Abstract
Two 21 d-experiments were conducted to determine the optimum standardized ileal digestible (SID) threonine:lysine ratio (Thr:Lys) for weaned piglets reared under clean (Exp. 1) or unclean (Exp. 2) sanitary conditions and fed antibiotic-free diets. In each experiment, 90 mixed-sex pigs (Duroc × [Yorkshire × Landrace]; initial BW 7.2 ± 0.3 kg) were randomly assigned to 5 dietary treatments each with 6 replicates (3 pigs per pen). The dietary treatments were 5 graded levels of SID Thr:Lys (55, 59, 63, 67 and 71%). Diets were corn-wheat-soybean meal-based with a constant SID Lys of 1.18% that was set to be second limiting amino acid. In Exp. 1 and Exp. 2, plasma-free Thr increased (P = 0.05) with increasing dietary SID Thr:Lys. In Exp. 1, the SID Thr:Lys for gain-to-feed ratio (G:F) was optimized at 65%. In Exp. 2, the estimated optimal SID Thr:Lys for overall G:F was 66.5%. In conclusion, an average optimal SID Thr:Lys of 65 and 66.5% could be used to optimize feed efficiency for weaned pigs under clean and unclean sanitary conditions, respectively.
Keywords: Threonine:lysine ratio, Sanitation, Weaned pigs, Growth performance
1. Introduction
Threonine (Thr) is the second-limiting amino acid (AA) after lysine (Lys) in pigs when fed wheat and barley-based diets and the third-limiting AA in corn-based diets (Adeola et al., 1994, Saldana et al., 1994). Besides protein synthesis, the major functions of Thr include maintenance of gut integrity and immunity (Ruth and Field, 2013). Hence, the requirement for Thr is likely to vary according to the importance of each of its functions. The NRC (2012) recommends a diet with a standardized ileal digestible (SID) threonine-to-lysine ratio (Thr:Lys) of 59% for pigs between 7 and 25 kg, however, it does not consider the health status of the pig. These levels might not be sufficient for pigs reared in commercial production conditions, where the chances of clinical and sub-clinical infections occur.
Weaned pigs reared under unclean sanitary conditions was used as a model to provoke a low-grade inflammatory response (Le Floc’h et al., 2009, Kahindi et al., 2014a) and those pigs had reduced growth performance (Kahindi et al., 2014a) and activated immune system (Williams et al., 1997) that would interfere with growth because of competition between protein deposition in structural tissues and immune function (Le Floc'h et al., 2009).
A current interest in the nutritional management of nursery pigs is to utilize nutritional programs without in-feed antibiotic growth promoter (AGP) (Nyachoti et al., 2006, de Lange et al., 2010), which may alter the Thr:Lys requirement for optimal performance, especially when piglets are exposed to an immunological challenge as is often the case under unsanitary conditions. For example, it has been shown that metabolism and requirement for Thr changes in pigs during an immune challenge (Le Floc'h and Seve, 2004, Ren et al., 2014). Furthermore, exposing piglets to unclean conditions is considered as a predisposing factor for weaning disorders (Williams et al., 1997).
Information about Thr:Lys for piglets fed AGP-free diets and subjected to an immunological challenge is limited. Thus, it was hypothesized that the Thr:Lys required to optimize piglet performance is higher when subjected to unclean sanitary conditions. The objective of this study was to determine the optimum SID Thr:Lys for weaned piglets reared in clean or unclean sanitary conditions.
2. Materials and methods
2.1. Animal care
The experimental protocol (F10-041/1/2) was reviewed and approved by the Animal Care Committee of University of Manitoba and pigs were cared for in accordance with the guidelines of Canadian Council on Animal Care (2009).
2.2. Experimental diets
Diets were corn (Zea mays), wheat (Triticum aestivum) and soybean (Glycine max) meal-based with a constant SID Lys of 1.18% that was set to be second limiting AA (Table 1). Ingredients contributing AA (corn, wheat and soybean meal) were analyzed for AA composition and the values were used in diet formulation. The diets contained graded levels of SID Thr:Lys (55, 59, 63, 67 and 71%). All other nutrients were provided in quantities meeting or exceeding National Research Council (2012) recommendations for a 6 – 10 kg pig. All diets were fed in mash form and did not contain any AGP.
Table 1.
Ingredient and nutrient composition of experimental diets (as-is basis).
| Item | Dietary SID Thr:Lys, % |
||||
|---|---|---|---|---|---|
| 55 | 59 | 63 | 67 | 71 | |
| Ingredients, % | |||||
| Corn | 46.20 | 46.20 | 46.20 | 46.20 | 46.20 |
| Wheat | 15.00 | 15.00 | 15.00 | 15.00 | 15.00 |
| Soybean meal | 30.00 | 30.00 | 30.00 | 30.00 | 30.00 |
| Vegetable oil | 3.85 | 3.85 | 3.85 | 3.85 | 3.85 |
| Corn starch | 0.50 | 0.45 | 0.40 | 0.36 | 0.31 |
| Monocalcium phosphate | 1.50 | 1.50 | 1.50 | 1.50 | 1.50 |
| Limestone | 1.08 | 1.08 | 1.08 | 1.08 | 1.08 |
| Salt | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 |
| Min-Vit premix1 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| L-Lys HCl | 0.35 | 0.35 | 0.35 | 0.35 | 0.35 |
| L-Trp | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 |
| DL-Met | 0.14 | 0.14 | 0.14 | 0.14 | 0.14 |
| L-Thr | – | 0.05 | 0.09 | 0.14 | 0.19 |
| Calculated nutrient composition, %2 | |||||
| NE, Kcal/kg | 2,484 | 2,484 | 2,484 | 2,484 | 2,484 |
| SID Lys | 1.18 | 1.18 | 1.18 | 1.18 | 1.18 |
| SID Met | 0.49 | 0.49 | 0.49 | 0.49 | 0.49 |
| SID Cys | 0.23 | 0.23 | 0.23 | 0.23 | 0.23 |
| SID Met + Cys | 0.72 | 0.72 | 0.72 | 0.72 | 0.72 |
| SID Trp | 0.26 | 0.26 | 0.26 | 0.26 | 0.26 |
| SID Thr | 0.65 | 0.70 | 0.75 | 0.79 | 0.84 |
| SID Ile | 0.76 | 0.76 | 0.76 | 0.76 | 0.76 |
| SID Val | 0.85 | 0.85 | 0.85 | 0.85 | 0.85 |
SID = standardized ileal digestible; Thr:Lys = threonine-to-lysine ratio.
Supplied the following per kg of diet: vitamin A, 8,250 IU; vitamin D3, 835 IU; vitamin E, 40 IU; vitamin B12, 25 μg; vitamin K, 4 mg; niacin, 25 μg; choline, 600 mg; riboflavin, 12 mg; biotin, 200 μg; pyridoxine, 4.5 mg; folic acid, 4 mg; thiamin, 2 mg; Mn, 50 mg; Zn, 150 mg; Fe, 120 mg; Cu, 25 mg; Se, 0.35 mg; I, 0.4 mg.
The calculated total contents of Lys, Thr, Met + Cys and Trp in the basal diet were 1.32, 0.77, 0.82, and 0.30%, respectively.
2.3. Animals and experimental design
A total of 180 piglets (Duroc × [Yorkshire × Landrace]; mixed sex) were used for the two experiments. In each of the two experiments, 90 pigs (weaned at 21 ± 1 d of age) were fed a corn-soybean meal-based starter diet (20% CP; 59% SID Thr:Lys) for 6 d adaptation period and then, pigs (initial BW 7.36 ± 0.2 kg) were randomly assigned to 1 of 5 dietary treatments with SID Thr:Lys (55, 59, 63, 67 and 71%) each with 6 replicates (3 pigs per pen). In Exp. 1, 90 piglets were housed in a clean room that had been cleaned and disinfected before the arrival of the piglets and the room was cleaned once weekly. In Exp. 2, 90 piglets were housed in the same room which was not cleaned and disinfected at the end of wk 3 from the first batch to allow the build-up of manure. Moreover, manure from swine herd was added (5 kg per pen) to the pens on d 0 and 7 of the Exp. 2. In both studies, pigs were provided ad libitum access to feed and water. Body weight and pen feed disappearance were recorded weekly to determine ADG, ADFI and gain-to-feed ratio (G:F) calculated on per pen basis. Blood was collected on d 0 and 14 and analyzed for plasma urea nitrogen (PUN) and plasma-free Thr and Lys. Faecal consistency scoring (0 = normal, 1 = soft faeces, 2 = mild diarrhoea, and 3 = severe diarrhoea) was done as described by Marquardt et al. (1999) by 2 independent trained individuals with no prior knowledge of the treatment allocation.
2.4. Sample preparation and laboratory analyses
Diet samples were ground through a 1-mm mesh screen. The DM content was determined according to AOAC (2000). The N content of the diets was determined with a gas combustion method using a Leco FP-2000 Nitrogen analyzer (Leco Corp., St. Joseph, MI). Amino acid analyses were carried out at the lab of Evonik Industries AG, Hanau-Wolfgang, Germany. Dietary concentrations of all essential and non-essential AA, except for tryptophan and tyrosine, were determined by ion-exchange chromatography with post-column derivatization with ninhydrin. Amino acids were oxidized with performic acid, which was neutralized with Na metabisulfite (Llames and Fontaine, 1994, Commission Directive, 1998). Amino acids were liberated from the protein by hydrolysis with 6 mol/L HCl for 24 h at 110°C and quantified with the internal standard by measuring the absorption of reaction products with ninhydrin at 570 nm. Tryptophan was determined by HPLC with fluorescence detection (extinction 280 nm, emission 356 nm), after alkaline hydrolysis with barium hydroxide octahydrate for 20 h at 110°C (Commission Directive, 2000). Tyrosine was not determined.
2.5. Plasma urea nitrogen
Blood sampling and PUN analysis were performed according to Nyachoti et al. (2006). Briefly, on d 0 and 14, a 10-mL blood sample was collected from one pig per pen via jugular venipuncture into heparinized vacutainer tube (Becton Dickinson, Rutherford, US) and stored on ice for 20 min before being centrifuged at 2,000 × g for 10 min at 4°C to recover plasma. Plasma samples were stored at −20°C until used for further analysis. Plasma samples were thawed and then analyzed for PUN using a Nova Stat Profile M blood gas and electrolyte analyzer (Nova Biomedical Corporation, Waltham, MA).
2.6. Plasma-free threonine and lysine
Plasma-free Thr and Lys concentrations were determined using amino acid analyzer (Skykam Amino Acid Analyzer, Germany) after being deproteinized with 4% sulfosalicylic acid.
2.7. Statistical analysis
Data were subjected to ANOVA using the Proc mixed procedure of SAS 9.2 (SAS Inst. Cary. NC). The data were analyzed as completely randomized design. Orthogonal polynomial contrasts were used to determine the linear and quadratic effects of increasing levels of SID Thr:Lys. Statistical significance was accepted at P < 0.05 and 0.05 < P < 0.10 was considered a trend. To determine the optimal SID Thr:Lys level, data were subjected to broken-line analysis (Robbins et al., 2006) using the Proc NLIN of SAS (SAS Inst. Inc. Cary, NC). Each pen was considered as an experimental unit.
3. Results
The analyzed AA and crude protein contents of the experimental diets were presented in Table 2. The SID Thr:Lys were then corrected based on the analyzed contents using the following formula: corrected SID Thr:Lys = (calculated SID Thr:Lys × analyzed total Thr:Lys)/calculated total Thr:Lys. The corrected SID Thr:Lys in the diets were 61, 64, 67, 69 and 72% which were used for the regression analysis.
Table 2.
Analyzed crude protein and amino acid composition of experimental diets.
| Item | Dietary SID Thr:Lys, %1 |
||||
|---|---|---|---|---|---|
| 55 | 59 | 63 | 67 | 71 | |
| Crude protein | 21.29 | 22.19 | 21.68 | 22.01 | 22.26 |
| Lys | 1.26 | 1.29 | 1.30 | 1.29 | 1.31 |
| Thr | 0.77 | 0.82 | 0.87 | 0.89 | 0.94 |
| Met + Cys | 0.77 | 0.79 | 0.80 | 0.80 | 0.81 |
| Ile | 0.89 | 0.91 | 0.91 | 0.90 | 0.92 |
| Val | 0.99 | 1.01 | 1.01 | 0.99 | 1.01 |
| Trp | 0.28 | 0.29 | 0.29 | 0.29 | 0.29 |
| SID Thr:Lys2 | 61.00 | 64.00 | 67.00 | 69.00 | 72.00 |
SID = standardized ileal digestible; Thr:Lys = threonine-to-lysine ratio.
Calculated dietary SID Thr:Lys.
Corrected dietary SID Thr:Lys = (calculated SID Thr:Lys × analyzed total Thr:Lys)/calculated total Thr:Lys.
3.1. Experiment 1
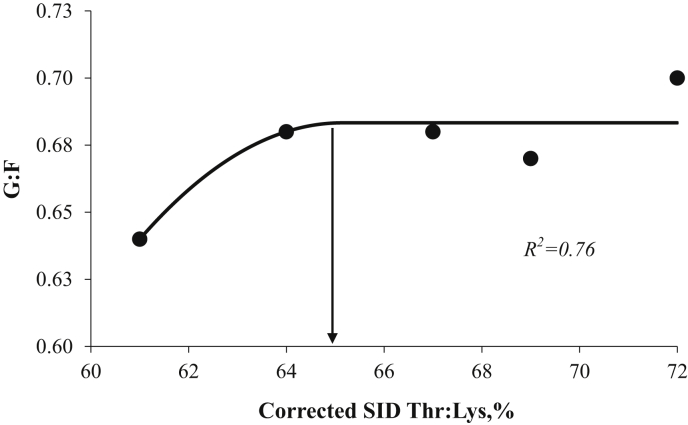
All animals remained healthy throughout the experimental period. No signs of diarrhea or any other clinical illness were observed for piglets. The initial and final BW (Table 3) were similar (P > 0.10) for the pigs fed the different dietary SID Thr:Lys. During wk 1, there was an increase (P < 0.05) in G:F, with increasing SID Thr:Lys, whereas there was no effect (P > 0.10) on ADG and feed intake (Table 3). On d 14, dietary SID Thr:Lys did not influence (P > 0.10) PUN and plasma-free Lys concentrations (Table 4), whereas there was an increase (P = 0.05; linear) in plasma-free Thr concentration with increasing dietary SID Thr:Lys. Based on overall G:F, the SID Thr:Lys was optimized at 65% using broken line quadratic (BLQ) model (Fig. 1).
Table 3.
Effects of different standardized ileal digestible threonine:lysine ratios (Thr:Lys) on growth performance of weaned pigs reared under clean and unclean sanitary conditions.1
| Item | Clean sanitary condition2 |
Unclean sanitary condition3 |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Corrected SID Thr:Lys, % |
SEM |
P-value4 |
Corrected SID Thr:Lys, % |
SEM |
P-value |
|||||||||||||
| 61 | 64 | 67 | 69 | 72 | Thr | Lin | Quad | 61 | 64 | 67 | 69 | 72 | Thr | Lin | Quad | |||
| Initial BW, kg | 7.37 | 7.16 | 7.33 | 7.40 | 7.30 | 0.39 | 7.18 | 7.03 | 7.05 | 7.11 | 7.12 | 0.33 | ||||||
| Final BW, kg | 17.5 | 17.3 | 17.7 | 17.6 | 18.0 | 0.34 | 0.598 | 0.166 | 0.508 | 15.7 | 16.0 | 15.1 | 15.7 | 15.8 | 0.63 | 0.896 | 0.940 | 0.637 |
| wk 1 (d 0 to 7) | ||||||||||||||||||
| ADG, g | 299 | 320 | 313 | 317 | 354 | 24.0 | 0.582 | 0.171 | 0.638 | 209 | 214 | 221 | 234 | 197 | 18.5 | 0.714 | 0.947 | 0.283 |
| ADFI, g | 449 | 447 | 430 | 444 | 432 | 31.4 | 0.987 | 0.712 | 0.922 | 322 | 349 | 303 | 344 | 299 | 26.0 | 0.558 | 0.533 | 0.576 |
| G:F | 0.66 | 0.73 | 0.73 | 0.73 | 0.82 | 0.04 | 0.178 | 0.033 | 0.835 | 0.66 | 0.65 | 0.73 | 0.69 | 0.65 | 0.05 | 0.738 | 0.879 | 0.343 |
| wk 2 (d 8 to 14) | ||||||||||||||||||
| ADG, g | 501 | 442 | 459 | 512 | 507 | 27.7 | 0.317 | 0.352 | 0.166 | 411 | 415 | 403 | 439 | 381 | 19.4 | 0.35 | 0.555 | 0.313 |
| ADFI, g | 828 | 749 | 797 | 835 | 808 | 44.9 | 0.714 | 0.750 | 0.575 | 580 | 537 | 535 | 599 | 568 | 25.3 | 0.354 | 0.655 | 0.358 |
| G:F | 0.60 | 0.59 | 0.58 | 0.62 | 0.62 | 0.03 | 0.464 | 0.487 | 0.710 | 0.71 | 0.78 | 0.75 | 0.73 | 0.67 | 0.03 | 0.263 | 0.247 | 0.066 |
| wk 3 (d 15 to 21) | ||||||||||||||||||
| ADG, g | 665 | 707 | 736 | 664 | 687 | 33.4 | 0.509 | 0.997 | 0.265 | 544 | 570 | 574 | 583 | 596 | 26.9 | 0.731 | 0.184 | 0.842 |
| ADFI, g | 1,002 | 980 | 1,011 | 981 | 964 | 39.9 | 0.921 | 0.556 | 0.732 | 876 | 804 | 774 | 808 | 878 | 31.4 | 0.102 | 0.929 | 0.007 |
| G:F | 0.67 | 0.72 | 0.73 | 0.68 | 0.71 | 0.03 | 0.409 | 0.581 | 0.283 | 0.63 | 0.71 | 0.75 | 0.72 | 0.68 | 0.04 | 0.170 | 0.322 | 0.024 |
| Overall (d 0 to 21) | ||||||||||||||||||
| ADG, g | 487 | 487 | 500 | 499 | 521 | 16.9 | 0.640 | 0.154 | 0.651 | 388 | 399 | 400 | 423 | 387 | 15.9 | 0.508 | 0.651 | 0.233 |
| ADFI, g | 758 | 720 | 741 | 757 | 744 | 28.8 | 0.899 | 0.928 | 0.684 | 592 | 564 | 538 | 594 | 571 | 20.8 | 0.340 | 0.870 | 0.245 |
| G:F | 0.64 | 0.68 | 0.68 | 0.67 | 0.70 | 0.02 | 0.407 | 0.138 | 0.822 | 0.66 | 0.71 | 0.75 | 0.72 | 0.70 | 0.02 | 0.071 | 0.440 | 0.006 |
SID = standardized ileal digestible; SEM = standard error of mean.
Values are least square means; n = 6 per treatment.
Clean sanitary condition: piglets were housed in cleaned and disinfected rooms and fed antibiotic-free diets.
Unclean sanitary conditions: piglets were housed in a room where cleaning and disinfection was not done, moreover, manure from swine herd was added (5 kg per pen) to the pens on d 0 and 7 of the experiment.
Probability values of linear (Lin) and quadratic (Quad) effects for dietary SID Thr:Lys (%).
Table 4.
Effects of different standardized ileal digestible threonine:lysine ratios (Thr:Lys) on plasma threonine, lysine and urea nitrogen concentrations (mmol/L) of weaned pigs reared under clean and unclean sanitary conditions.1
| Item | Clean sanitary condition2 |
Unclean sanitary condition3 |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Analyzed SID Thr:Lys, % |
SEM |
P-value4 |
Analyzed SID Thr:Lys, % |
SEM |
P-value |
|||||||||||||
| 61 | 64 | 67 | 69 | 72 | Thr | Lin | Quad | 61 | 64 | 67 | 69 | 72 | Thr | Lin | Quad | |||
| Plasma-free Thr | 523 | 617 | 622 | 652 | 634 | 38.4 | 0.199 | 0.050 | 0.185 | 415 | 517 | 629 | 592 | 639 | 55.4 | 0.070 | 0.010 | 0.241 |
| Plasma-free Lys | 138 | 117 | 127 | 119 | 123 | 15.0 | 0.896 | 0.570 | 0.581 | 199 | 200 | 168 | 171 | 209 | 33.0 | 0.848 | 0.938 | 0.399 |
| Plasma urea nitrogen | 3.55 | 3.73 | 2.92 | 2.64 | 3.37 | 0.33 | 0.110 | 0.226 | 0.207 | 3.74 | 4.10 | 3.40 | 3.99 | 3.81 | 0.33 | 0.616 | 0.980 | 0.871 |
SID = standardized ileal digestible; SEM = standard error of mean.
Values are least square means; n = 6 per treatment.
Clean sanitary condition: piglets were housed in cleaned and disinfected rooms and fed antibiotic-free diets.
Unclean sanitary conditions: piglets were housed in a room where cleaning and disinfection was not done, moreover, manure from swine herd was added (5 kg per pen) to the pens on d 0 and 7 of the experiment.
Probability values of linear (Lin) and quadratic (Quad) effects for dietary SID Thr:Lys (%).
Fig. 1.
The optimal dietary standardized ileal digestible (SID) threonine:lysine ratio (Thr:Lys) for gain-to-feed ratio (G:F) in weaned pigs reared under clean sanitary conditions (Exp. 1) determined using quadratic broken-line analysis was 65% [Y = 0.68−0.0025 (65−x)2]. Data points (●) represent least square means of dietary treatments (n = 6).
3.2. Experiment 2
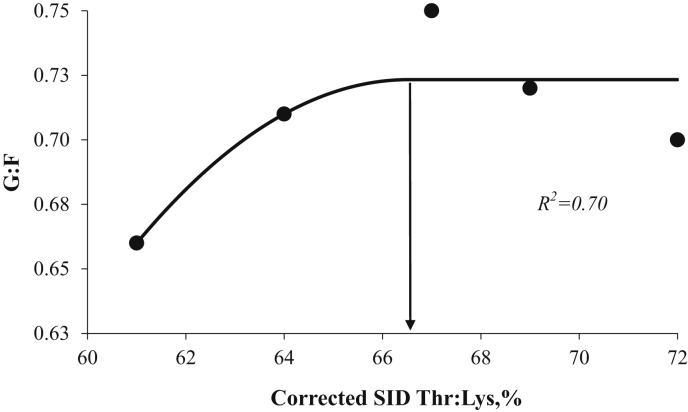
The initial and final BW was similar (P > 0.10) for the pigs from the different dietary SID Thr:Lys. During the overall experimental period, dietary SID Thr:Lys increased (P < 0.05; quadratic) G:F, whereas there was no difference (P > 0.10) in ADG and feed intake (Table 3). During wk 1, growth performance was not affected (P > 0.10) by different SID Thr:Lys. During wk 2 and 3, there was an increase (P < 0.05; quadratic) in G: F with increasing dietary SID Thr:Lys, however, there was no difference (P > 0.10) in ADG. During wk 3, feed intake increased (P < 0.05; quadratic) due to different SID Thr:Lys. On d 14, plasma-free Thr concentration increased (P < 0.05; linear) with increasing SID Thr:Lys, whereas PUN and plasma Lys concentrations were not affected (P > 0.10) by dietary treatments (Table 4). Based on overall G:F, the SID Thr:Lys was optimized at 66.5% using BLQ model (Fig. 2).
Fig. 2.
The optimal dietary standardized ileal digestible (SID) threonine:lysine ratio (Thr:Lys) for gain-to-feed ratio (G:F) in weaned pigs reared under unclean sanitary conditions (Exp. 1) determined using quadratic broken-line analysis was 66.5% [Y = 0.72−0.0021 (66.5−x)2]. Data points (●) represent least square means of dietary treatments (n = 6).
4. Discussion
The goal of this study was to determine the optimal SID Thr:Lys for weaned pigs reared under clean or unclean sanitary conditions using growth rate and PUN as response criteria. In this study, diets were corn-wheat-soybean meal-based with a constant SID Lys content of 1.18% was set to be 10% lower than the requirement level that was established in our lab (Kahindi et al., 2014b).
In Exp. 1, increasing the SID Thr:Lys improved the overall G:F but had no effect on ADG during wk 1, which is in agreement with Fernandez and Strathe (2009). The specific role of Thr could have been directed to intestinal integrity and function compared to protein accretion, which might be the reason for not showing significant difference in daily weight gain (Fernandez and Strathe, 2009). Based on overall feed efficiency, SID Thr:Lys of 65% was optimized using BLQ model (Fig. 1), which is consistent with previous studies (James et al., 2003, Lenehan et al., 2004).
Weaned pigs subjected to unclean sanitary condition was used as a model of moderate immune system stimulation (Le Floc'h et al., 2006), which was in turn anticipated to have effect on partitioning of AA between lean tissue deposition and supporting the immune system (Williams et al., 1997). In Exp. 2, piglets raised under unclean sanitary conditions had reduced growth performance compared with the values reported by other studies (335 g, NRC, 2012; 500 g, Zhang and Kim, 2014). This suggests that the sanitation model of immune challenge was effective, which is consistent with the findings of others (Lee et al., 2005, Le Floc’h et al., 2006, Kahindi et al., 2014a) that showed decreased growth performance when piglets subjected to poor sanitary conditions.
Under unclean sanitary conditions, based on G:F, SID Thr:Lys of 66.5% was optimized using BLQ model (Fig. 2). The optimal SID Thr:Lys estimated in this study was in agreement with previous studies (Baker, 2000, Trevisi et al., 29 August-2 September, 2011) using feed efficiency as response criteria. The estimated SID Thr:Lys requirement for weaned pigs in the current study is higher (11%) than NRC (2012) recommendations (59% SID Thr:Lys) for the 7 to 25 kg pigs, which implies that during general immune challenge conditions, such as unclean sanitary conditions, Thr requirements might be increased for weaned pigs.
Plasma urea nitrogen has been often used as a response criterion for determining AA requirements since PUN can be used as an indicator of protein utilization efficiency (Coma et al., 1995). When there is an excess AA, PUN is known to increase because excess AA cannot be stored and therefore they are degraded with the production of urea (Heo et al., 2009, Waguespack et al., 2011). If there is decrease in PUN, it would indicate that either an increase in nitrogen use efficiency or decrease in protein breakdown, (Shen et al., 2012). In this study, both under clean and unclean sanitary conditions, dietary Thr:Lys did not affect PUN concentration. In this study, the optimal SID Thr:Lys could not be determined using PUN as response criteria which is contradictory to previous studies (Defa et al., 1999; Wang et al., 2006, Zhang et al., 2013) reporting that by increasing Thr levels in the diet, there was a decrease in PUN.
Under both clean and unclean sanitary conditions, increasing levels of dietary SID Thr:Lys increased concentration of plasma-free Thr. This result is consistent with the previous studies (Defa et al., 1999, Wang et al., 2006) who also reported that increasing levels of dietary Thr increased serum free Thr. The increase in plasma-free Thr could be due to increasing levels of dietary SID Thr:Lys which is in agreement with Zhang et al. (2013).
5. Conclusions
In this study, under clean sanitary conditions, the optimal SID Thr:Lys based on G:F was 65% using BLQ model. Under unclean sanitary conditions, the estimated optimal SID Thr:Lys for overall G:F using BLQ model was 66.5%. In conclusion, an average optimal SID Thr:Lys of 65 and 66.5% could be used to optimize feed efficiency for weaned pigs under clean and unclean sanitary conditions, respectively.
Conflict of interest
The authors declare that there are no conflict of interest.
Acknowledgments
Funding for this study from Evonik Industries and Natural Science and Engineering Research Council is greatly appreciated. The authors would like to thank Robert Stuski, TK Cheung Animal Science Research Unit for physical assistance.
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- Adeola O., Lawrence B.V., Cline T.R. Availability of amino acids for 10- to 20- kilogram pigs: lysine and threonine in soybean meal. J Anim Sci. 1994;72:2061–2067. doi: 10.2527/1994.7282061x. [DOI] [PubMed] [Google Scholar]
- Association of Official Analytical Chemists . 17th ed. Association of Official Analytical Chemists; Arlington, VA: 2000. Official methods of analysis. [Google Scholar]
- Baker D.H. Recent advances in use of the ideal protein concept for swine feed formulation. Asian-Aus J Anim Sci. 2000;13:294–301. [Google Scholar]
- Canadian Council on Animal Care . CCAC; Ottawa, ON: 2009. Guidelines on: the care and use of experimental animals in research, teaching and testing. [Google Scholar]
- Coma J., Carrion D., Zimmerman D.R. Use of plasma urea nitrogen as a rapid response criterion to determine the lysine requirement of pigs. J Anim Sci. 1995;73:472–481. doi: 10.2527/1995.732472x. [DOI] [PubMed] [Google Scholar]
- Commission Directive Establishing community methods for the determination of amino-acids, crude oils and fats, and olanquindox in feeding stuff and amending directive 71/393/EEC, Annex Part A: determination of amino acids. Off J Eur Comm. 1998;L257:14–23. [Google Scholar]
- Commission Directive Establishing community methods for the determination of vitamin A, vitamin E and Trp, Annex Part C: determination of tryptophan. Off J Eur Comm. 2000;L174:45–50. [Google Scholar]
- Defa L., Changting X., Shiyan Q., Jinhui Z., Johnson E.W., Thacker P.A. Effects of dietary threonine on performance, plasma parameters and immune function of growing pigs. Anim Feed Sci Tech. 1999;78:179–188. [Google Scholar]
- de Lange C.F.M., Pluske J., Gong J., Nyachoti C.M. Strategic use of feed ingredients and feed additives to stimulate gut health and development in young pigs. Livest Sci. 2010;134:124–134. [Google Scholar]
- Fernandez J.A., Strathe A. Dietary tryptophan and threonine supply to 28 days old weaned piglets. Anim Feed Sci Tech. 2009;154:265–270. [Google Scholar]
- Heo J., Kim J., Hansen J.R., Mullan B., Hampson D., Pluske J. Feeding a diet with decreased protein content reduces indices of protein fermentation and the incidence of post-weaning diarrhoea in weaned pigs challenged with an enterotoxigenic strain of Escherichia coli. J Anim Sci. 2009;87:2833–2843. doi: 10.2527/jas.2008-1274. [DOI] [PubMed] [Google Scholar]
- James B.W., Tokach M.D., Goodband R.D., Dritz S.S., Nelssen J.L., Usry J.L. The optimal true ileal digestible threonine requirement for nursery pigs between 11 to 22 kg. J Anim Sci. 2003;81:42. [Google Scholar]
- Kahindi R.K., Htoo J.K., Nyachoti C.M. Effect of dietary lysine content and sanitation conditions on performance of weaned pigs fed antibiotic-free diets. Can J Anim Sci. 2014;94:115–118. [Google Scholar]
- Kahindi R.K., Htoo J.K., Nyachoti C.M. AVTRW/BSAS/WPSA Conference - Innovation from animal science - Science into Practice University of Nottingham, Jubilee Campus, Wollaton Road, Nottingham, UK 29-30 April. 2014. The standardised ileal digestible lysine requirement of 7 to 16 kg weaned pigs fed antibiotic-free diet; p. 121. [Google Scholar]
- Lee C.L., Giles R., Bryden W.L., Downing J.L., Owens P.C., Kirby A.C. Performance and endocrine responses of group housed weaner pigs exposed to the air quality of a commercial environment. Livest Prod Sci. 2005;93:255–262. [Google Scholar]
- Le Floc'h N., Jondreville C., Matte J.J., Seve B. Importance of sanitary environment for growth performance and plasma nutrient homeostasis during the postweaning period in piglets. Arch Anim Nutr. 2006;60:23–34. doi: 10.1080/17450390500467810. [DOI] [PubMed] [Google Scholar]
- Le Floc'h N., Lebellago L., Matte J.J., Melchior D., Seve B. The effect of sanitary status degradation and dietary tryptophan content on growth rate and tryptophan metabolism in weaning pigs. J Anim Sci. 2009;87:1686–1694. doi: 10.2527/jas.2008-1348. [DOI] [PubMed] [Google Scholar]
- Le Floc'h N., Seve B. Modification of protein and amino acid metabolism during inflammation and immune system activation. Livest Prod Sci. 2004;87:37–45. [Google Scholar]
- Lenehan N.A., Tokach M.D., Dritz S.S., Goodband R.D., Nelssen J.L., Usry J.L. The optimal true ileal digestible lysine and threonine requirement for nursery pigs between 10 and 20 kg. J Anim Sci. 2004;82:293. [Google Scholar]
- Llames C.R., Fontaine J. Determination of amino acids in feeds: collaborative study. J Assoc Off Anal Chem. 1994;77:1362–1402. [Google Scholar]
- Marquardt R.R., Jin R.L., Kim J.W., Fang A., Frolich A., Baidoo S.K. Passive protective effect of egg-yolk antibodies against enterotoxigenic Escherichia coli K88+ infection in neonatal and early-weaned piglets. FEMS Immunol Med Microbiol. 1999;23:283–288. doi: 10.1111/j.1574-695X.1999.tb01249.x. [DOI] [PubMed] [Google Scholar]
- National Research Council . 11th ed. National Academy Press; Washington, DC: 2012. Nutrient requirements of swine. [Google Scholar]
- Nyachoti C.M., Omogbenigun F.O., Rademacher M., Blank G. Performance responses and indicators of gastrointestinal health in early-weaned pigs fed low-protein amino acid-supplemented diets. J Anim Sci. 2006;84:125–134. doi: 10.2527/2006.841125x. [DOI] [PubMed] [Google Scholar]
- Ren M., Liu X.T., Wang X., Zhang G.Z., Qiao S.Y., Zeng X.F. Increasing levels of standardized ileal digestible threonine attenuates intestinal damage and immune response in E. coli K88+ challenge in weaned pigs. Anim Feed Sci Tech. 2014;195:67–75. [Google Scholar]
- Robbins K.R., Saxton A.M., Southern L.L. Estimation of nutrient requirements using broken-line regression analysis. J Anim Sci. 2006;84:155–165. doi: 10.2527/2006.8413_supple155x. [DOI] [PubMed] [Google Scholar]
- Ruth M.R., Field C.J. The immune modifying effects of amino acids on gut-associated lymphoid tissue. J Anim Sci Biotech. 2013;4:27. doi: 10.1186/2049-1891-4-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saldana C.I., Knabe D.A., Owen K.Q., Burgoon K.G., Gregg E.J. Digestible threonine requirement of starter and finisher pigs. J Anim Sci. 1994;72:144–150. doi: 10.2527/1994.721144x. [DOI] [PubMed] [Google Scholar]
- Shen Y.B., Voilque G., Kim J.D., Odle J., Kim S.W. Effects of increasing tryptophan intake on growth and physiological changes in nursery pigs. J Anim Sci. 2012;90:2264–2275. doi: 10.2527/jas.2011-4203. [DOI] [PubMed] [Google Scholar]
- Trevisi P., Simongiovanni A., Casini L., Mazzoni M., Messori S., Priori D. Book of abstracts of the 62nd Annual Meeting of the European Federation of Animal Science, 29 August-2 September. 2011. Effect of dietary threonine on weaned piglets susceptible or not to Escherichia coli K88, under E. coli K88 challenge; p. 158. Stavanger. [Google Scholar]
- Waguespack A.M., Powell S., Roux M.L., Fruge E.D., Bidner T.D., Payne R.L. Technical note: effect of determining baseline plasma urea nitrogen concentrations on subsequent post-treatment plasma urea nitrogen concentrations in 20-59-kilogram pigs. J Anim Sci. 2011;89:4116–4119. doi: 10.2527/jas.2011-4328. [DOI] [PubMed] [Google Scholar]
- Wang X., Qiao S.Y., Liu M., Ma Y.X. Effects of graded levels of true ileal digestible threonine on performance, serum parameters and immune function of 10-25 kg pigs. Anim Feed Sci Tech. 2006;129:264–278. [Google Scholar]
- Williams N.H., Stahly T.S., Zimmerman D.R. Effect of chronic immune system activation on body nitrogen retention, partial efficiency of lysine utilization, and lysine needs of pigs. J Anim Sci. 1997;75:2472–2480. doi: 10.2527/1997.7592472x. [DOI] [PubMed] [Google Scholar]
- Zhang Z.F., Kim I.H. Effects of dietary threonine:lysine ratios on growth performance, blood urea nitrogen and nitrogen balance in weaned pigs. J Appl Anim Res. 2014;42:440–444. [Google Scholar]
- Zhang G.J., Xie C.Y., Thacker P.A., Htoo J.K., Qiao S.Y. Estimation of the ideal ratio of standardized ileal digestible threonine to lysine for growing pigs (22–50 kg) fed low crude protein diets supplemented with crystalline amino acids. Anim Feed Sci Tech. 2013;180:83–91. [Google Scholar]