Abstract
Cognitive impairment in HIV-1 infection is associated with the induction of chronic proinflammatory responses in the brains of infected individuals. The risk of HIV-related cognitive impairment is increased by cigarette smoking, which induces brain inflammation in rodent models. To better understand the role of smoking and the associated immune response on behavioral and motor function in HIV infection, wild-type F344 and HIV-1 transgenic (HIV1Tg) rats were exposed to either smoke from nicotine-containing (regular) cigarettes, smoke from nicotine-free cigarettes, or to nicotine alone. The animals were then tested using the rotarod test (RRT), the novel object recognition test (NORT), and the open field test (OFT). Subsequently, brain frontal cortex from the rats was analyzed for levels of TNF-α, IL-1, and IL-6. On the RRT, impairment was noted for F344 rats exposed to either nicotine-free cigarette smoke or nicotine alone and for F344 and HIV1Tg rats exposed to regular cigarette smoke. Effects from the exposures on the OFT were seen only for HIV1Tg rats, for which function was worse following exposure to regular cigarette smoke as compared to exposure to nicotine alone. Expression levels for all three cytokines were overall higher for HIV1Tg than for F344 rats. For HIV1Tg rats, TNF-α, IL-1, and IL-6 gene expression levels for all exposure groups were higher than for control rats. All F344 rat exposure groups also showed significantly increased TNF-α expression levels. However, for F344 rats, IL-1 expression levels were higher only for rats exposed to nicotine-free and nicotine-containing CS, and no increase in IL-6 gene expression was noted with any of the exposures as compared to controls. These studies, therefore, demonstrate that F344 and HIV1Tg rats show differential behavioral and immune effects from these exposures. These effects may potentially reflect differences in the responsiveness of the various brain regions in the two animal species as well as the result of direct toxicity mediated by the proinflammatory cytokines that are produced by HIV proteins and by other factors that are present in regular cigarette smoke.
Keywords: HIV-1, Transgenic rat, Cigarettes, Smoking, Cytokines, Behavioral testing
Introduction
Individuals with HIV-1 infection are at high risk for the development of neurological complications, with nearly half at risk for developing neurocognitive impairment (Heaton et al. 2011). Prominent among the abnormalities that develop among such individuals are cognitive and motor deficits, including impairment of attention, learning, memory, coordination, and motor speed (Heaton et al. 2004). In addition, HIV infection is associated with an increased incidence of behavioral abnormalities such as generalized anxiety and panic disorders (Brandt et al. 2017). Nervous system damage in HIV infection has been found to be mediated by neurotoxicity that results, in part, from by-stander effects from chronic inflammation induced by factors secreted by persistently infected mononuclear cells (Anderson et al. 2002). The secretion of such factors has been demonstrated to be enhanced due to the effects of substances such as stimulants (methamphetamine and cocaine) and opioids (Nath 2002).
Recent studies suggest that measures of attention, learning, and memory in HIV-infected individuals get even worse with cigarette smoking (Harrison et al. 2017). Cigarette smoke exposure has been linked to an increased risk of other neurological disorders, such as stroke, Alzheimer’s disease, and multiple sclerosis (Ascherio and Munger 2007; Cataldo et al. 2010; Shah and Cole 2010). HIV-infected smokers also have a higher mortality than infected nonsmokers and HIV-negative individuals (Helleberg et al. 2013), suggesting likely direct effects from smoking and interactions between smoking and HIV-1 infection on mortality. Also, although data may be conflicting as to whether cigarette smoking can increase progression to AIDS (Galai et al. 1997; Nieman et al. 1993), smoking has been associated with an increased HIV plasma viral load (Wojna et al. 2007), and an increased risk of developing neurocognitive impairment (Harrison et al. 2017; Wojna et al. 2007). Approximately 40% of HIV-positive individuals are cigarette smokers (Lifson and Lando 2012), which is more than twice the prevalence of smoking among adults in the general population (Jamal et al. 2016). Therefore, it is important to develop models that can be utilized to study mechanisms that may be linked to the development of nervous system impairment that may occur due to cigarette smoking or from other forms of cigarette smoke exposure.
In previous studies utilizing Lewis rats, we found that cigarette smoke exposure resulted in profound increases in cytokine gene expression and oxidative stress responses in the brains of the animals (Khanna et al. 2013). These responses included elevated levels of interferon (IFN)-γ, tumor necrosis alpha (TNF)-α, and interleukin (IL)-6 mRNA as well as increased levels of nitric oxide synthase and decreased levels of superoxide dismutase with no change in levels of thioredoxin gene expression. In order to study interactions between smoking and HIV infection, we exposed HIV-1 transgenic (HIV1Tg) and wild-type Fisher (F344) rats to smoke from nicotine-containing (regular) cigarettes, cigarettes containing negligible amounts of nicotine (nicotine-free cigarettes) and to nicotine alone. These studies revealed that such exposures result in specific effects on behavioral responses in the animals in association with enhanced activation of proinflammatory cytokine genes.
Materials and methods
Animals and Exposures
Male and female F344 and HIV1Tg rats, 3–6 months of age, were utilized for these studies. The production of the HIV1Tg rat has been previously described in detail (Reid et al. 2001). The rats were maintained on a 12-h light-dark cycle (lights on at 6 AM). Both F344 and HIV1Tg rats were randomly assigned to four separate treatments. First, two cohorts of rats were exposed to either smoke from four 3R4F research grade cigarettes containing 0.7 mg nicotine/cigarette (regular cigarettes; KTRDC Tobacco Biotechnology Group, University of Kentucky) or smoke from four Quest 3 Cigarettes, which contain trace amounts of nicotine (0.05 mg nicotine/cigarette; Vector Tobacco, Morrisville, NC) and are essentially nicotine-free (nicotine-free cigarettes). Over a 6-week period, the animals were exposed to two cigarettes twice per day, 5 days per week with no smoke exposures over the weekends. The exposures lasted 8–10 min/cigarette. Another group of rats was concurrently treated with subcutaneous injections of 0.5 mg/kg/day of nicotine tartrate salt (Sigma-Aldrich, St. Louis, MO) dissolved in sterile 0.9% NaCl with the same schedule as smoke exposure groups. The control rats were exposed using the same schedule to the experimental apparatus but not to cigarette smoke or nicotine. The numbers of rats in each group were as follows: 12 F344 and 12 HIV1Tg rats were exposed to control conditions, 7 F344 and 12 HIV1Tg rats were exposed to nicotine-free cigarette smoke, 9 F344 and 12 HIV1Tg were exposed to regular cigarette smoke, and 11 F344 and 12 HIV1Tg rats were treated with subcutaneous nicotine. The University of Maryland School of Medicine Institutional Animal Care and Use Committee approved all studies.
Behavioral and Motor Studies
Behavioral testing was performed during the light phase of the light-dark cycle, during the last week of study. For the testing, the rats were transported in their cages from the animal facility to the testing facility. One week before the start of the behavioral testing, the rats were transported daily to the testing facility and allowed at least 2 h of habituation time after the transfer. At the end of the habituation period, the animals were transported back to the animal facility. On the mornings of the testing, the behavioral tests were conducted at the end of the habituation period. The following tests were performed:
Rotarod test. Rotarod testing is used for assessing motor coordination and balance and for examining motor learning (Kralic et al. 2003; Taylor et al. 2003). In this test, the rats are placed on the top of a rotating drum and must walk at the same rotating speed of the drum, or they will fall. Rotarod testing was performed using an IITC Life Science (Woodland Hills, CA) apparatus, consisting of a circular drum (9.5-cm diameter). Animals were habituated to the testing apparatus and rotation of the drum through training trials administered three times per day over 3 days. During training, the rotations were started at 4 RPM and linearly accelerating up to 40 RPM over 5 min. The latency to fall from the rotating drum was the primary outcome and was automatically recorded.
Novel object recognition test. This is an object discrimination task that assesses recognition memory. The testing was conducted on two consecutive days in the same arena with the same type of conditions as described in the open field test section. On the first day, two identical objects (either two Pyrex bottles filled with sand or two small flower pots turned upside down, which were comparable in size and counterbalanced between the animals) were placed in the arena. Animals were allowed to interact with the objects, and the amount of time during which their head and the object were in close proximity was recorded over a period of 15 min. On following day (the testing day), in the same arena, one of the identical objects was replaced with the unfamiliar object. The primary outcome for this analysis was, for a 10-min testing period, the ratio of the time spent with the novel object versus the time spent with the familiar object.
Open field test. The open field test examines general locomotor activity as well as a willingness to explore the center portion of the arena, and both can be decreased due to increased anxiety (Rodriquiz and Wetsel 2006). Rats were placed in a large circular open field arena (140-cm diameter × 66-cm height), made of plastic, and allowed to freely move for 15 min. The chamber was illuminated at ~ 35 lx in the center. Behavior was recorded and then analyzed with the Top Scan (Cleversys) system. The recorded outcomes utilized for this analysis were time spent in the center (defined as 50% of the total area) and the total distance moved.
Cytokine Gene Expression Analyses
Total RNA was purified from lysates produced from frontal cortex and analyzed by reverse-transcriptase polymerase chain reaction, as previously described (Royal et al. 2012). The PCR primers that were used are as follows: (1) TNF-α: forward = 5′-TCT TCT CGA ACC CCG AGT GA-3′, reverse = 5′-CGG TTC AGC CAC T-3′; (2) IL-1β: forward = 5′-TGT GAT GAA AGA CGG CAC AC-3′; reverse = 5′-CTT CTT CTT TGG GTATTG TTT GG-3′ (3) IL-6: forward = 5′-TCA AGG GAA AAG AAC CAG ACA-3′; reverse = 5′-GGT TTC AAA TCA CTC ACC CAT AC-3′; (4) GAPDH (internal control): forward = 5′-CGA CCA CTT TGT CAA GCT CA-3′; reverse = 5′-AGG GGA GAT TCA GTG TGG TG-3′. Data analyses were performed employing the ΔΔCt method (Applied Biosystems 2008).
Statistical Analyses
For the behavioral and motor testing, the average of the mean time to fall for each day over the 3-day period for the rotarod test, the mean ratio of time spent with the novel object, and the mean time spent in the center area and mean total distance traveled on the open field test were analyzed by one-way analysis of variance (ANOVA) with post hoc testing of significant differences using Tukey’s multiple comparisons test. To analyze the combined effects of the different exposures and animal genotype on the test outcomes and the potential interaction between the two factors, a two-way ANOVA was also performed. Mean levels of cytokine gene expression were analyzed by two-way ANOVA with post hoc testing using the Bonferroni test to correct for multiple comparisons. All data analyses were performed using Prism 7 for Windows (GraphPad Software, Inc., San Diego, CA.).
Results
Effects of Cigarette Smoke and Nicotine Exposure on F344 and HIV1Tg Rat Motor, Memory, and Behavioral Function
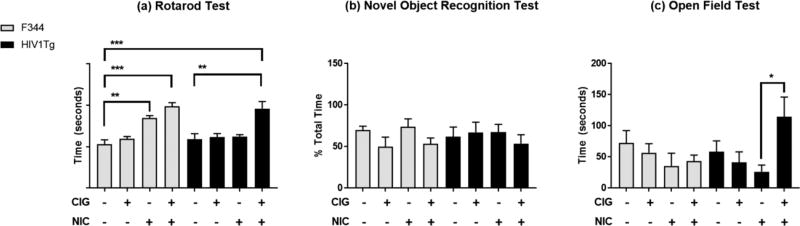
Rotarod. Performance on the rotarod test was assessed by measuring the mean latency to fall. Analysis of the data by two-way ANOVA showed a significant main effect for exposure type, but no significant main effect for genotype. There was also no significant interaction between these two factors (Table 1). Post hoc analysis revealed that, compared to control F344 rats, higher mean latency to fall times were measured for F344 rats either exposed to smoke from regular cigarettes (p < 0.001) or injected with subcutaneous nicotine (p < 0.01). Also, mean latency to fall times for HIV1Tg rats that had been exposed to smoke from regular cigarettes were higher than for control HIV1Tg rats (p < 0.01) as well as control F344 rats (p < 0.001) (Fig. 1a).
Table 1.
Analysis of effect of animal genotype and exposure on test outcomes
| Test | Feature | % of total variation |
F (DFn, DFd) | p value* |
|---|---|---|---|---|
| RRT | Genotype | 0.272 | F(1, 79) = 0.2637 | 0.609 |
| Exposure | 16.090 | F(2, 79) = 5.198 | 0.0025 | |
| Interaction | 2.034 | F(2, 79) = 0.657 | 0.581 | |
| NORT | Genotype | 0.021 | F(1, 50) = 0.01192 | 0.914 |
| Exposure | 6.273 | F(3, 50) = 1.162 | 0.334 | |
| Interaction | 3.616 | F(3, 50) = 0.6696 | 0.575 | |
| OFT | Genotype | 0.520 | F(1, 52) = 0.3502 | 0.557 |
| Exposure | 13.180 | F(3, 52) = 2.961 | 0.041 | |
| Interaction | 5.840 | F(3, 52) = 1.312 | 0.281 |
Adjusted for multiple comparisons using the Tukey test
OFT = open field test (small center time), RRT = rotarod test (average latency to fall), NORT = novel object recognition test (% of a 10-min period interacting with novel object)
Figure 1.
Analysis of HIV1Tg and F344 rat performance on a the rotarod test (total time on the rotating rod), b the novel object recognition test (mean percentage of time spent with an unfamiliar object), and c the openfield test (mean center time). The error bars represent the standard error of the mean values. *p < 0.05; **p < 0.01; ***p < 0.001 (adjusted for multiple comparisons using the Tukey test).
Novel object recognition. On the novel object test, two-way ANOVA revealed no main effect of either exposure type or genotype or their interaction and no significant differences were noted for the percentage of time that the rats in the different exposure groups spent observing the novel object (Table 1; Fig. 1b).
Open field testing. For open field testing, center times and total distance traveled were analyzed. Analysis of center times by two-way ANOVA revealed a significant main effect for exposure type, but no significant effect for genotype and no significant interaction between the two (Table 1). Post hoc analyses found that HIV1Tg rats exposed to regular cigarettes on average spent more time in the center than for HIV1Tg rats that were exposed to nicotine alone (p < 0.05) (Fig. 1c). No significant differences between the F344 and HIV1Tg rat groups were observed for total distance traveled (data not shown).
Effects of Cigarette Smoke and Nicotine Exposure on Proinflammatory Cytokine Gene Expression in HIV1Tg and F344 Rat Brain
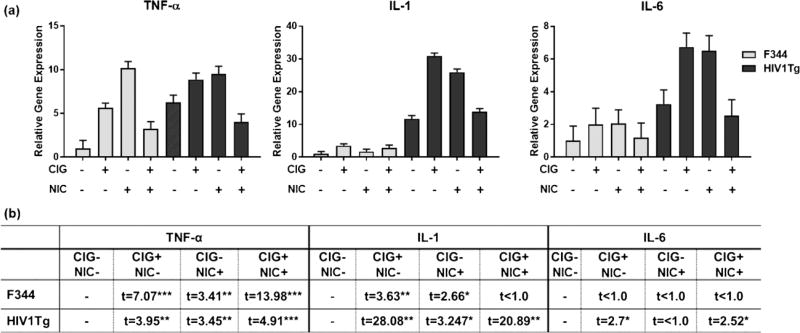
Gene expression for the proinflamatory cytokines IL-1, TNF-α, and IL-6 in frontal cortex was examined for the HIV1Tg and F344 rats that were either nonexposed or exposed to regular cigarettes, nicotine-free cigarettes, or to nicotine alone. Analysis of the expression levels by two-way ANOVA showed that, for all three cytokines, main effects of both genotype and exposure group were statistically significant. Interaction between exposure group and genotype was significant for IL-1 and TNF-α but not for IL-6 (Table 2). The levels of expression for these genes were overall higher for HIV1Tg rats relative to F344 rats (Fig. 2a). Subsequent Bonferroni-corrected pairwise comparisons between control and exposure groups in each genotype (Fig. 2b) showed that, compared to the control group, all exposure groups showed elevated gene expression levels for all three cytokine genes in HIV1Tg rats. However, for F344 rats, there was no change in IL-6 expression levels in any exposure groups. On the other hand, TNF-α expression levels significantly increased in all exposure groups in F344 rats. Also, for IL-1, F344 rats showed increased expression levels in both nicotine-free and nicotine-containing cigarettes compared to controls but not in the nicotine-alone condition.
Table 2.
Analysis of effect of animal strain and exposure on proinflammatory cytokine gene expression
| TNF-α | IL-1 | IL-6 | |
|---|---|---|---|
| Exposure | F(3,16) = 85.15, p < 0.0001 | F(3,16)* = 211.4, p < 0.0001 | F(3,16) = 4.402, p < 0.05 |
| Genotype | F(1,16) = 42.3, p < 0.0001 | F(1,16) = 2893, p < 0.0001 | F(1,16) = 24.66, p < 0.0001 |
| Interaction | F(3,16) = 16.1, p < 0.0001 | F(3,16) = 163.1, p < 0.0001 | F(3,16) = 1.641, p > 0.05 |
P-values adjusted for multiple comparisons using the Bonferroni correction
Figure 2.
F344 and HIV1Tg rat exposure group versus control group frontal cortex proinflammatory cytokine gene expression. a Plots of TNF-α, IL-1, and IL-6 gene expression levels, analyzed by RT-PCR (N = 3 rats pergroup). b Comparison of cytokine gene expression levels. CIG = cigarettes; NIC = nicotine; *p < 0.05; **p < 0.01; ***p < 0.001 (adjusted for multiple comparisons using the Bonferroni correction).
Discussion
The HIV-1 transgenic rat (HIV1Tg rat) was developed using a noninfectious transgene produced by deleting a portion of the viral gag-pol region from the infectious HIV-1 plasmid pNL4-3 (Reid et al. 2001). The transgene expresses seven of the nine HIV genes but does not produce infectious virus. The model mimics a number of abnormalities that have been demonstrated in humans with HIV infection. As we and others have previously demonstrated, these animals show evidence of (1) an increased proinflammatory state in brain and in peripheral immune cells (Cho et al. 2017; Gorantla et al. 2012; Royal et al. 2012); (2) expression of HIV proteins in macrophage/microglial cells and astrocytes in the brain with envelope glycoprotein and tat expressed in the macrophage/microglial cells (Royal et al. 2012), a pattern that is observed with HIV-1 infection in humans (Brack-Werner 1999; Glass et al. 1995); (3) neurodegenerative effects characterized by decreased numbers of frontal cortex parvalbumin + neurons (Sultana et al. 2010); and (4) the development of behavioral and motor abnormalities (June et al. 2009; Moran et al. 2013), all which are also observed in human HIV infection (Chana et al. 2006).
There are more than 4000 chemicals and toxic substances in cigarette smoke, many which have been shown to potentially cause serious and permanent negative health consequences due to the induction of inflammatory and oxidative damage (Burns 1991; Florek et al. 1999). In immunological studies, smokers have been found to have increased levels of soluble markers of inflammation in the circulation (Arnson et al. 2010). Due to nicotine’s effects on catecholamine secretion, smokers may develop an up to 30% increase in peripheral white blood cell counts (Arnson et al. 2010). For individuals with a < 50 pack-year history of cigarette smoking (light to moderate smokers), CD4+ and CD8+ T cell numbers are typically increased, whereas individuals with > 50 pack-year history of smoking (heavy smokers) have depressed CD4+ and increased CD8+ T cell numbers. Where such effects have been observed, changes in T cell numbers may be reversible; however, the improvement may be delayed for up to 2–4 years (Arnson et al. 2010). Therefore, smoking can have prolonged effects on inflammatory responses that can continue long after smoking cessation.
Nicotine promotes cigarette smoking through its addictive properties. Also, nicotine has been shown to suppress immune activation and proinflammatory responses through direct effects on immune cells and through effects on neurally mediated pathways (de Jonge and Ulloa 2007; Sopori 2002; Sopori et al. 1998). This would suggest that nicotine or its metabolites may be useful for treating neurocognitive impairment. However, chronic nicotine exposure can result in desensitization of the receptor in association with an increase in receptor numbers, and, paradoxically, an increase in proinflammatory responses (Marks et al. 1993). Similarly, HIV gp120 has been demonstrated to also upregulate microglial cell nicotinic acetyl choline receptor expression in association with downregulation of receptor function and suppression of anti-inflammatory cholinergic responses (Delgado-Velez et al. 2015). These effects could potentially result in nicotine inducing an overall enhancement of inflammatory responses, which could exacerbate the nervous system damage that can occur in HIV-infected individuals. Such effects would be consistent with studies that show that smoking has deleterious effects on cognitive functioning among HIV-infected individuals (Chang et al. 2017; Harrison et al. 2017; Wojna et al. 2007), although the specificity of such effects has been questioned (Bryant et al. 2013).
The studies described here are the first to analyze effects of both nicotine-containing and nicotine-free cigarette smoke exposure on inflammatory responses and behavior in the HIV-1 transgenic rat model. Previous studies of the effects of nicotine in the HIV1Tg rat model suggest that chronic nicotine exposure can have region-specific effects on nicotine-sensitive areas of brain (Song et al. 2016; Yang et al. 2016). In the studies reported here, where the rats were exposed to nicotine at a slightly higher dose and for a longer duration, as well as to potentially additional stress from behavioral and motor testing, we observed that TNF-α, IL-1, and IL-6 gene expression levels were all increased by smoke exposure in HIV1Tg rat brains, whereas IL-6 gene expression was not increased by the exposures in F344 rat brains, suggesting the existence of a greater susceptibility to such exposures for the transgenic rat. This is consistent with our previous findings where increased proinflammatory responses were demonstrated both systemically and in brains from the transgenic versus the wild-type animals and with other studies that demonstrated the presence of increased astrocyte and microglial activation the brains of transgenic rats that were produced on a Sprague-Dawley rat background (Repunte-Canonigo et al. 2014). Also, analysis of peripheral blood immune cell phenotypes in HIV1Tg rats showed increased numbers of circulating cytotoxic T cells as well as increased percentages of helper T cells and monocytes as compared to F344 rats (Abbondanzo and Chang 2014), although helper T cells and monocytes can be functionally either proinflammatory or anti-inflammatory (Geissmann et al. 2008).
Nicotine is associated with an increase in locomotor activity with chronic administration in rats (Clarke and Kumar 1983). In F344 rats, nicotine was found to improve performance on a series of learning tasks, but this effect was not observed in HIV1Tg rats (Vigorito et al. 2013). These findings could be explained by effects from viral proteins (Gonzalez-Lira et al. 2006), potentially inducing alterations in activation of signaling pathways in these nicotine-sensitive areas (Midde et al. 2011). In our studies, no significant differences were noted on the novel object recognition test for F344 and HIV1Tg rats and with exposure to the cigarette smoke and nicotine. However, on the rotarod test, exposure to nicotine alone and smoke from nicotine-containing regular cigarettes increased the average latency to fall for F344 rats, with the latter exposure inducing the most profound effects. In contrast, for HIV1Tg rats, an increase in average latency to fall was noted for only exposure to nicotine-containing regular cigarettes with no increase resulting from exposure to nicotine alone, although nicotine was found to induce the most prominent cytokine responses in the animals. The lack of response to nicotine alone in the HIV1Tg rats may be potentially due to dysfunction of nicotine-responsive regions in these animals. The similar responses on the RRT by the two species of rats to regular cigarette could be explained by the fact that an analysis of a dose response may be required in order to observe differences. For the open field test, the HIV1Tg rats exposed to regular cigarettes spent longer time in the center of the open field than HIV1Tg rats that were treated with nicotine alone. These responses were not observed among HIV1Tg rats exposed to either saline, nicotine alone, or nicotine-free cigarette smoke, which resulted in times for these rats that were similar to F344 control animals. Also, a similar effect was not seen for F344 rats subjected to these exposures or to regular cigarette smoke. The specific mechanisms that are involved in these responses are unclear; however, they are likely to be related to effects induced by products of the HIV-1 transgene.
There are some weaknesses to this study. They include the wide age range for the rats and that the cytokine responses were not measured specifically in prefrontal cortex or in other brain areas that are known to be primary targets of nicotine, where it is possible that different responses will be observed. A previous study demonstrated that older HIV1Tg rats perform worse on the rotarod and open field test than younger animals (Reid et al. 2016). However, the study showed the same for F344 control rats, and there was no interaction with the genotype of the animals. Also, we did not test whether the effect of the transgene, per se, might have been responsible for the differences observed for the F344 and HIV1Tg rats by measuring HIV-1 gene and protein expression, and we did not measure cytokine protein levels, which may differ from measures of gene expression. However, despite these concerns, the studies contained in this report provide valuable insight into possible mechanisms by which cigarette smoking and smoke exposure might impact the development of neurological disorders in HIV infection.
References
- Abbondanzo SJ, Chang SL. HIV-1 transgenic rats display alterations in immunophenotype and cellular responses associated with aging. PLoS One. 2014;9:e105256. doi: 10.1371/journal.pone.0105256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson E, Zink W, Xiong H, Gendelman HE. HIV-1-associated dementia: a metabolic encephalopathy perpetrated by virus-infected and immune-competent mononuclear phagocytes. J Acquir Immune Defic Syndr. 2002;31(Suppl 2):S43–S54. doi: 10.1097/00126334-200210012-00004. [DOI] [PubMed] [Google Scholar]
- Applied Biosystems. Guide to performing relative quantitation of gene expression using real-time quantitative PCR. 2008:1–70. [Google Scholar]
- Arnson Y, Shoenfeld Y, Amital H. Effects of tobacco smoke on immunity, inflammation and autoimmunity. J Autoimmun. 2010;34:J258–J265. doi: 10.1016/j.jaut.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Ascherio A, Munger KL. Environmental risk factors for multiple sclerosis. Part II: noninfectious factors. Ann Neurol. 2007;61:504–513. doi: 10.1002/ana.21141. [DOI] [PubMed] [Google Scholar]
- Brack-Werner R. Astrocytes: HIV cellular reservoirs and important participants in neuropathogenesis. AIDS. 1999;13:1–22. doi: 10.1097/00002030-199901140-00003. [DOI] [PubMed] [Google Scholar]
- Brandt C, Zvolensky MJ, Woods SP, Gonzalez A, Safren SA, O'Cleirigh CM. Anxiety symptoms and disorders among adults living with HIV and AIDS: a critical review and integrative synthesis of the empirical literature. Clin Psychol Rev. 2017;51:164–184. doi: 10.1016/j.cpr.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant VE, Kahler CW, Devlin KN, Monti PM, Cohen RA. The effects of cigarette smoking on learning and memory performance among people living with HIV/AIDS. AIDS Care. 2013;25:1308–1316. doi: 10.1080/09540121.2013.764965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns DM. Cigarettes and cigarette smoking. Clin. Chest Med. 1991;12:631–642. [PubMed] [Google Scholar]
- Cataldo JK, Prochaska JJ, Glantz SA. Cigarette smoking is a risk factor for Alzheimer’s disease: an analysis controlling for tobacco industry affiliation. J Alzheimers Dis. 2010;19:465–480. doi: 10.3233/JAD-2010-1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chana G, Everall IP, Crews L, Langford D, Adame A, Grant I, Cherner M, Lazzaretto D, Heaton R, Ellis R, Masliah E. Cognitive deficits and degeneration of interneurons in HIV+ methamphetamine users. Neurology. 2006;67:1486–1489. doi: 10.1212/01.wnl.0000240066.02404.e6. [DOI] [PubMed] [Google Scholar]
- Chang L, Lim A, Lau E, Alicata D. Chronic tobacco-smoking on psychopathological symptoms, impulsivity and cognitive deficits in HIV-infected individuals. J NeuroImmune Pharmacol. 2017;12:389–401. doi: 10.1007/s11481-017-9728-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho YE, Lee MH, Song BJ. Neuronal cell death and degeneration through increased nitroxidative stress and tau phosphorylation in HIV-1 transgenic rats. PLoS One. 2017;12:e0169945. doi: 10.1371/journal.pone.0169945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke PB, Kumar R. The effects of nicotine on locomotor activity in non-tolerant and tolerant rats. Br J Pharmacol. 1983;78:329–337. doi: 10.1111/j.1476-5381.1983.tb09398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jonge WJ, Ulloa L. The alpha7 nicotinic acetylcholine receptor as a pharmacological target for inflammation. Br J Pharmacol. 2007;151:915–929. doi: 10.1038/sj.bjp.0707264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado-Velez M, Baez-Pagan CA, Gerena Y, Quesada O, Santiago-Perez LI, Capo-Velez CM, Wojna V, Melendez L, Leon-Rivera R, Silva W, Lasalde-Dominicci JA. The alpha7-nicotinic receptor is upregulated in immune cells from HIV-seropositive women: consequences to the cholinergic anti-inflammatory response. Clin Transl Immunol. 2015;4:e53. doi: 10.1038/cti.2015.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florek E, Marszalek A, Biczysko W, Szymanowski K. The experimental investigations of the toxic influence of tobacco smoke affecting progeny during pregnancy. Hum Exp Toxicol. 1999;18:245–251. doi: 10.1191/096032799678839996. [DOI] [PubMed] [Google Scholar]
- Galai N, Park LP, Wesch J, Visscher B, Riddler S, Margolick JB. Effect of smoking on the clinical progression of HIV-1 infection. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;14:451–458. doi: 10.1097/00042560-199704150-00009. [DOI] [PubMed] [Google Scholar]
- Geissmann F, Auffray C, Palframan R, Wirrig C, Ciocca A, Campisi L, Narni-Mancinelli E, Lauvau G. Blood monocytes: distinct subsets, how they relate to dendritic cells, and their possible roles in the regulation of T-cell responses. Immunol Cell Biol. 2008;86:398–408. doi: 10.1038/icb.2008.19. [DOI] [PubMed] [Google Scholar]
- Glass JD, Fedor H, Wesselingh SL, McArthur JC. Immunocytochemical quantitation of human immunodeficiency virus in the brain: correlations with dementia. Ann Neurol. 1995;38:755–762. doi: 10.1002/ana.410380510. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Lira B, Rueda-Orozco PE, Galicia O, Montes-Rodriguez CJ, Guzman K, Guevara-Martinez M, Elder JH, Prospero-Garcia O. Nicotine prevents HIVgp120-caused electrophysiological and motor disturbances in rats. Neurosci Lett. 2006;394:136–139. doi: 10.1016/j.neulet.2005.10.021. [DOI] [PubMed] [Google Scholar]
- Gorantla S, Poluektova L, Gendelman HE. Rodent models for HIV-associated neurocognitive disorders. Trends Neurosci. 2012;35:197–208. doi: 10.1016/j.tins.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison JD, Dochney JA, Blazekovic S, Leone F, Metzger D, Frank I, Gross R, Hole A, Mounzer K, Siegel S, Schnoll RA, Ashare RL. The nature and consequences of cognitive deficits among tobacco smokers with HIV: a comparison to tobacco smokers without HIV. J Neurovirol. 2017;23:550–557. doi: 10.1007/s13365-017-0526-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Marcotte TD, Mindt MR, Sadek J, Moore DJ, Bentley H, McCutchan JA, Reicks C, Grant I. The impact of HIV-associated neuropsychological impairment on everyday functioning. J Int Neuropsychol Soc. 2004;10:317–331. doi: 10.1017/S1355617704102130. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, Corkran SH, Duarte NA, Clifford DB, Woods SP, Collier AC, Marra CM, Morgello S, Mindt MR, Taylor MJ, Marcotte TD, Atkinson JH, Wolfson T, Gelman BB, McArthur JC, Simpson DM, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neuro-Oncol. 2011;17:3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helleberg M, Afzal S, Kronborg G, Larsen CS, Pedersen G, Pedersen C, Gerstoft J, Nordestgaard BG, Obel N. Mortality attributable to smoking among HIV-1-infected individuals: a nationwide, population-based cohort study. Clin Infect Dis. 2013;56:727–734. doi: 10.1093/cid/cis933. [DOI] [PubMed] [Google Scholar]
- Jamal A, King BA, Neff LJ, Whitmill J, Babb SD, Graffunder CM. Current cigarette smoking among adults-United States, 2005–2015. MMWR Morb Mortal Wkly Rep. 2016;65:1205–1211. doi: 10.15585/mmwr.mm6544a2. [DOI] [PubMed] [Google Scholar]
- June HL, Tzeng Yang AR, Bryant JL, Jones O, Royal W., III Vitamin A deficiency and behavioral and motor deficits in the human immunodeficiency virus type 1 transgenic rat. J Neuro-Oncol. 2009;15:380–389. doi: 10.3109/13550280903350200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna A, Guo M, Mehra M, Royal W., III Inflammation and oxidative stress induced by cigarette smoke in Lewis rat brains. J Neuroimmunol. 2013;254:69–75. doi: 10.1016/j.jneuroim.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kralic JE, Wheeler M, Renzi K, Ferguson C, O'Buckley TK, Grobin AC, Morrow AL, Homanics GE. Deletion of GABAA receptor alpha 1 subunit-containing receptors alters responses to ethanol and other anesthetics. J Pharmacol Exp Ther. 2003;305:600–607. doi: 10.1124/jpet.102.048124. [DOI] [PubMed] [Google Scholar]
- Lifson AR, Lando HA. Smoking and HIV: prevalence, health risks, and cessation strategies. Curr HIV/AIDS Rep. 2012;9:223–230. doi: 10.1007/s11904-012-0121-0. [DOI] [PubMed] [Google Scholar]
- Marks MJ, Grady SR, Collins AC. Downregulation of nicotinic receptor function after chronic nicotine infusion. J Pharmacol Exp Ther. 1993;266:1268–1276. [PubMed] [Google Scholar]
- Midde NM, Gomez AM, Harrod SB, Zhu J. Genetically expressed HIV-1 viral proteins attenuate nicotine-induced behavioral sensitization and alter mesocorticolimbic ERK and CREB signaling in rats. Pharmacol Biochem Behav. 2011;98:587–597. doi: 10.1016/j.pbb.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran LM, Booze RM, Mactutus CF. Time and time again: temporal processing demands implicate perceptual and gating deficits in the HIV-1 transgenic rat. J NeuroImmune Pharmacol. 2013;8:988–997. doi: 10.1007/s11481-013-9472-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath A. Human immunodeficiency virus (HIV) proteins in neuropathogenesis of HIV dementia. J Infect Dis. 2002;186(Suppl 2):S193–S198. doi: 10.1086/344528. [DOI] [PubMed] [Google Scholar]
- Nieman RB, Fleming J, Coker RJ, Harris JR, Mitchell DM. The effect of cigarette smoking on the development of AIDS in HIV-1-seropositive individuals. AIDS. 1993;7:705–710. doi: 10.1097/00002030-199305000-00015. [DOI] [PubMed] [Google Scholar]
- Reid W, Sadowska M, Denaro F, Rao S, Foulke J, Jr, Hayes N, Jones O, Doodnauth D, Davis H, Sill A, O'Driscoll P, Huso D, Fouts T, Lewis G, Hill M, Kamin-Lewis R, Wei C, Ray P, Gallo RC, Reitz M, Bryant J. An HIV-1 transgenic rat that develops HIV-related pathology and immunologic dysfunction. Proc Natl Acad Sci U S A. 2001;98:9271–9276. doi: 10.1073/pnas.161290298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid WC, Casas R, Papadakis GZ, Muthusamy S, Lee DE, Ibrahim WG, Nair A, Koziol D, Maric D, Hammoud DA. Neurobehavioral abnormalities in the HIV-1 transgenic rat do not correspond to neuronal hypometabolism on 18F-FDG-PET. PLoS One. 2016;11:e0152265. doi: 10.1371/journal.pone.0152265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repunte-Canonigo V, Lefebvre C, George O, Kawamura T, Morales M, Koob GF, Califano A, Masliah E, Sanna PP. Gene expression changes consistent with neuroAIDS and impaired working memory in HIV-1 transgenic rats. Mol Neurodegener. 2014;9:26. doi: 10.1186/1750-1326-9-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriquiz RM, Wetsel WC. Assessments of cognitive deficits in mutant mice. In: Levin ED, Buccafusco JJ, editors. Animal models of cognitive impairment. CRC Press; Boca Raton: 2006. pp. 223–282. [PubMed] [Google Scholar]
- Royal W, III, Zhang L, Guo M, Jones O, Davis H, Bryant JL. Immune activation, viral gene product expression and neurotoxicity in the HIV-1 transgenic rat. J Neuroimmunol. 2012;247:16–24. doi: 10.1016/j.jneuroim.2012.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah RS, Cole JW. Smoking and stroke: the more you smoke the more you stroke. Expert Rev Cardiovasc Ther. 2010;8:917–932. doi: 10.1586/erc.10.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song G, Nesil T, Cao J, Yang Z, Chang SL, Li MD. Nicotine mediates expression of genes related to antioxidant capacity and oxidative stress response in HIV-1 transgenic rat brain. J Neuro-Oncol. 2016;22:114–124. doi: 10.1007/s13365-015-0375-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sopori M. Effects of cigarette smoke on the immune system. Nat Rev Immunol. 2002;2:372–377. doi: 10.1038/nri803. [DOI] [PubMed] [Google Scholar]
- Sopori ML, Kozak W, Savage SM, Geng Y, Soszynski D, Kluger MJ, Perryman EK, Snow GE. Effect of nicotine on the immune system: possible regulation of immune responses by central and peripheral mechanisms. Psychoneuroendocrinology. 1998;23:189–204. doi: 10.1016/s0306-4530(97)00076-0. [DOI] [PubMed] [Google Scholar]
- Sultana S, Li H, Puche A, Jones O, Bryant JL, Royal W. Quantitation of parvalbumin+ neurons and human immunodeficiency virus type 1 (HIV-1) regulatory gene expression in the HIV-1 transgenic rat: effects of vitamin A deficiency and morphine. J Neuro-Oncol. 2010;16:33–40. doi: 10.3109/13550280903555712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor BK, Joshi C, Uppal H. Stimulation of dopamine D2 receptors in the nucleus accumbens inhibits inflammatory pain. Brain Res. 2003;987:135–143. doi: 10.1016/s0006-8993(03)03318-3. [DOI] [PubMed] [Google Scholar]
- Vigorito M, Cao J, Li MD, Chang SL. Acquisition and long-term retention of spatial learning in the human immunodeficiency virus-1 transgenic rat: effects of repeated nicotine treatment. J Neuro-Oncol. 2013;19:157–165. doi: 10.1007/s13365-013-0154-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojna V, Robles L, Skolasky RL, Mayo R, Selnes O, de la Torre T, Maldonado E, Nath A, Melendez LM, Lasalde-Dominicci J. Associations of cigarette smoking with viral immune and cognitive function in human immunodeficiency virus-seropositive women. J Neuro-Oncol. 2007;13:561–568. doi: 10.1080/13550280701620747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Nesil T, Connaghan KP, Li MD, Chang SL. Modulation effect of HIV-1 viral proteins and nicotine on expression of the immune-related genes in brain of the HIV-1 transgenic rats. J NeuroImmune Pharmacol. 2016;11:562–571. doi: 10.1007/s11481-016-9679-4. [DOI] [PMC free article] [PubMed] [Google Scholar]