Abstract
Objectives
1) Measure the association between the Functional Comorbidity Index (range 0–18) and physical function health status (SF-36 Physical Function domain), general physical health status (SF-36 Physical Component Score), and general mental health status (SF-36 Mental Component Score) outcome measures in a cohort of sleep apnea patients. 2) Test if the Functional Comorbidity Index is more strongly associated (a better predictor) than the well-known Charlson Comorbidity Index (range 0–37) with these SF-36 outcome measures.
Study Design
Cross-sectional study.
Setting
University of Washington Sleep Center.
Subjects and Methods
In a cohort of newly diagnosed obstructive sleep apnea patients (N = 233), we obtained scores for the Functional Comorbidity Index, Charlson Comorbidity Index, and SF-36. We calculated Spearman correlations and adjusted coefficients of determination (R2) with multiple linear regression, adjusted for demographic and health covariates. Bootstrapping generated R2 distributions for statistical comparison.
Results
Functional Comorbidity Index scores (mean±standard deviation 2.4±1.7) were more widely distributed than Charlson Comorbidity Index scores (0.7±1.4). The Functional Comorbidity Index was significantly correlated with SF-36 Physical Function (−0.53, p<0.001), Physical Component Score (−0.44, p<0.001), and Mental Component Score (−0.38, p<0.001). The Functional Comorbidity Index was a better predictor than the Charlson Comorbidity Index of SF-36 Physical Function (R2 mean±standard error 0.27±0.05 versus 0.17±0.05, p<0.001), Physical Component Score (0.23±0.05 versus 0.17±0.05, p<0.001), and Mental Component Score (0.23±0.05 versus 0.13±0.05, p<0.001).
Conclusion
The Functional Comorbidity Index is a more robust predictor of general health status than the Charlson Comorbidity Index in obstructive sleep apnea patients.
Keywords: Functional Comorbidity Index, Charlson Comorbidity Index, Comorbidity, Health Status, Physical Function
INTRODUCTION
Patients with a chronic disease often have other diseases (comorbidities) that may impact the outcome after treatment for the chronic disease of interest1. For example, survival (outcome) after surgical resection (treatment) of cancer (disease of interest) is likely to be reduced in patients who also have severe unstable angina (comorbidity), as compared to patients without unstable angina. In other words, patients who are generally sicker tend to have worse overall treatment outcomes, regardless of treatment effectiveness.
When testing the effect of a treatment on a disease outcome, it is important to adjust for these baseline differences in comorbidities, which can confound, or alter, the disease outcome. This adjustment helps researchers determine the direct effect of the treatment on an outcome, independent of the effect of the baseline comorbidities on that same outcome. To account for multiple baseline comorbidities, often an index of relevant comorbidities is used to adjust for baseline health.
A relevant comorbidity is one that might impact the outcome of interest. For example, there are several comorbidity indexes that are useful for studies on mortality outcomes2–4 that include life-threatening comorbid conditions. The Charlson Comorbidity Index (Charlson Index) is a well-validated and widely-used example of a mortality comorbidity index2.
There is a paucity of comorbidity indexes designed to adjust for baseline conditions that impact quality of life (QOL) outcomes5,6. For example, arthritis does not impact mortality so is not included in mortality comorbidity indexes, yet this comorbidity impacts QOL outcomes. There is a need for comorbidity indexes that are relevant to QOL outcomes, in order to test the effects of treatments on QOL, independent of the effects of baseline comorbidities on QOL.
To address the issue of baseline health impacting the outcome of physical functional status, Groll et al. developed the Functional Comorbidity Index (Functional Index)7 to measure the impact of multiple comorbidities relevant to that outcome. The Functional Index includes common comorbidities that influence daily life and function, regardless of mortality risk. This index might be appropriate to adjust for baseline differences in comorbidties when assessing functional outcomes in other chronic diseases, such as obstructive sleep apnea (sleep apnea).
Sleep apnea is a common8 systemic chronic illness of repetitive upper airway obstructions during sleep for which disease burden is frequently assessed through patient-reported functional status and QOL9. Many who suffer from sleep apnea have multiple chronic comorbid conditions that can impact functional status and QOL.
The long-range goal of our research is to build on the Functional Index to develop and validate a new QOL comorbidity index that will be broadly applicable for QOL studies. For this study, we take the incremental step to further validate the Functional Index by testing it in a new population (sleep apnea) and with additional outcome measures (QOL).
The objectives of this study are: 1) Measure the association between the Functional Index and the SF-36 health status outcomes instrument, specifically the Physical Function domain, Physical Component Score (PCS), and Mental Component Score (MCS) in a cohort of sleep apnea patients; and 2) Test the hypotheses that the Functional Index is more strongly associated (and thus a better predictor) than the well-known Charlson Index with SF-36 Physical Function, PCS, and MCS in this population. We include the Physical Function domain outcome because the Functional Index was developed to predict this outcome originally. We include the PCS and MCS to test the instrument’s predictive ability for a broader measure of health status.
METHODS
Study Design and Sample
This cross-sectional study used prospectively collected data from the parent Seattle Sleep Cohort study10,11 of patients seen in the University of Washington Sleep Center at Harborview Medical Center in Seattle, Washington, during the evening of their initial overnight diagnostic polysomnography (sleep apnea test) between 2004–2007. This study had institutional review board approval from the University of Washington Human Subjects Division (IRB #24113).
Patients were eligible if they were at least 18 years of age, never previously diagnosed with sleep apnea, found to have sleep apnea during their diagnostic polysomnography (defined by apnea-hypopnea index of five or more events per hour of sleep, scored by AASM-accredited standards), fluent in verbal and written English, completed the SF-36 and comorbidity forms, and had medical records available for further comorbidity data extraction. We chose a random sample of 250 patients from the 669 eligible parent study patients, but 17 were retroactively excluded for an apnea-hypopnea index less than five events per hour of sleep in the finalized polysomnography report.
Data Collection
Primary Exposure Variables
The Functional Index is an 18-item list of distinct clinical comorbidities that stratify physical functional status burden measured on the SF-36 (items listed in Table 1)7. It was developed from self-reported conditions present in a database of over 9,000 randomly sampled Canadian adults (oversampling women) and was validated in a database of over 28,000 US adults seeking treatment for spine diseases. The Functional Index has been applied in only a small number of clinical populations12–17, and has been tested for prediction of PCS (yes) and MCS (no) in one rare life threatening condition.12 It has not been tested in sleep apnea or other common chronic otolaryngology conditions. The comorbidities are weighted evenly and each condition is given one point if present. The final score is the sum of all conditions in a continuous variable from 0–18.
Table 1.
Functional Comorbidity Index Items
| Conditions | |
|---|---|
| 1 | Arthritis (rheumatoid and osteoarthritis) |
| 2 | Osteoporosis |
| 3 | Asthma |
| 4 | Chronic obstructive pulmonary disease, acquired respiratory distress syndrome, or emphysema |
| 5 | Angina |
| 6 | Congestive heart failure (or heart disease) |
| 7 | Heart attack (myocardial infarction) |
| 8 | Neurological disease (Parkinson’s or multiple sclerosis) |
| 9 | Stroke or Transient Ischemic Attack |
| 10 | Peripheral vascular disease |
| 11 | Diabetes, Type I or Type II |
| 12 | Upper gastrointestinal disease (ulcer, hernia, reflux) |
| 13 | Depression |
| 14 | Anxiety or panic disorders |
| 15 | Visual impairment (such as cataracts or glaucoma) |
| 16 | Hearing Impairment (very hard of hearing) |
| 17 | Degenerative disc disease (back disease, spinal stenosis, or severe chronic back pain) |
| 18 | Obesity or Body Mass Index >30 kg/m2 |
Each condition is scored on a binary scale with 1 point given for the presence of a condition and 0 for the absence. The sum of the individual condition scores yields the Functional Comorbidity Index score, range 0–187.
The comorbidity data collected for the Charlson Index includes 19 separate conditions, each assigned a severity weighting of one through six, summed to a final Charlson Index score2. The Charlson Index was treated as a continuous variable with range 0–37.
All participants completed a self-reported comorbidity questionnaire, providing information on the comorbidities in the Charlson Index and the severity of those comorbidities (e.g., diabetes with or without disease related complications), as well as for most of the comorbidities in the Functional Index. The remaining Functional Index comorbidities were retrospectively extracted from medical records for the five years prior to study enrollment.
Outcome Variables
The SF-36 is a widely validated patient–reported health status instrument commonly used to measure health status outcomes. The 36 items cover eight domains that create a profile of health status and QOL. Physical function is one domain that includes questions on activities of daily living such as bathing, walking, lifting, etc. The four domains relevant to physical health are grouped into the Physical Component Score (PCS), and the four other domains relevant to mental health make up the Mental Component Score (MCS)18,19–21. For the domains scores (here, Physical Function domain), the raw score is transformed into a 100 point scaled score in which a minimum score of “0” represents the worst physical function and a maximum score of “100” represents the best physical function18. Each component score is calculated from normalized aggregate scores, where 50 ± 10 represents the normalized score and standard deviation of the general US 1998 population norms19–21; a lower score indicates worse health status than the 1998 norms.
Covariates
Adjustment was performed for age (continuous), gender (male/female), race (Caucasian/White, African American/Black, Asian, Native American, Native Hawaiian or Pacific Islander, Other), ethnicity (Hispanic yes/no), and sleep apnea severity (continuous apnea-hypopnea index). The self-reported comorbidity questionnaire provided information for several of the covariates (age, gender, ethnicity, and race). The apnea-hypopnea index was collected from the finalized polysomnography report after patient enrollment. The Epworth sleepiness scale, a measure of daytime sleepiness, was filled out by subjects at the time of enrollment.
Analysis
Descriptive data were reported as mean ± standard deviation. Spearman correlations were calculated between each exposure variable (Functional Index and Charlson Index) and each outcome variable (SF-36 Physical Function, PCS, and MCS). A P-value <0.05 was considered a statistically significant correlation different from zero.
The square of the correlation is the coefficient of determination, R2, which is the proportion of variability in the outcome variable explained by the exposure variable22. A priori, we defined a R2 value of 0.10 to be clinically important because it means that 10% of the variance in the outcome variable is explained by the exposure variable. This R2 corresponds to a clinically important correlation of 0.32.
Each exposure variable (Functional Index and Charlson Index) was entered into a separate simple linear regression analyses with each SF-36 outcome variable. Subsequent multiple linear regression models included the covariates age, gender, race, ethnicity, and sleep apnea severity. The R2 value was used to compare the strength of association for each exposure variable. The beta coefficient values were not used because they were on different scales between the two Indexes.
Bootstrapping, a method of randomly re-sampling data, was performed to generate distributions of the adjusted R2 point estimates. Random samples of 233 were drawn with re-sampling over 1000 iterations, sufficient iterations to ensure precision of the standard error to 0.01. The resultant means and confidence intervals permitted statistical comparison of the R2 estimates using Student’s t-test. A R2 difference of 0.10 was considered clinically important, and a P-value <0.05 was considered statistically significant. All statistical analyses were performed with Stata 11 (College Station, TX).
RESULTS
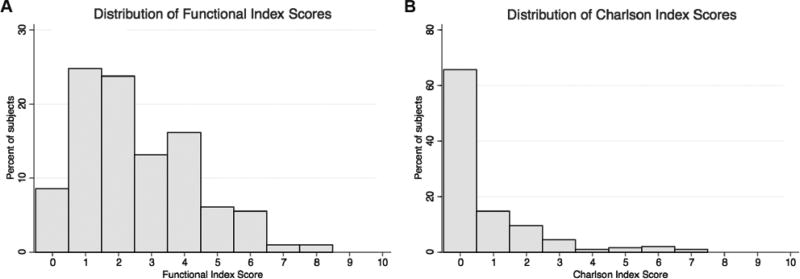
The study sample characteristics were consistent with sleep apnea and demographics in our catchment area (Table 2). The patients represent a heterogeneous sample of sleep apnea severity as measured by apnea-hypopnea index, lowest oxygen saturation, and sleepiness, with the full range of mild to severe represented on each measure (Table 2). On average, patients had severe sleep apnea by apnea-hypopnea index, mild by lowest oxygen saturation, and borderline normal by sleepiness. The Functional Index scores were more widely distributed than the Charlson Index. Most cohort patients had at least two comorbidities in the Functional Index compared to less than one comorbidity in the Charlson Index (Table 2, Figure 1A–B). There was a broad distribution of SF-36 Physical Function with mean 68 ± 30, median 80, and inter-quartile range (25th–75th percentile) 45 – 95. Mean PCS and MCS scores were below the 1998 general US population (Table 2).
Table 2.
Cohort Characteristics.
| Characteristic | Mean | Standard Deviation |
Range |
|---|---|---|---|
| Age (years) | 46 | 11 | 19–81 |
| Male (%) | 54 | ||
| Race: | |||
| White (%) | 79 | ||
| Black (%) | 10 | ||
| Asian (%) | 3 | ||
| Native American (%) | 4 | ||
| Pacific Islander (%) | 2 | ||
| Other (%) | 2 | ||
| Ethnicity (%Hispanic) | 4 | ||
| Body Mass Index (kg/m2) | 36 | 13 | 19–131 |
| Apnea Hypopnea Index (events/hour) | 56 | 34 | 9–185 |
| Lowest Oxygen Saturation (%) | 84 | 10 | 50–97 |
| Epworth Sleepiness Scale (0–24 scale, higher is worse) | 10 | 5 | 0–23 |
| Functional Index (0–18 scale, higher is worse) | 2.4 | 1.7 | 0–8 |
| Charlson Index (0–37 scale, higher is worse) | 0.7 | 1.4 | 0–7 |
| SF-36 Physical Function (0–100 scale, higher is better) | 68 | 30 | 15–57 |
| SF-36 Physical Component Score (50 normal, higher is better) | 42 | 12 | 15–57 |
| SF-36 Mental Component Score (50 normal, higher is better) | 42 | 9 | 10–68 |
Obstructive sleep apnea cohort characteristics.
Figure 1.
Histograms display the percent of the cohort subjects with each respective Index score. A: Functional Index histogram, mean = 2.4±1.7; range observed = 0–8 (potential range 0–18). B: Charlson Index histogram, mean = 0.7±1.4; range observed = 0–7 (potential range 0–37).
The bivariate analyses showed a clinically important and statistically significant correlation between each increasing Index (Functional and Charlson Indexes) score and each decreasing outcome (Physical Function, PCS, and MCS) score, except for the correlation between the Charlson Index and MCS (Tables 3–5). In other words, higher (worse) comorbidity is associated with lower (worse) physical function and general health status. Each correlation was stronger (larger absolute value) with the Functional Index than with the Charlson Index (Tables 3–5). Multivariate analysis confirmed the findings adjusted for the covariates (age, gender, race, ethnicity, and apnea-hypopnea index) (Tables 3–5). The differences in the bootstrapped mean adjusted R2 point estimates for the Functional Index were all clinically importantly and statistically significantly greater than for the Charlson Index (all p <0.001) (Tables 3–5).
Table 3.
Physical Function: Comparison of the Functional and Charlson Indexes.
| Functional Index | Charlson Index | |
|---|---|---|
| Spearman Correlation | −0.53* | −0.39* |
| Adjusted coefficient of determination (R2) | 0.27 | 0.17 |
| Bootstrapped distribution of the adjusted coefficient of determination (mean±standard error) | 0.27±0.05** | 0.17±0.05** |
Spearman correlation between each Index and the SF-36 Physical Function. Negative correlations indicate that as the number of comorbidities increases (higher Index score) the level of self-reported physical function decreases (lower SF-36 Physical Function score). Coefficients of determination generated by multiple linear regression, adjusted for age, gender, race, ethnicity, and apnea-hypopnea index.
Each correlation significantly different from zero, p<0.001.
Difference between Indexes statistically significant, p<0.001.
Table 5.
Mental Component Score: Comparison of the Functional and Charlson Indexes.
| MCS | Functional Index | Charlson Index |
|---|---|---|
| Spearman Correlation | −0.38* | −0.07 |
| Adjusted coefficient of determination (R2) | 0.23 | 0.13 |
| Bootstrapped distribution of the adjusted coefficient of determination (mean±standard error) | 0.23±0.05** | 0.13±0.05** |
Spearman correlation between each Index and the SF-36 Mental Component Score. Negative correlations indicate that as the number of comorbidities increases (higher Index score) the level of self-reported health status decreases (lower Mental Component Score). Coefficients of determination generated by multiple linear regression, adjusted for age, gender, race, ethnicity, and apnea-hypopnea index.
Each correlation significantly different from zero, p<0.001.
Difference between Indexes statistically significant, p<0.001.
DISCUSSION
Adjusting for baseline differences in comorbidities is of paramount importance in observational outcomes studies, whether measuring mortality outcomes or self-reported function and QOL. This study illustrates the importance of having a comorbidity index that can adjust for comorbidity confounding relevant to the outcome of interest. The relevant baseline health differences vary depending on the outcome one is trying to predict, as demonstrated by the different comorbidities contained in the Functional Index and Charlson Index. Though the Charlson Index is a widely validated and effective index at predicting one-year mortality, it is not as robust at predicting other non-survival outcomes as evidenced by the superiority of the Functional Index in predicting physical function and general QOL as measured by the SF-36.
We sought to validate and modify the Functional Index in a sleep apnea population. This population is distinct from the populations in which this index has previously been validated (acute respiratory distress syndrome, spine disease)7,12 Sleep apnea is a prevalent, systemic, chronic disease that has a strong impact on both QOL and survival. This population is also relevant because, due to the paucity of QOL comorbidity indexes, the Charlson Index or isolated comorbid conditions are most commonly used to adjust for baseline comorbidities in studies of sleep apnea patients. This study shows that the Charlson Index is a poor method of stratifying sleep apnea patients according to their baseline comorbidities when examining functional status or QOL outcomes, likely leaving significant residual confounding.
Our results for the SF-36 Physical Function and PCS in the sleep apnea population are similar to results in other clinical populations, which supports the generalizability (external validity) of the Functional Index to another disease. In their original validation of the Functional Index in a spine disorder population, Groll et al.7 reported the Spearman correlation of −0.53 between the Functional Index and the SF-36 Physical Function, identical to our results. Our study also corresponds with the results of a later longitudinal study by Groll et al.12, comparing the Functional Index to the Charlson Index as predictors of the SF-36 Physical Function in an acute respiratory distress syndrome survivor population. Likewise, the Functional Index was a significant predictor of PCS in their longitudinal study (R2 = 0.25 at 6 months), similar to our results (R2 = 0.23).
However, our results for the MCS in the sleep apnea population are different from other populations. In their longitudinal study of an acute respiratory distress syndrome survivor population12, the Functional Index was not a significant predictor of MCS over multiple longitudinal time points (data not given). In contrast, we found the Functional Index was a significant predictor of MCS in our sleep apnea cohort. This discrepancy might relate to the differences in MCS burden and changes with treatment between the populations23–29, and it supports the need to test the index across multiple diseases.
Despite the difference on MCS, the Functional Index generally behaves similarly in the very different populations of spine disease7, acute respiratory distress syndrome survivors12, and now sleep apnea demonstrated in this study. Though further research is needed to validate the Functional Index in other clinical and community-based populations, our results suggest the Functional Index may be generally useful as a method to adjust for baseline differences in health when studying outcomes that relate to self-reported general health status and QOL.
Our study has important limitations. The comorbidities in the Charlson Index were collected on the self-report forms and details confirmed by medical record extraction. However, the Functional Index was added retrospectively, requiring retrospective extraction of several items not collected by self-report (e.g., visual and hearing impairment, osteoporosis, some neurological diseases). These conditions are prone to under-reporting because they may not be documented as rigorously as conditions impacting mortality. This conservative bias would result in under-reported (lower) Functional Index scores compared to Charlson Index scores.
The study was performed in a sleep apnea population, which we feel is a good disease model in which to study future comorbidity indices given the multiple systemic, functional, and QOL effects of the disease. However, it is important to note that this study was performed in a clinic population and may not be generalizable to the whole community-based sleep apnea population. Clinic patients are more likely to have functional deficits that might have prompted clinical care. Nonetheless, the Functional Index will be valuable in studying health status and QOL in outcomes studies of the sleep apnea clinical population.
The Functional Index was originally developed specifically to predict physical function and might not include all the comorbidities relevant to predicting the SF-36 MCS and PCS in the sleep apnea population or in other populations. Conditions that are more common in populations with disordered sleep, such as migraine headaches, are not included in the Functional Index27,30. Thus, a future direction would be to modify the Functional Index to include important additional comorbidities and to validate this more robust index. Similar studies in other populations will enable development of a comorbidity instrument more generalizable to other populations and to related clinical outcomes.
CONCLUSION
The Functional Index is a valid tool to predict physical function, general physical health status (PCS), and general mental health status (MCS) in sleep apnea patients, and it performs better than the Charlson Index for this purpose.
Table 4.
Physical Component Score: Comparison of the Functional and Charlson Indexes.
| Functional Index | Charlson Index | |
|---|---|---|
| Spearman Correlation | −0.44* | −0.41* |
| Adjusted coefficient of determination (R2) | 0.23 | 0.17 |
| Bootstrapped distribution of the adjusted coefficient of determination (mean±standard error) | 0.23±0.05** | 0.17±0.05** |
Spearman correlation between each Index and the SF-36 Physical Component Score. Negative correlations indicate that as the number of comorbidities increases (higher Index score) the level of self-reported health status decreases (lower Physical Component Score). Coefficients of determination generated by multiple linear regression, adjusted for age, gender, race, ethnicity, and apnea-hypopnea index.
Each correlation significantly different from zero, p<0.001.
Difference between Indexes statistically significant, p<0.001.
Acknowledgments
This study was supported by NIH K23 HL68849 (Weaver), NIH T32 DC00018 (Weymuller), and a Triological Society Career Development Award (Weaver).
Footnotes
Poster Presentation pending at the Academy of Otolaryngology Meeting 2013 on 10/1/13
Contributor Information
Corinna G. Levine, Resident Department of Otolaryngology - Head and Neck Surgery, University of Washington, Seattle WA.
Edward M. Weaver, Chief of Sleep Surgery, Director of Outcomes Research, Department of Otolaryngology - Head and Neck Surgery, University of Washington, Seattle WA
References
- 1.Kaplan MH, Feinstein AR. The importance of classifying initial co-morbidity in evaluation the outcome of diabetes mellitus. J Chronic Dis. 1974 Sep;27(7–8):387–404. doi: 10.1016/0021-9681(74)90017-4. [DOI] [PubMed] [Google Scholar]
- 2.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 3.Walter LC, Brand RJ, Counsell SR, et al. Development and validation of a prognostic index for 1-year mortality in older adults after hospitalization. JAMA. 2001 Jun 20;285(23):2987–2994. doi: 10.1001/jama.285.23.2987. [DOI] [PubMed] [Google Scholar]
- 4.Piccirillo JF, Lacy PD, Basu A, Spitznagel EL. Development of a new head and neck cancer-specific comorbidity index. Arch Otolaryngol Head Neck Surg. 2002 Oct;128(10):1172–1179. doi: 10.1001/archotol.128.10.1172. [DOI] [PubMed] [Google Scholar]
- 5.Rijken M, van Kerkhof M, Dekker J, Schellevis FG. Comorbidity of chronic diseases: effects of disease pairs on physical and mental functioning. Qual Life Res. 2005 Feb;14(1):45–55. doi: 10.1007/s11136-004-0616-2. [DOI] [PubMed] [Google Scholar]
- 6.Gijsen R, Hoeymans N, Schellevis FG, Ruwaard D, Satariano WA, van den Bos GA. Causes and consequences of comorbidity: a review. J Clin Epidemiol. 2001 Jul;54(7):661–674. doi: 10.1016/s0895-4356(00)00363-2. [DOI] [PubMed] [Google Scholar]
- 7.Groll DL, To T, Bombardier C, Wright JG. The development of a comorbidity index with physical function as the outcome. J Clin Epidemiol. 2005 Jun;58(6):595–602. doi: 10.1016/j.jclinepi.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 8.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993 Apr 29;328(17):1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 9.Stucki A, Cieza A, Schuurmans MM, et al. Content comparison of health-related quality of life instruments for obstructive sleep apnea. Sleep Med. 2008 Jan;9(2):199–206. doi: 10.1016/j.sleep.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 10.Lam DJ, Jensen CC, Mueller BA, Starr JR, Cunningham ML, Weaver EM. Pediatric sleep apnea and craniofacial anomalies: a population-based case-control study. Laryngoscope. 2010 Oct;120(10):2098–2105. doi: 10.1002/lary.21093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Balakrishnan KJK, Weaver EM. Composite severity indices reflect sleep apnea disease burden more comprehensively than the apnea-hypopnea index. Otolaryngology—Head & Neck Surgery. 2013 Feb;148(2):324–330. doi: 10.1177/0194599812464468. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Groll DL, Heyland DK, Caeser M, Wright JG. Assessment of long-term physical function in acute respiratory distress syndrome (ARDS) patients: comparison of the Charlson Comorbidity Index and the Functional Comorbidity Index. Am J Phys Med Rehabil. 2006 Jul;85(7):574–581. doi: 10.1097/01.phm.0000223220.91914.61. [DOI] [PubMed] [Google Scholar]
- 13.Prince SA, Janssen I, Tranmer JE. Influences of body mass index and waist circumference on physical function in older persons with heart failure. Can J Cardiol. 2008 Dec;24(12):905–911. doi: 10.1016/s0828-282x(08)70697-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu-Ambrose T, Davis JC, Nagamatsu LS, Hsu CL, Katarynych LA, Khan KM. Changes in executive functions and self-efficacy are independently associated with improved usual gait speed in older women. BMC Geriatr. 2010;10:25. doi: 10.1186/1471-2318-10-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitchell SA, Leidy NK, Mooney KH, et al. Determinants of functional performance in long-term survivors of allogeneic hematopoietic stem cell transplantation with chronic graft-versus-host disease (cGVHD) Bone Marrow Transplant. 2010 Apr;45(4):762–769. doi: 10.1038/bmt.2009.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lahmek P, Berlin I, Michel L, Berghout C, Meunier N, Aubin HJ. Determinants of improvement in quality of life of alcohol-dependent patients during an inpatient withdrawal programme. Int J Med Sci. 2009;6(4):160–167. doi: 10.7150/ijms.6.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bjorgul K, Novicoff WM, Saleh KJ. Evaluating comorbidities in total hip and knee arthroplasty: available instruments. J Orthop Traumatol. 2010 Dec;11(4):203–209. doi: 10.1007/s10195-010-0115-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ware JE, Kosinski M, Keller SK. SF-36® Physical and Mental Health Summary Scales: A User's Manual. Boston, MA: The Health Institute; 1994. [Google Scholar]
- 19.Ware JE, Snow KK, Kosinski M, Gandek B. SF-36 Health Survey: manual & interpretation guide. Boston: New England Medical Ctr; 1993. [Google Scholar]
- 20.Ware JE, Gandek B. Overview of the SF-36 Health Survey and the International Quality of Life Assessment (IQOLA) Project. J Clin Epidemiol. 1998 Nov;51(11):903–912. doi: 10.1016/s0895-4356(98)00081-x. [DOI] [PubMed] [Google Scholar]
- 21.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992 Jun;30(6):473–483. [PubMed] [Google Scholar]
- 22.Beth Dawson, Trapp RG. Basic & Clinical Biostatistics. 4. McGraw-Hill Companies, Inc; 2004. [Google Scholar]
- 23.Herridge MS, Tansey CM, Matte A, et al. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011 Apr 7;364(14):1293–1304. doi: 10.1056/NEJMoa1011802. [DOI] [PubMed] [Google Scholar]
- 24.Wilcox ME, Herridge MS. Long-term outcomes in patients surviving acute respiratory distress syndrome. Semin Respir Crit Care Med. 2010 Feb;31(1):55–65. doi: 10.1055/s-0029-1246285. [DOI] [PubMed] [Google Scholar]
- 25.Ngai JC, Ko FW, Ng SS, To KW, Tong M, Hui DS. The long-term impact of severe acute respiratory syndrome on pulmonary function, exercise capacity and health status. Respirology. 2010 Apr;15(3):543–550. doi: 10.1111/j.1440-1843.2010.01720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heyland DK, Groll D, Caeser M. Survivors of acute respiratory distress syndrome: relationship between pulmonary dysfunction and long-term health-related quality of life. Crit Care Med. 2005 Jul;33(7):1549–1556. doi: 10.1097/01.ccm.0000168609.98847.50. [DOI] [PubMed] [Google Scholar]
- 27.Rains JC, Poceta JS. Headache and sleep disorders: review and clinical implications for headache management. Headache. 2006 Oct;46(9):1344–1363. doi: 10.1111/j.1526-4610.2006.00578.x. [DOI] [PubMed] [Google Scholar]
- 28.Jenkinson C, Stradling J, Petersen S. Comparison of three measures of quality of life outcome in the evaluation of continuous positive airways pressure therapy for sleep apnoea. J Sleep Res. 1997 Sep;6(3):199–204. doi: 10.1046/j.1365-2869.1997.00043.x. [DOI] [PubMed] [Google Scholar]
- 29.Smith IE, Shneerson JM. Is the SF 36 sensitive to sleep disruption? A study in subjects with sleep apnoea. J Sleep Res. 1995 Sep;4(3):183–188. doi: 10.1111/j.1365-2869.1995.tb00167.x. [DOI] [PubMed] [Google Scholar]
- 30.Rains JC, Poceta JS, Penzien DB. Sleep and headaches. Curr Neurol Neurosci Rep. 2008 Mar;8(2):167–175. doi: 10.1007/s11910-008-0027-9. [DOI] [PubMed] [Google Scholar]