Abstract
Species distribution models have been used to predict the distribution of invasive species for conservation planning. Understanding spatial transferability of niche predictions is critical to promote species-habitat conservation and forecasting areas vulnerable to invasion. Grain size of predictor variables is an important factor affecting the accuracy and transferability of species distribution models. Choice of grain size is often dependent on the type of predictor variables used and the selection of predictors sometimes rely on data availability. This study employed the MAXENT species distribution model to investigate the effect of the grain size on model transferability for an invasive plant species. We modelled the distribution of Rhododendron ponticum in Wales, U.K. and tested model performance and transferability by varying grain size (50 m, 300 m, and 1 km). MAXENT-based models are sensitive to grain size and selection of variables. We found that over-reliance on the commonly used bioclimatic variables may lead to less accurate models as it often compromises the finer grain size of biophysical variables which may be more important determinants of species distribution at small spatial scales. Model accuracy is likely to increase with decreasing grain size. However, successful model transferability may require optimization of model grain size.
Introduction
Species distribution models (SDMs) are becoming increasingly important in predicting spatial patterns of biological invasions, identification of hotspots for early detection and informing management of invasive species1. SDMs relate the presence/absence records of species to relevant environmental variables and subsequently project modelled relationships across geographical space using gridded layers of environmental data, producing a map indicating areas of potential species distribution2. One of the key features of gridded data is the ‘grain size’ – a term describing the geographical representation (spatial resolution) of the map layers. Grain size of predictor variables strongly affects the interpretation of biogeographic characteristics of modelled species3. Use of smaller or finer grain size allows for a more accurate representation of the effect of local environmental conditions and biotic interactions in model prediction4.
The challenge in using smaller grain size in SDMs is finding the optimum balance between data quality, data availability, and model performance5. Grain size represents the geographical space unit which contains all the information on characteristic attributes of the study area6. A decrease in grain size enhances the details of the landscape by sharpening the features it contains and by making the rare land use types in the landscape more prominent and distinguishable7. Conversely, coarse grain size of predictor variables in SDMs negatively affects the delineation of habitat features in a landscape, a feature of critical importance to modelling species presence. Selection of grain size and its relationship with habitat features is a crucial factor in SDM based studies3,7–9. Most literature to date reports on species distribution models built at a grain size of 1 km, a fact recently subjected to some scrutiny and critique7,10. Earlier observations indicate that the use of 1 km grain size may be too coarse to generate reliable SDM outputs7, especially for studies at small spatial scales. The challenge therefore, is to establish the threshold grain size at which predictor variables correctly describe local conditions and biotic interactions which play an important role in defining species’ range11.
The choice of grain size in SDM studies is sometimes based on data availability12 rather than relevant factors like species’ ecology and spatial scale of study. A review of more than 200 SDM-based research papers concluded that the choice of variables is ‘frequently opportunistic’ and that the majority of the studies, instead of making a tailored choice of variables, rely on a standard set of 19 bioclimatic variables13 which are available at a minimum of 1 km grain size. In a complementary analysis designed to provide an overview of current practice, we reviewed 59 recent SDM based studies published in peer-reviewed journals in 2016–2017 (Supplementary data S1). We confirmed that the most frequently used variables in MAXENT based ecological modelling studies are indeed, the 19 bioclimatic variables available from the ‘Global Climate Data’ (www.worldclim.org). We found that 55 out of the 59 studies selected the above-mentioned bioclimatic variables as input. Of these 55 studies 34 had used additional biophysical variables such as topography and land cover. These biophysical variables are available at a grain size as 100 meters or less. Since the grain size of all input variables in SDMs need to be harmonized, these biophysical variable are resampled to 1 km in when used in combination with the bioclimatic variables. Intriguingly, the results of 22 out of these 34 studies (which had both bioclimatic and biophysical variables) suggest that the variables critical to accurate species distribution prediction were the biophysical variables. Given the earlier argument that a finer grain size is more likely to improve model accuracy, the following speculation can be made: had these 22 studies not coarsened the biophysical variables – by avoiding the ‘customary’ choice of bioclimatic variables - this would have resulted in a more accurate prediction of species distribution. This speculation might appear to question the significance of bioclimatic variables in ecological models. It is a fact that bioclimatic variables are among the most frequently used variables in SDM based studies and rightly so as climate is a strong determinant of species’ distribution. However, an injudicious use of these variables without considering factors like species’ ecology, scale of study and optimal grain size is questionable13,14. Thus, we speculate that in many SDM based studies – especially at small spatial scale of study area - biophysical variables may be the more important ones and inclusion of bioclimatic variables in such cases may reduce the model accuracy.
One of the motivations for creating SDMs is to use them to predict the behaviour of a species colonizing new territory. Successful transferability of SDMs across space or time is extremely valuable in context of conservation planning. A basic assumption underlying SDMs is that the model is spatially and temporally transferable, i.e. the niche attributes are conserved across space and time2. Although the effect of grain size in SDMs is well documented15–17, its role in model transferability has not been put to sufficient scrutiny. There is evidence that although SDMs can accurately predict species distribution in the training area, their transferability to new areas is challenging due to numerous complex phenomena18,19. Among many factors, grain size has been reported as critical to satisfactory model performance and transferability20,21.
In this study we aim to test the role of grain size in SDMs both in the training and the transfer areas. Based on our review of literature, we speculate that over-reliance on easily available bioclimatic variables may lead to an unnecessary compromise on the grain size of critical variables, with potentially negative impact on the accuracy of model predictions and transferability. Specifically, we use a MAXENT modelling environment22 to model the distribution of Rhododendron ponticum (L.) in the Snowdonia National Park, Wales and then transfer the model to the Brecon Beacons National Park, Wales. The objectives of this study were to assess whether the decreasing the grain size improves model performance both in the training and the transfer area.
Methods
Species description
Rhododendron ponticum (L.) is an invasive plant species in the United Kingdom, having been introduced in the 18th century as an ornamental plant. The main ancestor is reported to be the population of R. ponticum resident in the southern tip of Spain23. It is a perennial, evergreen shrub that generally invades woodlands24, although it has been shown to colonize other types of habitat too. The UK invasion by this shrub has been more intense in Western and North-Western areas of Britain, which are comparatively cooler and wetter. We chose Wales as the study region because it is one of the most affected regions of the UK to be impacted by invasions of R. ponticum. In this study, we trained the model on the dataset for the Snowdonia National Park in Wales25 and then transferred the model to the Brecon Beacons National Park. Given the scale of the invasion, it is clear that the current environmental, topographic and land cover conditions both in Snowdonia and the Brecon Beacons represent a range of conditions very suitable for R. ponticum.
Species distribution modelling algorithm
We used MAXENT, a maximum-entropy based machine learning (presence/pseudo-absence) algorithm to model the distribution R. ponticum (L.) in Snowdonia National Park (the training area) and projected the model to the Brecon Beacons National Park (the transfer area). MAXENT predicts the probability distribution of a species on the basis of a given set of predictor variables and presence-only species occurrence data22. We selected MAXENT because, a) it does not require absence data26, b) it efficiently handles complex interactions between predictor and response variables27, c) being a generative model, it performs better than discriminative models when it comes to modelling with presence-only records, d) it can be run with both categorical and continuous data variables28 and, e) it efficiently transfers the model projections to another geographical area2. We used a reasonably large sample size29 and applied the recommended screening and verification of occurrence records.
Presence records for model training and validation
For the training area (Snowdonia National Park), presence-only occurrence records of R. ponticum (L.) were obtained from COFNOD (Local Environmental Records Centre in Wales, UK). A dataset of 152 occurrence records was created by a continuous field observation campaign between 1981 and 2000. COFNOD has confirmed that the entire area of Snowdonia National Park was thoroughly surveyed by ground surveys and remote sensing tools, thus minimizing the possibility of sampling bias in the dataset. Consequently, we targeted the entire area of the National Park, generating 10,000 random background points to be selected during each replicate run of the model. We used independent occurrence records of R. ponticum (L.) in the Brecon Beacons National Park downloaded from the National Biodiversity Network (NBN) online database (www.nbnatlas.org), yielding 100 observations. Spatial uncertainty of all occurrence records was addressed by removing all duplicate or non-geo-referenced occurrence points. Occurrence data were spatially rarefied using SDM toolbox 2.030 in ArcGIS 10.5 by eliminating all but one point present within a single grid cell of the predictor variable layers to avoid double counting of presence points.
Selection of predictor variables
Predictor variables were selected in the following three steps. In the first step, two categories of variables were compiled. The first category of variables comprised the most frequently used variables in SDM studies: ‘Bioclimatic Variables’ (BCV). The second category of variables was based on our expert knowledge and a review of literature on the ecology of R. ponticum (L.): ‘Biophysical Variables’ (BPV). A set of 19 bioclimatic variables from ‘Global Climate Data’ (www.worldclim.org, version 2, 1970–2000), identified as the most commonly used suite of variables in SDM research13, formed the BCV category. An extensive review of literature and background knowledge of the R. ponticum ecology yielded the most important biophysical variables, namely; topography (altitude, aspect and slope), land cover and ‘distance from water channels’ which formed the BPV category31–34. Although Rhododendron is sensitive to many other ecological factors, we kept the BPV category to the above mentioned variables as these variables were the most pertinent ones at the current spatial scale of study.
In the second step of variable selection, a sub-set of variables from the BCV and BPV categories was created on the basis of grain size. The first variable set (VS-1) included both BCV and BPV categories, with the latter resampled to a 1 km grain size which is the smallest cell size of BCV. The second variable set (VS-2) comprised the BPV at 300 m grain size. The third variable set (VS-3) consisted of the same BPV but at 50 m grain size (Tables 1 and 2). The VS-1 represents the commonly reported approach used in SDM studies and thus can be considered the ‘control’ scenario. The VS-2 & VS-3 represent scenarios where bioclimatic variables are excluded to conserve the finer grain size of BPV. All input data layers were re-sampled using nearest neighbour (for discrete variables) and bilinear interpolation (for continuous variables) resampling techniques35–37. Collinearity among predictor variables negatively impacts the model due to the substantial amount of information shared between collinear variables. Therefore, collinearity in variables makes it difficult to correctly interpret the relative contribution or importance of variables in the model predictions38. A Pearson correlation coefficient cut-off of r ≤ 0.70 was applied to select the variablesfor use in the final model runs38 for all three sets of variables (VS-1, VS-2 and VS-3). The aim of this step was to reduce the negative impact of multicollinearity and to conform to statistical assumptions39.
Table 1.
Predictor variables used in the study.
| VS-1 | VS-2 | VS-3 | |||
|---|---|---|---|---|---|
| Grain Size 1 km | Grain Size 300 m | Grain Size 50 m | |||
| Predictor Variable | Unit | Predictor Variable | Unit | Predictor Variable | Unit |
| Altitude | m | Altitude | m | Altitude | m |
| Aspect | ° | Aspect | ° | Aspect | ° |
| Slope | ° | Slope | ° | Slope | ° |
| Land Cover | Land Cover | Land Cover | |||
| Distance from water channels | m | Distance from water channels | m | Distance from water channels | m |
| Mean Diurnal Range (monthly (max temp - min temp)) | °C | ||||
| Isothermality (BIO2/BIO7)* 100 | |||||
| Mean Temperature of Driest Quarter | °C | ||||
| Precipitation Seasonality (Coefficient of Variation) | C of V | ||||
Acronyms VS-1, VS-2 & VS-3 refer to variable set 1, variable set 2 & variable set 3 respectively.
Table 2.
Allocation of predictor variables to ‘variable categories’ and ‘variable sets’.
| Predictor variable/s | Grain Size | Source | Variables Category | Variable Set |
|---|---|---|---|---|
| 19 bioclimatic variables | 1 km | WorldClim - Global Climate Data | BCV | VS-1 |
| Distance from water channels | 1 km | Edina Digimap Ordnance Survey | BCV | VS-1 |
| Land Cover | 300 m | Edina Digimap Ordnance Survey | BPV | VS-2 |
| Topography (Altitude, Aspect, Slope) | 300 m | Shuttle Radar Topography Mission USGS | BPV | VS-2 |
| Distance from water channels | 300 m | Edina Digimap Ordnance Survey | BPV | VS-2 |
| Land Cover | 50 m | Edina Digimap Ordnance Survey | BPV | VS-3 |
| Topography (Altitude, Aspect, Slope) | 50 m | Edina Digimap Ordnance Survey | BPV | VS-3 |
| Distance from water channels | 50 m | Edina Digimap Ordnance Survey | BPV | VS-3 |
Acronyms BCV, BPV, VS-1, VS-2 & VS-3 refer to Bioclimatic Variables, Biophysical Variables, Variable Set 1, Variable Set 2 & Variable Set 3 respectively.
Model calibration
All three modelling scenarios were run in MAXENT (version 3.3.3a) with a default convergence threshold of 10−6 and with 5000 iterations to allow the model scope for convergence while reducing the risk of over- or under-predicting modelled relationships. We processed 25 model replications with a bootstrap resampling method randomly allocating 75% of the occurrence records in the training area for calibration and 25% for validation. To avoid dubious projections by the model, we used the ‘fade-by-clamping’ feature which removes heavily clamped (clustered) pixels from the final predictions26. Rest of the MAXENT calibration was set to default settings.
Model Evaluation
Training area
Area Under the ROC (Receiver Operating Characteristic) Curve (AUC) was used to test the performance of the model against actual observations in the training area27. An AUC value of 0.5 shows that the model does not predict any better than random chance, whereas a value closer to 1 indicates a better performance of the model40. Permutation importance contribution was used to assess the relative significance of predictor variables. Fitted response curves were used to visually investigate the relationship between individual variables and predicted index of environmental suitability of R. ponticum. In addition to AUC, we used Continuous Boyce Index (CBI) as an additional assessment tool. The Boyce index requires presence data only and measures by how much model predictions differ from random distribution of observed presence across the prediction gradient. The continuous habitat suitability map is reclassified into i number of classes/bins. For each bin, Predicted and Expected frequencies are calculated. The Predicted Frequency is calculated by dividing the number of species’ occurrence points in the bin i, as forecasted by the model, by the total number of species’ occurrence points. The Expected Frequency is calculated by dividing the number of grid cells in bin i by the total number of grid cells. A P/E ratio is then calculated for each bin and a Spearman rank correlation coefficient rho (1-tailed test) evaluates if the ratio significantly increases as suitability increases (p < 0.05). The continuous values of the Boyce index vary between −1 and +1. Positive values indicate a model where predictions are consistent with the distribution of actual presence data, values close to zero mean that the model is no different from a random model and negative values indicate counter predictions (e.g. predicting no occurrence in areas where actual presence is recorded)41,42.
Transfer area (Model transferability)
MAXENT produces continuous probability maps of habitat suitability in the selected geographical area. We used R. ponticum (L.) presence records in the Brecon Beacons National Park to evaluate model projection in the transfer area. Continuous Boyce Index (CBI) was used to assess how well MAXENT has transferred the model to a different geographical area41,42. CBI is considered one of the most appropriate metrics for assessing model predictions applied to presence-only datasets. There is some indication that CBI is a more reliable metric than AUC when it comes to validating model transferability to a different geographical area43.
Data availability
Presence records of Rhododendron ponticum in Snowdonia National Park and Brecon Beacons National Park can be acquired from COFNOD (www.cofnod.org.uk) and NBN Atlas (www.nbnatlas.org) respectively.
Results
The AUC & CBI based evaluation of the three models in the training area, where each model used a different subset of predictor variables at different grain size, indicated variation in the degree of prediction accuracy. As shown in Fig. 1. AUCtrain, AUCtest and CBI values of VS-1, the variable set with the coarsest grain size are the lowest, indicating the least accurate predictions in the training area (Snowdonia). Variable sets VS-2 and VS-3, comprised of the same set of biophysical variables but at different grain size, indicate that the finer grain size is likely to yield better model predictions.
Figure 1.
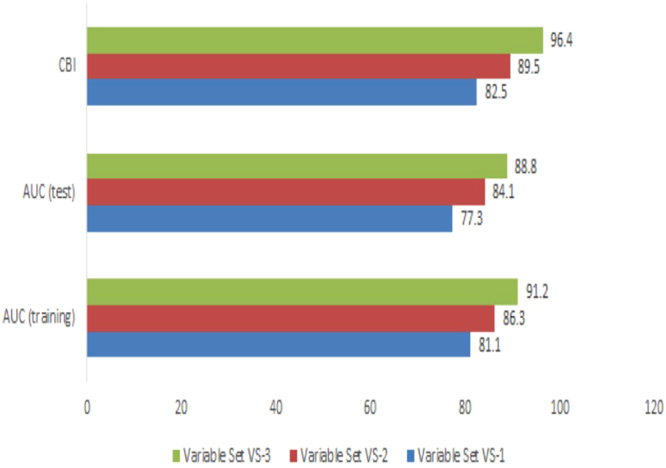
Area Under Curve (AUC) and Continuous Boyce Index (CBI) comparing prediction accuracy of Maxent-based models in Snowdonia National Park using three predictor variable sets at 1 km (VS-1), 300 m (VS-2) and 50 m (VS-3) resolution.
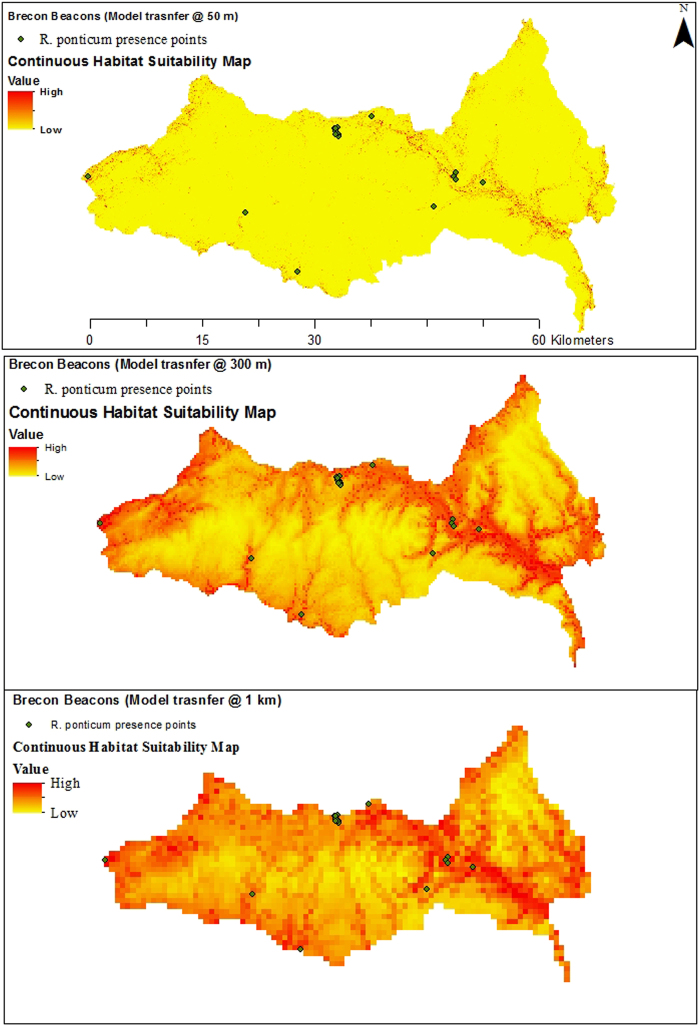
We used Continuous Boyce Index (CBI) to assess the transferability of the MAXENT models to an area not covered by the training dataset, in our case the Brecon Beacons National Park. The model comprising the VS-1 variables showed the poorest model transferability with a CBI value of 0.65. In comparison, the model based on the VS-2 dataset showed a high CBI of 0.90, while the third model based on VS-3 achieved a moderate CBI of 0.77. Analysis of the predictor variable contribution to model prediction (supplementary data S2) suggests that land cover and altitude were major contributors in all three models. Our results also suggest that the use of finer grain size improved model transferability (CBI value of Models VS-2 & VS-3 > VS-1). However, model transferability decreased at the finest grain size (50 m) of the predictor variables. Response curves for individual variables for all three modelling scenarios are provided in Supplementary data S3.
Discussion
A number of studies have highlighted the fact that coarse grain size of predictor variables in SDMs may obscure effects of biotic interactions, small-scale heterogeneity of abiotic factors and micro habitat of species44,45. A review of 149-peer reviewed publications concluded that the choice of grain size is a highly neglected aspect in species distribution modelling and is a factor that significantly impacts modelling outcomes12.
Model performance in the training area
The results from this study show that MAXENT model predictions in the training area are likely to improve with smaller grain size of predictor variables (AUC in the order of 50 m > 300 m > 1000 m grain size). The Snowdonia National Park is characterized by diverse topography, with altitude ranging from sea-level to above 1000 m over a relatively short distance. Altitude is one of the key factors affecting the invasive potential of alien species and the effect of altitude was shown to be most pronounced at fine grain size46. It has been claimed that too coarse a grain size in SDMs leads to spatial smoothing and thus obscures the connection between, for example, land cover types and species occurrence47. This occurs by homogenizing the dominant land types within a grid cell resulting in the loss of useful information for accurate modelling48. In accordance with this assertion, the accuracy of model predictions in our study improved with decreasing grain size of the predictor variables, possibly as the result of capturing small-scale ecological interactions critical for species distribution being maximized at a finer grain size11,45,49. In our case, the rugged topography of the area also affects factors such as soil physical and chemical properties, atmospheric humidity and wind speed/exposure over very short distances. With decreasing grain size, representation of these factors was more pronounced and improved model predictions. As grain size becomes finer, the number of mixed pixels decreases, leading to an increase in ‘distinct’ pixels which clearly separate different land cover, topographical or environmental units (or classes) and thus enables the algorithm to build more accurate species-habitat relationships7. This improvement becomes more relevant when the species being modelled is a habitat specialist. Since R. ponticum is considered one such species – in Wales it has a high preference for woodlands – better performance of models using small grain size data can be explained by improving representation of this community type.
As a habitat specialist, R. ponticum has repeatedly been shown to be strongly correlated with land cover type (Yang et al., 2013a). In Britain woodland is the most important land cover type in the context of R. ponticum invasion23, largely because of the availability of suitable micro-environments for seed germination33. For example, dead plant material and moss cover is critical to R. ponticum establishment50. Response curves in our study show that Forests are the most important land cover classes for R. ponticum distribution. Furthermores, R. ponticum is sensitive to topographic controls51–53. Response curves show that R. ponticum favors a northerly aspect for its establishment and growth as north-facing slopes at this latitude (Wales) are generally cooler, offering higher soil moisture and lower direct insulation intensity. Moreover, response curves suggest that R. ponticum distribution in Snowdonia is negatively correlated with slope. Shallow-slope areas are typically those with high soil moisture and nutrient availability, thus offering more favorable microenvironment for invasive species54. Distance from water channel was an important variable determining the habitat suitability of R. ponticum. This finding is compliments earlier studies suggesting that R. ponticum favors areas near water bodies55 primarily because soil in vicinity of water body is moist and often has dense vegetation. Many other invasive species have been reported to be negatively correlated with distance from water sources56.
Model performance in the transfer area
After assessing model performance in the training area, the second goal of the study was to test the effects of grain size on the spatial transferability of the model. The results suggest that a coarse grain size (1000 m) produced the poorest model transferability while a medium grain size (300 m) resulted in the most accurate transfer of the model. The poor model transferability at 1 km grain size (CBI = 0.65) may be explained by the fact that key environmental factors, which in our case were land cover and topography, are ‘averaged out’ at coarser grain size both in the training and the transfer areas44. We expected the best model transferability when using data with the finest grain size. This was not the case; our transferred model had the best predictive power at medium grain size. A possible explanation is that Snowdonia National Park (training area) and Brecon Beacons National Park (transfer area) differ in the range and the character of topographical features. Since topography and land cover are best represented at small grain size, a discrepancy in the typography of landscape features between the two areas will negatively affect model transferability. Similarly, it has been shown that species occurrence data needs to be highly accurate when modelled at very fine grain size as any location10,57 errors in the survey data may impact model performance.
In this study the CBI value of the SDM transferred at 300 m grain size was 0.90, a reasonably accurate prediction but which leaves room for improvement. We tested SDM transferability under the assumption that abiotic factors are the principal controls on species distribution. However, the distribution of any species is also likely to be constrained by biotic interactions58. These biotic interactions vary between geographical regions, just as topography, land cover and climatic factors differ. Even though the training and transfer areas used in the study are similar, any difference in the nature of the biotic interactions limiting R. ponticum may have constrained the degree of model transferability11. In this context, this invasive species may have occupied only a subset of its potential niche in the invaded area so far, known as the realized niche. A species may fail to occupy the entire potential niche due to factors such as intra-species competition, dispersal limitation, scarcity of resources and other spatial limitations59. The distribution of species is linked to a framework known as ‘Biotic Abiotic Mobility’ (BAM)60 which describes the potential niche yet to be inhabited by a species in the ‘unfilled niche’61. Thus, correct identification of this unfilled niche may help to identify areas vulnerable for future invasion and may prove helpful in understanding the invasive behavior of species under study62. Our results suggest therefore, that for habitat specialists, model transferability across geographical space becomes highly sensitive to the grain size when the model training and transfer areas differ in environmental and ecological features.
Although our study suggests that our model was transferred more accurately at 300 m grain size, it is important to mention that even at 50 m grain size, the model was also transferred with considerable success (CBI = 0.77). From an invasive species management point of view, a habitat suitability map at 50 m grain size with a lower prediction accuracy could still be more acceptable than a map with a better predictive ‘hit rate’ but at a six times coarser grain size. As an example, we include habitat suitability maps generated by model transfer to the Brecon Beacons National Park at three contrasting grain sizes (Fig. 2). The land cover map legend is provided in Supplementary data S3.
Figure 2.
Rhododendron ponticum habitat suitability maps at 1 km, 300 m and 50 m resolutions generated in ArcGIS 10.5 (ESRI, Redlands, CA, USA, www.esri.com). A spatial distribution model was trained in Snowdonia National Park and transferred to the Brecon Beacons National Park. Blue dots indicate verified occurrence records of the species.
Bioclimatic variables in SDMs – an inevitable choice?
In the context of our results it appears that unnecessary or ‘customary’ use of bioclimatic variables without considering the species’ ecology negatively affects the predictive potential of a SDM. Including these bioclimatic variables almost always comes at a cost of reducing the grain size of other variables, such as topography and land cover. However, as climate is likely to be one of the determinant of a species’ fundamental niche, we suggest that expert knowledge of species’ ecology and an extensive review of the literature should be carried out before deciding whether or not to include climatic variables in a SDM. Naturally, when modelling large-scale distributions (continental or global) or if the objective is a temporal prediction, perhaps to account for climate change, there currently may not be many alternatives to a 1 km grain size bioclimatic variables at a global scale. Choice of predictor variables is also a matter of the research question. If researches are strictly interested in estimating climatic suitability or sensitivity, then the climatic variables become an appropriate choice. Our results strictly refer to cases where researchers might be interested in mapping species’ distribution with high accuracy using the best possible combination of all the available predictor variables.
Limitations of the study and future recommendations
Our study suggests that a grain size smaller than 1 km should be preferred in SDM studies; however, models using finer grain size data should be trained and validated with carefully validated occurrence records. Training a model with predictor variables at very small grain size leads to a very specific species-habitat relationship and thus needs to be verified with accurate presence records. Our study modelled the distribution of R. ponticum, a habitat specialist species that showed a clear response to the changes in grain size. By contrast, generalist species may not be as sensitive to a change in grain size. Our study also suggests that there may not be a ‘gold standard’ for the grain size of predictor variables when it comes to model transferability across space. Ideally, transferring the model to another area requires the identification of optimum grain size by considering a range of grain sizes, perhaps on a sub-set of available occurrence data. Also, we considered only a small area for model training and transferability possibly explaining why climatic variables contributed the least in our models. For SDMs over large spatial scale, climatic variables may have greater effect in determining the distribution of species. In this study, we have only used two evaluation tools (AUC & CBI) which hint that the model with higher values might be better than the rest. For future studies we recommend applying more robust statistics to evaluate the significance of difference between modelling scenarios.
Electronic supplementary material
Analysis of Variables Contribution to Maxent-based Models
Response curves of Maxent based models and legends for land cover categories
Acknowledgements
We would like to thank Commonwealth Scholarship Commission for funding this PhD study. We also thank CONFOD who provided presence records of Rhododendron ponticum in Snowdonia National Park, Wales, U.K and Alaaeldin Soultan, MaxPlanck Institute for Ornithology, Germany for his advice in devising methodology of this study.
Author Contributions
All authors conceived the ideas and designed methodology; S.A.M. and G.G. analyzed the data; S.A.M. and M.L. led the writing of the manuscript. All authors contributed critically to the draft and gave final approval for publication.
Competing Interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-25437-1.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Václavík T, Meentemeyer RK. Invasive species distribution modeling (iSDM): Are absence data and dispersal constraints needed to predict actual distributions? Ecol. Modell. 2009;220:3248–3258. doi: 10.1016/j.ecolmodel.2009.08.013. [DOI] [Google Scholar]
- 2.Verbruggen H, et al. Improving Transferability of Introduced Species’ Distribution Models: New Tools to Forecast the Spread of a Highly Invasive Seaweed. PLoS One. 2013;8:1–13. doi: 10.1371/journal.pone.0068337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pearson RG, Dawson TP. Predicting the impacts of climate change on the distribution of species: Are bioclimate envelope models useful? Glob. Ecol. Biogeogr. 2003;12:361–371. doi: 10.1046/j.1466-822X.2003.00042.x. [DOI] [Google Scholar]
- 4.Soberón J. Grinnellian and Eltonian niches and geographic distributions of species. Ecol. Lett. 2007;10:1115–1123. doi: 10.1111/j.1461-0248.2007.01107.x. [DOI] [PubMed] [Google Scholar]
- 5.Menke SB, Holway DA, Fisher RN, Jetz W. Characterizing and predicting species distributions across environments and scales: Argentine ant occurrences in the eye of the beholder. Glob. Ecol. Biogeogr. 2009;18:50–63. doi: 10.1111/j.1466-8238.2008.00420.x. [DOI] [Google Scholar]
- 6.Wiens JA. Spatial Scaling in Ecology Spatial scaling in ecology1. Source Funct. Ecol. 1989;3:385–397. doi: 10.2307/2389612. [DOI] [Google Scholar]
- 7.Gottschalk TK, Aue B, Hotes S, Ekschmitt K. Influence of grain size on species-habitat models. Ecol. Modell. 2011;222:3403–3412. doi: 10.1016/j.ecolmodel.2011.07.008. [DOI] [Google Scholar]
- 8.Connor, T. et al. Effects of grain size and niche breadth on species distribution modeling. Ecography (Cop.). 1–12, 10.1111/ecog.03416 (2017).
- 9.Song W, Kim E, Lee D, Lee M, Jeon SW. The sensitivity of species distribution modeling to scale differences. Ecol. Modell. 2013;248:113–118. doi: 10.1016/j.ecolmodel.2012.09.012. [DOI] [Google Scholar]
- 10.Hanberry BB. Finer grain size increases effects of error and changes influence of environmental predictors on species distribution models. Ecol. Inform. 2013;15:8–13. doi: 10.1016/j.ecoinf.2013.02.003. [DOI] [Google Scholar]
- 11.Fernández M, Hamilton H. Ecological niche transferability using invasive species as a case study. PLoS One. 2015;10:1–17. doi: 10.1371/journal.pone.0119891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mayer AL, Cameron GN. Consideration of grain and extent in landscape studies of terrestrial vertebrate ecology. Landsc. Urban Plan. 2003;65:201–217. doi: 10.1016/S0169-2046(03)00057-4. [DOI] [Google Scholar]
- 13.Porfirio LL, et al. Improving the use of species distribution models in conservation planning and management under climate change. PLoS One. 2014;9:1–21. doi: 10.1371/journal.pone.0113749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang H, Liu D, Munroe D, Cao K, Biermann C. Study on selecting sensitive environmental variables in modelling species spatial distribution. Ann. GIS. 2016;22:57–69. doi: 10.1080/19475683.2015.1114523. [DOI] [Google Scholar]
- 15.Guisan A, et al. What matters for predicting the occurrences of trees: Techniques, data, or species’ characteristics? Ecol. Monogr. 2007;77:615–630. doi: 10.1890/06-1060.1. [DOI] [Google Scholar]
- 16.Venier La, Pearce J, McKee JE, McKenney DW, Niemi G. J. Climate and satellite-derived land cover for predicting breeding bird distribution in the Great Lakes basin. J. Biogeogr. 2004;31:315–331. doi: 10.1046/j.0305-0270.2003.01014.x. [DOI] [Google Scholar]
- 17.Guisan A, et al. Sensitivity of predictive species distribution models to change in grain size. Divers. Distrib. 2007;13:332–340. doi: 10.1111/j.1472-4642.2007.00342.x. [DOI] [Google Scholar]
- 18.Fitzpatrick MC, Weltzin JF, Sanders NJ, Dunn RR. The biogeography of prediction error: Why does the introduced range of the fire ant over-predict its native range? Glob. Ecol. Biogeogr. 2007;16:24–33. doi: 10.1111/j.1466-8238.2006.00258.x. [DOI] [Google Scholar]
- 19.Roach, N. S., Hunter, E. A., Nibbelink, N. P. & Barrett, K. Poor transferability of a distribution model for a widespread coastal marsh bird in the southeastern United States. Ecosphere8 (2017).
- 20.Khosravi R, Hemami MR, Malekian M, Flint AL, Flint LE. Maxent modeling for predicting potential distribution of goitered gazelle in central Iran: The effect of extent and grain size on performance of the model. Turkish J. Zool. 2016;40:574–585. doi: 10.3906/zoo-1505-38. [DOI] [Google Scholar]
- 21.Luoto M, Virkkala R, Heikkinen RK. The role of land cover in bioclimatic models depends on spatial resolution. Glob. Ecol. Biogeogr. 2007;16:34–42. doi: 10.1111/j.1466-8238.2006.00262.x. [DOI] [Google Scholar]
- 22.Phillips, S. J., Dudik, M. & Schapire, R. E. A maximum entropy approach to species distribution modeling. 655–662 (2004).
- 23.Dehnen-Schmutz K, Williamson M. Rhododendron ponticum in Britain and Ireland: Social, economic and ecological factors in its successful invasion. Environ. Hist. Camb. 2006;12:325–350. doi: 10.3197/096734006778226355. [DOI] [Google Scholar]
- 24.Tiedeken EJ, Stout JC. Insect-flower interaction network structure is resilient to a temporary pulse of floral resources from invasive Rhododendron ponticum. PLoS One. 2015;10:1–19. doi: 10.1371/journal.pone.0119733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jackson, P. Rhododendron in Snowdonia and a strategy for its control. Snowdownia Natl. Park Auth. (2008).
- 26.Phillips SB, Aneja VP, Kang D, Arya SP. Modelling and analysis of the atmospheric nitrogen deposition in North Carolina. Int. J. Glob. Environ. Issues. 2006;6:231–252. doi: 10.1504/IJGENVI.2006.010156. [DOI] [Google Scholar]
- 27.Elith J, et al. Novel methods improve prediction of species’ distributions from occurrence data. Ecography (Cop.). 2006;29:129–151. doi: 10.1111/j.2006.0906-7590.04596.x. [DOI] [Google Scholar]
- 28.Elith J, et al. A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 2011;17:43–57. doi: 10.1111/j.1472-4642.2010.00725.x. [DOI] [Google Scholar]
- 29.Wisz MS, et al. Effects of sample size on the performance of species distribution models. Divers. Distrib. 2008;14:763–773. doi: 10.1111/j.1472-4642.2008.00482.x. [DOI] [Google Scholar]
- 30.Brown JL. SDMtoolbox: A python-based GIS toolkit for landscape genetic, biogeographic and species distribution model analyses. Methods Ecol. Evol. 2014;5:694–700. doi: 10.1111/2041-210X.12200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harris CM, Stanford HL, Edwards C, Travis JMJ, Park KJ. Integrating demographic data and a mechanistic dispersal model to predict invasion spread of Rhododendron ponticum in different habitats. Ecol. Inform. 2011;6:187–195. doi: 10.1016/j.ecoinf.2011.03.004. [DOI] [Google Scholar]
- 32.Erfmeier A, Bruelheide H. Comparison of native and invasive Rhododendron ponticum populations: Growth, reproduction and morphology under field conditions. Flora - Morphol. Distrib. Funct. Ecol. Plants. 2004;199:120–133. doi: 10.1078/0367-2530-00141. [DOI] [Google Scholar]
- 33.Stephenson CM, MacKenzie ML, Edwards C, Travis JMJ. Modelling establishment probabilities of an exotic plant, Rhododendron ponticum, invading a heterogeneous, woodland landscape using logistic regression with spatial autocorrelation. Ecol. Modell. 2006;193:747–758. doi: 10.1016/j.ecolmodel.2005.09.007. [DOI] [Google Scholar]
- 34.Eşen D, Zedaker SM, Kirwan JL, Mou P. Soil and site factors influencing purple-flowered rhododendron (Rhododendron ponticum L.) and eastern beech forests (Fagus orientalis Lipsky) in Turkey. For. Ecol. Manage. 2004;203:229–240. doi: 10.1016/j.foreco.2004.07.052. [DOI] [Google Scholar]
- 35.Choudhury MR, Deb P, Singha H, Chakdar B, Medhi M. Predicting the probable distribution and threat of invasive Mimosa diplotricha Suavalle and Mikania micrantha Kunth in a protected tropical grassland. Ecol. Eng. 2016;97:23–31. doi: 10.1016/j.ecoleng.2016.07.018. [DOI] [Google Scholar]
- 36.Gibson L, McNeill A, Tores P, de, Wayne A, Yates C. Will future climate change threaten a range restricted endemic species, the quokka (Setonix brachyurus), in south west Australia? Biol. Conserv. 2010;143:2453–2461. doi: 10.1016/j.biocon.2010.06.011. [DOI] [Google Scholar]
- 37.Hu R, et al. A bird’s view of new conservation hotspots in China. Biol. Conserv. 2017;211:47–55. doi: 10.1016/j.biocon.2017.03.033. [DOI] [Google Scholar]
- 38.Dormann CF, et al. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography (Cop.). 2013;36:027–046. doi: 10.1111/j.1600-0587.2012.07348.x. [DOI] [Google Scholar]
- 39.Syfert, M. M., Smith, M. J. & Coomes, D. A. The Effects of Sampling Bias and Model Complexity on the Predictive Performance of MaxEnt Species Distribution Models. PLoS One8, (2013). [DOI] [PMC free article] [PubMed]
- 40.Swets JA. Measuring the accuracy of diagnostic systems. Science. 1988;240:1285–1293. doi: 10.1126/science.3287615. [DOI] [PubMed] [Google Scholar]
- 41.Boyce MS, Vernier PR, Nielsen SE, Schmiegelow FKA. Evaluating resource selection functions. Ecol. Modell. 2002;157:281–300. doi: 10.1016/S0304-3800(02)00200-4. [DOI] [Google Scholar]
- 42.Hirzel AH, Le Lay G, Helfer V, Randin C, Guisan A. Evaluating the ability of habitat suitability models to predict species presences. Ecol. Modell. 2006;199:142–152. doi: 10.1016/j.ecolmodel.2006.05.017. [DOI] [Google Scholar]
- 43.Cianfrani C, Le Lay G, Hirzel AH, Loy A. Do habitat suitability models reliably predict the recovery areas of threatened species? J. Appl. Ecol. 2010;47:421–430. doi: 10.1111/j.1365-2664.2010.01781.x. [DOI] [Google Scholar]
- 44.Baniya CB, Solhøy T, Gauslaa Y, Palmer MW. Richness and Composition of Vascular Plants and Cryptogams along a High Elevational Gradient on Buddha Mountain, Central Tibet. Folia Geobot. 2012;47:135–151. doi: 10.1007/s12224-011-9113-x. [DOI] [Google Scholar]
- 45.Özesmi U, Mitsch WJ. A spatial habitat model for the marsh-breeding red-winged blackbird (Agelaius phoeniceus L.) in coastal Lake Erie wetlands. Ecol. Modell. 1997;101:139–152. doi: 10.1016/S0304-3800(97)01983-2. [DOI] [Google Scholar]
- 46.Palmer MW. Scale dependence of native and alien species richness in North American floras. Preslia. 2006;78:427–436. [Google Scholar]
- 47.Lawes MJ, Piper SE. There is less to binary maps than meets the eye: The use of species distribution data in the southern African sub-region. S. Afr. J. Sci. 1998;94:207–210. [Google Scholar]
- 48.Saura S. Effects of minimum mapping unit on land cover data spatial configuration and composition. Int. J. Remote Sens. 2002;23:4853–4880. doi: 10.1080/01431160110114493. [DOI] [Google Scholar]
- 49.Ödland A, Birks HJB. The altitudinal gradient of vascular plant richness in Anrland, western Norway. Ecography (Cop.). 1999;22:548–566. doi: 10.1111/j.1600-0587.1999.tb01285.x. [DOI] [Google Scholar]
- 50.Cross JR. The Establishment of Rhododendron Ponticum in the Killarney Oakwoods, S. W. Ireland Author (s): J. R. Cross Published by: British Ecological Society Stable http://www.jstor.org/stable/2259638 JSTOR is a not-for-profit service that helps scho. J. Ecol. 1981;69:807–824. doi: 10.2307/2259638. [DOI] [Google Scholar]
- 51.Taylor SL, Hill RA, Edwards C. Characterising invasive non-native Rhododendron ponticum spectra signatures with spectroradiometry in the laboratory and field: Potential for remote mapping. ISPRS J. Photogramm. Remote Sens. 2013;81:70–81. doi: 10.1016/j.isprsjprs.2013.04.003. [DOI] [Google Scholar]
- 52.Francon L, Corona C, Roussel E, Lopez Saez J, Stoffel M. Warm summers and moderate winter precipitation boost Rhododendron ferrugineum L. growth in the Taillefer massif (French Alps) Sci. Total Environ. 2017;586:1020–1031. doi: 10.1016/j.scitotenv.2017.02.083. [DOI] [PubMed] [Google Scholar]
- 53.Christiaens A, et al. Determining the minimum daily light integral for forcing of azalea (Rhododendron simsii) Sci. Hortic. (Amsterdam). 2014;177:1–9. doi: 10.1016/j.scienta.2014.07.028. [DOI] [Google Scholar]
- 54.Kang W, Minor ES, Lee D, Park CR. Predicting impacts of climate change on habitat connectivity of Kalopanax septemlobus in South Korea. Acta Oecologica. 2016;71:31–38. doi: 10.1016/j.actao.2016.01.005. [DOI] [Google Scholar]
- 55.Evangelista, P. et al. Mapping Habitat and Potential Distributions of Invasive Plant Species on USFWS National Wildlife Refuges. 34 (2012).
- 56.Crall AW, et al. Using habitat suitability models to target invasive plant species surveys. Ecol. Appl. 2013;23:60–72. doi: 10.1890/12-0465.1. [DOI] [PubMed] [Google Scholar]
- 57.Guisan A, Thuiller W. Predicting species distribution: Offering more than simple habitat models. Ecol. Lett. 2005;8:993–1009. doi: 10.1111/j.1461-0248.2005.00792.x. [DOI] [PubMed] [Google Scholar]
- 58.Lenoir J, et al. Going against the flow: Potential mechanisms for unexpected downslope range shifts in a warming climate. Ecography (Cop.). 2010;33:295–303. [Google Scholar]
- 59.Mott CL. Environmental Constraints to the Geographic Expansion of Plant and Animal Species. Nat. Educ. Knowl. 2010;3:72. [Google Scholar]
- 60.Soberon J, Nakamura M. Niches and distributional areas: Concepts, methods, and assumptions. Proc. Natl. Acad. Sci. 2009;106:19644–19650. doi: 10.1073/pnas.0901637106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Broennimann O, Guisan A. Predicting current and future biological invasions: both native and invaded ranges matter. Biol. Lett. 2008;4:585–589. doi: 10.1098/rsbl.2008.0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Seoane J, Carrascal LM, Alonso CL, Palomino D. Species-specific traits associated to prediction errors in bird habitat suitability modelling. Ecol. Modell. 2005;185:299–308. doi: 10.1016/j.ecolmodel.2004.12.012. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Analysis of Variables Contribution to Maxent-based Models
Response curves of Maxent based models and legends for land cover categories
Data Availability Statement
Presence records of Rhododendron ponticum in Snowdonia National Park and Brecon Beacons National Park can be acquired from COFNOD (www.cofnod.org.uk) and NBN Atlas (www.nbnatlas.org) respectively.