Abstract
Zearalenone (ZEA), an estrogenic mycotoxin, is produced mainly by Fusarium fungi. Previous studies have indicated that acute ZEA exposure induced various damages in different species; however, its transparent hematotoxicity in female piglets at dietary levels of 1.1 to 3.2 mg/kg has not been shown. The present study was conducted to investigate the effects of dietary ZEA (1.1–3.2 mg/kg) on hematology, T lymphocyte subset, immunoglobulin, antibody titer, lymphocyte proliferation rate (LPR), and interleukin-2 (IL-2) in peripheral blood of post-weaning gilts. A total of 20 female piglets (Landrace × Yorkshire × Duroc), weaned at 42 d with an average body weight of 10.36 ± 1.21 kg were used in the study. Female piglets were kept in a temperature controlled room, divided into four treatments, and fed a diet based on corn-soybean meal-fishmeal-whey, with an addition of 0, 1.1, 2.0, or 3.2 mg/kg purified ZEA for 18 d ad libitum. Feed intake and refusal were measured daily and individual pigs were weighed weekly. Blood and serum samples were collected for selected immunological measurements. Female piglets fed different levels of dietary ZEA grew similarly with no difference in feed intake. Hematological values including leukocytes, platelets, lymphocytes, hematocrit, and mean corpuscular hemoglobin (MCH) decreased linearly (P < 0.05) as dietary ZEA increased. Female piglets fed diets containing 2.0 mg/kg ZEA or greater showed significantly decreased CD4+CD8+, CD4+, and CD4+/CD8+ in comparison to the control (P < 0.05), whereas CD8+ was significantly increased (P = 0.026) in the gilts which were fed the diet containing 3.2 mg/kg ZEA. Serum immunoglobulin G (IgG) and the antibody titer on d 18 were reduced linearly as dietary ZEA levels increased (P < 0.001). Linear decrease in LPR was observed (P < 0.05). Female piglets fed diets containing 2.0 mg/kg ZEA or more showed significantly decreased IL-2 in comparison to the control (P < 0.05). The results suggested that dietary ZEA at the levels of 1.1 to 3.2 mg/kg can induce different degrees of hematotoxicity and negatively affect immune function in female piglets.
Keywords: Zearalenone, Immune function, Piglets
1. Introduction
Zearalenone (ZEA) is a mycotoxin produced mainly by Fusarium fungi growing on grains and its derived products throughout the world (Luo et al., 1990, Schollenberger et al., 2006). Among farm animals, pigs, especially female pigs, are susceptible to ZEA (EFSA, 2004, Guan et al., 2011), resulting in maximum limits of 0.1 mg ZEA/kg in the diets of piglets and gilts (EC, 2006). However, investigations have revealed that the amount of feedstuffs contaminated by ZEA worldwide exceeded the maximum limits of EC (2006) (Zinedine et al., 2007).
The major toxicity of ZEA and its metabolites, such as α-zearalonol (α-ZOL), is attributed to their estrogenic effects on the genital organs and reproduction in gilts (Chen et al., 2015, Fushimi et al., 2015, Jiang et al., 2010a, Jiang et al., 2011). In addition, ZEA has been shown to be toxic to multiple tissues in animals, such as hepatotoxicity in rabbits (Conkova et al., 2001) and piglets (Jiang et al., 2010b, Jiang et al., 2012), haematotoxicity in rats (Cheraghi et al., 2015), oxidative stress in mice (Ben Salah-Abbès et al., 2009) and piglets (Jiang et al., 2011, Yin et al., 2014, Wu et al., 2013, Wu et al., 2015, Li et al., 2015), and to have cytotoxic effects on cultured Vero cells (Othmen et al., 2008).
Notwithstanding, the effects of ZEA on immune functions have been well established in mice (Abbès et al., 2006a, Ben Salah-Abbès et al., 2008), humans (Gao et al., 2013) and in vitro (Berek et al., 2001). However, studies of ZEA on immune response of pigs mostly have been conducted with respect to feeding grains naturally cocontaminated with ZEA and other Fusarium mycotoxins (Swamy et al., 2004). Moreover, several changes of immunological parameters were induced by high ZEA concentrations (Abbès et al., 2006a, Abbès et al., 2006b, Ben Salah-Abbès et al., 2008, Li et al., 2013), but such high doses are usually not found in cereals used for animal feed. Therefore, an experiment was conducted to examine whether or not the feeding of a purified ZEA-contaminated (1.1–3.2 mg/kg) diet to postweanling piglets will influence hematological values, T lymphocyte subset, immune globulin, antibody titer, lymphocyte proliferation rate (LPR), and interleukin-2 (IL-2) production in post-weaning gilts.
2. Materials and methods
2.1. Preparation of zearalenone-contaminated diet
Purified ZEA (Fermentek, Israel) was dissolved in acetic ether, and then poured onto talcum powder. A ZEA premix was prepared by blending ZEA-contaminated talcum powder with ZEA-free corn, which was subsequently mixed at the appropriate levels with a corn-soybean meal diet to create the experimental diets. All diets were prepared in one batch, and then stored in covered containers prior to feeding. A composite sample of each experimental diet was prepared for analysis of ZEA and other mycotoxins by the Asia Mycotoxin Analysis Center (Chaoyang University of Technology, Taiwan), before and at the end of the feeding experiment. Deoxynivalenol (DON) was analyzed using high performance liquid chromatography (HPLC). Enzyme linked immunosorbent assay (ELISA) and fluorometry techniques were used to measure ZEA, fumonisins (FUM), and aflatoxin (AFL) levels. The detection limits of these mycotoxins were 1 μg/kg for AFL, 0.1 mg/kg for ZEA, 0.1 mg/kg for DON, including 3-acetyl deoxynivalenol, 15-acetyl deoxynivalenol, and nivalenol, and 0.25 mg/kg for FUM (Chen et al., 2015).
2.2. Experimental design, animals and management
Animals used for all experiments were cared for in accordance with guidelines of the Animal Nutrition Research Institute of Shandong Agricultural University and the Ministry of Agriculture of China for the care and use of laboratory animals. A total of twenty post-weaning female piglets (Landrace × Yorkshire × Duroc) with an average body weight of 10.36 ± 1.21 kg were used in the study. Gilts were randomly allocated into four treatments after seven days of adaptation. The pigs were fed a basal mash diet (Table 1) supplemented with addition of 0, 1.1 ± 0.02, 2.0 ± 0.01 and 3.2 ± 0.02 mg/kg purified ZEA for 18 d. Aflatoxin, DON, and FUM were not detected in the test diets (Jiang et al., 2011).
Table 1.
Ingredients and composition of the basal diet (air-dry basis).
| Ingredients | Content, % | Nutrients composition, % | Analyzed values |
|---|---|---|---|
| Corn | 53.00 | Gross energy, MJ/kg | 17.12 |
| Wheat middling | 5.00 | Crude protein | 19.40 |
| Whey powder | 6.50 | Calcium | 0.84 |
| Soybean oil | 2.50 | Total phosphorus | 0.73 |
| Soybean meal | 24.76 | Lysine | 1.36 |
| Fish meal | 5.50 | Methionine | 0.46 |
| L-Lysine HCl | 0.30 | Sulfur amino acid | 0.79 |
| DL-Methionine | 0.10 | Threonine | 0.90 |
| L-Threonine | 0.04 | Tryptophan | 0.25 |
| Calcium phosphate | 0.80 | ||
| Limestone, pulverized | 0.30 | ||
| Sodium chloride | 0.20 | ||
| Premix1 | 1.00 |
Supplied per kg of diet: vitamin A, 3,300 IU; vitamin D3, 330 IU; vitamin E, 24 IU; vitamin K3, 0.75 mg; vitamin B1, 1.50 mg; vitamin B2, 5.25 mg; vitamin B6, 2.25 mg; vitamin B12, 0.02625 mg; pantothenic acid, 15.00 mg; niacin, 22.5 mg; biotin, 0.075 mg; folic acid, 0.45 mg; Mn (from MnSO4), 6.00 mg; Fe (from FeSO4·H2O), 150 mg; Zn (from ZnSO4), 150 mg; Cu (from CuSO4), 9.00 mg; I (from KI), 0.21 mg; Se (from Na2SeO3), 0.45 mg.
The diets used in the study were isocaloric and isonitrogenous with the only difference being ZEA levels. All nutrient concentrations were formulated to meet or exceed minimal requirements according to the NRC (1998). The pigs were housed in cages equipped with one nipple drinker and one brick-shaped feeder in a temperature controlled room at Jinzhuyuan Farm (Yinan, Shandong, China). During the experimental period, the temperature in the nursery room was maintained between 26 and 28°C. The mean relative humidity was approximately 65%. The gilts were fed ad libitum and allowed access to water freely through the entire experiment period.
2.3. Blood sampling
Blood samples were taken from piglets of all treatments via the jugular vein after the pigs had fasted for 12 h at the end of the experimental period. Samples of 10 mL were collected into a test tube containing an anticoagulant (K2EDTA) for hematological values, T lymphocyte subset, lymphocyte proliferation rate and IL-2 production test, and the other 10 mL were collected into non-heparinized tubes, incubated at 37°C for 2 h, and centrifuged at 1,500 × g for 10 min, then the serum was separated and stored in 1.5 mL Eppendorf tubes at −20°C for serum immunoglobulin and swine plague antibody titer analysis.
2.4. Determination of hematological parameters
Leukocytes, erythrocytes, platelets, lymphocyte ratio, hemoglobin, hematocrit, mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), and mean corpuscular hemoglobin concentration (MCHC) were determined using a blood counter (KX-21 model, automatic blood analyzer, SYSMEX East Asia Co., Japan) with adapted dilutions.
2.5. Phenotyping of peripheral blood lymphocytes
Blood (5 mL) was mixed with 10 mL of Dulbecco's Phosphate Buffered Saline (DPBS, Sigma–Aldrich, USA), then underlayed with 4 mL of Lympholyte-Mammal (Cedarlane Laboratories Limited, Burlington, Canada) for density gradient separation of peripheral blood mononuclear cells (PBMC). The blood samples were centrifuged at 400 × g at room temperature for 30 min. The cell layer at the lympholyte interface was transferred, washed three times with DPBS, and suspended in 1 mL of DPBS. More than 95% of the cells were viable and assessed using trypan blue staining.
Samples of 50 μL of suspended viable PBMC (1 × 106 cells) were transferred to a 96-well round-bottom plate, followed by the addition of 50 μL of the appropriate antibody (Southern Biotechnology Associates, Birmingham, USA). The cells were triple stained with mouse anti-pig CD3ε antibody (CT-3 clone, mouse [BALB/c] IgG1κ, conjugated to fluorescein isothyocyanate, 1 μg/106 cells), either mouse anti-pig CD4a antibody (CT-4 clone, mouse IgG1κ, conjugated to R-phycoerythrin [RPE], 0.2 μg/106 cells), or mouse anti-pig CD8 antibody (CT-8 clone, mouse IgG1κ, conjugated to RPE, 0.2 μg/106 cells) to label CD3+, CD4+ and CD8+ T cells, respectively. The plate was shaken manually for 15 s at the outset and incubated at 4°C for 30 min in the dark for each step of antibody labeling. The plate was subsequently washed three times with DPBS containing 1% bovine serum albumin (BSA). The cells were suspended at the end in 500 μL of the DPBS-BSA. A total of 10,000 cells were acquired and analyzed using a Becton Dickinson FACScan flow cytometer (FACSCALIBVR, USA) and Cell Quest software (Becton–Dickinson Immunocytometry System, San Jose, CA). For data analysis, a size (FSC) vs. internal complexity (SSC) dot-plot was generated, and a region was drawn around the small, live cell population containing the lymphocyte population. Due to the fact that this region may include variable amounts of thrombocytes and red blood cells, the cell population data obtained from the quadrant statistics (two-color staining) or histogram statistics (one-color staining) were standardized for the proportion of lymphocytes using the sum of T cells (CD3+ cells). The lymphocyte subset data were then adjusted by calculation to yield an estimate of the percentage of CD3+CD4+CD8+, CD3+CD4+, and CD3+CD8+ T lymphocytes, respectively. In order to estimate the concentrations of these lymphocyte subsets in the blood circulation, the concentration of lymphocytes throughout the blood was multiplied by the proportion of various lymphocyte subsets obtained from the flow cytometric analysis.
2.6. Lymphocyte proliferation rate of peripheral blood
Pretreatment of blood: whole blood was diluted by D-hanks at a ratio of 1:1, and then was added into three times with DPBS, and suspended in 1 mL of DPBS. More than 95% of the lymphocyte separation liquid, and the mixture were subjected to centrifugation at a speed of 295 × g for 30 min. Next, leukocytes in the intermediate layer were harvested. The cell mixture including erythrocytes was lysed with red blood cell lysates, and then they were washed three times with RPMI-1640 (Hyclone, USA). The washed cells were subjected to centrifugation for 5 min at a speed of 295 × g, then the number of live cells was counted with trypan-blue staining (the number of live cells should exceed 95%). Finally, the lymphocytes were suspended in an RPMI-1640 complete medium and the cells were adjusted to a cell concentration of 2 × 106 cells/mL.
Measurement of cell proliferation rate of lymphocytes: the above cell suspensions were added into 96 well cell culture plates with 190 μL cell suspension in each well, and in the meantime 10 μL ConA was added to each well. The cell mixtures were placed in a 5% CO2 incubator for 72 h under 37°C. Four hours before the culturing of cells was complete, 100 μL MTT was added into every well and the cells continued culturing. After culturing was completed, 100 μL supernatant was removed from every well, and 100 μL dimethyl sulfoxide (DMSO) was added to dissolve purple crystallized products. The absorbance of each well (OD value) was measured at a wavelength of 570 nm using an ELISA reader. The absorptance value of the control group was 1, and the absorptance values of the other experiment groups were compared with that of the control, then the percentages of cell numbers in the experiment groups which were relative to the control group were obtained (Cetin and Bullerman, 2005).
2.7. IL-2 Production of peripheral blood
Induction of IL-2 in vitro: preparation of peripheral blood lymphocytes was as described above. Five hundred microliter cell suspensions were added to each well of the 48 well cell culture plates. The plates were placed in a 5% CO2 incubator for 24 h for static culture under 37°C. The cell culture plates were removed after culturing; supernatants were harvested sterilely on a super-clean workbench, and the harvested supernatants were stored in a refrigerator at −80°C for further detection.
Preparation of targeted cells for detection of induced IL-2 activity: 1 mL cell suspensions and 1 mL ConA solution were placed in every well of the 24 well cell culture plates, and then placed in a 5% CO2 incubator under 37° C for culturing for 48 h. The cell culture plates were removed from the incubator, and lymphocytes were collected with lymphocyte separation solution by means of density gradient equilibrium centrifugation (664 × g, centrifugation for 20 min). The harvested cells were washed twice with an RPMI-1640 complete medium, and then the cells were additionally washed three times with α-mannosidosis. Finally, the targeted cells were adjusted to a concentration of 8 × 106 cells/mL with RPMI-1640 complete medium containing α-mannosidosis for further detection.
Measurement of IL-2 content: a 144 μL RPMI-1640 complete medium was placed in every well of the 96 well cell culture plates, then 6 μL samples (diluted with an RPMI-1640 complete medium at a ratio of 1:16) was added to each well, and 50 μL targeted cells were also added to each well. Every sample included three repetition wells, and the RPMI-1640 complete medium was set as a blank control. The prepared cells in the plates were placed in an incubator with 5% CO2 at 37°C for culturing for 30 h. After culturing was complete, a 100 μL MTT solution was placed in each well, and the cells were further cultured for 4–6 h. One hundred microliter supernatant was removed from each well and 100 μL DMSO was added to the supernatant. Optical density value was measured at a wavelength of 570 nm using an ELISA reader.
2.8. Serum immunoglobulins
The total concentrations of the different immunoglobulin subsets (immunoglobulin G [IgG], IgA, and IgM) were measured by ELISA. All antibodies and reference sera used in the assay were purchased from Southern Biotechnology Associates (Birmingham, USA). Goat anti-pig IgA (alpha chain-specific, 0.01 mg/mL), goat anti-pig IgG (Fc fragment specific, 2 ng/mL), and goat anti-pig IgM (mu chain-specific, 2 ng/mL) were used as capture antibodies. The concentrations of IgM, IgG, and IgA in the reference sera were 0.6, 6.0, and 0.4 mg/mL, respectively, and standard curves were constructed for immunoglobulin by diluting the reference sera. Horseradish peroxidase (HRP)-conjugated antibodies included 1/60,000 goat anti-pig IgM-HRP (mu chain-specific), 1/125,000 goat anti-pig IgG-HRP (Fc-fragment specific), and 1/100,000 goat anti-pig IgA-HRP (alpha chain-specific). The efficiency of immunoglobulin detection in the serum was quantified with 50 ng/mL of purified pig IgG, IgM, or IgA. Absorbance was read at 450 nm using an ELISA plate reader (Spectra thermo, Tecan, Trappes, France).
2.9. Antibody response to swine plague
On the first day of the ZEA challenged trial, all piglets were immunized by subcutaneous inoculation with one dose of swine plague vaccine (Harbin Veterinary Research Institute, CAAS, China). Blood samples (5 mL) were taken on the day before the ZEA challenged trial and on experiment days 6, 12 and 18 from each piglet via the jugular vein using the same methods described above. All antibodies and reference sera used in the assay were purchased from Harbin Veterinary Research Institute, CAAS (China). The swine plague antibody titers were measured by the hemagglutination (HA) and hemagglutination-inhibition (HI) test (microtiter). Then 0.05 mL of serum were serially diluted with phosphate-buffered saline (0.005 mol/L phosphates) (NaH2PO4·H2O + Na2HPO4 anhydrous), 0.146 mol/L NaCl (pH 7.2) on 96-well plates after being inactivated for 30 min at 56°C, then an additional 0.05 mL of virus dilution containing 4 HA units per well was added. The plates were oscillated for 3 min and incubated for 10 min at room temperature, and then 0.05 mL RBC suspension was added per well, oscillated for 3 min and reinsulated for 15 to 60 min at room temperature. The results of the swine plague antibody titers were read at the end point of hemagglutination inhibition.
2.10. Statistical analyses
All data were subjected to analysis of variance using the GLM procedure of SAS (SAS 9.1, 2003). The data were first analyzed as a completely randomized design with individual piglet as a random factor to examine the overall effect of the treatments. Orthogonal polynomial contrasts were then used to determine linear responses to ZEA levels of treatments. The significances of differences among treatments were tested using Duncan's multiple range tests. All statements of significance were based on P < 0.05.
3. Results
No significant differences were observed in the average daily feed intake (ADFI), average daily gain (ADG) or feed conversion ratio (FCR, gain: feed ratio) throughout the testing period of piglets among all treatments.
3.1. Hematological values
Gilts fed the diet containing 1.1 mg/kg ZEA or greater had reduced (P < 0.05) platelets compared with the control (Table 2). Gilts fed diets containing 2.0 mg/kg ZEA or greater had decreased (P < 0.05) blood levels of leukocytes, lymphocytes and hematocrit compared with the control. Increasing dietary ZEA linearly decreased (P < 0.05) leukocytes, platelets, lymphocytes, hematocrit, and MCH.
Table 2.
Hematological values of gilts fed diet with or without zearalenone (ZEA) supplementation.1
| Item | Control2 | ZEA12 | ZEA22 | ZEA32 | SEM | Effects (P-value) |
|
|---|---|---|---|---|---|---|---|
| Treatment | Linear | ||||||
| Leukocytes, × 109/L | 18.68a | 17.51a | 14.27b | 14.13b | 0.09 | <0.001 | <0.001 |
| Erythrocytes, × 1012/L | 6.47 | 5.98 | 6.21 | 5.93 | 0.03 | 0.094 | 0.154 |
| Platelets, × 109/L | 296.87a | 269.73b | 261.26b | 220.17c | 1.55 | <0.001 | <0.001 |
| Lymphocytes, % | 30.28a | 29.73a | 24.46b | 18.74c | 0.05 | <0.001 | <0.001 |
| Hemoglobin, g/L | 96.77 | 94.28 | 92.71 | 90.33 | 0.35 | 0.089 | 0.009 |
| Hematocrit, L/L | 39.31a | 37.37ab | 36.74b | 34.58c | 0.14 | 0.001 | <0.001 |
| MCV, fl | 59.48 | 59.66 | 59.84 | 58.92 | 0.12 | 0.728 | 0.519 |
| MCH, pg | 16.72 | 16.13 | 15.88 | 15.56 | 0.08 | 0.255 | 0.043 |
| MCHC, g/L | 265.86 | 262.97 | 265.15 | 266.07 | 0.49 | 0.734 | 0.846 |
MCV = mean corpuscular volume; MCH = mean corpuscular hemoglobin; MCHC = mean corpuscular hemoglobin concentration; SEM = standard error of the means.
a, b, c Means within a row with different letters differ significantly (P < 0.05).
Data are means for 5 replicates per treatment. Data per piglet were run in triplicate in a single assay to avoid inter-assay variation.
Zearalenone was not detectable in the control diet; ZEA1, ZEA2 and ZEA3 represent the control diet with an addition of 1.1, 2.0 and 3.2 mg/kg ZEA, respectively, in the experiment treatments.
3.2. T lymphocyte subset of peripheral blood
The T lymphocyte subsets of CD4+CD8+, CD4+, and CD4+/CD8+ of piglets supplemented with 2.0 mg/kg ZEA were significantly lower than those of the control piglets (P < 0.05) (Table 3), whereas CD8+ was significantly increased (P = 0.026) in gilts fed the diet containing 3.2 mg/kg ZEA.
Table 3.
T lymphocyte subset of peripheral blood in gilts fed diet with or without zearalenone (ZEA) supplementation.1
| Item | Control2 | ZEA12 | ZEA22 | ZEA32 | SEM | Effects (P-value) |
|
|---|---|---|---|---|---|---|---|
| Treatment | Linear | ||||||
| CD8+ | 42.49b | 48.32b | 48.33b | 63.02a | 1.28 | 0.026 | 0.331 |
| CD4+CD8+ | 17.82a | 16.54a | 13.32b | 13.27b | 0.69 | 0.038 | 0.254 |
| CD4+ | 38.11a | 37.28a | 32.83b | 32.79b | 1.23 | 0.016 | 0.108 |
| CD4+/CD8+ | 0.90a | 0.83ab | 0.73b | 0.54c | 0.01 | 0.001 | 0.002 |
SEM = standard error of the means.
a, b, c Means within a row with different letters differ significantly (P < 0.05).
Data are means for 5 replicates per treatment. Data per piglet were run in triplicate in a single assay to avoid inter-assay variation.
Zearalenone was not detectable in the control diet; ZEA1, ZEA2 and ZEA3 represent the control diet with an addition of 1.1, 2.0 and 3.2 mg/kg ZEA, respectively, in the experiment treatments.
3.3. Lymphocyte proliferation rate and IL-2 production of peripheral blood
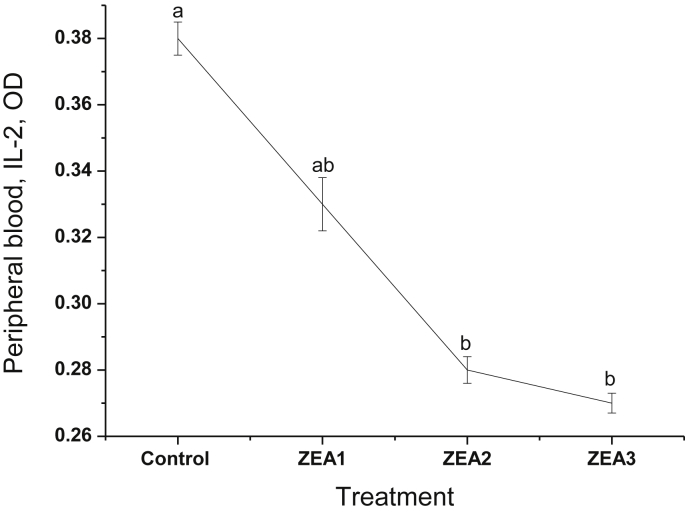
Linear decrease in LPR was observed (P < 0.05) in the gilts fed greater than or equal to 1.1 mg/kg ZEA-contaminated diet (Fig. 1). Gilts fed diets containing 2.0 mg/kg ZEA or greater had significantly decreased (P < 0.05) IL-2 compared with the control (Fig. 2).
Fig. 1.
Effects of different levels of zearalenone (ZEA) on lymphocyte proliferation rate (LPR) in peripheral blood of gilts. Data are means for 5 replicates per treatment. Zearalenone was not detectable in the control diet; ZEA1, ZEA2 and ZEA3 represent the control diet with an addition of 1.1, 2.0 and 3.2 mg/kg ZEA, respectively. Different letters above standard error bars indicate statistically significant differences between treatments (P < 0.05).
Fig. 2.
Effects of different levels of zearalenone (ZEA) on interleukin-2 (IL-2) production in peripheral blood of gilts. Data are means for 5 replicates per treatment. Zearalenone was not detectable in the control diet; ZEA1, ZEA2 and ZEA3 represent the control diet with an addition of 1.1, 2.0 and 3.2 mg/kg ZEA, respectively. Different letters above standard error bars indicate statistically significant differences between treatments (P < 0.05).
3.4. Immunoglobulin concentrations
Gilts fed the diet containing 2.0 mg/kg ZEA or greater had reduced (P < 0.001) serum IgG compared with the control (Table 4), and the IgG concentrations were reduced linearly as dietary ZEA levels increased (P < 0.001). The feeding of ZEA-contaminated diets to pigs did not cause any changes in serum IgA or IgM concentrations (P > 0.05).
Table 4.
Immunoglobulin (mg/mL) of gilts fed diet with or without zearalenone (ZEA) supplementation.1
| Item | Control2 | ZEA12 | ZEA22 | ZEA32 | SEM | Effects (P-value) |
|
|---|---|---|---|---|---|---|---|
| Treatment | Linear | ||||||
| IgA | 1.28 | 1.22 | 1.34 | 1.24 | 0.02 | 0.302 | 0.501 |
| IgG | 8.55a | 8.00a | 6.60b | 6.29b | 0.07 | <0.001 | <0.001 |
| IgM | 5.08 | 5.00 | 4.45 | 4.28 | 0.07 | 0.296 | 0.077 |
IgA = immunoglobulin A; IgG = immunoglobulin G; IgM = immunoglobulin M; SEM = standard error of the means.
a, b Means within a row with different letters differ significantly (P < 0.05).
Data are means for 5 replicates per treatment. Data per piglet were run in triplicate in a single assay to avoid inter-assay variation.
Zearalenone was not detectable in the control diet; ZEA1, ZEA2 and ZEA3 represents the control diet with an addition of 1.1, 2.0 and 3.2 mg/kg ZEA, respectively, in the experiment treatments.
3.5. Antibody titer to swine plague
Gilts which ingested 2.0 or greater ZEA-contaminated diets decreased (P < 0.05) antibody titer to swine plague at both 12 and 18 d of the experiment (Table 5), whereas the treatment effects on antibody titer to swine plague were not observed at 6 d of the experiment.
Table 5.
Antibody titer of swine plague in gilts fed diet with or without zearalenone (ZEA) supplementation.1
| Item | Control2 | ZEA12 | ZEA22 | ZEA32 | SEM | Effects (P-value) |
|
|---|---|---|---|---|---|---|---|
| Treatment | Linear | ||||||
| d 1 pre-immune | 4.17 | 4.67 | 4.50 | 4.17 | 0.07 | 0.675 | 0.915 |
| d 6 post-immune | 4.50 | 4.83 | 4.17 | 4.33 | 0.07 | 0.580 | 0.455 |
| d 12 post-immune | 5.16a | 5.67a | 5.33b | 5.50b | 0.07 | 0.201 | 0.441 |
| d 18 post-immune | 7.33a | 7.33a | 6.00b | 5.83b | 0.06 | 0.002 | <0.001 |
SEM = standard error of the means.
a, b Means within a row with different letters differ significantly (P < 0.05).
Data are means for 5 replicates per treatment. Data per piglet were run in triplicate in a single assay to avoid inter-assay variation.
Zearalenone was not detectable in the control diet; ZEA1, ZEA2 and ZEA3 represent the control diet with an addition of 1.1, 2.0 and 3.2 mg/kg ZEA, respectively, in the experiment treatments.
4. Discussion
4.1. Hematological values
It has been confirmed by many studies that ZEA-induced hematotoxicity appears in many types of animals. Specifically, it has also been verified that high doses of ZEA (40 mg/kg BW) lead to increased levels of hemoglobin and decreased numbers of platelets in mice (Abbès et al., 2006a). Maaroufi et al. (1996) reported that ZEA treatments (3 and 5 mg/kg) resulted in significantly reduced numbers of platelets and dramatically increased numbers of leucocytes in female mice. Swamy et al. (2004) also confirmed that ZEA-contaminated daily foods linearly decreased the numbers of B cells of turkeys. Jiang et al. (2010b) confirmed that the numbers of platelets and content of hemoglobin significantly decreased in weaned pigs treated with 1.3 mg/kg ZEA. However, it has also been shown that numbers of blood cells including lymphocytes, neutrophilic polymorphonuclear leukocytes, monocytes and eosinophils were not affected in mice treated with 10 mg/kg ZEA (Forsell et al., 1986). In the present study, ZEA treatments significantly altered the compositions of piglet cells, and decreased numbers of leucocytes, platelets and lymphocytes in a dose-dependent manner further confirmed the hematologic toxicity effects of ZEA in piglets.
4.2. T lymphocyte subset of peripheral blood
The stability of the T lymphocyte subsets was essential for the maintenance of the normal immune regulation of bodies, and was also an important index for evaluating the cellular immune functions of bodies. High doses of ZEA (40 mg/kg BW) significantly decreased the numbers of CD3+, CD4+, CD8+ and CD56+ cells in the peripheral blood of mice (Abbès et al., 2006b). Karagezian (2000) confirmed that intravenous injection of ZEA (15 mg/kg BW) in mice led to significant abnormality of phospholipid metabolism in the lymphocyte membranes, which may explain why the T lymphocyte subsets reduced. The results of the present study demonstrated that numbers of CD4+, CD4+CD8+ and CD4+/CD8+ cells in the peripheral bloods in the group with piglets treated with ZEA (2.0 and 3.2 mg/kg) were all significantly lower than those of the control group, and there was a dose dependent effect of ZEA on the numbers of CD4+/CD8+ cells, suggesting that there was an inhibition effect of ZEA (2.0–3.2 mg/kg) on the proliferation of the peripheral blood T lymphocyte subsets in weaning piglets, thus showing that the cellular immune functions of the bodies were affected.
4.3. Lymphocyte proliferation rate and IL-2 production of peripheral blood
The proliferation of lymphocytes was a normal protective and physiological reaction when body immune systems were stimulated by foreign antigens, which had a great significance in resisting foreign microbial infection for organisms (Sean et al., 2005). Zearalenone, α-ZOL and β-ZOL could induce cytotoxicity of immune cells, and it was confirmed that 10−5 mol/L ZEA and its metabolites inhibited the proliferation of neutrophil (Muratori et al., 2003). Even low concentrations of ZEA (10 μmol/L) could inhibit the proliferation of thymoma Jurkat T cells, and it was confirmed that the inhibition effect was induced by ZEA-induced T cell apoptosis (Luongo et al., 2007). Zearalenone could inhibit proliferation of human peripheral blood lymphocytes stimulated by phytohemagglutin phytolectin, and ZEA also could inhibit the formation of B cells and T cells stimulated by Concanavalin A and pokeweed mitogen (Vlata et al., 2006). Abid-Essefi et al. (2004) confirmed that ZEA could suppress synthesis of proteins and DNA, block cell cycle proceeding at the G2/M stage, block DNA replication, and thereby inhibit cell proliferation. It had been verified that if there were heavy loads in the DNA repair system due to excessive damage, cell apoptosis or other types of cell death occurred, which may explain why cell viability decreased (Orren et al., 1997). In the present study, the MTT method was used to uncover the inhibition effects of ZEA on cell proliferation of peripheral blood lymphocytes, which thus further affected the immune functions of lymphocytes in piglets.
In vivo, cells producing IL-2 were mainly T cells, but some precursors of T cells and B cells also could generate IL-2. In vitro, mitogen, some cytokines and some drugs were all able to induce production of IL-2 in lymphocytes. Recently, IL-2, as a molecular vaccine adjuvant, has often been used to enhance immune effects, especially in enhancing cellular immune responses. Studies have shown that α-ZOL (40 and 80 μmol/L) significantly decreased the expression level of IL-2 in Thymoma T cells (Luongo et al., 2007). Polluted daily diets (DON: 1 mg/kg, ZEA: 0.25 mg/kg) dramatically decreased the expression of cytokine IL-2 of spleen in weaning pigs (Cheng et al., 2006). In the current experiment, levels of IL-2 in the peripheral blood of piglets of the group treated with 3.2 mg/kg ZEA were dramatically lower than those of the control group, and levels of IL-2 decreased linearly with the increased levels of ZEA. Due to the fact that IL-2 is a cytokine which is essential for the proliferation and differentiation of T cells, the reasons of decreased levels of IL-2 in peripheral bloods induced by 3.2 mg/kg ZEA may be explained by the fact that there was a decreased proliferation rate of peripheral blood lymphocytes.
4.4. Immunoglobulin concentrations
Immunoglobulins are important products of immune responses against antigen materials for organisms. Immunoglobulins may block detriments of pathogens on bodies, thus leading to loss of pathogenic roles for pathogens. Immunoglobulin G is a major antibody in mediating humoral immunity with a main force in resisting infection immunity for animal bodies. In addition, IgG is also a main antibody in diagnostic serology and monitoring after vaccine immunization. Monitoring IgM antibodies may aid in conducting early diagnosis of vaccine serology. High doses of ZEA (40 mg/kg BW) have been shown to significantly decrease levels of IgA and IgG in mice, and affected humoral immune response (Abbès et al., 2006b). Feeding the weaned piglets with toxin-polluted diets (DON: 1 mg/kg, ZEA: 0.25 mg/kg) dramatically reduced levels of serum IgG (Cheng et al., 2006). In the present experiment, levels of serum IgG in the group treated with ZEA (2.0 and 3.2 mg/kg) were significantly lower than those of the control group in a dose-dependent manner. Levels of IgM showed a trend of decreasing linearly with increased levels of ZEA, indicating that ZEA (3.2 mg/kg) had inhibitive effects on humoral immunity in piglets, consistent with the results that ZEA decreased levels of serum globulin, which indicated that ZEA may affect protein metabolisms.
4.5. Antibody titer to swine plague
Hog cholera vaccine in the present experiments was a live vaccine which was regarded as a safe and effective hog cholera vaccine internationally produced by a lapinized virus C strain. Classical swine fever antibody titer was an index reflecting the specific humoral immunity function of organisms. Aflatoxin (2.5 mg/kg) could clearly inhibit the immune response of broiler against Newcastle disease vaccine, and lowered immunization in organisms (Ibrahim et al., 2000). Feeding weaned piglets with toxin-contaminated diets (DON: 1 mg/kg, ZEA: 0.25 mg/kg) dramatically reduced titer of antibody in porcine pseudorabies when they were immunized for 28 d, whereas it showed no significant effect on titer of antibody in piglets Pseudorabies when they were immunized for 14 d (Cheng et al., 2006). The results in the present study demonstrated that antibody titers of hog cholera vaccine in the group treated with ZEA (2.0 and 3.2 mg/kg) when they were immunized for 18 d were significantly lower than those of the control group in a dose-dependent manner. Therefore, the present investigation showed that ZEA may deactivate macrophages to some degree, and inhibit production of antibodies in the blood of weaned piglets in a dose-dependent manner. The effects of single toxin ZEA on classical swine fever antibody titer were not reported. Therefore, the underlying mechanism still requires further exploration.
5. Conclusions
The current study demonstrated that ZEA feeding at 1.1 to 3.2 mg/kg for 18 d induced different degrees of hematotoxicity in post-weaning gilts, as indicated by the changes in hematological values, CD4+CD8+, CD4+, and CD4+/CD8+, lymphocyte proliferation rate, IL-2, serum IgG, and the antibody titer tested in this study. Most of the parameters were linearly affected as dietary ZEA concentrations increased. In addition to its estrogenic effects, the study also showed that the increase of hematotoxicity in gilts fed ZEA contaminated feeds provides another possible pathway of ZEA toxicity in pigs. However, more cellular and molecular studies are required to thoroughly understand ZEA biological mode of actions and the toxicity of ZEA.
Acknowledgments
This research was financed by National Nature Science Foundation of China (Project No. 31572441). The authors declare that there are no competing financial interests in the work described.
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
Contributor Information
Shuzhen Jiang, Email: shuzhen305@163.com.
Zaibin Yang, Email: yzb204@163.com.
References
- Abbès S., Salah-Abbès J.B., Ouanes Z., Houas Z., Othman O., Bacha H. Preventive role of phyllosilicate clay on the immunological and biochemical toxicity of zearalenone in Balb/c mice. Int Immunopharmacol. 2006;6:1251–1258. doi: 10.1016/j.intimp.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Abbès S., Ouanes Z., Salah-Abbès J.B., Houas Z., Oueslati R., Bacha H. The protective effect of hydrated sodium calcium aluminosilicate against haematological, biochemical and pathological changes induced by zearalenone in mice. Toxicon. 2006;47:567–574. doi: 10.1016/j.toxicon.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Abid-Essefi S., Ouanes Z., Hassen W. Cytotoxicity, inhibition of DNA and protein syntheses and oxidative damage in cultured cells exposed to zearalenone. Toxicol Vitro. 2004;18:467–474. doi: 10.1016/j.tiv.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Ben Salah-Abbès J., Abbès S., Houas Z., Abdel-Wahhab M.A., Ridha O. Zearalenone induces immunotoxicity in mice: possible protective effects of Radish extract (Raphanus Sativus) J Pharm Pharmacol. 2008;60:1–10. doi: 10.1211/jpp.60.6.0012. [DOI] [PubMed] [Google Scholar]
- Ben Salah-Abbès J., Abdel-Wahhab M.A., Oueslati R. Raphanus sativus extract protects against zearalenone induced reproductive toxicity, oxidative stress and mutagenic alterations in male Balb/c mice. Toxicon. 2009;53:525–533. doi: 10.1016/j.toxicon.2009.01.013. [DOI] [PubMed] [Google Scholar]
- Berek L., Petri I.B., Mesterhazy A., Téren J., Molnál J. Effects of mycotoxins on human immune functions in vitro. Toxicol Vitro. 2001;15:25–30. doi: 10.1016/s0887-2333(00)00055-2. [DOI] [PubMed] [Google Scholar]
- Cetin Y., Bullerman L.B. Cytotoxicity of Fusarium mycotoxins to mammalian cell cultures as determined by the MTT bioassay. Food Chem Toxicol. 2005;43:755–764. doi: 10.1016/j.fct.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Chen X.X., Yang C.W., Huang L.B., Niu Q.S., Jiang S.Z., Chi F. Zearalenone altered the serum hormones, morphologic and apoptotic measurements of genital organs in post-weaning gilts. Asian Austr J Anim Sci. 2015;28(28):171–179. doi: 10.5713/ajas.14.0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y.H., Weng C.F., Chen B.J., Chang M.H. Toxicity of different Fusarium mycotoxins on growth performance, immune responses and efficacy of a mycotoxin degrading enzyme in pigs. Anim Res. 2006;55:579–590. [Google Scholar]
- Cheraghi S., Razi M., Malekinejad H. Involvement of cyclin D1 and E2f1 in zearalenone-induced DNA damage in testis of rats. Toxicon. 2015;106:108–116. doi: 10.1016/j.toxicon.2015.09.018. [DOI] [PubMed] [Google Scholar]
- Conkova E., Laciakova A., Pastorova B., Seidel H., Kovac G. The effect of zearalenone on some enzymatic parameters in rabbits. Toxicol Lett. 2001;121:145–149. doi: 10.1016/s0378-4274(01)00312-5. [DOI] [PubMed] [Google Scholar]
- EC. Commission recommendation of 17 August 2006 On the presence of deoxynivalenol, zearalenone, ochratoxin A, T-2 and HT-2 and fumonisins in products intended for animal feeding. Off J Eur Union. 2006;576(L229):7–9. [Google Scholar]
- EFSA Opinion of the Scientific Panel on Contaminants in the Food Chain on a request from the Commission related to zearalenone as undesirable substance in animal feed. EFSA J. 2004;89:1–35. [Google Scholar]
- Forsell J.H., Witt M.F., Tai J.H., Jensen R., Pestka J.J. Effects of 8-week exposure of the B6C3F1 mouse to dietary deoxynivalenol (vomitoxin) and zearalenone. Food Chem Toxicol. 1986;24:213–219. doi: 10.1016/0278-6915(86)90231-0. [DOI] [PubMed] [Google Scholar]
- Fushimi Y., Takagi M., Monniaux D., Uno S., Kokushi E., Shinya U. Effects of dietary contamination by zearalenone and its metabolites on serum anti-müllerian hormone: impact on the reproductive performance of breeding cows. Reprod Domest Anim. 2015;50(5):834–839. doi: 10.1111/rda.12599. [DOI] [PubMed] [Google Scholar]
- Gao F., Jiang L.P., Chen M., Geng C.Y., Yang G., Ji F. Genotoxic effects induced by zearalenone in a human embryonic kidney cell line. Mutat Res-Gen Tox En. 2013;755(1):6–10. doi: 10.1016/j.mrgentox.2013.04.009. [DOI] [PubMed] [Google Scholar]
- Guan S., Zhou T., Yin Y.L., Young J.C. Microbial strategies to control aflatoxins in food and feed. World Mycotoxin J. 2011;4(4):413–424. [Google Scholar]
- Ibrahim I.K., Shareef A.M., Al-Joubory K.M.T. Ameliorative effects of sodium bentonite on phagocytosis and Newcastle disease antibody formation in broiler chickens during aflatoxicosis. Res Vet Sci. 2000;69:119–122. doi: 10.1053/rvsc.2000.0390. [DOI] [PubMed] [Google Scholar]
- Jiang S.Z., Yang Z.B., Yang W.R., Yao B.Q., Zhao H., Liu F.X. Effects of feeding purified zearalenone contaminated diets with or without clay enterosorbent on growth, nutrient availability, and genital organs in post-weaning female pigs. Asian Austr J Anim Sci. 2010;23:74–81. [Google Scholar]
- Jiang S.Z., Yang Z.B., Yang W.R., Jing Gao, Liu F.X., Chen C.C. Physiopathological effects of zearalenone in post-weaning female piglets with or without montmorillonite clay adsorbent. Livest Sci. 2010;131:130–136. [Google Scholar]
- Jiang S.Z., Yang Z.B., Yang W.R., Gao J., Liu F.X., Broomhead J. Effects of purified zearalenone on growth performance, organ size, serum metabolites, and oxidative stress in postweaning gilts. J Anim Sci. 2011;89(10):3008–3015. doi: 10.2527/jas.2010-3658. [DOI] [PubMed] [Google Scholar]
- Jiang S.Z., Yang Z.B., Yang W.R., Wang S.J. Effect of purified zearalenone with or without modified montmorillonite on nutrient availability, genital organs and serum hormones in post-weaning piglets. Livest Sci. 2012;144:110–118. [Google Scholar]
- Karagezian M.K. Effect of the mycotoxin zearalenone on the metabolism of membrane phospholipids of rat lymphocytes. Ukr Biokhim Zh. 2000;72:105–109. [PubMed] [Google Scholar]
- Li W., Wence W., Kang Y., Ting Z., Jie Y., Tiejun L. Effects of dietary arginine and glutamine on alleviating the impairment induced by deoxynivalenol stress and immune relevant cytokines in growing pigs. Plos One. 2013;8(7) doi: 10.1371/journal.pone.0069502. e69502–e69502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Peng L., Liuqin H., Wenkai R., Jie Y., Jielin D. Growth performance, serum biochemical profile, jejunal morphology, and the expression of nutrients transporter genes in deoxynivalenol (DON)- challenged growing pigs. BMC Veterinary Res. 2015;11(1):1–10. doi: 10.1186/s12917-015-0449-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y., Yoshizawa T., Katayama T. Comparative study on the natural occurrence of Fusarium mycotoxins (trichothecenes and zearalenone) in corn and wheat from high- and low-risk areas for human esophageal cancer in China. Appl Environ Microbiol. 1990;56:3723–3726. doi: 10.1128/aem.56.12.3723-3726.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luongo D., Severino L., Bergamo P., Luna R.D., Lucisano A., Rossi M. Interactive effects of fumonisin B1 and α-zearalenol on proliferation and cytokine expression in Jurkat T cells. Toxicol Vitro. 2007;20:1403–1410. doi: 10.1016/j.tiv.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Maaroufi K., Chekir L., Creppy E.E. Zearalenone induces modifications of haematological and biochemical parameters in rats. Toxicon. 1996;34(5):535–540. doi: 10.1016/0041-0101(96)00008-6. [DOI] [PubMed] [Google Scholar]
- Muratori M., Maggi M., Spinelli S., Filimberti E., Forti G., Baldi E. Spontaneous DNA fragmentation in swim-up selected human spermatozoa during long term incubation. J Androl. 2003;24:253–262. doi: 10.1002/j.1939-4640.2003.tb02670.x. [DOI] [PubMed] [Google Scholar]
- NRC . 10th ed. National Academy Press; Washington, DC: 1998. Nutrient requirements of swine. [Google Scholar]
- Orren D.K., Petersen L.N., Bohr V.A. Persistent DNA damage inhibits S-phase and G2 progression, and results in apoptosis. Mol Biol Cell. 1997;8:1129–1142. doi: 10.1091/mbc.8.6.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Othmen O.B., Golli E.E., Abid-Essefi S., Bacha H. Cytotoxicity effects induced by Zearalenone metabolites, α Zearalenol and β Zearalenol, on cultured Vero cells. Toxicology. 2008;252(1–3):72–77. doi: 10.1016/j.tox.2008.07.065. [DOI] [PubMed] [Google Scholar]
- SAS Institute . 1st ed. SAS Institute Inc.; Cary, North Carolina: 2003. SAS/STAT User's guide: version 9. [Google Scholar]
- Schollenberger M., Muller H.M., Rufle M., Suchy S., Plank S., Drochner W. Natural occurrence of 16 Fusarium toxins in grains and feedstuffs of plant origin from Germany. Mycopathologia. 2006;161:43–52. doi: 10.1007/s11046-005-0199-7. [DOI] [PubMed] [Google Scholar]
- Sean X.L., Xue Q.L., Huang Y., Ferrucci L., Fried L.P., Walston J.D. Baseline total and specific differential white blood cell counts and 5-year all-cause mortality in community dwelling older women. Exp Gerontol. 2005;40:982–987. doi: 10.1016/j.exger.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Swamy H.V.L.N., Smith T.K., Karrow N.A., Boermans H.J. Effects of feeding blends of grains naturally contaminated with Fusarium mycotoxins on growth and immunological parameters of broiler chickens. Poult Sci. 2004;83:533–543. doi: 10.1093/ps/83.4.533. [DOI] [PubMed] [Google Scholar]
- Vlata Z., Porichis F., Tzanakakis G. A study of zearalenone cytotoxicity on human peripheral blood mononuclear cells. Toxicol Lett. 2006;165(3):274–281. doi: 10.1016/j.toxlet.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Wu M., Xiao H., Ren W., Yin J., Hu J., Duan J. An NMR-based metabolomic approach to investigate the effects of supplementation with glutamic acid in piglets challenged with deoxynivalenol. Plos One. 2013;9(12) doi: 10.1371/journal.pone.0113687. e113687–e113687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L., Liao P., He L., Feng Z., Ren W., Yin J. Dietary l-arginine supplementation protects weanling pigs from deoxynivalenol-induced toxicity. Toxins. 2015;7(4):1341–1354. doi: 10.3390/toxins7041341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J., Ren W., Duan J., Wu L., Chen S., Li T.J. Dietary arginine supplementation enhances intestinal expression of SLC7A7 and SLC7A1 and ameliorates growth depression in mycotoxin-challenged pigs. Amino Acids. 2014;46(4):883–892. doi: 10.1007/s00726-013-1643-5. [DOI] [PubMed] [Google Scholar]
- Zinedine A., Soriano J.J., Manes J. Review on the toxicity, occurrence, metabolism, detoxification, regulations and intake of zearalenone: an oestrogenic mycotoxin. Food Chem Toxicol. 2007;45:1–18. doi: 10.1016/j.fct.2006.07.030. [DOI] [PubMed] [Google Scholar]