Abstract
A L9(34) orthogonal experiment was conducted to evaluate the influence of 9 mixtures which consisted of Cu2+, Zn2+, Fe2+ and I− ions at different ion concentrations on fungal diversity and toxigenic fungal growth in a Bacillus subtilis-fermented liquid feed (FLF) using pyrosequencing. The maximal Chao estimator and Shannon index were achieved in the FLF with a mixture of Cu2+ (200 mg/kg), Zn2+ (160 mg/kg), Fe2+ (150 mg/kg) and I− (2.4 mg/kg). The minimal relative abundance of Aspergillus was achieved when a mixture of Cu2+ (200 mg/kg), Zn2+, Fe2+ and I− was added to the FLF. Compared with Zn2+, Fe2+ and I−, Cu2+ was the most important ion in inhibiting Aspergillus growth. Adding Zn2+ (160 mg/kg), Cu2+, Fe2+ and I− to the FLF minimized the relative abundance of Fusarium. It was Zn2+ instead of Cu2+ played a critical role in suppressing the growth of Fusarium. The proper use of the mixture of Cu2+, Zn2+, Fe2+ and I− in FLF contributes to inhibit the growth of mycotoxin-producing fungi during storage. The new findings of this study help farmers properly use the mixture of Cu2+, Zn2+, Fe2+ and I− to inhibit the growth of mycotoxin-producing fungi in the production of high quality FLF and alleviate mycotoxins damages to animals and humans.
Keywords: Trace elements, Fungal growth, Mixture of ions, Fermented liquid feed, Pyrosequencing
1. Introduction
The use of fermented liquid feed (FLF) is becoming more and more popular in piglets feeding, because it keeps high and regular feed and water intake of piglets (Missottena et al., 2010), alleviates the stress associated with dietary change and improves piglet health and performance (Maslowski and Mackay, 2011, Wang et al., 2011). However, how to effectively inhibit the growth of mycotoxigenic fungi during the storage of FLF has been a common concern, because mycotoxigenic fungi could grow well in high-moisture feed and produce mycotoxins.
Mycotoxins are toxic secondary metabolites produced by many species of Aspergillus, Fusarium, Penicillium and Alternaria. The most extensively investigated mycotoxins in animal feed are aflatoxin B1 (AFB1), zearalenone (ZEN) and deoxynivalenol (DON; also known as vomitoxin) (Yiannikouris and Jouany, 2002, Streit et al., 2012, Li et al., 2015). The aflatoxins (B, G and M) are natural toxins produced by the filamentous fungi Aspergillus flavus, Aspergillus parasiticus, Aspergillus bombycis, Aspergillus nomius, Aspergillus ochraceoroseus, Aspergillus pseudotamarii and Aspergillus tamari (Mishra and Das, 2003, Guan et al., 2011a, Guan et al., 2011b). When animals continuously ingest aflatoxins through contaminated feed, gastrointestinal dysfunction, reduced feed utilization, anaemia and jaundice often occur (Streit et al., 2012). Zearalenone is mainly produced by Fusarium graminearum and Fusarium culmorum (Labuda et al., 2005). Ingesting ZEN usually results in genital problems including hyperaemia, swelling and reddening of the vulva, uterine enlargement, vaginal and rectal prolapse, enlargement of mammary glands, and reproductive disorders of sows (Kakeya et al., 2002). Deoxynivalenol is a trichothecene mycotoxin, which is primarily associated with F. graminearum and F. culmorum (Stroia et al., 2010). The toxicosis of DON in pigs is manifested by vomiting, feed refusal, abortion, stillbirths and weak offspring (Williams et al., 1989, Tirado et al., 2010, Xiao et al., 2013). Thus, it is a high priority to develop measures to alleviate mycotoxins contamination and toxicity in animal feed industry (Wu et al., 2013, Duan et al., 2014, Yin et al., 2014, Wu et al., 2014).
Trace elements (copper, zinc, iron, molybdenum, magnesium, etc.) play important roles in controlling the growth and secondary metabolism of fungi. The effect of some trace elements on the biosynthesis of aflatoxin by A. flavus and A. parasiticus has been reviewed. It was found that iron, copper, zinc, manganese, molybdenum, calcium and magnesium under certain concentrations enhanced the growth of A. flavus and A. parasiticus (Lee et al., 1966, Lillehoj et al., 1974, Aziz et al., 2000, Datsugwai et al., 2013). However, when the concentrations of trace elements are beyond optimum concentrations, trace elements could inhibit the growth of fungi progressively (Rawla, 1969).
To promote the performance of pigs and decrease the incidence of intestinal diseases, Cu2+, Zn2+, Fe2+ and I− are often added to the feed of pigs at higher concentrations than the normal required concentrations, relative to other trace elements. No studies have investigated whether fungal diversity and toxigenic fungal growth are influenced by a mixture of Cu2+, Zn2+, Fe2+ and I−. To find an effective method to decrease the growth of toxigenic fungi during the storage period of FLF, we designed the present study to survey the difference in fungal diversity and growth in 9 Bacillus subtilis FLF containing different concentrations of Cu2+, Zn2+, Fe2+ and I− by pyrosequencing.
2. Materials and methods
2.1. Experimental design and sample preparation
We selected Cu2+, Zn2+, Fe2+ and I− as experimental factors. Each factor had 3 concentrations which are listed in Table 1 (these ions are currently added to diets of piglets in China at concentrations more than 0 mg/kg, thus the concentration of 0 mg/kg was excluded). In addition, the selected concentrations were based on the analyzed values of 6 commercial diets for suckling piglets and 6 commercial diets for early weanling piglets. Table 2 lists 9 mineral mixtures which were prepared according to the L9(34) orthogonal design. Each mineral mixture was added to the basal diet to produce 9 experimental diets. The basal diet is shown in Table 3.
Table 1.
Three concentrations of trace element ions1 (mg/kg) to be added to the basal diet.
| Item | Concentration 1 | Concentration 2 | Concentration 3 |
|---|---|---|---|
| Cu2+ | 200 | 150 | 100 |
| Zn2+ | 160 | 110 | 60 |
| Fe2+ | 150 | 100 | 50 |
| I- | 2.4 | 1.2 | 0.6 |
Cu2+ of CuSO4·5H2O, Zn2+ of ZnSO4·H2O, Fe2+ of FeSO4·H2O and I− of KI.
Table 2.
Trace element mixtures to be added to the basal diet (mg/kg) in a L9 (34) orthogonal experiment design.
| Item | Cu2+ | Zn2+ | Fe2+ | I- |
|---|---|---|---|---|
| Mixture 1 | 200 | 160 | 150 | 2.4 |
| Mixture 2 | 200 | 110 | 100 | 1.2 |
| Mixture 3 | 200 | 60 | 50 | 0.6 |
| Mixture 4 | 150 | 160 | 100 | 0.6 |
| Mixture 5 | 150 | 110 | 50 | 2.4 |
| Mixture 6 | 150 | 60 | 150 | 1.2 |
| Mixture 7 | 100 | 160 | 50 | 1.2 |
| Mixture 8 | 100 | 110 | 150 | 0.6 |
| Mixture 9 | 100 | 60 | 100 | 2.4 |
Table 3.
Ingredients and nutrient levels of the basal diet (air-dry basis).
| Item | Content |
|---|---|
| Ingredient, % | |
| Corn | 52.0 |
| Wheat bran | 7.0 |
| Extruded soybean | 30.0 |
| Fishmeal (Peru) | 3.0 |
| Lactose | 4.0 |
| Premix1 | 4.0 |
| Total | 100.0 |
| Nutrient levels,2% | |
| Digestible energy, MJ/kg | 13.71 |
| Crude protein | 19.75 |
| Calcium | 1.05 |
| Total phosphorus | 0.66 |
| Lysine | 1.32 |
| Methionine + Cystine | 0.78 |
Premix provided per kilogram diet: VA 450,000 IU, VD3 72,000 IU, VE 2750 IU, VK3 100 mg, VB1 90 mg, VB2 280 mg, VB6 190 mg, VB12 0.8 mg, Niacin 1,450 mg, Pantothenic acid 950 mg, Biotin 3 mg, Choline chloride 10,500 mg, Lysine 40,000 mg, Mn 2,000 mg, Co 38 mg, Se 10.5 mg, Ca 137,000 mg, P 40,800 mg, NaCl 80,000 mg, Wole200 (heat-resistant Bacillus subtilis HEWD113, effective live bacteria ≥ 2 × 1010 CFU/g) 7,500 mg.
Nutrient levels in the table were analyzed values except digestible energy.
Each experimental diet (100 g) and tap water (300 g) were mixed into a polypropylene bag (size: 18 cm × 15 cm; thickness: 80 μm; 6 bags per experimental diet). These bags were sealed with heat sealer and heated in a steam container at 80 °C for 30 min under normal pressure to kill undesirable microorganisms to make B. subtilis grow well. And then, they were placed in room temperature (22.5 to 33.9 °C) for a 21-d storage. We randomly selected 4 bags of FLF from each treatment on day 22, mixed the FLF and then sampled them. All samples were stored at −80 °C before genomic DNA extraction.
2.2. DNA extraction and pyrosequencing
We used the E.Z.N.A Soil DNA kit (OMEGA, USA) to extract genomic DNA. The triplicate DNA extracts for each sample were pooled prior to PCR. Samples were amplified using the forward primer (A-ITS1) and reverse primer (B-ITS4). The forward primer (A-ITS1) was 5′-CCATCTCATCCCTGCGTGTCTCCGACGACTNNNNNNNNNNTCCGTAGGTGAACCTGCGG-3′, where the sequence of adaptor A is shown in italics and underlined, and the Ns represent a ten-base sample specific barcode sequence. The reverse primer (B-ITS4) was 5′-CCTATCCCCTGTGTGCCTTGGCAGTCGACTTCCTCCGCTTATTGATATGC-3′, where the sequence of adaptor B is shown in italics and underlined.
The polymerase chain reaction (PCR) was carried out in a 20-mL reaction volume which was containing 0.4 μL TransStart Fastpfu DNA Polymerase (Beijing TransGen Biotech Co., Ltd, China), 4 μL FastPfu buffer (5×), 2 μL dNTPs (2.5 mmol/L), 0.8 μL Forward Primer (5 μmol/L), 0.8 μL reverse primer (5 μmol/L), 0.4 μL Fastpfu Polymerase (5 μmol/L), 10 ng DNA template and de-ionized ultrapure water. The PCR protocol was performed on ABI GeneAmp 9700 Cycler in following conditions: initial denaturation for 2 min at 95 °C, followed by 30 cycles of denaturation for 30 s at 95 °C, annealing for 30 s at 55 °C and extension for 30 s at 72 °C, then with a final extension for 5 min at 72 °C. Amplification products were visualized on 2% agarose gels, then purified using AxyPrepDNA PCR purification kit (Axygen, China), quantified using the QuantiFluor-ST system (Promega) and pooled in equimolar ratios based on concentration and subjected to emulsion PCR (Roche GS FLX Titanium emPCR Kits) to generate amplicon libraries. Amplicon pyrosequencing was performed from the A-end using a 454/Roche GS-FLX Titanium platform.
2.3. Sequences processing and bioinformatic analysis
Raw sequences that were obtained from 454/Roche GS-FLX Titanium pyrosequencer were processed with Mothur software (http://sourceforge.net/projects/seqclean/ & http://www.mothur.org/wiki/Main_Page) and the unqualified sequences were removed according to following criteria: <200 nucleotides in length (not including sample specific barcodes), contained ambiguous bases, had an imperfect match to a sample-specific barcode and a read quality score <25. The chimeric sequences were also excluded by Usearch software (version6.1, http://drive5.com/usearch/). The unique sequences were clustered into operational taxonomic units (OTUs) at 97% sequence identity by using Qiime software (http://qiime.org/scripts/assign_taxonomy.html, Naïve Bayesian Classifier). A taxonomy assignment was conducted using the ITS database (http://its2.bioapps.biozentrum.uni-wuerzburg.de/) with a confidence level of 0.7. The abundance coverage-based estimator (http://www.mothur.org/wiki/Ace) and Shannon index (http://www.mothur.org/wiki/Shannon) were calculated by Mothur, and microbial community barplot and heatmap were generated by R packages.
3. Results
3.1. Reads, OTUs and alpha-diversity
After processing and quality filtering of raw data, the reads (qualified sequences) and OTUs of samples from different FLF are listed in Table 4. The data of reads were deposited in the sequence read archive (SRA) database (accession number: SRP044186). Ace and Chao 1 are the indexes of microbial richness. The higher values of Ace (or, Chao 1), the more microbial communities. Thereby, the fungi richness in FLF was ranked in this order: 1 > 8 > 4 > 6 > 3 > 2 > 5 > 9 > 7, as shown in Table 4. Shannon and Simpson are the indexes of microbial diversity. The higher Shannon value (or the lower Simpson value) means the higher microbial diversity. Thus, the fungal diversity in FLF was ranked in this order: 1 > 8 > 4 > 6 > 3 > 2 > 9 > 5 > 7, as shown in Table 4.
Table 4.
Reads, operational taxonomic units (OTUs), richness and diversity of fungi in fermented liquid feeds (FLF) samples.
| Item | Reads | 0.97 (Sequence identity) |
||||
|---|---|---|---|---|---|---|
| OTUs | Ace | Chao 1 | Shannon | Simpson | ||
| FLF 1 | 6748 | 826 | 1249 | 1258 | 4.86 | 0.0303 |
| FLF 2 | 5704 | 317 | 525 | 445 | 2.88 | 0.2097 |
| FLF 3 | 6085 | 397 | 580 | 574 | 3.32 | 0.1326 |
| FLF 4 | 5586 | 609 | 853 | 860 | 4.24 | 0.0786 |
| FLF 5 | 6459 | 309 | 498 | 424 | 2.64 | 0.2491 |
| FLF 6 | 5790 | 560 | 785 | 784 | 3.98 | 0.0953 |
| FLF 7 | 6600 | 283 | 376 | 395 | 2.50 | 0.2761 |
| FLF 8 | 6638 | 704 | 1026 | 1011 | 4.30 | 0.0790 |
| FLF 9 | 6740 | 326 | 418 | 416 | 2.72 | 0.2688 |
3.2. Community composition of fungi in samples of different FLF
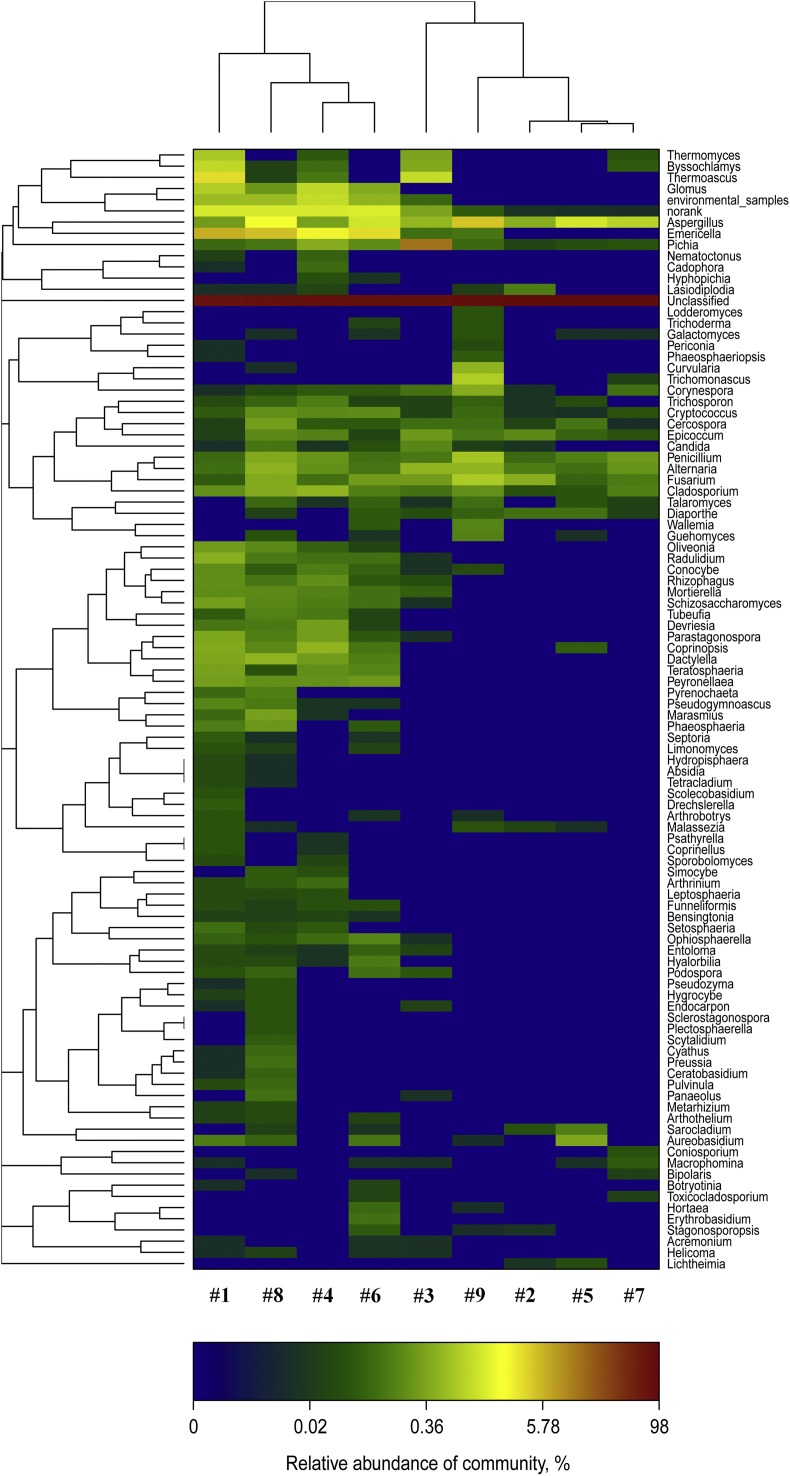
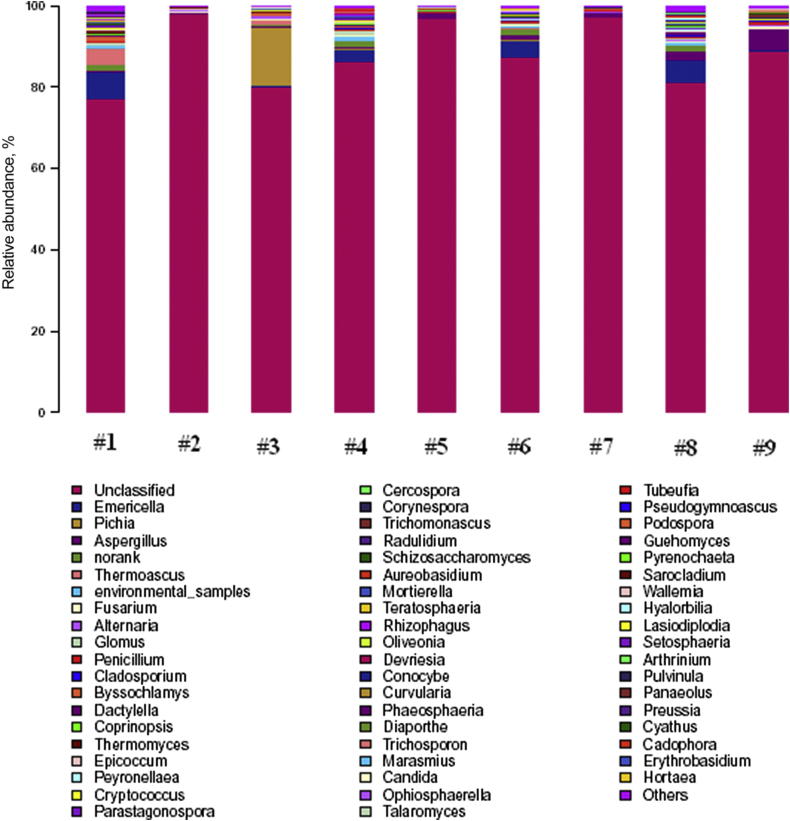
The hierarchical heatmap was based on the top 100 abundant fungi community at genus level (as shown in Fig. 1). This heatmap disclosed that samples of FLF 1 to 9 were numerically dominated by unclassified genus. The highest similarity of fungi community was found between samples of FLF 4 and 6, FLF 5 and 7, respectively. Fig. 2 shows the fungi community compositions of the 9 FLF samples. The relative abundance of toxigenic fungi, Aspergillus and Fusarium, in the 9 FLF samples are listed as follows: for Aspergillus, 0.31, 0.46, 0.56, 0.36, 1.41, 1.30, 0.95, 2.21 and 4.94; for Fusarium, 0.07, 0.46, 0.31, 0.13, 0.09, 0.31, 0.15, 0.42 and 0.80. Samples from FLF 1 had the minimal relative abundance of Aspergillus and Fusarium, but samples from FLF 9 had the maximal relative abundance of Aspergillus and Fusarium.
Fig. 1.
Hierarchical dendrogram of the top 100 fungi in different fermented liquid feed (FLF). Distribution of the top 100 abundant fungi genus in different FLF. The phylogenetic tree was calculated using the neighbour-joining method and the relationship among samples was determined by Bray–Curtis distance. The heatmap plot depicted the relative percentage of each fungal genus within each sample. The relative values for fungal genus were indicated by colour intensity.
Fig. 2.
Genus-level taxonomic compositions of fungi in different FLF. Genus-level taxonomic compositions of fungi in FLF. Sequences that could not be classified into any known group were named as No-rank. Sequences that could not be matched to any known sequences were designated as unclassified. Sequences that had relative abundance of less than 1% were grouped into others.
3.3. Effect of trace elements on relative abundance of toxigenic fungi
The best concentration and significance order of the trace elements for the relative abundance of Aspergillus and Fusarium were different (as shown in Table 5). Compared with Zn2+, Fe2+ and I−, Cu2+ is the most important ion in controlling the growth of Aspergillus. The best concentration of Cu2+ in inhibiting the growth of Aspergillus is 200 mg/kg (Concentration 1). It was Zn2+ instead of Cu2+ plays a dominant role in promoting the growth of Fusarium, and the addition of Zn2+ at 160 mg/kg (Concentration 1) minimized Fusarium growth. The comprehensive analysis showed that the growth of Aspergillus, Fusarium, Alternaria and Penicillium in the FLF can be inhibited when liquid feed was fermented with a mixture of Cu2+, Zn2+, I− and Fe2+ at 200, 160, 1.2 and 50 mg/kg, respectively.
Table 5.
Effect of trace elements on the relative abundance of toxigenic fungi in FLF.
| Item | Factor | Mean |
Range1 | Best concen.2 | Significance order | ||
|---|---|---|---|---|---|---|---|
| Concen. 1 | Concen. 2 | Concen. 3 | |||||
| Aspergillus | Cu2+ | 0.44 | 1.02 | 2.70 | 2.26 | Concen. 1 | Cu2+ > Zn2+ > I− > Fe2+ |
| Zn2+ | 0.54 | 1.36 | 2.26 | 1.72 | Concen. 1 | ||
| Fe2+ | 1.27 | 1.92 | 0.97 | 0.94 | Concen. 3 | ||
| I− | 2.22 | 0.90 | 1.04 | 1.32 | Concen. 2 | ||
| Fusarium | Cu2+ | 0.28 | 0.18 | 0.46 | 0.28 | Concen. 2 | Zn2+ > Fe2+ = Cu2+ > I− |
| Zn2+ | 0.12 | 0.32 | 0.47 | 0.36 | Concen. 1 | ||
| Fe2+ | 0.27 | 0.46 | 0.19 | 0.28 | Concen. 3 | ||
| I− | 0.32 | 0.31 | 0.29 | 0.04 | Concen. 3 | ||
| Alternaria | Cu2+ | 0.26 | 0.17 | 0.42 | 0.25 | Concen. 2 | Cu2+ > I− > Zn2+ > Fe2+ |
| Zn2+ | 0.21 | 0.25 | 0.38 | 0.17 | Concen. 1 | ||
| Fe2+ | 0.25 | 0.30 | 0.30 | 0.05 | Concen. 1 | ||
| I− | 0.25 | 0.19 | 0.41 | 0.22 | Concen. 2 | ||
| Penicillium | Cu2+ | 0.12 | 0.19 | 0.48 | 0.36 | Concen. 1 | Cu2+ > Fe2+ = I− > Zn2+ |
| Zn2+ | 0.23 | 0.23 | 0.33 | 0.10 | Concen. 2 | ||
| Fe2+ | 0.22 | 0.36 | 0.21 | 0.15 | Concen. 3 | ||
| I− | 0.33 | 0.18 | 0.28 | 0.15 | Concen. 2 | ||
Range = maximal relative abundance minus minimal relative abundance.
Concen. = concentration. The best concentration is the concentration to achieve the minimal relative abundance.
4. Discussion
Trace elements (e.g., Cu, Zn, Fe and I) are crucial for the proper growth and metabolism of fungi. A lack or overly concentrated addition of trace elements is adverse to the growth of fungi. No information is available for the influence of Cu2+, Zn2+, Fe2+ and I− mixtures on the growth of fungi in FLF. Results in the current study showed that the mixture of Cu2+, Zn2+, Fe2+ and I− added at different concentrations affected the growth and diversity of fungi in FLF during storage, and the growth and diversity of fungi in FLF decreased with decreasing single Cu2+, Zn2+ or Fe2+ concentration.
Toxigenic fungi grow well in liquid feeds with abundant moistures and other nutrients and produce mycotoxins at optimal temperatures. Thus, how to suppress the growth of toxigenic fungi in FLF is highly concerned by the producers of animal husbandry industry. Toxigenic fungi that are most extensively investigated in animal feed are Aspergillus and Fusarium (Streit et al., 2012, Stroia et al., 2010, Guan et al., 2011a, Guan et al., 2011b). Previous results found that trace elements affect the growth of Aspergillus and Fusarium (Adiga et al., 1962, Jackson et al., 1989, Cuero et al., 2003, Cuero and Ouellet, 2005). The influence of trace elements is different depending on the type of trace element ions and whether they were used alone or in a mixture (Cuero and Ouellet, 2005). Some trace elements such as Cu2+ and Zn2+ are essential micronutrients. However, when they exceed certain threshold levels, they are toxic to fungi; the optimal concentrations of Fe2+, Cu2+ and Zn2+ vary much widely with different strains (Hartikainen et al., 2012).
Data of the present study indicated that the relative abundance of Aspergillus in FLF varied with different mixtures of Cu2+, Zn2+, Fe2+ and I−. Aspergillus showed the lowest relative abundance in the FLF with a mixture of Cu2+ (200 mg/kg) and Zn2+, Fe2+, I−. The ability of mixtures in inhibiting Aspergillus growth was ranked as 1 > 4 > 2 > 3 > 7 > 6 > 5 > 8 > 9. This showed that addition of Cu2+ at 200 mg/kg combined with Zn2+, Fe2+ and I− to the FLF could minimize the growth of Aspergillus.
The significance of single Cu2+, Zn2+, Fe2+ and I− for the relative abundance of Aspergillus was different and ranked as Cu2+ > Zn2+ > I– > Fe2+ in the FLF according to the value of range. The best concentrations of single Cu2+, Zn2+, Fe2+ and I− ions in inhibiting the growth of Aspergillus were 200, 160, 50 and 1.2 mg/kg, respectively. Elevated concentration (from 100 to 200 mg/kg) of Cu2+ decreased the growth of Aspergillus, which is consistent with the previous result (Al Abboud and Alawlaqi, 2011). Elevated concentration (from 60 to 160 mg/kg) of Zn2+ suppressed the relative abundance of Aspergillus. Aziz et al. (2000) also found that elevated Zn2+ (from 100 to 300 mg/kg) decreased the growth of A. flavus in a 14-d incubation, but increased the growth of A. flavus in a 28-d incubation, and the increase in Cu2+ or Fe2+ concentration numerically decreased the growth of A. flavus (Aziz et al., 2000). Our results also showed high Fe2+ concentration inhibited the growth of Aspergillus, and high I− concentration stimulated the growth of Aspergillus during a 21-d storage.
Fusarium showed minimal relative abundance in the FLF with a combination of Zn2+ (110 mg/kg) and Cu2+, Fe2+, I−. The ability of Cu2+, Zn2+, Fe2+ and I− mixtures in suppressing the growth of Fusarium was ranked as 1 > 5 > 4 > 7 > 3 = 6 > 8 > 2 > 9. This indicated that high concentration of Zn2+ combined with Cu2+, Fe2+ and I− decreased the growth of Fusarium in FLF.
Compared with Cu2+, Fe2+ and I−, Zn2+ was the most important ion in inhibiting the growth of Fusarium in the FLF. Elevated Zn2+ concentration suppressed Fusarium growth. Thind and Madan (1977) found that Zn2+ was necessary for the growth and sporulation of Fusarium moniliforme, and Zn2+ from 0.0001 to 10 mg/kg supplementation increased the growth of the fungus, but from 10 to 400 mg/kg decreased it progressively, and the optimal concentration of Zn2+ for F. moniliforme is 1.0 mg/kg. Cuero and Ouellet (2005) reported that the supplementation of Zn2+, Cu2+ or Fe2+ to F. graminearum liquid cultures stimulated the growth of F. graminearum, and single Fe2+ ion had the largest effect, but single Cu2+ ion had the smallest effect. The triple mixture of Zn2+, Cu2+ and Fe2+ also stimulated the growth of F. graminearum (Cuero and Ouellet, 2005). Our results validated that Zn2+ instead of Cu2+ played a dominant role in the growth of Fusarium, and the best concentration for Zn2+ to decrease the growth of Fusarium was 110 mg/kg.
5. Conclusions
The diversity and relative abundance of fungi in FLF are influenced by a mixture of Cu2+, Zn2+, Fe2+ and I− at different concentrations. The most important ion in inhibiting Aspergillus growth is Cu2+, and Zn2+ plays a critical role in suppressing the growth of Fusarium. The supplementation of Cu2+ at 200 mg/kg, Zn2+ at 160 mg/kg, Fe2+ at 150 mg/kg and I− at 2.4 mg/kg to a B. subtilis FLF could minimize the relative abundance of Aspergillus and Fusarium.
Acknowledgement
The study was supported by Jiangxi Provincial Key Technology R&D Program (20121BBF60032 and 20132BBF60039).
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
Contributor Information
Wei Lu, Email: lw20030508@163.com.
Huadong Wu, Email: whd0618@163.com.
References
- Al Abboud M.A., Alawlaqi M.M. Biouptake of copper and their impact on fungal fatty acids. AJBAS. 2011;5:283–290. [Google Scholar]
- Adiga P.R., Sastry K.S., Sarma P.S. The influence of iron and magnesium on the uptake of heavy metals in metal toxicities in Aspergillus niger. Biochim Biophys Acta. 1962;64:546–548. doi: 10.1016/0006-3002(62)90313-x. [DOI] [PubMed] [Google Scholar]
- Aziz N.H., Shahin A.A.M., Abou-Zeid A.A.M., El-Zeany S.A. Correlation of growth and aflatoxin production by Aspergillus flavus with some essential metals in gamma irradiated crushed corn. Food. 2000;44:354–359. doi: 10.1002/1521-3803(20001001)44:5<354::AID-FOOD354>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Cuero R., Ouellet T. Metal ions modulate gene expression and accumulation of the mycotoxins aflatoxin and zearalenone. J Appl Microbiol. 2005;98:598–605. doi: 10.1111/j.1365-2672.2004.02492.x. [DOI] [PubMed] [Google Scholar]
- Cuero R., Ouellet T., Yu J., Mogongwa N. Metal ion enhancement of fungal growth, gene expression and aflatoxin synthesis in Aspergillus flavus: RT-PCR characterization. J Appl Microbiol. 2003;94:953–961. doi: 10.1046/j.1365-2672.2003.01870.x. [DOI] [PubMed] [Google Scholar]
- Datsugwai M.S.S., Ezekiel B., Audu Y., Legbo M.I., Azeh Y., Gogo M.R. Mycotoxins: toxigenic fungal compounds – a review. ARPN J Sci Technol. 2013;3:687–692. [Google Scholar]
- Duan J.L., Yin J., Wu M.M., Liao P., Deng D., Liu G. Dietary glutamate supplementation ameliorates mycotoxin-induced abnormalities in the intestinal structure and expression of amino acid transporters in young pigs. PLoS One. 2014;9:e112357. doi: 10.1371/journal.pone.0112357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan S., Gong M., Yin Y.L., Huang R.L., Ruan Z., Zhou T. Occurrence of mycotoxins in feeds and feed ingredients in China. J Food Agric Environ. 2011;9:163–167. [Google Scholar]
- Guan S., Zhou T., Yin Y., Xie M., Ruan Z., Young J.C. Microbial strategies to control aflatoxins in food and feed. World Mycotoxin J. 2011;4:413–424. [Google Scholar]
- Hartikainen E.S., Lankinen P., Rajasärkkä J., Koponen H., Virta M., Hatakka A. Impact of copper and zinc on the growth of saprotrophic fungi and the production of extracellular enzymes. Boreal Environ Res. 2012;17:210–218. [Google Scholar]
- Jackson M., Slinger P.J., Bothas R.J. Effects of zinc, iron, cobalt, and manganese on Fusarium moniliforme NRRL 1316 growth and fusarin C. biosynthesis in submerged cultures. Appl Environ Microb. 1989;55:649–655. doi: 10.1128/aem.55.3.649-655.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakeya H., Takahashi-Ando N., Kimura M., Onose R., Yamaguchi I., Osada H. Biotransformation of the mycotoxin, zearalenone, to a non-estrogenic compound by a fungal strain of Clonostachys sp. Biosci Biotechnol Biochem. 2002;66:2723–2726. doi: 10.1271/bbb.66.2723. [DOI] [PubMed] [Google Scholar]
- Labuda R., Parich A., Berthiller F., Tancinova D. Incidence of trichothecenes and zearalenone in poultry feed mixtures from Slovakia. Int J Food Microbiol. 2005;105:19–25. doi: 10.1016/j.ijfoodmicro.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Lee E.G., Townsley H., Walden C.C. Effect of bivalent metals on the production of aflatoxins in submerged cultures. J Food Sci. 1966;31:432–436. [Google Scholar]
- Li W., Liao P., He L.Q., Feng Z.M., Ren W.K., Yin J. Dietary L-arginine supplementation protects weanling pigs from deoxynivalenol-induced toxicity. Toxins. 2015;7:1341–1354. doi: 10.3390/toxins7041341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillehoj E.B., Garcia W.J., Lambrow M. Aspergillus flavus infection and aflatoxin production in corn: influence of trace elements. Appl Environ Microb. 1974;28:763–767. doi: 10.1128/am.28.5.763-767.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslowski K.M., Mackay C.R. Diet, gut microbiota and immune responses. Nat Immunol. 2011;12:5–9. doi: 10.1038/ni0111-5. [DOI] [PubMed] [Google Scholar]
- Mishra H.N., Das C. A review on biological control and metabolism of aflatoxin. Crit Rev Food Sci. 2003;43:245–264. doi: 10.1080/10408690390826518. [DOI] [PubMed] [Google Scholar]
- Missottena J.A.M., Michielsb J., Ovyna A., De Smeta S., Dierick N.A. Fermented liquid feed for pigs. Arch Anim Nutr. 2010;6:437–466. doi: 10.1080/1745039X.2010.512725. [DOI] [PubMed] [Google Scholar]
- Rawla G.S. A note on trace elements for the growth of Nigrospora oryzae (B. and BR.) petch. New Phytol. 1969;68:941–943. [Google Scholar]
- Streit E., Schatzmayr G., Tassis P., Tzika E., Marin M., Taranu I. Current situation of mycotoxin contamination and co-occurrence in animal feed-focus on Europe. Toxins. 2012;4:788–809. doi: 10.3390/toxins4100788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroia C., Tabuc C., Neacsu A. Incidence of Fusarium species and its mycotoxins in cereals from western Romania. Res J Agric Sci. 2010;42:302–309. [Google Scholar]
- Thind K.S., Madan M. Effect of various trace elements on the growth and sporulation of four fungi. Proc Indian Natl Sci Acad. 1977;43:115–124. [Google Scholar]
- Tirado M.C., Clarke R., Jaykus L.A., Mcquatters-Gollop A., Frank J.M. Climate change and food safety: a review. Food Res Int. 2010;43:1745–1765. [Google Scholar]
- Wang S.P., Yang L.Y., Tang X.S., Cai L.C., Liu G., Kong X.F. Dietary supplementation with high-dose Bacillus subtilis or Lactobacillus reuteri modulates cellular and humoral immunities and improves performance in weaned piglets. J Food Agric Environ. 2011;9:181–187. [Google Scholar]
- Williams K.C., Blaney B.J., Magee M.H. Responses of pigs fed wheat naturally infected with Fusarium graminearum and containing the mycotoxins 4-deoxynivalenol and zearalenone. Aust J Agric Res. 1989;39:1095–1105. [Google Scholar]
- Wu L., Wang W.C., Yao K., Zhou T., Yin J., Li T.J. Effects of dietary arginine and glutamine on alleviating the impairment induced by deoxynivalenol stress and immune relevant cytokines in growing pigs. PLoS One. 2013;8:e69502. doi: 10.1371/journal.pone.0069502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M.M., Xiao H., Ren W.K., Yin J., Hu J.Y., Duan J.L. An NMR-based metabolomic approach to investigate the effects of supplementation with glutamic acid in piglets challenged with deoxynivalenol. PLoS One. 2014;9:e113687. doi: 10.1371/journal.pone.0113687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao H., Wu M.M., Tan B.E., Yin Y.L., Li T.J., Xiao D.F. Effects of composite antimicrobial peptides in weanling piglets challenged with deoxynivalenol: I. Growth performance, immune function, and antioxidation capacity. J Anim Sci. 2013;91:4772–4780. doi: 10.2527/jas.2013-6426. [DOI] [PubMed] [Google Scholar]
- Yiannikouris A., Jouany J.P. Mycotoxins in feeds and their fate in animals: a review. Anim Res. 2002;51:81–99. [Google Scholar]
- Yin J., Ren W.K., Duan J.L., Wu L., Chen S., Li T.J. Dietary arginine supplementation enhances intestinal expression of SLC7A7 and SLC7A1 and ameliorates growth depression in mycotoxin-challenged pigs. Amino Acids. 2014;46:883–892. doi: 10.1007/s00726-013-1643-5. [DOI] [PubMed] [Google Scholar]