Abstract
Short-term exposures to air pollution are associated with acute effects on respiratory health. This study aimed to describe 10-year temporal trends in respiratory mortality in the urban areas of Shenyang, China, according to gender and age and estimate the effects of air pollution on respiratory diseases (ICD-10J00-J99) and lung cancer (ICD-10 C33-C34) using a case-crossover design. During the study period 2013–2015, the exposure-response relationship between ambient air pollutants and mortality data was fitted by a quasi-Poisson model. Age-standardized mortality rates for a combined number of respiratory diseases and for lung cancer declined in Shenyang; however, death counts increased with aging. Deaths from respiratory diseases increased by 4.7% (95% CI, 0.00–9.9), and lung cancer mortality increased by 6.5% (95% CI, 1.2–12.0), both associated with a 10 μg/m3 increase in exposure to particulate matter < 2.5 μg in diameter (PM2.5). Moreover, males in Shenyang’s urban areas were more susceptible to the acute effects of PM2.5 and SO2 exposure; people aged ≥ 65 years had a high susceptibility to ozone, and those aged < 65 years were more susceptible to other air pollutants. These results provided an updated estimate of the short-term effects of air pollution in Shenyang. Since population aging is also associated with increasing mortality from respiratory diseases and lung cancer, reinforcing air quality control measures and health-promoting behaviors is urgent and necessary in Shenyang.
Electronic supplementary material
The online version of this article (10.1007/s11356-018-1270-5) contains supplementary material, which is available to authorized users.
Keywords: Air pollution, Mortality, Respiratory disease, Lung cancer, Case-crossover, Particulate matter, Population aging
Introduction
Over the recent decades, the air quality in China has worsened due to rapid economic development, accelerated urbanization, and industrialization (Zhang and Cao 2015). China now ranks as one of the top polluted countries in the world. Disability-adjusted life years attributed to outdoor particulate matter (PM) exposure has been increasing in China over the past years (Guan et al. 2016). Epidemiological studies indicated that patients with chronic obstructive pulmonary disease are at an increased risk of death associated with the exposure to particle air pollutants (Sunyer et al. 2000). Previous evidences indicated that ambient air pollutants have long-term and short-term adverse effects on mortality and morbidity for cardiopulmonary diseases in developing countries, especially in China (Li et al. 2017). Several reviews of the adverse health effects of air pollution in the Chinese population yield similar results (Lu et al. 2015; Shang et al. 2013). In China, the short-term effect of the air pollution on the health burden has been studied in several cities such as Beijing, Shanghai, Wuhan, and Shenzhen (Lai and Brimblecombe 2017; Li et al. 2015; Liang et al. 2017; Lin et al. 2017; Zhang et al. 2017). A systematic review of 33 time series and case-crossover studies provided additional insights into the heterogeneity in effects on daily mortality after exposure to air pollution in China (Shang et al. 2013). The combined estimates in this meta-analysis indicated that exposures to all pollutants of interest significantly enhanced the risks of mortality in Chinese population, and the effects demonstrated heterogeneity in relation to the air pollution sources and the chemical composition of ambient particle.
Because of the regional or seasonal heterogeneity, the urban air in northern China was generally more polluted than that in the south mainly due to the coal smoke. Shenyang is an old industrial city in Northeast China. Since the winter is almost a half-year long, the use of coal-burning heating systems is very common in the city (Geng et al. 2013). Haze or smog episodes over the urban areas have become a common feature of winter, with levels of PM2.5 frequently exceeding 500 μg/m3. Associations between exposure to air pollution and mortality were observed in epidemiological studies in Shenyang (Ma et al. 2011; Zhang et al. 2011). However, updated air pollution epidemiological study on all pollutants of interest, especially on PM2.5 in Shenyang city, is still very limited. Meanwhile, rapid population aging is occurring in Shenyang city. The fraction of population aged ≥ 65 years in Shenyang’s urban areas has increased by 13% since 2013, compared with only 9% in 2005. Increases in natural mortality from PM exposure have been found among susceptible subgroups who were elderly persons (aged ≥ 65 years) and with chronic morbidity (Alessandrini et al. 2016). To better understand the association between air pollutants and mortality in Shenyang, especially in different age or gender groups, we evaluated 10-year temporal changes in respiratory mortality and lung cancer mortality, and also we examined the association between air pollutant exposure and daily respiratory death in Shenyang from 2013 to 2015 using a case-crossover analysis.
Materials and methods
Setting
Shenyang had an urban population of 3.8 million in 2014. The city typically has a sub-humid temperate continental climate, with an average temperature of 8 °C (46 °F). Its summer is not very hot, and the hottest month is July, with an average temperature of 23 °C (73 °F). The winter is cold and dry, with the coldest month being January, presenting an average temperature of − 16 °C (3 °F). Being one of the old cradles of industry in China, Shenyang is experiencing serious air pollution problems in recent years.
Data collection
Data regarding average daily air mass index (Air Quality Index, AQI) in Shenyang were obtained from the China National Environmental Monitoring Centre from January 2013 to December 2015. Since AQI was not routinely monitored in early 2013, data from the Mission China air quality monitoring program of the USA were collected. The 13 monitoring stations established in Shenyang were fully automated and routinely monitored levels of six criteria pollutants, including particulate matter < 2.5 μm in diameter (PM2.5), PM < 10 μm in aerodynamic diameter (PM10), sulfur dioxide (SO2), nitrogen dioxide (NO2), carbon monoxide (CO), and ozone (O3). The daily average concentration of pollutants at these urban stations was represented by the mean of the daily average concentrations of atmospheric pollutants of 13 national fixed-site stations, converted from the concentrations of multiple pollutants based on the Ambient Air Quality Standard (GB 3095-2012).
Daily meteorological data were obtained for the same period from the China Meteorological Administration from 1 January 2013 to 31 December 2015, including information on daily average temperature, barometric pressure, and relative humidity.
Daily mortality data of residents living in the urban areas of Shenyang were collected from the death registration system of Shenyang Center for Disease Control and Prevention during the same period. Causes of deaths were coded according to the International Classification of Diseases, 10th Revision (ICD-10); respiratory deaths were classified under codes J00-J99, and lung cancer under codes C33-C34. In this study, death counts for a combined number of respiratory diseases (J00-J99), lung cancer (C33-C34), and three common respiratory diseases (chronic lower respiratory disease [CLRD, J40-J47], pneumonia [J18], and pneumoconiosis [J61-J62]) were analyzed.
Data analysis
Mortality rates were age-standardized using the direct method based on the Chinese standard population of 2010. The average annual percentage of change (AAPC) and its 95% confidence interval (CI) were calculated using joinpoint regression analysis (Kim et al. 2000).
A time-stratified case-crossover design was used to evaluate the associations between the daily mean concentration of pollutants and the daily mortality count of each outcome, with adjustment for same-day meteorological factors including daily average temperature and relative humidity introduced as concomitant variables and considering the lag effects of air pollutant increments. Control days were chosen such that cases and controls were matched on the calendar month and day of the week. Air pollutant concentrations were examined in single-pollutant models for each disease category and the effects of lagging exposure for 0, 1, and 2 days (lag 0, lag l, and lag 2 days, respectively) as well as cumulative lags (lag 01 and lag 02) were assessed. Multiple-pollutant models (in which three pollutants of PM2.5, SO2 and NO2 were included together) examined the independence of any significant or marginally significant (P values ≈0.05) associations observed in single-pollutant models. PM10 and CO were excluded from the multiple-pollutant models due to their high correlation with other pollutants. For each pollutant, odds ratios (ORs) and 95% CIs were calculated using the Poisson regression. The regression model was modified by a quasi-Poisson model, which accounted for over dispersion in utilizing the GLM function of R3.3.2 in the stats package.
Results
Data description
The demographic characteristics of the study population for respiratory deaths are shown in Table 1. From 1 January 2005 to 31 December 2015, a total of 29,693 respiratory deaths and 29,112 lung cancer deaths were recorded. Pneumonia and CLRD accounted for 79.08% (23,482) of the total number of respiratory deaths.
Table 1.
Demographic characteristics of the study population
| Characteristics | Respiratory diseases (J00-J99) |
Pneumonia (J18) |
CLRD (J40-J47) |
Pneumoconiosis (J61-J62) |
Lung cancer (C33-C34) |
|
|---|---|---|---|---|---|---|
| Gender | Male deaths (%) | 16,402 (55.24%) | 7465 (58.90%) | 5325 (49.26%) | 252 (87.20%) | 17,656 (60.65%) |
| Female deaths (%) | 13,291 (44.76%) | 5208 (41.10%) | 5484 (50.74%) | 37 (12.80%) | 11,456 (39.35%) | |
| Age | Mean ± SD | 78.14 ± 11.86 | 78.72 ± 12.53 | 78.30 ± 10.30 | 80.24 ± 12.28 | 70.87 ± 11.60 |
CLRD chronic lower respiratory disease
Death rates were age-standardized based on the Chinese standard population of the Sixth National Population Census. The age-standardized mortality rates (per 100,000 per year) of a combined number of respiratory diseases decreased from 82.87 in 2005 to 37.05 in 2015 (Table 2). Respiratory mortality has decreased significantly since 2006 by − 5.1 (95% CI, − 7.2 to − 3.0) per 100,000 per year in men and by − 8.1 (95% CI, − 10.6 to − 5.6) per 100,000 per year in women. The AAPC from 2005 to 2015 also indicates a continued decrease in lung cancer mortality (male, − 4.2 [95% CI, − 7.2 to − 3.0]; female, − 5.7 [95% CI, − 7.4 to − 4.0]).
Table 2.
Respiratory disease and lung cancer mortality in the urban areas of Shenyang, China, in 2005–2015 (per 100,000 per year)
| Respiratory diseases (J00-J99) |
Pneumonia (J18) |
CLRD (J40-J47) |
Pneumoconiosis (J61-J62) |
Lung cancer (C33-C34) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Year | Count | Crude rate | ASR | Count | Crude rate | ASR | Count | Crude rate | ASR | Count | Crude rate | ASR | Count | Crude rate | ASR | |
| 2005 | 2428 | 70.16 | 82.87 | 638 | 18.44 | 22.14 | 1225 | 35.40 | 41.21 | 39 | 1.13 | 1.14 | 2304 | 66.58 | 65.97 | |
| 2006 | 2216 | 63.78 | 57.17 | 558 | 16.06 | 14.78 | 1018 | 29.30 | 26.02 | 27 | 0.78 | 0.65 | 2386 | 68.67 | 56.33 | |
| 2007 | 2305 | 65.92 | 63.01 | 577 | 16.50 | 16.12 | 1021 | 29.20 | 27.39 | 21 | 0.60 | 0.54 | 2450 | 70.07 | 58.70 | |
| Total | 2008 | 2479 | 70.68 | 58.82 | 900 | 25.65 | 21.76 | 958 | 27.30 | 22.36 | 14 | 0.40 | 0.30 | 2561 | 72.99 | 56.19 |
| 2009 | 2461 | 69.91 | 57.22 | 995 | 28.26 | 23.51 | 912 | 25.91 | 20.85 | 24 | 0.68 | 0.50 | 2482 | 70.51 | 54.52 | |
| 2010 | 2686 | 73.79 | 44.31 | 1171 | 32.17 | 19.47 | 1107 | 30.41 | 18.02 | 28 | 0.77 | 0.43 | 2769 | 76.07 | 48.16 | |
| 2011 | 2654 | 70.57 | 39.13 | 1144 | 30.42 | 16.89 | 1046 | 27.81 | 15.26 | 24 | 0.64 | 0.33 | 2742 | 72.91 | 43.77 | |
| 2012 | 3021 | 79.93 | 42.22 | 1402 | 37.09 | 19.49 | 1051 | 27.81 | 14.54 | 38 | 1.01 | 0.51 | 2929 | 77.50 | 45.49 | |
| 2013 | 2957 | 78.58 | 39.96 | 1528 | 40.60 | 20.48 | 758 | 20.14 | 9.97 | 32 | 0.85 | 0.39 | 2598 | 69.04 | 38.63 | |
| 2014 | 3362 | 88.35 | 42.69 | 2164 | 56.89 | 27.50 | 881 | 23.14 | 10.96 | 25 | 0.66 | 0.29 | 2995 | 78.66 | 43.00 | |
| 2015 | 3124 | 81.42 | 37.05 | 1596 | 41.60 | 18.89 | 777 | 20.25 | 8.86 | 17 | 0.44 | 0.19 | 2896 | 75.51 | 39.69 | |
| AAPC, 95% CI | − 6.6 [− 8.8, − 4.3] | 1.9 [− 2.2, 6.2] | − 13.1 [− 15.0, − 11.1] | − 11.2 [− 16.5, − 5.5] | − 6.6 [− 8.8, − 4.3] | |||||||||||
| 2005 | 1268 | 73.64 | 21.89 | 377 | 33.92 | 28.52 | 584 | 34.11 | 42.28 | 35 | 2.03 | 2.22 | 1345 | 78.11 | 82.61 | |
| 2006 | 1216 | 70.40 | 18.64 | 322 | 30.17 | 18.11 | 521 | 30.32 | 28.52 | 24 | 1.39 | 1.26 | 1469 | 85.05 | 74.50 | |
| 2007 | 1235 | 71.20 | 19.37 | 336 | 28.59 | 20.45 | 496 | 28.79 | 28.27 | 18 | 1.04 | 0.97 | 1528 | 88.09 | 78.05 | |
| 2008 | 1336 | 76.95 | 31.04 | 539 | 26.49 | 28.02 | 460 | 26.67 | 23.17 | 14 | 0.81 | 0.65 | 1565 | 90.14 | 74.38 | |
| Male | 2009 | 1358 | 78.29 | 33.50 | 581 | 25.31 | 29.59 | 439 | 25.48 | 22.08 | 19 | 1.10 | 0.84 | 1465 | 84.46 | 70.17 |
| 2010 | 1474 | 82.24 | 38.67 | 693 | 30.19 | 25.4 | 541 | 30.41 | 19.47 | 24 | 1.34 | 0.81 | 1625 | 90.67 | 62.14 | |
| 2011 | 1502 | 81.19 | 38.11 | 705 | 28.38 | 23.08 | 525 | 28.60 | 16.98 | 21 | 1.14 | 0.63 | 1661 | 89.78 | 58.67 | |
| 2012 | 1698 | 91.42 | 45.87 | 852 | 27.73 | 26.39 | 515 | 27.98 | 16.13 | 34 | 1.83 | 1.03 | 1752 | 94.33 | 60.04 | |
| 2013 | 1684 | 91.16 | 49.75 | 919 | 19.92 | 27.76 | 368 | 20.09 | 11.00 | 26 | 1.41 | 0.70 | 1624 | 87.91 | 53.54 | |
| 2014 | 1872 | 100.30 | 66.99 | 1250 | 23.03 | 36.18 | 430 | 23.28 | 12.33 | 23 | 1.23 | 0.60 | 1808 | 96.82 | 57.54 | |
| 2015 | 1759 | 93.95 | 47.59 | 891 | 22.00 | 24.04 | 412 | 22.30 | 11.05 | 14 | 0.75 | 0.35 | 1815 | 96.94 | 55.36 | |
| AAPC, 95% CI | − 5.1 [− 7.2, − 3.0] | 2.4 [− 1.7, 6.6] | − 11.9 [− 13.7, − 11.0] | − 11.0 [− 16.5, − 5.1] | − 4.2 [− 7.2, − 3.0] | |||||||||||
| 2005 | 1160 | 66.72 | 15.01 | 261 | 36.87 | 16.93 | 641 | 37.06 | 35.22 | 4 | 0.23 | 0.17 | 959 | 55.16 | 51.25 | |
| 2006 | 1000 | 57.23 | 13.51 | 236 | 28.44 | 11.91 | 497 | 28.58 | 30.23 | 3 | 0.17 | 0.12 | 917 | 52.48 | 39.92 | |
| 2007 | 1070 | 60.73 | 13.68 | 241 | 29.80 | 12.61 | 525 | 29.99 | 25.94 | 3 | 0.17 | 0.16 | 922 | 52.33 | 41.31 | |
| 2008 | 1143 | 64.55 | 20.37 | 361 | 28.10 | 16.37 | 498 | 28.27 | 22.27 | 0 | 0.00 | 0.00 | 996 | 56.20 | 40.19 | |
| Female | 2009 | 1103 | 61.77 | 23.18 | 414 | 26.49 | 18.20 | 473 | 26.65 | 19.11 | 5 | 0.28 | 0.19 | 1017 | 56.95 | 40.71 |
| 2010 | 1212 | 65.60 | 25.87 | 478 | 30.63 | 14.40 | 566 | 30.84 | 16.40 | 4 | 0.22 | 0.11 | 1144 | 61.91 | 35.57 | |
| 2011 | 1152 | 60.29 | 22.97 | 439 | 27.27 | 11.74 | 521 | 27.46 | 14.08 | 3 | 0.16 | 0.08 | 1081 | 56.57 | 30.73 | |
| 2012 | 1323 | 68.83 | 28.61 | 550 | 27.88 | 13.81 | 536 | 28.11 | 12.08 | 4 | 0.21 | 0.10 | 1177 | 61.23 | 32.75 | |
| 2013 | 1273 | 66.44 | 31.79 | 609 | 20.36 | 14.41 | 390 | 20.51 | 10.37 | 6 | 0.31 | 0.14 | 974 | 50.84 | 25.47 | |
| 2014 | 1490 | 76.85 | 47.16 | 914 | 23.25 | 20.32 | 451 | 23.47 | 8.90 | 2 | 0.10 | 0.04 | 1187 | 61.18 | 30.51 | |
| 2015 | 1365 | 69.49 | 35.89 | 705 | 18.58 | 14.75 | 365 | 19.00 | 7.64 | 3 | 0.15 | 0.06 | 1082 | 55.08 | 26.59 | |
| AAPC, 95% CI | −8.1 [−10.6, −5.6] | 1.5 [−2.7, 5.9] | −14.2 [−16.2, −12.1] | – | −5.7 [−7.4, −4.0] | |||||||||||
CLRD chronic lower respiratory disease, ASR age-standardized rate, per 100,000 per year, AAPC average annual percentage of change, CI confidence interval
Despite decreases in age-standardized death rates, the absolute number of respiratory deaths, occurring more frequently at older ages, continues to increase. The death counts for a combined number of respiratory diseases increased by 29% between 2005 and 2015 and were mainly associated with population aging. The number of deaths from pneumonia increased more than twofold during the same period, but a non-significantly increased age-standardized rate was found (1.9; 95% CI, − 2.2 to 6.2).
From 2013 to 2015, a daily average of 8.54 persons died from respiratory diseases and 7.67 from lung cancer in the urban areas of Shenyang (Table 3). During the study period, the average concentration of PM2.5 and PM10 was 70.71 and 117.08 μg/m3, respectively.
Table 3.
Mortality, air pollution, and meteorological measurements in Shenyang, China, 2013–2015
| Mean | SD | IQR | Min | Q1 | Q2 | Q3 | Max | |
|---|---|---|---|---|---|---|---|---|
| PM2.5 (μg/m3) | 70.71 | 60.36 | 53.00 | 10.00 | 34.00 | 53.00 | 87.00 | 848.00 |
| PM10 (μg/m3) | 117.08 | 76.94 | 77.00 | 19.00 | 69.00 | 97.00 | 146.00 | 912.00 |
| SO2 (μg/m3) | 68.45 | 69.50 | 81.00 | 3.00 | 19.00 | 38.00 | 100.00 | 379.00 |
| NO2 (μg/m3) | 44.63 | 18.01 | 24.00 | 10.00 | 31.00 | 41.00 | 55.00 | 125.00 |
| CO (mg/m3) | 1.18 | 0.62 | 0.62 | 0.32 | 0.73 | 0.99 | 1.35 | 4.93 |
| O3 (μg/m3) | 56.19 | 30.77 | 46.00 | 6.00 | 31.00 | 53.00 | 77.00 | 178.00 |
| Mean temperature (°C) | 8.91 | 13.17 | 24.00 | − 21.00 | − 3.00 | 11.00 | 21.00 | 29.00 |
| Mean humidity (%) | 61.75 | 15.65 | 22.5 | 21.00 | 51.00 | 63.00 | 73.50 | 95.00 |
| Respiratory disease deaths (J00-J99) | 8.54 | 3.17 | 4 | 1 | 6 | 8 | 10 | 21 |
| Lung cancer deaths (C33-C34) |
7.67 | 2.94 | 5 | 0 | 5 | 8 | 10 | 20 |
SD standard deviation, IQR interquartile range, Min minimum, Q quartile, Max maximum, PM2.5 particulate matter < 2.5 μm in diameter, PM10 particulate matter < 10 μm in diameter
Spearman’s rank correlation coefficients between pollutants and weather variables are presented in Table 4. All five pollutants (PM2.5, PM10, SO2, NO2, CO) were highly correlated, especially between PM2.5 and PM10 (r = 0.915); however, they were all negatively correlated with ozone. Furthermore, the daily mean temperature is negatively correlated with air pollutants except for ozone; the largest correlation coefficient was found in SO2 with − 0.767.
Table 4.
Spearman’s rank correlation coefficients between pollutants and weather variables
| PM2.5 | PM10 | SO2 | NO2 | CO | O3 | Mean humidity | Mean temperature | |
|---|---|---|---|---|---|---|---|---|
| PM2.5 (μg/m3) | 1.000 | |||||||
| PM10 (μg/m3) | 0.915* | 1.000 | ||||||
| SO2 (μg/m3) | 0.671* | 0.648* | 1.000 | |||||
| NO2 (μg/m3) | 0.666* | 0.610* | 0.585* | 1.000 | ||||
| CO (mg/m3) | 0.828* | 0.777* | 0.673* | 0.595* | 1.000 | |||
| O3 (μg/m3) | − 0.256* | − 0.208* | − 0.555* | − 0.514* | − 0.253* | 1.000 | ||
| Mean humidity (%) | 0.037 | − 0.135* | − 0.182* | − 0.039 | 0.129* | − 0.052 | 1.000 | |
| Mean temperature (°C) | − 0.362* | − 0.334* | − 0.767* | − 0.320* | − 0.322* | 0.690* | 0.222* | 1.000 |
*P < 0.01
Associations between air pollutants and respiratory deaths
The effect estimates of each air pollutant on daily respiratory mortality after controlling for meteorological and seasonal influences are shown in Table 5. Increased mortality was observed to be associated with elevated concentrations of PM2.5 on the same day. A 10 μg/m3 increment of PM2.5 was associated with a 4.5% increase in daily mortality for respiratory diseases and lung cancer (95% CI, 1.1–8.1%). The 10 μg/m3 increment of PM10 and and 1 mg/m3 increment of CO were associated with a 4.8% (95% CI, 0.7–9.2%) and a 1.9% (95% CI, 0.3–3.7%) increase in overall deaths, respectively. Moreover, with a 24-h lag period, associations between mortality and PM2.5 or CO levels still existed, with excess risks of 3.6% (95% CI, 0.1–7.2%) and 2.1% (95% CI, 0.4–3.8%), respectively. In addition, increments in SO2 exposure with a 1-day lag were associated with overall respiratory mortality, with an OR of 5.2% (1.3%, 9.3%). A 10 μg/m3 increase in 2-day mean PM2.5, PM10, and SO2 concentrations was associated with a 4.7% (95% CI, 0.5–9.0%), 5.1% (95% CI, 0.0–10.4%), and 5.9% [95% CI, 1.1–10.9%) increased risk of overall respiratory death, respectively. Same-day PM2.5 exposure or 2-day mean PM2.5 concentrations were also statistically significantly associated with lung cancer mortality, with 6.5% (95% CI, 1.2–12.0%) and 6.8% (95% CI, 0.5–13.6%) increases in mortality. For death counts attributed to respiratory diseases, CO exposure with a 1-day lag showed a significant association, with an elevated OR of 3.8% (95% CI, 1.3–6.3%) per 1 mg/m3 increase in CO concentration. PM2.5 exposure has a positive but weak association with respiratory diseases mortality (4.7%, 95% CI, 0–9.9%). Lung cancer mortality was also associated with PM10 and SO2 with 7.3 and 8.1% for an increase of 10 μg/m3. It was affected by PM10 level on the day of death, whereas more affected by the average level of the day and previous day’s SO2 concentration. For multiple-pollutant (adjusted for SO2 and NO2) models, increased mortality of lung cancer and the overall respiratory mortality were significantly associated with PM2.5, with the ORs for every 10 μg/m3 increase in PM2.5 being 1.087 (95% CI, 1.008–1.172) and 1.067 (95% CI, 1.015–1.122), respectively, at lag 0 day in multiple-pollutant model. SO2 also had certain effects on overall respiratory mortality with evident lag effects. The ORs (95% CIs) with a 10 μg/m3 increase in concentration of SO2, were 1.070 (95% CI, 1.005–1.141) and 1.084 (95% CI, 1.01–1.164) for lag 01 and lag 02, respectively. We also observed that a 10 μg/m3 change in NO2 of single-lag (lag 2) and cumulative-lag values (measured as a two or three-day average of lag 0, lag 1, and lag 2) was associated with a weak decline in daily mortality of respiratory diseases.
Table 5.
Associations between air pollutants and mortality controlled by meteorological and seasonal influences
| Overall respiratory mortality (including lung cancer) OR (95% CI) |
Respiratory disease deaths OR (95% CI) |
Lung cancer deaths OR (95% CI) |
|||||
|---|---|---|---|---|---|---|---|
| Single-pollutant | Multiple-pollutant | Single-pollutant | Multiple-pollutant | Single-pollutant | Multiple-pollutant | ||
| PM2.5 | Lag 0 | 1.045 (1.011, 1.081) | 1.067 (1.015, 1.122) | 1.028 (0.981, 1.077) | 1.049 (0.978, 1.126) | 1.065 (1.012, 1.12) | 1.087 (1.008, 1.172) |
| Lag 1 | 1.036 (1.001, 1.072) | 1.021 (0.97, 1.074) | 1.047 (1.000, 1.099) | 1.054 (0.982, 1.131) | 1.024 (0.973, 1.078) | 0.987 (0.915, 1.064) | |
| Lag 2 | 1.006 (0.972, 1.042) | 1.028 (0.977, 1.082) | 0.994 (0.948, 1.043) | 1.045 (0.974, 1.121) | 1.021 (0.970, 1.075) | 1.011 (0.936, 1.092) | |
| Lag 01 | 1.047 (1.005, 1.090) | 1.046 (0.989, 1.105) | 1.027 (0.970, 1.088) | 1.046 (0.968, 1.13) | 1.068 (1.005, 1.136) | 1.046 (0.963, 1.137) | |
| Lag 02 | 1.028 (0.982, 1.076) | 1.033 (0.974, 1.097) | 1.002 (0.940, 1.068) | 1.037 (0.954, 1.126) | 1.056 (0.987, 1.131) | 1.029 (0.942, 1.125) | |
| PM10 | Lag 0 | 1.048 (1.007, 1.092) | 1.027 (0.970, 1.086) | 1.073 (1.009, 1.140) | |||
| Lag 1 | 1.027 (0.986, 1.070) | 1.04 (0.982, 1.101) | 1.014 (0.954, 1.078) | ||||
| Lag 2 | 1.001 (0.960, 1.043) | 0.993 (0.937, 1.051) | 1.011 (0.950, 1.076) | ||||
| Lag 01 | 1.051 (1.000, 1.104) | 1.03 (0.961, 1.104) | 1.073 (0.997, 1.156) | ||||
| Lag 02 | 1.037 (0.981, 1.097) | 1.016 (0.940, 1.099) | 1.06 (0.977, 1.151) | ||||
| SO2 | Lag 0 | 1.033 (0.995, 1.073) | 1.02 1(0.965, 1.079) | 1.027 (0.975, 1.082) | 1.042 (0.963, 1.127) | 1.039 (0.983, 1.099) | 0.998 (0.919, 1.085) |
| Lag 1 | 1.052 (1.013, 1.093) | 1.06 (1.002, 1.122) | 1.052 (0.998, 1.109) | 1.061 (0.980, 1.147) | 1.052 (0.994, 1.113) | 1.06 (0.975, 1.153) | |
| Lag 2 | 1.002 (0.964, 1.041) | 1.015 (0.959, 1.074) | 0.982 (0.931, 1.035) | 1.012 (0.936, 1.095) | 1.025 (0.968, 1.085) | 1.018 (0.936, 1.107) | |
| Lag 01 | 1.059 (1.011, 1.109) | 1.070 (1.005, 1.141) | 1.052 (0.994, 1.113) | 1.079 (0.987, 1.179) | 1.081 (1.009, 1.158) | 1.062 (0.967, 1.167) | |
| Lag 02 | 1.051 (0.996, 1.109) | 1.084 (1.01, 1.164) | 1.025 (0.968, 1.085) | 1.102 (0.998, 1.216) | 1.081 (0.998, 1.171) | 1.066 (0.959, 1.185) | |
| NO2 | Lag 0 | 1.017 (0.963, 1.075) | 0.926 (0.851, 1.008) | 0.991 (0.918, 1.070) | 0.900 (0.800, 1.013) | 1.047 (0.964, 1.138) | 0.955 (0.841, 1.083) |
| Lag 1 | 1.035 (0.979, 1.094) | 0.953 (0.875, 1.038) | 1.022 (0.946, 1.103) | 0.908 (0.807, 1.022) | 1.050 (0.966, 1.140) | 1.005 (0.885, 1.140) | |
| Lag 2 | 0.975 (0.922, 1.032) | 0.931 (0.854, 1.015) | 0.931 (0.861, 1.006) | 0.874 (0.776, 0.985) | 1.028 (0.946, 1.118) | 0.998 (0.877, 1.135) | |
| Lag 01 | 1.019 (0.951, 1.092) | 0.912 (0.827, 1.006) | 0.968 (0.879, 1.065) | 0.860 (0.749, 0.987) | 1.079 (0.973, 1.196) | 0.972 (0.839, 1.126) | |
| Lag 02 | 0.987 (0.911, 1.069) | 0.888 (0.793, 0.994) | 0.907 (0.812, 1.014) | 0.802 (0.685, 0.938) | 1.081 (0.96, 1.218) | 0.991 (0.837, 1.173) | |
| CO | Lag 0 | 1.019 (1.003, 1.037) | 1.02 (0.997, 1.044) | 1.018 (0.994, 1.044) | |||
| Lag 1 | 1.021 (1.004, 1.038) | 1.038 (1.013, 1.063) | 1.005 (0.981, 1.03) | ||||
| Lag 2 | 1.001 (0.985, 1.018) | 0.995 (0.973, 1.018) | 1.008 (0.983, 1.034) | ||||
| Lag 01 | 1.028 (0.998, 1.058) | 1.028 (0.987, 1.071) | 1.027 (0.984, 1.072) | ||||
| Lag 02 | 1.004 (0.966, 1.043) | 0.987 (0.935, 1.041) | 1.023 (0.965, 1.084) | ||||
| O3 | Lag 0 | 1.032 (0.980, 1.087) | 1.049 (0.976, 1.128) | 1.013 (0.937, 1.095) | |||
| Lag 1 | 1.000 (0.949, 1.054) | 1.027 (0.955, 1.104) | 0.971 (0.897, 1.051) | ||||
| Lag 2 | 1.029 (0.976, 1.085) | 1.065 (0.989, 1.146) | 0.991 (0.916, 1.073) | ||||
| Lag 01 | 1.029 (0.970, 1.092) | 1.060 (0.976, 1.152) | 0.996 (0.911, 1.089) | ||||
| Lag 02 | 1.046 (0.979, 1.118) | 1.092 (0.996, 1.197) | 0.998 (0.904, 1.103) | ||||
OR odds ratio, CI confidence interval, PM2.5 particulate matter < 2.5 μm in diameter, PM10 particulate matter < 10 μm in diameter
Italic ORs are statistically significant (P < 0.05). Measurement for every 10 μg/m3 increment of PM2.5, PM10, SO2, NO2, and O3 and 1 mg/m3 increment of CO
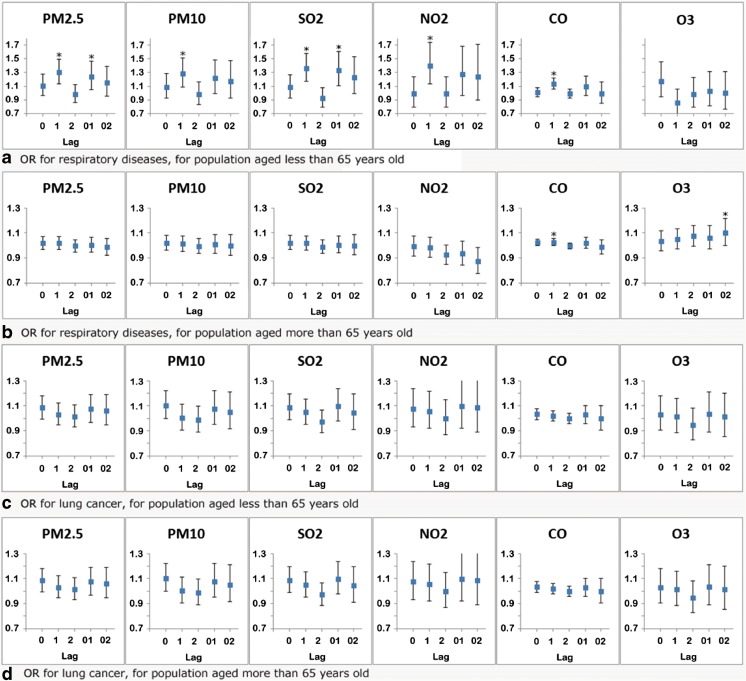
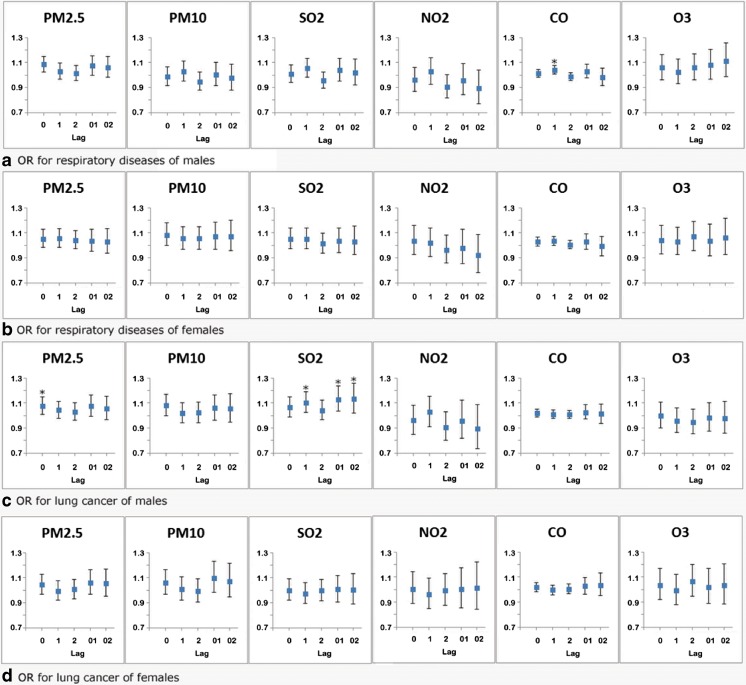
As shown in Fig. 1 for the stratified analysis, five major air pollutants (PM2.5, PM10, SO2, NO2, and CO) significantly increased the mortality risk of respiratory diseases in persons aged < 65 years, while in elderly persons, the effects were attenuated and no longer significant except for that of CO. Increased daily mortality of respiratory diseases in persons aged over 65 years was associated with increase in ambient ozone on lag 02 (OR = 1.102, 95% CI,1.001–1.214),whereas NO2 level of lag 02 showed an negative association (OR = 0.873; 95% CI, 0.776–0.981). The results also showed a significant association of respiratory diseases with CO exposure in men with OR of 1.041(95% CI, 1.009–1.075) but not in women (Fig. 2).
Fig. 1.
Effect estimates of air pollutants on daily mortality of lung cancer and respiratory diseases in different age groups using single-pollutant models (*P < 0.05). Measurement for every 10 μg/m3 increment of PM2.5, PM10, SO2, NO2, and O3 and 1 mg/m3 increment of CO
Fig. 2.
Effect estimates of air pollutants on daily mortality of lung cancer and respiratory diseases of different gender groups using single-pollutant models (*P < 0.05). Measurement for every 10 μg/m3 increment of PM2.5, PM10, SO2, NO2, and O3 and 1 mg/m3 increment of CO
An estimated increase of 7.7% (95% CI, 0.8–15.0%) in male lung cancer mortality was observed for a 10 μg/m3 increase in PM2.5 concentration on the same day, and a delayed effect of SO2 exposure was observed with a 10.4% (95% CI, 2.6–18.9%) increment in male lung cancer mortality in a 1-day lag. SO2 concentrations with a mean of 2–3 days also showed stronger effects (see Figs. 1 and 2 and Table 6, detailed data see Supplemental Material).
Table 6.
Effect estimates of air pollutants on daily mortality of three specific respiratory diseases in single-pollutant models
| CLRD | Pneumonia | Pneumoconiosis | ||
|---|---|---|---|---|
| PM2.5 | Lag 0 | 0.969 (0.887, 1.058) | 1.022 (0.958, 1.091) | 0.891 (0.688, 1.153) |
| Lag 1 | 1.002 (0.916, 1.096) | 1.044 (0.978, 1.115) | 0.801 (0.625, 1.027) | |
| Lag 2 | 0.979 (0.895, 1.07) | 1.005 (0.941, 1.073) | 0.709 (0.558, 0.900) | |
| Lag 01 | 0.953 (0.856, 1.061) | 1.029 (0.950, 1.113) | 0.618 (0.452, 0.845) | |
| Lag 02 | 0.922 (0.817, 1.039) | 1.019 (0.933, 1.113) | 0.561 (0.395 ,0.798) | |
| PM10 | Lag 0 | 0.957 (0.861, 1.063) | 1.017 (0.941, 1.100) | 0.941 (0.683, 1.294) |
| Lag 1 | 0.973 (0.873, 1.085) | 1.027 (0.949, 1.112) | 0.697 (0.526, 0.924) | |
| Lag 2 | 0.984 (0.884, 1.095) | 0.982 (0.907, 1.063) | 0.745 (0.562, 0.988) | |
| Lag 01 | 0.926 (0.812, 1.055) | 1.021 (0.927, 1.124) | 0.617 (0.423, 0.900) | |
| Lag 02 | 0.917 (0.792, 1.062) | 1.010 (0.907, 1.125) | 0.612 (0.407, 0.919) | |
| SO2 | Lag 0 | 1.018 (0.923, 1.123) | 0.993 (0.924, 1.068) | 1.118 (0.843, 1.484) |
| Lag 1 | 1.024 (0.926, 1.132) | 1.030 (0.958, 1.108) | 0.914 (0.674, 1.239) | |
| Lag 2 | 0.990 (0.896, 1.093) | 0.971 (0.903, 1.044) | 0.957 (0.725, 1.263) | |
| Lag 01 | 1.019 (0.903, 1.15) | 0.997 (0.912, 1.090) | 0.870 (0.596, 1.271) | |
| Lag 02 | 1.000 (0.869, 1.151) | 0.989 (0.890, 1.098) | 0.891 (0.570, 1.392) | |
| NO2 | Lag 0 | 0.933 (0.810, 1.075) | 0.988 (0.888, 1.099) | 1.098 (0.721, 1.673) |
| Lag 1 | 1.001 (0.867, 1.156) | 0.993 (0.893, 1.105) | 0.862 (0.556, 1.336) | |
| Lag 2 | 0.915 (0.791, 1.058) | 0.930 (0.836, 1.035) | 0.708 (0.340, 1.460) | |
| Lag 01 | 0.914 (0.766, 1.091) | 0.950 (0.832, 1.085) | 0.703 (0.410, 1.206) | |
| Lag 02 | 0.833 (0.677, 1.025) | 0.903 (0.774, 1.053) | 0.846 (0.233, 1.554) | |
| CO | Lag 0 | 1.018 (0.974, 1.063) | 1.015 (0.983, 1.047) | 0.897 (0.804, 1.001) |
| Lag 1 | 1.058 (1.01, 1.108) | 1.032 (0.999, 1.066) | 0.971 (0.86, 1.095) | |
| Lag 2 | 0.983 (0.943, 1.025) | 1.019 (0.987, 1.052) | 0.839 (0.748, 0.941) | |
| Lag 01 | 1.018 (0.941, 1.101) | 1.042 (0.985, 1.103) | 0.752 (0.578, 0.978) | |
| Lag 02 | 0.942 (0.854, 1.039) | 1.042 (0.964, 1.126) | 0.691 (0.491, 0.973) | |
| O3 | Lag 0 | 1.059 (0.924, 1.213) | 1.023 (0.926, 1.130) | 1.141 (0.767, 1.697) |
| Lag 1 | 1.015 (0.886, 1.163) | 0.991 (0.897, 1.096) | 1.505 (1.020, 2.220) | |
| Lag 2 | 1.123 (0.976, 1.292) | 1.058 (0.956, 1.171) | 1.040 (0.719, 1.504) | |
| Lag 01 | 1.013 (0.868, 1.182) | 1.037 (0.925, 1.162) | 1.477 (0.929, 2.348) | |
| Lag 02 | 1.054 (0.888, 1.252) | 1.071 (0.944, 1.215) | 1.300 (0.805, 2.099) |
CLRD chronic lower respiratory disease, PM2.5 particulate matter < 2.5 μm in diameter, PM10 particulate matter < 10 μm in diameter
Italic ORs are statistically significant (P < 0.05). Measurement for every 10 μg/m3 increment of PM2.5, PM10, SO2, and O3 and 1 mg/m3 increment of CO
Discussion
Our study’s results showed a decline in mortality from respiratory diseases and lung cancer in Shenyang, China. However, the respiratory and lung cancer death counts are continuing to increase with the aging of Shenyang’s population. The death count due to pneumonia has increased nearly threefold in 10 years. Our results suggest the effects of air pollution exposure on the number of deaths due to respiratory illness and lung cancer on subsequent days. We found a statistically significant increase in lung cancer mortality of 8.7% (95% CI, 0.8–17.2%) for a 10 μg/m3 increase in PM2.5; the increase was 10.4% (2.6–18.9%) in men for an average 10 μg/m3 increase in SO2 the previous day, and 13.2% (2–25.6%) for a 10 μg/m3 increase in SO2 at lag 02 days. For deaths due to respiratory diseases, the effect estimates were 4.7% (0–9.9%) for a 10 μg/m3 increase in PM2.5 on the same day, and 3.8% (1.3–6.3%) for a 1 mg/m3 increase of CO the previous day. The relationship between ozone exposure and respiratory mortality was also significant in people aged more than 65 years, with 10.2% (0.1–21.4%) increase in mortality from respiratory diseases.
Air pollution levels in China have been increasing rapidly. An analysis of data from the Global Burden of Diseases Study 2015 indicated that ambient PM2.5 accounted for 7.6% of total global deaths (about 4.2 million deaths), 59% of which being in East and South Asia (Cohen et al. 2017). Studies have investigated the short-term associations between daily increases in PM (PM2.5 and PM10) and mortality, especially in China (Tao et al. 2014; Xu et al. 2016; Yang et al. 2015). PM2.5 was ranked fifth in the risk factors of mortality in 2015 (Cohen et al. 2017). In this present study, we observed an elevated risk of dying from respiratory diseases associated with a high PM2.5 concentration at lag 1 day. Our findings also provided some evidence for an increased risk of lung cancer mortality caused by PM2.5 (Colao et al. 2016). A meta-analysis indicated that the meta-estimate for lung cancer mortality associated with PM2.5 was greater for males than for females (Huang et al. 2017). Similarly, our study demonstrated a positive relationship between PM2.5 and lung cancer mortality in males but not in females. PM2.5 mass concentration also showed increasing associations with both total respiratory-related mortality including lung cancer and lung cancer mortality only of the same day in the multiple-pollutant model. Our findings here corroborated the findings in previous studies (Crouse et al. 2015; Dominici et al. 2015).
Although ambient PM10 was significantly and positively associated with PM2.5 (Spearman’s correlation: r = 0.915, P < 0.01) and long-term exposure to elevated PM10 levels has been reported to generate a relative risk of all-cause and cause-specific mortality (Chen et al. 2016; Heinrich et al. 2013), significant positive correlation with respiratory diseases mortality risk was found only in population younger than 65 years with short-term exposure to PM10 in this study. Some studies in China obtained significant associations relating respiratory diseases risk to PM10; whereas, some studies conducted in city nearby Shenyang did not find the same association (Chen et al. 2010; Shang et al. 2013). Similar results were obtained in a study of 235,000 population, suggesting that expected short-term exposure to PM10 appears to have a limited impact on mortality (Carugno et al. 2014). Meanwhile, it has been reported that PM exposure tends to increase the natural death risk among people with chronic morbidity but has no significant risk among healthy persons (Alessandrini et al. 2016). RRs of lung cancer mortality were reported increases substantially among men in relation to long-term ambient concentrations of PM10 in a non-smoking cohort (Abbey et al. 1999). In our study, the mean PM10 concentration was not associated with lung cancer mortality in men or women. Increased OR was only observed in total population with every 10 μg/m3 increment of PM10 in the same day exposure. Since limited individual data were collected in this study, further research is needed to identify subgroups susceptible to elevated PM10 exposure.
Epidemiological studies indicated that daily changes in ambient concentrations of NO2 and SO2 trigger negative health effects on cardiopulmonary function, with both long-term and short-term exposure being associated with mortality risk (Brunekreef et al. 2009; Ghozikali et al. 2015; Int Panis et al. 2017; Miri et al. 2016). Our study found a relationship between lung cancer mortality and elevated SO2 level. Diurnal average SO2 concentrations with 2 or 3 previous-day exposure was associated with an increased lung cancer mortality risk, especially in males. Furthermore, single-day effects of the previous day (lag 1) of PM2.5, PM10, SO2, NO2, and CO, or the moving averages over the same day and previous day (lag 01) of PM2.5 and SO2, exposure substantially increased the risk of respiratory diseases mortality in the population younger than 65 years. NO2 and SO2 are often considered as indicators of traffic-related air pollution; the harmful effects of NO2 and SO2 would last with the increase in traffic intensity. Elevated risk of total respiratory-related mortality was also observed for SO2 (lag 01 and lag 02) when adjusted for the other pollutants. Results from a time series study (Zhang et al. 2015) confirmed that short-term exposure to SO2 was associated with 1.34% increases in respiratory disease emergency admissions in Beijing. However, in our study, no significant (P < 0.05) effects of SO2 were found for any respiratory disease deaths or lung cancer deaths in the multiple-pollutant model, except for the overall deaths combining respiratory diseases and lung cancer. Also, both respiratory disease deaths and lung cancer deaths had no significant association with exposure to NO2 in the single-pollutant model, but for population aged over 65 years, NO2 on lag 02 days seem to decline the daily mortality of respiratory disease. Inexplicably, in the multiple-pollutant model the average concentration of lag days for NO2 was associated with lower risk of respiratory disease deaths. The effect may be due in part to the potential for exposure misclassification, residual confounding, other unmeasured component of traffic pollution, and co-pollutant effects between NO2 and other pollutants (Hesterberg et al. 2009). Since lack of suitable evidence for personal exposure, the health effect that could result from exposure to the combination of the pollutants rather than individual pollutants could not be determined based on our data. It has been proved that ambient concentrations were not associated with their corresponding personal exposures for gaseous pollutants (Sarnat et al. 2001). Measured information of ambient air pollutants concentrations in more detail with the hour-to-hour variations rather than 24-h integrated were needed to describe the health risk associated with human exposure to air pollutants.
The results of this study also suggested that the mortality rate of respiratory diseases has direct significant correlations with CO concentrations in the air, in both older and younger populations. The acute effects of CO exposure occur with a 1-day lag. Similar studies have reported that increment concentrations of urban CO were related to respiratory mortality, especially during the warmer months (Atkinson et al. 2016). In the present study, about 85.95 and 91.86% of respiratory deaths were observed in males and females aged ≥ 65 years, respectively. Elderly persons in Shenyang may be at high risk for respiratory diseases because of the long-term exposure to industrial dust at their early life and the short-term exposure to air pollutants. As a traffic-related air pollutant, CO requires ample attention for its health impacts. Gender-stratified analyses were also performed to calculate the ORs of mortality associated with CO; a significant mortality impact attributable to CO was found in men, and only a low marginal effect (95% CI, 0.997–1.07) was found in women. Several epidemiological studies have shown that men were more susceptible to CO levels (Qorbani et al. 2012) or other air pollutant exposure than women (Xu et al. 2016). Whether mask usage is the key reason for females in Shenyang to be less susceptible to the harmful effects of CO remains unclear. Thus, further studies that assess exposure to the individual level are necessary.
Short-term ozone exposure has been reported to be associated with transient decrements in lung functions and increased respiratory symptoms (Chen et al. 2017; Ito et al. 2005). There is growing evidence that adverse effects of ozone exposure induce increased mortality risks in the aging population (Chen et al. 2017; Nuvolone et al. 2017). Our findings suggest that ozone exposure could lead to more deaths from respiratory diseases in people aged ≥ 65 years in Shenyang. However, the ozone-mortality association was not significant in the younger population. We found ozone daily concentration was negatively related with the other pollutants. Ozone is mainly produced from its major anthropogenic precursors such as the nitrogen oxides (NOx) and volatile organic compounds (VOCs). Previous study suggested that emission changes in VOCs might have played a more important role in the observed increase of surface ozone (Ma et al. 2016). The variation of reactivates of VOCs in morning traffic rush time at weekend or weekday may consequently change the concentration of surface ozone (Qin et al. 2004). The correlation between daily respiratory death and ozone were also different in winter and summer (Lindgren et al. 2009). To further understand the effect of ozone contributing to respiratory mortality, more detailed data and analysis were necessary.
The hazards of pneumoconiosis, a systemic occupational disease caused by long-term dust inhalation, are still serious in the old industrial city of Shenyang. Total of 289 individuals died from pneumoconiosis in the period of 2005–2015 in Shenyang. Pneumoconiosis is generally caused by long-term inhalation of dust (CDC 2012). So far, there is no evidence from previously published information in the association between mortality of pneumoconiosis and ambient air pollutants. We found every 10 μg/m3 of lag 1 day O3 concentration result a 50.5% increases of pneumoconiosis death and a confusing results that PM2.5, PM10, and CO show a small response shift effect to pneumoconiosis. It is unclear whether the association is due to a few outliers that occurred since every day death sample sizes were small or is attributable to confounding by some individual unknown or unmeasured factors. Most of the pneumoconiosis cases in this study were aged 80 and over. Additional analyses by cause of death are needed to examine the causal association between excess mortality and exposure to air pollution.
Multi-pollutant models which include terms of estimated population exposure for several pollutants were commonly used to identify the pollutant responsible for the observed effects (Vedal and Kaufman 2011). Multi-pollutant approach had been applied to examine the effect of multi-pollutant mixtures (Dominici et al. 2010). In this study, the main three pollutants of PM2.5, SO2, and NO2 were introduced into multiple-pollutant models to examine the independence of any associations observed in single-pollutant models. Due to the presence of highly collinear components among the ambient concentrations of air pollutant, it is important to conduct the multi-pollutant models to examine the role of these pollutants in multi-pollutant models rather than single-pollutant model. And we found that increase in the number of overall respiratory deaths were related with every 10 μg/m3 increases in the same day ambient concentrations of PM2.5 or the mean concentrations of SO2 of the same day and the previous 1 or 2 days.
This study has several limitations. A primary limitation of this study, and other similar studies in this field, is that air pollution exposure in the population is not assessed at the individual level, which may lead to aggregation bias. Furthermore, personal behaviors such as breathing mask usage and amount of time spent outdoors may also affect personal exposures. Females are more likely to use masks than males, resulting in the underestimation of air pollution effects. Younger groups spend more time outdoors than the elderly, particularly in the winter; hence, the association with mortality may be overestimated. In addition, this study was conducted in a sub-humid temperate continental city with a long winter period, where people need 5 months of coal burning. Moreover, the specific location of Shenyang may also limit the generalizability of findings.
Conclusions
In this study, we have found positive associations between daily concentrations of air pollutants and mortality from respiratory diseases and lung cancer in Shenyang, China. We conclude that PM2.5, SO2, and CO exposures are significant risk factors for mortality from respiratory diseases and lung cancer in Shenyang, noting that younger people are more susceptible to the effects of particulate pollutants. PM2.5 and SO2 are also associated with increasing death counts due to lung cancer, especially in men. Our results confirm those of previous studies on possible acute adverse effects of air pollution exposure and further indicate which population subgroups are more susceptible to different air pollutants.
Electronic supplementary material
(DOC 68 kb)
Acknowledgements
We thank the China National Environmental Monitoring Centre and the Mission China air quality monitoring program of the USA for publicly sharing the air pollution data, as well as the Chinese Meteorological Data Sharing Service System for providing meteorology data.
Authors’ contributions
X.X. Xue and J.P. Chen performed the data analyses, and X.L. Li revised the manuscript. B.S Zhou and B.J. Sun conceived and designed the experiments.
Funding information
This study was supported by the National Key Research and Development Program of China (No. 2016YFC1302500).
Compliance with ethical standards
Conflicts of interest
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s11356-018-1270-5) contains supplementary material, which is available to authorized users.
References
- Abbey DE, Nishino N, McDonnell WF, Burchette RJ, Knutsen SF, Lawrence Beeson W, Yang JX. Long-term inhalable particles and other air pollutants related to mortality in nonsmokers. Am J Respir Crit Care Med. 1999;159(2):373–382. doi: 10.1164/ajrccm.159.2.9806020. [DOI] [PubMed] [Google Scholar]
- Alessandrini ER, Stafoggia M, Faustini A, Berti G, Canova C, De Togni A, Di Biagio K, Gherardi B, Giannini S, Lauriola P, Pandolfi P, Randi G, Ranzi A, Simonato L, Zauli Sajani S, Cadum E, Forastiere F, On behalf of the EpiAir2 Study Group (2016) Association between short-term exposure to PM2.5 and PM10 and mortality in susceptible subgroups: a multisite case-crossover analysis of individual effect modifiers. Am J Epidemiol. 10.1093/aje/kww078 [DOI] [PubMed]
- Atkinson RW, Analitis A, Samoli E, Fuller GW, Green DC, Mudway IS, Anderson HR, Kelly FJ. Short-term exposure to traffic-related air pollution and daily mortality in London, UK. J Expo Sci Environ Epidemiol. 2016;26(2):125–132. doi: 10.1038/jes.2015.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunekreef B, Beelen R, Hoek G, Schouten L, Bausch-Goldbohm S, Fischer P, Armstrong B, Hughes E, Jerrett M, van den Brandt P (2009) Effects of long-term exposure to traffic-related air pollution on respiratory and cardiovascular mortality in the Netherlands: the NLCS-AIR study. Res Rep Health Eff Inst (139):5–71;discussion 73–89 [PubMed]
- Carugno M, Randi G, Campagnolo D, Cattaneo A, Cavallo DM, Bertazzi PA. 0257 Mortality and morbidity health impact assessment of expected exposure to PM10 due to the major construction site for a large international exhibition. Occup Environ Med. 2014;71(Suppl 1):A33–A34. doi: 10.1136/oemed-2014-102362.104. [DOI] [Google Scholar]
- CDC (Centers for Disease Control and Prevention) Pneumoconiosis and advanced occupational lung disease among surface coal miners--16 states, 2010–2011. MMWR Morb Mortal Wkly Rep. 2012;61:431–434. [PubMed] [Google Scholar]
- Chen R, Pan G, Kan H, Tan J, Song W, Wu Z, Xu X, Xu Q, Jiang C, Chen B. Ambient air pollution and daily mortality in Anshan, China: a time-stratified case-crossover analysis. Sci Total Environ. 2010;408(24):6086–6091. doi: 10.1016/j.scitotenv.2010.09.018. [DOI] [PubMed] [Google Scholar]
- Chen X, Zhang LW, Huang JJ, Song FJ, Zhang LP, Qian ZM, Trevathan E, Mao HJ, Han B, Vaughn M, Chen KX, Liu YM, Chen J, Zhao BX, Jiang GH, Gu Q, Bai ZP, Dong GH, Tang NJ. Long-term exposure to urban air pollution and lung cancer mortality: a 12-year cohort study in Northern China. Sci Total Environ. 2016;571:855–861. doi: 10.1016/j.scitotenv.2016.07.064. [DOI] [PubMed] [Google Scholar]
- Chen K, Zhou L, Chen X, Bi J, Kinney PL. Acute effect of ozone exposure on daily mortality in seven cities of Jiangsu Province, China: no clear evidence for threshold. Environ Res. 2017;155:235–241. doi: 10.1016/j.envres.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AJ, Brauer M, Burnett R, Anderson HR, Frostad J, Estep K, Balakrishnan K, Brunekreef B, Dandona L, Dandona R, Feigin V, Freedman G, Hubbell B, Jobling A, Kan H, Knibbs L, Liu Y, Martin R, Morawska L, Pope CA, 3rd, Shin H, Straif K, Shaddick G, Thomas M, van Dingenen R, van Donkelaar A, Vos T, Murray CJL, Forouzanfar MH. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the Global Burden of Diseases Study 2015. Lancet. 2017;389(10082):1907–1918. doi: 10.1016/S0140-6736(17)30505-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colao A, Muscogiuri G, Piscitelli P. Environment and health: not only cancer. Int J Environ Res Public Health. 2016;13(7):724. doi: 10.3390/ijerph13070724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouse DL, Peters PA, Hystad P, Brook JR, van Donkelaar A, Martin RV, Villeneuve PJ, Jerrett M, Goldberg MS, Pope CA, 3rd, Brauer M, Brook RD, Robichaud A, Menard R, Burnett RT. Ambient PM(2.5), O(3), and NO(2) exposures and associations with mortality over 16 years of follow-up in the Canadian Census Health and Environment Cohort (CanCHEC) Environ Health Perspect. 2015;123:1180–1186. doi: 10.1289/ehp.1409276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominici F, Peng RD, Barr CD, Bell ML. Protecting human health from air pollution: shifting from a single-pollutant to a multipollutant approach. Epidemiology. 2010;21(2):187–194. doi: 10.1097/EDE.0b013e3181cc86e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominici F, Wang Y, Correia AW, Ezzati M, Pope CA, Dockery DW. Chemical composition of fine particulate matter and life expectancy: in 95 US counties between 2002 and 2007. Epidemiology (Cambridge, Mass) 2015;26:556–564. doi: 10.1097/EDE.0000000000000297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng Y, Sarkis J, Wang X, Zhao H, Zhong Y. Regional application of ground source heat pump in China: a case of Shenyang. Renew Sust Energ Rev. 2013;18:95–102. doi: 10.1016/j.rser.2012.10.015. [DOI] [Google Scholar]
- Ghozikali MG, Mosaferi M, Safari GH, Jaafari J. Effect of exposure to O3, NO2, and SO2 on chronic obstructive pulmonary disease hospitalizations in Tabriz, Iran. Environ Sci Pollut Res Int. 2015;22(4):2817–2823. doi: 10.1007/s11356-014-3512-5. [DOI] [PubMed] [Google Scholar]
- Guan W-J, Zheng X-Y, Chung K-F, Zhong N-S. Impact of air pollution on the burden of chronic respiratory diseases in China: time for urgent action. Lancet. 2016;388(10054):1939–1951. doi: 10.1016/S0140-6736(16)31597-5. [DOI] [PubMed] [Google Scholar]
- Heinrich J, Thiering E, Rzehak P, Krämer U, Hochadel M, Rauchfuss KM, Gehring U, Wichmann HE. Long-term exposure to NO2 and PM10 and all-cause and cause-specific mortality in a prospective cohort of women. Occup Environ Med. 2013;70(3):179–186. doi: 10.1136/oemed-2012-100876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesterberg TW, Bunn WB, McClellan RO, Hamade AK, Long CM, Valberg PA. Critical review of the human data on short-term nitrogen dioxide (NO2) exposures: evidence for NO2 no-effect levels. Crit Rev Toxicol. 2009;39(9):743–781. doi: 10.3109/10408440903294945. [DOI] [PubMed] [Google Scholar]
- Huang F, Pan B, Wu J, Chen E, Chen L. Relationship between exposure to PM2.5 and lung cancer incidence and mortality: a meta-analysis. Oncotarget. 2017;8(26):43322–43331. doi: 10.18632/oncotarget.17313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Int Panis L, Provost EB, Cox B, Louwies T, Laeremans M, Standaert A, Dons E, Holmstock L, Nawrot T, De Boever P. Short-term air pollution exposure decreases lung function: a repeated measures study in healthy adults. Environ Health. 2017;16(1):60. doi: 10.1186/s12940-017-0271-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, De Leon SF, Lippmann M. Associations between ozone and daily mortality. Epidemiology. 2005;16(4):446–457. doi: 10.1097/01.ede.0000165821.90114.7f. [DOI] [PubMed] [Google Scholar]
- Kim H-J, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19(3):335–351. doi: 10.1002/(SICI)1097-0258(20000215)19:3<335::AID-SIM336>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Lai Y, Brimblecombe P. Regulatory effects on particulate pollution in the early hours of Chinese New Year, 2015. Environ Monit Assess. 2017;189(9):467. doi: 10.1007/s10661-017-6167-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Ma Z, Zheng C, Shang Y. Ambient temperature enhanced acute cardiovascular-respiratory mortality effects of PM2.5 in Beijing, China. Int J Biometeorol. 2015;59(12):1761–1770. doi: 10.1007/s00484-015-0984-z. [DOI] [PubMed] [Google Scholar]
- Li H, Wu S, Pan L, Xu J, Shan J, Yang X, Dong W, Deng F, Chen Y, Shima M, Guo X. Short-term effects of various ozone metrics on cardiopulmonary function in chronic obstructive pulmonary disease patients: results from a panel study in Beijing. China Environ Pollut. 2017;232:358–366. doi: 10.1016/j.envpol.2017.09.030. [DOI] [PubMed] [Google Scholar]
- Liang H, Qiu H, Tian L. Short-term effects of fine particulate matter on acute myocardial infraction mortality and years of life lost: a time series study in Hong Kong. Sci Total Environ. 2017;615:558–563. doi: 10.1016/j.scitotenv.2017.09.266. [DOI] [PubMed] [Google Scholar]
- Lin Z, Niu Y, Chen R, Xu W, Li H, Liu C, Cai J, Zhao Z, Kan H, Qiao L. Fine particulate matter constituents and blood pressure in patients with chronic obstructive pulmonary disease: a panel study in Shanghai, China. Environ Res. 2017;159:291–296. doi: 10.1016/j.envres.2017.08.024. [DOI] [PubMed] [Google Scholar]
- Lindgren A, Stroh E, Montnemery P, Nihlen U, Jakobsson K, Axmon A. Traffic-related air pollution associated with prevalence of asthma and COPD/chronic bronchitis. A cross-sectional study in Southern Sweden. Int J Health Geogr. 2009;8(1):2. doi: 10.1186/1476-072X-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu F, Xu D, Cheng Y, Dong S, Guo C, Jiang X, Zheng X. Systematic review and meta-analysis of the adverse health effects of ambient PM2.5 and PM10 pollution in the Chinese population. Environ Res. 2015;136:196–204. doi: 10.1016/j.envres.2014.06.029. [DOI] [PubMed] [Google Scholar]
- Ma Y, Chen R, Pan G, Xu X, Song W, Chen B, Kan H. Fine particulate air pollution and daily mortality in Shenyang. China Sci Total Environ. 2011;409(13):2473–2477. doi: 10.1016/j.scitotenv.2011.03.017. [DOI] [PubMed] [Google Scholar]
- Ma Z, Xu J, Quan W, Zhang Z, Lin W, Xu X. Significant increase of surface ozone at a rural site, north of eastern China. Atmos Chem Phys. 2016;16(6):3969–3977. doi: 10.5194/acp-16-3969-2016. [DOI] [Google Scholar]
- Miri M, Derakhshan Z, Allahabadi A, Ahmadi E, Oliveri Conti G, Ferrante M, Aval HE. Mortality and morbidity due to exposure to outdoor air pollution in Mashhad metropolis, Iran. The AirQ model approach. Environ Res. 2016;151:451–457. doi: 10.1016/j.envres.2016.07.039. [DOI] [PubMed] [Google Scholar]
- Nuvolone D, Petri D, Voller F (2017) The effects of ozone on human health. Environ Sci Pollut Res Int. 10.1007/s11356-017-9239-3 [DOI] [PubMed]
- Qin Y, Tonnesen GS, Wang Z. Weekend/weekday differences of ozone, NOx, Co, VOCs, PM10 and the light scatter during ozone season in southern California. Atmos Environ. 2004;38(19):3069–3087. doi: 10.1016/j.atmosenv.2004.01.035. [DOI] [Google Scholar]
- Qorbani M, Yunesian M, Fotouhi A, Sadeghian S. Effect of air pollution on onset of acute coronary syndrome in susceptible subgroups. East Mediterr Health J. 2012;18(6):550–555. doi: 10.26719/2012.18.6.550. [DOI] [PubMed] [Google Scholar]
- Sarnat JA, Schwartz J, Catalano PJ, Suh HH. Gaseous pollutants in particulate matter epidemiology: confounders or surrogates? Environ Health Perspect. 2001;109(10):1053–1061. doi: 10.1289/ehp.011091053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang Y, Sun Z, Cao J, Wang X, Zhong L, Bi X, Li H, Liu W, Zhu T, Huang W. Systematic review of Chinese studies of short-term exposure to air pollution and daily mortality. Environ Int. 2013;54:100–111. doi: 10.1016/j.envint.2013.01.010. [DOI] [PubMed] [Google Scholar]
- Sunyer J, Schwartz J, Tobías A, Macfarlane D, Garcia J, Antó JM. Patients with chronic obstructive pulmonary disease are at increased risk of death associated with urban particle air pollution: a case-crossover analysis. Am J Epidemiol. 2000;151(1):50–56. doi: 10.1093/oxfordjournals.aje.a010121. [DOI] [PubMed] [Google Scholar]
- Tao Y, Mi S, Zhou S, Wang S, Xie X. Air pollution and hospital admissions for respiratory diseases in Lanzhou, China. Environ Pollut. 2014;185:196–201. doi: 10.1016/j.envpol.2013.10.035. [DOI] [PubMed] [Google Scholar]
- Vedal S, Kaufman JD. What does multi-pollutant air pollution research mean? Am J Respir Crit Care Med. 2011;183(1):4–6. doi: 10.1164/rccm.201009-1520ED. [DOI] [PubMed] [Google Scholar]
- Xu Q, Li X, Wang S, Wang C, Huang F, Gao Q, Wu L, Tao L, Guo J, Wang W, Guo X. Fine particulate air pollution and hospital emergency room visits for respiratory disease in urban areas in Beijing, China, in 2013. PLoS One. 2016;11(4):e0153099. doi: 10.1371/journal.pone.0153099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Cao Y, Li W, Li R, Wang M, Wu Z, Xu Q. Multi-site time series analysis of acute effects of multiple air pollutants on respiratory mortality: a population-based study in Beijing, China. Sci Total Environ. 2015;508:178–187. doi: 10.1016/j.scitotenv.2014.11.070. [DOI] [PubMed] [Google Scholar]
- Zhang YL, Cao F. Fine particulate matter (PM 2.5) in China at a city level. Sci Rep. 2015;5(1):14884. doi: 10.1038/srep14884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Dong G, Sun B, Zhang L, Chen X, Ma N, Yu F, Guo H, Huang H, Lee YL, Tang N, Chen J. Long-term exposure to ambient air pollution and mortality due to cardiovascular disease and cerebrovascular disease in Shenyang, China. PLoS One. 2011;6(6):e20827. doi: 10.1371/journal.pone.0020827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Wang SG, Ma YX, Shang KZ, Cheng YF, Li X, Ning GC, Zhao WJ, Li NR. Association between ambient air pollution and hospital emergency admissions for respiratory and cardiovascular diseases in Beijing: a time series study. Biomed Environ Sci : BES. 2015;28(5):352–363. doi: 10.3967/bes2015.049. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Peng M, Yu C, Zhang L. Burden of mortality and years of life lost due to ambient PM10 pollution in Wuhan, China. Environ Pollut. 2017;230:1073–1080. doi: 10.1016/j.envpol.2017.07.053. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 68 kb)