Abstract
Objective
1) Define the minimal clinically important difference (MCID) of the Velopharyngeal Insufficiency (VPI) Effects on Life Outcomes (VELO) instrument, and 2) test for change in quality of life after VPI surgery.
Study Design
Prospective observational cohort.
Setting
VPI clinic at a tertiary pediatric medical center.
Subjects and Methods
Children with VPI and their parents completed the VELO instrument (higher score is better quality of life) at enrollment and then underwent VPI surgery (Furlow palatoplasty or sphincter pharyngoplasty, n=32), other treatments (obturator or oronasal fistula repair, n=7), or no treatment (n=18). They completed the VELO instrument again and an instrument of global rating of change in quality of life at one year. MCID was anchored to the global change instrument scores corresponding to “a little” or “somewhat” better.
Within-group (paired t-test) and between-group (Student’s t-test) changes in VELO were tested for the VPI surgery and no treatment groups. The association between treatment group and VELO change was tested with multivariate linear regression, adjusting for confounders.
Results
Follow up was obtained on 37/57 (65%) of patients. The mean (SD) VELO change scores corresponding to the MCID anchor was 15 (13). The VELO score improved significantly more in the VPI surgery group (change 22 [15]), p<0.001) than in the no-treatment group (change 9 [12], p=0.04), after adjusting for confounders (p=0.007 between groups).
Conclusion
VPI surgery using the Furlow palatoplasty or sphincter pharyngoplasty improves VPI specific quality-of-life, and the improvement is clinically important.
Keywords: Velopharyngeal insufficiency, velopharyngeal dysfunction, quality-of-life, minimal clinically important difference, minimal important change, responsiveness, sphincter pharyngoplasty, Furlow palatoplasty
Introduction
Velopharyngeal insufficiency (VPI) affects speech, swallowing and many psychosocial aspects of life. The VPI Effects on Life Outcomes (VELO) instrument, with youth and parent versions, is a quality of life (QOL) instrument that was developed to capture the effects of VPI on children’s lives.1 Previous studies have documented the VELO instrument’s validity, reliability and responsiveness,1–3 but the minimal clinically important difference (MCID)4 has not yet been defined for this instrument. MCID analysis defines the smallest change in QOL that reaches clinical importance and is an important step in instrument assessment.5
A primary goal for the VELO instrument is to enable rigorous measurement of the effects of VPI treatment on QOL. Previous studies of life effects of VPI treatment have shown improvement in a small sample with a functional status measure.6 Additionally in a previous short-term case series, we found significant improvements in short-term QOL measured by the VELO.2 While these studies have provided a foundation for future analyses, neither study utilized a control group. Data from a comparison group provides further evidence that measured changes in QOL are associated with the intervention rather than just temporal trends or natural course of the disorder, and it allows adjustment for confounders.7
Surgical treatment of VPI includes palatal and/or pharyngeal procedures. Furlow palatoplasty utilizes mucosal and myomucosal z-plasty to lengthen the palate and re-orient the levator veli palatini.8 Sphincter pharyngoplasty utilizes rotational myomucosal flaps based on the palatopharyngeus and superior constrictors to augment the posterior pharyngeal wall and laterally narrow the velopharynx. Pharyngeal flap utilizes a superiorly based myomucosal flap to obturate the central velopharynx. The pharyngeal flap is not used at this institution. Nonsurgical management typically involves use of an intraoral appliance (i.e., obturator) or conservative measures like speech therapy alone.
The primary aims of this study were to 1) define the VELO instrument MCID, and 2) test the effects of VPI surgery on QOL one year after treatment using the validated VELO instrument. Secondary aims included analyses of subgroups and on VELO instrument subscales.
Methods
Study Subjects
This prospective cohort study enrolled subjects with VPI from Seattle Children’s Hospital VPI Clinic as previously described.2 English-speaking children (ages 3–22 years) were enrolled from January 2010 to February 2012. Exclusion criteria included severe intellectual disability (n=3), VPI surgery within 6-months prior to enrollment (n=2), treatment with maxillo-mandibular advancement procedures (n=4) and incomplete follow up (i.e., not reaching 12 month time point) at the close of the study (n=23). The study was approved by the Institutional Review Board at Seattle Children’s Hospital and all parents provided written informed consent.
Subjects completed questionnaires at enrollment during a VPI clinic visit. Medical records were monitored to identify subjects’ treatment dates. Follow up questionnaires were sent by mail 12 months after treatment for those treated with surgery or an obturator. The mean time from enrollment to surgical or obturator treatment was 3 months for the first 45 subjects treated. Therefore, follow up questionnaires were sent by mail 15 months after enrollment for those not receiving surgery or obturator treatment, to match the outcome time point on the time from enrollment.
VPI Treatment Groups
After initial evaluation, surgical and nonsurgical treatment options were discussed, and patients proceeded in one of three treatment groups: 1) VPI Surgery, 2) Other Treatment, or 3) No Treatment. The VPI Surgery group included patients receiving either Furlow palatoplasty or sphincter pharyngoplasty (n=32). Details about our institution’s diagnosis and treatment protocols have been described.9 The Other Treatment group included patients treated with an obturator or oronasal fistula repair (n=7). Subjects who elected prosthetic treatment were referred to the dental service for obturator fitting.
Obturators were adjusted until the speech and language pathologist felt the VPI was adequately treated. Some subjects had no functional palatal surgery, but rather only oronasal fistula repair and were also grouped in the Other Treatment group. The No Treatment group included patients (and families) who deferred these specific VPI treatments for a variety of reasons such as perceived mild speech dysfunction or social reasons (n=18). All subjects with velopharyngeal mislearning were eligible for speech therapy, regardless of specific VPI treatment.
Patient-Reported Outcomes
The VELO instrument is a VPI-specific QOL measure that was developed using focus groups which provide face validity.1 It includes a 26-item parent version (VELO-P) and a 23-item youth version (VELO-Y) for children 8 years and older. The total score (VELO-P Total or VELO-Y Total) ranges from 0 to 100 with 100 representing the highest QOL. Subscales are scored similarly and include Speech, Swallow, Situational Difficulty, Perception, and Emotional for both the VELO-P and VELO-Y, and also includes Caregiver Impact for the VELO-P. The instrument was previously tested for reliability, validity and responsiveness to change in QOL 3 months after treatment.1–3
Parents were asked if there had been interval change in their child’s QOL with a Global Ratings of Change instrument. On this instrument, parents reported how much better or worse their child's QOL was on a 13-point Likert-type scale ranging from “A great deal better” (+6) to “A great deal worse” (−6). A score of 0 indicated the QOL was “about the same” over the interval. A similar instrument has been previously used to determine MCID in other QOL instruments.4,10,11 Parents also reported specific Speech Ratings of Change, Swallowing Ratings of Change, and Situational & Social Interactions Ratings of Change measures analogous to the Global Ratings of Change.
Data Analysis
MCID Analysis
The primary MCID analysis used the Global Ratings of Change as an anchor for the MCID of the VELO-P Total score. The Global Ratings of Change measure was used to categorize subjects according to the degrees of clinical change in QOL at follow up. Subjects with Global Ratings of Change scores of −1, 0 and +1 were considered to have no clinically important change in QOL. Subjects with Global Ratings of Change scores of −3, −2, +2, and +3 were considered to have a minimal clinically important change in QOL, and these scores were used to anchor our definition of MCID.11 The mean magnitude change in the VELO-P Total scores of these subjects, regardless of treatment group, were defined as the MCID of the VELO instrument. We used the VELO-P Total for the primary assessment of MCID because parents completed the instrument for all children regardless of age (n=32, missing data n=5) while the VELO-Y was completed only for a subset of subjects with age 8 years and older (n=8). The association between change in VELO-P Total score and Global Ratings of Change was tested with the test for trend and modeled with multivariate linear regression.
Secondary MCID analyses were performed in the subgroup of youth scores (VELO-Y Total) and on the subscales of the VELO-P using the specific ratings of change instruments for speech, swallowing, and situational and social interactions. For example the MCID for the VELO-P Speech Limitations subscale was calculated with anchors (−3, −2, +2, +3) from the Speech Ratings of Change instrument.
As a tertiary MCID analysis, we calculated MCID using a statistical distribution method12 as 0.2 – 0.5 times the baseline VELO-P Total standard deviation. In other words, using this distribution method, we defined MCID as an effect size of 0.2, (small but meaningful effect) to an effect size of 0.5 (moderate effect).13
Change in VELO with VPI Surgery
VELO-P Total was tested for change from baseline to 12 month follow up with paired t-test for the VPI Surgery group and for the No Treatment group. The change in VELO-P Total was calculated for each subject as the difference between baseline and follow up where a positive change score denotes improvement. The difference in VELO-P Total change scores was tested between the VPI Surgery group and the No Treatment group with Student’s t-test. Secondary analyses were similarly conducted for each of the subscales of the VELO-P. We do not present analyses of change in VELO-Y with VPI Surgery because the sample of youth-completed questionnaires in that group are small.
The association between VPI surgery status (VPI Surgery versus No Treatment) and change in VELO-P Total was tested using multivariate linear regression adjusting for a priori hypothesized confounders. Potential confounders include age, VPI severity, compensatory misarticulations, size of velopharyngeal gap on nasendoscopy, and subjects’ need for special education. Confounders were included in the final multivariate model if their inclusion appreciably altered (≥10%) the association of VPI surgery status and change in VELO-P Total score, a validated method of adjusting for confounders in explanatory regression models.14
All statistical analyses were performed with Stata 12 (College Station, TX). For primary analyses, p-values < 0.05 were considered statistically significant. For secondary analyses, the Bonferroni p-value correction was applied.
Results
Eighty nine patients were potentially eligible for participation. Thirty two patients were excluded based upon the criteria noted in the methods section. Enrollment included 57 patients (mean age 6.8 [4.0] years) and their parents. All parents completed the VELO-P (n=57), and patients older than 8 years also completed the VELO-Y (n=15). The demographic and clinical characteristics of each treatment group were similar (Table 1) except for VPI severity (p<0.05). A large proportion of subjects in each group had a history of cleft palate with or without cleft lip. Follow up was obtained for 22/32 (69%) of the VPI Surgery group, 4/7 (57%) of the Other Treatment group, 11/18 (61%) of the No Treatment group, and 8/15 (53%) patients older than 8 years.
Table 1.
Characteristics of Cohort
| Parameter | VPI Surgery n=32 |
Other Treatmenta n=7 |
No Treatment n=18 |
|---|---|---|---|
| Child's Age; years | 6.3 (3.7) | 6.7 (4.6) | 7.6 (4.3) |
| Child's Sex; n (%) Female | 19, 58% | 4, 57% | 9, 50% |
| Hispanic Ethnicity | 4, 13% | 0, 0% | 2, 11% |
| Race | |||
| Caucasian | 24, 75% | 7, 100% | 11, 65% |
| Asian | 6, 19% | 0, 0% | 3, 18% |
| American Indian | 1, 3% | 0, 0% | 0, 0% |
| African American | 0, 0% | 0, 0% | 1, 6% |
| Other | 1, 3% | 0, 0% | 2, 12% |
| Other characteristics | |||
| Cleft Lip & Palate | 13, 39% | 1, 17% | 7, 39% |
| Cleft Palate Alone | 7, 21% | 2, 33% | 2, 11% |
| No Cleft | 13, 39% | 3, 50% | 9, 50% |
| Child with Syndrome | 13, 39% | 4, 67% | 5, 29% |
| In Special Education | 19, 66% | 4, 57% | 6, 40% |
| VPI Severity | |||
| None | 0, 0%b, c | 0, 0%c | 0, 0%c |
| Minimal | 1, 3%b | 2, 29% | 6, 33% |
| Mild | 11, 35%b | 3, 43% | 5, 28% |
| Moderate | 12, 35%b | 0, 0% | 4, 22% |
| Severe | 8, 26%b | 2, 29% | 3, 17% |
| Misarticulation | |||
| None | 16, 48% | 4, 57% | 12, 67% |
| Minimal | 6, 18% | 0, 0% | 2, 11% |
| Mild | 7, 21% | 0, 0% | 1, 6% |
| Moderate | 2, 6% | 2, 29% | 2, 11% |
| Severe | 2, 6% | 1, 14% | 1, 6% |
| VELO-P Total | 56 (14) | 45 (22) | 54 (15) |
| VELO-Y Total | 69 (14) | 26 (0)b | 57 (11) |
| Follow up | 22, 68% | 4, 57% | 11, 61% |
Velopharyngeal Insufficiency (VPI); VPI Effects on Life Outcomes-Parent(VELO-P) and Youth (VELO-Y).
Data presented as mean (SD) and n, %
Includes Obturation (n=5) and Fistula repair (n=2),
t-test versus No Treatment p-value less than 0.05, remainder of p-values greater than 0.05; VPI severity and misarticulation tested with Wilcoxon rank sum-test.
Inclusion in the cohort required presence of at least minimal VPI.
MCID and the Relationship Between the VELO and the Global Ratings of Change
Subjects from all treatment groups were included in the MCID analysis. The distribution of the VELO-P and VELO-Y scores across the Global Ratings of Change scores is shown in Table 2. The mean Global Ratings of Change score was 2.6 (2.6) and mean change in VELO-P Total score was 16 (15). Eleven parents scored the Global Ratings of Change as −1, 0, or +1 corresponding to no change in QOL, and their mean change in VELO-P Total score was 11 (14). Six parents scored the Global Ratings of Change as −3 (n=0), −2 (n=0), +2 (n=2), or +3 (n=4) corresponding to the anchor for MCID, and their mean magnitude change in VELO-P Total score was 15 (13).
Table 2.
Global Rating of Change Instrument and Change in VELO at One Year for all treatment categories
| Change in VELO-P | Change in VELO-Y | ||||
|---|---|---|---|---|---|
| Global Rating of Change | Score | Mean (SD) | n | Mean (SD) | n |
| A great deal worse | −6 | - | - | - | - |
| A good deal worse | −5 | - | - | - | - |
| Moderately worse | −4 | 11 (−) | 1 | 10 (−) | 1 |
| Somewhat worse | −3 | - | - | - | - |
| A little worse | −2 | - | - | - | - |
| Hardly worse at all | −1 | - | - | - | - |
| About the same | 0 | 11 (14) | 10 | 15 (22) | 3 |
| Hardly better at all | 1 | 15 (−) | 1 | 10 (−) | 1 |
| A little better | 2 | 12 (16) | 2 | - | - |
| Somewhat better | 3 | 17 (14) | 4 | 11 (15) | 2 |
| Moderately better | 4 | 17 (8) | 3 | 21 (−) | 1 |
| A good deal better | 5 | 22 (15) | 8 | - | - |
| A great deal better | 6 | 43 (5) | 3 | - | - |
| p-valuea | 0.02 | 0.54 | |||
Velopharyngeal Insufficiency Effects on Life Outcomes – Parent (VELO-P) and Youth (VELO-Y).
Test for trend.
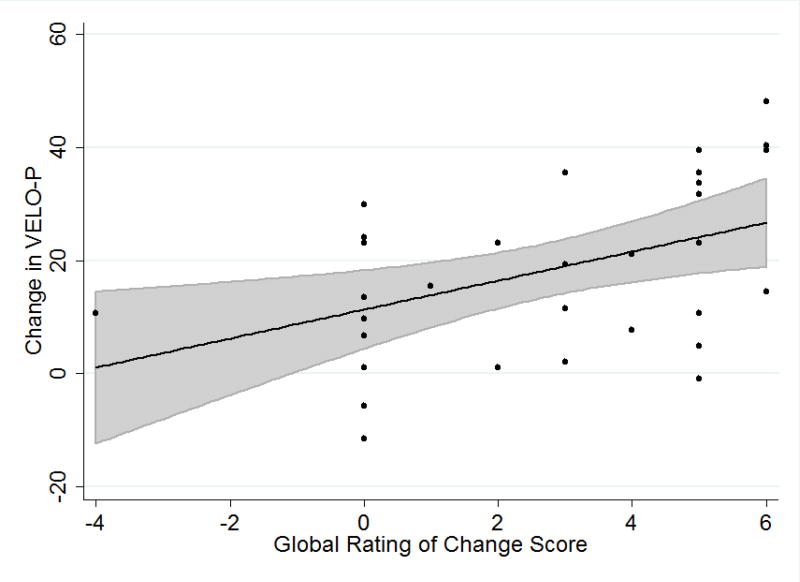
Higher Global Ratings of Change scores were associated with larger improvements in mean VELO-P Total score (p=0.02). Linear regression of change in VELO-P Total with Global Ratings of Change as the independent variable showed each increment in Global Ratings of Change was associated with a 3-point improvement (95% CI 1 – 5, p=0.006) in VELO-P Total (Figure 1). The mean (95% CI) change in VELO-P Total for the MCID group based on the linear regression was 21 (14– 28).
Figure 1.
Change in VELO-P total versus Global Ratings of Change. Data points shown in black, black line identifies the mean linear regression estimate with 95% CI for the regression estimate shown in grey. Global Ratings of Change score (Table 2) with higher value representing more improvement
For the patients at least 8 years old, the mean parental scoring of the Global Ratings of Change score was 0.9 (2.5) and mean change in youth scoring of the VELO-Y Total score was 12 (13). In this subgroup, four parents scored the Global Ratings of Change as −1, 0, or +1 corresponding to no change in QOL, and the youth mean change in VELO-Y Total score was 14 (18). Two parents scored the Global Ratings of Change as −3, −2, +2, or +3 corresponding to the anchor for MCID, and the youth mean magnitude change in VELO-Y Total score was 11 (15). Secondary VELO-P subscale MCID analyses are shown in Table 3. By the statistical distribution method, the VELO-P Total MCID was 3 – 7.5.
Table 3.
Distribution of Change in VELO-P Subscales and Corresponding Ratings of Change Scales for all treatment categories
| VELO-P Speech Change |
VELO-P Swallow Change |
VELO-P Situational Change |
||||
|---|---|---|---|---|---|---|
| Subscale Categoriesa | Mean (SD) | n | Mean (SD) | n | Mean (SD) | n |
| A great deal worse | - | - | - | - | - | - |
| A good deal worse | - | - | - | - | 15 (−) | 1 |
| Moderately worse | - | - | - | - | - | - |
| Somewhat worse | 7 (−) | 1 | −8 (−) | 1 | - | - |
| A little worse | - | - | - | - | - | - |
| Hardly worse at all | 25 (−) | 1 | - | - | - | - |
| About the same | 7 (18) | 9 | 2 (12) | 21 | 19 (18) | 17 |
| Hardly better at all | - | - | - | - | 43 (18) | 2 |
| A little better | 4 (−) | 1 | 4 | 1 | 25 (−) | 1 |
| Somewhat better | 20 (10) | 5 | - | - | 13 (4) | 2 |
| Moderately better | 27 (8) | 2 | 25 (−) | 1 | 18 (4) | 2 |
| A good deal better | 25 (17) | 8 | 6 (8) | 4 | 30 (39) | 3 |
| A great deal better | 52 (19) | 5 | 31 (24) | 4 | 60 (17) | 3 |
| p-value | 0.001 | 0.01 | 0.007 | |||
Velopharyngeal Insufficiency Effects on Life Outcomes-Parent (VELO-P).
Categories of Speech, Swallowing and Social & Interactions Ratings of Change in QOL scales for each respective analysis
Test for trend
Change in VELO with VPI Surgery
Subjects in the VPI Surgery group reported improvement in mean (SD) VELO-P Total from 55 (13) to 76 (15) (p<0.0001) with improvement in all subscales except Perception by Others (Bonferroni adjusted p<0.05, Table 4). VELO-P Total scores for the No Treatment group also improved from mean (SD) score of 49 (13) at baseline to 58 (20) (p=0.04). Improvements in most subscale in the No Treatment group were not statistically significant, (Bonferroni adjusted p>0.05, Table 4).
Table 4.
Change in VELO-P at Baseline and One-Year
| Baseline mean (SD) |
12 month mean (SD) |
p-valuea | |
|---|---|---|---|
| VPI Surgery | |||
| VELO-P Total (n=22) | 55 (13) | 76 (15) | <0.0001 |
| Speech Limitation | 41 (18) | 70 (18) | <0.001 |
| Swallowing | 86 (17) | 93 (14) | 0.05b |
| Situational Difficulty | 31 (19) | 63 (22) | <0.001 |
| Emotional Impact | 69 (17) | 83 (13) | 0.005 |
| Perception by Others | 75 (16) | 86 (2.9) | 0.10 |
| Caregiver Impact | 50 (19) | 79 (20) | <0.001 |
| No Treatment | |||
| VELO-P Total (n=11) | 49.3 (13.3) | 58.0 (19.8) | 0.04 |
| Speech Limitation | 35 (20) | 42 (23) | 0.42 |
| Swallowing | 92 (15) | 92 (15) | 0.99b |
| Situational Difficulty | 29 (18) | 46 (26) | 0.04 |
| Emotional Impact | 56 (23) | 60 (24) | 0.99 |
| Perception by Others | 64 (27) | 68 (26) | 0.99 |
| Caregiver Impact | 45 (24) | 63 (24) | 0.06 |
Velopharyngeal Insufficiency Effects on Life Outcomes-Parent (VELO-P).
paired t-test with Bonferroni adjustment for subscale analyses.
Mann-Whitney U-test for non-parametric data.
VELO-P Total improved more in the VPI Surgery group than in the No Treatment group (Table 5), and this relationship persisted after adjusting for confounders (p=0.007). VELO-P subscales all improved more in the VPI Surgery group than in the No Treatment group, but these differences were not statistically significant in this sample (Table 5).
Table 5.
Change in VELO-P in VPI Surgery and No Treatment Groups
| VPI Surgery Change in VELO mean (95% CI) |
No Treatment Change in VELO mean (95% CI) |
p-valuea | |
|---|---|---|---|
| VELO-P Total | 21.9 (15.1 – 28.6) | 8.6 (0.6 –16.7) | 0.02 |
| Speech Limitation | 28.8 (19.4 – 38.1) | 7.8 (−0.8 – 16.3) | 0.03 |
| Swallowing | 7.0 (−0.4 – 14.4) | 0.0 (−8.3 – 8.3) | 0.99 |
| Situational Difficulty | 31.8 (21.5 – 42.1) | 17.3 (5.9 – 28.7) | 0.45 |
| Emotional Impact | 14.6 (6.8 – 22.3) | 3.4 (−18.4 – 25.2) | 0.99 |
| Perception by Others | 10.5 (2.1 – 18.9) | 4.5 (−6.1 – 15.2) | 0.99 |
| Caregiver Impact | 29.1 (20.4 – 37.9) | 17.4 (5.3 – 29.5) | 0.65 |
Velopharyngeal Insufficiency Effects on Life Outcomes-Parent (VELO-P).
t-test with unequal variance and Bonferroni adjustment for subscale analyses
Discussion
One goal of measuring QOL is to understand how treatments affect patients. Patient centered assessments, such as QOL instruments, are potentially powerful tools for providers and researchers. Understanding how instruments function and how to interpret results is essential. While statistical tests allow for confirming or refuting hypotheses, they do not provide clinical context for the relevance of changes in QOL scores. Determining the MCID helps identify changes in VELO-P Total score that are clinically important rather than just statistically significant. This study builds on the previously conducted validation, reliability and responsiveness testing1–3 to determine the MCID for the VELO instrument and rigorously test for change in QOL with VPI surgery.
The MCID analysis is limited by the small sample size in the MCID group. The inclusion of subjects with less definitive treatments (i.e., no surgery) or no treatment provided more patients with minimal improvements to supplement the sample for MCID determination. The MCID value determined by the anchor method and the linear regression were in good agreement. The difference between this value and that defined in the VELO-Y analysis is likely related to the sample sizes. Because of the larger sample size of the parent group and possibly increased variability among self report in younger subjects, it may be wise to place more emphasis on the VELO-P MCID results. The distribution method resulted in a smaller MCID value but is of less clear clinical importance. The correlation of change in VELO and Global Ratings of Change measure provides further construct validity for the VELO instrument by showing it measures what we intend it to measure. Based on the analyses, the MCID for the VELO-P is likely between 15 and 21. Until further work is done to evaluate the MCID of the VELO-P in a larger sample, it will be considered approximately 15 as that was the primary a priori identified endpoint for the study.
VELO subscale MCID analysis is also limited by the small sample size. The MCID values are supported by the association with subscale transition measures (p<0.01, Table 3). The VELO swallowing subscale was skewed limiting the potential for improvement. Further studies with larger samples are needed to understand change in VELO subscales.
While previous studies have identified a change in QOL or functional status with surgery,2,6 the favorable change in VELO score in the No Treatment group from baseline to 12 months highlights the importance of a control group. Improvement in the No Treatment group was driven by the VELO Situational Difficulty and Caregiver Impact subscales. Having a diagnosis may improve some of the psychosocial aspects of QOL and may be reflected in the improvements in these subscales. Some of subjects in the No Treatment group received speech therapy, as indicated, that may have improved their speech related QOL (albeit not statistically significantly).
Subjects who had VPI surgery had improvement of the VELO-P Total and all subscale scores, most statistically significant even after applying a correction for multiple testing (paired t-test p<0.05). The change in VELO-P Total was greater for the VPI Surgery group than the No Treatment group (p=0.02) and remained so after adjusting for confounders (p<0.01). The measured change in VELO-P Total was above the MCID defined in this study. The MCID and associated analyses provides context for interpreting this change in VELO-P Total. The mean change in VELO-P Total after adjusting for confounders (20.3) is near the mean of those in the "a good deal better" category of the Global Ratings of Change measure (22).
This observational study has important limitations. It is possible that the subjects undergoing surgery had more severe VPI and greater room for improvement in VPI specific QOL; however, the baseline VELO-P Total scores were comparable between the VPI Surgery and No Treatment groups (Table 1) and we adjusted for baseline VPI severity when comparing the groups with multiple linear regression. The risk of unmeasured confounders remains present as with any observational study, but we were deliberate in our inclusion of potential confounders. The improvement in the VPI Surgery group compared to the No Treatment group remained large and statistically significant after adjusting for confounders. Only a randomized trial can remove entirely the concern of confounding. The follow up rate of 65% may have introduced bias if those lost to follow up were systematically different from those followed. However, the baseline characteristics between those followed and those lost to follow up were not significantly different (data not shown). While it is possible that patients with worse outcomes were more likely not to complete the follow up questionnaires, we did not observe a pattern of worse outcomes in the clinical follow up these patients. Additionally, we mailed our 12 month follow up questionnaires to help minimize this bias.
The present study sought to test the change in self reported VPI QOL with surgery with the VELO-Y but the small sample size in the VPI Surgery group (n=3) and the No Treatment group (n=5) limited the power and interpretation. Self report with VELO-Y is important and should be measured when possible. In addition to providing the patient perspective, it allows for an assessment of differences between parent- and youth-reported VPI QOL. These differences might impact treatment decisions, for example when a child reports impaired swallowing related QOL about which the parent was otherwise unaware. The full VELO-Y instrument is 23 questions and may be too burdensome to younger patients. Future studies developing a short form instrument appropriate for youth under 8 would be of benefit.
The two surgical procedures included in the VPI Surgery group (Furlow palatoplasty and sphincter pharyngoplasty) may affect VPI related QOL differently. The cohort was not powered to detect a difference between the surgical techniques. Future studies are needed to test for difference between surgical techniques for treating VPI.
Conclusions
The VELO instrument provides a VPI specific QOL instrument that was developed to capture the way VPI impacts children's lives. It has previously been tested for validity, reliability, and responsiveness, and now it's MCID is defined. Furlow palatoplasty and sphincter pharyngoplasty both improve VPI specific QOL at a clinically important level when compared to no treatment (or speech therapy alone). The VELO instrument provides a rigorously evaluated QOL instrument for future investigations of VPI treatments with a focus on patient-centered outcomes.
Acknowledgments
This study was supported by Clinical and Translational Science Awards Grant Number 1 UL1 RR025014 from the National Center for Research Resources (NCRR), a component of the NIH and by the Resident Research Award from the American Academy of Otolaryngology – Head & Neck Surgery Foundation (AAO-HNSF). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR, NIH or AAO-HNSF.
Footnotes
This work was presented, in part, at the 2013 American Society of Pediatric Otolaryngology Spring Meeting; April 28, 2013; Arlington, VA.
References
- 1.Skirko JR, Weaver EM, Perkins J, Kinter S, Sie KC. Modification and evaluation of a Velopharyngeal Insufficiency Quality-of-Life instrument. Arch Otolaryngol Head Neck Surg. 2012 Oct;138(10):929–935. doi: 10.1001/2013.jamaoto.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Skirko JR, Weaver EM, Perkins JA, Kinter S, Eblen L, Sie KC. Validity and responsiveness of VELO: a velopharyngeal insufficiency quality of life measure. Otolaryngol Head Neck Surg. 2013 Aug;149(2):304–311. doi: 10.1177/0194599813486081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barr L, Thibeault SL, Muntz H, de Serres L. Quality of life in children with velopharyngeal insufficiency. Arch Otolaryngol Head Neck Surg. 2007 Mar;133(3):224–229. doi: 10.1001/archotol.133.3.224. [DOI] [PubMed] [Google Scholar]
- 4.Jaeschke R, Singer J, Guyatt GH. Measurement of health status. Ascertaining the minimal clinically important difference. Control Clin Trials. 1989 Dec;10(4):407–415. doi: 10.1016/0197-2456(89)90005-6. [DOI] [PubMed] [Google Scholar]
- 5.Terwee CB, Bot SD, de Boer MR, et al. Quality criteria were proposed for measurement properties of health status questionnaires. J Clin Epidemiol. 2007 Jan;60(1):34–42. doi: 10.1016/j.jclinepi.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 6.Boseley ME, Hartnick CJ. Assessing the outcome of surgery to correct velopharyngeal insufficiency with the pediatric voice outcomes survey. Int J Pediatr Otorhinolaryngol. 2004 Nov;68(11):1429–1433. doi: 10.1016/j.ijporl.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 7.Howick JCI, Glasziou P, Greenhalgh T, Henneghan C, Liberati A, Moschetti I, Phillips B, Thornton H, Goddard O, Hodgkinson M. [Accessed 2/18/2013];Oxford Centre for Evidence-based Medicine - Levels of Evidence (March 2009) 2011 http://www.cebm.net/index.aspx?o=1025. 2013.
- 8.Furlow LT., Jr Cleft palate repair by double opposing Z-plasty. Plast Reconstr Surg. 1986 Dec;78(6):724–738. doi: 10.1097/00006534-198678060-00002. [DOI] [PubMed] [Google Scholar]
- 9.Sie KC, Chen EY. Management of velopharyngeal insufficiency: development of a protocol and modifications of sphincter pharyngoplasty. Facial Plast Surg. 2007 May;23(2):128–139. doi: 10.1055/s-2007-979282. [DOI] [PubMed] [Google Scholar]
- 10.Flemons WW, Reimer MA. Development of a disease-specific health-related quality of life questionnaire for sleep apnea. Am J Respir Crit Care Med. 1998 Aug;158(2):494–503. doi: 10.1164/ajrccm.158.2.9712036. [DOI] [PubMed] [Google Scholar]
- 11.Juniper EF, Guyatt GH, Willan A, Griffith LE. Determining a minimal important change in a disease-specific Quality of Life Questionnaire. J Clin Epidemiol. 1994 Jan;47(1):81–87. doi: 10.1016/0895-4356(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 12.Guyatt GH, Osoba D, Wu AW, Wyrwich KW, Norman GR. Methods to explain the clinical significance of health status measures. Mayo Clin Proc. 2002 Apr;77(4):371–383. doi: 10.4065/77.4.371. [DOI] [PubMed] [Google Scholar]
- 13.Kazis LE, Anderson JJ, Meenan RF. Effect sizes for interpreting changes in health status. Med Care. 1989 Mar;27(3 Suppl):S178–189. doi: 10.1097/00005650-198903001-00015. [DOI] [PubMed] [Google Scholar]
- 14.Maldonado G, Greenland S. Simulation study of confounder-selection strategies. Am J Epidemiol. 1993 Dec 1;138(11):923–936. doi: 10.1093/oxfordjournals.aje.a116813. [DOI] [PubMed] [Google Scholar]