Abstract
This experiment was conducted to investigate the effects of branched-chain amino acids (BCAA) supplemented in protein-restricted diets on the growth performance and the expression profile of amino acid transporters and energy metabolism related regulators in the white adipose tissue (WAT) of different regional depots including dorsal subcutaneous adipose (DSA) and abdominal subcutaneous adipose (ASA). A total of 24 crossbred barrows (7.40 ± 0.70 kg) were randomly divided into 4 groups and were fed the following isocaloric diets for 33 days: 1) a recommended adequate protein diet (AP, 20% CP, as a positive control); 2) a low protein diet (LP, 17% CP); 3) the LP diet supplemented with BCAA (LP + B, 17% CP) to reach the same level of the AP diet group; 4) the LP diet supplemented with 2 times the amount of BCAA (LP + 2B, 17% CP). The daily gain and daily feed intake of the LP diet group were the lowest among all the treatments (P < 0.01). The feed conversion was improved markedly in the group of LP + B compared with the LP diet group (P < 0.05). No significant difference was noted for the serum biochemical parameter concentrations of glucose, triglyceride, nonesterified fatty acid and insulin among the groups (P > 0.05). Moreover, BCAA supplementation down-regulated the expression levels of amino acid transporters including L-type amino acid transporter 1 and sodium-coupled neutral amino acid transporter 2 in DSA, but up-regulated the expression level of L-type amino acid transporter 4 in ASA (P < 0.05). Meanwhile, the energy sensor AMP-activated protein kinase α was activated in the DSA of pigs fed LP diet and in the ASA of the pigs fed AP or LP + 2B diets (P < 0.05). The mRNA expression profile of the selected mitochondrial component and mitochondrial biogenesis associated regulators in DSA and ASA also responded differently to dietary BCAA supplementation. These results suggested that the growth performance of growing pigs fed protein restricted diets supplemented with BCAA could catch up to that of the pigs fed AP diets. The results also partly demonstrated that the regulation mechanisms of BCAA are different in the adipose tissues of different depots.
Keywords: Branched-chain amino acid, Protein-restricted diet, Energy metabolism, White adipose tissue, Pig
1. Introduction
The overall metabolic profile of pigs is similar to that of humans, thus pigs may be an optimal animal model for investigating lipid metabolism and metabolic disorders (Douglas, 1972, Spurlock and NK, 2008). Currently, adipose tissue has been perceived predominantly as an active fuel reservoir in role of energy balance, instead of a metabolism inertness depot in the last decade (Valenzuela and Sanhueza, 2009). Sufficient in vitro and in vivo evidence has pointed out that adipose tissue is capable of metabolizing significant quantities of branch chain amino acids (BCAA), including leucine, isoleucine and valine (Rosenthal et al., 1974, Tischler and Goldberg, 1980, Layman, 2003, Herman et al., 2010). The critical roles of BCAA in protein synthesis and turnover have been widely documented, especially in skeletal muscle (Corporation HP, 2014). Now, the potential relationship between BCAA function and energy metabolism in adipose tissue is of great interest. It is beneficial for us to know how energy metabolism is regulated and coordinated by BCAA in white adipose tissue (WAT). The oxidation of BCAA seems to be advantageous to human metabolic health as their catabolism increases fatty acid oxidation as well as controls obesity (Corporation HP, 2014). Nishimura et al. (2010) have observed that isoleucine supplementation leads to a decrease in weight gain and a reduction in lipid mass. In a double-blind, placebo-control, cross-over study on human volunteers, Gualano et al. (2011) noticed that BCAA supplementation increases lipid oxidation during exercise and helps to overcome fatigue. Qin et al. (2011) investigated on middle aged healthy adults and found that there is an inverse relation between BCAA intake and obesity. All of these findings suggest that BCAA have a large influence on energy metabolism.
Energy metabolism and mitochondrial biogenesis are inextricably linked. The AMP-activated protein kinase α (AMPKα) is a crucial metabolic fuel gauge and a signal transducer for maintaining energy homeostasis and regulating mitochondrial biogenesis. Notably, the expression of multiple genes, such as peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) and silent information regulator 1 (SIRT1) involved in the regulation of energy metabolism, appears to be associated with mitochondrial biogenesis (Koves et al., 2005, Bastin et al., 2008). The impact of BCAA on energy balance and mitochondrial function in skeletal muscle has been widely studied (Scarpulla et al., 2012, Liang et al., 2014), while only several in vitro experiments have indicated that leucine mediates the energy metabolism of adipocyte partly through mitochondrial biogenesis (Sun and Zemel, 2009). The BCAA-specific transporters play critical roles in this process, which are present on membranes to sense amino acid availability and relay nutrient signals to the cell interior (Hundal and Taylor, 2009, Nicklin et al., 2009, Duan et al., 2015) Several predominant transporters that are recently reported to be directly or indirectly associated with BCAA have been studied (Evans, 2007).
In the present study, we attempted to address whether BCAA affect the growth performance and the expression levels of selected genes that are involved in AA transporters and energy metabolism in WAT including dorsal subcutaneous adipose (DSA) tissue and abdominal subcutaneous adipose (ASA) tissue in vivo using the pig as an animal model.
2. Materials and methods
2.1. Animals and experimental diets
All procedures outlined in this experiment were approved by the Animal Care and Use Committee of the Chinese Academy of Sciences (Fugui et al., 2010).
A total of 24 crossbred barrows (Landrace × Large White, 7.40 ± 0.70 kg BW) were randomly divided into 4 treatments. Each treatment had 6 replicates (n = 6). Pigs were housed individually in cages (Tan et al., 2011) and fed diets based on maize, soybean meal, fish meal and whey powder (Table 1). The dietary treatments were as follows: 1) a recommended adequate protein (AP) diet containing 20% CP, considered as the positive control group; 2) a low protein (LP) diet containing 17% CP, considered as the negative control group; 3) the LP diet supplemented with BCAA (LP + B) to contain the same level as that of the AP diet; 4) the LP diet supplemented with 2 times amount of the BCAA (LP + 2B). All experimental diets were formulated to be isocaloric, and the limiting AA, including lysine, methionine, threonine and tryptophan, were all designed to meet the National Research Council (NRC, 2012) recommendations. The pigs had ad libitum access to diets and drinking water throughout the study (Tan et al., 2009). All pigs were weighed at the start and the end of this 33-day experiment, and feed intakes were recorded on a daily basis to calculate final body weight (FBW), average daily gain (ADG), average daily feed intake (ADFI) and feed conversion ratio (FCR) (Yin et al., 2010).
Table 1.
Ingredients and nutrient levels of the diets (as-fed basis).1
| Item | AP | LP | LP + B | LP + 2B |
|---|---|---|---|---|
| Ingredient, % | ||||
| Maize | 59.86 | 70.09 | 70.09 | 70.09 |
| Dehulled soybean meal | 22.00 | 10.70 | 10.40 | 9.60 |
| Whey powder | 5.00 | 5.00 | 5.00 | 5.00 |
| Fish meal | 4.00 | 4.00 | 4.00 | 4.00 |
| Concentrated soybean protein | 5.00 | 5.00 | 5.00 | 5.00 |
| Soybean oil | 0.79 | 0.40 | 0.50 | 0.70 |
| L-Lysine HCl | 0.22 | 0.48 | 0.48 | 0.50 |
| DL-Methionine | 0.14 | 0.23 | 0.23 | 0.24 |
| Threonine | 0.08 | 0.22 | 0.23 | 0.24 |
| Tryptophan | 0.01 | 0.06 | 0.06 | 0.06 |
| Isoleucine | – | – | 0.17 | 0.34 |
| Leucine | – | – | 0.24 | 0.48 |
| Valine | – | – | 0.16 | 0.32 |
| Alanine | – | 0.42 | – | – |
| Dicalcium phosphate | 1.00 | 1.30 | 1.30 | 1.30 |
| Limestone | 0.60 | 0.60 | 0.60 | 0.60 |
| Salt | 0.30 | 0.30 | 0.30 | 0.30 |
| Bentonite | – | 0.20 | 0.24 | 0.23 |
| Premix2 | 1.00 | 1.00 | 1.00 | 1.00 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 |
| Nutrient levels, % | ||||
| NE, MJ/kg | 10.37 | 10.38 | 10.38 | 10.37 |
| CP | 19.50 | 16.70 | 16.70 | 17.20 |
| SID amino acid3 | ||||
| Lys | 1.23 | 1.23 | 1.23 | 1.23 |
| Met + Cys | 0.68 | 0.68 | 0.68 | 0.68 |
| Thr | 0.73 | 0.73 | 0.73 | 0.73 |
| Trp | 0.20 | 0.20 | 0.20 | 0.20 |
| Leu | 1.56 | 1.32 | 1.56 | 1.77 |
| Ile | 0.75 | 0.58 | 0.75 | 0.90 |
| Val | 0.84 | 0.68 | 0.84 | 0.98 |
| His | 0.47 | 0.39 | 0.38 | 0.38 |
| Phe | 1.09 | 0.93 | 0.92 | 0.91 |
| Arg | 1.11 | 0.84 | 0.83 | 0.81 |
SID = standardized ileal digestible.
AP = adequate protein diet; LP = low protein diet; LP + B = LP diet supplemented with BCAAs to contain the same level as that of the AP diet; LP + 2B = LP diet supplemented with two times amount of BCAAs.
Supplied per kilogram of diet: CuSO4·5H2O 19.8 mg; KI 0.20 mg; FeSO4·7H2O 400 mg; NaSeO3 0.56 mg; ZnSO4·7H2O 359 mg; MnSO4·H2O 10.2 mg; Vitamin K (menadione) 5 mg; Vitamin B1 2 mg; Vitamin B2 15 mg; Vitamin B12 30 μg; Vitamin A 5400 IU; Vitamin D3 110 IU; Vitamin E 18 IU; Choline chloride 80 mg; Antioxidants 20 mg; Fungicide 100 mg.
Calculated nutrient levels.
2.2. Sample collection
Blood samples (about 5 mL from each pig) were collected into 10-mL tubes and centrifuged at 3,000 × g at 4 °C for 15 min. Then, the supernatants (serum) were collected and stored at −20 °C until required for analysis. Immediately after blood sampling, pigs were electrically stunned (250 V, 0.5 A, 5 or 6 s), exsanguinated and eviscerated in a slaughterhouse (Liu et al., 2012; Tan et al., 2011). Adipose tissue samples including DSA and ASA were rapidly excised from the right side of the carcasses. Samples were immediately frozen in liquid nitrogen and then stored at −80 °C until further analysis (Liu et al., 2015).
2.3. Serum chemical parameters
We determined the serum concentrations of glucose (Glu) and triacylglycerols (TG) using a Biochemical Analytical Instrument (Beckman CX4) and commercial kits (Sino-German Beijing Leadman Biotech Ltd., Beijing, China). We analyzed the nonesterified fatty acid (NEFA) concentration using colorimetric assays according to the manufacturer's instructions of the NEFA detection kit (Nanjing Jianchen Bioengineering Institute, China). Six samples of each group were measured.
2.4. RNA extraction and cDNA synthesis
We isolated the total RNA from DSA and ASA using the TRIZOL reagent (100 mg tissue per 1 mL Trizol; Invitrogen, Carlsbad, USA). The integrity of RNA was checked by 1% agarose gel electrophoresis, stained with 10 μg/mL ethidium bromide. We determined the quality and quantity of RNA by ultraviolet spectroscopy using a spectrophotometer (NanoDrop ND-1000; Thermo Fisher Scientific, DE, USA). The RNA was treated with DNase I (Invitrogen, CA, USA) according to the manufacturer's instructions. Thereafter, about 1.0 μg of total RNA was incubated with DNase I (Fermentas, WI, USA). Later we synthesized cDNA using the First-Strand cDNA Synthesis Kit (Fermentas) according to the manufacturer's protocol. The cDNA was synthesized with Oligo dT and superscript II reverse-transcriptase. The cDNA were stored at −80 °C before further processing (Huang et al., 2016).
2.5. Quantitative real-time PCR analysis
We previously determined the changes in the mRNA expression of the selected genes using real-time PCR (Li et al., 2014a, Li et al., 2014b). The primer sequences for selected genes are listed in Table 2. We performed real-time PCR for each cDNA sample in duplicate, using SYBR Green I as PCR core reagents in a final volume of 10 μL. Polymerase chain reaction conditions were as follows: incubation for 10 min at 95 °C, followed by 40 cycles of denaturation for 15 s at 95 °C, annealing and extension for 60 s at (56 to 64 °C). We acquired the target genes mRNA expression levels in arbitrary units from the value of the threshold cycle (Ct) of the real-time PCR as related to that of β-actin using the comparative Ct method through the formula 2−ΔΔCt [ΔΔCt = (Ct gene of interest − Ct β-actin)treat − (Ct gene of interest − Ct β-actin)untreat] (Pfaffl, 2001). We used β-actin house-keeping gene as an internal control to normalize the expression of target genes (Zhang et al., 2013a).
Table 2.
Primers used for real-time PCR analysis.
| Target genes | Primer sequences (5′–3′) | Products, bp | Genbank accession No. |
|---|---|---|---|
| LAT1/SLC7A7 | F: TTTGTTATGCGGAACTGG | 155 | NM_001110421 |
| R: AAAGGTGATGGCAATGAC | |||
| LAT4/SLC43A2 | F: ACGGAGCAAGTAACCCCAGC | 235 | XM_003358191 |
| R: GCCACGAGGATGACGATGAA | |||
| SNAT2/SLC38A2 | F: TACTTGGTTCTGCTGGTGTCC | 212 | XM_003126626 |
| R: GTTGTGGGCTGTGTAAAGGTG | |||
| AMPKα | F: CAGACAGCCCTAAAGCAAGA | 311 | NM_214266 |
| R: CTCCAGCACCTCATCATCAA | |||
| PGC-1α | F: GCCCAGTCTGCGGCTATTT | 265 | GU991077 |
| R: GTTCAGCTCGGCTCGGATTT | |||
| SIRT1 | F: GGTTTGAAGAATGTTGCCTG | 114 | NM_001145750 |
| R: CCGTTTACTAATCTGCTCCT | |||
| Cyt c | F: CTGCGAGTGGTGGATTGT | 222 | NM_001129970 |
| R: ATGCCTTTGTTCTTGTTGG | |||
| ATPase 6 | F: CTATTCCCAACACCCAAACG | 196 | AJN90987 |
| R: TGGGTGTGAATGAGTGTGGT | |||
| UCP2 | F: CACCAAGGGCTCTGAGCATG | 387 | XM_005667098 |
| R: TCTACAGGGGAGGCGATGAC | |||
| UCP3 | F: GACGTGGTGAAGGTTCGATT | 330 | DQ530368 |
| R: CGAGTTCATGTACCGGGTCT | |||
| NRF-1 | F: TGTGTTGAATGTGTCCCCCAA | 136 | AY496013 |
| R: CTCCCAAAGGGCAACAATGC | |||
| TFAM | F: GACTACTGCGTCTGCACCTT | 116 | NM_001130211 |
| R: GCAACTCTTCAGACCTCGCT | |||
| β-actin | F: TGCGGGACATCAAGGAGAAG | 216 | XM_003357928.2 |
| R: AGTTGAAGGTGGTCTCGTGG |
LAT1 = anti-L-type amino acid transporter 1; LAT4 = L-type amino acid transporter 4; SNAT2 = sodium-coupled neutral amino acid transporter 2; AMPKα = AMP-activated protein kinase α; PGC-1α = peroxisome proliferator-activated receptor gamma coactivator 1-alpha; SIRT1 = silent information regulator 1; Cyt c = cytochrome c; UCP2 = uncoupling protein 2; UCP3 = uncoupling protein 3; NRF-1 = nuclear respiratory factors-1; TFAM = transcription factor A.
2.6. Western blotting analysis
Western blot analysis was conducted according to previous studies (Li et al., 2014a, Li et al., 2014b). Briefly, about 30 to 50 μg of the total protein extracted from DSA and ASA was separated by a reducing SDS-PAGE electrophoresis (Wu et al., 2013b). After blocking with 5% nonfat milk, the blots were incubated overnight at 4 °C with gentle agitation in dilutions of primary antibodies. The following antibodies were used: rabbit anti-phospho (P)-AMPKα (Thr172) (Cell Signaling Technology, MA, USA, 1:1000); anti-L-type amino acid transporter 1 (LAT1) (Santa Cruz Biotechnology, CA, USA, 1:200), L-type amino acid transporter 4 (LAT4) (Santa Cruz Biotechnology, CA, USA, 1:200) and sodium-coupled neutral amino acid transporter 2 (SNAT2) (Santa Cruz Biotechnology, CA, USA, 1:200). The membranes were then rinsed in Tris-buffered saline containing 0.1% Tween 20 and incubated with second antibody peroxidase-conjugated anti-rabbit or anti-goat IgG (Santa Cruz) for 1 h at a dilution of 1:5,000. Mouse anti-β-actin (Santa Cruz), or rabbit anti-AMPKα (Cell Signaling) diluted at 1:1,000 were used as an internal control. The bands of the protein were visualized with a chemiluminescent reagent (Pierce, Rockford, IL, USA) by a digital luminescent image analyzer LAS-1000 (Fujifilm, Japan). We quantified the resultant signals using Alpha Imager 2200 software (Alpha Innotech Corporation, San Leandro, CA, USA).
2.7. Statistical analysis
We analyzed all obtained data using one-way analysis of variance (ANOVA) with the aid of SAS 8.2 software package (SAS Institute Inc, North Carolina, USA). We separated differences between significant mean values using Duncan's multiple range tests and considered it to be statistically significant at P < 0.05.
3. Results
3.1. Growth performance of the pigs
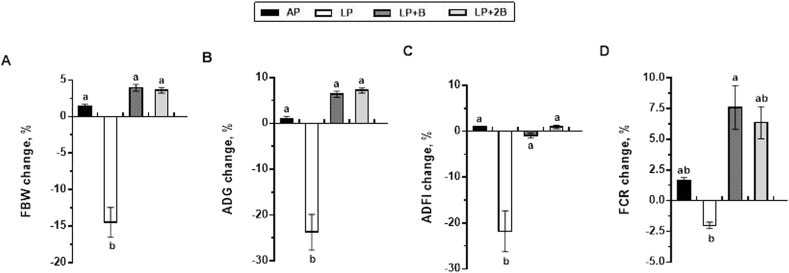
The growth performance of the pigs fed the different diets is presented in Fig. 1. The LP diet group resulted in an approximate 15% reduction in FBW and 25% reduction in ADG compared with the AP diet group (positive control) (Fig. 1A and B). Consistent with the rapid weight loss, ADFI were also significantly decreased over 20% in the LP diet group compared with the AP diet group (Fig. 1C). Furthermore, when the LP diet supplemented with BCAAs (the LP + B or LP + 2B group), FBW, ADG and ADFI were all significantly improved compared with the LP diet group catching up to the growth parameters of the AP diet group. Notably, the LP + B diet group had higher FCR in comparison with the AP diet group, and it had even slightly higher FCR than the LP + 2B diet group, whereas the LP diet group showed about a 2.5% reduction in FCR relative to the AP diet group (Fig. 1D).
Fig. 1.
Protein-restricted diets supplemented with BCAA affected the growth performance of the growing pigs. The pigs were fed an adequate protein (AP) diet, a low protein (LP) diet, a LP diet supplemented with BCAA (LP + B) diet, and a LP diet supplemented with 2 times amount of BCAAs (LP + 2B). Data were calculated based on the values of the LP, LP + B, LP + 2B diet groups versus the AP diet group (positive control). Data are means ± SE (n = 6). A: Final body weight (FBW) change; B: Average daily gain (ADG) change; C: Average daily feed intake (ADFI) change; D: Feed conversion ratio (FCR) change.
3.2. Serum concentrations of the biochemical parameters
The serum concentrations of the biochemical parameters are shown in Table 3. No significant differences in serum GLU, TG, NEFA and insulin concentrations among the 4 diet groups were noted.
Table 3.
Serum concentrations of the biochemical parameters.1
| Item | AP | LP | LP + B | LP + 2B | P-value |
|---|---|---|---|---|---|
| GLU, mmol/L | 5.63 ± 0.39 | 4.44 ± 0.25 | 5.04 ± 0.67 | 5.79 ± 0.83 | 0.34 |
| TG, mmol/L | 0.64 ± 0.07 | 0.85 ± 0.09 | 0.70 ± 0.13 | 0.67 ± 0.07 | 0.44 |
| NEFA, mmol/L | 1.34 ± 0.15 | 1.13 ± 0.12 | 1.11 ± 0.14 | 1.42 ± 0.15 | 0.41 |
| Insulin, μIU/mL | 9.10 ± 1.03 | 10.30 ± 1.23 | 10.38 ± 1.33 | 10.15 ± 1.44 | 0.87 |
GLU = glucose; TG = triglyceride; NEFA = nonesterified fatty acid.
Data are expressed as means ± SEM, n = 6.
AP = adequate protein diet; LP = low protein diet; LP + B = LP diet supplemented with BCAAs to contain the same level as that of the AP diet; LP + 2B = LP diet supplemented with two times amount of BCAAs.
3.3. Effects of dietary BCAAs on the expression levels of genes involved in BCAA transporters in different adipose depots
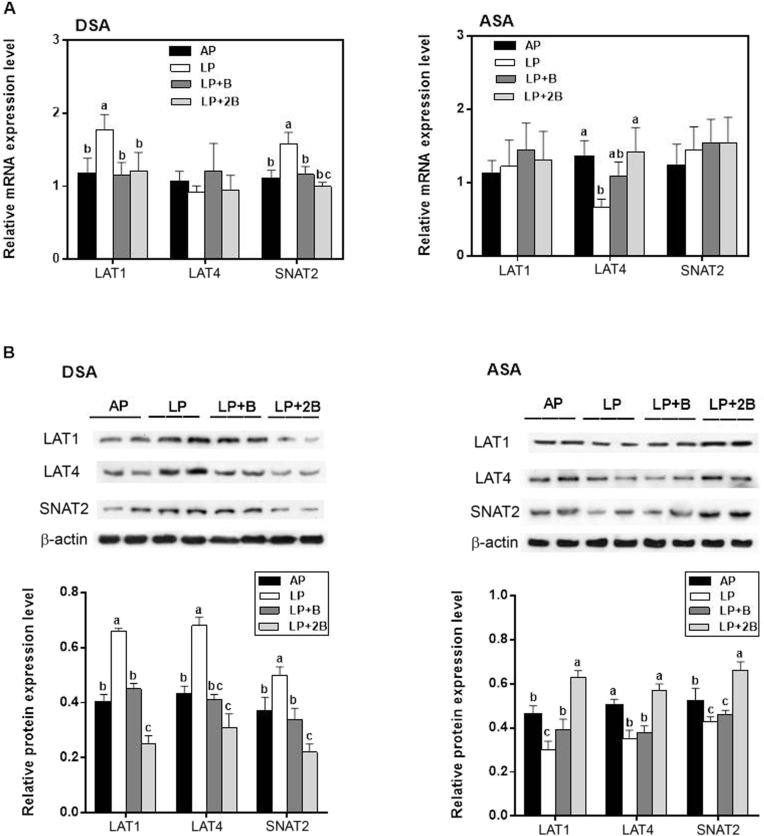
The mRNA and protein expression levels of LAT1, LAT4 and SNAT2 in DSA and ASA tissues of the pigs are shown in Fig. 2. In DSA tissue, the mRNA expression levels of LAT1 and SNAT2 significantly increased in the pigs fed the LP diet (P < 0.05) compared with the other 3 treatments. In ASA tissue, the mRNA expression level of LAT4 in the pigs fed the LP diet was the lowest among the groups; however, no differences were detected in the mRNA expression levels of LAT1 and SNAT2 among the 4 treatments (P > 0.05).
Fig. 2.
The gene transcript (A) and protein expression (B) levels of amino acid transporters (LAT1, LAT4 and SNAT2) regulated by dietary BCAA in DSA and ASA tissues of the growing pigs. We used β-actin as an internal control. Data were represented as means ± SE (n = 6); bars with different letters (a, b, c) are considered as significant difference (P < 0.05). DSA = dorsal subcutaneous adipose; ASA = abdominal subcutaneous adipose; AP = adequate protein diet; LP = low protein diet; LP + B = LP diet supplemented with BCAAs; LP + 2B = LP diet supplemented with two times amount of BCAAs.
As shown in Fig. 2B, the analysis of DSA tissue revealed a pronounced promotion in LAT1, LAT4 and SNAT2 protein expression levels in the pigs fed the LP diet relative to the other 3 treatments (P < 0.05), and the protein-restricted diet supplemented with BCAA markedly reduced the value (P < 0.05). This effect was further promoted by adding double amounts of BCAA, the magnitude of the reduction was greater, and the protein expression levels of the BCAA transporters were even significantly lower than those of the AP diet group (P < 0.05); however in the ASA tissue, the LAT1 protein expression level was the lowest in the LP diet group but the highest in the LP + 2B group (P < 0.05). The LP and LP + B diets suppressed the value of LAT4 and SNAT2, and the LP + 2B diet restored the protein expression levels of the transporters compared with the AP diet group (P < 0.05).
3.4. Effects of dietary BCAA on the expression levels of energy metabolism related regulators in different adipose depots
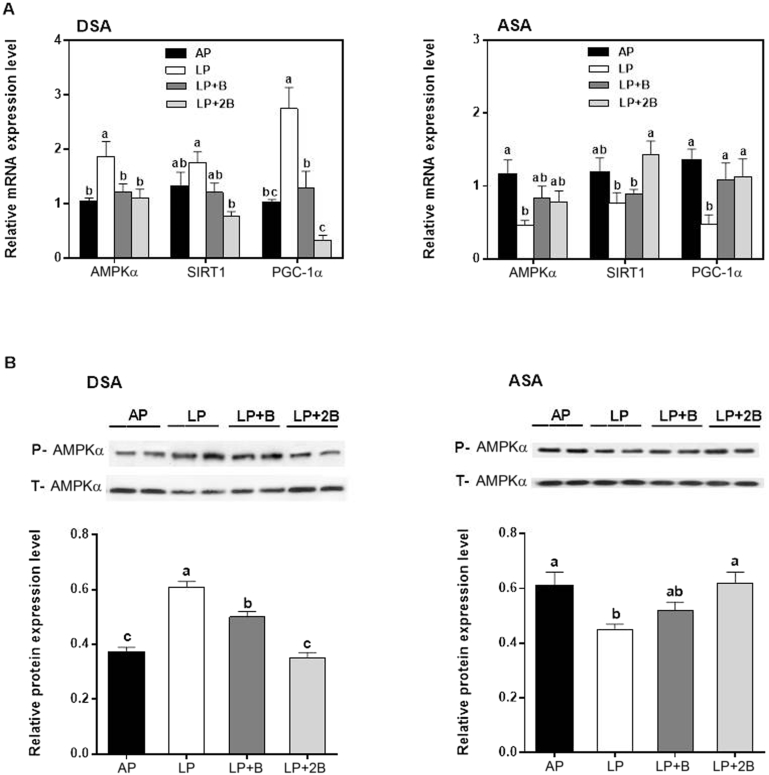
As shown in Fig. 3A, in DSA tissue, the mRNA expression levels of AMPKα and PGC-1α were the highest in the pigs fed the LP diet, and their values were decreased in the pigs fed the BCAA supplementation diets compared with that of the LP diet group (P < 0.05). The mRNA expression level of SIRT1 was decreased in the LP + 2B group relative to that of the LP diet group (P < 0.05). However, in ASA tissue, the trend of expression profile was almost reverse. The mRNA expression level of AMPKα was decreased in the pigs fed the LP diet relative to that of the AP diet group, and the value of SIRT1 was increased in the pigs fed the LP + 2B diet compared with that of the pigs fed the LP diet or LP + B diet (P < 0.05). Furthermore, the LP diet decreased the mRNA expression level of PGC-1α compared with that of the AP diet group, but BCAAs supplementation restored the value (P < 0.05).
Fig. 3.
The gene transcript (A) and protein expression (B) levels of energy metabolism related factors (AMPKα, SIRT1 and PGC-1α) regulated by dietary BCAAs in DSA and ASA tissues of the growing pigs. We used β-actin as an internal control. Data were represented as mean ± SE (n = 6); Values with different letters (a, b, c) are considered as significant difference (P < 0.05). DSA = dorsal subcutaneous adipose; ASA = abdominal subcutaneous adipose; AP = adequate protein diet; LP = low protein diet; LP + B = the LP diet supplemented with BCAAs; LP + 2B = the LP diet supplemented with two times amount of BCAAs.
As shown in Fig. 3B, the phosphorylation level of AMPKα in DSA of pigs was promoted in the LP diet group relative to the AP diet group, but the value was then decreased with the supplementation of BCAA (P < 0.05). In contrast, in ASA, the phosphorylation level of AMPKα was reduced in the LP diet group compared with the AP diet group, but then gradually increased with the supplementation of BCAA, and there were no difference among the AP, LP + B and LP + 2B diets (P > 0.05).
3.5. Effects of dietary BCAA on the transcript levels of the genes involved in mitochondrial function
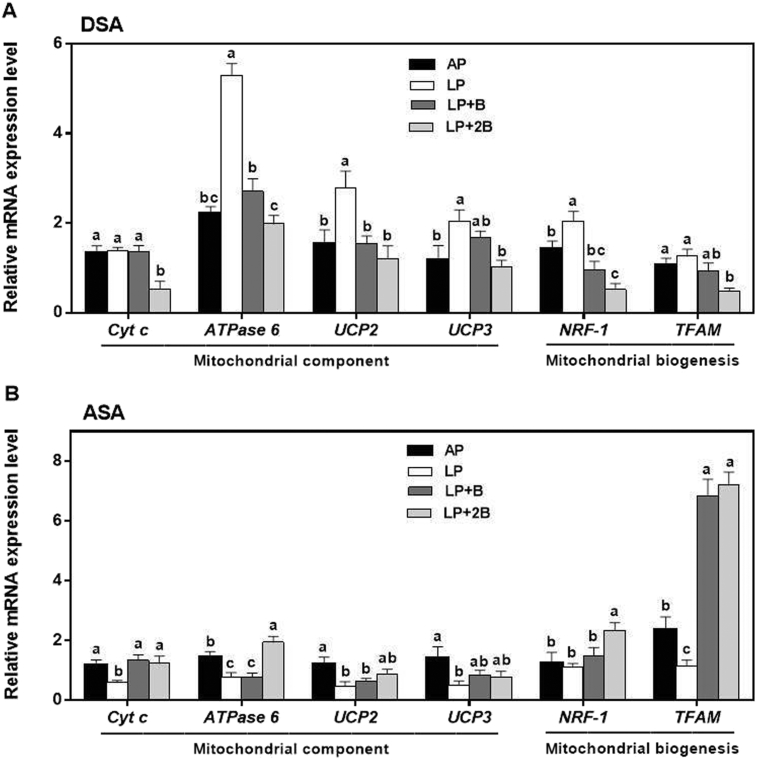
As shown in Fig. 4A, in DSA tissue, the LP + 2B diet group showed lower cytochrome c (Cyt c) mRNA expression level than the other treatments (P < 0.05). For ATPase 6, uncoupling protein 2 (UCP2), uncoupling protein 3 (UCP3) and nuclear respiratory factors-1 (NRF-1), the gene transcripts were the highest in the LP diet group, and the value was decreased after adding BCAA. The mRNA expression level of mitochondrial transcription factor A (TFAM) was higher in the AP and LP diet groups than that in the LP + 2B diet group (P < 0.05).
Fig. 4.
The gene transcript levels of mitochondrial component (Cyt c, ATPase6, UCP2 and UCP3) and mitochondrial biogenesis (NRF-1 and TFAM) related factors regulated by dietary BCAA in DSA (A) and ASA (B) tissues of the growing pigs. We used β-actin as an internal control. Data were represented as means ± SE (n = 6); bars with different letters (a, b, c) are considered as significant difference (P < 0.05). DSA = dorsal subcutaneous adipose; ASA = abdominal subcutaneous adipose; AP = adequate protein diet; LP = low protein diet; LP + B = LP diet supplemented with BCAAs; LP + 2B = LP diet supplemented with two times amount of BCAAs.
In contrast, in ASA tissue, gene transcripts of the above mentioned genes including Cyt c, ATPase 6, UCP2, UCP3, and TFAM were slightly or significantly reduced in the LP diet group compared with the AP diet group (P < 0.05), but the values of Cyt c, ATPase 6, and TFAM were increased in the BCAA supplementation groups. The expression level of NRF-1 in the LP + 2B diet group was higher than the other 3 treatments (Fig. 4B).
4. Discussion
Adipose tissue is not a homogeneous organ, and in mammals it develops in diverse sites throughout the body and generally occurs in subcutaneous layers between muscle and dermis. Regional depots of adipose tissue are different in structural organization, cellular size and biological function (Kim et al., 2011). Few investigations have assessed the role of dietary BCAAs in regulating energy metabolism in adipose tissue in vivo. Thus, we compared the response of the two depots of WAT, including DSA and ASA, to dietary BCAA supplementation in protein-restricted diets. Our study confirmed previous findings that supplementation of BCAA to the LP diet (17% CP) obtained improved growth response and feed conversion efficiency to a level that was similar to pigs fed the AP diet (Lordelo et al., 2008, Figueroa et al., 2003, Zhang et al., 2013b, Liu et al., 2014). However, dietary BCAA did not influence the serum concentration of the biochemical parameters including GLU, TG, NEFA and insulin in the present study.
Branched-chain amino acids are substrates for tissue protein synthesis. An increasing volume of data supports that BCAA play vital roles as nutrient signals and energy sources in the physiological functions regulating metabolism and maintaining homeostasis (Nair and Short, 2005, Kim et al., 2007, Herman et al., 2010, Hou et al., 2015, Wu et al., 2007, Wu et al., 2009, Wu et al., 2013a). Interest in the contribution of adipose tissues to sense extracellular BCAA levels and modulate cellular metabolism has been renewed within recent observations. Some studies indicate that doubled dietary leucine intake results in a decrease in adiposity, also reverse many of the metabolite abnormalities caused by a high-fat diet (Macotela et al., 2011). This immediately sparked the speculation that adipose tissue could respond to the leucine content in diets. But there is another report demonstrates that mice maintained on a leucine-deficient diet experienced a dramatic reduction in abdominal fat mass, and similar data are obtained after isoleucine or valine-deprivation, suggesting that WAT can sense the change of BCAA content and then elicit lipolysis in response to BCAA deprivation. However, based on the above studies, the BCAA sensing mechanisms in adipose tissue are complex and the results reported are controversial. In this experiment, we attempted to determine the relationship between the expression profiles of lipid metabolism related regulators of adipose tissue and dietary BCAA in vivo.
Amino acid transporters, which are ubiquitously present in membranes of many cell types including adipocyte, are gatekeepers of the cells (Hundal and Taylor, 2009, Duan et al., 2015). The L-type amino acid transporter (LAT) family is responsible for signal perception and transmitting of the majority of cellular BCAA, and launching a series of cascade responses. The widely-known one is the LAT1 (SLC7A5), which is form heteromeric complexes with the heavy chain of 4F2 antigen (Kanai et al., 1998, Wang and Holst, 2015). As a newly identified LAT, LAT4 (SLC43A2), does not appear to require a binding partner (Bodoy et al., 2005). Another class of BCAA-associated transporter is the system A-type including the sodium-coupled neutral AA transporters (i.e., SNAT2/SLC38A2). The increased activity of AA transporters is mainly due to their up-regulation of respective expression levels (Hundal and Taylor, 2009). In this study, we noticed that all of the selected genes encoding for BCAA transporters exhibited greater protein expression levels in the DSA of pigs fed the LP diet than other groups, partly suggesting higher transmitting capacity of the transporters. The mRNA and protein expression levels of LAT1 and SNAT2 in the DSA of pigs fed the LP + B diet were not different from those of the pigs fed the AP diet. Moreover, the reduction in the protein expression levels of LAT1 and SNAT2 was greater in the LP + 2B group compared with that of the LP + B diet group. The LP diet decreased feed intake, which resulted in AA deprivation. Several studies have found that some of the transporters gene expression levels were increased following AA deprivation (e.g., starvation, feed-restriction) in cells (Palii et al., 2009) of rats (Ihara et al., 2000), chickens (Chen et al., 2005), and humans (Palii et al., 2004). Therefore, compared with AP, LP + B or LP + 2B diet groups which have adequate or excess BCAAs in diet, the LP diet group with AA deprivation may lead to an increase in LAT1, SNAT2 mRNA and protein expression levels to efficiently sense and transport BCAAs in adipocytes of DSA tissue. The result is in agreement with the previous study demonstrating that the bioavailability of dietary amino acids is higher in animals fed a LP diet compared with those given an AP diet (Rezaei et al., 2013). Another possibility is that elevating transporter expression may be a mechanism to transport more AA out of cells for body survival when AA are deprived (Bode, 2001). However, we found that the expression levels of LAT1, LAT4 and SNAT2 changed differently in response of the dietary BCAA intake in ASA. It was convinced that BCAA might mediate important and different regulators through affecting the expression of AA transporters in different depots of WAT to maintain metabolism homeostasis.
It is well known that a higher expression of LAT1, LAT4 and SNAT2 contributed to absorb BCAA resulted in activation of the protein synthesis signaling pathway (Peyrollier et al., 2000, Durante et al., 2004, Baird et al., 2009, Wang and Holst, 2015). Protein synthesis is also an energy-consuming process. As a crucial cellular energy sensor, AMPKα, is activated by ATP production and it transduces signals for maintaining energy homeostasis (Gwinn et al., 2008). As the protein expression level of transporters is described above, P-AMPKα exhibited the highest phosphorylation level in the DSA of the pigs fed the LP diet, but the addition of BCAAs to the LP diet markedly inhibited P-AMPKα activity, an effect which was more pronounce in BCAA excess diets (LP + 2B) to a level that was similar to that of the AP diet group. It is in agreement with a previous study where dietary leucine supplementation suppressed AMPK phosphorylation in rat skeletal muscle (Shi et al., 2012).
Another metabolic sensor is SIRT1 (Chan and Arany, 2014), an important upstream regulator of PGC-1α, well recognized to stimulate the expression of mitochondrial biogenesis genes in WAT, such as NRF-1, and TFAM (Scarpulla et al., 2012). Studies observed that the SIRT1 mRNA level is obviously correlated with PGC-1α, NRF-1, TFAM gene expression (Larsson et al., 1998, Tcherepanova et al., 2000, Jarno et al., 2010). Both mitochondrial biogenesis and mitochondrial component associated genes are the indicators of mitochondrial function (Sun and Zemel, 2009). As per previous studies, we also noticed that it was characterized by an increased transcript level of SIRT1, PGC-1α, ATPase 6, UCP2, UCP3 and NRF-1 in the DSA of the pigs fed the LP diet relative to that of the pigs fed the AP diet. It was indicated that BCAA deprivation provoked mitochondrial biogenesis capacity and fatty acid oxidation in DSA tissue and tended to restitute and maintain the energy homeostasis of the body. In contrast, the addition of BCAA to the LP diet attenuated these effects. This may aid in explaining the concept that excess BCAA are stored in the form of lipid by stimulating the lipogenic response (Felig et al., 1969). Of note, the mRNA expression alterations of the genes associated with mitochondrial function were consistent with P-AMPKα protein expression level change. Thus we speculated that BCAAs might have an indirect effect in mediating energy metabolism in DSA through their roles in mitochondrial function.
It should also be noted that unlike in DSA, the transcript levels of most selected marker genes of mitochondrial function and energy metabolism were the lowest in the ASA of pigs fed the LP diet. However, the supplementation of BCAA to the LP diet, especially the diet of LP + 2B, distinctly promoted the transcript levels of those above-mentioned genes except for UCP2 and UCP3. It appears that the LP + 2B diet was likely benefit for the function of mitochondria and the activation of cellular energy metabolism in ASA. There are other reports supporting that leucine is a key amino acid that can increase mitochondrial biogenesis and fatty acid oxidation in both muscle cells and adipose cells (Sun and Zemel, 2009). Moreover, BCAA are more energy efficient than Glu. The complete oxidation of leucine in muscle produces more energy than that of Glu in the form of ATP. It is possible that in ASA, the oxidation of excess BCAA increases to meet some special energy demands of body, for example, to support the acceleration of protein synthesis (Corporation HP, 2014). Canto and Auwerx (2009) reported that AMPKα, SIRT1 and PGC-1α are all tightly interconnected and might act as an orchestrated network to improve metabolic fitness and mitochondrial flexibility, indicating the activation of an energy sensing pathway in the ASA of pigs fed the LP diet supplemented with BCAA. The sense and transmit of AA including BCAA are an extremely complicated physiological process influenced by multiple factors (Hundal and Taylor, 2009). It is not surprising that adipose tissue is exquisitely designed to respond to acute or chronic changes in nutritional cues. Typically, different WAT depots ordinarily exhibit different alterations in the molecular levels of factors responding to nutritional (e.g., BCAA) stimulation.
5. Conclusions
This study demonstrated that a low protein (17% CP) diet supplemented with BCAAs could enable growing pigs to catch up to the growth performance of the pigs fed adequate dietary protein (20%) diets. Dietary BCAAs differently regulated the AA transporters including LAT1, LAT4 and SNAT2 in DSA and ASA tissues accompanied by the activation of AMPKα, and the mitochondrial biogenesis and component related regulators were also involved. The findings provided novel insight into the expression profile of energy homeostasis and mitochondrial function associated factors in different adipose depots of growing pigs fed protein-restricted diets supplemented with BCAA. Primary Cells isolated from DSA and ASA will be cultured in our following studies to further determine the exact function of BCAAs in energy metabolism of porcine adipocyte.
Acknowledgments
This research was jointly supported by National Basic Research Program of China (2013CB127305, 2012CB124704), National Nature Science Foundation of China (31110103909, 31330075), Nature Science Foundation of Hunan (2015JJ2146), The Chinese Academy of Sciences STS Project (KFJ-EW-STS-063), and Key Projects in the National Science & Technology Pillar Program (2013BAD21B04) and Hunan Province project (2014GK1007).
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
Contributor Information
Fengna Li, Email: lifengna@isa.ac.cn.
Yulong Yin, Email: yinyulong@isa.ac.cn.
References
- Baird F.E., Bett K.J., Maclean C., Tee A.R., Hundal H.S., Taylor P.M. Tertiary active transport of amino acids reconstituted by coexpression of system A and L transporters in Xenopus oocytes. Am J Physiol Endocrinol Metab. 2009;297 doi: 10.1152/ajpendo.00330.2009. [DOI] [PubMed] [Google Scholar]
- Bastin J., Aubey F., Rötig A., Munnich A., Djouadi F. Activation of peroxisome proliferator-activated receptor pathway stimulates the mitochondrial respiratory chain and can correct deficiencies in patients' cells lacking its components. J Clin Endocrinol Metab. 2008;93:1433–1441. doi: 10.1210/jc.2007-1701. [DOI] [PubMed] [Google Scholar]
- Bode B.P. Recent molecular advances in mammalian glutamine transport. J Nutr. 2001;131:2475S–2485S. doi: 10.1093/jn/131.9.2475S. discussion 2486S–2477S. [DOI] [PubMed] [Google Scholar]
- Bodoy S., Martin L., Zorzano A., Palacin M., Estevez R., Bertran J. Identification of LAT4, a novel amino acid transporter with system L activity. J Biol Chem. 2005;280:12002–12011. doi: 10.1074/jbc.M408638200. [DOI] [PubMed] [Google Scholar]
- Canto C., Auwerx J. PGC-1alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr Opin Lipidol. 2009;20:98–105. doi: 10.1097/MOL.0b013e328328d0a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan M.C., Arany Z. The many roles of PGC-1alpha in muscle – recent developments. Metabolism. 2014;63:441–451. doi: 10.1016/j.metabol.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Pan Y., Wong E.A., Webb K.E., Jr. Dietary protein level and stage of development affect expression of an intestinal peptide transporter (cPepT1) in chickens. J Nutr. 2005;135:193–198. doi: 10.1093/jn/135.2.193. [DOI] [PubMed] [Google Scholar]
- Corporation HP Metabolic and physiological roles of branched-chain amino acids. Adv Mol Biol. 2014;2014 [Google Scholar]
- Douglas W.R. Of pigs and men and research: a review of applications and analogies of the pig, sus scrofa, in human medical research. Space Life Sci. 1972;3:226–234. doi: 10.1007/BF00928167. [DOI] [PubMed] [Google Scholar]
- Duan Y., Li F., Liu H., Li Y., Liu Y., Kong X. Nutritional and regulatory roles of leucine in muscle growth and fat reduction. Front Biosci. 2015;20:796–813. doi: 10.2741/4338. [DOI] [PubMed] [Google Scholar]
- Durante W., Reyna S.V., Ensenat D., Peyton K.J., Wang H., Schafer A.I. Platelet-derived growth factor stimulates LAT1 gene expression in vascular smooth muscle: role in cell growth. FASEB J. 2004;18:126. doi: 10.1096/fj.03-0886fje. [DOI] [PubMed] [Google Scholar]
- Evans K. Acidosis-sensing glutamine pump SNAT2 determines amino acid levels and mammalian target of rapamycin signalling to protein synthesis in L6 muscle cells. J Am Soc Nephrol JASN. 2007;18:1426–1436. doi: 10.1681/ASN.2006091014. [DOI] [PubMed] [Google Scholar]
- Felig P., Marliss E., Cahill G.F., Jr. Plasma amino acid levels and insulin secretion in obesity. N Engl J Med. 1969;281:811–816. doi: 10.1056/NEJM196910092811503. [DOI] [PubMed] [Google Scholar]
- Figueroa J.L., Lewis A.J., Miller P.S., Fischer R.L., Diedrichsen R.M. Growth, carcass traits, and plasma amino acid concentrations of gilts fed low-protein diets supplemented with amino acids including histidine, isoleucine, and valine. J Anim Sci. 2003;81:1529–1537. doi: 10.2527/2003.8161529x. [DOI] [PubMed] [Google Scholar]
- Fugui Y., Zhenzhen Z., Ju H., Yulong Y. Digestion rate of dietary starch affects systemic circulation of amino acids in weaned pigs. Br J Nutr. 2010;103:1404–1412. doi: 10.1017/S0007114509993321. [DOI] [PubMed] [Google Scholar]
- Gualano A.B., Bozza T., Lopes D.C.P., Roschel H., Dos S.C.A., Luiz M.M. Branched-chain amino acids supplementation enhances exercise capacity and lipid oxidation during endurance exercise after muscle glycogen depletion. J Sports Med Phys Fit. 2011;51:82–88. [PubMed] [Google Scholar]
- Gwinn D.M., Shackelford D.B., Egan D.F., Mihaylova M.M., Mery A., Vasquez D.S. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman M.A., She P., Peroni O.D., Lynch C.J., Kahn B.B. Adipose tissue branched chain amino acid (BCAA) metabolism modulates circulating BCAA levels. J Biol Chem. 2010;285:11348–11356. doi: 10.1074/jbc.M109.075184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Y., Yin Y., Wu G. Dietary essentiality of “nutritionally non-essential amino acids” for animals and humans. Exp Biol Med. 2015;240 doi: 10.1177/1535370215587913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hundal H.S., Taylor P.M. Amino acid transceptors: gate keepers of nutrient exchange and regulators of nutrient signaling. AJP Endocrinol Metab. 2009;296:E603–E613. doi: 10.1152/ajpendo.91002.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B., Xiao D., Tan B., Xiao H., Wang J., Yin J. Chitosan oligosaccharide reduces intestinal inflammation that 2 involves calcium-sensing receptor (CaSR) activation in lipopolysaccharide (LPS)-challenged piglets. J Agric Food Chem. 2016;64:245–252. doi: 10.1021/acs.jafc.5b05195. [DOI] [PubMed] [Google Scholar]
- Ihara T., Tsujikawa T., Fujiyama Y., Bamba T. Regulation of PepT1 peptide transporter expression in the rat small intestine under malnourished conditions. Digestion. 2000;61:59–67. doi: 10.1159/000007736. [DOI] [PubMed] [Google Scholar]
- Jarno R., Nagendra Y., Shalem M., Jussi P.K., Markku V.N., Paula I. SIRT1 mRNA expression may be associated with energy expenditure and insulin sensitivity. Diabetes. 2010;59:829–835. doi: 10.2337/db09-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai Y., Segawa H., Miyamoto K., Uchino H., Takeda E., Endou H. Expression cloning and characterization of a transporter for large neutral amino acids activated by the heavy chain of 4F2 antigen (CD98) J Biol Chem. 1998;273:23629–23632. doi: 10.1074/jbc.273.37.23629. [DOI] [PubMed] [Google Scholar]
- Kim S.W., Mateo R.D., Yin Y.L., Wu G., Kim S.W., Mateo R.D. Functional amino acids and fatty acids for enhancing production performance of sows and piglets. Asian-Australas J Anim Sci. 2007;20:295–306. [Google Scholar]
- Kim E.J., Kim Y.K., Kim J.E., Kim S., Kim M.K., Park C.H. UV modulation of subcutaneous fat metabolism. J Invest Dermatol. 2011;131:1720–1726. doi: 10.1038/jid.2011.106. [DOI] [PubMed] [Google Scholar]
- Koves T.R., Li P., An J., Akimoto T., Slentz D., Ilkayeva O. Peroxisome proliferator-activated receptor-gamma co-activator 1alpha-mediated metabolic remodeling of skeletal myocytes mimics exercise training and reverses lipid-induced mitochondrial inefficiency. J Biol Chem. 2005;280:33588–33598. doi: 10.1074/jbc.M507621200. [DOI] [PubMed] [Google Scholar]
- Larsson N.G., Wang J., Wilhelmsson H., Oldfors A., Rustin P., Lewandoski M. Mitochondrial transcription factor A is necessary for mtDNA maintenance and embryogenesis in mice. Nat Genet. 1998;18:231–236. doi: 10.1038/ng0398-231. [DOI] [PubMed] [Google Scholar]
- Layman D.K. The role of leucine in weight loss diets and glucose homeostasis. J Nutr. 2003;133:261S–267S. doi: 10.1093/jn/133.1.261S. [DOI] [PubMed] [Google Scholar]
- Li F., Li Y., Tang Y., Lin B., Kong X., Oladele O.A. Protective effect of myokine IL-15 against H2O2-mediated oxidative stress in skeletal muscle cells. Mol Biol Rep. 2014;41:7715–7722. doi: 10.1007/s11033-014-3665-9. [DOI] [PubMed] [Google Scholar]
- Li Y., Li F., Lin B., Kong X., Tang Y., Yin Y. Myokine IL-15 regulates the crosstalk of co-cultured porcine skeletal muscle satellite cells and preadipocytes. Mol Biol Rep. 2014;41:7543–7553. doi: 10.1007/s11033-014-3646-z. [DOI] [PubMed] [Google Scholar]
- Liang C., Curry B.J., Brown P.L., Zemel M.B. Leucine modulates mitochondrial biogenesis and SIRT1-AMPK signaling in C2C12 myotubes. J Nutr Metab. 2014;2014:239750. doi: 10.1155/2014/239750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X.D., Wu X., Yin Y.L., Liu Y.Q., Geng M.M., Yang H.S. Effects of dietary l-arginine or N-carbamylglutamate supplementation during late gestation of sows on the miR-15b/16, miR-221/222, VEGFA and eNOS expression in umbilical vein. Amino Acids. 2012;42:2111–2119. doi: 10.1007/s00726-011-0948-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.J., Ren M., Zeng X.F., Qiao S.Y. Low protein diet supplemented with branched chain amino acids improves growth performance and nitrogen utilization in weaned piglet. Chin J Anim Sci. 2014 [Google Scholar]
- Liu Y., Li F., He L., Tan B., Deng J., Kong X. Dietary protein intake affects expression of genes for lipid metabolism in porcine skeletal muscle in a genotype-dependent manner. Br J Nutr. 2015;113:1069–1077. doi: 10.1017/S0007114514004310. [DOI] [PubMed] [Google Scholar]
- Lordelo M.M., AM G., L L.B., JP F. Isoleucine and valine supplementation of a low-protein corn–wheat–soybean meal-based diet for piglets: growth performance and nitrogen balance. J Anim Sci. 2008;86:2936–2941. doi: 10.2527/jas.2007-0222. [DOI] [PubMed] [Google Scholar]
- Macotela Y., Emanuelli B., Bång A.M., Espinoza D.O., Boucher J., Beebe K. Dietary leucine – an environmental modifier of insulin resistance acting on multiple levels of metabolism. PLoS One. 2011;6:e21187. doi: 10.1371/journal.pone.0021187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair K.S., Short K.R. Hormonal and signaling role of branched-chain amino acids. J Nutr. 2005;135:1547S–1552S. doi: 10.1093/jn/135.6.1547S. [DOI] [PubMed] [Google Scholar]
- Nicklin P., Bergman P., Zhang B., Triantafellow E., Wang H., Nyfeler B. Bidirectional transport of amino acids regulates mTOR and autophagy. Cell. 2009;136 doi: 10.1016/j.cell.2008.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura J., Masaki T., Arakawa M., Seike M., Yoshimatsu H. Isoleucine prevents the accumulation of tissue triglycerides and upregulates the expression of PPARalpha and uncoupling protein in diet-induced obese mice. J Nutr. 2010;140:496–500. doi: 10.3945/jn.109.108977. [DOI] [PubMed] [Google Scholar]
- NRC . Natl. Acad. Press; Washington, DC: 2012. Nutrient requirements of swine. 11th rev. ed. [Google Scholar]
- Palii S.S., Hong C., Kilberg M.S. Transcriptional control of the human sodium-coupled neutral amino acid transporter system A gene by amino acid availability is mediated by an intronic element. J Biol Chem. 2004;279:3463–3471. doi: 10.1074/jbc.M310483200. [DOI] [PubMed] [Google Scholar]
- Palii S.S., Kays C.E., Deval C., Bruhat A., Fafournoux P., Kilberg M.S. Specificity of amino acid regulated gene expression: analysis of genes subjected to either complete or single amino acid deprivation. Amino Acids. 2009;37:79–88. doi: 10.1007/s00726-008-0199-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyrollier K., Hajduch E., Blair A.S., Hyde R., Hundal H.S. L-leucine availability regulates phosphatidylinositol 3-kinase, p70 S6 kinase and glycogen synthase kinase-3 activity in L6 muscle cells: evidence for the involvement of the mammalian target of rapamycin (mTOR) pathway in the L-leucine-induced up-regulation of system A amino acid transport. Biochem J. 2000;350(2):361–368. [PMC free article] [PubMed] [Google Scholar]
- Pfaffl M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L.Q., Xun P., Bujnowski D., Daviglus M.L., Van H.L., Stamler J. Higher branched-chain amino acid intake is associated with a lower prevalence of being overweight or obese in middle-aged East Asian and Western adults. J Nutr. 2011;141:249–254. doi: 10.3945/jn.110.128520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezaei R., Wang W., Wu Z., Dai Z., Wang J., Wu G. Biochemical and physiological bases for utilization of dietary amino acids by young pigs. J Anim Sci Biotechnol. 2013;4:90–101. doi: 10.1186/2049-1891-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal J., Angel A., Farkas J. Metabolic fate of leucine: a significant sterol precursor in adipose tissue and muscle. Am J Physiol. 1974;226:411–418. doi: 10.1152/ajplegacy.1974.226.2.411. [DOI] [PubMed] [Google Scholar]
- Scarpulla R.C., Vega R.B., Kelly D.P. Transcriptional integration of mitochondrial biogenesis. Trends Endocrinol Metab. 2012;23:459–466. doi: 10.1016/j.tem.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi R., Whitworth H.B., Mixon W.T., Gordon S.E. A leucine-enriched diet enhances overload-induced growth and markers of protein synthesis in aged rat skeletal muscle. Int J Exerc Sci Conf Abstr Submiss. 2012;2 [Google Scholar]
- Spurlock M.E., NK G. The development of porcine models of obesity and the metabolic syndrome. J Nutr. 2008;138:397–402. doi: 10.1093/jn/138.2.397. [DOI] [PubMed] [Google Scholar]
- Sun X., Zemel M.B. Leucine modulation of mitochondrial mass and oxygen consumption in skeletal muscle cells and adipocytes. Nutr Metab. 2009;6:1722–1726. doi: 10.1186/1743-7075-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan B., Yin Y., Liu Z., Li X., Xu H., Kong X. Dietary L-arginine supplementation increases muscle gain and reduces body fat mass in growing-finishing pigs. Amino Acids. 2009;37:169–175. doi: 10.1007/s00726-008-0148-0. [DOI] [PubMed] [Google Scholar]
- Tan B.E., Yin Y.L., Liu Z.Q., Tang W.J., Xu H.J., Konga X.F. Dietary L-arginine supplementation differentially regulates expression of fat-metabolic genes in porcine adipose tissue and skeletal muscle. J Nutr Bioc. 2011;22:441–445. doi: 10.1016/j.jnutbio.2010.03.012. [DOI] [PubMed] [Google Scholar]
- Tcherepanova I., Puigserver P., Norris J.D., Spiegelman B.M., McDonnell D.P. Modulation of estrogen receptor-alpha transcriptional activity by the coactivator PGC-1. J Biol Chem. 2000;275:16302–16308. doi: 10.1074/jbc.M001364200. [DOI] [PubMed] [Google Scholar]
- Tischler M.E., Goldberg A.L. Leucine degradation and release of glutamine and alanine by adipose tissue. J Biol Chem. 1980;255:8074–8081. [PubMed] [Google Scholar]
- Valenzuela B.A., Sanhueza C.J. The adipose tissue: something more than a reservoir of energy. Grasas Aceites. 2009;60:437–450. [Google Scholar]
- Wang Q., Holst J. L-type amino acid transport and cancer: targeting the mTORC1 pathway to inhibit neoplasia. Am J Cancer Res. 2015;5:1281–1294. [PMC free article] [PubMed] [Google Scholar]
- Wu G., Bazer F.W., Davis T.A., Jaeger L.A., Johnson G.A., Kim S.W. Important roles for the arginine family of amino acids in swine nutrition and production. Livest Sci. 2007;112:8–22. [Google Scholar]
- Wu G., Bazer F.W., Davis T.A., Kim S.W., Peng L., Rhoads J.M. Arginine metabolism and nutrition in growth, health and disease. Amino Acids. 2009;37:153–168. doi: 10.1007/s00726-008-0210-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G., Wu Z., Dai Z., Yang Y., Wang W., Liu C. Dietary requirements of “nutritionally non-essential amino acids” by animals and humans. Amino Acids. 2013;44:1107–1113. doi: 10.1007/s00726-012-1444-2. [DOI] [PubMed] [Google Scholar]
- Wu X., Xie C., Yin Y., Li F., Li T., Huang R. Effect of L-arginine on HSP70 expression in liver in weanling piglets. BMC Vet Res. 2013;9:63. doi: 10.1186/1746-6148-9-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y., Kang Y., Liu Z., Min G., Zheng R., Deng D. Supplementing L-leucine to a low-protein diet increases tissue protein synthesis in weanling pigs. Amino Acids. 2010;39:1477–1486. doi: 10.1007/s00726-010-0612-5. [DOI] [PubMed] [Google Scholar]
- Zhang J., Yin Y., Shu X.G., Li T., Li F., Tan B. Oral administration of MSG increases expression of glutamate receptors and transporters in the gastrointestinal tract of young piglets. Amino Acids. 2013;45:1169–1177. doi: 10.1007/s00726-013-1573-2. [DOI] [PubMed] [Google Scholar]
- Zhang S., Qiao S., Man R., Zeng X., Xi M., Wu Z. Supplementation with branched-chain amino acids to a low-protein diet regulates intestinal expression of amino acid and peptide transporters in weanling pigs. Amino Acids. 2013;45:1191–1205. doi: 10.1007/s00726-013-1577-y. [DOI] [PubMed] [Google Scholar]