Abstract
Background
Mixed adenoneuroendocrine carcinoma (MANEC) is defined as a neoplasm composed of both exocrine and endocrine carcinomas, each comprising at least 30% of the tumor. MANEC can occur in various organs of the gastrointestinal tract, including the esophagus, stomach, and colon. We herein provide the first case report of surgically resected MANEC arising in Barrett’s esophagus (BE).
Case presentation
A 70-year-old man presenting with abdominal pain was referred to our hospital. Upper endoscopy showed a type 0-IIa + IIc elevated lesion adjacent to BE. According to a biopsy specimen, the elevated lesion was diagnosed as adenocarcinoma with neuroendocrine differentiation. No lymphatic or distant metastasis was detected in the preoperative examination. Laparoscopic distal esophagectomy and proximal gastrectomy were performed, and a diagnosis of MANEC in BE was determined according to the surgically resected specimen.
Conclusions
A very rare case of MANEC in BE was detected. BE can be the origin of esophageal MANEC as well as adenocarcinoma. Due to the small number of esophageal or esophagogastric MANEC cases reported, further accumulation of such cases is necessary to recommend an optimal management strategy for esophageal or esophagogastric MANEC.
Keywords: Mixed adenoneuroendocrine carcinoma (MANEC), Barrett’s esophagus, Neuroendocrine tumor
Background
Since Oberndorfer first reported a case of “karzinoide” in 1907, carcinoid tumors or neuroendocrine tumors (NETs) have been debated, and the treatment strategy for these tumors has been a contentious subject [1]. In the 2010 World Health Organization (WHO) classification of neuroendocrine neoplasms in the digestive system, mixed adenoneuroendocrine carcinoma (MANEC) is defined as a neoplasm composed of both exocrine and endocrine carcinomas, each comprising at least 30% of the tumor [2]. MANECs in the gastrointestinal tract can occur in various organs such as the esophagus [3], stomach [4, 5], and colon [6].
Barrett’s esophagus (BE) is an esophageal lesion exhibiting Barrett’s mucosa, which is a columnar epithelial metaplasia that extends from the stomach to the esophagus, as confirmed by endoscopy. Histologically, Barrett’s mucosa is characterized by the following three features: proper esophageal glands or ducts beneath the overlying columnar epithelium, squamous epithelial islets in the columnar epithelium, and double-layered lamina muscularis mucosae [7]. The malignant progression of BE to adenocarcinoma has been confirmed [8], and the incidence of esophageal adenocarcinoma has increased recently, especially in Western countries [9]. To the best of our knowledge, there has been only one brief case report of MANEC developing in BE, which was resected by endoscopic submucosal dissection [10].
The incidence of esophageal carcinoma in Japan is 31.0/100,000 person-years according to the National Cancer Center, Japan [11]. Among esophageal carcinomas, Barrett’s adenocarcinoma and endocrine cell carcinoma are extremely rare, with rates of 1.1 and 0.2%, respectively, among 5878 total esophageal cancer cases, according to the Comprehensive Registry of Esophageal Cancer in Japan, 2010 [12].
In this report, we present a case of MANEC that arose in histologically confirmed BE, along with a brief literature review. We herein provide the first report of surgically resected MANEC in BE.
Case presentation
A 70-year-old man presenting with abdominal pain was referred to our hospital for further examination. He had no past history without appendicitis operated during his childhood. He claimed no family history of cancer or any genetic disorders. Laboratory data revealed no significant abnormalities. Serum levels of tumor markers such as carbohydrate antigen 19-9 (25.7 U/ml, normal range < 37 U/ml) and neuron-specific enolase (9.4 ng/ml, normal range < 16.3 ng/ml) were within the normal ranges, while the carcinoembryonic antigen level was slightly increased (5.6 ng/ml, normal range < 5.0 ng/ml). The patient underwent upper endoscopy, which revealed BE and a type 0-IIa + IIc elevated lesion adjacent to the BE lesion (Fig. 1). The tumor biopsy specimen confirmed well to poorly differentiated adenocarcinoma with neuroendocrine differentiation. Esophagography showed a type 0-IIa + IIc elevated lesion in the left wall of the lower esophagus, with a size of 15 mm and an estimated depth invading into the submucosa (Fig. 2). Thoracic and abdominal contrast-enhanced computed tomography revealed no evidence of an esophageal mass, lymphatic metastasis, or distant metastasis.
Fig. 1.
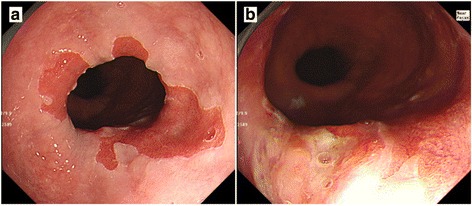
Upper endoscopy. Upper endoscopy showed an elongated columnar epithelium from the squamocolumnar junction indicating BE (a) and a type 0-IIa + IIc elevated lesion adjacent to the BE lesion (b)
Fig. 2.
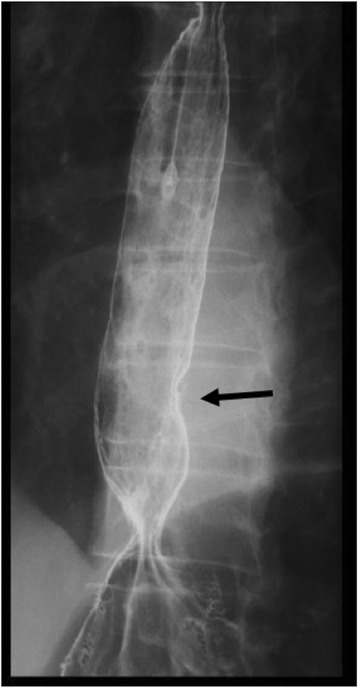
Esophagography. Esophagography showed a type 0-IIa + IIc elevated lesion (15 mm in size) on the left wall of the lower esophagus, and the tumor exhibited arcuate change suggesting submucosal invasion
The patient was clinically diagnosed with adenocarcinoma developing in BE. He subsequently underwent laparoscopic distal esophagectomy, proximal gastrectomy, regional lymph node dissection, and double-tract reconstruction with curative intent. Intraoperative findings revealed no tumor invasion into the esophageal adventitia.
In the resected specimen, a type 0-IIa + IIc lesion measuring 25 × 10 mm was macroscopically observed in the esophagogastric junction. Histological examination revealed that the neoplastic lesion was composed of well to moderately differentiated adenocarcinoma invading the submucosa and an area of proliferation of round-shaped carcinoma cells in a nested or sheeted pattern (Fig. 3). The round-shaped carcinoma cells contained hyperchromatic small nuclei with scant cytoplasm indicating small cell NEC [13]. Histological examination also revealed some islands of squamous epithelium, esophageal glands beneath the columnar epithelium, and double-layered muscularis mucosae, indicating BE near the neoplastic lesion (Fig. 4). Immunohistochemically, the adenocarcinoma cells were negative for chromogranin A and synaptophysin, whereas the round-shaped carcinoma cells were diffusely positive for synaptophysin but negative for chromogranin A and p40. The Ki67 labeling index was 50% (Fig. 5). Based on these findings, a diagnosis of MANEC arising in BE was made. No metastasis of the carcinoma cells into resected regional lymph nodes was detected. The final pathological stage was T1 N0 M0 stage I according to the seventh edition of the Union for International Cancer Control classification.
Fig. 3.
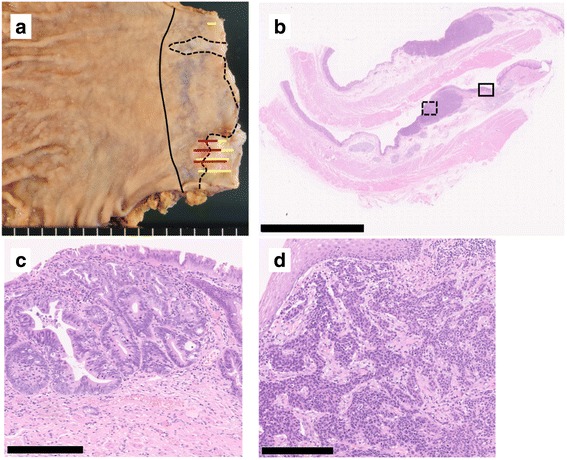
Histological findings of MANEC. Macroscopically, a type 0-IIa + IIc lesion measuring 25 × 10 mm was detected in the esophagogastric junction. The black solid line indicates the esophagogastric junction, the black dotted line the squamocolumnar junction, the yellow line the adenocarcinoma component, and the red line the NEC component (a). A loupe image of the lesion is shown (b, scale bar 10 nm). The solid rectangle indicates well differentiated adenocarcinoma (c scale bar 250 μm), and the dotted rectangle indicates an area of proliferation of round-shaped carcinoma cells with hyperchromatic nuclei and scant cytoplasm in a nested pattern, indicative of small cell NEC (d scale bar 250 μm)
Fig. 4.
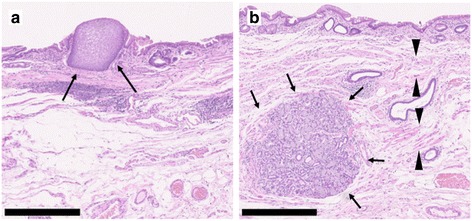
Histological findings of Barrett’s esophagus. BE was recognized histologically. Some islands of squamous epithelium (a, arrow), esophageal glands beneath the columnar epithelium (b, arrow), and double-layered muscularis mucosae (b, arrow head) were observed. All scale bars—500 μm
Fig. 5.
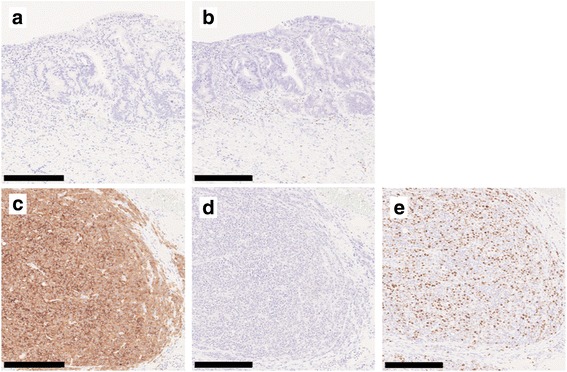
Immunohistochemical findings. Immunohistochemically, adenocarcinoma cells were negative for synaptophysin (a) and chromogranin A (b), while the round-shaped carcinoma cells were diffusely positive for synaptophysin (c), but negative for chromogranin A (d). The Ki67 labeling index was 50% (e). All scale bars—250 μm
The postoperative course was uneventful. The patient was discharged on postoperative day 19 and has been followed carefully without any adjuvant chemotherapy and no evidence of tumor recurrence 4 months after surgical resection.
Discussion
The 2010 WHO classification categorizes NETs into grade 1 and 2 NETs, neuroendocrine carcinoma (NEC), MANEC, and hyperplastic and preneoplastic lesion [2]. In this classification, grade 1 NETs (mitotic count < 2 per 10 high-power fields [HPFs]), grade 2 NETs (mitotic count 2–20 per 10 HPFs and/or Ki67 index > 20%), NEC (mitotic count > 20 per 10 HPFs and/or Ki67 index > 20%), and MANEC is defined as neoplasms comprised of both adenocarcinoma and NEC components in at least 30% of the tumor [2]. The tumor architecture is the most important diagnostic feature for MANEC, which is then confirmed by immunohistochemical findings such as chromogranin A, synaptophysin, CD56, and neuron-specific enolase expression [14]. Chromogranin A and synaptophysin expression is seen in 69.1 and 90.2%, respectively, of gastroenteropancreatic neuroendocrine neoplasms [15]. In the present case, almost equal proportions of the two components (adenocarcinoma and NEC) were confirmed. Immunohistochemically, the NEC component was positive for synaptophysin but negative for chromogranin A and p40. The Ki67 index was 50%.
La Rosa et al. categorized mixed exocrine–neuroendocrine neoplasms of the gastrointestinal tract into three subgroups: high-grade malignant neoplasms composed of mixed adenoma/adenocarcinoma and NEC, intermediate-grade malignant neoplasms composed of adenocarcinoma and grade 1/2 NET or amphicrine carcinoma, and mixed adenoneuroendocrine tumor composed of adenoma and NET [16]. According to this classification [16], our case fit the criteria of high-grade malignant MANEC.
BE is a precancerous condition in the development of esophageal adenocarcinoma, and it increases the risk of cancer by 11-fold [17]. Barrett’s mucosa exhibits the three pathological characteristics mentioned above [7]. In this case, some islands of squamous epithelium, esophageal glands beneath the columnar epithelium, and double-layered muscularis mucosae were confirmed histologically. Based on these features, MANEC arising in BE was diagnosed.
In the literature published to date, eight MANEC cases, including our case of MANEC located in the esophagus or esophagogastric junction, have been reported [3, 10, 18–21]. The clinicopathological features of the eight cases are shown in Table 1. Seven patients were male, and one patient was female. The mean age of the patients was 64 years (range, 57–70 years). The mean size of the tumors was 5.0 cm (range, 1.7–9.7 cm). Four cases developed in the esophagogastric junction and the other four in the esophagus. Seven cases were resected surgically, and one case with limited invasion to the submucosa was treated by endoscopic submucosal dissection. Lymph node metastases were observed in five cases. MANEC in BE was reported in only one case [10].
Table 1.
Previously reported cases of esophageal or esophagogastric MANEC
| Case | Age | Sex | Location | Treatment | Tumor size (cm) | Tumor depth | LN metastasis | Adjuvant chemotherapy | Recurrence | Prognosis | Author, year |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 64 | Male | Ut | Surgery | 1.7 | T1 | N0 | None | None | Alive (16 months) | Kitajima, 2013 |
| 2 | 63 | Male | EGJ | Surgery | 9.7 | T3 | N2 | NAC (CDDP + CPT-11), adjuvant (S-1 + CDDP) | None | Alive (24 months) | Nakai, 2013 |
| 3 | 68 | Male | EGJ | ESD | ND | T1 | ND | ND | ND | ND | Veits, 2013 |
| 4 | 68 | Male | Lt | Surgery | 9.5 | T4 | Positive | ND | ND | ND | Kadhim, 2016 |
| 5 | 57 | Male | EGJ | Surgery | ND | T3 | N3 | Chemoradiation (details ND) | None | Alive (8 months) | Juanmartinena, 2017 |
| 6 | 64 | Female | Mt | Surgery | 4.0 | T2 | N1 | Platinum and VP-16 | Supraclavicular lymph nodes and liver (4 months) | Dead (8 months) | Yuan, 2017 |
| 7 | 62 | Male | Mt | Surgery | 6.0 | T2 | N2 | Platinum and VP-16 | Pleural effusion (2 months) | Dead (19 months) | Yuan, 2017 |
| Our case | 70 | Male | EGJ | Surgery | 2.5 | T1 | N0 | None | None | Alive (4 months) |
Mt middle esophagus, EGJ esophagogastric junction, Lt lower esophagus, Ut upper esophagus, ESD endoscopic submucosal dissection, NAC neoadjuvant chemotherapy, CDDP cisplatin, VP-16 etoposide, ND not described
Regarding the origin of esophageal NETs, Merkel cells and stem cells are reported candidates [22, 23]. Egashira et al. showed positive staining for CK20, a marker of Merkel cells, in 14.3% of esophageal MANECs and suggested c-kit and p53 to be potential markers of the origin of NEC cells [22]. On the other hand, it was suggested that gastric NECs arise from adenocarcinomas rather than from non-neoplastic neuroendocrine cells [24]. If the origin of the tumor of our case was Merkel cells, the tumor would not be expected to exhibit adenocarcinoma differentiation but to exhibit squamous cell carcinoma differentiation, so in the present case, the tumor might have originated from esophageal adenocarcinoma or stem cells rather than Merkel cells. It is suggested that the BE–esophageal adenocarcinoma–MANEC sequence is a potential mechanism in the pathway of MANEC carcinogenesis.
The specific and optimal treatment for MANEC in the esophagus is unknown due to the number of esophageal MANEC cases reported. According to previous reports, surgical treatment with regional lymph node dissection may be a treatment strategy for localized esophageal MANEC. Because the efficacy of lymph node dissection has not been demonstrated, further accumulation of cases is required. Adjuvant chemotherapy can be considered after resection, but there is no sufficient evidence [20]. While surgery may be the treatment of choice according to the WHO [2], multidisciplinary modalities can improve the prognosis of esophageal NEC even for resectable limited diseases [25]. The chemotherapy regimen administered for esophageal NEC is the same as that for small cell lung cancer, consisting of cisplatin, etoposide (VP-16), and irinotecan (CPT-11) [26].
Patients with mixed NEC in the esophagus (NEC plus adenocarcinoma or squamous cell carcinoma components) are more likely to be diagnosed at an earlier stage, and they showed a significantly longer survival, compared with patients with pure NEC (median survival, 28 vs. 15 months) [27]. The prognosis of high-grade MANEC was reported to depend on tumor stage and type [16].
Conclusions
We have demonstrated a very rare case of MANEC developing in BE. BE can be the origin of esophageal MANEC as well as adenocarcinoma. The clinical and prognostic features of MANEC in BE are still unclear. Further studies are necessary to determine the optimal management strategy for esophageal or esophagogastric MANEC.
Acknowledgments
Availability of data and materials
Data sharing is not applicable to this article, as no datasets were generated or analyzed during the current study.
Abbreviations
- BE
Barrett’s esophagus
- HPF
High-power field
- MANEC
Mixed adenoneuroendocrine carcinoma
- NEC
Neuroendocrine carcinoma
- NET
Neuroendocrine tumor
- WHO
World Health Organization
Authors’ contributions
TK and HS conceived the case presentation and drafted the manuscript. KE, SK, DT, KK, RN, NK, KA, and YN participated in the treatment of the patient. MF determined the pathological diagnosis of the patient. EO, YO, and YM revised the article. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The present study was conducted in accordance with the ethical standards of our institution.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Tetsuro Kawazoe, Email: ktetsuro@surg2.med.kyushu-u.ac.jp.
Hiroshi Saeki, Phone: +81-92-642-5466, Email: h-saeki@surg2.med.kyushu-u.ac.jp.
Keitaro Edahiro, Email: edahiro@surg2.med.kyushu-u.ac.jp.
Shotaro Korehisa, Email: s_kore@surg2.med.kyushu-u.ac.jp.
Daisuke Taniguchi, Email: dtanigu@surg2.med.kyushu-u.ac.jp.
Kensuke Kudou, Email: k_kudo@surg2.med.kyushu-u.ac.jp.
Ryota Nakanishi, Email: naka-r@surg2.med.kyushu-u.ac.jp.
Nobuhide Kubo, Email: nobkubo@surg2.med.kyushu-u.ac.jp.
Koji Ando, Email: k-ando@surg2.med.kyushu-u.ac.jp.
Yuichiro Nakashima, Email: naka0215@surg2.med.kyushu-u.ac.jp.
Eiji Oki, Email: okieiji@surg2.med.kyushu-u.ac.jp.
Minako Fujiwara, Email: mi-hira@surgpath.med.kyushu-u.ac.jp.
Yoshinao Oda, Email: oda@surgpath.med.kyushu-u.ac.jp.
Yoshihiko Maehara, Email: maehara@surg2.med.kyushu-u.ac.jp.
References
- 1.Kunz PL. Carcinoid and neuroendocrine tumors: building on success. J Clin Oncol. 2015;33(16):1855–1863. doi: 10.1200/JCO.2014.60.2532. [DOI] [PubMed] [Google Scholar]
- 2.Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO classification of tumours of the digestive system. 4th ed. Geneva: World Health Organization; 2010. https://www.cabdirect.org/cabdirect/abstract/20113051318.
- 3.Kadhim MM, Jespersen ML, Pilegaard HK, Nordsmark M, Villadsen GE. Mixed adenoneuroendocrine carcinoma is a rare but important tumour found in the oesophagus. Case Rep Gastrointest Med. 2016;2016:9542687. doi: 10.1155/2016/9542687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamauchi H, Sakurai S, Nakazawa N, Yoshida T, Tabe Y, Saitoh K, et al. A case of mixed adenoneuroendocrine carcinoma of the stomach with focal intestinal metaplasia and hypergastrinemia. Int Surg. 2015;100(3):562–567. doi: 10.9738/INTSURG-D-14-00074.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kwok CM. Mixed adenoneuroendocrine carcinoma of the stomach. Case Rep Gastroenterol. 2015;9(2):241–245. doi: 10.1159/000437293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ito H, Kudo A, Matsumura S, Ban D, Irie T, Ochiai T, et al. Mixed adenoneuroendocrine carcinoma of the colon progressed rapidly after hepatic rupture: report of a case. Int Surg. 2014;99(1):40–44. doi: 10.9738/INTSURG-D-13-00161.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuwano H, Nishimura Y, Oyama T, Kato H, Kitagawa Y, Kusano M, et al. Guidelines for diagnosis and treatment of carcinoma of the esophagus April 2012 edited by the Japan Esophageal Society. Esophagus. 2015;12:1–30. doi: 10.1007/s10388-014-0465-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhat S, Coleman HG, Yousef F, Johnston BT, McManus DT, Gavin AT, et al. Risk of malignant progression in Barrett’s esophagus patients: results from a large population-based study. J Natl Cancer Inst. 2011;103(13):1049–1057. doi: 10.1093/jnci/djr203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gindea C, Birla R, Hoara P, Caragui A, Constantinoiu S. Barrett esophagus: history, definition and etiopathogeny. J Med Life. 2014;7(Spec No. 3):23–30. [PMC free article] [PubMed]
- 10.Veits L, Lang-Schwarz C, Volkholz H, Falkeis C, Vieth M, Schulz H. Mixed adenoneuroendocrine carcinoma (MANEC) of the esophagogastric junction predominantly consisting of poorly differentiated neuroendocrine carcinoma. Endoscopy. 2013;45(Suppl 2 UCTN):E16–E17. doi: 10.1055/s-0032-1326113. [DOI] [PubMed] [Google Scholar]
- 11.Hori M, Matsuda T, Shibata A, Katanoda K, Sobue T, Nishimoto H. Cancer incidence and incidence rates in Japan in 2009: a study of 32 population-based cancer registries for the Monitoring of cancer Incidence in Japan (MCIJ) project. Jpn J Clin Oncol. 2015;45(9):884–891. doi: 10.1093/jjco/hyv088. [DOI] [PubMed] [Google Scholar]
- 12.Tachimori Y, Ozawa S, Numasaki H, Ishihara R, Matsubara H, Muro K, et al. Comprehensive registry of esophageal cancer in Japan, 2010. Esophagus. 2017;14(3):189–214. doi: 10.1007/s10388-017-0578-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang Q, Wu H, Nie L, Shi J, Lebenthal A, Chen J, et al. Primary high-grade neuroendocrine carcinoma of the esophagus: a clinicopathologic and immunohistochemical study of 42 resection cases. Am J Surg Pathol. 2013;37(4):467–483. doi: 10.1097/PAS.0b013e31826d2639. [DOI] [PubMed] [Google Scholar]
- 14.Gurzu S, Kadar Z, Bara T, Bara T, Jr, Tamasi A, Azamfirei L, et al. Mixed adenoneuroendocrine carcinoma of gastrointestinal tract: report of two cases. World J Gastroenterol. 2015;21(4):1329–1333. doi: 10.3748/wjg.v21.i4.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang YH, Lin Y, Xue L, Wang JH, Chen MH, Chen J. Relationship between clinical characteristics and survival of gastroenteropancreatic neuroendocrine neoplasms: a single-institution analysis (1995-2012) in South China. BMC Endocr Disord. 2012;12:30. doi: 10.1186/1472-6823-12-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.La Rosa S, Marando A, Sessa F, Capella C. Mixed adenoneuroendocrine carcinomas (MANECs) of the gastrointestinal tract: an update. Cancers (Basel) 2012;4(1):11–30. doi: 10.3390/cancers4010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chandra S, Gorospe EC, Leggett CL, Wang KK. Barrett’s esophagus in 2012: updates in pathogenesis, treatment, and surveillance. Curr Gastroenterol Rep. 2013;15(5):322. doi: 10.1007/s11894-013-0322-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kitajima T, Kaida S, Lee S, Haruta S, Shinohara H, Ueno M, et al. Mixed adeno(neuro)endocrine carcinoma arising from the ectopic gastric mucosa of the upper thoracic esophagus. World J Surg Oncol. 2013;11:218. doi: 10.1186/1477-7819-11-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakai M, Kawasaki H, Wajima N, Kimura A, Nakayama Y, Muroya T, et al. A case of mixed adenoneuroendocrine carcinoma of the esophagogastric junction treated with neoadjuvant chemotherapy. Gan To Kagaku Ryoho. 2013;40(12):2301–2303. [PubMed] [Google Scholar]
- 20.Juanmartinena JF, Fernandez-Urien I, Cordoba A, Miranda C, Borda A. Mixed adenoneuroendocrine carcinoma (MANEC) of the gastroesophageal junction: a case report and review of the literature. Rev Esp Enferm Dig. 2017;109(2):160–162. doi: 10.17235/reed.2016.4315/2016. [DOI] [PubMed] [Google Scholar]
- 21.Yuan W, Liu Z, Lei W, Sun L, Yang H, Wang Y, et al. Mutation landscape and intra-tumor heterogeneity of two MANECs of the esophagus revealed by multi-region sequencing. Oncotarget. 2017;8(41):69610–69621. doi: 10.18632/oncotarget.18678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Egashira A, Morita M, Kumagai R, Taguchi KI, Ueda M, Yamaguchi S, et al. Neuroendocrine carcinoma of the esophagus: clinicopathological and immunohistochemical features of 14 cases. PLoS One. 2017;12(3):e0173501. doi: 10.1371/journal.pone.0173501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harmse JL, Carey FA, Baird AR, Craig SR, Christie KN, Hopwood D, et al. Merkel cells in the human oesophagus. J Pathol. 1999;189(2):176–179. doi: 10.1002/(SICI)1096-9896(199910)189:2<176::AID-PATH416>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 24.Ishida M, Sekine S, Fukagawa T, Ohashi M, Morita S, Taniguchi H, et al. Neuroendocrine carcinoma of the stomach: morphologic and immunohistochemical characteristics and prognosis. Am J Surg Pathol. 2013;37(7):949–959. doi: 10.1097/PAS.0b013e31828ff59d. [DOI] [PubMed] [Google Scholar]
- 25.Hou X, Wei JC, Wu JX, Wang X, Fu JH, Lin P, et al. Multidisciplinary modalities achieve encouraging long-term survival in resectable limited-disease esophageal small cell carcinoma. PLoS One. 2013;8(7):e69259. doi: 10.1371/journal.pone.0069259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takeoka T, Hirao M, Fujitani K, Yamamoto K, Asaoka T, Ikenaga M, et al. A case of neuroendocrine cell carcinoma of the esophagus with lung metastases successfully treated with CPT-11/CDDP. Gan To Kagaku Ryoho. 2013;40(12):2121–2123. [PubMed] [Google Scholar]
- 27.Maru DM, Khurana H, Rashid A, Correa AM, Anandasabapathy S, Krishnan S, et al. Retrospective study of clinicopathologic features and prognosis of high-grade neuroendocrine carcinoma of the esophagus. Am J Surg Pathol. 2008;32(9):1404–1411. doi: 10.1097/PAS.0b013e31816bf41f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article, as no datasets were generated or analyzed during the current study.


