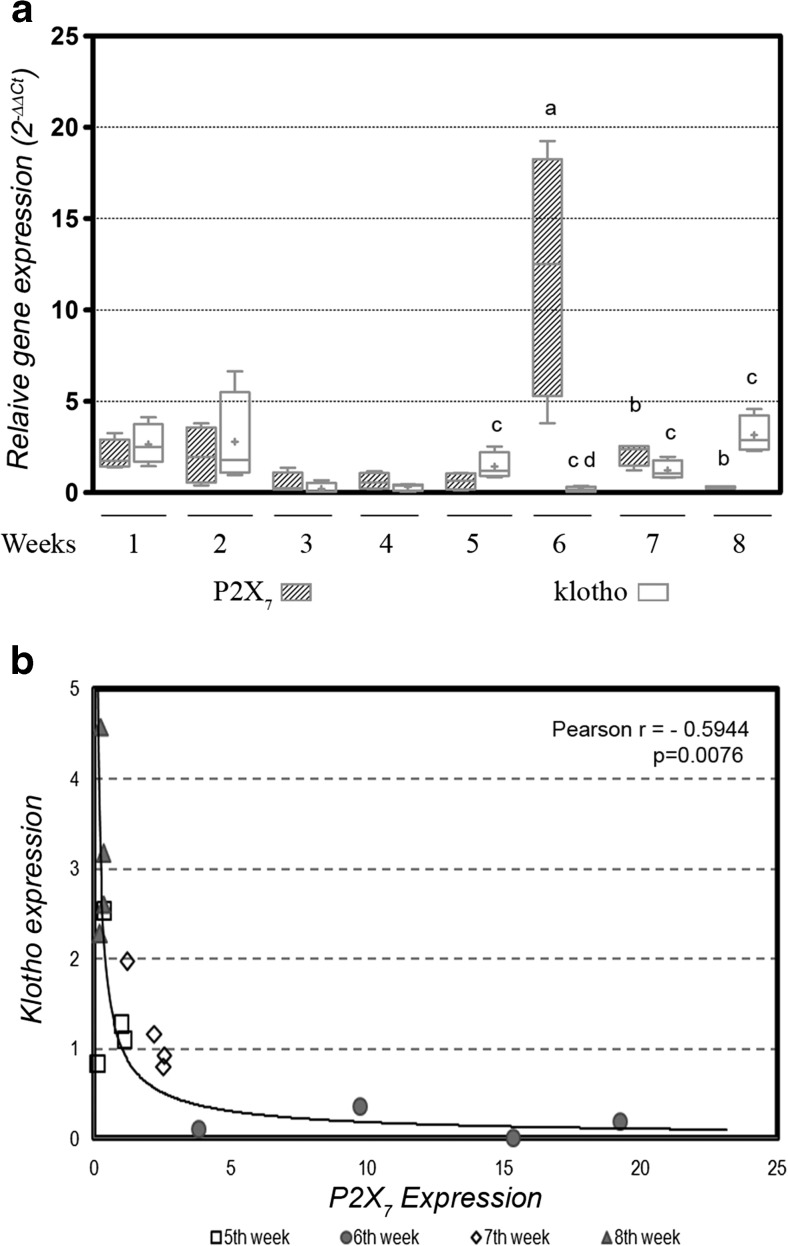
In response to this redox imbalance, the P2X7 receptors were analyzed, and in the previous study in our laboratory, we showed that this receptor was correlated with oxidative stress levels. Now, we wished to understand how the expression of this receptor is related to the expression of klotho in diabetes, and we observed that until the 4th week, the levels of both of the mRNAs were paired and unchanged, and since then, they showed an antagonist behavior. In other words, when there were high levels of klotho mRNA, the P2X7 expression was reduced (observed at the 5th and 8th weeks) (1.54 ± 0.3 vs 0.52 ± 0.2, p = 0.0281, and 3.16 ± 0.5 vs 0.29 ± 0.1, p = 0.0013, respectively) and vice versa (observed at the 6th and 7th weeks) (0.17 ± 0.1 vs 12.02 ± 3.7, p = 0.0125, and 1.03 ± 0.1 vs 2.13 ± 0.3, p = 0.0155; respectively) (Fig. 3a). Thus, to evaluate the relationship between the mRNAs of these proteins, we analyzed the period from the 5th to 8th weeks due to oscillation between them, which showed a moderate and inverse correlation between the P2X7 and klotho expression (p = 0.0076 and Pearson r = 0.5944) (Fig. 3b).
Fig. 3.
a Relative gene expression of P2X7 and klotho from renal cortex of first to eighth weeks of diabetes. The values are expressed in the bar graphics with the average and SEM; significance is set at p < 0.05; n = 5 reactions. One-way ANOVA with repeated measures and Newman-Keuls post-hoc test: a vs all previous weeks of diabetes; b vs seventh week of P2X7; d vs first and second weeks of klotho. Unpaired Student’s t test: c vs P2X7 of the same week. SEM = standard error of the mean. b The correlation analysis of the period from the fifth to eighth weeks, which showed a moderate and inverse correlation of the P2X7 and klotho expressions (p = 0.0076 and Pearson r = 0.5944)
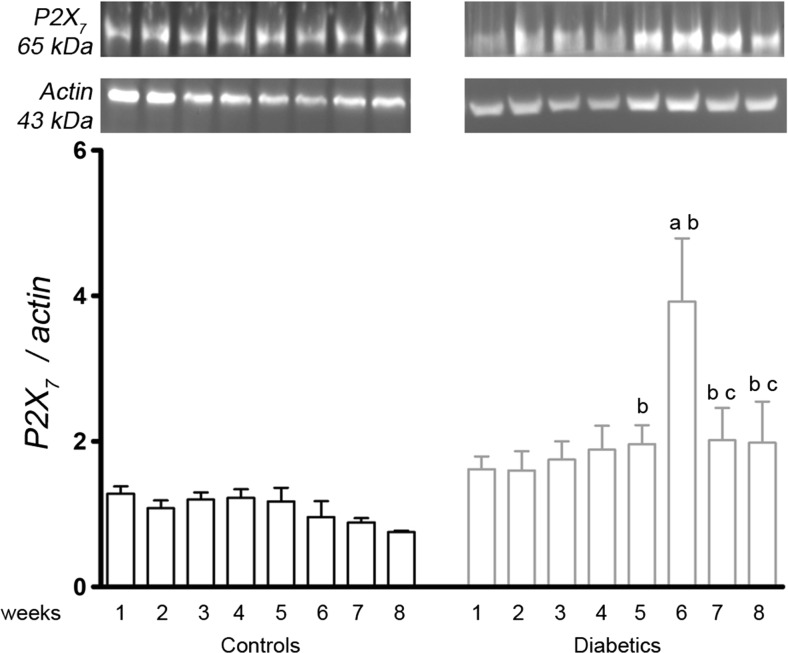
The Western blotting analysis of the P2X7 protein content showed that the diabetic animals had an increase of the protein content at the 6th week compared with the previous weeks (3.9 ± 0.9 vs 1.62 ± 0.2, p = 0.0187; 1.60 ± 0.3, p = 0.0215; 1.75 ± 0.2, p = 0.0279; 1.89 ± 0.3, p = 0.0427; and 1.96 ± 0.3, p = 0.0434; respectively, 1st, 2nd, 3rd, 4th, and 5th weeks). The protein levels for P2X7 at the 6th, 7th, and 8th weeks were significantly increased compared to those of the respective weekly controls (0.96 ± 0.2, p = 0.0056; 0.88 ± 0.1, p = 0.0305; and 0.75 ± 0.1, p = 0.05, for the control groups at the 6th, 7th, and 8th weeks, respectively) (Fig. 4).
Fig. 4.
A representative image of the P2X7 protein bands in the renal cortex from the first to eighth weeks of diabetes. The values are expressed in bar graphics as the average and SEM; significance is set at p < 0.05; n = 6 reactions. One-way ANOVA with repeated measures and Newman-Keuls post-hoc test: a vs all previous weeks of diabetes; c vs sixth week of diabetes. Unpaired Student’s t test: b vs control of the same week. kDa kilodalton
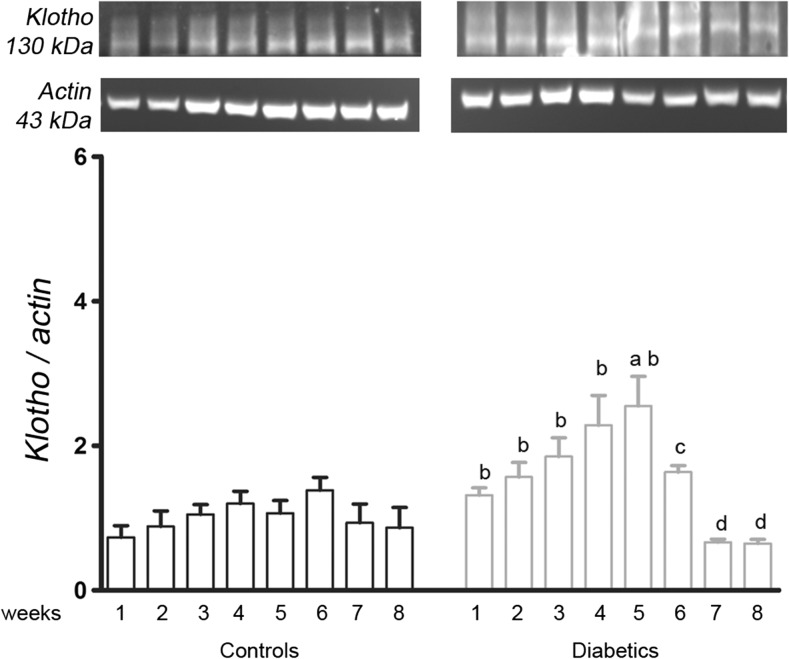
The klotho protein analysis showed that the diabetic kidneys presented a progressive increase in this protein, which became relevant at the 5th week (2.55 ± 0.4 vs 1.07, p = 0.0076). Until the 5th week, it was significantly higher in all of the diabetic samples compared to the respective controls of the same week (1.32 ± 0.1 vs 0.73 ± 0.2, p = 0.0118; 1.57 ± 0.2 vs 0.88 ± 0.2, p = 0.0434; 1.85 ± 0.3 vs 1.1 ± 0.1, p = 0.0218; 2.28 ± 0.4 vs 1.2 ± 0.2, p = 0.0355; respectively). However, in the 6th, 7th, and 8th weeks, there was a reduction of the klotho protein (1.64 ± 0.1, p = 0.05; 0.67 ± 0.05, p = 0.0010; and 0.65 ± 0.06, p = 0.0010; respectively) to the lowest values observed in diabetic group (Fig. 5).
Fig. 5.
A representative image of the klotho protein bands in the renal cortex from the first to eighth weeks of diabetes. The values are expressed in bar graphics as the average and SEM; significance is set at p < 0.05; n = 6 reactions. One-way ANOVA with repeated measures and Newman-Keuls post-hoc test: a vs all previous weeks of diabetes; c vs 6th week of diabetes. Unpaired Student’s t test: b vs control of the same week. kDa kilodalton
Discussion
Diabetes is a serious health problem that affects more people each year, and once it is developed, it promotes microvascular and macrovascular lesions, resulting in the damage of several organs [33]. Therefore, an early therapeutic approach is important for maintaining a good quality of life. Many mechanisms have been proposed in the pathophysiology of DM, including the participation of purinergic receptors such as P2X7. Until the present study, it was not yet clear as to the exact moment when the P2X7 becomes expressed in this disease.
To our knowledge, this study identified, for the first time, the moment when P2X7 expression becomes relevant throughout the development of DM in rats. In addition, other important changes occurred. For example, the expression of klotho, a co-receptor related to longevity, showed a negative correlation relative to that of P2X7, and this inverse relationship was also reflected in other important metabolic changes, such as in the renal parameters and redox balance. Current studies have shown that klotho preserves the glomerular filtration rate in both AKI and CKD, as well as that it reduces albuminuria by inhibition of the ATP-stimulated actin cytoskeletal remodeling in podocytes [34, 35]. If nothing else, klotho upregulates the antioxidant defenses though Nrf-2, thus preserving the redox balance [36].
In diabetes, glucose is not used by cells as source of energy, due to the failure of insulin action and/or its receptors. However, the energy is recruited from proteins and fatty acids of adipose tissue, through proteolysis and lipolysis, respectively. The utilization of these alternative sources causes a reduction in body weight [37], as was observed in this study. Excessive thirst (polydipsia) and increased diuresis (polyuria) and hunger (polyphagia) are other classic symptoms of diabetes [38], which were reproduced in this study, demonstrating the efficacy of this experimental model.
In this disease, the kidneys work overtime to filter and reabsorb excess filtrate, which is mainly glucose, and this overload of renal work can cause injury and other damages triggered by hyperglycemia, such as podocyte detachment, death of epithelial and mesangial cells, and intense production of extracellular mesangial matrix. All of these factors result in glomerular sclerosis and contribute to diabetic nephropathy [39]. Our data showed that in diabetic animals, the creatinine clearance was reduced at the 5th week and remained low until the end of the protocol and that the plasma creatinine progressively increased and became significant at the 6th week, as well as did the plasma urea, another parameter of renal function. These alterations in association with proteinuria characterize diabetic nephropathy.
The glycemia of diabetic rats, as expected, was higher than that of the respective controls, showing a rising profile over the weeks. A study showed that the direct and indirect pathways of hyperglycemia are responsible for the excessive production of oxidative stress in the blood [40], demonstrating that the high blood glucose and lipid peroxidation work as a cause and effect mechanism, which corroborates with our data in diabetic animals.
In our study, the control animals, at the 8th week, presented a low food intake. This likely occurs due to a gradual, physiological reduction of their growth, which initiates at this point. Furthermore, the health of the animals at the 8th week presented significantly low TBARS levels compared to the ones at the 1st to 7th weeks of the protocol. This agrees with other researchers who demonstrated this relationship [41, 42]. The main product of hyperglycemia is oxidative stress, which is a redox imbalance, i.e., an excess of oxidizing agents compared to the levels of antioxidants. In this condition, damage to important molecules, such as proteins and DNA, occurs changing the bioavailability of certain molecules. Among these molecules is NO, a potent vasodilator, which forms peroxynitrite, an extremely cytotoxic free radical, in the presence of a superoxide anion [43].
The kidney is one of the most perfused organs of the body. We believe that this is the reason for the high plasma concentrations of oxidizing particles in the kidneys, which is responsible for NO depletion. NO and superoxide anions participate in a rapid reaction (k = 7 × 109 M−1 s−1), in the formation of peroxynitrite [44], whereas the natural dismutation of superoxide anions is seven times slower (k = 109 M−1 s−1) [45]. This may explain our findings of the NO bioavailability in diabetic animals, which was reduced in the plasma at the end of the protocol. It was also reduced in the urine and in the renal tissue. In all situations of nitric oxide reduction, there was an increase of lipid peroxidation.
In a previous publication by our laboratory, we observed renal damage in diabetic rats at the 8th week of the protocol [27]. In the present study, we observed that lipid peroxidation levels increase in renal tissue simultaneously with the reduction of nitric oxide, at the 7th week after diabetes induction.
The redox imbalance can promote relevant changes in the cell signaling pathways that cause damage or cell death. Physiologically, some cells release ATP into the extracellular medium, and in renal tissue, this is done mainly through vesicles [5]. In the condition of hyperglycemia, several mechanisms are inactivated, including ecto-nucleotidases, which are enzymes that rapidly hydrolyze extracellular ATP to adenosine [46]. This could explain the accumulation of ATP in the bloodstream in diabetes, which activates P2X7 expression. This agrees with our results because we showed a peak of P2X7 mRNA production in the 6th week of diabetes. From this initial activation, P2X7 initiates the cellular swelling process, which at millimolar levels begins to open other channels and allows the exit of metabolites and electrolytes, among them being the ATP molecule, to the extracellular medium and results in positive feedback to the receptor.
In our study, there was a moment between the 5th and 6th weeks of diabetes when, due to partial ATP degradation, there was a generation of inorganic phosphate that was enough to activate klotho, which in turn modulates the levels of phosphate through the kidneys. In fact, a moderate elevation of klotho reduces serum phosphate and a high elevation of klotho can induce hypophosphatemia [47]. As an outcome, this can trigger a range of responses. For Lichtman et al. [48], the lack of phosphate induced a reduction of 3-diphosphoglycerate, an important molecule that regulates the release of oxygen from hemoglobin, and therefore, all procedures that involve the use of oxygen would be committed. Among them, we mainly cited ATP synthesis, because beyond the need for oxygen in the mitochondria, ATP requires phosphate too. Klotho causing phosphate depletion can downregulate P2X7 levels, explaining our results, showing that klotho expression was decreased in the 6th to 8th weeks of diabetes, while P2X7 had the highest levels of expression during these weeks.
Many studies have attributed other functions for klotho, besides those of anti-aging. Among them are its anti-inflammatory action [20], phosphaturia modeling [21], cell-surface ion channel stabilization, anti-apoptotic properties [22], reduction of oxidative stress [23], and production of NO [24]. However, this is the first time, to our knowledge, that klotho was investigated as a modulator of P2X7 expression. As a limitation of this study, we think that the phosphate levels in plasma and urine need to be determined in these animals.
Conclusion
These results suggest that there is likely an antagonist effect between the expressions of P2X7 and klotho in the evolution of diabetic nephropathy, but more studies are needed to clarify the role of both proteins in diabetes to contribute to the reduction its complications.
Acknowledgements
The authors acknowledge Margaret G Mouro and Deyse Y Lima for their technical assistance and Professor Sergio R R Araujo for the histological analysis. This study was supported by Coordenaçao de Aperfeiçoamento de Pessoal de Nivel Superior (CAPES) and Fundaçao de Apoio a Pesquisa da UNIFESP (FAP).
Compliance with ethical standards
Conflicts of interest
A. M. Rodrigues declares that he has no conflict of interest.
R. S. Serralha declares that he has no conflict of interest.
C. Farias declares that she has no conflict of interest.
G. R. Punaro declares that she has no conflict of interest.
M. J. S. Fernandes declares that she has no conflict of interest.
Elisa Mieko Suemitsu Higa declares that she has no conflict of interest.
Ethical approval
The protocol was approved by the Ethics Committee in Research of Universidade Federal de Sao Paulo under protocol #2056100314.
References
- 1.Burnstock G. A basis for distinguishing two types of purinergic receptor. In: Straub RW, Bolis CL, editors. Cell membrane receptors for drugs and hormones: a multidisciplinary approach. New York: Raven Press; 1978. pp. 107–118. [Google Scholar]
- 2.Kennedy C, Burnstock G. Evidence for two types of P2-purinoceptor in longitudinal muscle of the rabbit portal vein. Eur J Pharmacol. 1985;111:49–56. doi: 10.1016/0014-2999(85)90112-8. [DOI] [PubMed] [Google Scholar]
- 3.Kaczmarek-Hajek K, Lorinczi E, Hausmann R, et al. Molecular and functional properties of P2X receptors—recent progress and persisting challenges. Purinergic Signalling. 2012;8:375–417. doi: 10.1007/s11302-012-9314-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Surprenant A, Rassendren F, Kawashima E, North RA, Buell G. The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7) Science. 1996;272:735–738. doi: 10.1126/science.272.5262.735. [DOI] [PubMed] [Google Scholar]
- 5.Bjaelde RG, Arnadottir SS, Overgaard MT, et al. Renal epithelial cells can release ATP by vesicular fusion. Front Physiol. 2013;4:238. doi: 10.3389/fphys.2013.00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Solini A, Usuelli V, Fiorina P. The dark side of extracellular ATP in kidney diseases. J Am Soc Nephrol. 2014;26:1007–1016. doi: 10.1681/ASN.2014070721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kwak SH, Park KS, Lee KU, Lee HK. Mitochondrial metabolism and diabetes. J Diabetes Investig. 2010;1:161–169. doi: 10.1111/j.2040-1124.2010.00047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rucker B, Abreu-Vieira G, Bischoff LB, et al. The nucleotide hydrolysis is altered in blood serum of streptozotocin-induced diabetic rats. Arch Physiol Biochem. 2010;116:79–87. doi: 10.3109/13813451003777067. [DOI] [PubMed] [Google Scholar]
- 9.Payne BA, Chinnery PF. Mitochondrial dysfunction in aging: much progress but many unresolved questions. Biochim Biophys Acta. 2015;1847:1347–1353. doi: 10.1016/j.bbabio.2015.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gross JL, De Azevedo MJ, Silveiro SP, et al. Diabetic nephropathy: diagnosis, prevention, and treatment. Diabetes Care. 2005;28:164–176. doi: 10.2337/diacare.28.1.164. [DOI] [PubMed] [Google Scholar]
- 11.Burnstock G, Novak I. Purinergic signalling and diabetes. Purinergic Signalling. 2013;9:307–324. doi: 10.1007/s11302-013-9359-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hansen PB, Schnermann J. Vasoconstrictor and vasodilator effects of adenosine in the kidney. Am J Physiol. 2003;285:F590–F599. doi: 10.1152/ajprenal.00051.2003. [DOI] [PubMed] [Google Scholar]
- 13.Sallstrom J, Carlsson PO, Fredholm BB, et al. Diabetes-induced hyperfiltration in adenosine A(1)-receptor deficient mice lacking the tubuloglomerular feedback mechanism. Acta Physiol (Oxford, England) 2007;190:253–259. doi: 10.1111/j.1748-1716.2007.01705.x. [DOI] [PubMed] [Google Scholar]
- 14.Kretschmar C, Oyarzun C, Villablanca C, et al. Reduced adenosine uptake and its contribution to signaling that mediates profibrotic activation in renal tubular epithelial cells: implication in diabetic nephropathy. PLoS One. 2016;11:e0147430. doi: 10.1371/journal.pone.0147430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen K, Zhang J, Zhang W, Zhang J, Yang J, Li K, He Y. ATP-P2X4 signaling mediates NLRP3 inflammasome activation: a novel pathway of diabetic nephropathy. Int J Biochem Cell Biol. 2013;45:932–943. doi: 10.1016/j.biocel.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 16.Menzies RI, Booth JWR, Mullins JJ, Bailey MA, Tam FWK, Norman JT, Unwin RJ. Hyperglycemia-induced renal P2X7 receptor activation enhances diabetes-related injury. EBioMedicine. 2017;19:73–83. doi: 10.1016/j.ebiom.2017.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuro-O M. Klotho. Pflugers Arch. 2010;459:333–343. doi: 10.1007/s00424-009-0722-7. [DOI] [PubMed] [Google Scholar]
- 18.Asai O, Nakatani K, Tanaka T, Sakan H, Imura A, Yoshimoto S, Samejima KI, Yamaguchi Y, Matsui M, Akai Y, Konishi N, Iwano M, Nabeshima Y, Saito Y. Decreased renal alpha-klotho expression in early diabetic nephropathy in humans and mice and its possible role in urinary calcium excretion. Kidney Int. 2012;81:539–547. doi: 10.1038/ki.2011.423. [DOI] [PubMed] [Google Scholar]
- 19.Maltese G, Fountoulakis N, Siow RC, Gnudi L, Karalliedde J. Perturbations of the anti-ageing hormone klotho in patients with type 1 diabetes and microalbuminuria. Diabetologia. 2017;60:911–914. doi: 10.1007/s00125-017-4219-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao Y, Banerjee S, Dey N, LeJeune WS, Sarkar PS, Brobey R, Rosenblatt KP, Tilton RG, Choudhary S. Klotho depletion contributes to increased inflammation in kidney of the db/db mouse model of diabetes via RelA (serine)536 phosphorylation. Diabetes. 2011;60:1907–1916. doi: 10.2337/db10-1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang CL, Moe OW. Klotho: a novel regulator of calcium and phosphorus homeostasis. Pflugers Arch. 2011;462:185–193. doi: 10.1007/s00424-011-0950-5. [DOI] [PubMed] [Google Scholar]
- 22.Chang Q, Hoefs S, Van Der Kemp AW, et al. The beta-glucuronidase klotho hydrolyzes and activates the TRPV5 channel. Science. 2005;310:490–493. doi: 10.1126/science.1114245. [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto M, Clark JD, Pastor JV, Gurnani P, Nandi A, Kurosu H, Miyoshi M, Ogawa Y, Castrillon DH, Rosenblatt KP, Kuro-o M. Regulation of oxidative stress by the anti-aging hormone klotho. J Biol Chem. 2005;280:38029–38034. doi: 10.1074/jbc.M509039200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saito Y, Yamagishi T, Nakamura T, Ohyama Y, Aizawa H, Suga T, Matsumura Y, Masuda H, Kurabayashi M, Kuro-o M, Nabeshima YI, Nagai R. Klotho protein protects against endothelial dysfunction. Biochem Biophys Res Commun. 1998;248:324–329. doi: 10.1006/bbrc.1998.8943. [DOI] [PubMed] [Google Scholar]
- 25.Vergani A, Fotino C, D'addio F, et al. Effect of the purinergic inhibitor oxidized ATP in a model of islet allograft rejection. Diabetes. 2013;62:1665–1675. doi: 10.2337/db12-0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vergani A, Tezza S, Fotino C, Visner G, Pileggi A, Chandraker A, Fiorina P. The purinergic system in allotransplantation. Am J Transplant. 2014;14:507–514. doi: 10.1111/ajt.12567. [DOI] [PubMed] [Google Scholar]
- 27.Rodrigues AM, Bergamaschi CT, Fernandes MJ, et al. P2x(7) receptor in the kidneys of diabetic rats submitted to aerobic training or to N-acetylcysteine supplementation. PLoS One. 2014;9:e97452. doi: 10.1371/journal.pone.0097452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ochodnicky P, De Zeeuw D, Henning RH, et al. Endothelial function predicts the development of renal damage after combined nephrectomy and myocardial infarction. J Am Soc Nephrol. 2006;17:S49–S52. doi: 10.1681/ASN.2005121322. [DOI] [PubMed] [Google Scholar]
- 29.Hampl V, Walters CL, Archer SL. Determination of nitric oxide by the chemiluminescence reaction with ozone. In: Feelisch M, Stamler JS, editors. Methods in nitric oxide research. Chichester: Wiley; 1996. pp. 310–318. [Google Scholar]
- 30.Bernheim F, Bernheim ML, Wilbur KM. The reaction between thiobarbituric acid and the oxidation products of certain lipides. J Biol Chem. 1948;174:257–264. [PubMed] [Google Scholar]
- 31.Shimizu MH, Danilovic A, Andrade L, et al. N-acetylcysteine protects against renal injury following bilateral ureteral obstruction. Nephrol Dial Transplant. 2008;23:3067–3073. doi: 10.1093/ndt/gfn237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 33.Idf (2012) IDF diabetes atlas. In: The global burden. International Diabetes Federation, Brussels
- 34.Hu MC, Kuro-O M, Moe OW. Klotho and kidney disease. J Nephrol. 2010;23(Suppl 16):S136–S144. [PMC free article] [PubMed] [Google Scholar]
- 35.Kim JH, Xie J, Hwang KH, et al. Klotho may ameliorate proteinuria by targeting TRPC6 channels in podocytes. J Am Soc Nephrol. 2016;28:140–151. doi: 10.1681/ASN.2015080888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maltese G, Psefteli PM, Rizzo B, et al. The anti-ageing hormone klotho induces Nrf2-mediated antioxidant defences in human aortic smooth muscle cells. J Cell Mol Med. 2016;21:621–627. doi: 10.1111/jcmm.12996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Misra S (2016) Explaining common symptoms. In: Reversing diabetes—the high 5 way. Educreation Publishing, New Delhi, p 41–46
- 38.Unger Rh Fd (1992) Diabetes mellitus. In: Wilson JD FD (ed) Williams textbook of endocrinology. WB Saunders Company, Philadelphia, p 1255–1355
- 39.Fakhruddin S, Alanazi W, Jackson KE. Diabetes-induced reactive oxygen species: mechanism of their generation and role in renal injury. J Diabetes Res. 2017;2017:8379327. doi: 10.1155/2017/8379327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Vriese AS, Verbeuren TJ, Van De Voorde J, et al. Endothelial dysfunction in diabetes. Br J Pharmacol. 2000;130:963–974. doi: 10.1038/sj.bjp.0703393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walsh ME, Shi Y, Van Remmen H. The effects of dietary restriction on oxidative stress in rodents. Free Radic Biol Med. 2014;66:88–99. doi: 10.1016/j.freeradbiomed.2013.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Masoro EJ. Caloric restriction and aging: an update. Exp Gerontol. 2000;35:299–305. doi: 10.1016/S0531-5565(00)00084-X. [DOI] [PubMed] [Google Scholar]
- 43.Guzik TJ, West NE, Pillai R, et al. Nitric oxide modulates superoxide release and peroxynitrite formation in human blood vessels. Hypertension. 2002;39:1088–1094. doi: 10.1161/01.HYP.0000018041.48432.B5. [DOI] [PubMed] [Google Scholar]
- 44.Habib S, Ali A. Biochemistry of nitric oxide. Indian J Clin Biochem. 2011;26:3–17. doi: 10.1007/s12291-011-0108-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miwa S, Muller FL, Beckman KB. The basics of oxidative biochemistry. In: Miwa S, editor. Aging medicine: oxidative stress in aging: from model systems to human diseases. New Jersey: Humana Press; 2008. pp. 11–38. [Google Scholar]
- 46.Capiotti KM, Siebel AM, Kist LW, Bogo MR, Bonan CD, da Silva RS. Hyperglycemia alters E-NTPDases, ecto-5′-nucleotidase, and ectosolic and cytosolic adenosine deaminase activities and expression from encephala of adult zebrafish (Danio rerio) Purinergic Signalling. 2016;12:211–220. doi: 10.1007/s11302-015-9494-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bian A, Xing C, Hu MC (2014) Alpha klotho and phosphate homeostasis. J Endocrinol Investig 37:1121–112648 [DOI] [PMC free article] [PubMed]
- 48.Lichtman Ma, Miller Dr, Cohen J et al. (1971) Reduced red cell glycolysis, 2, 3-diphosphoglycerate and adenosine triphosphate concentration, and increased hemoglobin-oxygen affinity caused by hypophosphatemia. Ann of intern med 74:562–568 [DOI] [PubMed]