Abstract
Animal gastrointestinal tract is not only a digestive organ, but also a nutrient sensing organ which detects luminal nutrient and thus can regulate food intake. There are many amino acid sensing receptors and transporters in the gut. Amino acids sensing by these receptors and transporters can stimulate the intestinal endocrine cells to release a variety of gut hormones. These hormones trigger a series of physiological effects via the nerve system. This review summarized the recent advance on the amino acid sensing receptors and transporters in the gastrointestinal tract, the gut hormones released from the intestinal endocrine cells and the hormones-induced signal transduction between the gut and brain. A better understanding of these processes may help to gain further insight into the specific role of amino acids in digestion and provide guidelines in developing strategy for the better use of amino acids in the diet.
Keywords: Amino acid sensing, Gut hormones, Sensing receptor, Amino acid transporter, Gut-brain signaling
1. Introduction
Animal gastrointestinal (GI) tract is the largest digestive and immune organ in the body. In addition, as a nutrient sensor, the GI tract is also involved in regulating glucose and energy homeostasis. Gastrointestinal tract serves as a sensing organ, which was confirmed by Bayliss and Starling (1902) when they discovered the first gut-derived hormone secretin. Recent advances highlighted that intestinal luminal nutrients (such as carbohydrate, fat and protein) are sensed by specific ‘taste’ receptors or transporters located in the membrane of cells in the intestinal epithelium. Among the receptors, G-protein-coupled receptor family C group 6 member A (GPRC6A), the taste receptor 1 family (T1Rs), calcium-sensing receptor (CaSR) can sense luminal protein and amino acids. Gut hormones are produced due to sensing the amino acids by endocrine cells. After secreted, the hormones enter into lamina propria and recognize respective receptors on the vagal afferent nerve, which signals to the brain. This process establishes the basis for regulating appetite and energy balance by the gut-brain axis. The progress in nutrient sensing indicates a promising approach to treating obesity and diabetes by targeting nutrient-induced hormone production.
2. Amino acids sensing receptor and transporter
2.1. Amino acids sensing receptor
Amino acids are signaling ligands for sensory receptors. Some of G-protein-coupled receptors (GPCRs) expressed on the enteroendocrine cells (EECs) or brush cells participate in the luminal amino acids sensing. Moreover, the specific amino acids transporters on the cell membrane also play an important role in the amino acids sensing.
G-protein-coupled receptors, including T1Rs, GPRC6A and CaSR, are the major amino acids sensing receptors.
The T1R family consists of three different subtypes (T1R1, T1R2 and T1R3) which were originally found in oral epithelial cells. Subsequent research demonstrates that they are also expressed on the intestinal brush cells and enteric endocrine cells of different species (Shirazi-Beechey et al., 2014). The T1R1 and T1R3 form a heterodimer to recognize most of the L-type amino acids except tryptophan. The responses are strictly dependent on the combined presence of T1R1 and T1R3, and are highly selective for L-amino acids; D-amino acids do not activate the T1R1/T1R3 heterodimer. The T1R1/T1R3 can also detect umami tastants such as monosodium glutamate (MSG), L-2-amino-4-phosphono-butyric acid (L-AP4), but the signal mediated by the transduction pathway involving T1R1/T1R3 may be different from that involving metabotropic glutamate receptor (mGluRs) (Temussi, 2009). As a typical G-protein-coupled receptor, T1R1/T1R3 is activated only when α-Gustducin (a G protein) exists.
The CaSR is a class C G-protein-coupled receptor which was firstly found in bovine parathyroid gland and is involved in extracellular calcium homeostasis in mammals. Lately, CaSR has been identified in the GI endocrine G, I and D cells where it acts as an amino acids sensor. Calcium-sensing receptor is not only activated by extracellular calcium but is also activated by L-aromatic amino acids (such as L-phenylalanine, L-tryptophan). The CaSR senses aromatic L-amino acids only when intracellular calcium concentration is higher than 1 mmol/L. Oral administration of L-phenylalanine (L-Phe) stimulated gastrin secretion in wild type but not in CaSR knockout mice. However, when CaSR knockout mice were treated with cinacalcet (an agonist of CaSR), the effect of gastrin secretion would occur (Feng et al., 2010), suggesting that L-Phe stimulated cholecystokinin (CCK) release via CaSR. In addition, some small peptides are also the ligands of CaSR. Several γ-glutamyl peptides, such as γ-Glu-Cys-Gly (GSH) and γ-Glu-Val-Gly, are involved in CaSR activation (Ohsu et al., 2010). Calcium-sensing receptor was involved in the CCK secretion induced by various protein hydrolysate; CCK secretions induced by protein hydrolysate were significantly decreased by the presence of CaSR antagonist compared with vehicle (Nakajima et al., 2012). This study indicated the significant role of CaSR in mediating CCK secretion by peptides stimulation in enteroendocrine cells.
The GPRC6A is a member of G protein-coupled receptor and expresses in gastric G cells, small intestinal and colonic L cells (Oya et al., 2013). It can sense many kinds of amino acids, especially basic amino acids (such as L-lysine, L-arginine and L-ornithine) and small neutral amino acids (such as L-alanine, L-glycine and L-serine), but the affinities of these amino acids are different: L-arginine > L-ornithine ≥ L-lysine = L-alanine ≥ glycine > serine (Wellendorph et al., 2005). G-protein-coupled receptor family C group 6 has a high homology with CaSR and its activation requires the presence of extracellular calcium. In HEK293 cells GPRC6A could be activated by an extracellular calcium concentrations of 5 mmol/L (Pi and Quarles, 2012). Interestingly, the CaSR agonist NPSR-568 can also activate GPRC6A (Pi et al., 2005).
2.2. Amino acid transporters
Amino acid transporters are widely expressed on the cell membranes, monitoring the amino acids concentration and mediating extra- and intra-cellular amino acids exchange. Amino acids transport may be coupled to movements of ions, including Na+, H+, K+, and/or Cl−, as well as movement of other amino acids by anti-port. However, the mechanisms of different amino acid transporters are almost different. Bröer (2008) summarized the amino acids transporters in the apical and basolateral membrane (Table 1).
Table 1.
Part of the amino acid transport system in mammalian intestinal epithelial cells.
| System | cDNA | Gene | AA substrate | Mechanism | Iron | Expression |
|---|---|---|---|---|---|---|
| ASC | ASCT2 | SLC1A5 | A,S,C,T,Q | A | Na+ | AM |
| B0 | B0AT1 | SLC6A19 | AA0 | S | Na+ | AM |
| B0,+ | ATB0,+ | SLC6A14 | AA0, AA+, β-Ala | S | Na+, Cl− | AM |
| b0,+ | rBAT/b0,+AT | SLC3A1/SLC7A9 | R,K,O | A | – | AM |
| IMINO | IMINO | SLC6A20 | P,HO-P | S | Na+, Cl− | AM |
| L | 4F2hc/LAT2 | SLC3A2/SLC7A8 | AA0 except P | A | – | BM |
| PAT | PAT1 | SLC36A1 | P,G,A,β-Ala | S | H+ | BM |
| T | TAT1 | SLC16A10 | F,Y,W | U | – | BM |
| X−AG | EAAT3 | SLC1A1 | E,D | S | Na+, H+, K+ | AM |
| y+L | 4F2hc/y+LAT1 | SLC3A2/SLC7A7 | K,R,Q,H,M,L | A | Na+ | BM |
| y+L | 4F2hc/y+LAT2 | SLC3A2/SLC7A6 | K,R,Q,H,M,L,A,C | A | Na+ | BM |
| A | SNAT2 | SLC38A2 | G,P,A,S,C,Q,N,H | S | Na+ | AM, BM |
| X−c | 4F2 hc/xCT | SLC3A2/SLC7A11 | E | A | – | AM, BM |
| y+ | CAT-1 | SLC7A1 | R, K、O, H | U | – | AM, BM |
A = anti-port; AM = apical membrane; AA0 = neutral amino acids; S = symport; AA+ = cationic amino acids; BM = basolateral membrane; U = uni-port.
Several lines of evidence have shown that some of the amino acid transporters themselves can function as an amino acid sensor and are essential for amino acids sensing. Sodium dependent neural amino acids transporter 2 (SNAT2), a major amino acid transporter, is one of the best characterized amino acid transceptor. Amino acids uptake via SNAT2 are coupled with the inward movement of Na+, which helps down its electrochemical gradient. It has been proved that this transport mechanism is involved in the stimulation of gut hormone release through consequential increases in intracellular Ca2+ (Young et al., 2010), and specifically in glutamine stimulated GLP-1 release from intestinal L cells (Tolhurst et al., 2011). In addition, studies have shown that like SNAT2, the B0AT1 is also related to trigger GLP-1 release from GLUTgs cells in presence of glutamine (Reimann et al., 2004). Some other transporters, like EAAT3, y+LAT1 and CAT-1, may act by initiating downstream signaling and modulating gene expression in response to amino acids availability. However, whether they are involved in gut hormone release is remain unclear.
3. Amino acid sensing mediated GI hormone release
The EECs are derived from multipotent stem cells, located towards the base of the intestinal crypts. Although they represent less than 1% of the epithelial population, they constitute the largest endocrine organ of the human body. It is thought that EECs are the primary chemosensory cells. There are at least 15 subtypes of enteroendocrine cells that react to changes in gut contents by releasing peptide hormones, which then enter blood vessels and activate extrinsic or intrinsic afferent nerves or other nearby target cells. Sensing of amino acids will stimulate EECs to release glucagon-like peptide-1(GLP-1), cholecystokinin (CCK) and peptide tyrosine–tyrosine (PYY).
Glucagon-like peptide-1(GLP-1) is released from L cells of the jejunum, ileum and colon. It increases after a meal to increase satiety. Glucagon-like peptide-1 decreases gastric emptying rate and is an important incretin. Free amino acids, including glutamine (Gln), phenylalanine (Phe) and tryptophan (Trp), have been shown to stimulate GLP-1 secretion using intestinal epithelial cell models (Mace et al., 2012). Moreover, meat hydrolysate, as well as a mixture of essential amino acids (EAAs) can potently stimulate GLP-1 secretion from NCI-H716 cells, but it has no effect on non-essential amino acids (NEEAs) (Reimann, 2006). Studies have shown that oral glutamine increases circulating GLP-1, in lean, obese and type 2 diabetic subjects.
Cholecystokinin, a hormone that circulates in different molecular forms (CCK8, CCK33/39, CCK58), is released post-prandially from the K cells in the small intestine in response to amino acids or other nutrients. Cholecystokinin causes the release of digestive enzymes and bile from the pancreas and gallbladder. In addition to its role in digestion, CCK is also a satiety signal that delays gastric emptying. As for humans, dogs and many other rodent animals, amino acids can exert a direct stimulatory effect on CCK secretion in the I cells, especially aromatic amino acids. The L-Phe and L-Try can stimulate releasing CCK through CaSR in I cells (Wang et al., 2011). In addition, the soybean β51–63 peptide (an arginine-rich fragment) stimulates CCK secretion in enteroendocrine STC-1 cells (Nakajima et al., 2010).
Peptide tyrosine–tyrosine is a peptide of 36 amino acids released from the enteroendocrine L cells in the ileum and colon and is usually co-localized with GLP-1. It was initially isolated from porcine intestine and can be cleaved by dipeptidyl peptidase-4 resulting in 2 circulating isoforms, PYY1–36 and PYY3–36. Peptide YY is an important mediator of the ileal break, a primary inhibitory feedback mechanism that controls the transit of a meal through the gut to optimize nutrient digestion and absorption. It has been found that PYY was released from L cells in response to some amino acids. The I cells release both GLP-1 and PYY when treated with L-glutamine (Joshi et al., 2013). Moreover, after intake of high-protein, the release of PYY in normal-weight and obese subjects both increased greatly, and long-term high-protein diets reduce weight gain and enhance PYY synthesis and secretion in mice. The PYY knockout mice, in contrast, had no corresponding effects (Batterham et al., 2006).
4. Amino acid sensing in the gut-brain signaling
4.1. Intestinal primary sensory neurons
Two classes of primary afferent neurons are associated with the GI tract: intrinsic primary afferent neurons (IPANs) and extrinsic primary afferent neurons. Intrinsic primary afferent neurons are derived either from the myenteric or submucosal plexus. They are parts of the enteric nervous system and are necessary for the generation of reflexes in reaction to intestinal contents, blood flow and water/electrolyte secretion. Intrinsic primary afferent neurons can monitor the intestinal luminal chemical changes. In addition, they contact with each other and form a dense network that communicates to interneurons to provide the enteric nervous system with the information required for the autonomic mediate of digestion. Extrinsic primary afferent neurons consist of spinal primary afferent neurons and vagal primary afferent neurons, and both of them innervate the entire length of the GI tract. The vagus nerve is the major component of the extrinsic afferent pathway which has been testified that it can sense amino acid. Using electrophysiological recordings to detect the discharge of vagal afferent fibers, Iggo (1957) found that many kinds of nutrients can activate the vagus from the gut. Steinetrt and Beglinger (2011) found that the process of CCK, PYY and other gut hormones has an effect on controlling food intake, and this may be accompanied by the activation of the vagus nerve, which is implying neuronal vagal activation are likely to rely on secondary substances released from the mucosal epithelium. Recent studies have identified receptors of CCK, PYY and GLP-1 on the afferent fibers (Koda et al., 2005, Date et al., 2002).
4.2. Gut hormones-induced signal transduction between the gut and brain
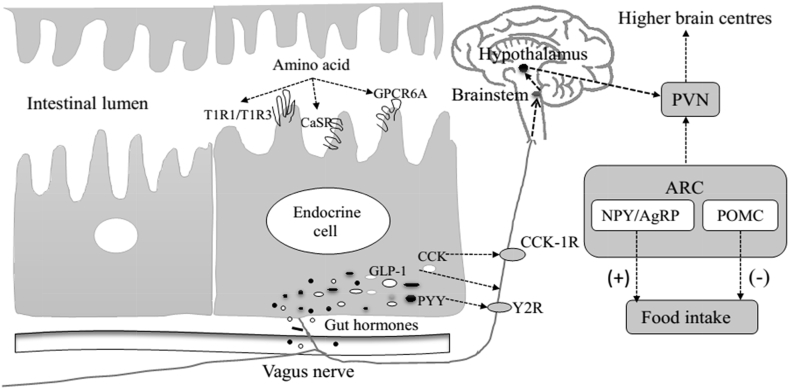
Enteroendocrine as primary chemical sensors, secrete GLP-1, CCK, PYY and other gut hormones after sensing amino acids. Gastrointestinal vagal afferents are activated by these hormones, and converge in the nucleus tractus solitarius (NTS) of the brainstem. These signals are then feed signals forward the NTS to the hypothalamic arcuate nucleus (ARC). Neuronal projections from the ARC in turn carry signals to the hypothalamic paraventricular nucleus (PVN) to regulate food intake (Sam et al., 2012) (as shown in Fig. 1).
Fig. 1.
Consequences of amino acids sensing on the gut-brain signaling involved in the regulation of food intake (Ueno and Nakazato, 2016, Baggio and Drucker, 2014). CaSR = Ca sensing receptor; GPCR6A = G-protein-coupled receptor family C group 6 member A; CCK = Cholecystokinin; GLP-1 = Glucagon-like peptide-1; PYY = Peptide tyrosine–tyrosine; CCK-1R = CCK1 receptor; Y2R = Y2 receptor; PVN = paraventricular nucleus; ARC = arcuate nucleus; NPY = Neuropeptide Y; AgRP = Agouti-related peptide; POMC = pro-opiomelanocortin; (+) increase; (−) decrease.
Glucagon-like peptide-1 is a satiety signal that decreases meal size and delays gastric emptying. Studies have shown that both acute peripheral and central administration of GLP-1 reduce food intake in rats, exenatide and liraglutide (GLP-1 analogues) decrease food intake and body weight in a dose-dependent manner in rats (Jelsing et al., 2012). Peripheral administration of GLP-1 to rats activates neurons within the brainstem. However, this increase in neuronal activity and the anorectic effect of GLP-1 are abolished following vagotomy (Yegen et al., 1997). In addition, peripheral administration of GLP-1 causes signal intensity change within neuronal populations in the ventral medial hypothalamus (VMH) and the PVN when functional magnetic resonance imaging (fMRI) is used (Parkinson et al., 2009). Collectively, these findings draw a conclusion that appetite is regulated by GLP-1 via these hypothalamic and brainstem areas.
Like GLP-1, CCK also exerts its satiety action primarily through the activation of vagal afferent neurons. There are two types of CCK receptor: CCK1R and CCK2R. The CCK1R has been proved to be expressed on the intestinal vagal nerve, and pharmacological and gene tests showed CCK1R is involved in the regulation of food intake. Rats lack of the CCK1 receptors suffer from hyperphagia and obesity (Bi and Moran, 2002). Bilateral midbrain transections block the behavioral effects of CCK on feeding, and the increasing effects of neural activity also disappear in rats (Crawley et al., 1984). In addition, Blevins et al. (2000) microinjected CCK into one of several different brain sites of rats and found its impact on subsequent food intake was decreased. When the majority of the medial and commissural subnuclei of the NTS as well as the area postrema (AP) were lesioned, there is a significant attenuation of the satietogenic effect of CCK. These studies suggested that CCK may transmit the signal to the brain either through direct or indirect way to regulate food intake.
Peptide tyrosine–tyrosine is another satiety hormone and it acts through multiple receptor subtypes, Y1 through Y5, and the Y2 receptor (Y2R) is selectively activated by PYY3-36. Peripheral injection of PYY3-36 in animals and humans inhibits food intake and reduces weight gain in both lean and obese individuals, suggesting that Y2R may be involved in this process (Gautier-Stein et al., 2013). Y2 receptor (Y2R) is an auto-inhibitory pre-synaptic receptor found on neuropeptide Y (NPY) neurons within the ARC. Studies have shown that deficiency of the Y2R abolishes the anorectic effects of PYY (Scott et al., 2005). In addition, Y2R can significantly decreased the release of NPY, with a concomitant increase in alpha melanocyte stimulating hormone (a-MSH) released from hypothalamic explants (Batterham et al., 2002). Researchers proposed that circulating PYY3-36 may inhibit NPY/agouti-related peptide (AGRP) neurons through Y2R, thus activating the pro-opiomelanocortin (POMC) which is deterred by the NPY/AGRP. Moreover, Y2R is also expressed on the terminal intestinal vagus nerve. Peripheral administration of PYY3–36 significantly reduces food intake and body weight in rodents, meanwhile the neurons in ARC are activated. However, these effects are abolished following either bilateral sub-diaphragmatic total truncal vagotomy or brainstem–hypothalamic pathway transectioning (Abbott et al., 2005).
5. Summary
Dietary amino acids are sensed by the epithelium in the gastrointestinal tract which stimulates the release of gut hormones from endocrine cells, then these signals are sent to the brain via gut-brain axis. This, in return, regulates the intestinal physiology and other physical activities. In addition, amino acids sensing receptors and their ligands are further identified. The amino acid sensing pathway that is involved in animal digestive process and the host gut health needs further study, which has a profound value of developing new therapies to fight nutrition metabolic diseases.
Acknowledgments
This work was supported by the National Key Basic Research Program of China (2013CB127300), Natural Science Foundation of China (31430082) and Jiangsu Province Natural Science Foundation (BK20130058). WZ also thanks to the Collaborative Innovation Center of Meat Production and Processing.
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- Abbott C.R., Monteiro M., Small C.J., Sajedi A., Smith K.L., Parkinson J.R. The inhibitory effects of peripheral administration of peptide YY 3-36 and glucagon-like peptide-1 on food intake are attenuated by ablation of the vagal-brainstem-hypothalamic pathway. Brain Res. 2005;1044:127–131. doi: 10.1016/j.brainres.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Baggio L.L., Drucker D.J. Glucagon-like peptide-1 receptors in the brain: controlling food intake and body weight. J Clin Invest. 2014;124(10):4223–4226. doi: 10.1172/JCI78371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayliss W.M., Starling T.E.H. The mechanism of pancreatic secretion. J Physiol. 1902;8:325–353. doi: 10.1113/jphysiol.1902.sp000920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batterham R.L., Cowley M.A., Small C.J., Herzog H., Cohen M.A., Dakin C.L. Gut hormone PYY3-36 physiologically inhibits food intake. Nature. 2002;418:650–654. doi: 10.1038/nature00887. [DOI] [PubMed] [Google Scholar]
- Batterham R.L., Heffron H., Kapoor S., Chivers J.E., Chandarana K., Herzog H. Critical role for peptide YY in protein-mediated satiation and body-weight regulation. Cell Metab. 2006;4:223–233. doi: 10.1016/j.cmet.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Bi S., Moran T.H. Actions of CCK in the controls of food intake and body weight: lessons from the CCK-A receptor deficient OLETF rat. Neuropeptides. 2002;36:171–181. doi: 10.1054/npep.2002.0895. [DOI] [PubMed] [Google Scholar]
- Blevins J.E., Stanley B.G., Reidelberge R.D. Brain regions where cholecystokinin suppresses feeding in rats. Brain Res. 2000;860(1):1–10. doi: 10.1016/s0006-8993(99)02477-4. [DOI] [PubMed] [Google Scholar]
- Bröer S. Amino acid transport across mammalian intestinal and renal epithelia. Physiol Rev. 2008;88:249–286. doi: 10.1152/physrev.00018.2006. [DOI] [PubMed] [Google Scholar]
- Crawley J.N., Kiss J., Mezey E. Bilateral midbrain transections block the behavioral effects of cholecystokinin on feeding and exploration in rats. Brain Res. 1984;322:316–321. doi: 10.1016/0006-8993(84)90124-0. [DOI] [PubMed] [Google Scholar]
- Date Y., Murakami N., Toshinai K., Matsukura S., Niijima A., Matsuo H. The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology. 2002;123:1120–1128. doi: 10.1053/gast.2002.35954. [DOI] [PubMed] [Google Scholar]
- Feng J., Petersen C.D., Coy D.H., Jiang J.K., Thomas C.J., Pollak M.R. Calcium-sensing receptor is a physiologic multimodal chemosensor regulating gastric G-cell growth and gastrin secretion. Proc Natl Acad Sci. 2010;107(41):17791–17796. doi: 10.1073/pnas.1009078107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier-Stein A., Mithieux G. A role for PYY 3-36 in GLP1-induced insulin secretion. Mol Metab. 2013;2(3):123–125. doi: 10.1016/j.molmet.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iggo A. Gastric mucosal chemoreceptors with vagal afferent fibres in the cat. Q J Exp Physiol Cogn Med Sci. 1957;42(4):398–409. doi: 10.1113/expphysiol.1957.sp001284. [DOI] [PubMed] [Google Scholar]
- Jelsing J., Vrang N., Hansen G. Liraglutide: short-lived effect on gastric emptying-long lasting effects on body weight. Diabet Obes Metab. 2012;14(6):531–538. doi: 10.1111/j.1463-1326.2012.01557.x. [DOI] [PubMed] [Google Scholar]
- Joshi S., Tough I., Cox H. Endogenous PYY and GLP-1 mediate l-glutamine responses in intestinal mucosa. Br J Pharmacol. 2013;170(5):1092–1101. doi: 10.1111/bph.12352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koda S., Date Y., Murakami N., Shimbara T., Hanada T., Toshinai K. The role of the vagal nerve in peripheral PYY3-36-induced feeding reduction in rats. Endocrinology. 2005;146(5):2369–2375. doi: 10.1210/en.2004-1266. [DOI] [PubMed] [Google Scholar]
- Mace O.J., Schindler M., Patel S. The regulation of K-and L-cell activity by GLUT2 and the calcium-sensing receptor CasR in rat small intestine. J Physiol. 2012;590(12):2917. doi: 10.1113/jphysiol.2011.223800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima S., Hira T., Hara H. Calcium-sensing receptor mediates dietary peptide-induced CCK secretion in enter endocrine STC-1 cells. Mol Nutr Food Res. 2012;56:753–760. doi: 10.1002/mnfr.201100666. [DOI] [PubMed] [Google Scholar]
- Nakajima S., Hira T., Eto Y., Asano K., Hara H. Soybean β51-63 peptide stimulates cholecystokinin secretion via a calcium-sensing receptor in enteroendocrine STC-1 cells. Regul Pept. 2010;159:148–155. doi: 10.1016/j.regpep.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Ohsu T., Amino Y., Nagasaki H., Yamanaka T., Takeshita S., Hatanaka T. Involvement of the calcium-sensing receptor in human taste perception. J Biol Chem. 2010;285:1016–1022. doi: 10.1074/jbc.M109.029165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oya M., Kitaguchi T., Pais R. The G protein-coupled receptor family C group 6 subtype A (GPRC6A) receptor is involved in amino acid-induced glucagon-like peptide-1 secretion from GLUTag cells. J Biol Chem. 2013;288(7):4513–4521. doi: 10.1074/jbc.M112.402677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson J.R.C., Chaudhri O.B., Kuo Y.T., Field B.C., Herlihy A.H., Dhillo W.S. Differential patterns of neuronal activation in the brainstem and hypothalamus following peripheral injection of GLP-1,oxyntomodulin and lithium chloride in mice detected by manganese-enhanced magnetic resonance imaging(MEMRI) Neuroimage. 2009;44:1022–1031. doi: 10.1016/j.neuroimage.2008.09.047. [DOI] [PubMed] [Google Scholar]
- Pi M., Quarles L.D. Multiligand specificity and wide tissue expression of GPRC6A reveals new endocrine networks. Endocrinology. 2012;153:2062–2069. doi: 10.1210/en.2011-2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pi M., Faber P., Ekeme G., Jackson P.D., Ting A., Wang N. Identification of a novel extracellular cation-sensing G-protein-coupled receptor. J Biol Chem. 2005;280(48):40201–40209. doi: 10.1074/jbc.M505186200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimann F., Williams L., da Silva Xavier G., Rutter G.A., Gribble F.M. Glutamine potently stimulates glucagon-like peptide-1 secretion from GLUTag cells. Diabetologia. 2004;47(9):1592–1601. doi: 10.1007/s00125-004-1498-0. [DOI] [PubMed] [Google Scholar]
- Reimann R.A. Meat hydrolysate and essential amino acid-induced glucagon-like peptide-1 secretion, in the human NCI-H716 enteroendocrine cell line, is regulated by extracellular signal-regulated kinase1/2 and p38 mitogen-activated protein kinases. J Endocrinol. 2006;191:159–170. doi: 10.1677/joe.1.06557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sam A.H., Troke R.C., Tan T.M., Rutter G.A., Gribble F.M. The role of the gut/brain axis in modulating food intake. Neuropharmacology. 2012;63:46–56. doi: 10.1016/j.neuropharm.2011.10.008. [DOI] [PubMed] [Google Scholar]
- Scott V., Kimura N., Stark J.A., Luckman S.M. Intravenous peptide YY3-36 and Y2 receptor antagonism in the rat: effects on feeding behavior. J Neuroendocrinol. 2005;17:452–457. doi: 10.1111/j.1365-2826.2005.01330.x. [DOI] [PubMed] [Google Scholar]
- Shirazi-Beechey S.P., Daly K., Al-Rammahi M. Role of nutrient-sensing taste 1 receptor (T1R) family members in gastrointestinal chemosensing. Br J Nutr. 2014;111(S1):S8–S15. doi: 10.1017/S0007114513002286. [DOI] [PubMed] [Google Scholar]
- Steinetrt R.E., Beglinger C. Nutrient sensing in the gut: interactions between chemosensory cells, visceral afferents and the secretion of satiation peptides. Physiol Behav. 2011;105:62–70. doi: 10.1016/j.physbeh.2011.02.039. [DOI] [PubMed] [Google Scholar]
- Temussi P.A. Sweet, bitter and umami receptors: a complex relationship. Trends Biochem Sci. 2009;34:296–302. doi: 10.1016/j.tibs.2009.02.005. [DOI] [PubMed] [Google Scholar]
- Tolhurst G., Zheng Y., Parker H.E. Glutamine triggers and potentiates glucagon-like peptide-1 secretion by raising cytosolic Ca2+ and cAMP. Endocrinology. 2011;152(2):405–413. doi: 10.1210/en.2010-0956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno H., Nakazato M. The mechanistic relationship between the vagal afferent pathway, central nervous system, and peripheral organs in appetite regulation. J Diabet Invest. 2016 doi: 10.1111/jdi.12492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Chandra R., Samsa L.A., Gooch B., Fee B.E., Cook J.M. Amino acids stimulate cholecystokinin release through the Ca2+-sensing receptor. Am J Physiol-Gastrointest Liver Physiol. 2011;300:528–537. doi: 10.1152/ajpgi.00387.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellendorph P., Hansen K.B., Balsgaard A., Greenwood J.R., Egebjerg J., Bräuner-Osborne H. Deorphanization of GPRC6A: a promiscuous L-α-amino acid receptor with preference for basic amino acids. Mol Pharmacol. 2005;67(3):589–597. doi: 10.1124/mol.104.007559. [DOI] [PubMed] [Google Scholar]
- Yegen B.C., Bozkurt A., Coskun T., Villanueva-Peñacarrillo M.L., Ulusoy N.B. Glucagon-like peptide-1 inhibits gastric emptying via vagal afferent-mediated central mechanisms. Am J Physiol-Gastrointest Liver Physiol. 1997;273:G920–G927. doi: 10.1152/ajpgi.1997.273.4.G920. [DOI] [PubMed] [Google Scholar]
- Young S.H., Rey O., Sternini C. Amino acid sensing by enteroendocrine STC-1 cells: role of the Na+-coupled neutral amino acid transporter 2. Am J Physiol Cell Physiol. 2010;298(6):C1401–C1413. doi: 10.1152/ajpcell.00518.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]