Abstract
Objective
To compare two composite indices of sleep apnea disease burden to the commonly used apnea-hypopnea index with regard to baseline measurement of subjective and objective disease burden.
Study design
Cross-sectional study.
Setting
Tertiary academic medical center sleep laboratory.
Subjects and methods
Patients with suspected diagnosis of sleep apnea undergoing first diagnostic polysomnography. Subjective data were collected via validated questionnaires; objective data were obtained by standardized physical examination, chart extraction, and polysomnography. Four subjective (patient-reported) disease burden measures and three objective (anatomic and physiologic) disease burden measures were used for validation. Associations between composite indices or apnea-hypopnea index and these seven construct validation measures were compared using bootstrapped correlation coefficients.
Results
216 subjects were included in the final analysis. Both composite disease burden indices showed clinically important or nearly important associations with three of four subjective validation measures and all three objective validation measures, while apnea-hypopnea index was associated only with the objective validation measures.
Conclusion
Composite indices of sleep apnea disease burden may capture the breadth of baseline sleep apnea disease burden, particularly subjective disease burden, better than the apnea-hypopnea index.
Keywords: sleep apnea, sleep disordered breathing, patient-centered, composite measure, conjunctive consolidation
Introduction/Background
Obstructive sleep apnea is defined by a single polysomnography measure, the apnea-hypopnea index (AHI). Diagnosis of obstructive sleep apnea syndrome requires AHI ≥ 5 (at least five apneas + hypopneas per hour of sleep) associated with subjective symptoms1. However, AHI cutoffs used in practice are variable, as is the definition of hypopnea2. Differences in equipment between sleep laboratories also contributes to AHI variability. This lack of standardization hinders comparisons of disease severity and therapeutic outcomes between different clinical and research settings.
Further, AHI appears to be an incomplete measure of disease severity, correlating poorly with measures of health status, quality of life, function, and symptoms at baseline and after treatment, thus not fully reflecting sleep apnea health burden or treatment effects3,4. Similarly, the utility of AHI in research is unclear, particularly in evaluating surgical interventions4,5.
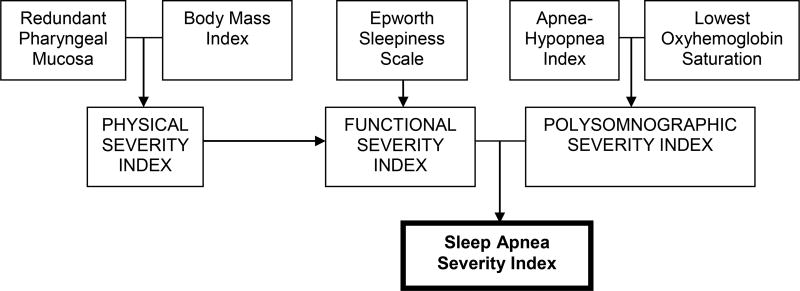
For chronic disorders like sleep apnea, it is important to include both subjective (i.e., patient-perceived) and objective measures of disease burden. Few such conceptual models for measuring sleep apnea disease burden comprehensively exist in the literature6,7. In keeping with this need, and as an alternative to AHI, we studied the more comprehensive Sleep Apnea Severity Index (SASI) proposed by Piccirillo et al. in a 1998 study funded by the American Academy of Otolaryngology – Head & Neck Surgery8. SASI incorporates diverse aspects of sleep apnea, including anatomic (redundant pharyngeal mucosa), anthropometric (body mass index), subjective (daytime sleepiness reflected by the Epworth Sleepiness Scale), and physiologic (AHI and lowest oxyhemoglobin saturation) elements (Figure 1). By considering both objective and patient-centered subjective data, SASI may offer a more holistic view of a sleep apnea patient's status. Despite this potential, it has not been evaluated further and has not been incorporated in clinical practice or research.
Figure 1.
Schematic derivation of the Sleep Apnea Severity Index (SASI) and modified Sleep Apnea Severity Index (Modified SASI). The scoring algorithm was generated through a conjunctive consolidation process. Modified SASI uses tonsil size in place of SASI’s redundant pharyngeal mucosa element.
This study aimed to evaluate SASI through a cross-sectional construct validation. We also evaluated a modified SASI, first proposed here, in which redundant pharyngeal mucosa is replaced by tonsil size. Redundant pharyngeal mucosa lacks a standardized definition or measurement and risks decreasing reliability between examiners, whereas tonsil size grading is standardized9 and shows intra- and interobserver reproducibility10. We hypothesized that SASI and Modified SASI would each be more strongly associated than AHI with seven measures (constructs) of sleep apnea disease burden, reflecting more completely this complex disease.
Methods
Study Design
This cross-sectional study analyzed Seattle Sleep Cohort participants at the University of Washington Sleep Disorders Center at Harborview Medical Center, Seattle, Washington. Participants were recruited between August 2006 and October 2007. All participants were recruited and all data collected on the night of their first diagnostic polysomnography.
Participants
Patients were eligible if they presented to the sleep laboratory for overnight polysomnography for undiagnosed but suspected obstructive sleep apnea, successfully completed polysomnography, had linguistic and cognitive ability to answer questionnaires and give informed consent, and were age 18 or older. Patients were excluded for prior diagnosis of sleep apnea, other upper airway obstructive disease, or neurologic, neuromuscular, or pulmonary disease. Patients with either full-night or split-night polysomnography were included in the study. This study was approved by the University of Washington institutional review board.
Data Collection
Consenting participants answered the Sleep Apnea Quality of Life Index, Short Form – 36 (SF-36), Epworth Sleepiness Scale, and Pittsburgh Sleep Quality Index. Trained research assistants administered these instruments in a standardized fashion and performed standardized physical examinations including height, weight, presence or absence of redundant pharyngeal mucosa, and tonsil size. Body mass index was calculated from height and weight measurements. Blood pressures were obtained from the chart for each patient’s most recent sleep clinic visit or from automated blood pressure measurements as part of the physical examination at the time of enrollment. Following physical examination, patients underwent scheduled diagnostic polysomnography, from which AHI, lowest oxyhemoglobin saturation, and desaturation index data were extracted. All patients underwent overnight, monitored, in-lab polysomnography. On the morning after polysomnography, patients underwent phlebotomy for a high-sensitivity serum C-reactive protein assay.
SASI scores were calculated as described previously8, producing a score of I (lowest sleep apnea severity) to III (highest sleep apnea severity). Modified SASI scores were calculated in similar fashion, substituting tonsil size for redundancy of pharyngeal mucosa. Tonsil size was graded as 0 for surgically absent tonsils and 1–4 for percent distance from faucial arch to midline (1=0–25%, 2=25–50%, 3=50–75%, and 4=75–100%). Tonsil size was converted to a binary variable, with 0–1 coded as normal and 2–4 coded as enlarged.
When split-night (combined diagnostic and continuous positive airway pressure titration) polysomnography studies were performed (N = 15), only data from the diagnostic portion (mean sleep time ± standard deviation = 145 ± 23 minutes) were analyzed for this study. All polysomnography tests were performed in the sleep laboratory and monitored, and they included recordings of sleep state parameters (4-lead electroencephalogram, bilateral electro-oculogram, and submental and bilateral leg electromyogram), breathing (oronasal airflow by nasal pressure transducer and oronasal thermister, and thoracic and abdominal excursion by strain gauge), oximetry, electrocardiogram, and infrared video. All studies were manually scored in standard fashion by trained technicians and confirmed by board-certified sleep physicians.
Apnea was defined as 80–100% reduction in airflow lasting ≥10 seconds. Hypopnea was defined as 30–80% reduction in airflow lasting ≥10 seconds (an associated desaturation or arousal was not required). AHI was defined as the number of apnea and hypopneas per hour of sleep and was categorized as normal (0 – 4.9), mild (5 – 14.9), moderate (15 – 29.9), or severe (≥30). Lowest oxyhemoglobin saturation was defined as lowest oxygen saturation during sleep. Desaturation index was defined as number of desaturations ≥3% from baseline per hour of sleep.
Measures of Sleep Apnea Burden (Contruct Validation Measures)
Seven construct validation measures were used to measure sleep apnea disease burden. Four measures addressed subjective, patient-centered aspects of disease burden, while three addressed objective, physiological burden. Because the subjective experience of sleep apnea drives patients to seek care, we decided a priori to place greater importance on subjective measures. In order of priority, these measures were: 1) Sleep Apnea Quality of Life Index11, 2) Vitality domain from the SF-36, 3) Mental Health Component Score from the SF-36, 4) Pittsburgh Sleep Quality Index12, 5) 3% desaturation index, 6) Mean arterial pressure, and 7) serum C-reactive protein. The latter three, all objective, are surrogate physiological measures of cardiovascular risk and mortality risk associated with sleep apnea. As surrogate measures, they were given lower priority in ranking of importance.
Analysis
Statistical analysis used Stata/SE 9 software (StataCorp LP, College Station, TX). Descriptive summary for continuous (or near continuous variables) were reported as mean ± standard deviation. Distributions of index scores were compared using the Mantel-Haenszel chi-square test and Cuzick’s nonparametric test for trend across ordered groups. Associations between index scores (SASI, Modified SASI, and AHI) and the sleep apnea burden construct validation measures were tested using Spearman correlation coefficients. Spearman correlations were compared for each construct variable, to determine whether SASI (or Modified SASI) had a stronger correlation than AHI. Sample size was based on finding important correlations (|r| > 0.25) between SASI (or Modified SASI) and the construct variables. To compare correlations statistically, we used bootstrapping (seed=12345, 200 repetitions) to generate a normal distribution of correlations for each association, thus providing a mean and standard deviation13. The bootstrapped distribution data enabled us to test the hypothesis that the SASI (or Modified SASI) had a stronger correlation than AHI for each construct, using the paired t-test. A p-value < 0.01 was considered statistically significant and a p-value 0.01 – 0.10 was considered a statistical trend; we used a significance level lower than the usual 0.05 to minimize the potential problem of multiple comparisons.
Role of the funding source
Funding sources had no involvement in study design, data collection, analysis, or interpretation. The timing and final decision to submit this manuscript for publication was based solely on the authors’ judgment.
Results
Participants included 292 consecutive patients who consented and enrolled in this study. One patient was excluded due to a diagnosis of Rosai-Dorfman disease, which might confound associations with C-reactive protein levels. Necessary data were available for 216 participants included in the final analysis. The study sample demographic characteristics included age 46 ± 11 years, 56% male, and 87% white. The components of the SASI and Mondified SASI for the sample included redundant pharyngeal mucosa present in 19%, enlarged tonsils (size ≥ 2) present in 34%, body mass index 34 ± 8 kg/m2, Epworth Sleepiness Scale 10 ± 5, AHI 54 ± 31 events per hour, and lowest oxyhemoglobin saturation 86 ± 9%. The final sample was not significantly different from the total consenting population in any of these characteristics, annual income, or education level (data not shown). The distribution of sleep apnea severity was more balanced for the SASI and the Modified SASI than for AHI (Table 1).
Table 1.
Distribution of sleep apnea severity by each measure studied.
| Apnea-Hypopnea Index | Sleep Apnea Severity Index | Modified Sleep Apnea Severity Index | |
|---|---|---|---|
| Mild, N (%) | 12 (6%) | 98 (45%) | 92 (43%) |
| Moderate, N (%) | 44 (20%) | 58 (27%) | 61 (28%) |
| Severe, N (%) | 160 (74%) | 60 (28%) | 63 (29%) |
The construct validation measures summarized in Table 2 showed some associations with each other. Most notably, the four subjective validation variables appeared to be somewhat correlated (absolute value of mean correlation strength 0.44 ± 0.24, range |0.13| – |0.70|). Subjective and objective measures did not appear to be associated (absolute value of mean correlation strength 0.11 ± 0.12, range |0.01| – |0.39|), and objective validation measures were not clearly associated with each other (absolute value of mean correlation strength 0.17 ± 0.08, range 0.08 – 0.24).
Table 2.
Summary of observed values for validation measures.
| Validation Measure | Possible Range of Values | Observed Range | Mean ± SD |
|---|---|---|---|
|
| |||
| Sleep Apnea Quality of Life Index | 1.0 – 7.0 (7.0 best) | 1.6 – 6.4 | 4.5 ± 1.0 |
|
| |||
| SF-36 Mental Health component | 0 – 100 (100 best) | 16 – 100 | 68 ± 17 |
|
| |||
| SF-36 Vitality status | 0 – 100 (100 best) | 10 – 80 | 43 ±16 |
|
| |||
| Pittsburgh Sleep Quality Index | 0 – 21 (21 worst) | 3 – 20 | 10 ± 3 |
|
| |||
| 3% desaturation index | ≥ 0 (higher worse) | 0 – 156 desaturations/hour | 17 ± 23 |
|
| |||
| Mean arterial pressure | 70 – 93 (normal range) | 65 – 125 mmHg | 96 ± 12 |
| 93 – 107 (prehypertension range) | |||
| >107 (hypertension range) | |||
|
| |||
| Serum C-reactive protein | 0.0 – 10.0 (> 3.0 mg/L associated with high cardiovascular disease risk) | 0.2 – 44.8 mg/L | 4.6 ± 5.9 |
The association between each measure of sleep apnea severity and each sleep apnea disease burden construct is shown in Table 3. AHI was not significantly associated with any subjective construct validation measures but was associated (statistical significance or trend) with all objective construct measures. Both SASI and Modified SASI were associated (statistical significance or trend) with three out of four subjective construct measures and all three objective construct measures. Both SASI and Modified SASI showed clinically important associations (r ≥ 0.25) or nearly-important associations (r ≥ 0.15) with six of seven construct validation measures, whereas AHI did so only with the three objective construct validation measures.
Table 3.
Associations between measures of sleep apnea severity and constructs of sleep apnea disease burden. Coefficients are presented with standard errors generated by bootstrapping with 200 repetitions, each of n=216. Bold indicates associations with statistical significance (p < 0.01) or trend (p = 0.01 – 0.10). Dark shading indicates clinically important associations (defined as |r| ≥ 0.25); light shading indicates associations approaching importance (defined as |r| ≥ 0.15).
| Sleep apnea- specific quality of life |
SF-36 vitality domain |
SF-36 mental health status |
Sleep quality | 3% desaturation index |
Mean arterial pressure |
Serum C- reactive protein |
|
|---|---|---|---|---|---|---|---|
|
| |||||||
| Apnea-Hypopnea Index | −0.09 ± 0.07 | −0.01 ± 0.07 | 0.06 ± 0.07 | 0.05 ± 0.06 | 0.69 ± 0.05 | 0.22 ± 0.06 | 0.16 ± 0.07 |
| (p = 0.17) | (p = 0.90) | (p = 0.79) | (p = 0.47) | (p < 0.001) | (p < 0.001) | (p = 0.03) | |
|
| |||||||
| Sleep Apnea Severity Index | −0.26 ± 0.06 | −0.16 ± 0.07 | −0.03 ± 0.07 | 0.19 ± 0.06 | 0.50 ± 0.06 | 0.18 ± 0.06 | 0.32 ± 0.06 |
| (p < 0.001) | (p = 0.03) | (p = 0.73) | (p = 0.003)) | (p < 0.001) | (p = 0.002) | (p < 0.001) | |
|
| |||||||
| Modified Sleep Apnea Severity Index | −0.24 ± 0.06 | −0.17 ± 0.07 | −0.005 ± 0.08 | 0.20 ± 0.07 | 0.52 ± 0.06 | 0.21 ± 0.06 | 0.31 ± 0.06 |
| (p < 0.001) | (p = 0.02) | (p = 0.95) | (p = 0.003) | (p < 0.001) | (p < 0.001) | (p < 0.001) | |
Bootstrapping allowed direct comparison between strengths of association of AHI, SASI, and Modified SASI with each construct validation measure (Table 4). Comparisons were performed only for validation measures with which at least one overall disease severity measure (SASI, Modified SASI, or AHI) showed significant association, because comparison between insignificant associations was not thought useful or meaningful. SASI and Modified SASI were significantly more strongly associated than AHI with sleep apnea-specific quality of life, vitality status, sleep quality, and serum C-reactive protein. AHI was significantly more strongly associated than SASI or Modified SASI with mental health status and desaturation index and more strongly than SASI with mean arterial pressure. However, there was no significant difference between Modified SASI and AHI in strength of association with mean arterial pressure.
Table 4.
Comparisons of sleep apnea severity indexes and apnea-hypopnea index as measures of sleep apnea disease burden. Comparisons were done via a 2-tailed t-test, with p < 0.01 considered statistically significant. Dark shading indicates stronger associations for SASI or Modified SASI than AHI; light shading indicated stronger associations for AHI than SASI or Modified SASI. SASI = Sleep Apnea Severity Index. AHI = Apnea-Hypopnea Index. Modified SASI = Modified Sleep Apnea Severity Index.
| Sleep apnea- specific quality of life |
SF-36 vitality status |
SF-36 mental health status |
Sleep quality | 3% desaturation Index |
Mean arterial pressure |
Serum C- reactive protein |
|
|---|---|---|---|---|---|---|---|
|
| |||||||
| SASI vs AHI | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 |
| SASI better | SASI better | AHI better | SASI better | AHI better | AHI better | SASI better | |
|
| |||||||
| Modified SASI vs AHI | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p = 0.10 | p < 0.0001 |
| Modified SASI better | Modified SASI better | AHI better | Modified SASI better | AHI better | Modified SASI better | ||
Sensitivity analysis was performed for bootstrapping with different numbers of repetitions, using the same seed value and per-repetition sample size as the main analysis. Bootstrapping with 10, 50, 1000, and 10000 repetitions produced nearly identical correlation coefficient estimates, confidence intervals, and p-values (data not shown).
Discussion
The current measure used to assess obstructive sleep apnea severity is AHI, a physiologic measure. While some studies support relationships between AHI and self-reported health status14 or daytime sleepiness15, others show poor associations between AHI and subjective and objective measures of disease burden3,4. AHI also has limitations as an outcomes measure. A meta-analysis of surgical interventions for sleep apnea, for example, found that AHI outcomes and subjective outcomes followed different patterns5. Previous studies of various therapies have showed little association between change in AHI and change in quality of life or other subjective and objective outcomes4,16. Thus, AHI may be an incomplete measure of disease severity for clinicians and researchers interested in disease burden and therapeutic effects as experienced by patients. Our results support this conclusion, suggesting that physiologic measures are not consistently associated with each other or with subjective measures.
However, AHI has some prognostic value. It is associated with hypertension17,18,19, other markers of cardiovascular risk20, and cardiovascular outcomes21,22. In this study, AHI showed significant associations with serum C-reactive protein and mean arterial blood pressure, two surrogate measures of cardiovascular risk and mortality risk23,24.
Meanwhile, commonly used subjective disease burden measures may also not reflect the breadth of this disease. As mentioned, associations are inconsistent between AHI and patients’ subjective complaints, and the same holds true for other combinations of objective and subjective measures25.
Ideally, assessment of disease severity, burden, and outcome should incorporate multiple relevant variables. For example, cancer staging uses disease-specific combinations of tumor size and nodal and distant metastasis. The SASI and Modified SASI may play a similar role in assessing baseline sleep apnea disease burden, combining diverse subjective and objective elements into a simple score. SASI has been shown to have some such utility; in its original description, scores showed dose-response relationships with multiple aspects of health status measured by the SF-368. Meanwhile, because SASI incorporates anatomic and physiologic variables including AHI, it may associate with physiologic aspects of sleep apnea and their clinical sequelae.
To our knowledge, this study is the first to validate any composite adult sleep apnea severity indices across subjective and objective measures of disease burden. Furthermore, it is the first to test the prognostic value of composite indices for the broader disease burden of sleep apnea, particularly potential cardiovascular disease risk and mortality risk as reflected by the surrogate measures 3% desaturation index, mean arterial pressure, and serum C-reactive protein.
This study supported our hypothesis that SASI and Modified SASI, as composite measures, reflect sleep apnea disease burden more thoroughly than the isolated physiologic AHI measure. Relationships with subjective construct measures were statistically significant and also clinically important (especially with sleep apnea-specific quality of life, the most disease-specific of our subjective measures, and our primary construct defined a priori) or nearly so. Meanwhile, these two indices also reflected the breadth of objective disease burden well.
Modified SASI, using tonsil size instead of pharyngeal mucosal redundancy, may be more useful, because it is easier to use and is based on a standardized measure of anatomic upper airway obstruction. Anecdotally, our research assistants found assessment of pharyngeal mucosal redundancy more vague, and thus more challenging, than standardized tonsil grading, despite having written and photographic descriptions of each. Dichotomization of tonsil size in Modified SASI was based on a preliminary analysis demonstrating that none of the construct variables, AHI, or body-mass index had significantly different distributions when comparing 0–1 versus 2–4 or 0–2 versus 3–4 dichotomization, and based on evidence that AHI is similar in tonsil grades 0 and 1 but rises between 1 and 226.
We addressed the complexity of sleep apnea as a disease by validating SASI, Modified SASI, and AHI across seven validation measures selected a priori to reflect disease-specific and general subjective burden, short-term physiologic burden, and long-term cardiovascular and mortality risk. The use of seven validation measures raises the specter of false positive findings due to multiple testing, but by choosing these measures in advance and by using a stricter significance level, the problems of multiple testing are minimized.
In interpreting our findings, it is important to distinguish between statistically significant and clinically important results. In this study, our predetermined cutoff for clinical importance was |r| ≥ 0.25. The two composite indices exceed or approach this number more than does AHI for most of our validation measures. Indeed, the composite indices appear to maintain all or most of AHI’s association with desaturation index and mean arterial pressure while capturing additional subjective burden. Along the same lines, while AHI was statistically more significantly associated with SF-36 mental health status than either SASI or Modified SASI, the absolute strengths of all three associations were very weak, so this comparison is not useful.
The three-point scale of SASI and Modified SASI is a potential limitation, lacking the granularity of a continuous variable. However, for disease staging, a three-point scale may be clinically easy and useful. Similarly, while TNM staging produces many combinations of tumor, nodal, and metastatic status, it is reduced to a limited ordinal scale (I–IV). While a more granular scale might be produced for SASI, it would likely require very large samples to stratify multiple combinations of the scoring elements.
The study has other limitations. Complete data were not available for all participants, creating possible reporting bias by analyzing only those with complete data. However, the analyzed sample appears similar to those not analyzed. Our AHI definitions were based on criteria at the time in our Sleep Center. Since these data were collected, our Center has adopted the definitions since recommended by the American Academy of Sleep Medicine27. It is possible that the newer definitions translate to a more robust AHI measure of disease burden. However, since the definitions did not change drastically and since SASI and Modified SASI also include AHI, it appears unlikely that this change will differentially improve AHI performance over SASI or Modified SASI. Our objective measures of disease burden are all surrogate measures of clinical outcome. It would be better to measure the clinical outcomes themselves (e.g. future incident cardiovascular disease and mortality); however, those long-term prospective measurements were beyond the scope of this cross-sectional study.
With regard to this study's generalizability, our study sample is similar to the general sleep apnea population; a random telephone survey matched to US-wide regional age distributions found that men were generally at higher risk than women, ages 50–64 were at highest risk, and risk rose markedly with BMI ≥ 3028. However, our predominantly white sample may not reflect sleep apnea prevalence patterns in other racial groups29,30.
Conclusion
Sleep apnea is a complex disease with important subjective and objective components. While patients seek care for subjective complaints, physicians and researchers often focus on objective measures, specifically AHI. While AHI has value in the diagnosis and evaluation of sleep apnea, this study suggests that composite indices may be useful adjuncts in baseline assessment, prognostication, and outcomes assessment of these patients by clinicians and researchers. Studies currently underway are examining the utility of such indices in predicting and assessing therapeutic effects and disease changes over time.
Acknowledgments
The research assistants of the Sleep Apnea Research Group and staff at the University of Washington Sleep Center at Harborview Medical Center were invaluable in data collection. This research was supported by an American Academy of Otolaryngology—Head & Neck Surgery Foundation Health Services Research Grant (KB), NIH F32 HL090226 (KB), NIH K23 HL068849 (EMW), and the Triological Research Career Development Award (EMW).
Footnotes
Presented at the American Academy of Otolaryngology – Head & Neck Surgery Annual Meeting, September 2007, Washington, DC
References
- 1.American Academy of Sleep Medicine Task Force. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. Sleep. 1999;22:667–689. [PubMed] [Google Scholar]
- 2.Redline S, Sanders M. Hypopnea, a floating metric: implications for prevalence, morbidity, estimates, and case finding. Sleep. 1997;20:1209–1217. doi: 10.1093/sleep/20.12.1209. [DOI] [PubMed] [Google Scholar]
- 3.Weaver EM, Kapur V, Yueh B. Polysomnography vs self-reported measures in patients with sleep apnea. Arch Otolaryngol Head Neck Surg. 2004;130:453–458. doi: 10.1001/archotol.130.4.453. [DOI] [PubMed] [Google Scholar]
- 4.Weaver EM, Woodson BT, Steward DL. Polysomnography indices are discordant with quality of life, symptoms, and reaction times in sleep apnea patients. Otolaryngol Head Neck Surg. 2005;132:255–262. doi: 10.1016/j.otohns.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Sundaram S, Bridgman SA, Lim J, Lasserson TJ. Surgery for obstructive sleep apnoea. Cochrane Database Syst Rev. 2005;4:CD001004. doi: 10.1002/14651858.CD001004.pub2. [DOI] [PubMed] [Google Scholar]
- 6.Kutner NG, Bliwise DL, Zhang R. Linking race and well-being within a biopsychosocial framework: variation in subjective sleep quality in two racially diverse older adult samples. J Health Soc Behav. 2004;45:99–113. doi: 10.1177/002214650404500107. [DOI] [PubMed] [Google Scholar]
- 7.Johns MW. Daytime sleepiness, snoring, and obstructive sleep apnea: the Epworth Sleepiness Scale. Chest. 1993;103:30–36. doi: 10.1378/chest.103.1.30. [DOI] [PubMed] [Google Scholar]
- 8.Piccirillo JF, Gates GA, White DL, Schectman KB. Obstructive sleep apnea treatment outcomes pilot study. Otolaryngol Head Neck Surg. 1998;118:833–844. doi: 10.1016/S0194-5998(98)70277-3. [DOI] [PubMed] [Google Scholar]
- 9.Brodsky L. Modern assessment of tonsils and adenoids. Pediatr Clin North Am. 1989;36:1551–1569. doi: 10.1016/s0031-3955(16)36806-7. [DOI] [PubMed] [Google Scholar]
- 10.Ng SK, Lee DLY, Li AM, Wing YK, Tong MCF. Reproducibility of clinical grading of tonsillar size. Arch Otolaryngol Head Neck Surg. 2010;136:159–162. doi: 10.1001/archoto.2009.170. [DOI] [PubMed] [Google Scholar]
- 11.Flemons WW, Reimer MA. Development of a disease-specific health-related quality of life questionnaire for sleep apnea. Am J Respir Crit Care Med. 1998;158:494–503. doi: 10.1164/ajrccm.158.2.9712036. [DOI] [PubMed] [Google Scholar]
- 12.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 13.Haukoos JS, Lewis RJ. Advanced statistics: bootstrapping confidence intervals for statistics with "difficult" distributions. Acad Emerg Med. 2005;12:360–365. doi: 10.1197/j.aem.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 14.Yang EH, Hla KM, McHorney CA, Havighurst T, Badr MS, Weber S. Sleep apnea and quality of life. Sleep. 2000;23:535–541. [PubMed] [Google Scholar]
- 15.Goncalves MA, Paiva T, Ramos E, Guilleminault C. Obstructive sleep apnea syndrome, sleepiness, and quality of life. Chest. 2004;125:2091–2096. doi: 10.1378/chest.125.6.2091. [DOI] [PubMed] [Google Scholar]
- 16.Lloberes P, Marti S, Sampol G, et al. Predictive factors of quality-of-life improvement and continuous positive airway pressure use in patients with sleep apnea-hypopnea syndrome: study at 1 year. Chest. 2004;126:1241–1247. doi: 10.1378/chest.126.4.1241. [DOI] [PubMed] [Google Scholar]
- 17.Kraiczi H, Peker Y, Caidahl K, Samuelssohn A, Hedner J. Blood pressure, cardiac structure and severity of obstructive sleep apnea in a sleep clinic population. J Hypertens. 2001;19:2071–2078. doi: 10.1097/00004872-200111000-00019. [DOI] [PubMed] [Google Scholar]
- 18.Peppard PE, Young T, Skatrud J. Prospective study of the association between sleep disordered breathing and hypertension. N Engl J Med. 2000;342:1378–84. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 19.Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA. 2000;283:1829–36. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 20.Can M, Acikgoz S, Mungan G, et al. Serum cardiovascular risk factors in obstructive sleep apnea. Chest. 2006;129:233–237. doi: 10.1378/chest.129.2.233. [DOI] [PubMed] [Google Scholar]
- 21.Peker Y, Hedner J, Norum J, Kraiczi H, Carlson J. Increased incidence of cardiovascular disease in middle-aged men with obstructive sleep apnea: a 7-year follow-up. Am J Respir Crit Care Med. 2002;166:159–165. doi: 10.1164/rccm.2105124. [DOI] [PubMed] [Google Scholar]
- 22.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long- term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–1053. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 23.Pai JK, Pischon T, Ma J, et al. Inflammatory markers and the risk of coronary heart disease in men and women. N Engl J Med. 2004;351:2599–2610. doi: 10.1056/NEJMoa040967. [DOI] [PubMed] [Google Scholar]
- 24.Kengne A-P, Czernichow S, Huxley R, et al. Blood pressure variables and cardiovascular risk: new findings from ADVANCE. Hypertension. 2009;54:399–404. doi: 10.1161/HYPERTENSIONAHA.109.133041. [DOI] [PubMed] [Google Scholar]
- 25.Chervin RD, Aldrich MS. The Epworth Sleepiness Scale may not reflect objective measures of sleepiness or sleep apnea. Neurology. 1999;52:125–131. doi: 10.1212/wnl.52.1.125. [DOI] [PubMed] [Google Scholar]
- 26.Friedman M, Tanyeri H, LaRosa M, et al. Clinical predictors of obstructive sleep apnea. Laryngoscope. 1999;102:1901–1907. doi: 10.1097/00005537-199912000-00002. [DOI] [PubMed] [Google Scholar]
- 27.Iber C, Ancoli-Israel S, Chesson AL, Quan SF. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 28.Hiestand DM, Britz P, Goldman M, Phillips B. Prevalence of symptoms and risk of sleep apnea in the the US population: results from the National Sleep Foundation Sleep in America 2005 poll. Chest. 2006;130:780–786. doi: 10.1378/chest.130.3.780. [DOI] [PubMed] [Google Scholar]
- 29.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–1239. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 30.Arias MA, Alonso-Fernandez A, Garcia-Rio F. Obstructive sleep apnea in minorities. Am J Med. 2007;120:e17. doi: 10.1016/j.amjmed.2006.01.026. [DOI] [PubMed] [Google Scholar]