Abstract
Increasing evidence has shown that Chinese herbal medicine (CHM) has promising therapeutic effects in colorectal cancer (CRC); however, the active ingredients and potential targets remain unclear. In this study, we aimed to investigate the relative molecular targets of the Chinese herbs that have been found effective in treating metastatic CRC (mCRC) based on clinical data and network pharmacology. In multivariate analysis CHM resulted an independent prognostic factor. The hazard ratio was 0.103 (95% confidence interval = 0.064–0.164; P < 0.001). Compared with the non-CHM group, the median survival time of the CHM group was also improved (40 versus 12 months; P < 0.001). Eighteen out of 295 herbs showed significant correlation with survival results (P < 0.05). Bioinformatics analysis indicated that the 18 herbs realize anti-CRC activity mainly through suppressing the proliferative activity of ERBB2, peroxisome proliferator-activated receptor gamma, and retinoid X receptor, suppressing angiogenesis via inhibition of VEGFR and VEGFA expression, inhibiting the phosphatidylinositol-3-kinase/AKT1 signaling pathway directly through SRC and AKT1, and reducing tumor necrosis factor-induced inflammation.
Introduction
Colorectal cancer (CRC) is one of the most commonly diagnosed cancers and the third leading cause of cancer-related deaths worldwide1. Approximately 20–25% of the patients have distant metastases at the time of diagnosis; in addition, surgery becomes non-beneficial in a large proportion of these patients because of the inconspicuous and nonspecific early symptoms2–5. Complete resection (R0) of metastases and primary cancer focus is the main therapy to achieve long-term survival in patients with metastatic CRC (mCRC)6. However, patients with unresectable mCRC have a life expectancy of 8 months only7. Combination therapy with chemotherapy and targeted therapy could prolong the median survival time of patients with mCRC8–10. However, long-term therapy could result in serious adverse reactions and reduce the quality of life. Therefore, the prognosis and quality of life of mCRC patients are still below expectations.
Chinese herbal medicine (CHM), a widely used supplementary and alternative medicine therapies in China, has been shown to reduce the toxic and side effects of radiotherapy and chemotherapy, improve the immune function, reduce postoperative metastasis and recurrence, and relieve tumor-related symptoms11–13. In addition, oral CHM could improve the quality of life, prolong the survival rate, enhance the immediate tumor response, and increase the effectiveness of chemotherapy in patients with CRC14–16. However, the herbs in the formula that are directly related to survival, as well as their active ingredients and potential targets remain unclear.
Network pharmacology, which clarifies the synergistic effects and underlying mechanisms of multi-component and multi-target agents using the analysis of networks, is a suitable approach to measure the efficacy and to reveal the functional mechanisms of multi-target drugs17,18. In recent years, network pharmacology has been developed rapidly, and especially, the concept of “network target” has brought a new era in the field of CHM19. It provides a new research paradigm for translating CHM from an experience-based medicine to an evidence-based medicine system, which will accelerate CHM drug discovery, and also improve current drug discovery strategies20–22.
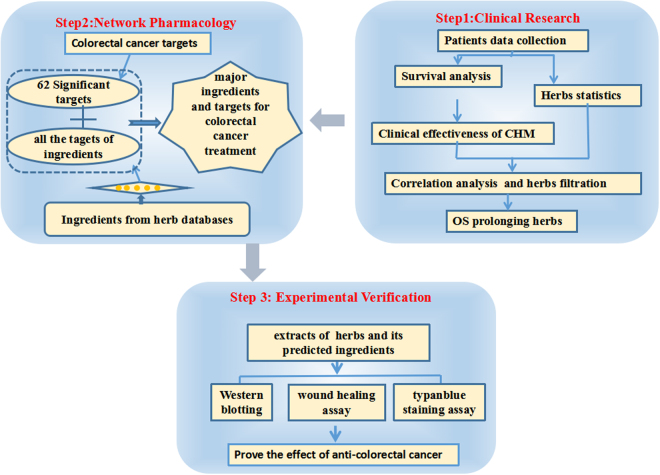
In the present study (Fig. 1), we investigated 222 patients with mCRC to evaluate the efficiency of CHM and identify the effective herbs that were closely correlated with survival. Furthermore, we investigated the underlying pharmacological mechanisms of the effective herbs using bioinformatics approaches.
Figure 1.
Process overview.
Results
Survival characteristics
In this research, we retrospectively studied 222 patients who were diagnosed with mCRC. Among them, 78 patients received CHM treatment, and 144 patients received non-CHM treatment. All patients exhibited metachronous or simultaneous distant metastases, and complete resection of the cancer was performed in 71 patients. The baselines of the patient demographics were equal between patients with and without CHM treatment (Table S1). Statistical results demonstrated that the patients’ age, gender, smoking (yes/no), family history (yes/no), tumor site (colon/rectum), primary tumor size (≤4/>4 cm), differentiated degree (high/middle/poor), carbohydrate antigen 199 (high/normal), carcinoembryonic antigen (high/normal), lymph node metastases, systemic chemotherapy (yes/no), radiotherapy (yes/no) and R0 after metastasis (yes/no) didn’t differ obviously between the two groups.
Kaplan-Meier analysis indicated that smoking history (P = 0.028), primary tumor size (P = 0.023), invasion of the serous membrane (P < 0.001), primary tumor differentiation (P < 0.001), pathological classification (P = 0.005), lymph node metastasis (P < 0.001), and carcinoembryonic antigen (CEA) >5 ng/mL (P = 0.010) were obviously correlated with decreased median survival time. In contrast, systemic chemotherapy (P < 0.001), radiotherapy (P < 0.001), complete resection after metastasis (P < 0.001), and CHM (P < 0.001) were considered to be beneficial factors for median survival time. Cox regression analysis showed that complete resection after metastasis and CHM were independent protective factors. The hazard ratio (HR) was 0.103 (95% CI 0.064∼0.164; P < 0.001). Detailed data are presented in Table 1.
Table 1.
Univariate and multivariate analyses of variables affecting the survival of 222 patients with mCRC.
| Characteristics | Univariate Analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| N (%) | P Value | Β | Exp(β) | 95% CI for Exp(β) | P | |
| Age(year) | 0.320 | — | — | — | — | |
| ≤40 | 14 (6.3) | |||||
| 40–60 | 116 (52.3) | |||||
| ≥60 | 92 (41.4) | |||||
| Gender | 0.815 | — | — | — | — | |
| Male | 136 (61.3) | |||||
| Female | 86 (38.7) | |||||
| Smoking history | 0.028 | — | — | — | — | |
| Yes | 70 (31.5) | |||||
| No | 152 (68.5) | |||||
| Primary tumor size | 0.023 | — | — | — | — | |
| ≤4 | 76 (34.2) | |||||
| >4 cm | 146 (65.8) | |||||
| Primary tumor location | 0.078 | — | — | — | — | |
| Rectum | 115 (51.8) | |||||
| Colon | 107 (48.2) | |||||
| Invaded the serous membrane | <0.001 | 0.794 | 2.212 | 1.412–3.464 | 0.001 | |
| Yes | 169 (76.1) | |||||
| No | 53 (23.9) | |||||
| Tumor differentiation | <0.001 | 0.446 | 1.561 | 1.108–2.199 | 0.011 | |
| High | 10 (4.5) | |||||
| Middle | 171 (77.0) | |||||
| Poor | 41 (18.5) | |||||
| General classification | 0.653 | — | — | — | — | |
| Ulcer type | 188 (84.7) | |||||
| Uplift type | 29 (13.0) | |||||
| Infiltrating type | 5 (2.3) | |||||
| Pathological classification | 0.005 | — | — | — | — | |
| Tubular adenocarcinoma | 176 (79.3) | |||||
| Mucinous adenocarcinoma | 34 (15.3) | |||||
| Papillary adenocarcinoma | 12 (5.4) | |||||
| Lymph node metastases | <0.001 | 0.134 | 1.144 | 0.925–1.415 | 0.216 | |
| No | 108 (48.6) | |||||
| 1–3 | 79 (35.6) | |||||
| ≥ 4 | 35 (15.8) | |||||
| CEA | 0.010 | 0.428 | 1.534 | 1.069–2.199 | 0.020 | |
| High | 129 (58.1) | |||||
| Normal | 93 (41.9) | |||||
| CA19-9 | 0.001 | — | — | — | — | |
| High | 75 (33.8) | |||||
| Normal | 147 (66.2) | |||||
| CA24-2 | <0.001 | — | — | — | — | |
| High | 105 (47.3) | |||||
| Normal | 117 (52.7) | |||||
| CA72-4 | <0.001 | — | — | — | — | |
| High | 75 (33.8) | |||||
| Normal | 147 (66.2) | |||||
| Systemic Chemotherapy | <0.001 | −0.823 | 0.439 | 0.278–0.695 | <0.001 | |
| Yes | 193 (86.9) | |||||
| No | 29 (13.1) | |||||
| Radiotherapy | <0.001 | −1.058 | 0.347 | 0.207–0.581 | <0.001 | |
| Yes | 45 (20.3) | |||||
| No | 177 (79.7) | |||||
| Complete resection after metastasis | <0.001 | −1.988 | 0.137 | 0.086–0.218 | <0.001 | |
| Yes | 71 (32.0) | |||||
| No | 151 (68.0) | |||||
| CHM | <0.001 | −2.275 | 0.103 | 0.064–0.164 | <0.001 | |
| Yes | 78 (35.1) | |||||
| No | 144 (64.9) | |||||
CHM, Chinese herbal medicine; CEA, carcinoembryonic antigen; CA-199, carbohydrate antigen 199; CA-242, carbohydrate antigen 242; CA-724: carbohydrate antigen 724.
Patients in the CHM group had a longer median survival time (40 months) compared with the non-CHM group (12 months) (P < 0.001). In addition, the 1-, 2-, 3-, and 5-year survival rates were 96.1, 84.3, 56.3, and 29.2% in the CHM group versus 46.3, 24.5, 13.8, and 7.3% in the non-CHM group, respectively (Fig. 2).
Figure 2.
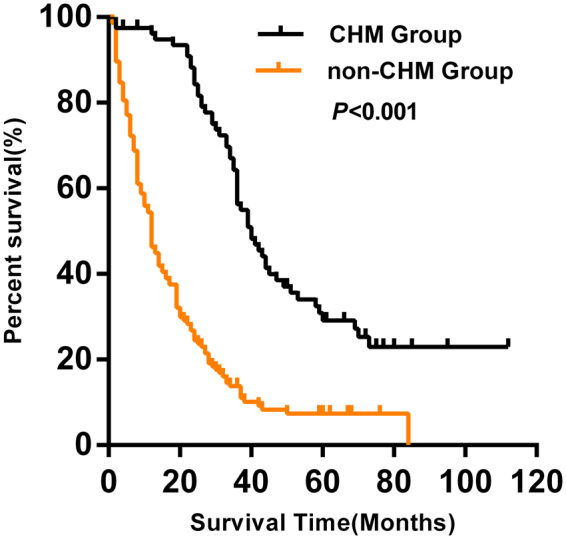
Kaplan–Meier curve between CHM and non-CHM groups. Patients who received CHM treatment had a longer median survival time than those without CHM treatment (40 vs. 12 months, P < 0.001). CHM, Chinese herbal medicine.
Candidate targets associated with CRC
Although hundreds of significant genes and proteins have been shown to be differently expressed or to exhibit genetic variations in CRC, only a few of hub genes and proteins were identified as candidate targets. Therapeutic Target Database (TTD) provides information about the known and explored therapeutic protein and nucleic acid targets. Sixty-two significant targets were obtained from the TTD. As shown in Fig. 3, these targets were primarily involved in cell proliferation, cancer metastasis, and immunity. They included the RAS, phosphoinositide 3-kinase (PI3K)/AKT1, vascular endothelial growth factor (VEGF), and interleukin signaling pathways, as well as pathways involved in focal adhesion.
Figure 3.
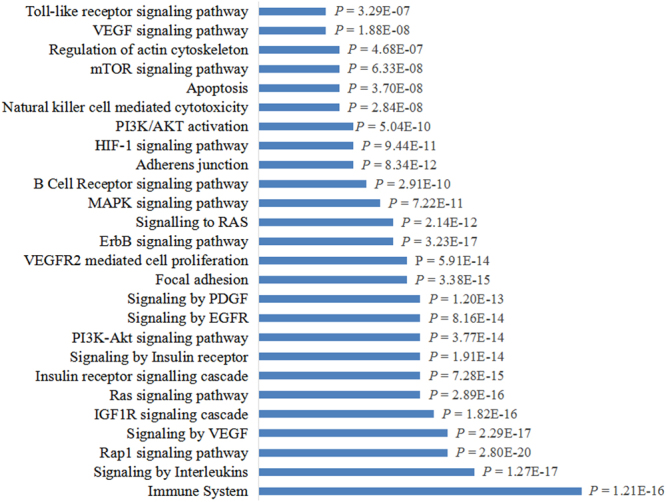
Enrichment analysis of candidate targets for colorectal cancer treatment. Enrichment analysis showed that candidate targets for colorectal cancer treatment were frequently involved in intracellular signaling cascades and immune responses.
Candidate herbs related to CRC and their putative major ingredients and targets
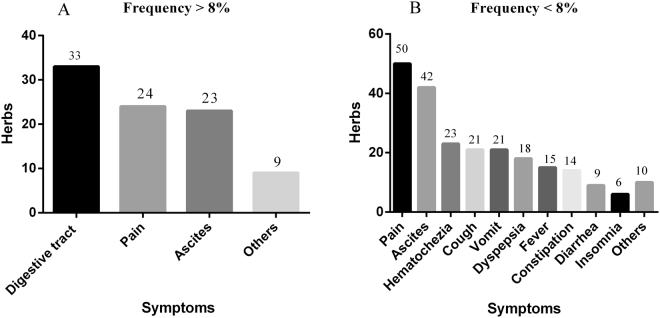
A total of 78 patients received CHM treatment, and all the CHM prescriptions used by them were counted; these prescriptions contained 295 herbs. Among the 295 types of herbs, we distinguished the commonly used herbs from the uncommonly used herbs according to the single herb frequency/total frequency. A total of 92 herbs with using frequency/total frequency >8% were defined as commonly used herbs, and 18 herbs of these were closely associated with survival. Other 74 commonly used herbs were applied to relieve the major complications of CRC mainly through relieving pain, diuresis and alleviating digestive tract symptoms (Fig. 4A). Furthermore, a total of 203 herbs with using frequency <8% were defined as uncommonly used herbs, which were frequently administered to relieve various uncomfortable symptoms, such as pain, ascites, vomit, hematochezia, cough, expectoration, fever, dyspepsia, constipation, diarrhea, insomnia and so on (Fig. 4B). The maximum value of single herb frequency was 1570. And 92 herbs with using frequency >125.6 (1570 × 8%) were selected for correlation analysis. Statistical results indicated that 18 herbs were closely related to improving survival(P < 0.05, correlation coefficients ≧ 0.23). These 18 herbs were Lycii Fructus (LF), Magnolia officinalis Rehd Et Wils (MO), Radix Clematidis (RC), Aucklandiae Radix (AR), Angelicae sinensis Radix (ASR), Xanthii Fructus (XF), Eriocauli Flos (EF), Cassiae Semen (CaS), Fallopia multiflora (FM), Selaginella doederleinii Hieron (SDH), Herba Patriniae (HP), Portulacae Herba (PH), Coicis Semen (CoS), Taraxacum mongolicum Hand (TM), Agrimonia eupatoria (AE), Ranunculi ternati Radix (RTR), Schisandrae chinensis Fructus (SCF), Radix Paeoniae rubra (RPR). Furthermore, 165 ingredients present in these 18 herbs were suggested to be related to CRC treatment. To further elucidate the underlying molecular mechanisms of these herbal medicines, targets of the proposed active ingredients were identified based on a comprehensive method. These candidate ingredients yielded 41 potential targets involved in CRC.
Figure 4.
Analysis of the herbs unrelated to survival. (A) Seventy-four commonly used herbs unrelated to survival were applied to relieve the major complications of CRC mainly through relieving pain, diuresis and alleviating digestive tract symptoms. (B) 203 herbs uncommonly used herbs were administered to relieve various uncomfortable symptoms.
Targets prediction in the candidate ingredient-target network
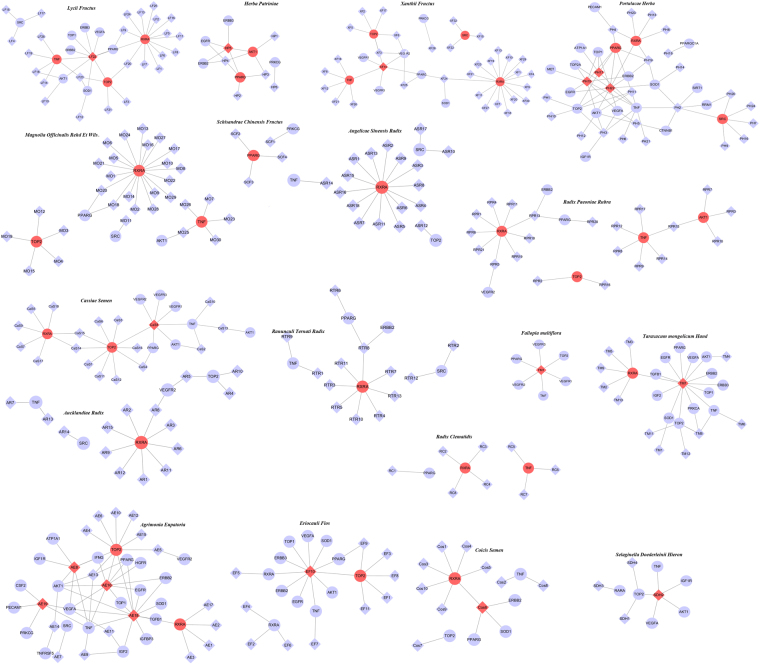
The 18 herbs contain 309 compounds, 165 of which have certain effect on CRC. Among them, 18 ingredients corresponded to the most targets and exhibited high scores, and each of them hit the 41 major putative colorectal cancer targets. Ingredient-target networks of the herbs are shown in Fig. 5. Targets in the outer circle had much fewer interactions with the candidate ingredients than those in the inner circle, which also indicated that many candidate targets were affected by only one candidate ingredient. Alternatively, some targets could be modulated by multiple rather than a single ingredient. We constructed a general network of all the candidate ingredients and candidate protein targets of the 18 herbs. As shown in Fig. 6, the major ingredients and targets involved in CRC treatment include SRC, AKT1, VEGFA, VEGFR, TNF, TOP2, PPARG, RXRA, which are represented by the nodes with red color. The putative major ingredients were determined by analyzing the topological parameters of the networks (Table 2). Quercetin might play an important role in CRC treatment since it is the major ingredient of five herbs (Lycii Fructus, Eriocauli Flos, Portulacae Herba, Taraxacum mongolicum Hand, and Phytolaccae Radix). Ingredients such as emodin, stigmasterol, apigenin, and oleic acid may also play significant roles, because these ingredients were present in more than two herbs. The number of ingredients, serial number of each ingredient, DL values, and number of validated/predicted targets are shown in Table S2.
Figure 5.
The ingredient-target networks. The diamond nodes represent ingredients, the circular nodes represent targets, and the nodes with red color are the major ingredients and targets involved in CRC treatment. LF, Lycii Fructus; MO, Magnolia officinalis Rehd Et Wils; RC, Radix Clematidis; AR, Aucklandiae Radix; ASR, Angelicae sinensis Radix; XF, Xanthii Fructus; EF, Eriocauli Flos; CaS, Cassiae Semen; FM, Fallopia multiflora; SDH, Selaginella doederleinii Hieron; HP, Herba Patriniae; PH, Portulacae Herba; CoS, Coicis Semen; TM, Taraxacum mongolicum Hand; AE, Agrimonia eupatoria; RTR, Ranunculi ternati Radix; SCF, Schisandrae chinensis Fructus; RPR, Radix Paeoniae rubra.
Figure 6.
Network of all the candidate ingredients and candidate protein targets of the 18 herbs for CRC treatment. The diamond nodes represent ingredients, the circular nodes represent targets, and the nodes with red color are the major ingredients and targets involved in CRC treatment.
Table 2.
The major ingredients and major targets of the 18 herbs involved in CRC treatment.
| Chinese Name | Latin name | Major ingredients | Number of targets | Major targets | Frequency (%) |
Average dosage(g) | Correlation coefficient | P value |
|---|---|---|---|---|---|---|---|---|
|
|
Lycii Fructus | Quercetin (LF22) | 11 | TNF RXRA TOP2 |
12.6 | 10 | 0.35 | 0.002 |

|
Magnolia Officinalis Rehd Et Wils. | Honokiol (MO18) Magnolol (MO20) |
6 | TNF RXRA TOP2 |
15.2 | 6 | 0.346 | 0.003 |
|
|
Radix Clematidis | Nonane (RC6) Palmitic acid (RC7) Stigmasterol (RC8) |
3 | TNF RXRA PPARG |
24.2 | 10 | 0.315 | 0.007 |

|
Aucklandiae Radix | (R)-linalool (AR5) | 5 | RXRA | 43.5 | 5 | 0.282 | 0.017 |

|
Angelicae Sinensis Radix | Stigmasterol (ASR16) | 4 | RXRA | 52.0 | 10 | 0.28 | 0.018 |
|
|
Xanthii Fructus | Emodin (XF14) | 10 | TNF RXRA TOP2 SRC |
11.5 | 9 | 0.274 | 0.021 |
|
|
Eriocauli Flos | Quercetin (EF10) | 12 | TOP2 | 18.5 | 30 | 0.274 | 0.021 |
|
|
Cassiae Semen | Emodin (XF8) | 9 | RXRA TOP2 TNF |
16.6 | 10 | 0.274 | 0.021 |

|
Fallopia multiflora | Emodin (FM1) | 6 | TOP2 TNF |
10.2 | 30 | 0.274 | 0.021 |
|
|
Selaginella Doederleinii Hieron | Apigenin (SDH2) | 6 | TOP2 | 31.1 | 30 | 0.272 | 0.022 |
|
|
Herba Patriniae | luteolin (EF4) | 6 | AKT1 PPARG |
71.0 | 30 | 0.271 | 0.022 |
|
|
Portulacae Herba | Luteolin (PH15) Myricetin (PH17) Quercetin (PH22) |
20 | RXRA PPARG SRC |
33.6 | 30 | 0.263 | 0.027 |
|
|
Coicis
Semen |
Oleic acid (CoS6) | 6 | RXRA | 14.7 | 30 | 0.254 | 0.032 |
|
|
Taraxacum mongolicum Hand | Quercetin (TM7) | 14 | RXRA TOP2 |
15.3 | 10 | 0.247 | 0.038 |
|
|
Phytolaccae Radix | Quercetin (PR8) Apigenin (PR16) Luteolin (PR18) Ursolic acid (PR19) |
23 | RXRA TOP2 |
71.8 | 30 | 0.246 | 0.039 |
|
|
Ranunculi Ternati Radix | Oleic acid (RTR8) | 5 | RXRA | 60.4 | 30 | 0.244 | 0.04 |
|
|
Schisandrae Chinensis Fructus | Protocatechuic acid (SCF1) Arnebin 7 (SCF2) Gomisin T (SCF3) Schisanhenol (SCF4) |
2 | PPARG | 42.0 | 6 | 0.244 | 0.041 |

|
Radix Paeoniae Rubra | Oleic acid (RPR13) Paeonol (RPR15) |
7 | RXRA TOP2 TNF AKT1 |
8.9 | 20 | 0.236 | 0.048 |
Holistic mechanisms of anti-CRC medicinal herbs
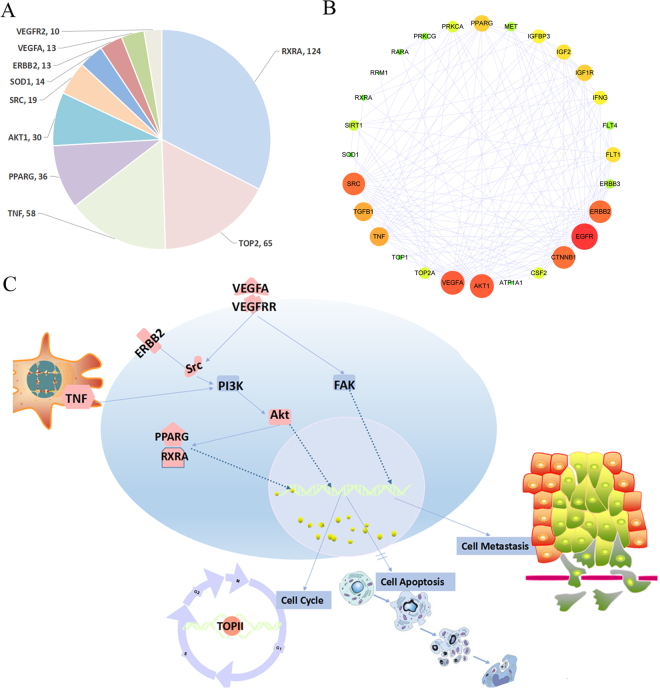
Cancer is a complicated disease, wherein many parallel signaling pathways are involved in the development and maintenance of tumors. In this research, 41 tumor-associated proteins involved in tumorigenesis were identified as targets of 18 herbs using network analysis. Interestingly, RXRA, TOP2, TNF, PPARγ, AKT1, SRC, ERBB2, VEGFR, and VEGFA had the most of the direct interactions with these herbs, suggesting that these proteins might play important roles in the treatment of CRC. Based on the network and multi-target computational approach, we found that simultaneous manipulation of multiple targets involved in proliferation, such as epidermal growth factor receptor (EGFR), hepatocyte growth factor receptor (HGFR), peroxisome proliferator-activated receptors (PPARs), ERBB2, and insulin-like growth factor 1 receptor (IGF1R), as well as angiogenesis, such as VEGF receptor (VEGFR), might underlie the beneficial effects of the 18 herbs in CRC. Retinoid X receptor A (RXRA), topoisomerase 2 (TOP2), tumor necrosis factor (TNF), PPARγ, AKT1, SRC, ERBB2, VEGFR, and VEGFA were the most important targets because they were inhibited by multiple components of 18 herbs. The major targets and the number of the related ingredients were shown in Fig. 7A. The protein-protein interaction of all the candidate protein targets is shown in Fig. 7B. It indicated that VEGFA, AKT1, EGFR, ERBB2 and SRC played central roles in the protein interactions. Interestingly, these proteins also the major targets of the 18 herbs and play important roles in CRC treatment. To better understand the target functions associated with the 18 herbs, we mapped the targets to the canonical signaling pathways identified in the Kyoto Encyclopedia of Genes and Genomes (KEGG) and summarized the most relevant pathways (Fig. 7C).
Figure 7.
Signaling pathways involved in the actions of the 18 herbs against colorectal cancer. (A) Major predicted targets (could be targeted by more than 10 ingredients) were listed according to the scores from high to low. (B) Protein-protein interaction of all the candidate protein targets. The map node size gets larger with increased degree and the map color gets bright from green to red with increased degree. This figure was drew using cytoscape with the protein-protein interactions getting from STRING48,49. (C) The 18 herbs might exhibit anti-colorectal cancer activity mainly via 1) inhibition of the proliferative activity of ErbB2, PPARγ, and RXR, 2) suppression of angiogenesis by inhibiting VEGFR and VEGFA expression, 3) inhibition of the PI3K/Akt signaling pathway directly through Src and Akt, and 4) reduction of TNF-induced inflammation.
Experimental validation
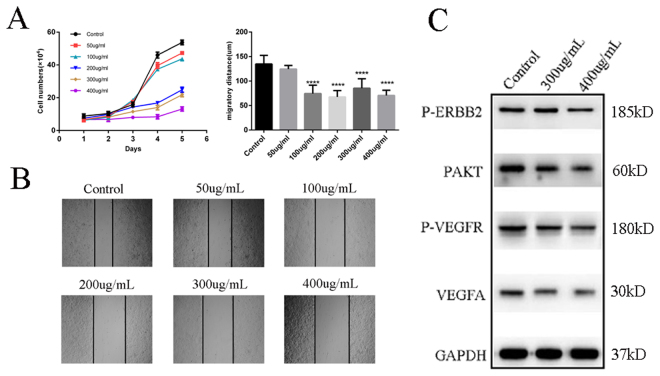
The survival closely associated 18 herbs and their putative targets were validated by experiments. The anti-proliferation cancer effects of 18 herbs were evaluated using typan blue staining assay. The anti-migration effects were evaluated by wound healing assay. The activity of the core predicted targets were tested using western blotting. Figure 8A shows a statistical chart of anti-proliferation and anti-migration of 18 herbs; and the effects of herbs on wound healing assay were shown in Fig. 8B. The experimental results indicated that aqueous extracts of 18 herbs showed a significant suppression effect on cell proliferation after 36 hours in dose of 200 ug/mL, 300 ug/mL and 400 ug/mL in vitro. And the aqueous extract could also significantly suppress cell migration at 12 hours in dose of 100 ug/mL, 200 ug/mL, 300 ug/mL and 400 ug/mL. Interestingly, we also found that aqueous extracts of 18 herbs could obviously inhibit cell migration at the 12th hour, but it can only obviously inhibit cell proliferation after 36 hours. It is indicated that aqueous extracts of 18 herbs could affect cell migration in a short time, but it takes enough time to play the role of anti-cell proliferation. Furthermore, aqueous extracts of 18 herbs affected the expression of VEGFA as well as the phosphorylation progress of ERBB2, AKT and VEGFR. As shown in Fig. 8C, aqueous extracts of 18 herbs obviously decreased VEGFA, p-ERBB2, p-AKT and p-VEGFR at the dose of 300 ug/mL and 400 ug/mL.
Figure 8.
Effect of aqueous extract of 18 herbs on cell proliferation, cell migration and predicted targets. (A)The statistical views of cell proliferation (left) and cell migration (right). P-values are represented as asterisks (****P < 0,001). (B) The result of wound healing assay. (C) Western blot assay analyzed P-ERBB2, P-AKT1, VEGFA and P-VEGFR after the treatment of aqueous extract of 18 herbs. No grouping of gels/blots cropped from diferent parts of the same gel, or from diferent gels, felds, or exposures was performed.
Discussion
Previous studies have shown that chemotherapy is usually combined with CHM to improve the resistance of patients, adjust their nutritional imbalance, play an anticancer effect, and facilitate the implementation of the chemotherapy process23,24. In addition, previous clinical studies have suggested that CHM treatment in CRC has promising effects. In line with this, the present study showed encouraging results of CHM treatment for colorectal cancer patients. Patients in the CHM group had a longer median survival time (40 months) than those in the non-CHM group (12 months). Compared with non-CHM group, the overall survival rate of CHM group improved significantly. The effects of CHM in mCRC have been previously reported; however, the molecular mechanisms remain to be clarified.
From the TTD, we determined 62 targets related to CRC. And these targets are frequently involved in intracellular signaling cascades that are related to cancer proliferation and metastasis. These pathways included RAP, IGF1R, RAS, and PI3K/AKT1 signaling pathways. These candidate targets are also involved in immune responses, such as natural killer cell-mediated cytotoxicity, B cell receptor signaling pathway, and toll-like receptor signaling pathway.
Clinically, 18 herbs obviously improved the survival of patients with mCRC. 309 complex components were contained in these 18 herbs, and 165 components with potential effects on CRC were preserved for further study. Among the 165 chemical ingredients, 18 ingredients corresponded to the most targets and exhibited high scores. These ingredients were linalool, apigenin, arnebin, emodin, gomisin T, honokiol, luteolin, magnolol, myricetin, nonane, oleic acid, paeonol, palmitic acid, protocatechuic acid, quercetin, schisanhenol, stigmasterol, and ursolic acid. Some of these ingredients, such as quercetin25,26, ursolic acid27,28, and stigmasterol29, were previously shown to exert favorable anticancer activities.
Among the 41 major putative targets of the 18 herbs, RXRA, TOP2, TNF, PPARγ, AKT1, SRC, ERBB2, VEGFR, and VEGFA had the most of the direct interactions with these herbs, suggesting that these proteins might play important roles in the treatment of CRC. RXR and PPARγ are potential candidate targets for cancer prevention and treatment. Once activated, PPARγ binds to RXR to form PPAR–RXR heterodimer. The activation of PPARγ results in growth arrest of colon carcinoma cells via induction of cell-cycle arrest or/and apoptosis30–32. TOP2 is involved in critical processes in the cell, including DNA replication, transcription, and chromosome segregation. Interfering with TOP2 and generating enzyme-mediated DNA damage are effective strategies for cancer therapy33. Chronic inflammatory diseases are associated with an increased risk of CRC34. TNF is crucial for the initiation and progression of colitis-associated colon carcinogenesis35. TNF antagonists were shown to inhibit inflammatory cytokines, matrix metalloproteinases (MMPs), angiogenesis, and leucocyte trafficking to the sites of inflammation. All these effects could be useful in the treatment of cancer36. Activation of AKT1 signaling and inhibition of the expression of phosphatase and tensin homolog (PTEN), a negative regulator of AKT1, have been reported in 60–70% of patients with CRC37. Inhibitors of PI3K/AKT1 signaling pathway have been suggested as potential therapeutic agents in CRC38. In addition, SRC is elevated in the premalignant tissues in CRC, which could result in induction of apparent loosening of the clusters of colon cancer cells39. Overexpression of cytoplasmic ERBB2 plays an important role in the progression of CRC, where its expression correlates with the tumor size, subserosal invasion, liver metastasis, and Dukes’ classification40. Moreover, these targets were mapped to the canonical signaling pathways identified in the KEGG. Collectively, our results showed that 18 herbs in CHM might exhibit anti-CRC activity mainly via 1) inhibition of the proliferative activity of ERBB2, PPARγ, and RXR; 2) suppression of angiogenesis by inhibiting VEGFR and VEGFA expression; 3) inhibition of the PI3K/AKT1 signaling pathway directly through SRC and AKT1; and 4) Reduction of TNF-induced inflammation.
The anti-colorectal cancer effects of 18 herbs were evaluated by wound healing assay and typanblue staining assay. The experimental results showed that aqueous of 18 herbs had obvious inhibitory effects on cell proliferation and the effects were improved with increased dosages. Cell migration was significantly inhibited (in 12 hours) when the dose of aqueous reached 100 ug/mL in vitro. Furthermore, the results of western blotting confirmed the effects of these herbs on predicting targets.
Network pharmacology is a suitable approach to measure the efficacy and to reveal the functional mechanisms of multi-target drugs. At the same time, we must recognize its shortcomings. (i) The components of Chinese medicine screened out by DL value may be incompatible with the exact components; (ii) Because the accuracy of targets prediction tools is different, the results obtained by different prediction tools may be incompatible. Moreover, the number of statistical clinical cases in the present study is not large enough; and the Spearman’s bivariate correlate analysis was adopted to obtain the strong correlated herbs for positive effectiveness, which may not accurately reflect the real clinical situation.
Conclusion
In the present study, we showed that CHM treatment could significantly improve the survival of patients with mCRC; and correlation analysis identified 18 herbs with positive effects on survival. Moreover, we performed a network pharmacological approach to investigate the underlying mechanisms, which provides a helpful method for herbal research based on clinical data.
Materials and Methods
Patient characteristics
The present research was approved by the Ethics Committee of Tianjin Medical University(The certificate no. Tmuhmec2015007). All methods were in accordance with the relevant guidelines and regulations. The ethics committee approved the exemption from informed consent, because this is a retrospective study and most of the patients died before conducting the research. The patients were included to our research by the following criteria: age ≥ 18 years old, clear pathological diagnosis of surgery or colonoscopy, and Chinese herbal medicine treatment in CHM group ≥ 2 months. And the cases were excluded through the following criteria: serious disease, concurrent cancer, incomplete medical records, lack of accurate documentation of the recurrence time, no distant metastasis, and loss to follow-up.
Finally, the medical records of 222 patients diagnosed with mCRC between November 2007 and April 2012 were retrospectively reviewed. Seventy-eight patients who received CHM ≥ two months were assigned to the CHM group, and 144 patients who did not receive CHM were included in the non-CHM group.
Treatment
CHM group patients received both traditional Chinese medicine (TCM) and Western medicine (WM), and non-CHM group patients received WM only. Radical resection was offered to patients with resectable hepatic metastases. In the CHM group, CHM was administered according to the syndrome differentiation, wherein the formula was administered orally three times daily 30 minutes after meals for 2 months or longer.
Generally, each formula for mCRC included 20–30 kinds of herbs. We counted the prescriptions of CHM from 78 patients, who received TCM treatment with CHM for 24850 days, including 295 herbs. Among the 295 types of herbs, some herbs were frequently used, while some herbs were not commonly used. The commonly used herbs were shared among most patients and were closely associated with survival. The uncommonly used herbs were frequently administered to relieve various uncomfortable symptoms, such as pain, ascites, vomit, hematochezia, cough, expectoration and so on. We adopted the using frequency to identify commonly used herbs. The herbs with frequency >8% were selected for bivariate correlation analysis. In addition, we calculated the coefficients of correlation between each separate herb and survival time. These herbs were used in a further network pharmacology dissection according to the following criteria: single medicinal substance frequency/total frequency >8% and P value < 0.05 based on the results of the correlation analysis.
Significant targets of CRC
Candidate targets related to CRC were obtained from the Therapeutic Target Database (http://bidd.nus.edu.sg/group/cjttd/ttd_home.asp, Version 4.3.02 release on Sep 15th, 2013)41.
Herb formulation ingredient collection, target fishing, and function scoring
The chemical ingredients of herbs and their predicted targets were obtained from the TCM Systems Pharmacology (TCMSP) Database (http://lsp.nwu.edu.cn/tcmsp.php)42, then they were selected for further research. DL is a qualitative concept used in drug design for an estimate on how ‘drug-like’ a prospective compound is. The ‘drug-like’ level of the compounds is 0.18, which is used as a selection criterion for the ‘drug-like’ compounds in the traditional Chinese herbs43,44. Therefore, the chemical ingredients with DL values ˃ 0.18 were selected for further research.
Network construction and analysis
The methods applied for network construction and analysis were similar to those used in our previous studies45–47. Briefly, the ingredient-target networks of herbs and herb-target networks were constructed using Cytoscape software48 (Version 3.2.2) and were analyzed by using Cytoscape plugin CentiScaPe49. We finally predicted the main components and targets through calculating the optimal topological structure and analyzing statistical properties of network.
Experimental validation
Colorectal carcinoma cell line HT29 was used. The anti-colorectal cancer effects of aqueous extract of 18 herbs were tested. In this study, we extracted the aqueous extracts of 18 herbs together. Cell proliferation and cell migration were evaluated using typanblue staining assay and wound healing assay, respectively. The following antibodies were used: p-ERBB2 (Immuno Way, USA), GAPDH (Immuno Way, USA), p-AKT1 (Immuno Way, USA), p-VEGFR (Immuno Way, USA), VEGFA (Abcam, USA) to prove the predicted targets with western blotting. No grouping of gels/blots cropped from diferent parts of the same gel, or from diferent gels, felds, or exposures was performed.
Statistical analysis
The overall survival (OS) was defined as the time from the diagnosis of mCRC to the day of death or the last follow-up of patients with CRC. Baseline characteristics were compared by the chi-square test. Kaplan Meier method was used for survival rate. Prognostic factors were predicted by multivariate Cox regression analyses. The herbs related to survival were determined by Spearman’s bivariate correlation analysis. P < 0.05 was considered statistically significant. Statistical analyses were carried out by SPSS 21.0.
Ethical statement
All experimental protocols were under the approval of the Ethics Committee of Tianjin Medical University. (Study number: Tmuhmec2015007).
Electronic supplementary material
Acknowledgements
This study was supported by the National Science Foundation of China, No.81173376 and 81473441.
Author Contributions
Hongxu Zhu, Jian Hao substantially contributed to the acquisition and analysis of data, conception and design of the study; all authors drafted the article and made critical revisions related to the intellectual content of the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Hongxu Zhu, Jian Hao and Yangyang Niu contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-25500-x.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Toiyama Y, et al. Serum miR-200c is a novel prognostic and metastasis-predictive biomarker in patients with colorectal cancer. Ann. Surg. 2014;259:735–743. doi: 10.1097/SLA.0b013e3182a6909d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lykoudis PM, O’Reilly D, Nastos K, Fusai G. Systematic review of surgical management of synchronous colorectal liver metastases. Br. J. Surg. 2014;101:605–612. doi: 10.1002/bjs.9449. [DOI] [PubMed] [Google Scholar]
- 3.Nordlinger B, et al. Does chemotherapy prior to liver resection increase the potential for cure inpatients with metastatic colorectal cancer? A report from the European Colorectal Metastases Treatment Group. Eur. J. Cancer. 2007;43:2037–2045. doi: 10.1016/j.ejca.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 4.Nordlinger B, et al. Combination of surgery and chemotherapy and the role of targeted agents in the treatment of patients with colorectal liver metastases: recommendations from an expert panel. Ann. Oncol. 2009;20:985–992. doi: 10.1093/annonc/mdn735. [DOI] [PubMed] [Google Scholar]
- 5.Simmonds DC, et al. Surgical resection of hepatic metastases from colorectal cancer: a systematic review of published studies. Br. J. Cancer. 2006;94:982–999. doi: 10.1038/sj.bjc.6603033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van CE, Cervantes A, Nordlinger B, Arnold D. ESMO Guidelines Working Group. Metastatic colorectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2014;25:1–9. doi: 10.1093/annonc/mdt521. [DOI] [Google Scholar]
- 7.Kelly C, Cassidy J. Chemotherapy in metastatic colorectal cancer. Surgical. Oncol. 2007;16:65–70. doi: 10.1016/j.suronc.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 8.Glimelius B, Cavalli-Bjorkman N. Metastatic colorectal cancer: current treatment and future options for improved survival. Scand. J. Gastroenterol. 2012;47:296–314. doi: 10.3109/00365521.2012.640828. [DOI] [PubMed] [Google Scholar]
- 9.De GA, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. Clin. Oncol. 2000;18:2938–2947. doi: 10.1200/JCO.2000.18.16.2938. [DOI] [PubMed] [Google Scholar]
- 10.Van CE, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastaticcolorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J. Clin. Oncol. 2011;29(15):2011–2019. doi: 10.1200/JCO.2010.33.5091. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y, Liang Y, He C. Anticancer activities and mechanisms of heat-clearing and detoxicating traditional Chineseherbal medicine. Chin. Med. 2017;12:20. doi: 10.1186/s13020-017-0140-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qi F, et al. The advantages of using traditional Chinese medicine as an adjunctive therapy in the whole course of cancer treatment instead of only terminal stage of cancer. Bioscience. Trends. 2015;9(1):16–34. doi: 10.5582/bst.2015.01019. [DOI] [PubMed] [Google Scholar]
- 13.Li X, et al. Traditional Chinese medicine in cancer care: a review of controlled clinical studies published in Chinese. PloS. One. 2013;8(4):e60338. doi: 10.1371/journal.pone.0060338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu T, Munro AJ, Guanjian L. Chinese medical herbs for chemotherapy side effects in colorectal cancer patients. The. Cochrane. Library. 2012;25(1):CD004540. doi: 10.1002/14651858.CD004540.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tan KY, et al. The role of traditional Chinese medicine in colorectal cancer treatment. Tech. Coloproct. 2008;12:1–6. doi: 10.1007/s10151-008-0392-z. [DOI] [PubMed] [Google Scholar]
- 16.Tao L, et al. Clinical study on survival benefit for elderly patients with resected stage II or III colorectal cancer based on traditional Chinese medicine syndrome differentiation and treatment. J. Chin. Integr. Med. 2010;8:1159–1164. doi: 10.3736/jcim20101208. [DOI] [PubMed] [Google Scholar]
- 17.Hopkins AL. Network pharmacology: the next paradigm in drug discovery[J] Nat. Chem. Bio. 2008;4:682–690. doi: 10.1038/nchembio.118. [DOI] [PubMed] [Google Scholar]
- 18.Poornima P, Kumar JD, Zhao Q. Network pharmacology of cancer: From understanding of complex interactomes to the design of multi-target specific therapeutics from nature. Pharmacological Research. 2016;111:290–302. doi: 10.1016/j.phrs.2016.06.018. [DOI] [PubMed] [Google Scholar]
- 19.Li S, Zhang B, Zhang NB. Network target for screening synergistic drug combinations with application to traditional Chinese medicine. BMC. Systems. Biology. 2011;5(S1):S10. doi: 10.1186/1752-0509-5-S1-S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu CW, Lu L, Liang SW, Chen C, Wang SM. Application of drug-target prediction technology in network pharmacology of traditional Chinese medicine. Zhongguo Zhong Yao Za Zhi. 2016;41(3):377–382. doi: 10.4268/cjcmm20160303. [DOI] [PubMed] [Google Scholar]
- 21.Zhang GB, et al. Network pharmacology: a new approach for chinese herbal medicine research. Evid. Based. Complement. Alternat. Med. 2013;2013:621423. doi: 10.1155/2013/621423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li S, Zhang B. Traditional Chinese medicine network pharmacology: theory, methodology and application. Chinese Journal of Natural Medicines. 2013;11(2):110–120. doi: 10.1016/S1875-5364(13)60037-0. [DOI] [PubMed] [Google Scholar]
- 23.Sandler A, et al. An openlabel multicenter three-stage phase II study of s-l in combination with cisplatin as first -line therapy for patients with advanced non-small cell lung cancer. J. Thorac. Oncol. 2011;6:1400–1406. doi: 10.1097/JTO.0b013e31820d7805. [DOI] [PubMed] [Google Scholar]
- 24.Sanoff HK, et al. Five -year data andprognostic factor analysis of oxaliplatin and irinotecan combinations for advanced colorectal cancer. J. Clin. Oncol. 2008;26:5721–5727. doi: 10.1200/JCO.2008.17.7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jeong JH, An JY, Kwon YT, Rhee JG, Lee YJ. Effects of low dose quercetin: Cancer cell‐specific inhibition of cell cycle progression. J. Cell. Biochem. 2009;106:73–82. doi: 10.1002/jcb.21977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park CH, et al. Quercetin, a potent inhibitor against β-catenin/Tcf signaling in SW480 colon cancer cells. Biochem. Biophy. Res. Commun. 2005;328:227–234. doi: 10.1016/j.bbrc.2004.12.151. [DOI] [PubMed] [Google Scholar]
- 27.Hsu YL, Kuo PL, Lin CC. Proliferative inhibition, cell-cycle dysregulation, and induction of apoptosis by ursolic acid in human non-small cell lung cancer A549 cells. Life. Sci. 2004;75:2303–2316. doi: 10.1016/j.lfs.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 28.Yan S, Huang C, Wu S, Yin MC. Oleanolic acid and ursolic acid induce apoptosis in four human liver cancer cell lines. Toxicol. In. Vitro. 2010;24:842–848. doi: 10.1016/j.tiv.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 29.Ali H, et al. Isolation and evaluation of anticancer efficacy of stigmasterol in a mouse model of DMBA-induced skin carcinoma. Drug. Des. Devel. Ther. 2015;9:2793–2800. doi: 10.2147/DDDT.S83514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fiatte C, et al. Genetic analysis of peroxisome proliferator-activated receptor γ1 splice variants in human colorectal cell lines. Internat. J. Oncol. 2009;29:1601–1610. [PubMed] [Google Scholar]
- 31.Yaacob NS, Darus HM, Norazmi MN. Modulation of cell growth and PPARγ expression in human colorectal cancer cell lines by ciglitazone. Experi. Toxicol. Pathol. 2008;60:505–512. doi: 10.1016/j.etp.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 32.Lin MS, Chen WC, Bai X, Wang YD. Activation of peroxisome proliferator‐activated receptor γ inhibits cell growth via apoptosis and arrest of the cell cycle in human colorectal cancer. J. Digest. Dis. 2007;8:82–88. doi: 10.1111/j.1443-9573.2007.00290.x. [DOI] [PubMed] [Google Scholar]
- 33.Nitiss JL. Targeting DNA topoisomerase II in cancer chemotherapy. Nat. Rev. Cancer. 2009;9:338. doi: 10.1038/nrc2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Terzi J, Grivennikov S, Karin E, Karin M. Inflammation and colon cancer. Gastroenterol. 2010;138:2101–2114. doi: 10.1053/j.gastro.2010.01.058. [DOI] [PubMed] [Google Scholar]
- 35.Balkwill F. TNF in promotion and progression of cancer. Cancer. Metast. Rev. 2006;25:409–416. doi: 10.1007/s10555-006-9005-3. [DOI] [PubMed] [Google Scholar]
- 36.Feldmann M. Development of anti-TNF therapy for rheumatoid arthritis. Nat. Rev. Immunol. 2002;2:364–371. doi: 10.1038/nri802. [DOI] [PubMed] [Google Scholar]
- 37.Colakoglu T, et al. Clinicopathological significance of PTEN loss and the phosphoinositide 3-kinase/Akt pathway in sporadic colorectal neoplasms: is PTEN loss predictor of local recurrence. Am. J. Surg. 2008;195(6):719–725. doi: 10.1016/j.amjsurg.2007.05.061. [DOI] [PubMed] [Google Scholar]
- 38.Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat. Rev. Cancer. 2009;9:550–562. doi: 10.1038/nrc2664. [DOI] [PubMed] [Google Scholar]
- 39.Avizienyte E, et al. Src-induced de-regulation of E-cadherin in colon cancer cells requires integrin signalling. Nat. Cell. Biol. 2002;4:632. doi: 10.1038/ncb829. [DOI] [PubMed] [Google Scholar]
- 40.Osako T, et al. Immunohistochemical study of c-erbB-2 protein in colorectal cancer and the correlation with patient survival. Oncol. 1998;55:548–555. doi: 10.1159/000011911. [DOI] [PubMed] [Google Scholar]
- 41.Yang H, et al. Therapeutic target database update 2016: enriched resource for bench to clinical drug target and targeted pathway information. Nucleic. Acids. Res. 2016;44(D1):D1069–1074. doi: 10.1093/nar/gkv1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ru J, et al. TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. J. Cheminformatics. 2014;6:13. doi: 10.1186/1758-2946-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tao W, et al. Network pharmacology-based prediction of the active ingredients and potential targets of Chinese herbal Radix Curcumae formula for application to cardiovascular disease. J. Ethnopharmacol. 2013;145:1–10. doi: 10.1016/j.jep.2012.09.051. [DOI] [PubMed] [Google Scholar]
- 44.Liu J, et al. Systems-pharmacology dissection of traditional chinese medicine compound saffron formula reveals multi-scale treatment strategy for cardiovascular diseases. Sci. Rep. 2016;2016:6. doi: 10.1038/srep19809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gao L, et al. Molecular targets of Chinese herbs: a clinical study of hepatoma based on network pharmacology. Sci. Rep. 2016;6:24944. doi: 10.1038/srep24944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mao Y, et al. Network pharmacology-based and clinically relevant prediction of the active ingredients and potential targets of Chinese herbs in metastatic breast cancer patients. Oncotarget. 2017;8(16):27007–27021. doi: 10.18632/oncotarget.15351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gao L, et al. Network pharmacology dissection of multiscale mechanisms of herbal medicines in stage IV gastric adenocarcinoma treatment. Medicine. 2016;95(35):e4389. doi: 10.1097/MD.0000000000004389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smoot ME, Ono K, Ruscheinski J, Wang PL, Ideker T. Cytoscape 2.8: new features for data integration and network visualization. Bioinformatics. 2011;27:431–432. doi: 10.1093/bioinformatics/btq675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scardoni G, Petterlini M, Laudanna C. Analyzing biological network parameters with CentiScaPe. Bioinformatics. 2009;25:2857–2859. doi: 10.1093/bioinformatics/btp517. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.