Abstract
The stability of chloroplastic glutamine synthetase (GS; EC 6.3.1.2) was investigated under photooxidative stress using wheat (Triticum aestivum L.) leaves, chloroplasts, and chloroplast lysates. Illuminated seedlings sprayed with the superoxide radical (O2⨪) propagator methyl viologen showed rapid GS decline dependent on MV concentration and exposure time. Degradation products of approximately 39 and 31 kD were detected when chloroplast lysates containing both stroma and thylakoids were illuminated in the presence of MV or H2O2. In all cases, GS cleavage was prevented by the addition of the electron transport inhibitor 3-(3,4-dichlorophenyl)-1,1-dimethylurea. Full protection against degradation could also be obtained by the incorporation of chelators or antioxidant enzymes. Maximal rates of degradation required the presence of transition metals and reducing compounds such as NADPH or dithiothreitol. Similar patterns of GS cleavage were obtained when seedlings were exposed to high doses of irradiation. The results indicate that chloroplastic GS is extremely prone to oxidative cleavage, and that reduced transition metals, presumably resulting from the destruction of iron-sulfur clusters by light-generated O2⨪, play a crucial role in the degradation process. The physiological implications of GS lability to oxidative stress are discussed.
Adverse environmental conditions such as drought, chilling, or excess light can limit plant productivity (Allen, 1995). One common feature of these conditions is the development of oxidative processes mediated by reactive oxygen species (ROS). ROS can be generated in chloroplasts by direct transfer of excitation energy from chlorophyll to produce singlet oxygen, or by univalent oxygen reduction at PSI, in the Mehler reaction (Foyer et al., 1994; Allen, 1995; Asada et al., 1998). The latter process results in the formation of the superoxide anion radical (O2⨪), and eventually H2O2 and the highly toxic hydroxyl radical (·OH) (Cadenas, 1989). The rate of this reaction can be greatly increased by the use of redox cycling compounds such as MV, which efficiently mediates electron transfer from PSI to oxygen.
During foliar senescence or under unfavorable environmental conditions, ROS concentrations can increase to toxic levels (Foyer et al., 1994; Allen, 1995; Asada et al., 1998), resulting in protein and DNA damage and lipid peroxidation (Cadenas, 1989). The direct effect of ROS on isolated proteins has been thoroughly documented (Davies, 1987; Stadtman, 1993). These molecules can increase protein hydrophobicity, modify sensitive residues, and induce intra- and intermolecular cross-linking and peptide fragmentation (Stadtman, 1993, and refs. therein), as well as an increased susceptibility to proteolysis (Davies et al., 1987). In chloroplasts, ROS are known to cause extensive modifications in a wide variety of stromal and thylakoid proteins, including inactivation and degradation of Rubisco (Mehta et al., 1992; Casano et al., 1994; Desimone et al., 1996; Ishida et al., 1997) and other components of the Calvin cycle (Asada et al., 1998), aggregation and breakdown of thylakoid proteins, including D1 (Aro et al., 1990; Roberts et al., 1991), and membrane solubilization of Fd-NADP+ reductase (Palatnik et al., 1997).
To cope with the harmful effects of ROS toxicity, plants have developed a highly complex and intertwined antioxidant defense barrier composed of both enzymatic and nonenzymatic constituents. A number of enzymes involved in antioxidant protection are normally induced in response to a variety of oxidative challenges, including catalases, peroxidases, superoxide dismutases, and oxidoreductases (Foyer et al., 1994; Allen, 1995; Asada et al., 1998). These enzymes play different and complementary roles in the concerted cell defense, such as direct scavenging of ROS, re-establishment of the redox homeostasis once the challenge has subsided, and repair of the damage caused by the oxidative condition. Whenever the balance between pro-oxidants and antioxidants is displaced in favor of the former, either by an increase of the oxidative input or by a disruption of the defense systems, the outcome is a condition known as oxidative stress (Scandalios, 1993). The final consequences of such a condition (e.g. survival or death) depend on the proper expression pattern (in time and space) of several components of the defense system, while the effects introduced by the manipulation of any individual component are not always predictable (Sen Gupta et al., 1993; Foyer et al., 1994). For instance, the transient increase of NADPH levels resulting from early oxidative inhibition of Calvin cycle enzymes (Asada et al., 1998) paradoxically increases the risks of oxidative damage by at least two possible mechanisms: electron diversion from PSI to oxygen due to NADP+ shortage, with a concomitant accumulation of O2⨪ (Polle, 1996), and direct reduction of transition metals such as iron and copper, which are required for the conversion of H2O2 into ·OH via Fenton-type chemistry (Cadenas, 1989).
Several lines of evidence have suggested that photorespiration, the metabolism of phosphoglycolate produced by the oxygenase activity of Rubisco, might be instrumental in the survival of C3 plants under conditions of photooxidative stress by dissipating excess photochemical energy and recycling NADP+ (Osmond et al., 1997). Indeed, transgenic plants overexpressing plastidic GS, a key enzyme that catalyzes the rate-determining step in the photorespiratory pathway, displayed an improved tolerance to over-irradiation (Kozaki and Takeba, 1996). The protective role of photorespiration in vivo has, however, been challenged by the observation that GS is readily degraded in illuminated chloroplasts through oxidative processes (Stieger and Feller, 1997).
As part of a concerted effort to clarify the role of this enzyme in the defensive system of plants to photooxidation, we evaluated the effects of high levels of irradiation, oxidants, and redox cycling compounds on GS stability using wheat seedlings, isolated chloroplasts, and plastid lysates. In the present study, we show that chloroplastic GS is particularly prone to degradation under oxidative stress conditions. Furthermore, we provide evidence indicating that both transition metals and reducing equivalents produced by illuminated thylakoids are required for this fragmentation.
MATERIALS AND METHODS
Wheat (Triticum aestivum L. cv Oasis) plants were grown for 8 d in a phytotron with day/night temperatures of 24°C/19°C and 75% RH. The photoperiod was 16 h, with a PPFD of 200 μmol quanta m−2 s−1.
Preparation of Intact Chloroplasts and Chloroplast Lysates
Wheat chloroplasts were isolated by mechanical disruption of 8-d-old seedlings with a polytron homogenizer (Bachofer GmBH, Reutlingen, Germany), followed by filtration through Miracloth (Calbiochem-Novabiochem, San Diego) and Percoll gradient centrifugation, as described previously (Palatnik et al., 1997). Plastids were finally suspended in 50 mm HEPES-NaOH (pH 7.5), 330 mm sorbitol, 1 mm EDTA, 1 mm DTT, and 5 mm MgCl2. Intact chloroplasts were centrifuged (2 min × 2,000g) and then lysed by resuspension in 50 mm HEPES-NaOH (pH 7.5).
Light and Oxidative Treatments
Chemical stress was imposed to wheat plants by spraying 40 seedlings with 20 mL of a 0.05% (v/v) Tween 20 solution containing various concentrations of MV and, when indicated, 100 μm DCMU. Plants were then illuminated (200 μmol quanta m−2 s−1), and leaves were homogenized at different times in 50 mm Tris-HCl (pH 7.5), 5 mm MgCl2, 1 mm DTT, and 1 mm PMSF. Soluble fractions were obtained by centrifugation (10 min × 10,000g). The effect of high light intensities was assayed by exposing the seedlings to a source of actinic light (950 ± 60 μmol quanta m−2 s−1) at 10°C. Samples were taken at various times and processed as indicated above.
When intact or lysed chloroplast suspensions were subjected to oxidative conditions, samples corresponding to 100 μg chlorophyll mL−1 were incubated for 1 h at 22°C with the different oxidants and effectors under continuous illumination (600 μmol quanta m−2 s−1).
Analytical Procedures
Chlorophyll content and total soluble proteins were determined according to the method of Whatley and Arnon (1963) and Peterson (1977), respectively. After the different treatments, lysates were subjected to SDS-PAGE on 12% (w/v) acrylamide gels and transferred to nitrocellulose membranes. Immunodetection methods were carried out as described previously (Krapp et al., 1997) using antisera raised in rabbits against maize chloroplastic GS (Sakakibara et al., 1992). Molecular masses of GS fragments were estimated using marker proteins (catalog no. 161–0305, Bio-Rad Laboratories, Hercules, CA).
RESULTS
Effect of MV Treatment on GS Stability in Wheat Seedlings
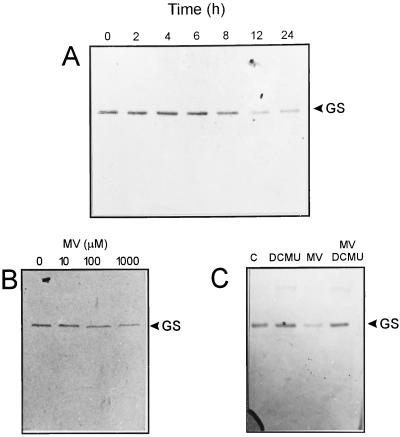
To investigate the stability of GS in plants subjected to oxidative stress conditions, we sprayed 8-d-old wheat shoots with 500 μm MV. Plants were illuminated for various times after treatment, and the presence of GS was determined in total leaf extracts by SDS-PAGE and immunoblotting. As shown in Figure 1A, a single protein band of approximately 44 kD reacted toward GS antisera. The level of this band began to diminish after 8 h of the initial challenge, reaching a minimum at 12 h. More prolonged incubations failed to produce a further decrease (Fig. 1A), presumably reflecting detoxification by the cellular defense systems. Control plants sprayed with Tween 20 showed essentially the same GS levels throughout the 12-h illumination period (data not shown). GS decline was already evident at 100 μm MV, and increased as the concentration of the herbicide was raised (Fig. 1B). Stieger and Feller (1997) have shown that GS degradation in illuminated chloroplasts requires the function of the photosynthetic electron transport chain. In good agreement, inclusion of the electron transport inhibitor DCMU in the spraying solution totally abolished the effect of MV, suggesting that ROS generated at the reducing side of PSI were involved in the observed GS decline (Fig. 1C). Under similar conditions, GS proved to be more sensitive than Rubisco (data not shown), which is normally used as an early indicator of chloroplast oxidative damage at the protein level (Mehta et al., 1992; Casano et al., 1994; Desimone et al., 1996; Ishida et al., 1997).
Figure 1.
Influence of MV on GS degradation in wheat seedlings. A, Eight-day-old plants were sprayed in the light with 500 μm MV and sampled at the indicated times. B, Seedlings were sprayed with 0 to 1 mm MV and illuminated with a PPFD of 200 μmol quanta m−2 s−1 for 8 h prior to sampling. C, DCMU and/or MV concentrations in the spraying solution were both 100 μm and plants were incubated for 8 h in the light. Lane C, Control plants sprayed with 0.05% (v/v) Tween 20. Sampled leaves were homogenized as indicated in “Materials and Methods.” Soluble fractions corresponding to 20 μg of protein were subjected to SDS-PAGE and blotted onto nitrocellulose membranes for immunodetection of GS.
GS Degradation in Chloroplast Lysates
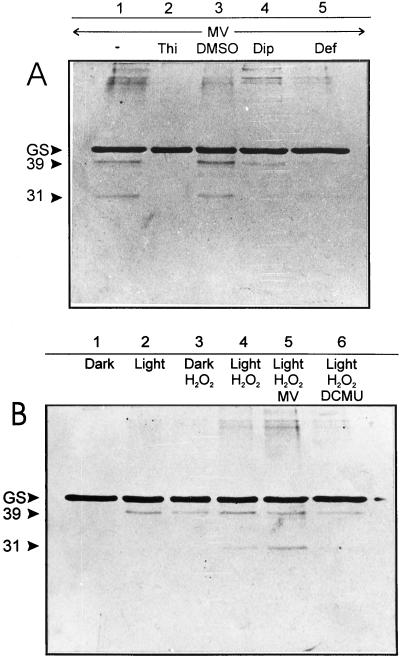
Illumination of intact wheat chloroplasts resulted in a progressive decrease in the stromal GS levels, which was further stimulated by the addition of MV (data not shown). The pattern and time course of GS decline, presumably reflecting protein degradation, closely resembled those reported for intact pea chloroplasts under similar conditions (Stieger and Feller, 1997). Once again, GS displayed higher sensitivity than Rubisco, the levels of which remained stable for several minutes when isolated chloroplasts were illuminated in the absence of effectors (not shown, but see Stieger and Feller, 1997). In an attempt to further characterize the features and requirements of this presumptive degradation, isolated chloroplasts were disrupted by osmotic shock, and complete lysates containing soluble fractions and thylakoid membranes were illuminated in the presence of various oxidants and scavengers. When incubation was carried out in the light, GS decline was accompanied by the appearance of a degradation product of approximately 39 kD (Fig. 2B, lanes 1 and 2), whereas the addition of MV led to the accumulation of a second subfragment of approximately 31 kD (Fig. 2A, lane 1). A certain degree of GS aggregation was also evident under the latter condition (Fig. 2A, lane 1).
Figure 2.
Oxidative cleavage of stromal GS by MV (A) and H2O2 (B) in wheat chloroplast lysates. Freshly broken chloroplasts were incubated for 1 h at 22°C in the presence of the following compounds (as indicated on the top of each lane): 100 μm MV, 2.5 mm H2O2, 100 mm thiourea (Thi), 5% (v/v) DMSO, 5 mm 2,2′-dipyridyl (Dip), 0.1 mm deferoxamine (Def), and 100 μm DCMU. Unless otherwise stated, incubations were carried out in the light (600 μmol quanta m−2 s−1). Aliquots corresponding to 3 μg of chlorophyll were fractionated by SDS-PAGE and analyzed by immunoblotting. The relative positions of GS and the 39- and 31-kD cleavage products are indicated on the left.
Similar degradation patterns were obtained by treatment of the lysates with H2O2, even when incubated in the dark (Fig. 2B, lanes 3 and 4). The extent of GS modification was strictly dependent on the intensity of the stress imposed (Fig. 2B, lanes 3–5). Interestingly enough, the damaging effects of H2O2 were partially prevented by the addition of DCMU in the light (Fig. 2B, lane 6), indicating that maximal rates of GS fragmentation by H2O2 still required the function of the photosynthetic electron transport chain.
GS destruction by light and MV could be mitigated by 2,2′-dipyridyl, and totally prevented by deferoxamine, two chelating agents that preferentially bind iron (Fig. 2A, lanes 4 and 5). Thiourea, which displays the properties of a metal chelator and a scavenger of both ·OH and H2O2, also provided full protection (Fig. 2A, lane 2). Although more specific ·OH scavengers such as DMSO (Fig. 2A, lane 3) and mannitol (data not shown) failed to prevent GS degradation, we cannot completely rule out the involvement of ·OH. The overall results suggest the participation of transition metals in the light-mediated degradation of this enzyme.
Participation of the Photosynthetic Electron Transport Chain in GS Degradation
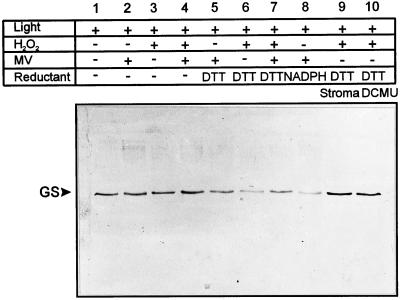
The effects of DCMU reported in Figures 1 and 2 prompted us to evaluate whether reducing equivalents generated through the photosynthetic electron transport chain could be involved in the light-dependent degradation of GS. The addition of NADPH or DTT greatly accelerated GS fragmentation in illuminated chloroplast lysates (Fig. 3). Detection levels chosen for these immunoblots were insufficient to recognize degradation products, but a dramatic decline of full-size GS was evident when the reductants were combined with either MV or H2O2 (Fig. 3). Stimulation of GS cleavage by NADPH or DTT could be due to reduction of trace amounts of transition metals required for ·OH production. In the presence of H2O2 and DTT, MV protected to some extent against GS degradation (Fig. 3, lanes 3–7). However, the role of photosynthetic electron transport is not limited to the provision of reducing equivalents. The addition of DTT (Fig. 3, lane 10) or NADPH (data not shown) to illuminated lysates supplemented with H2O2 failed to overcome the effects of DCMU blockade, despite the ample provision of reducing equivalents. Also, no decline in the GS level could be detected when H2O2 was added in cleared stromal fractions from which thylakoids had been removed by centrifugation, even in the presence of DTT (Fig. 3, lane 9).
Figure 3.
Reductants stimulate light-dependent GS degradation in the presence of MV or H2O2. Chloroplast lysates were incubated under the conditions described in Figure 2 with the compounds shown above each lane: 100 μm MV, 2.5 mm H2O2, 1 mm DTT, 0.5 mm NADPH, and/or 100 μm DCMU. Samples corresponding to 0.5 μg of chlorophyll were analyzed by SDS-PAGE and immunoreaction. Lane 9 (Stroma), Supernatant of chloroplast lysates after centrifugation for 10 min at 10,000g. The relative mobility of GS is indicated on the left.
The Role of O2⨪ in GS Degradation
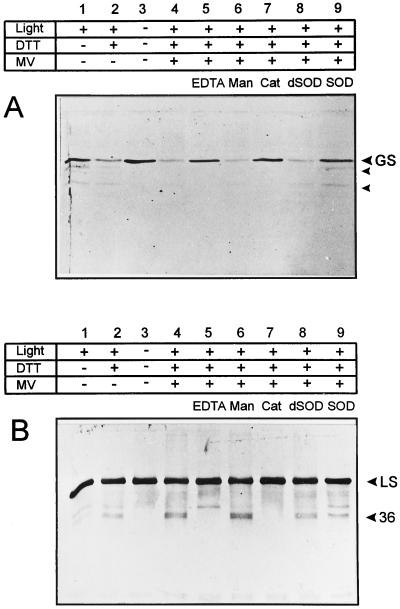
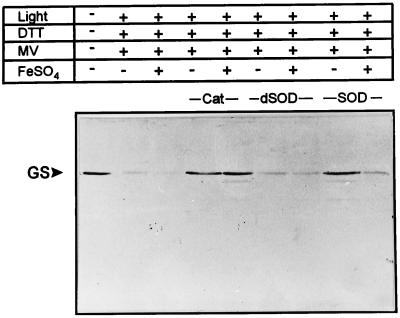
To further elucidate the requirements for the light-induced degradation of GS, we compared the stability of GS and Rubisco in chloroplast lysates illuminated in the presence of MV, DTT, and various scavengers. Under these conditions, mannitol failed to protect GS or Rubisco, whereas the general chelator EDTA or the H2O2-scavenging enzyme catalase provided full protection to both proteins, as judged by the level of the full-size GS or the appearance of 36-kD Rubisco fragmentation product (Fig. 4, A and B). As already reported (Desimone et al., 1996; Ishida et al., 1997), the addition of active SOD did not inhibit Rubisco degradation (Fig. 4B, lane 9). On the other hand, the presence of this metallo-enzyme largely prevented oxidative damage to GS (Fig. 4A, lane 9), suggesting that the accumulation of O2⨪ is a prerequisite for GS decline in illuminated chloroplast lysates. The protective role of SOD could not be attributed to a potential function as an alternative protein target, since heat-inactivated SOD was without effect (Fig. 4A, lane 8).
Figure 4.
Effect of scavengers and chelators on the light-induced degradation of GS (A) and Rubisco (B) in chloroplast lysates. Concentrations of the various effectors were: 100 μm MV, 1 mm DTT, 5 mm EDTA, 100 mm mannitol (Man), 100 units mL−1 catalase (Cat; Sigma C–40), and 100 units mL−1 SOD (Sigma S–4636). dSOD represents heat-inactivated SOD, obtained by boiling a solution of 2,000 units mL−1 SOD in 50 mm HEPES-NaOH (pH 7.5) for 5 min. All other conditions were as described in the legend to Figure 2. Samples corresponding to 1 μg (A) and 0.1 μg of chlorophyll (B) were analyzed. The electrophoretic mobilities of GS, the large subunit of Rubisco (LS), and the corresponding major degradation products are indicated on the right.
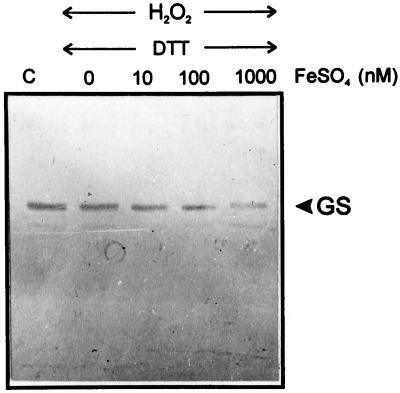
The protection afforded by SOD might shed some light on the possible role of the photosynthetic electron transport chain in GS degradation, which is related to the generation of O2⨪ in situ. The reasons for the cellular toxicity of O2⨪, which is by itself a poorly reactive compound, are 2-fold: it is the source of more active oxygen derivatives by successive univalent reductions (Cadenas, 1989) and it causes metal release from susceptible metallo-proteins, particularly those containing iron-sulfur clusters (Keyer et al., 1995). The key role played by these released metals during subsequent oxidative damage is well documented in bacteria (Fridovich, 1997). To probe this possibility, the stromal fraction of chloroplast lysates, which failed to promote GS degradation in the presence of H2O2 and DTT (Fig. 3, lane 9), was further supplemented with various concentrations of FeSO4. Under all conditions tested, incubation with the transition metal resulted in an increase of GS degradation in a time- (data not shown) and concentration-dependent manner (Fig. 5). Moreover, the addition of FeSO4 to illuminated lysates in the presence of MV resulted in further breakdown of stromal GS. The addition of catalase still prevented GS decline, whereas the protective effect of SOD was largely overcome by the presence of iron (Fig. 6).
Figure 5.
FeSO4 stimulates degradation of soluble GS by H2O2 and DTT. Cleared stromal fractions were exposed for 1 h at 22°C to the indicated concentrations of FeSO4 in the presence of 2.5 mm H2O2 and 1 mm DTT. Lane C, Extracts were incubated under the same conditions but in the absence of effectors. Analysis of the samples (equivalent to 0.5 μg of chlorophyll per lane) was carried out as described in Figure 2. The position of GS is shown on the right.
Figure 6.
Effect of FeSO4 on the light-induced degradation of GS by chloroplast lysates. FeSO4 was assayed at 1 μm. All other conditions and concentrations were as in Figure 3. The position of GS is shown on the left. Cat, Catalase; dSOD, heat-inactivated SOD.
Over-Irradiation Causes GS Cleavage in Wheat Plants
Kozaki and Takeba (1996) have shown that overexpression of chloroplastic GS in transgenic tobacco (2-fold GS activity over control plants) provided increased tolerance toward high doses of irradiation (2,000 μmol quanta m−2 s−1). The extreme sensitivity to oxidants displayed by GS, however, casts doubts on the actual contribution of this enzyme in the dissipation of excess photons in vivo. Since both our results and those of Stieger and Feller (1997) were obtained using isolated chloroplasts, plastid lysates, or plants subjected to artificial oxidative conditions, it was interesting to probe the effect of over-irradiation on protein stability in untreated wheat plants. Seedlings were thus subjected to continuous illumination at 10°C, since high irradiation doses (950 μmol quanta m−2 s−1 versus growth levels of 200 μmol quanta m−2 s−1) at low temperature may cause irreversible damage to the photosynthetic capacity of the leaves (Sen Gupta et al., 1993).
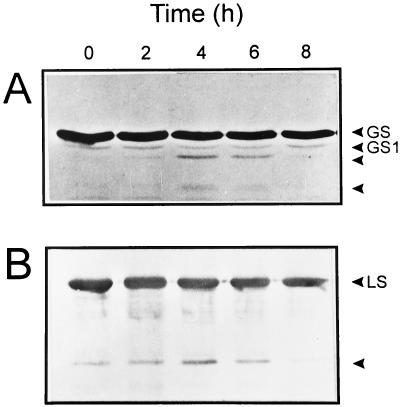
Even under these moderate stress conditions, both GS and Rubisco began to decline after 2 h, with concomitant accumulation of cleavage products. Time courses for the degradation of the two enzymes were very similar under the conditions assayed (Fig. 7, A and B). This contrasts with the higher lability displayed by GS in chloroplasts (Stieger and Feller, 1997) and in MV-treated plants (this work). The accumulation of GS cleavage products reached a maximum at 4 h of irradiation; then declined to undetectable levels after 8 h of treatment, presumably reflecting extensive degradation to small fragments that were not amenable to immunological detection methods (Fig. 7A). Taken together, our results indicate that GS is a very sensitive target to oxidative stress, both in vitro and in vivo.
Figure 7.
High light intensities cause GS degradation in wheat seedlings. Eight-day-old plants were illuminated by an actinic lamp (950 ± 60 μmol quanta m−2 s−1) at 10°C during the times indicated on top of each lane. Samples were analyzed as described in the legend to Figure 1, using 60 and 2 μg of total soluble protein for GS (A) and Rubisco (B) immunodetection, respectively. GS1 represents the homologous cytosolic Gln synthetase, which reacts weakly with the chloroplastic GS antiserum (Sakakibara et al., 1992). LS, Large subunit of Rubisco.
DISCUSSION
The present work documents the participation of ROS in the aggregation and cleavage of chloroplastic GS under oxidative stress conditions. The evidence presented here confirms and extends previous observations indicating that GS and other stromal proteins are rapidly degraded in isolated pea chloroplasts exposed to light, whereas in the dark these proteins remain stable for hours (Stieger and Feller, 1997). A number of experiments, based mainly on the use of inhibitors and uncouplers of photophosphorylation, suggested that the transport of electrons through the thylakoids and the oxidative events associated with it were responsible for the observed degradation (Stieger and Feller, 1997). Our results further indicate that these processes also occur in irradiated whole plants (Figs. 1 and 7), and could be accomplished even in the dark by the addition of H2O2 (Fig. 2B). Chloroplastic GS appears to be an early target for oxidative damage, displaying higher sensitivity than Rubisco in plastid lysates (Fig. 4) and seedlings (not shown) illuminated in the presence of redox-cycling herbicides. Degradation of GS and Rubisco was also evident in the absence of oxidants, when plants were exposed to high light intensities, although in this case both enzymes declined with a similar time course (Fig. 7).
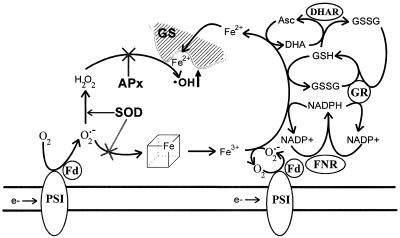
Using chloroplast lysates, we evaluated the requirements for GS degradation. Both O2⨪ generated by the light-dependent reduction of oxygen at PSI and H2O2 when added in the dark were able to promote GS cleavage (Fig. 2). A direct relationship between the amount of degradation products detected and the intensity of the provoked stress was observed in all cases. Maximal rates of degradation required the presence of reductants and transition metals (Figs. 3–6), suggesting that the formation of oxygen (·OH)-centered radicals produced by Fenton-type reactions are involved in this process. Although the lack of protection from the radical scavengers DMSO and mannitol (Figs. 2A and 4) argues against participation of ·OH in this process, it is conceivable that H2O2 could react with an enzyme-bound metal to produce a “crypto” ·OH in the metal-binding site of GS (Fig. 8).
Figure 8.
Proposed mechanism for the light-induced degradation of chloroplastic GS. Under oxidative conditions (drought, excess light), NADP+ levels decline and oxygen becomes the preferred acceptor for the photosynthetic electron transport chain. The O2⨪ thus generated is converted into H2O2, either spontaneously or through the action of chloroplast SODs. The O2⨪ molecules that escape SOD detoxification can react with soluble and membrane-bound iron-sulfur clusters releasing transition metals into the chloroplast stroma. Transition metal ions reduced by chloroplast metabolites (NADPH, GSH, and ascorbate) and by O2⨪ may then bind to the metal ligand site(s) of GS (Fucci et al., 1983; Kim et al., 1985; Stadtman, 1993), reacting locally with H2O2 to produce ·OH in situ. APx, Ascorbate peroxidase; DHAR, dehydroascorbate reductase; GR, glutathione reductase; FNR, Fd-NADP+ reductase.
This type of site-specific oxidative cleavage has been demonstrated for a number of proteins, including the yeast and bacterial GS counterparts (Fucci et al., 1983; Kim et al., 1985; Stadtman, 1993; Berlett and Stadtman, 1997). These reactions are viewed as “caged” processes in which the bound metal undergoes redox cycling through H2O2-dependent oxidation and protein-dependent reduction (Stadtman, 1993). Oxygen free radical intermediates thus generated (i.e. ·OH) would then react with any amino acid residue located within 5 to 10 molecular diameters from their place of formation (Cadenas, 1989). Since the oxygen radicals are produced in situ, they would not be able to escape the cage to encounter soluble scavengers such as mannitol, nor would the scavengers (even small lipophilic molecules such as DMSO) have easy access to the cage and compete with amino acid side chains for reaction with the radical (Stadtman, 1993). The inability of DMSO to prevent these processes is well documented (Fucci et al., 1983; Stadtman, 1993).
Chloroplastic GS being degraded by a site-specific metal-catalyzed oxidation is preferred because of several observations. First, homologous GS enzymes from other organisms have been shown to undergo this type of oxidative cleavage under conditions similar to those described here (Fucci et al., 1983; Kim et al., 1985). The site-specific mechanism is also supported by the observation that a discrete number of similar fragmentation products are generated by treatments such as light and MV (Fig. 2), H2O2 plus NADPH or DTT (Fig. 3), and intense irradiation of whole plants (Fig. 7). The inhibitory effect exerted by metal chelators (Figs. 2A and 4), which are expected to sequester the metal in the bulk solution, is also consistent with this type of mechanism.
Within this context, the effect of EDTA deserves special consideration, taking into account that metal complexes of this compound are known to promote oxidative fragmentation of proteins under certain conditions (Stadtman, 1993). The multifaceted responses of protein oxidation to EDTA are in part due to differences in the metal- and EDTA-binding capacities of the proteins themselves (Stadtman, 1993). Compelling evidence has been gathered to indicate that EDTA behaves as an inhibitor rather than a promoter of site-specific oxidative cleavage of proteins, with GS representing a conspicuous example of this behavior (Fucci et al., 1983). Finally, protease inhibitors such as PMSF and aprotinin provided little or no protection to the light-induced GS degradation (data not shown), suggesting that proteolytic enzymes are not involved in the cleavage process.
As indicated above, the major source of ROS in plants is the reduction of oxygen by PSI, particularly when NADP(H) pools are reduced (Asada et al., 1998). Experiments with various illumination times (Figs. 1A and 7), MV concentrations (Fig. 1B), and DCMU (Fig. 1C) clearly indicate that an increase in the amount of ROS generated by the photosynthetic electron transport chain stimulated GS degradation. However, the role of electron transport is not limited to the formation of ROS via the Mehler reaction; it also provides additional factors required for maximal rates of GS fragmentation. Accordingly, illuminated thylakoids still cause a strong stimulation of protein cleavage, even in the presence of ample amounts of H2O2 and reductants (Fig. 3). Data shown in Figures 5 and 6 strongly suggest that this extra contribution is related to the provision of reduced transition metals, presumably Fe2+, resulting from destruction of iron-sulfur proteins by O2⨪ (Keyer et al., 1995; Fridovich, 1997). Indeed, accumulation of free catalytic iron in pea plants exposed to MV or water deficit has been recently documented by Iturbe-Ormaetxe et al. (1998).
A comprehensive pathway for the oxidative degradation of chloroplastic GS, compatible with the data reported here, is proposed in Figure 8. This model summarizes the multi-step contributions of the photosynthetic electron transport to GS cleavage, and integrates a number of existing observations, such as the proposed mechanisms for metal-induced GS rupture in other organisms (Fucci et al., 1983; Kim et al., 1985) and the role played by various effectors in degradation of the chloroplastic enzyme (Stieger and Feller, 1997). According to this scheme, PSI is expected to donate electrons to different acceptors providing O2⨪, which is both a precursor of H2O2 and a reagent for iron release from proteins, and reducing equivalents (NADPH, GSH, ascorbate) that keep transition metals in a reduced state. The combination of H2O2 and Fe2+ at the GS metal binding site leads to ·OH radical generation in situ, contributing directly to the specific GS fragmentation. Ascorbate is included in the scheme as an electron donor for Fe3+ reduction based on its high concentration in illuminated chloroplasts (Asada et al., 1998), although this metabolite is expected to be readily oxidized by the photogenerated O2⨪ and ·OH in the presence of MV under the conditions shown in Figure 1.
Many environmental factors such as drought and high light lead to an excess of absorbed photons that can overcome the photosynthetic capacity of the plant cell. Although a substantial part of the excess excitation in the PSII antenna can be dissipated by non-radiative decay processes in the antennae pigment matrices (Brestic et al., 1995), the failure of the Calvin cycle to use the NADPH generated may still cause overreduction of the electron transport chain components and increase the risks of photoinhibition (Asada et al., 1998). Under these conditions, two alternative electron sinks to CO2 assimilation have been suggested: the photorespiratory pathway, which can supply CO2 for the Calvin cycle and recycle NADP+ for the photosynthetic electron transport chain, and the water-water cycle, which uses O2 as an electron acceptor to generate O2⨪ and involves the activity of SOD and ascorbate peroxidase for its detoxification (Osmond et al., 1997; Asada et al., 1998). The relative protection contributed by each of these pathways within the metabolic context of the stressed cell has been a matter of controversy. Different lines of evidence suggest that either photorespiration (Wu et al., 1991; Heber et al., 1996) or the water-water cycle (Biehler and Fock, 1996) is the predominant electron sink in the stressed plant.
Taking into account that GS catalyzes the rate-limiting step in photorespiration (Kozaki and Takeba, 1996), and given the extreme sensitivity to oxidants displayed by this enzyme, we postulate that the alternative electron sink employed by the plant under conditions of NADP+ shortage will be determined by the extent of the concomitant oxidative challenge. Under mild conditions that allow the survival of substantial amounts of GS, photorespiration will supply the NADP+ needed for the last step of the photosynthetic electron transport, assuming that the oxygenase activity of Rubisco is high enough to produce the substrates needed for photorespiration. However, when ROS levels increase beyond a certain threshold, GS decline would become rate-limiting, preventing further NADP+ recycling and leading to the use of oxygen as the ultimate electron acceptor at PSI. The water-water cycle would then take over to dissipate the excess electrons. Research is currently in progress to evaluate this hypothesis.
ACKNOWLEDGMENTS
Wheat seeds were kindly supplied by the Instituto Nacional de Tecnología Agropecuaria (Pergamino, Argentina). We wish to thank Dr. Tatsuo Sugiyama (Nagoya University, Japan) for the generous gift of the maize chloroplastic GS antisera.
Footnotes
This work was supported by grant no. BID 802/0C–AR PICT 01–00000–01363 from the National Research Agency of Argentina. N.C. and E.M.V. are members of the National Research Council of Argentina and J.F.P. is a fellow of the same institution.
LITERATURE CITED
- Allen RD. Dissection of oxidative stress tolerance using transgenic plants. Plant Physiol. 1995;107:1049–1054. doi: 10.1104/pp.107.4.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aro E-M, Hundal T, Carlberg I, Andersson B. In vitro studies on light-induced inhibition of photosystem II and D1-protein degradation at low temperature. Biochim Biophys Acta. 1990;1019:269–275. [Google Scholar]
- Asada K, Endo T, Mano J, Miyake C. Molecular mechanism for relaxation of and protection from light stress. In: Sato K, Murata N, editors. Stress Responses of Photosynthetic Organisms. Amsterdam: Elsevier Science Publishing; 1998. pp. 37–52. [Google Scholar]
- Berlett BS, Stadtman ER. Protein oxidation in aging, disease and oxidative stress. J Biol Chem. 1997;272:20313–20316. doi: 10.1074/jbc.272.33.20313. [DOI] [PubMed] [Google Scholar]
- Biehler K, Fock H. Evidence for the contribution of the Mehler-peroxidase reaction in dissipating excess electrons in drought-stressed wheat. Plant Physiol. 1996;112:265–272. doi: 10.1104/pp.112.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brestic M, Cornic G, Fryer MJ, Baker NR. Does photorespiration protect the photosynthetic apparatus in French bean leaves from photoinhibition during drought stress? Planta. 1995;196:450–457. [Google Scholar]
- Cadenas E. Biochemistry of oxygen toxicity. Annu Rev Biochem. 1989;58:79–110. doi: 10.1146/annurev.bi.58.070189.000455. [DOI] [PubMed] [Google Scholar]
- Casano LM, Lascano HR, Trippi VS. Hydroxyl radicals and a thylakoid-bound endopeptidase are involved in light- and oxygen-induced proteolysis in oat chloroplasts. Plant Cell Physiol. 1994;35:145–152. [Google Scholar]
- Davies KJ. Protein damage and degradation by oxygen radicals. I. General aspects. J Biol Chem. 1987;262:9895–9901. [PubMed] [Google Scholar]
- Davies KJ, Lin SW, Pacifici RE. Protein damage and degradation by oxygen radicals. IV. Degradation of denatured protein. J Biol Chem. 1987;262:9914–9920. [PubMed] [Google Scholar]
- Desimone M, Henke A, Wagner E. Oxidative stress induces partial degradation of the large subunit of ribulose-1,5-bisphosphate carboxylase/oxygenase in isolated chloroplasts of barley. Plant Physiol. 1996;111:789–796. doi: 10.1104/pp.111.3.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyer CH, Descourviéres P, Kunert KJ. Protection against oxygen radicals: an important defense mechanism studied in transgenic plants. Plant Cell Environ. 1994;17:507–523. [Google Scholar]
- Fridovich I. Superoxide anion radical (O2⨪), superoxide dismutases, and related matters. J Biol Chem. 1997;272:18515–18517. doi: 10.1074/jbc.272.30.18515. [DOI] [PubMed] [Google Scholar]
- Fucci L, Oliver CN, Coon MJ, Stadtman ER. Inactivation of key metabolic enzymes by mixed-function oxidation reactions: possible implication in protein turnover and aging. Proc Natl Acad Sci USA. 1983;80:1521–1525. doi: 10.1073/pnas.80.6.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heber U, Bligny R, Streb P, Douce R. Photorespiration is essential for the protection of the photosynthetic apparatus of C3 plants against photoinactivation under sunlight. Bot Acta. 1996;109:307–315. [Google Scholar]
- Ishida H, Nishimori Y, Sugisawa M, Makino A, Mae T. The large subunit of ribulose-1,5-bisphosphate carboxylase/oxygenase is fragmented into 37-kDa and 16-kDa polypeptides by active oxygen in the lysates of chloroplasts from primary leaves of wheat. Plant Cell Physiol. 1997;38:471–479. doi: 10.1093/oxfordjournals.pcp.a029191. [DOI] [PubMed] [Google Scholar]
- Iturbe-Ormaetxe I, Escuredo PR, Arrese-Igor C, Becana M. Oxidative damage in pea plants exposed to water deficit or paraquat. Plant Physiol. 1998;116:173–181. [Google Scholar]
- Keyer K, Gort AS, Imlay JA. Superoxide and the production of oxidative DNA damage. J Bacteriol. 1995;177:6782–6790. doi: 10.1128/jb.177.23.6782-6790.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Rhee SG, Stadtman ER. Nonenzymatic cleavage of proteins by reactive oxygen species generated by dithiothreitol and iron. J Biol Chem. 1985;260:15394–15397. [PubMed] [Google Scholar]
- Kozaki A, Takeba G. Photorespiration protects C3 plants from photooxidation. Nature. 1996;384:557–560. [Google Scholar]
- Krapp AR, Tognetti VB, Carrillo N, Acevedo A. The role of ferredoxin-NADP+ reductase in the concerted cell defense against oxidative damage: studies using Escherichia coli mutants and cloned plant genes. Eur J Biochem. 1997;249:556–563. doi: 10.1111/j.1432-1033.1997.00556.x. [DOI] [PubMed] [Google Scholar]
- Mehta RA, Fawcett TW, Porath D, Mattoo AR. Oxidative stress causes rapid membrane translocation and in vivo degradation of ribulose-1,5-bisphosphate carboxylase/oxygenase. J Biol Chem. 1992;267:2810–2816. [PubMed] [Google Scholar]
- Osmond B, Badger M, Maxwell K, Bjorkman O, Leegood R. Too many photons: photorespiration, photoinhibition and photooxidation. Trends Plant Sci. 1997;2:119–121. [Google Scholar]
- Palatnik JF, Valle EM, Carrillo N. Oxidative stress causes ferredoxin-NADP+ reductase solubilization from the thylakoid membranes in methyl viologen-treated plants. Plant Physiol. 1997;115:1721–1727. doi: 10.1104/pp.115.4.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson GL. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977;83:346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Polle A. Mehler reaction: friend or foe in photosynthesis? Bot Acta. 1996;109:84–89. [Google Scholar]
- Roberts DR, Kristie DN, Thompson JE, Dumbroff EB, Gepstein S. In vitro evidence for the involvement of activated oxygen in light-induced aggregation of thylakoid proteins. Physiol Plant. 1991;82:389–396. [Google Scholar]
- Sakakibara H, Kawabata S, Takahashi H, Hase T, Sugiyama T. Molecular cloning of the family of glutamine synthetase genes from maize: expression of genes for glutamine synthetase and ferredoxin-dependent glutamate synthase in photosynthetic and non-photosynthetic tissues. Plant Cell Physiol. 1992;33:49–58. [Google Scholar]
- Scandalios JG. Oxygen stress and superoxide dismutase. Plant Physiol. 1993;101:7–12. doi: 10.1104/pp.101.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen Gupta A, Heinen JL, Haladay AS, Burke JJ, Allen RD. Increased resistance to oxidative stress in transgenic plants that overexpress chloroplastic Cu/Zn superoxide dismutase. Proc Natl Acad Sci USA. 1993;90:1629–1633. doi: 10.1073/pnas.90.4.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtman ER. Oxidation of free amino acids and amino acid residues in proteins by radiolysis and by metal-catalyzed reactions. Annu Rev Biochem. 1993;62:797–821. doi: 10.1146/annurev.bi.62.070193.004053. [DOI] [PubMed] [Google Scholar]
- Stieger PA, Feller U. Requirements for the light-stimulated degradation of stromal proteins in isolated pea (Pisum sativum L.) chloroplasts. J Exp Bot. 1997;48:1639–1645. [Google Scholar]
- Whatley FR, Arnon DI. Photosynthetic phosphorylation in plants. Methods Enzymol. 1963;6:308–313. [Google Scholar]
- Wu J, Neimanis S, Heber U. Photorespiration is more effective than the Mehler reaction in protecting the photosynthetic apparatus against photoinhibition. Bot Acta. 1991;104:283–291. [Google Scholar]