Abstract
The objective of the current study is to evaluate the effects of dietary supplementation with arginine (ARG), N-carbamylglutamate (NCG), and glutamine (GLN) on rat intestinal morphology and antioxidant status under oxidative stress. Rats were fed for 30 d with one of the following iso-nitrogenous diets: basal diet (BD), BD plus 1% ARG, BD plus 0.1% NCG, and BD plus 1% GLN. On day 28, half of the rats fed BD were intraperitoneally injected with 12 mg/kg body weight of diquat (DT; i.e., the DT group) and the other half was intraperitoneally injected with sterile solution (i.e., the control group). The other diet groups were intraperitoneally injected with 12 mg/kg body weight of DT (i.e., DT + 1% GLN [DT + GLN], DT + 1% ARG [DT + ARG], and DT + 0.1% NCG [DT + NCG]). Rat jejunum samples obtained at 48 h after DT injection were analyzed. Results showed that DT significantly decreased catalase (CAT) activity and glutathione (GSH) content by 58.25% and 56.57%, respectively, and elevated malondialdehyde (MDA) content and crypt depth (CD) by 19.39% and 22.13%, respectively, in the jejunum (P < 0.05, relative to the control group). Compared with the DT group, the DT + GLN group exhibited significantly improved villus height (VH), villus width (VW), villus surface area (VSA), CD and total antioxidant capacity (T-AOC) activity (P < 0.05); the DT + ARG group exhibited significantly increased the ratio of VH to CD (H:D) and T-AOC activity (P < 0.05); the DT + GLN, DT + ARG and DT + NCG groups exhibited significantly enhanced CAT activity and GSH content as well as decreased MDA content (P < 0.05). Moreover, VH, VW, VSA, CD and GSH content in the DT + GLN group were higher whereas MDA content was lower compared with the corresponding values observed in both the DT + ARG and the DT + NCG groups (P < 0.05). The H:D ratio in the DT + ARG group significantly increased compared with that in the DT + NCG and DT + GLN groups (P < 0.05). Collectively, this study suggested that dietary supplementation with 1% GLN, 0.1% NCG, and 1% ARG was effective in enhancing the antioxidant status and maintaining the morphological structure of rat jejunum under oxidative stress; of these supplements, 1% GLN exerted the greatest effects on mitigating oxidative stress.
Keywords: Arginine, Glutamine, N-Carbamylglutamate, Oxidative stress, Jejunum, Antioxidant status
1. Introduction
Numerous factors, such as weaning, environmental factors, infection, and pro-oxidants, can induce oxidative stress, resulting in tremendous economic loss during livestock production (Liu et al., 2014, Yin et al., 2013). Oxidative stress is a general term that describes the imbalance between the relative levels of reactive oxygen species (ROS) and intra- and extracellular antioxidants wherein the amount of ROS is in excess (Reuter et al., 2010). Low and moderate quantities of ROS are beneficial to some physiological processes, including pathogen elimination, tissue repair, and wound healing. However, abundant quantities of ROS will lead to the loss of cell functions through oxidative damage of several cell constituents, including DNA, RNA, lipids, and proteins, and can result in organ- and pathway-specific toxicity related to processes such as alteration of membrane permeability, promotion of inappropriate apoptosis, and reduction of the antioxidant defense ability of the body (Veskoukis et al., 2012, Zhu et al., 2012, Jones, 2006). Oxidative stress can also disrupt the normal architecture of the small intestine, including enhancing crypt depth (CD) and reducing villus height (VH) and the ratio of villus height to crypt depth (H:D) (Wei et al., 2015).
The small intestine provides the main defense barrier against hazardous substances, such as carcinogens, dietary-derived mutagens, oxidants, and is the dominant interface responsible for substance and energy exchange between an animal and its environment (Aw, 2005, Wang et al., 2007). The small intestine can trigger signals to the central nervous system, guarantee energy homeostasis, and structurally accommodate nutrient digestion and absorption (Konturek et al., 2004, Bischoff, 2011). Besides these functions, several health problems during animal growth are related to the structures of the small intestine (Huang et al., 2016, Cao et al., 2015). Therefore, protecting the integrity of small intestinal structures from the damage induced by oxidative stress is important to ensure its absorptive and protective functions. Previous work has discovered that dietary supplementation with antioxidants or bioactive substances can be an effective approach to decrease oxidative stress (Liu et al., 2014, Mao et al., 2014).
L-Arginine (ARG) serves as a nutritionally significant amino acid for most animals under stressful conditions and exerts the beneficial effects of treating developmental and health problems (Yin et al., 2014). Recent evidences show that ARG can improve reproductive performance (Gao et al., 2012, Ren et al., 2012, Ren et al., 2013, Wu et al., 2012, Liu et al., 2012), promote intestinal cell proliferation (Yin et al., 2014, Tan et al., 2010), increase immune function (Ren et al., 2012, Tan et al., 2015), regulate intestinal gene expression (Yin et al., 2014), accelerate wound healing through the effects of nitric oxide (Wu et al., 2009), alter the metabolic profile (Tan et al., 2012), stimulate signal transduction and protein synthesis (Kong et al., 2012), improve the intestinal morphology, such as VH and CD (Yin et al., 2014), and attenuate oxidative stress (Ma et al., 2010).
Scientific evidences indicate that glutamine (GLN) can be converted into ARG via complex inter-organ metabolism in animals. Glutamine is traditionally considered as a non-essential amino acid with physical functions similar to those of ARG. For instance, GLN can maintain intestinal function and integrity by ameliorating intestinal mucosal atrophy (Wang et al., 2014), mitigate oxidative damage challenged with anesthesia or intraabdominal pressure (Tihan et al., 2011), modulate immune response (Li et al., 2007), and promote animal survival and growth (Liu et al., 2015a, Liu et al., 2015b, Liu et al., 2015b). In particular, GLN is the main energy substrate for intestinal cells (Wu et al., 2011).
N-Carbamylglutamate (NCG), an effective precursor of ARG, has been clinically utilized to treat several diseases (Schwahn et al., 2010, Ucar et al., 2009). N-Carbamylglutamate supplementation can improve ARG synthesis in enterocytes (Zeng et al., 2012), increase muscle protein synthesis (Frank et al., 2007), regulate signaling pathway (Zeng et al., 2012), improve reproductive performance (Zeng et al., 2012), and enhance intestinal growth as well as heat shock protein-70 expression (Wu et al., 2010). At present and to the best of our knowledge, little is known about the effect of NCG supplementation on the intestinal antioxidant ability of animals. Moreover, few studies have reported the difference in small intestinal protection associated with ARG, GLN, and NCG.
This study is part of a larger study that involved determining the metabolomic effects of ARG and NCG in combination with DT, which is a compound that causes oxidative stress (Liu et al., 2016). This experiment was conducted to study the effects of dietary supplementation with ARG, GLN, and NCG on the morphology and antioxidant capacity of rat jejunum under oxidative stress. The results of this work may provide scientific evidence of the capacity of ARG, GLN, and NCG to enhance the antioxidant status of the jejunum and will help to determine the optimal dietary dosage of these supplements.
2. Materials and methods
2.1. Animal feeding and sample collection
The animal experiment was approved by the Animal Care and Use Committee of Sichuan Agricultural University and conducted according to the established guidelines (Laboratory Animals of the National Research Council) for animal welfare and use. A total of 65 eight-week-old male Sprague-Dawley rats (Dossy Experimental Animals Co., Ltd., Chengdu, China) with initial weights of 230 ± 15 g were used in the current study and acclimatized to the testing environment for 3 d before the experiment was begun. All of the rats were randomly distributed into five treatment groups with 13 replicates per group and housed in individual cages of the Animal Nutrition Institute, Sichuan Agricultural University. The rats were fed for 30 d with one of the four iso-nitrogenous diets: basal diet (BD), BD plus 1% ARG, BD plus 0.1% NCG, and BD plus 1% GLN. On day 28, half of the rats fed BD were intraperitoneally injected with 12 mg/kg body weight of diquat (DT) (Sigma Chemical Co., St. Louis, MO, USA; DT group) and the other half was intraperitoneally injected with sterile saline solution of the same quantity (i.e., the control group). The other diet groups were intraperitoneally injected with 12 mg/kg body weight (i.e., DT + 1% GLN [DT + GLN], DT + 1% ARG [DT + ARG], or DT + 0.1% NCG [DT + NCG]). L-Arginine and GLN were purchased from Jiakangyuan Technology Development Co., Ltd. (Beijing, China) and NCG was supplied by Asia Pacific Xingmu Technology Co. Ltd. (Beijing, China). Diets were formulated in accordance with recommendations for rodent diets, as described by Reeves et al. (1993), and featured equivalent nitrogen and energy contents.
The rats were reared in a controlled room at temperatures ranging from 24 to 25°C, relative air humidity of 50% to 70%, and a light:dark cycle of 12:12 (lights off from 08:00 to 20:00) and had access to drinking water and their respective food ad libitum throughout the entire experiment. Clinical features (e.g., mental and appetite states) were observed during the experimental period. The rats were slaughtered under ether anesthesia at the end of the experiment (i.e., 48 h after DT injection). A small piece of the jejunum was washed in cold saline (0.9% NaCl; 4°C), rapidly harvested from the small intestine, and prepared for histological analysis. The remaining segments of the jejunum sample were stored at −80°C for detection of antioxidant parameters. The dosages of ARG, GLN, NCG, and DT were selected according to previous studies (Liu et al., 2014, Yin et al., 2014, Wu et al., 2011, Zeng et al., 2012).
2.2. Measurement of jejunum
To evaluate jejunal histomorphology, 2 cm-long jejunum samples were maintained in 10% buffered neutral formaldehyde. The fixed jejunum segments were processed for dehydration, cleared, and then embedded in paraffin block. Transverse semi-serial sections of 5 mm thickness were acquired using a microtome and stained with hematoxylin and eosin. Ultrathin specimens were used to determine the VH, VW, and CD via an image processing and analysis system (Image Pro Plus, Media Cybernetics, Bethesda, MD, USA). Villus height was measured from the tip of the villi to the villus–crypt junction, and VW was examined at the midpoint of the VH. Crypt depth was defined as the invaginated depth between the villus–crypt junction and the distal limit of the crypt (Liu et al., 2015a). Ten intact well-oriented crypt-villus units selected from each segment per rat and the mean value was calculated. The villus surface area (VSA) of the jejunum was calculated using the following formula: VSA (mm2) = 2π (VW/2) × VH.
2.3. Biochemical analysis of jejunum
2.3.1. Tissue preparation
About 0.1 g of a fragment of the frozen jejunum sample in ice-cold saline solution was weighed. The proportion of normal saline (mL) to jejunum sample (g) was 9:1. The mixture of jejunum and phosphate-buffered saline was homogenized using a tissue homogenizer (Bullet Blender, Next Advance, Inc. NY, USA), and the jejunum tissue homogenate was then centrifuged at 3,500 × g for 10 min at 4°C. Finally, the supernatant was collected and stored at −20°C for detection.
2.3.2. Antioxidant-related enzyme activities assay in jejunum
Malondialdehyde (MDA) content was quantified by using a thiobarbituric acid (TBA) test kit (Nanjing Jiancheng Bio-Engineering Institute, China) according to the manufacturer's instructions. Malondialdehyde reacts with TBA to develop a pink composite that can be readily examined through a spectrophotometer at 532 nm to indicate the degree of lipid peroxidation. The results were translated into nanomoles of MDA per milligram protein. The protein concentration of the jejunum samples was determined according to the Bradford method (Bradford, 1976) using a protein quantification kit (Coomassie brilliant blue) and bovine serum albumin as the protein standard. Concentrations obtained were converted into milligrams of protein per gram of wet weight of the intestine.
Anti-superoxide anion (ASA) activity was measured by using the method described by Jiang et al. (2009). Anti-superoxide anion ability can be spectrophotochemically tested using Griess reagent after addition of the electron acceptor from the reaction of xanthine and xanthine oxidase, which are sources of superoxide radicals; the absorbance of this reaction system was read at 550 nm. One ASA unit was defined as the amount of superoxide anion free radicals necessary to scavenge 1 g of tissue protein within 40 min at 37°C, which is equivalent to 1 g of vitamin C-scavenging under identical conditions.
Anti-hydroxyl radical (AHR) capacity was assayed according to a previous study (Jiang et al., 2009). Hydroxyl free radicals (OH−) can be generated via the Fenton reaction (Fe2+ + H2O2 → Fe3+ + OH− + OH), and a coloration reaction was carried out using the Griess reagent at 550 nm followed by addition of the electron acceptor. The amount of hydroxyl radicals in the reaction can be directly revealed by the coloration level of this system. One unit of AHR capacity was defined as the quantity of ARH in the reagent mixture that can decrease 1 mmol/L H2O2 within 1 min per milligram of tissue protein.
Superoxide dismutase (SOD) activity was assayed following the procedure described by Hou et al. (2013). Superoxide dismutase prevents decreases in nitro blue tetrazolium via the product (superoxide ions) of the xanthine/xanthine oxidase system, and activity was analyzed at 550 nm. Superoxide dismutase activity was expressed in terms of units per milligram of protein. Here, one unit of SOD was defined as the quantity of enzyme required to inhibit superoxide ion production in the reaction by 50%.
Catalase (CAT) activity was detected using a spectrophotometric assay kit (Nanjing Jiancheng Bio-Engineering Institute, China) according to the colorimetric method of Özmen et al. (2002). The jejunum supernatant was incubated in H2O2 substrate, and the enzymatic reaction was terminated by addition of ammonium molybdate. The reaction forms a light-yellow compound, which can be measured at 405 nm. Catalase activity was presented in terms of units per milligram of protein, and one CAT unit was defined as the quantity of enzyme required to exhaust 1 mmol/L H2O2 within 1 s per milligram of tissue protein at 37°C.
Glutathione (GSH) content was quantified spectrophotometrically at 412 nm via reaction with 5,5′-dithiobis-p-nitrobenzoic acid to generate the yellow-colored 5-thio-2-nitrobenzoic acid. Results were expressed as milligrams of GSH per gram of protein; here, commercial GSH was used as a standard.
The total antioxidant capacity (T-AOC) of the jejunum was examined at 550 nm; here, T-AOC refers to the concentration of antioxidants, such as vitamin C, vitamin E, glutathione, polyphenol complex, and protein thiol groups that can reduce Fe3+ to Fe2+. Ferrous ion can combine with phenanthroline to generate stable and colored chelates. Total antioxidant capacity was expressed in terms of units per milligram protein and one T-AOC unit corresponded to a 0.01 increase in absorbance within 1 min per milligram of tissue protein.
2.4. Statistical analysis
Data for all parameters determined were statistically analyzed by single factorial variance analysis employing the general linear model procedure of SPSS 21.0 software (SPSS Inc., Chicago, IL, USA). Duncan's multiple range test was used to compare statistical differences among treatments. Data were expressed as means ± SEM. The P < 0.05 was employed to show statistical significance.
3. Results
3.1. Jejunum morphology parameters
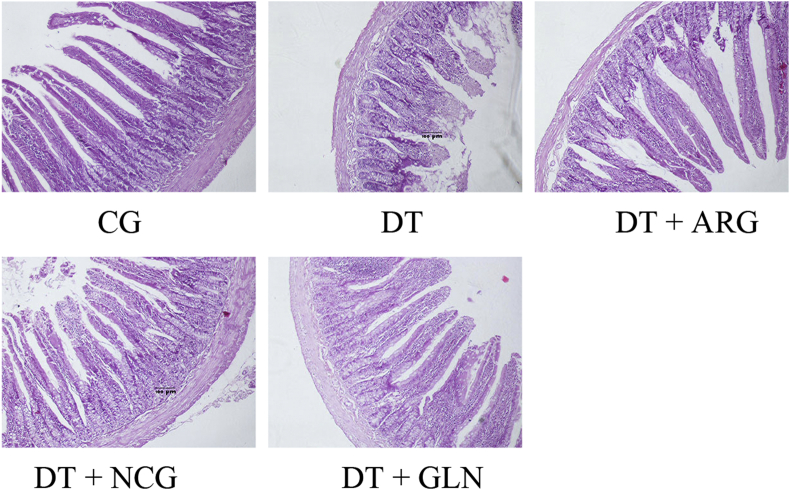
Fig. 1 shows that rat jejunal architecture and epithelial continuity were damaged by DT injection, which was recovered at different levels by supplementation of ARG and GLN. Results from Table 1 indicated that CD significantly increased by 22.13% (P < 0.05) whereas VH, VW, VSA, and H:D ratio did not differ (P > 0.05) in the DT group relative to the control group. Compared with the DT group, the DT + GLN group exhibited significant enhancements in VH, VW, VSA, and CD by 62.32%, 46.96%, 136.81%, and 87.14%, respectively (P < 0.05). The DT + ARG group exhibited a significant increase in H:D ratio by 12.75% (P < 0.05) compared with the DT group. Villus height, VW, VSA, and CD in the DT + GLN group increased by 47.96%, 48.13%, 116.87%, and 92.35%, respectively, compared with the corresponding values in the DT + ARG group (P < 0.05) and were enhanced by 58.46%, 52.90%, 139.02%, and 65.94%, respectively, relative to the results of the DT + NCG group (P < 0.05). The ratio of villus height to crypt depth was 22.61% lower in the DT + GLN group and 20.29% lower in the DT + NCG group compared with the DT + ARG group (P < 0.05).
Fig. 1.
Effect of arginine, N-carbamylglutamate and glutamine on jejunal morphology of rats under oxidative stress. CG: control group, DT: diquat treatment, DT + ARG: diquat plus 1% arginine treatment, DT + NCG: diquat plus 0.1% N-carbamylglutamate treatment, DT + GLN: diquat plus 1% glutamine treatment. The jejunum was cut off and maintained in 10% buffered neutral formaldehyde, and then stained with hematoxylin and eosin (HE). Hematoxylin and eosin staining with original magnification ×100.
Table 1.
Effect of arginine, N-carbamylglutamate and glutamine on the jejunum morphology of rats under oxidative stress.1
| Item | CG2 | DT2 | DT + ARG2 | DT + NCG2 | DT + GLN2 |
|---|---|---|---|---|---|
| VH, μm | 463.24 ± 16.35 | 492.24 ± 44.70b | 540.01 ± 47.10b | 504.22 ± 46.11b | 799.01 ± 35.13a |
| CD, μm | 133.37 ± 2.85 | 162.89 ± 18.23b∗ | 158.48 ± 12.98b | 183.70 ± 12.82b | 304.84 ± 22.28a |
| H:D ratio | 3.48 ± 0.11 | 3.06 ± 0.14b | 3.45 ± 0.23a | 2.75 ± 0.18b | 2.67 ± 0.11b |
| VW, μm | 43.61 ± 0.52 | 48.13 ± 2.10b | 47.75 ± 2.58b | 46.26 ± 3.14b | 70.73 ± 5.08a |
| VSA, μm2 | 63,459 ± 2512 | 75,826 ± 1049b | 82,799 ± 1183b | 75,127 ± 1132b | 179,567 ± 1796a |
VH = villus height; CD = crypt depth; H:D = the ratio of villus height to crypt depth; VW = villus width; VSA = villus surface area.
Mean values within a row with the letter (*) are significantly difference (P < 0.05) for comparison between DT and CG. Mean values within a row with different letters (a and b) are significantly difference (P < 0.05) for comparison between DT, DT + ARG, DT + NCG and DT + GLN. Data are stated as mean ± SEM.
CG: control group, DT: diquat treatment, DT + ARG: diquat plus 1% arginine treatment, DT + NCG: diquat plus 0.1% N-carbamylglutamate treatment, DT + GLN: diquat plus 1% glutamine treatment.
3.2. Oxidative stress parameters of jejunum in the rats
Table 2 shows the antioxidant parameters of the jejunum. Glutathione content and CAT activity decreased by 56.57% and 58.25%, respectively, in the group treated with DT compared with values obtained from the control group (P < 0.05). By contrast, DT treatment evoked improvements in MDA content (by 19.39%) relative to the control group (P < 0.05). The DT + ARG, DT + NCG, and DT + GLN groups respectively exhibited 141.13%, 151.61%, and 189.52% increases in CAT activity; 166.28%, 144.44%, and 219.54% increases in GSH contents; and 10.26%, 9.40%, and 17.09% decreases in MDA content. The DT + ARG and DT + GLN groups also respectively showed 35.90% and 28.21% increases in T-AOC activity compared with the DT group (P < 0.05). Moreover, in comparison with the DT + ARG group, GSH and MDA contents in the DT + GLN group increased by 20.00% and decreased by 7.62%, respectively (P < 0.05). Glutathione amount was higher by 30.72%, but MDA content was lower by 8.49% in the DT + GLN group compared with the DT + NCG group (P < 0.05).
Table 2.
Effect of arginine, N-carbamylglutamate and glutamine on antioxidant status in the jejunum of rats under oxidative stress.1
| Item | CG2 | DT2 | DT + ARG2 | DT + NCG2 | DT + GLN2 |
|---|---|---|---|---|---|
| T-AOC, U/mg protein | 0.96 ± 0.06 | 0.78 ± 0.11c | 1.06 ± 0.02ab | 0.85 ± 0.04bc | 1.00 ± 0.08a |
| GSH, mg/mg protein | 6.01 ± 0.42 | 2.61 ± 0.17c∗ | 6.95 ± 0.61b | 6.38 ± 0.40b | 8.34 ± 0.37a |
| ASA, U/g protein | 92.87 ± 4.48 | 89.48 ± 4.60 | 92.03 ± 6.02 | 93.48 ± 7.32 | 90.91 ± 7.01 |
| CAT, U/mg protein | 2.97 ± 0.28 | 1.24 ± 0.08b∗ | 2.99 ± 0.23a | 3.12 ± 0.21a | 3.59 ± 0.34a |
| MDA, nmol/mg protein | 0.98 ± 0.09 | 1.17 ± 0.02a∗ | 1.05 ± 0.03b | 1.06 ± 0.02b | 0.97 ± 0.02c |
| T-SOD, U/mg protein | 0.61 ± 0.03 | 0.58 ± 0.02 | 0.65 ± 0.03 | 0.62 ± 0.04 | 0.62 ± 0.05 |
| AHR, U/mg protein | 72.14 ± 4.24 | 64.60 ± 6.00 | 72.18 ± 6.25 | 76.60 ± 4.87 | 75.44 ± 5.52 |
T-AOC = total antioxidant capacity; GSH = glutathione; ASA = anti-superoxide anion; CAT = catalase; MDA = malondialdehyde; T-SOD = total superoxide dismutase; AHR = anti-hydroxyl radical.
Mean values within a row with the letter (*) are significantly difference (P < 0.05) for comparison between DT and CG. Mean values within a row with different letters (a–c) are significantly difference (P < 0.05) for comparison between DT, DT + ARG, DT + NCG and DT + GLN. Data are stated as mean ± SEM.
CG: control group, DT: diquat treatment, DT + ARG: diquat plus 1% arginine treatment, DT + NCG: diquat plus 0.1% N-carbamylglutamate treatment, DT + GLN: diquat plus 1% glutamine treatment.
4. Discussion
4.1. Effects of diquat on the morphological structure and antioxidant status of the jejunum
Diquat, as an effective pro-oxidant, can destroy the redox balance of the body in different animal models (e.g., pigs, rats) because of its ability to utilize molecular oxygen to generate more superoxide anion radicals and, subsequently, hydrogen peroxide (Osbum et al., 2006, Yin et al., 2015). Thus, in the present study, to determine whether or not rats suffer from oxidative stress induced by DT injection, we investigated alterations in jejunum morphology following DT treatment. Diquat treatment caused significant damage to the jejunum CD but showed no marked effects on VH, VW, VSA, and H:D ratio. This observation is consistent with the previous study which suggested that the jejunum is not the primary target organ attacked by DT (Burk et al., 1995). Crypt depth, an additional index of the small intestinal morphology, is considered as the villus factory, and its measure indicates rates of tissue turnover, enterocyte renewal, and demand for tissue growth (Burk et al., 1995). Increases in CD can cause poor nutrient absorption, decreased disease resistance, and lower performance (Xu et al., 2003). Hence, the results show that DT can inhibit jejunum development.
Diquat can lead to intracellular production of ROS, which is directly or indirectly associated with the antioxidant status. Thus, antioxidant parameters related to oxidative stress were also examined in this work. This work showed that CAT activity and GSH content were significantly decreased, whereas MDA content was increased in the jejunum by DT injection compared with the corresponding values in the control group. These results suggest that DT treatment can decrease the antioxidant ability of the jejunum (Jiang et al., 2011, Hung et al., 2006, Das et al., 2005, Liu et al., 2007).
In view of the findings above, our study indicated that the oxidative stress model of the rat intestine was successfully established by using DT.
4.2. Effects of arginine, N-carbamylglutamate, and glutamine on rat jejunal morphology under oxidative stress
The small intestine has been indicated to play a crucial role in promoting immunologic, metabolic, and barrier functions aside from nutrient digestion and absorption. The small intestinal architecture is intimately concerned with its functional status, and its destruction often leads to a number of diseases, such as food allergies, intestinal mucositis, and celiac diseases (DeMeo et al., 2002). Oxidative stress can undermine the normal architecture of the small intestine (Wei et al., 2015). The integrity of the intestinal morphology is often considered as a basis for evaluating the normal physiological functions of the small intestine; Therefore, protecting the small intestinal morphology from oxidative stress is necessary to improve animal performance and health. Intestinal epithelial cells are derived from the crypt and move along the villus surface up to the villus tip. Longer and wider villi are closely related to activate cell mitosis and can improve nutrient absorption in the small intestine because of the large surface area available for this function (Samanya and Yamauchi, 2002, Ilsley et al., 2005). Inversely, shorter intestinal villi impair absorption in the intestine, decrease the absorption ability of an animal, and result in low performance (Ilsley et al., 2005). Crypt depth enhancement is associated with fast tissue turnover and a high energy demand for intestine maintenance relative to other organs (Xu et al., 2003). Any additional tissue turnover must improve nutrient requirements for maintenance and will result in poor feed efficiency in livestock. In view of the foregoing, H:D ratio can be deemed as an indicator of the absorption capacity of animals (Hou et al., 2010). The VSA of the small intestine is directly concerned with absorption and serves as the main morphological parameter for nutrient digestion and absorption (Caspary, 1992). Consequently, according to above-mentioned findings, we detected the protective effects of ARG, NCG, and GLN on the jejunal morphology in rats under oxidative stress and found that dietary supplementation with 1% GLN can significantly enhance VH, VW, VSA, and CD, likely because of intensive epithelial cell turnover; 1% ARG significantly elevated H:D ratio. These findings are in agreement with those of previous researchers (Ma et al., 2010, Wang et al., 2014, Bauchart-Thevret et al., 2010). Moreover, CD in 1% GLN group was significantly improved, which may be associated with the huge increases in VH and VW, and this assumption needs further study. Our results show that dietary supplementation with 1% GLN can provide effective protection to the morphological structure of jejunum against oxidative stress, and 1% ARG can also partially protect the jejunal architecture from oxidative stress caused by DT injection.
Additionally, VH, VW, VSA, and CD in the 1% GLN group were higher compared with those in both the 1% ARG group and the 0.1% NCG group. The ratio of villus height to crypt depth evidently increased in the 1% ARG group compared with those of the 1% GLN and 0.1% NCG groups. Based on these results, we conclude that 1% GLN supplementation followed by 1% ARG yields the greatest influences on maintenance of the integrity of the jejunum.
4.3. Effects of arginine, N-carbamylglutamate, and glutamine on antioxidant protection of the rat jejunum under oxidative stress
Oxidative stress as the end-process of numerous stressors such as weaning and birth provokes excessive production of ROS. This ROS excess is harmful to an animal's antioxidant status and may cause damage to the cellularity of the digestive organs, animal health, and depression in production efficiency. In addition, ROS can cause lipid peroxidation of the cytomembrane. Malondialdehyde is used as a principal indicator of oxidative stress damage in the body (Liu et al., 2007). Supplementation of amino acids, including ARG, NCG, and GLN, has been previously demonstrated to be a possible approach for mitigating oxidative damage (Ma et al., 2010, Tihan et al., 2011, Wu et al., 2010). Malondialdehyde content was evaluated after supplementation with 1% ARG, 0.1% NCG, and 1% GLN under oxidative stress, and our study indicated that 1% ARG, 0.1% NCG, and 1% GLN can significantly decrease MDA content in the rat jejunum, which are consistent with the findings of previous studies (Ma et al., 2010, Liu et al., 2015a, Liu et al., 2015b, Liu et al., 2015b, Hou et al., 2010). These findings demonstrate that supplementation of 1% ARG, 0.1% NCG, and 1% GLN can improve the intestinal antioxidant status by anti-lipid peroxidation.
The mechanisms behind the antioxidant functions of dietary supplementation with ARG, NCG, and GLN may be also attributed to their regulatory effects on antioxidant and non-antioxidant enzymes. Catalase is the downstream antioxidant enzyme in charge of inhibiting the formation of hydroxyl radicals by virtue of its ability to convert H2O2 into oxygen and water in antioxidant defense systems (Jiang et al., 2011). In the current study, we found that dietary supplementation with 1% ARG, 0.1% NCG, and 1% GLN can enhance CAT activity of the rat jejunum, which shows that 1% ARG, 0.1% NCG, and 1% GLN can protect the rat intestine from oxidative stress through enzymatic antioxidant system.
In addition to the important roles of the anti-lipid peroxidation and enzymatic antioxidant defense systems, nonenzymatic antioxidant defense systems are also essential for the body's protection. In the present study, nonenzymatic antioxidant substances (GSH, T-AOC) were detected. Glutathione is the most significant defense mechanism that can eliminate the damage induced by free radicals both intracellularly and extracellularly, because it is associated with numerous enzymatic processes that reduce H2O2 through GSH metabolism into oxidized glutathione and other hybrid disulfides, and thus frequently regarded as an appropriate biomarker of cellular antioxidant defense capacity (Hung et al., 2006, Das et al., 2005). Total antioxidant capacity correlates with free radical-scavenging ability, reflecting the defensive capacity in the non-enzymatic antioxidant defense system, and can be generally used as an integrative indicator of the body's total antioxidant capacity (Ren et al., 2012). According to our study, correlation analysis reveal that supplementation with 1% ARG, 0.1% NCG, and 1% GLN can significantly improve GSH amount in the rat jejunum, but only supplementation of 1% ARG and 1% GLN can significantly increase the activity of T-AOC, which are consistent with the findings of previous studies (Ma et al., 2010, Liu et al., 2015a, Liu et al., 2015b, Liu et al., 2015b, Hou et al., 2010). Based on our foregoing findings, this study suggests that dietary supplementation with 1% ARG, 0.1% NCG, and 1% GLN can enhance the intestinal antioxidant status through nonenzymatic antioxidant system.
What is more, we also found that dietary supplementation with 1% GLN significantly increased GSH amount and decreased MDA content compared with 1% ARG or 0.1% NCG supplementation. Considering these findings, our research reveals that dietary supplementation with 1% GLN exhibits the largest influence on improving the antioxidant capacity of the jejunum under oxidative stress caused by DT injection and that the effect of 1% ARG is second to that of 1% GLN, thereby supporting previous findings on morphology. Such a phenomenon may be attributed to the fact that GLN serves as the main substrate for synthesizing GSH, which can mediate the mechanisms related to the protective effects of GLN (Flaring et al., 2003). Moreover, GLN is the carbon skeleton for the in vivo conversion of various substances that aid in generating antioxidants to protect the intestine, and its catabolized level is much higher than those of NCG or ARG in intestinal epithelial cells.
5. Conclusion
This work demonstrates that oxidative stress induced by DT injection can disrupt the intestinal morphology and reduce antioxidant abilities. Dietary supplementation with 0.1% NCG, 1% GLN, and 1% ARG enhanced the antioxidant status of rat jejunum under oxidative stress. Moreover, glutamine and ARG prevented intestinal dysfunction. Specifically, 1% GLN exerted the largest influence on improving the antioxidant capacity and maintaining the integrity of the jejunum. To the best of our knowledge, this research is the first to confirm that 0.1% NCG elevates the intestinal antioxidant status in vivo and determine the optimal dosage among several supplements to protect the intestinal architecture and antioxidant status associated with oxidative stress. Further research is essential to evaluate the mechanism of the effects exerted by dietary NCG supplementation on the animal antioxidant capacity and gut morphology.
Acknowledgments
We would like to express the deep gratitude for the ongoing help of our teammates. We also hope to thank the grants from National Natural Science Foundation of China (No.31301986), the Academy of Kechuang Feed Industry in Sichuan, and Specific Research Supporting Program for Discipline Construction in Sichuan Agricultural University (to G. Liu).
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- Aw T.Y. Intestinal glutathione: determinant of mucosal peroxide transport, metabolism, and oxidative susceptibility. Toxicol Appl Pharm. 2005;204:320–328. doi: 10.1016/j.taap.2004.11.016. [DOI] [PubMed] [Google Scholar]
- Bauchart-Thevret C., Cui L.W., Wu G.Y., Burrin D.G. Arginine-induced stimulation of protein synthesis and survival in IPEC-J2 cells is mediated by mTOR but not nitric oxide. Am J Physiol Endocrinol Metab. 2010;299:E899–E909. doi: 10.1152/ajpendo.00068.2010. [DOI] [PubMed] [Google Scholar]
- Bischoff S.C. 'Gut health': a new objective in medicine. BMC Med. 2011;9:24. doi: 10.1186/1741-7015-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Burk R.F., Hill K.E., Awad J.A., Morrow J.D., Kato T., Cockell K.A. Pathogenesis of diquat-induced liver necrosis in selenium-deficient rats: assessment of the roles of lipid peroxidation and selenoprotein P. Hepatology. 1995;21:561–569. [PubMed] [Google Scholar]
- Cao W., Liu G.M., Fang T.T., Wu X.J., Jia G., Zhao H. Effects of spermine on the morphology, digestive enzyme activities, and antioxidant status of jejunum in suckling rats. RSC Adv. 2015;5:76607–76614. [Google Scholar]
- Caspary W.F. Physiology and pathophysiology of intestinal absorption. Am J Clin Nutr. 1992;55:299–308. doi: 10.1093/ajcn/55.1.299s. [DOI] [PubMed] [Google Scholar]
- Das S.C., Isichei U.P., Mohammed A.Z., Otokwula A.A., Emokpae A. Impact of iodine deficiency on thyroid function in pregnant African women—a possible factor in the genesis of ‘small for dates’ babies. Ind J Clin Biochem. 2005;20:35–42. doi: 10.1007/BF02867398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMeo M.T., Mutlu E.A., Keshavarzian A., Tobin M.C. Intestinal permeation and gastrointestinal disease. J Clin Gastroenterol. 2002;34:385–396. doi: 10.1097/00004836-200204000-00003. [DOI] [PubMed] [Google Scholar]
- Flaring U.B., Rooyackers O.E., Wernerman J., Hammarqvist F. Glutamine attenuates post-traumatic glutathione depletion in human muscle. Clin Sci. 2003;104:275–282. doi: 10.1042/CS20020198. [DOI] [PubMed] [Google Scholar]
- Frank J.W., Escobar J., Nguyen H.V., Jobgen S.C., Jobgen W.S., Davis T.A. Oral Ncarbamylglutamate supplementation increases protein synthesis in skeletal muscle of piglets. J Nutr. 2007;137:315–319. doi: 10.1093/jn/137.2.315. [DOI] [PubMed] [Google Scholar]
- Gao K.G., Jiang Z.J., Lin Y.C., Zheng C.T., Zhou G.L., Chen F. Dietary l-arginine supplementation enhances placental growth and reproductive performance in sows. Amino Acids. 2012;42:2207–2214. doi: 10.1007/s00726-011-0960-9. [DOI] [PubMed] [Google Scholar]
- Huang B., Xiao D.F., Tan B., Xiao H., Wang J., Yin J. Chitosan oligosaccharide reduces intestinal inflammation that involves CaSR activation in LPS challenged-piglets. J Agric Food Chem. 2016;64:245–255. doi: 10.1021/acs.jafc.5b05195. [DOI] [PubMed] [Google Scholar]
- Hou F.L., Zhang R.F., Zhang M.W., Su D.X., Wei Z.C., Deng Y.Y. Hepatoprotective and antioxidant activity of anthocyanins in black rice bran on carbon tetrachloride-induced liver injury in mice. J Funct Foods. 2013;5:1705–1713. [Google Scholar]
- Hou Y.Q., Wang L., Ding B.Y., Liu Y.L., Zhu H.L., Liu J. Dietary a-ketoglutarate supplementation ameliorates intestinal injury in lipopolysaccharide-challenged piglets. Amino Acids. 2010;39:555–564. doi: 10.1007/s00726-010-0473-y. [DOI] [PubMed] [Google Scholar]
- Hung M.Y., Fu T.Y.C., Shih P.H., Lee C.P., Yen G.C. Du-Zhong (Eucommia ulmoides Oliv.) leaves inhibits CCl 4-induced hepatic damage in rats. Food Chem Toxicol. 2006;44:1424–1431. doi: 10.1016/j.fct.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Ilsley S.E., Miller H.M., Kamel C. Effects of dietary quillaja saponin and curcumin on the performance and immune status of weaned piglets. J Anim Sci. 2005;83:82–88. doi: 10.2527/2005.83182x. [DOI] [PubMed] [Google Scholar]
- Jiang J., Zheng T., Zhou X.Q., Liu Y., Feng L. Influence of glutamine and vitamin E on growth and antioxidant capacity of fish enterocytes. Aquacult Nutr. 2009;15:409–414. [Google Scholar]
- Jiang M.Z., Yan H., Wen Y., Li X.M. In vitro and in vivo studies of antioxidant activities of flavonoids from Adiantum capillus-veneris L. Afr J Pharm Pharmacol. 2011;5:2079–2085. [Google Scholar]
- Jones D.P. Redefining oxidative stress. Antioxid Redox Signal. 2006;8:1865–1879. doi: 10.1089/ars.2006.8.1865. [DOI] [PubMed] [Google Scholar]
- Kong X.F., Tan B., Yin Y.L., Gao H.J., Li X.L., Jaeger L.A. L-arginine stimulates the mTOR signaling pathway and protein synthesis in porcine trophectoderm cells. J Nutr Biochem. 2012;23:1178–1183. doi: 10.1016/j.jnutbio.2011.06.012. [DOI] [PubMed] [Google Scholar]
- Konturek S.J., Konturek J.W., Pawlik T., Brzozowki T. Brain–gut axis and its role in the control of food intake. J Physiol Pharmacol. 2004;55:137–154. [PubMed] [Google Scholar]
- Li P., Yin Y.L., Li D.F., Kim S.W., Wu G.Y. Amino acids and immune function. J Nutr. 2007;98:237–252. doi: 10.1017/S000711450769936X. [DOI] [PubMed] [Google Scholar]
- Liu C.S., Chen N.H., Zhang J.T. Protection of PC12 cells from hydrogen peroxide-induced cytotoxicity by salvianolic acid B, a new compound isolated from Radix Salviae miltiorrhizae. Phytomedicine. 2007;14:492–497. doi: 10.1016/j.phymed.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Liu G.M., Fang T.T., Yan T., Jia G., Zhao H., Chen X.L. Systemic responses of weaned rats to spermine against oxidative stress revealed by a metabolomic strategy. RSC Adv. 2014;4:56766–56778. [Google Scholar]
- Liu X.D., Wu X., Yin Y.L., Liu Y.Q., Geng M.M., Yang H.S. Effects of dietary L-arginine or N-carbamylglutamate supplementation during late gestation of sows on the miR-15b/16, miR-221/222, VEGFA and eNOS expression in umbilical vein. Amino Acids. 2012;42:2111–2119. doi: 10.1007/s00726-011-0948-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G.M., Xiao L., Cao Wei, Fang T.T., Jia G., Chen X.L. Changes in the metabolome of rats after exposure to arginine and N-carbamyglutamate in combination with diquat, a compound that causes oxidative stress, assessed by 1H NMR spectroscopy. Food Funct. 2016;7:964–974. doi: 10.1039/c5fo01486g. [DOI] [PubMed] [Google Scholar]
- Liu G.M., Yan T., Fang T.T., Jia G., Chen X.L., Zhao H. Nutrimetabolomic analysis provides new insights into spermine-induced ileum-system alterations for suckling rats. RSC Adv. 2015;5:48769–48778. [Google Scholar]
- Liu J.W., Mai K.S., Xu W., Zhang Y.J., Zhou H.H., Ai Q.H. Effects of dietary glutamine on survival, growth performance, activities of digestive enzyme, antioxidant status and hypoxia stress resistance of half-smooth tongue sole (Cynoglossus semilaevis Günther) post larvae. Aquaculture. 2015;446:48–56. [Google Scholar]
- Ma X.Y., Lin Y.C., Jiang Z.Y., Zheng C.T., Zhou G.L., Yu D.Q. Dietary arginine supplementation enhances antioxidative capacity and improves meat quality of finishing pigs. Amino Acids. 2010;38:95–102. doi: 10.1007/s00726-008-0213-8. [DOI] [PubMed] [Google Scholar]
- Mao X.B., Lv M., Yu B., He J., Zheng P., Yu J. The effect of dietary tryptophan levels on oxidative stress of liver induced by diquat in weaned piglets. J Anim Sci Biotechnol. 2014;5:49. doi: 10.1186/2049-1891-5-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osbum W.O., Wakabayashi N., Misra V., Nilles T., Biswal S., Trush M.A. Nrf2 regulates an adaptive response protecting against oxidative damage following diquat-mediated formation of superoxide anion. Arch Biochem Biophys. 2006;454:7–15. doi: 10.1016/j.abb.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Özmen B., Özmen D., Erkin E., Güner I., Habif S., Bayındır O. Lens superoxide dismutase and catalase activities in diabetic cataract. Clin Biochem. 2002;35:69–72. doi: 10.1016/s0009-9120(01)00284-3. [DOI] [PubMed] [Google Scholar]
- Reeves PG, Nielsen FH, Fahey GC. AIN-93 purified diets for laboratory rodents: final reports of the American institute of nutrition ad hoc writing committee on reformulation of the AIN-76A rodents diets. J Nutr. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- Ren W.K., Yin Y.L., Liu G., Yu X.L., Li Y.H., Yang G. Effect of dietary arginine supplementation on reproductive performance of mice with porcine circovirus type 2 infection. Amino Acids. 2012;42:2089–2094. doi: 10.1007/s00726-011-0942-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren W.K., Luo W., Wu M.M., Liu G., Yu X.L., Fang J. Dietary L-glutamine supplementation improves pregnancy outcome in mice infected with type-2 porcine circovirus. Amino Acids. 2013;45:479–488. doi: 10.1007/s00726-011-1134-5. [DOI] [PubMed] [Google Scholar]
- Reuter S., Gupta S.C., Chaturvedi M.M., Aggarwal B.B. Oxidative stress, inflammation, and cancer: how are they linked. Free Radic Biol Med. 2010;49:1603–1616. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanya M., Yamauchi K. Histological alterations of intestinal villi in chickens fed dried Bacillus subtilis var natto. Comp Biochem Physiol A Mol Integr Physiol. 2002;133:95–104. doi: 10.1016/s1095-6433(02)00121-6. [DOI] [PubMed] [Google Scholar]
- Schwahn B.C., Pieterse L., Bisset W.M., Galloway P.G., Robinson P.H. Biochemical efficacy of N-carbamylglutamate in neonatal severe hyperammonaemia due to propionic acidaemia. Eur J Pediatr. 2010;169:133–134. doi: 10.1007/s00431-009-1036-7. [DOI] [PubMed] [Google Scholar]
- Tan B, Li XG, Wu GY, Kong XF, Liu ZQ, Li TJ. Dynamic changes in blood flow and oxygen consumption in the portal-drained viscera of growing pigs receiving acute administration of L-arginine. Amino Acids. 2012;43:2481–2489. doi: 10.1007/s00726-012-1328-5. [DOI] [PubMed] [Google Scholar]
- Tan B., Yin Y.L., Kong X.F., Li P., Li X.L., Gao H.J. L-Arginine stimulates proliferation and prevents endotoxin-induced death of intestinal cells. Amino Acids. 2010;38:1227–1235. doi: 10.1007/s00726-009-0334-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan B., Xiao H., Xiong X., Wang J., Li G., Yin Y. L-Arginine improves DNA synthesis in LPS-challenged enterocytes. Front Biosci. 2015;20:989–1003. doi: 10.2741/4352. [DOI] [PubMed] [Google Scholar]
- Tihan D.N., Erbil1 Y., Seven R., Arkaya S., Turkoglu U., Hepgul G. The effect of glutamine on oxidative damage in an experimental abdominal compartment syndrome model in rats. Ulus Travma Acil Cerrahi Derg. 2011;17:1–8. [PubMed] [Google Scholar]
- Ucar S.K., Coker M., Habif S., Saz E.U., Karapinar B., Ucar H. The first use of N-carbamylglutamate in a patient with decompensated maple syrup urine disease. Metab Brain Dis. 2009;24:409–414. doi: 10.1007/s11011-009-9155-4. [DOI] [PubMed] [Google Scholar]
- Veskoukis A.S., Tsatsakis A.M., Kouretas D. Dietary oxidative stress and antioxidant defense with an emphasis on plant extract administration. Cell Stress Chaperon. 2012;17:11–21. doi: 10.1007/s12192-011-0293-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B., Wu G.Y., Zhou Z.G., Dai Z.L., Sun Y.L., Ji Y. Glutamine and intestinal barrier function. Amino Acids. 2014:1–12. doi: 10.1007/s00726-014-1773-4. [DOI] [PubMed] [Google Scholar]
- Wang Y.L., Holmes E., Comelli E.M., Fotopoulos G., Dorta G., Tang H. Topographical variation in metabolic signatures of human gastrointestinal biopsies revealed by high-resolution magic-angle spinning 1H NMR spectroscopy. J Proteome Res. 2007;6:3944–3951. doi: 10.1021/pr0702565. [DOI] [PubMed] [Google Scholar]
- Wei H.K., Chen G., Wang R.J., Peng J. Oregano essential oil decreased susceptibility to oxidative stress-induced dysfunction of intestinal epithelial barrier in rats. J Funct Foods. 2015;18:1191–1199. [Google Scholar]
- Wu X., Yin Y.L., Liu Y.Q., Liu X.D., Liu Z.Q., Li T.J. Effect of dietary arginine and N-carbamoylglutamate supplementation on reproduction and gene expression of eNOS, VEGFA and PlGF1 in placenta in late pregnancy of sows. Anim Reprod Sci. 2012;132:187–192. doi: 10.1016/j.anireprosci.2012.05.002. [DOI] [PubMed] [Google Scholar]
- Wu G., Bazer F.W., Johnson G.A., Knabe D.A., Burghardt R.C., Spencer T.E. Important roles for l-glutamine in swine nutrition and production. J Anim Sci. 2011;89:2017–2030. doi: 10.2527/jas.2010-3614. [DOI] [PubMed] [Google Scholar]
- Wu G.Y., Bazer F.W., Davis T.A., Kim S.W., Li P., Rhoads J.M. Arginine metabolism and nutrition in growth, health and disease. Amino Acids. 2009;37:153–168. doi: 10.1007/s00726-008-0210-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Ruan Z., Gao Y.L., Yin Y.L., Zhou X.H., Wang L. Dietary supplementation with L-arginine or N-carbamylglutamate enhances intestinal growth and heat shock protein-70 expression in weanling pigs fed a corn- and soybean mealbased diet. Amino Acids. 2010;39:831–839. doi: 10.1007/s00726-010-0538-y. [DOI] [PubMed] [Google Scholar]
- Xu Z.R., Hu C.H., Xia M.S., Zhan X.A., Wang M.Q. Effects of dietary fructooligosaccharide on digestive enzyme activities, intestinal microflora and morphology of male broilers. Poult Sci. 2003;82:1030–1036. doi: 10.1093/ps/82.6.1030. [DOI] [PubMed] [Google Scholar]
- Yin J., Ren W., Liu G., Duan J., Yang G., Wu L. Birth oxidative stress and the development of an antioxidant system in newborn piglets. Free Radic Res. 2013;47:1027–1035. doi: 10.3109/10715762.2013.848277. [DOI] [PubMed] [Google Scholar]
- Yin J., Ren W.K., Duan J.L., Wu L., Chen S., Li T.J. Dietary arginine supplementation enhances intestinal expression of SLC7A7 and SLC7A1 and ameliorates growth depression in mycotoxin-challenged pigs. Amino Acids. 2014;46:883–892. doi: 10.1007/s00726-013-1643-5. [DOI] [PubMed] [Google Scholar]
- Yin J., Liu M., Ren W., Duan J., Yang G., Zhao Y. Effects of dietary supplementation with glutamate and aspartate on diquat-induced oxidative stress in piglets. PLoS One. 2015;10:e0122893. doi: 10.1371/journal.pone.0122893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X.F., Huang Z.M., Mao X.B., Wang J.J., Wu G.Y., Qiao S.Y. N-carbamylglutamate enhances pregnancy outcome in rats through activation of the PI3K/PKB/mTOR signaling pathway. PLoS One. 2012;7:e41192. doi: 10.1371/journal.pone.0041192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L.H., Zhao K.L., Chen X.L., Xu J.X. Impact of weaning and an antioxidant blend on intestinal barrier function and antioxidant status in pigs. J Anim Sci. 2012;90:2581–2589. doi: 10.2527/jas.2012-4444. [DOI] [PubMed] [Google Scholar]