Abstract
Although Tsukamurella infections have been increasingly reported in Europe, Asia, America, and Africa, indicating that diseases caused by this group of bacteria are emerging in a global scale, species identification within this genus is difficult in most clinical microbiology laboratories. Recently, we showed that groEL gene sequencing is useful for identification of all existing Tsukamurella species. Nevertheless, PCR sequencing is still considered expensive, time-consuming, and technically demanding, and therefore is yet to be incorporated as a routine identification method in clinical laboratories. Using groEL gene sequencing as the reference method, 60 Tsukamurella isolates were identified as five different Tsukamurella species [T. tyrosinosolvens (n = 31), T. pulmonis (n = 25), T. hongkongensis (n = 2), T. strandjordii (n = 1), and T. sinensis (n = 1)]. The most common source of the patient isolates were the eye (n = 18), sputum (n = 6), and blood (n = 6). None of the 60 isolates were identified correctly to species level by MALDI-TOF MS with the original Bruker database V.6.0.0.0. Using the Bruker database extended with 15 type and reference strains which covered all the currently recognized 11 Tsukamurella species, 59 of the 60 isolates were correctly identified to the species level with score ≥2.0. MALDI-TOF MS should be useful for routine species identification of Tsukamurella in clinical microbiology laboratories after optimization of the database. T. tyrosinosolvens was the most common species observed in patients with Tsukamurella infections and the predominant species associated with ocular infections.
Introduction
The genus Tsukamurella contains clinically relevant species and cases have been increasingly reported in Europe, Asia, America, and Africa, indicating a global distribution of the bacteria1–20. The taxonomy of this genus has been continuously updated over the past few years. Recently, we have reclassified Tsukamurella spongiae DSM 44990, Tsukamurella carboxydivorans JCM 15482, and Tsukamurella sunchonesis JCM 15929 as later heterotypic synonyms of Tsukamurella pulmonis, Tsukamurella tyrosinosolvens, and Tsukamurella pseudospumae, respectively, based on the results of DNA–DNA hybridization and whole-genome sequencing21, 22. As a result, only 11 Tsukamurella species should be included in this genus according to the current state of the taxonomy at the time of writing21, 22. Among these 11 species, 8 are known to be associated with human infections, with the most common infections being indwelling device-related infections, meningitis, pulmonary, and cutaneous infections23. The disease spectra of Tsukamurella were further extended to ophthalmologic infections in recent years1, 2, 24. We have also discovered two novel Tsukamurella species, Tsukamurella hongkongensis, and Tsukamurella sinensis, from patients with keratitis and conjunctivitis respectively25. In addition to the association with various human infections, Tsukamurella can also be found in different environmental sources and animals23, 26–29. We have recently described another novel species, Tsukamurella serpentis, from the oral cavity of Chinese cobras30.
Accurate and rapid identification of bacteria is of critical importance in the diagnosis and management of infections. Although traditional phenotypic methods and commercial kits allow identification of most commonly encountered bacterial species in clinical microbiology laboratories, they often fail to differentiate Tsukamurella from the other related genera of the order Corynebacteriales24, 31, such as Nocardia, Rhodococcus, and Gordonia. Species identification within these genera is difficult in most clinical microbiology laboratories, as they share similar phenotypic properties. With the increasing availability of polymerase chain reaction (PCR) and DNA sequencing facilities, amplification, and sequencing of universal gene targets represents an advanced technology that theoretically yields unambiguous identification results, especially in cases where bacterial isolates are difficult to identify by phenotypic tests. Among the various studied gene targets, the 16S ribosomal RNA (rRNA) gene has been the most widely used for bacterial identification and classification32, 33. However, our previous study showed that this gene target cannot be confidently used for species identification of Tsukamurella as highly similar 16S rRNA gene sequence can be shared by different Tsukamurella species34. Sequencing of an alternative gene target, the groEL gene, was shown to be useful and accurate for identification of all existing Tsukamurella species34. Nevertheless, PCR sequencing is still considered expensive, time-consuming and technically demanding, and therefore is yet to be incorporated as a routine identification method in clinical microbiology laboratories.
Matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS) has recently emerged as a revolutionary technique for identification of bacterial and fungal pathogens, yielding rapid, accurate and highly reproducible results at a lower price than any other methods routinely used in clinical microbiology laboratories35–39. The application of MALDI-TOF MS for identification of Tsukamurella species has not been fully explored to date. So far, there were only two studies that have reported on the evaluation of MALDI-TOF MS for identification of Tsukamurella, with only two Tsukamurella strains being included in each series40, 41. In this study, using groEL gene sequencing as the reference method, we evaluated the performance of MALDI-TOF MS using the Bruker Biotyper system for identification of 60 clinical Tsukamurella isolates.
Results
groEL gene sequence analysis of Tsukamurella isolates
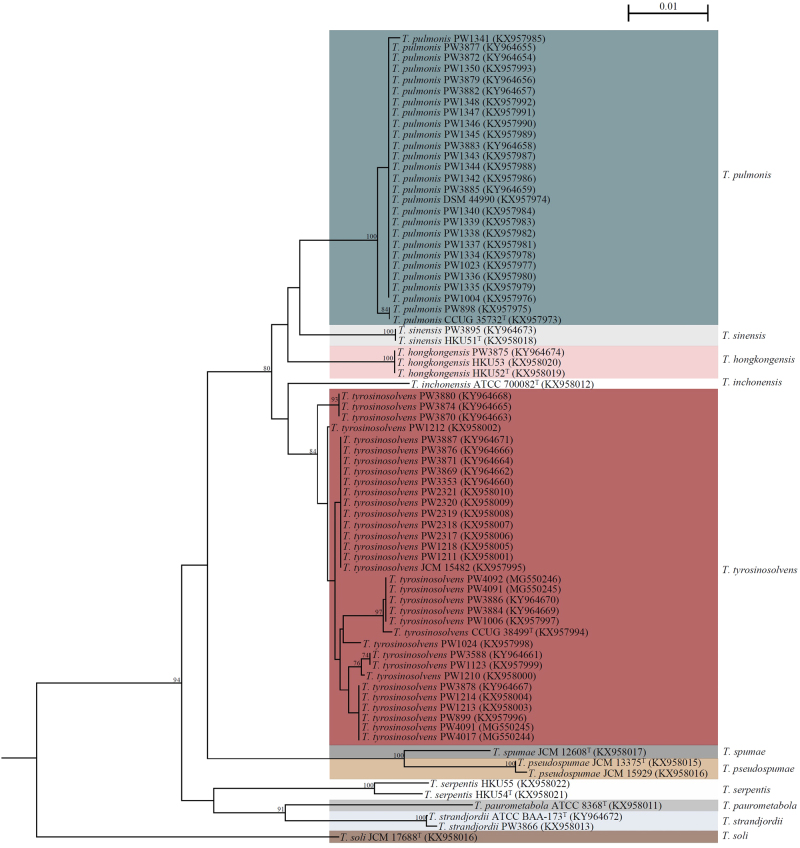
Using groEL gene sequencing as the reference method, the 60 Tsukamurella isolates included in this study were identified as five different Tsukamurella species, including T. tyrosinosolvens (n = 31, 51.7%), T. pulmonis (n = 25, 41.7%), T. hongkongensis (n = 2, 3.3%), T. strandjordii (n = 1, 1.7%), and T. sinensis (n = 1, 1.7%). Among the 25 T. pulmonis strains identified, 16 (64.0%) were isolated from the oral cavities of Chinese cobras while the remaining 9 (36.0%) were isolated from patients. groEL gene sequence analysis showed that intraspecies nucleotide identities among the 27 strains of T. tyrosinosolvens, the 25 strains of T. pulmonis and the two strains of T. hongkongensis ranged from 98.7 to 100.0%, 99.7 to 100.0% and 100.0%, respectively. Phylogenetic tree construction based on the groEL gene sequences revealed that all the 60 isolates clustered correctly with their corresponding type and reference strains of the same species (Fig. 1).
Fig. 1. Phylogenetic trees showing the relationship of Tsukamurella strains included in this study, including 15 type and reference strains and 60 Tsukamurella isolates.
The tree was inferred from partial groEL sequence data (677 nucleotide positions of the trimmed sequence alignments respectively) by the maximum-likelihood method using the model GTR + I + G and Mycobacterium smegmatis MC2 155 (CP009494.1) as the outgroup. The scale bar indicates the estimated number of substitutions per base. Numbers at nodes indicate levels of bootstrap support calculated from 1000 trees and expressed as percentage. All names and accession numbers are given as cited in the GenBank database
Clinical spectra of Tsukamurella infections in Hong Kong
Including the clinical isolates that we previously reported in our locality1, 2, 34, the most common Tsukamurella species observed in our patients was T. tyrosinosolvens (n = 31), followed by T. pulmonis (n = 9), T. hongkongensis (n = 3), T. sinensis (n = 2), and T. strandjordii (n = 1). These Tsukamurella isolates were recovered from different types of clinical specimens, most commonly eye (n = 18), sputum (n = 6), and blood (n = 6) (Table 1).
Table 1.
Summary of Tsukamurella species isolated from different clinical specimens
| Type of specimen | T. tyrosinosolvens (n = 31) | T. pulmonis (n = 9) | T. hongkongensis (n = 3) | T. sinensis (n = 2) | T. strandjordii (n = 1) |
|---|---|---|---|---|---|
| Eye (n = 18) | 11 | 4 | 1 | 2 | 0 |
| Sputum (n = 6) | 4 | 0 | 1 | 0 | 1 |
| Blood (n = 6) | 4 | 1 | 1 | 0 | 0 |
| Others (n = 9) | |||||
| Axillary lymph node biopsya | 1 | 0 | 0 | 0 | 0 |
| Plantar granulation tissuea | 2 | 0 | 0 | 0 | 0 |
| Lung tissuea | 1 | 0 | 0 | 0 | 0 |
| Peritoneal tissuea | 1 | 0 | 0 | 0 | 0 |
| Peritoneal dialysis fluid | 1 | 0 | 0 | 0 | 0 |
| Pus swab from submandibular skin | 0 | 1 | 0 | 0 | 0 |
| Superficial wound swab from hand | 1 | 1 | 0 | 0 | 0 |
| Unknown specimens (n = 7) | 5 | 2 | 0 | 0 | 0 |
a The recovery of these isolates was due to laboratory contamination during processing of the tissue specimens [previously reported in ref. 29]
Species identification of Tsukamurella by MALDI-TOF MS
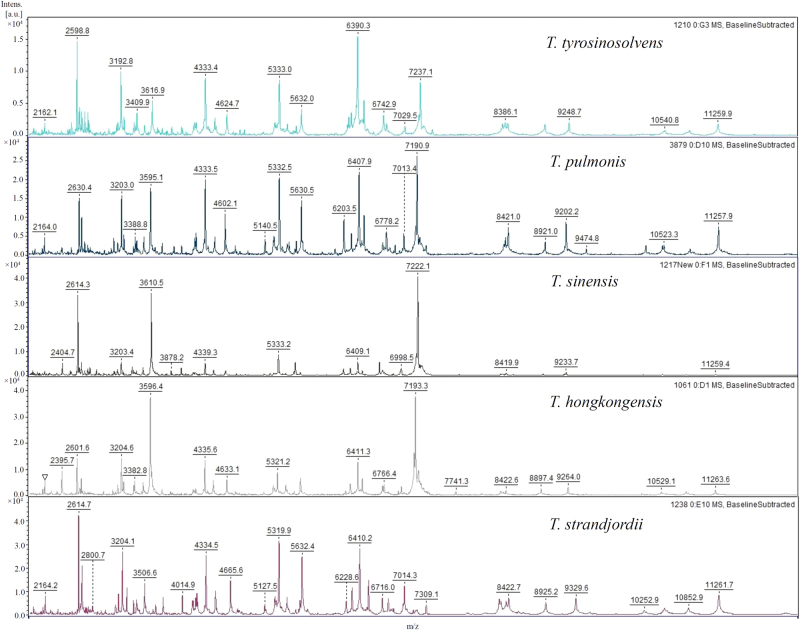
The MALDI-TOF MS results of the 15 Tsukamurella type and reference strains using the Bruker reference library V.6.0.0.0 (6903 spectra) were shown in Table 2. Using groEL gene sequencing as the reference method for species identity, 14 (93.3%) of the 15 isolates were identified correctly to the genus level but only 6 (40.0%) showed the correct genus with score ≥1.7. One isolate showed incorrect genus identification. T. soli was misidentified as Corynebacterium jeikeium with score <1.7 (Table 2). Of the 14 isolates identified to the genus level, only 2 (14.3%) showed the correct species with score ≥2.0 (Table 2). Of the 13 strains which showed incorrect species identification, all were due to the absence of the corresponding species in the database. Therefore, reference spectra which covered all the currently recognized 11 Tsukamurella species were included in the reference library. The spectra of some commonly observed Tsukamurella species, including T. tyrosinosolvens, T. pulmonis, T. hongkongensis, T. strandjordii, and T. sinensis, were shown in Fig. 2. Using the extended in-house Bruker database, all the 15 type and reference strains were identified correctly to the species level with score ≥2.0 (Table 2).
Table 2.
MALDI-TOF MS results of 75 Tsukamurella species and 14 species of closely related genera using Bruker database and extended in-house database
| Bacterial species | Total no. of isolates (n = 89) | No. of isolates resulting in the indicated score attained using: | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Bruker Reference Library V.6.0.0.0 | Extended in-house database | ||||||||
| ≥2.0 | 1.7–1.9 | <1.7 | Incorrect species identification | ≥2.0 | 1.7–1.9 | <1.7 | Incorrect species identification | ||
| Type and reference (n = 15)a | |||||||||
| T. paurometabola | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| T. inchonensis | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| T. strandjordii | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 |
| T. pulmonis | 2 | 0 | 1 | 1 | 2 | 2 | 0 | 0 | 0 |
| T. tyrosinosolvens | 2 | 2 | 0 | 0 | 2 | 2 | 0 | 0 | 0 |
| T. pseudospumae | 2 | 0 | 0 | 2 | 2 | 2 | 0 | 0 | 0 |
| T. spumae | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 |
| T. soli | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 |
| T. serpentis | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 |
| T. hongkongensis | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 |
| T. serpentis | 2 | 0 | 0 | 2 | 2 | 2 | 0 | 0 | 0 |
| Clinical or veterinary (n = 74) | |||||||||
| T. tyrosinosolvens | 31 | 20 | 10 | 1 | 31 | 31 | 0 | 0 | 0 |
| T. pulmonis | 25 | 0 | 9 | 16 | 25 | 25 | 0 | 0 | 0 |
| T. hongkongensis | 2 | 0 | 0 | 2 | 2 | 1 | 1 | 0 | 1 |
| T. sinensis | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 |
| T. strandjordii | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 |
| N. nova | 3 | 3 | 0 | 0 | 0 | 3 | 0 | 0 | 0 |
| N. cyriacigeorgica | 2 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 |
| N. brasiliensis | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 |
| N. farcinica | 2 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 |
| R. equi | 2 | 2 | 0 | 0 | 0 | 2 | 0 | 0 | 0 |
| R. erythropolis | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| G. sputi | 2 | 2 | 0 | 0 | 0 | 2 | 0 | 0 | 0 |
| G. bronchialis | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
a Among the 15 type and reference strains, only 6 (40.0%) showed the correct genus with score ≥1.7 by MALDI-TOF MS using the Bruker Reference Library V.6.0.0.0
Fig. 2. MALDI-TOF MS spectra of T. tyrosinosolvens, T. pulmonis, T. hongkongensis, T. strandjordii, and T. sinensis.
The intensity in arbitrary units [a.u.] are shown on the y axis, and the masses (m/z) of the ions are shown on the x axis. The m/z values represent the mass-to-charge ratios
The MALDI-TOF MS results of the 60 Tsukamurella isolates, including patient (n = 44) and veterinary (n = 16) isolates, using the Bruker reference library V.6.0.0.0 (6903 spectra), was shown in Table 2. Using groEL gene sequencing as the reference method for species identification, all isolates were identified correctly to the genus level but only 39 (65.0%) showed the correct genus with score ≥1.7, and none of the 60 isolates were identified correctly to the species level (Table 2). Using the extended in-house Bruker database, 59 (98.3%) of the 60 isolates were correctly identified to the species level with score ≥2.0 [54 (90.0%) with score of top match ≥2.0 and score of second match lower by ≥10%]. One of the two strains of T. hongkongensis was misidentified as T. inchonensis (score <2.0) though the MSP of T. hongkongensis has been already included in the extended in-house database.
Using both the Bruker reference library V.6.0.0.0 and the extended in-house database, all the 14 clinical isolates belonging to genera that are closely related to Tsukamurella, including Nocardia [N. nova (n = 3); N. cyriacigeorgica (n = 2); N. brasiliensis (n = 1); N. farcinica (n = 2)], Rhodococcus [R. equi (n = 2); R. erythropolis (n = 1)], and Gordonia [G. sputi (n = 2); G. bronchialis (n = 1)], were identified correctly to species level. Among these, 11 (78.6%) showed correct species with score ≥2.0 (Table 2).
Clustering analysis of the spectra of Tsukamurella generated by the bruker biotyper
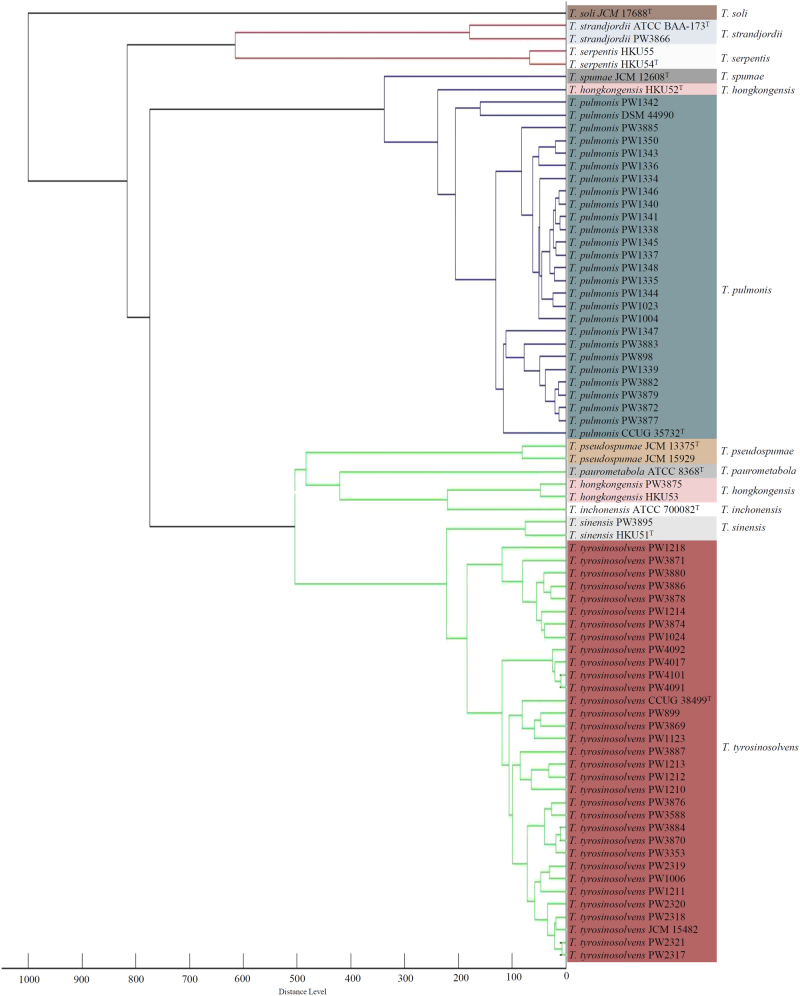
Dendrogram generated from hierarchical cluster analysis of MALDI-TOF MS showed that the spectra of the 15 Tsukamurella type and reference strains and 60 Tsukamurella isolates were able to form distinct clusters for each Tsukamurella species, except for one strain of T. hongkongensis, which was not able to cluster with the other two strains of T. hongkongensis (Fig. 3). Except this particular T. hongkongensis strain, the topology of the dendrogram was completely concordant to that of the phylogenetic tree constructed based on groEL gene sequence (Fig. 1).
Fig. 3. Dendrogram generated from hierarchical cluster analysis of MALDI-TOF MS spectra of 15 type and reference strains and 60 Tsukamurella isolates included in this study.
Distances are displayed in relative units
GenBank nucleotide sequence accession numbers
The nucleotide sequences of the partial groEL gene sequences of Tsukamurella isolates obtained in the present study have been lodged within the Genbank sequence database under accession numbers KY964654-KY964674 and MG550244-MG550247.
Discussion
MALDI-TOF MS should be useful for routine species identification of Tsukamurella in clinical microbiology laboratories after optimization of the database by adding reference MSPs of all the known Tsukamurella species. Differentiation of Tsukamurella from other related genera of the order Corynebacteriales, such as Nocardia, Rhodococcus, and Gordonia; and species identification within these genera are difficult in most clinical microbiology laboratories, as they share similar phenotypic properties. Recently, we showed that groEL gene sequencing is a reliable molecular diagnostic method for species identification of Tsukamurella34. However, identification by sequencing is still beyond the reach of many routine clinical laboratories. In contrast, MALDI-TOF MS is user-friendly, rapid, and cost-effective. Most importantly, this method allows the addition of MSPs of bacterial species not included in the database to improve its performance for bacterial species identification. In this study, all the 60 isolates were identified correctly to the genus level using the Bruker database V.6.0.0.0 (6903 spectra). However, all of them were misidentified at the species level, which is in line with the results of the two previous studies where none of the four Tsukamurella strains tested, including two strains of T. tyrosinosolvens and two strains of unknown species identity, could be identified correctly to the species level40, 41. This is most likely due to the absence of a comprehensive Tsukamurella spectral database in the Bruker system which, at the time of writing, includes only the spectra of two species, T. paurometabola and T. inchonensis. In view of this problem, we optimized the database by adding 15 MSPs covering all the known Tsukamurella species. Using the expanded in-house database, 59 (98.3%) of the 60 Tsukamurella isolates can be correctly identified to species level with score ≥2.0. This marked increase in accuracy after adding MSPs in the database is in line with results of our previous study, which also showed that by including 21 B. pseudomallei MSPs in the Bruker database, all 31 clinical, veterinary and environmental isolates of B. pseudomallei were correctly identified42. In addition to the improved identification accuracy, such in-house database can also facilitate the rapid species identification of Tsukamurella. We previously reported a study of Tsukamurella pseudo-outbreak due to environmental contamination of laboratory device29. The species identities of the concerned Tsukamurella isolates (n = 5) were determined as T. tyrosinosolvens after 10 days of extensive diagnostic workup29. In contrast, using the expanded in-house Bruker database developed in this study, all the concerned isolates could be rapidly identified as T. tyrosinosolvens within an hour, highlighting this technology could markedly shorten the turnaround time for identification of Tsukamurella.
The number of strains for each species in MALDI-TOF MS databases should also be expanded to cover intraspecies variability to improve the performance and accuracy for species identification. Although we have added one reference spectrum of T. hongkongensis in the MALDI-TOF MS database, not all the strains of T. hongkongensis could be correctly identified. There were two test strains of T. hongkongensis included in the present study, one showed correct species identification with high score (score ≥2.0), whereas the other test strain was misidentified as T. inchonensis with score <2.0 (Table 2). Consistently, dendrogram also revealed that one T. hongkongensis strain was separated from the T. hongkongensis cluster, being more related to the T. pulmonis cluster (Fig. 3). We speculate that the wrong identification or clustering may be due to the inclusion of just one T. hongkongensis MSP in the database. We anticipate that correct identification of this test strain can be achieved when more MSPs belonging to the species of T. hongkongensis is included. This is also in line with our previous observation that our three Burkholderia thailandensis isolates were originally misidentified as B. pseudomallei when the database contained only one B. thailandensis MSP, but expansion of the database with one additional B. thailandensis reference strain enabled correct identification of two other B. thailandensis isolates42. Increasing the number of available spectra for each species is essential to cover intraspecies variability, thereby improving the accuracy of MALDI-TOF MS for identification of Tsukamurella.
T. tyrosinosolvens was the most common species observed in patients with Tsukamurella infections, especially ocular infections, in our locality. Among the 46 patient isolates included in the present study, 31 (67.4%) were identified as T. tyrosinosolvens, followed by T. pulmonis (n = 9, 19.6%). The predominance of T. tyrosinosolvens is similar to that of Taiwan where T. tyrosinosolvens was also the most commonly encountered Tsukamurella species in their population24. Among the cases reported in the literature during 1992–2016, T. tyrosinosolvens also appeared to be more prevalent than other Tsukamurella species1–14, 18–20. However, most of these studies were relied on phenotypic tests and/or 16S rRNA gene sequencing for species identification, which may not offer sufficient discriminative power for species discrimination3, 5–8, 10, 13, 14, 20. Similar studies in other countries using groEL gene sequencing or MALDI-TOF MS with an expanded in-house Bruker database for species identification of Tsukamurella are required to more accurately assess their relative clinical importance and ascertain the emergence and pathogenic potential of T. tyrosinosolvens.
Materials and methods
Type and reference strains
A total of 15 type and reference strains of the genus Tsukamurella, representing 11 species, were obtained from four public culture collections, with T. pulmonis CCUG 35732T and T. tyrosinosolvens CCUG 38499T obtained from Culture Collection, University of Göteborg, T. paurometabola ATCC 8368T, T. strandjordii ATCC BAA-173T, and T. inchonensis ATCC 700082T from American Type Culture Collection, T. pulmonis DSM 44990 from Leibniz Institute DSMZ – German Collection of Microorganisms and Cell Cultures and T. hongkongensis JCM 30715T, T. sinensis JCM 30714T, T. serpentis JCM 31017T, T. serpentis JCM 31018, T. soli JCM 17688T, T. pseudospumae JCM 15929, T. pseudospumae JCM 13375T, T. spumae JCM 12608T, and T. tyrosinosolvens JCM 15482 from Japan Collection of Microorganisms.
Patient and veterinary isolates
In addition to the 15 Tsukamurella type and reference strains, 60 Tsukamurella clinical or veterinary isolates were included in this study. Among these, 44 were isolated from patients whereas 16 were isolated from the oral cavities of healthy Chinese cobras (Naja atra). Of the 44 human isolates, they were recovered from different types of specimens from in-patients of four different hospitals during 2004 to 2017. The presumptive identification of all the 60 isolates to the genus level was initially made by conventional biochemical methods (i.e., displayed as typical dry and rough colonies on horse blood agar, appeared as Gram-positive rods, and negative for Ziehl–Neelsen stain but positive for modified Ziehl-Neelsen stain) prior to performing groEL gene sequencing for confirmation of their species identities34, 43. The species identities of the 34 isolates were confirmed in a previous study34, whereas those of the remaining 26 isolates were confirmed in the present study by groEL gene sequencing using the validated threshold value of 98.2% for species identification34. Fourteen clinical isolates belonging to genera that are closely related to Tsukamurella, including Nocardia [N. nova (n = 3); N. cyriacigeorgica (n = 2); N. brasiliensis (n = 1); N. farcinica (n = 2)], Rhodococcus [R. equi (n = 2); R. erythropolis (n = 1)], and Gordonia [G. sputi (n = 2); G. bronchialis (n = 1)] were used to evaluate the extended in-house database.
DNA extraction
Bacterial DNA extraction was modified from our previous published protocol25. Briefly, bacterial cells were harvested and suspended in sterile distilled water. One microliter of RNase A (10 mg/mL) (QIAGEN, Hilden, Germany) and 2 μL of lysozyme (100 mg/mL) (Sigma Aldrich, St Louis, MO, USA) were added into the cell suspension and the mixture was incubated at 37 °C for 60 min to break down the bacterial cell wall. Two microliters of proteinase K (600 mAU/mL) (QIAGEN) and 120 μL of SDS (10%) (Sigma Aldrich) were then added into the mixture and incubated at 65 °C for 60 min to lyse the cells. Subsequently, 420 μL of ammonium acetate (8 M) (Sigma Aldrich) was added into the cell lysate and the mixture was chilled on ice immediately for 5 min followed by centrifuged at 16,100 × g for 20 min. The supernatant was collected, mixed with an equal volume of isopropanol to precipitate DNA overnight at 4 °C. On the following day, the cell pellet was washed with 70% ethanol and DNA was re-dissolved in 25 μL of sterile distilled water. The concentration was measured by a micro-volume UV-visible light spectrophotometer (NanoDrop 2000) (Thermo Scientific, Waltham, MA, USA). The resultant mixture was diluted 100× and 0.5 μL of the diluted extract was used for PCR.
PCR amplification and sequencing
Extracted DNA from the 25 isolates of Tsukamurella was used as the template for amplification of groEL gene using primers LPW34162 (5′-GAC GCT CAT CGT CAA CAA GAT CC-3′) and LPW33894 (5′-GGA CTY AGA AGT CCA TGC CGC CCA T-3′). The PCR mixture (25 μL) contained denatured Tsukamurella genomic DNA, 4% of DMSO (Bio-Rad, Hercules, CA, USA), PCR buffer (10 mM Tris-HCl pH 8.3 and 50 mM KCl), 2 mM MgCl2, 10 μM forward and reverse primers, 200 μM of each deoxynucleoside triphosphates and 2.5 U AmpliTaq Gold DNA polymerase (Applied Biosystems, Foster City, CA, USA). The sample was amplified in 40 cycles of 94 °C for 1 min, 55 °C for 1.5 min and 72 °C for 2 min, and with a final extension at 72 °C for 7 min in an automated thermal cycler (Applied Biosystems). Five microliters of each amplified product was electrophoresed in 2% (w/v) agarose gel, with a molecular size marker (GeneRuler™ 50 bp DNA ladder, MBI Fermentas, Canada). Electrophoresis in Tris-borate-EDTA buffer was performed at 100 volts for 45 min. The gel was stained with ethidium bromide (0.5 μg/mL) for 20 min, rinsed and photographed under ultraviolet light illumination.
The PCR product was gel-purified using the QIAquick PCR purification kit (QIAGEN). Both strands of the PCR product were sequenced using BigDye Terminator Cycle Sequencing kit version 3.1 with an ABI Prism 3730XL Analyzer according to manufacturer’s instructions (Applied Biosystems) and the PCR primers of respective gene targets.
Phylogenetic analyses
The sequences of the PCR products were compared with sequences of all currently recognized Tsukamurella species by multiple sequence alignment using MUSCLE 3.844 and the aligned sequences were trimmed using BioEdit 7.2.045. Comparative gene sequence analysis was performed using BioEdit 7.2.0. Phylogenetic tree was constructed by maximum-likelihood method using MEGA version 646.
MALDI-TOF MS analysis
Sample preparation for MALDI-TOF MS analysis using Bruker Biotyper was performed as previously described with modifications47. Briefly, bacterial strains were grown on blood agar at 37 °C for 48 h. Two to three colonies were harvested in 500 μL of sterile water and boiled for 30 min followed by centrifugation at 13,000 × g for 2 min to remove the supernatant. Sterile water (300 μL) and pure ethanol (900 μL) were added to the suspension and mixed. The suspensions were centrifuged twice at 13,000 × g for 2 min to remove the supernatant. The pellets were dried at room temperature and suspended in 50 μL of 70% formic acid (Sigma-Aldrich) and 50 μL of acetonitrile (Sigma-Aldrich). After centrifugation at 13,000 × g for 2 min, 1 μL of supernatant was spotted on a polished steel target plate. Immediately after drying, spots were overlaid with 1 μL of matrix solution. Samples were processed in Bruker Biotyper (Bruker Daltonics, GmbH, Bremen, Germany) system. Spectra within the range of m/z 2000–20,000 Da were obtained with an accelerating voltage of 20 kV in linear mode and analyzed with MALDI Biotyper 3.1 and Reference Library V.6.0.0.0 (6903 spectra). Bruker bacterial test standard (BTS, no. 255343, Bruker Daltonics) was used for calibration and quality control in each run. Thresholds for species and genus identification were ≥2.0 and ≥1.7, respectively. Results were presented with the species and genus identification and compared to identification results by groEL gene sequencing. Scores below the cutoff were considered invalid results with the conclusion “not reliable identification”. Furthermore, the mass spectrum profile (MSP) created from spectra of each strain was used for hierarchical cluster analysis using the Bruker Biotyper software with default parameters, where distance values were relative and normalized to a maximum value of 100048.
Establishment of a Tsukamurella database
The procedures for establishing an in-house Tsukamurella database was performed as previously described with modifications47. One microliter of the final extraction product of the Tsukamurella strain was spotted four times onto a steel target and each spot was measured six times for collecting a total of 24 individual, high-quality spectra per isolate. The spectra were then analyzed in the flexAnalysis software (version 3.0, Bruker Daltonics) to generate a single mean spectrum for each Tsukamurella reference strain using the Biotyper MSP creation standard method as described previously49.
Acknowledgements
This work is partly supported by the Seed Fund for Basic Research, The University of Hong Kong; and the Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, the Ministry of Education of China.
Authors’ contribution:
J.T. designed the study, contributed to the interpretation of results, and wrote the manuscript. Y.T. performed the laboratory work, contributed to the interpretation of results, and revised the manuscript. S.W. designed the study, contributed reagents, and revised the manuscript. J.F., Z.Z., and C.P.W. performed the laboratory work. J.C. and A.N. contributed to the interpretation of results and revised the manuscript. A.W., K.F., and T.L.Q. gave advice on the study and revised the manuscript. S.L. and P.W. conceived the study, designed the study, contributed reagents, and revised the manuscript. All authors read and approved the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Contributor Information
Susanna K. P. Lau, Email: skplau@hku.hk
Patrick C. Y. Woo, Email: pcywoo@hku.hk
References
- 1.Woo PCY, et al. First report of Tsukamurella keratitis: association between T. tyrosinosolvens and T. pulmonis and ophthalmologic infections. J. Clin. Microbiol. 2009;47:1953–1956. doi: 10.1128/JCM.00424-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Woo PCY, Ngan AHY, Lau SKP, Yuen KY. Tsukamurella conjunctivitis: a novel clinical syndrome. J. Clin. Microbiol. 2003;41:3368–3371. doi: 10.1128/JCM.41.7.3368-3371.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elshibly S, et al. Central line-related bacteraemia due to Tsukamurella tyrosinosolvens in a haematology patient. Ulst. Med. J. 2005;74:43–46. [PMC free article] [PubMed] [Google Scholar]
- 4.Granel F, et al. Cutaneous infection caused by Tsukamurella paurometabolum. Clin. Infect. Dis. 1996;23:839–840. doi: 10.1093/clinids/23.4.839. [DOI] [PubMed] [Google Scholar]
- 5.Jones RS, Fekete T, Truant AL, Satishchandran V. Persistent bacteremia due to Tsukamurella paurometabolum in a patient undergoing hemodialysis: case report and review. Clin. Infect. Dis. 1994;18:830–832. doi: 10.1093/clinids/18.5.830. [DOI] [PubMed] [Google Scholar]
- 6.Lai KK. A cancer patient with central venous catheter-related sepsis caused by Tsukamurella paurometabolum (Gordona aurantiaca) Clin. Infect. Dis. 1993;17:285–287. doi: 10.1093/clinids/17.2.285-a. [DOI] [PubMed] [Google Scholar]
- 7.Maalouf R, Mierau SB, Moore TA, Kaul A. First case report of community-acquired pneumonia due to Tsukamurella pulmonis. Ann. Intern. Med. 2009;150:147–148. doi: 10.7326/0003-4819-150-2-200901200-00022. [DOI] [PubMed] [Google Scholar]
- 8.Maertens J, et al. Catheter-related bacteremia due to Tsukamurella pulmonis. Clin. Microbiol. Infect. 1998;4:51–53. doi: 10.1111/j.1469-0691.1998.tb00336.x. [DOI] [PubMed] [Google Scholar]
- 9.Matsumoto T, et al. Tsukamurella tyrosinosolvens cultured from sputum of a patient who received total gastrectomy for gastric cancer. Kekkaku. 2006;81:487–490. [PubMed] [Google Scholar]
- 10.Perez VA, Swigris J, Ruoss SJ. Coexistence of primary adenocarcinoma of the lung and Tsukamurella infection: a case report and review of the literature. J. Med. Case Rep. 2008;2:207. doi: 10.1186/1752-1947-2-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rey D, et al. Tsukamurella and HIV infection. AIDS. 1995;9:1379. doi: 10.1097/00002030-199512000-00014. [DOI] [PubMed] [Google Scholar]
- 12.Schwartz MA, et al. Central venous catheter-related bacteremia due to Tsukamurella species in the immunocompromised host: a case series and review of the literature. Clin. Infect. Dis. 2002;35:e72–e77. doi: 10.1086/342561. [DOI] [PubMed] [Google Scholar]
- 13.Shapiro CL, et al. Tsukamurella paurometabolum: a novel pathogen causing catheter-related bacteremia in patients with cancer. Clin. Infect. Dis. 1992;14:200–203. doi: 10.1093/clinids/14.1.200. [DOI] [PubMed] [Google Scholar]
- 14.Stanley T, et al. The potential misidentification of Tsukamurella pulmonis as an atypical Mycobacterium species: a cautionary tale. J. Med. Microbiol. 2006;55:475–478. doi: 10.1099/jmm.0.46355-0. [DOI] [PubMed] [Google Scholar]
- 15.Tsukamura M, Kawakami K. Lung infection caused by Gordona aurantiaca (Rhodococcus aurantiacus) J. Clin. Microbiol. 1982;16:604–607. doi: 10.1128/jcm.16.4.604-607.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Osoagbaka OU. Evidence for the pathogenic role of Rhodococcus species in pulmonary diseases. J. Appl. Bacteriol. 1989;66:497–506. doi: 10.1111/j.1365-2672.1989.tb04570.x. [DOI] [PubMed] [Google Scholar]
- 17.Takebe I, Sawabe E, Ohkusu K, Tojo N, Tohda S. Catheter-related bloodstream infection by Tsukamurella inchonensis in an immunocompromised patient. J. Clin. Microbiol. 2014;52:2251–2253. doi: 10.1128/JCM.00421-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bilbo, D. & Munson, E.. Photo quiz: a 47-year-old female with general fatigue, fever, and respiratory symptoms. Answer to photo quiz: bacteremia caused by Tsukamurella tyrosinosolvens. J. Clin. Microbiol.52, 711, 1026 (2014).. [DOI] [PMC free article] [PubMed]
- 19.Chen CH, Lee CT, Chang TC. Tsukamurella tyrosinosolvens bacteremia with coinfection of Mycobacterium bovis pneumonia: case report and literature review. Springerplus. 2016;5:2033. doi: 10.1186/s40064-016-3707-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karunakaran R, et al. Tsukamurella tyrosinosolvens intravascular catheter-related bacteremia in a haematology patient: a case report. Eur. Rev. Med. Pharmacol. Sci. 2011;15:1343–1346. [PubMed] [Google Scholar]
- 21.Teng JLL, et al. Phylogenomic analyses and reclassification of species within the genus Tsukamurella: insights to species definition in the post-genomic era. Front. Microbiol. 2016;7:1137. doi: 10.3389/fmicb.2016.01137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oren A, Garrity GM. Notification of changes in taxonomic opinion previously published outside the IJSEM. Int. J. Syst. Evol. Microbiol. 2017;67:7–8. doi: 10.1099/ijsem.0.001710. [DOI] [PubMed] [Google Scholar]
- 23.Goodfellow, M. & Kumar, Y. in Bergey’s Mannual of Systematic Bacteriology Vol. 5 (eds Goodfellow, M. et al.) 500–509 (Springer, 2012).
- 24.Liu CY, et al. Clinical characteristics of infections caused by Tsukamurella spp. and antimicrobial susceptibilities of the isolates. Int. J. Antimicrob. Agents. 2011;38:534–537. doi: 10.1016/j.ijantimicag.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 25.Teng JLL, et al. Tsukamurella hongkongensis sp. nov. and Tsukamurella sinensis sp. nov., isolated from patients with keratitis, catheter-related bacteraemia and conjunctivitis. Int. J. Syst. Evol. Microbiol. 2016;66:391–397. doi: 10.1099/ijsem.0.000733. [DOI] [PubMed] [Google Scholar]
- 26.Jiang Y, et al. Diversity of cultivable actinomycetes in 6 species of herbivore feces. Int. J. Microbiol. Res. 2013;1:76–84. [Google Scholar]
- 27.Maeda Y, et al. No evidence of transmission of bacteria between reptiles and a CF patient–a case report of a young adult CF patient and reptiles. Zoonoses Public Health. 2010;57:e47–e53. doi: 10.1111/j.1863-2378.2009.01293.x. [DOI] [PubMed] [Google Scholar]
- 28.Park SW, Kim SM, Park ST, Kim YM. Tsukamurella carboxydivorans sp. nov., a carbon monoxide-oxidizing actinomycete. Int. J. Syst. Evol. Microbiol. 2009;59:1541–1544. doi: 10.1099/ijs.0.005959-0. [DOI] [PubMed] [Google Scholar]
- 29.To KKW, et al. Characterization of a Tsukamurella pseudo-outbreak by phenotypic tests, 16S rRNA sequencing, pulsed-field gel electrophoresis, and metabolic footprinting. J. Clin. Microbiol. 2013;51:334–338. doi: 10.1128/JCM.02845-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang Y, et al. Tsukamurella serpentis sp. nov., isolated from the oral cavity of Chinese cobras (Naja atra) Int. J. Syst. Evol. Microbiol. 2016;66:3329–3336. doi: 10.1099/ijsem.0.001187. [DOI] [PubMed] [Google Scholar]
- 31.Alcaide ML, Espinoza L, Abbo L. Cavitary pneumonia secondary to Tsukamurella in an AIDS patient. First case and a review of the literature. J. Infect. 2004;49:17–19. doi: 10.1016/S0163-4453(03)00139-7. [DOI] [PubMed] [Google Scholar]
- 32.Fox GE, Magrum LJ, Balch WE, Wolfe RS, Woese CR. Classification of methanogenic bacteria by 16S ribosomal RNA characterization. Proc. Natl Acad. Sci. USA. 1977;74:4537–4541. doi: 10.1073/pnas.74.10.4537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gupta R, Lanter JM, Woese CR. Sequence of the 16S ribosomal RNA from Halobacterium volcanii, an Archaebacterium. Science. 1983;221:656–659. doi: 10.1126/science.221.4611.656. [DOI] [PubMed] [Google Scholar]
- 34.Teng JL, et al. The groEL gene is a promising target for species-level identification of Tsukamurella. J. Clin. Microbiol. 2017;55:649–653. doi: 10.1128/JCM.02260-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bizzini A, Durussel C, Bille J, Greub G, Prod’hom G. Performance of matrix-assisted laser desorption ionization-time of flight mass spectrometry for identification of bacterial strains routinely isolated in a clinical microbiology laboratory. J. Clin. Microbiol. 2010;48:1549–1554. doi: 10.1128/JCM.01794-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eigner U, et al. Performance of a matrix-assisted laser desorption ionization-time-of-flight mass spectrometry system for the identification of bacterial isolates in the clinical routine laboratory. Clin. Lab. 2009;55:289–296. [PubMed] [Google Scholar]
- 37.Seng P, et al. Ongoing revolution in bacteriology: routine identification of bacteria by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Clin. Infect. Dis. 2009;49:543–551. doi: 10.1086/600885. [DOI] [PubMed] [Google Scholar]
- 38.Stevenson LG, Drake SK, Shea YR, Zelazny AM, Murray PR. Evaluation of matrix-assisted laser desorption ionization-time of flight mass spectrometry for identification of clinically important yeast species. J. Clin. Microbiol. 2010;48:3482–3486. doi: 10.1128/JCM.00687-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yaman G, Akyar I, Can S. Evaluation of the MALDI TOF-MS method for identification of Candida strains isolated from blood cultures. Diagn. Microbiol. Infect. Dis. 2012;73:65–67. doi: 10.1016/j.diagmicrobio.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 40.Hsueh PR, et al. Bruker biotyper matrix-assisted laser desorption ionization-time of flight mass spectrometry system for identification of Nocardia, Rhodococcus, Kocuria, Gordonia, Tsukamurella, and Listeria species. J. Clin. Microbiol. 2014;52:2371–2379. doi: 10.1128/JCM.00456-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yarbrough ML, Lainhart W, Burnham CD. Identification of Nocardia, Streptomyces, and Tsukamurella using MALDI-TOF MS with the Bruker Biotyper. Diagn. Microbiol. Infect. Dis. 2017;89:92–97. doi: 10.1016/j.diagmicrobio.2017.06.019. [DOI] [PubMed] [Google Scholar]
- 42.Lau SK, et al. Matrix-assisted laser desorption ionization-time of flight mass spectrometry for rapid identification of Burkholderia pseudomallei: importance of expanding databases with pathogens endemic to different localities. J. Clin. Microbiol. 2012;50:3142–3143. doi: 10.1128/JCM.01349-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smibert, R. M. & Krieg, N. R. in Methods for General and Molecular Bacteriology (eds Gerhardt, P., Murray, R. G. E., Wood, W. A., & Krieg, N. R.) 607–654 (ASM Press, Washington, D.C, 1994).
- 44.Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999;41:95–98. [Google Scholar]
- 46.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Verroken A, et al. Evaluation of matrix-assisted laser desorption ionization-time of flight mass spectrometry for identification of Nocardia species. J. Clin. Microbiol. 2010;48:4015–4021. doi: 10.1128/JCM.01234-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ketterlinus, R., Hsieh, S. Y., Teng, S. H., Lee, H. & Pusch, W. Fishing for biomarkers: analyzing mass spectrometry data with the new ClinProTools software. Biotechniques38, S37–S40 (2005). [DOI] [PubMed]
- 49.Calderaro A, et al. MALDI-TOF MS analysis of human and animal Brachyspira species and benefits of database extension. J. Proteom. 2013;78:273–280. doi: 10.1016/j.jprot.2012.09.027. [DOI] [PubMed] [Google Scholar]