Abstract
The objective of the study was to investigate the possibility that tandem inclusions of a reducing agent and a protease may advantage chicken-meat production and to ascertain if the established benefits of including sodium metabisulphite in sorghum-based diets extend to wheat-based diets. The study comprised a 2 × 2 × 2 factorial array of treatments in which either nutritionally iso-nitrogenous and iso-energetic wheat- or sorghum-based diets, without and with sodium metabisulphite (2.75 g/kg), without and with protease (1,000 units/kg) were offered to broiler chickens from 7 to 28 days post–hatch. The effects of dietary treatments on growth performance, nutrient utilisation, protein (N) and starch digestibility coefficients and digestive dynamics were determined. A preliminary investigation into the effects of two treatments on concentrations of free amino acids and glucose in the portal circulation was conducted. There was significant feed grain by sodium metabisulphite interactions (P = 0.03 to 0.005) for parameters of nutrient utilisation (AME, ME:GE ratios, N retention, AMEn). For example, sodium metabisulphite inclusions in sorghum-based diets enhanced AME by 0.18 MJ (12.47 versus 12.29 MJ/kg) but depressed AME by 0.43 MJ (11.88 versus 12.31 MJ/kg) in wheat-based diets. There was a linear relationship between starch:protein disappearance rate ratios in the distal ileum with weight gain (r = −0.484; P = 0.0012) indicating that condensed ratios (or absorption of more protein relative to starch) advantaged growth performance. Concentrations of free amino acids in the portal circulation or the post-enteral availability of certain amino acids, including the branched-chain amino acids, methionine, phenylalanine and threonine, were significantly correlated to FCR. For example, threonine concentrations were negatively correlated to FCR (r = −0.773; P = 0.005). Finally, tandem inclusions of sodium metabisulphite and protease in sorghum-based diets may hold merit but it appears that the established ‘energy sparing’ effects of sodium metabisulphite inclusions in sorghum-based diets are not duplicated in wheat-based diets.
Keywords: Amino acids, Broiler chickens, Protease, Sodium metabisulphite
1. Introduction
The inclusion of the reducing agent sodium metabisulphite in sorghum-based broiler diets has been shown to enhance energy utilisation in broiler chickens (Liu et al., 2014, Selle et al., 2013a, Selle et al., 2014, Truong et al., 2016). For example, Truong et al. (2016) reported that 3.50 g/kg sodium metabisulphite increased N-corrected apparent metabolisable energy (AMEn) by 0.42 MJ from 11.43 to 11.85 MJ/kg in broiler diets based on five different sorghum varieties. This ‘energy sparing’ effect could be attributed to the sulphite reducing agent via either the depolymerisation of starch polysaccharides by oxidative–reductive reactions (Paterson et al., 1996, Paterson et al., 1997) and/or the reduction of disulphide cross-linkages in protein. In respect of the latter, the reduction of disulphide bonds in the periphery of kafirin protein bodies and the mitigation of starch–protein interactions in sorghum endosperm may hold particular relevance (Taylor and Emmambux, 2010).
In one feeding study completed at this institution, supplementation of a sorghum-based broiler diet with another exogenous protease displayed promise. This protease increased protein (N) and, to a lesser extent, starch digestibility coefficients (Selle et al., 2013b) and increased amino acid digestibility coefficients and the digestion rates of the majority of amino acids assessed (Liu et al., 2013). However, exogenous proteases hydrolyse proteins and almost certainly lack the capacity to reduce disulphide bonds. Thus, the assumption was made that inclusions of a protease and a reducing agent in tandem may be advantageous in sorghum-based broiler diets because of the distinct modes of action in their digestion of protein.
In Australia, wheat is more commonly used than sorghum as a feed grain in chicken-meat production. However, it has not been demonstrated if the advantages observed pursuant to the inclusion of sodium metabisulphite in sorghum-based diets extend to wheat-based diets. Thus the prime objectives of this study were two-fold. One was to investigate the possibility that inclusions of a reducing agent in wheat-based diets would be of benefit. The other was to explore the possibility that tandem inclusions of a reducing agent and an exogenous protease in wheat- and sorghum-based diets would be advantageous. Additionally, concentrations of free amino acids and glucose in the portal circulation for 2 treatment groups (wheat-based diets without and with sodium metabisulphite plus protease) were determined as a preliminary investigation.
2. Materials and methods
Wheat, sorghum and soybean meal were characterised and iso-nitrogenous and iso-energetic wheat- and sorghum-based diets were formulated to recommended nutrient specifications for Ross 308 grower (11 to 24 days) as shown in Table 1. Sodium metabisulphite was substituted for sodium bicarbonate in the 2 basal diets in order to maintain dietary sodium levels at 1.80 g/kg and adjustments for this and protease addition was made at the expense of Celite. The experimental diets were steam-pelleted at a temperature of 80 °C with a 14 s residence time in the conditioner, and crumbled after passing through a vertical cooler. The steam-pellet press (Palmer PP330 pellet press; Palmer Milling Engineering, Griffith, NSW, Australia) contained a 4.0 mm die. Wheat, sorghum and soybean meal had been ground through a 3.2 mm hammer-mill screen prior to being mixed into the diets. The trial design consisted of a 2 × 2 × 2 factorial arrangement of dietary treatments: wheat or sorghum-based diets, without or with 2.75 g/kg sodium metabisulphite, without or with exogenous protease. The selected enzyme was a serine protease [Ronozyme ProAct (CT); DSM] produced by a genetically modified strain of Bacillus licheniformis. The feed enzyme addition rate of 500 g/kg provided 1,000 units of protease activity per kg of feed.
Table 1.
Composition and nutrient specifications of the basal wheat- and sorghum-based diets (as is basis).
| Item | Wheat-based diet4 | Sorghum-based diet5 |
|---|---|---|
| Ingredient, g/kg | ||
| Wheat | 638.2 | – |
| Sorghum | – | 682.3 |
| Soybean meal | 255.0 | 235.0 |
| Canola oil | 45.0 | 20.0 |
| Limestone | 13.0 | 13.0 |
| Dicalcium phosphate | 12.0 | 12.0 |
| Sodium bicarbonate1 | 6.0 | 5.7 |
| Lysine HCl | 3.4 | 4.5 |
| Methionine | 2.6 | 3.0 |
| Threonine | 1.6 | 1.6 |
| Arginine | 5.6 | 5.0 |
| Choline chloride | 0.6 | 0.9 |
| Vitamin-trace mineral premix2 | 2.0 | 2.0 |
| Celite3 | 15.0 | 15.0 |
| Nutrient level, g/kg | ||
| Metabolisable energy, MJ/kg | 13.06 | 12.92 |
| Protein | 215.1 | 215.7 |
| Calcium | 8.05 | 7.81 |
| Total phosphorus | 6.54 | 6.22 |
| Available phosphorus | 4.05 | 3.85 |
| Sodium | 1.79 | 1.80 |
| Digestible amino acids | ||
| Lysine | 11.40 | 11.50 |
| Methionine | 5.40 | 5.78 |
| Methionine + cystine | 8.61 | 8.66 |
| Tryptophan | 2.13 | 2.13 |
| Arginine | 17.2 | 15.6 |
| Threonine | 7.68 | 7.68 |
| Leucine | 12.6 | 17.5 |
| Isoleucine | 8.18 | 8.31 |
Sodium metabisulphite (2.75 g/kg) replaced 2.34 g/kg sodium bicarbonate to maintain Na levels, difference corrected with Celite.
Vitamin-trace mineral premix supplied per tonne of feed; [million international units, MIU] retinol 12, cholecalciferol 5, [g] tocopherol 50, menadione 3, thiamine 3, riboflavin 9, pyridoxine 5, cobalamin 0.025, niacin 50, pantothenate 18, folate 2, biotin 0.2, copper 20, iron 40 manganese 110, cobalt 0.25, iodine 1, molybdenum 2, zinc 90, selenium 0.3.
Protease added at the expense of Celite.
On analysis, wheat-based diets contained 456 g/kg starch and 205 g/kg protein (N).
On analysis, sorghum-based diets contained 501 g/kg starch and 196 g/kg protein (N).
A total of 288 feather-sexed, male Ross 308 chicks were housed in an environmentally-controlled facility with unlimited access to feed and water under a “18 h on-6 h off” lighting schedule. There were 6 replicate cages (6 birds per cage) for each of the 8 dietary treatments and birds were initially offered a proprietary wheat-based starter diet prior to being allocated to dietary treatments from 7 to 28 days post–hatch. At 7 days post–hatch birds were weighed and allocated into 48 cages so that the mean and standard deviation of body-weight for each cage was nearly identical. Body weights were again determined at 28-days post hatch and caged feed intakes recorded over the entire 21-day period to calculate FCR with adjustments made from the weight of any dead or culled birds, which were monitored on a daily basis. At 28-days post hatch the birds were euthanised (intravenous injection of sodium pentobarbitone) and digesta samples were collected in their entirety from the proximal jejunum, distal jejunum, proximal ileum and distal ileum and pooled for each cage. This was in order to determine nutrient (starch and crude protein) digestibility coefficients and nutrient disappearance rates [g/(bird·day)].
Total excreta collection over a 48 h period at 25 days post–hatch was used to determine apparent metabolisable energy (AME) on a dry matter basis, metabolisable energy to gross energy (ME:GE) ratios, nitrogen (N) retention and AMEn. Total excreta were quantitatively collected from each cage and feed intakes recorded for the 48 h collection period. Excreta were dried in a forced-air oven at 80 °C for 24 h and the GE of excreta and diets were determined using an adiabatic bomb calorimeter. The AME values of the diets on a dry matter basis were calculated from the following equation:
ME:GE ratios were calculated by dividing AME by the GE of the appropriate diets. N contents of diets and excreta were determined using a nitrogen determinator (Leco Corporation, St Joseph, MI) and N retentions calculated from the following equation:
AMEn (MJ/kg DM) values were calculated by correcting N retention to zero using the factor of 36.54 kJ/g N retained in the body (Hill and Anderson, 1958).
Digesta samples were freeze-dried to determine apparent digestibilities of starch and crude protein (N) using Celite as acid insoluble ash (AIA) as the inert dietary marker. Starch concentrations in diets and digesta were determined by a procedure based on dimethyl sulphoxide, α-amylase and amyloglucosidase as described by Mahasukhonthachat et al. (2010). N concentrations were determined as already stated and AIA concentrations were determined by the method of Siriwan et al. (1993). The apparent digestibility coefficients for starch and protein (N) at four small intestinal sites were calculated from the following equation:
Protein (N) digestibility coefficients were determined in all four small intestinal segments while starch digestibility coefficients were determined in the proximal jejunum and distal ileum. Starch and protein (N) disappearance rates [g/(bird·day)] were deduced from feed intakes over the final phase of the feeding period from the following equation:
Ratios of starch to protein disappearance rates in the intestinal segments were calculated as this effectively eliminates the potential confounding influence of feed intake.
Three birds were selected at random from each caged replicate from the two wheat-based diets without and with sodium metabisulphite plus protease treatment groups. Immediately following euthanasia the abdominal cavity was opened and blood samples withdrawn from the anterior mesenteric vein, which drains the small intestine. Plasma samples were stored at −80 °C after centrifugation.
Concentrations of the twenty common proteinogenic amino acids in plasma taken from the anterior mesenteric vein were determined using precolumn derivatisation amino acid analysis with 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate (AQC; Waters AccQTag Ultra; Waters Australia PL; www.waters.com) followed by separation of the derivatives and quantification by reversed phase ultra-performance liquid chromatography (RP-UPLC; Cohen, 2001). All amino acids were detected by UV absorbance.
Plasma samples were thawed to room temperature and samples (50 μL) were diluted 1:1 with an internal standard (DL-Norvaline; Sigma Aldrich; www.sigmaaldrich.com) solution and deproteinised by ultrafiltration for 60 min at 13,800 RCF through a membrane with a 10 kDa nominal molecular weight cut-off (Amicon Ultra Centrifugal Filters, Merk Millipore; www.merkmillipore.com). The filtrate (20 μL) was then subjected to AQC derivatisation for 10 min at 50 °C and analysis based on the method of Cohen (2001) but adapted for use with a Waters ACQUITY Ultra Performance Liquid Chromatography (RP-UPLC) system. The column employed was an ACQUITY UPLC BEH C18 1.7 μm column (Waters) with a binary gradient flow rate of 0.7 mL/min at 60 °C with detection at 260 nm. This enabled a 12.2 min analysis time per sample. Chromatographic analysis and quantitation of the detected amino acids was undertaken using Empower software (Waters) using a set of prepared standards, with DL-Norvaline as the internal standard. The injected range of the standards was 0 to 50 pmol with a standard sample injection of 1.0 μL.
Triplicate analysis of each of the thawed plasma samples was undertaken using an Accu-Chek “Performa” blood glucometer and strips designed for human use (Roche; www.accu-chek.com.au). Twenty microliters of plasma was loaded onto the end of the strip and the measurements, in mmol/L recorded.
Experimental data were analysed using the IBM SPSS Statistics 20 program (IBM Corporation. Somers, NY,USA) and a probability level of less than 5% was considered to be statistically significant. Cage means were the experimental units and statistical procedures included univariate analyses of variance using the general linear models procedure and Pearson correlations were established. The feeding studies complied with specific guidelines approved by the Animal Ethics Committee of The University of Sydney.
3. Results
The effects of grain type and dietary inclusions of sodium metabisulphite and protease on growth performance are shown in Table 2. Relative to 2014 Ross 308 performance objectives, overall bird performance was satisfactory with a weight gain of 1,532 g/bird, feed intake of 2,370 g/bird, FCR of 1.549 from 7 to 28 days post–hatch with a low mortality/cull rate of 0.78%. Interestingly, birds offered control wheat-based diets numerically outperformed their sorghum counterparts in weight gain by 4.44% (1,553 versus 1,487 g/bird) and in FCR by 2.70% (1.513 versus 1.555). The only significant treatment effect observed for growth performance was that protease increased feed intake by 2.91% (2,408 versus 2,340 g/bird; P < 0.05). However, the interaction between grain type and sodium metabisulphite closely approached significance (P = 0.061) for FCR. This was because sodium metabisulphite tended to improve FCR of sorghum-based diets by 1.28% (1.541 versus 1.561) but depressed FCR of wheat-based diets by 3.68% (1.577 versus 1.521).
Table 2.
The effects of grain type and dietary inclusions of sodium metabisulphite (SMBS) and protease on parameters of growth performance from 7 to 28 days post–hatch.
| Treatments |
Growth performance |
|||||
|---|---|---|---|---|---|---|
| Grain | SMBS, g/kg | Protease, units/kg | Weight gain, g/bird | Feed intake, g/bird | FCR, g/g | Mortality/culls, % |
| Wheat | 0 | 0 | 1,553 | 2,347 | 1.513 | 0.00 |
| 0 | 1,000 | 1,589 | 2,430 | 1.529 | 3.33 | |
| 2.75 | 0 | 1,529 | 2,400 | 1.573 | 0.00 | |
| 2.75 | 1,000 | 1,541 | 2,436 | 1.582 | 0.00 | |
| Sorghum | 0 | 0 | 1,487 | 2,311 | 1.555 | 0.00 |
| 0 | 1,000 | 1,539 | 2,411 | 1.566 | 3.33 | |
| 2.75 | 0 | 1,493 | 2,304 | 1.545 | 0.00 | |
| 2.75 | 1,000 | 1,533 | 2,355 | 1.538 | 0.00 | |
| SEM | 24.459 | 44.725 | 0.0273 | 1.5373 | ||
| Main effects grain | ||||||
| Wheat | 1,553 | 2,403 | 1.549 | 0.83 | ||
| Sorghum | 1,513 | 2,345 | 1.551 | 0.83 | ||
| SMBS, g/kg | ||||||
| 0 | 1,542 | 2,374 | 1.541 | 1.67 | ||
| 2.75 | 1,524 | 2,374 | 1.559 | 0.00 | ||
| Protease, units/kg | ||||||
| 0 | 1,516 | 2,340a | 1.546 | 0.00 | ||
| 1,000 | 1,551 | 2,408b | 1.554 | 1.67 | ||
| Significance (P-value) | ||||||
| Grain | 0.067 | 0.075 | 0.916 | 1.000 | ||
| SMBS | 0.410 | 0.985 | 0.354 | 0.136 | ||
| Protease | 0.106 | 0.040 | 0.713 | 0.136 | ||
| Grain × SMBS | 0.401 | 0.338 | 0.061 | 1.000 | ||
| Grain × Protease | 0.600 | 0.798 | 0.788 | 1.000 | ||
| SMBS × Protease | 0.666 | 0.455 | 0.749 | 0.136 | ||
| Grain × SMBS × Protease | 0.895 | 0.992 | 0.882 | 1.000 | ||
a,b Means within columns not sharing a common suffix are significantly different at the 5% level of probability.
The effects of dietary treatments on parameters of nutrient utilisation are shown in Table 3. As a main effect, sorghum-based diets notionally supported better AME by 0.29 MJ (12.38 versus 12.09 MJ/kg; P < 0.01), ME:GE ratios by 3.99% (0.756 versus 0.727; P < 0.001), N retention by 1.77 percentage units (64.85% versus 63.08%; P < 0.04) and AMEn by 0.30 MJ (11.03 versus 10.73 MJ/kg; P < 0.001). However, there were significant grain type by sodium metabisulphite interactions (P = 0.015 to 0.005) that impacted on the 4 listed outcomes and the interactive data is tabulated. The inclusion of sodium metabisulphite in sorghum-based diets enhanced AME by 0.18 MJ (12.47 versus 12.29 MJ/kg), ME:GE ratios by 1.20% (0.761 versus 0.752), N retention by 2.96% (65.80 versus 63.91) and AMEn by 0.09 MJ (11.17 versus 11.08 MJ/kg). In contrast, sodium metabisulphite inclusion in wheat-based diets depressed AME by 0.43 MJ (11.88 versus 12.31 MJ/kg), ME:GE ratios by 3.65% (0.713 versus 0.740), N retention by 2.65 percentage units (61.75% versus 64.40%) and AMEn by 0.40 MJ (10.53 versus 10.93 MJ/kg).
Table 3.
The effects of grain type and dietary inclusions of sodium metabisulphite (SMBS) and protease on parameters of nutrient utilisation within 7 to 28 days post–-hatch.
| Treatments |
Nutrient utilisation |
|||||
|---|---|---|---|---|---|---|
| Grain | SMBS, g/kg | Protease, units/kg | AME, MJ/kg DM | ME:GE ratio | N retention, % | AMEn, MJ/kg DM |
| Wheat | 0 | 0 | 12.48 | 0.750 | 65.48 | 11.06 |
| 0 | 1,000 | 12.15 | 0.729 | 63.32 | 10.81 | |
| 2.75 | 0 | 12.04 | 0.722 | 62.04 | 10.71 | |
| 2.75 | 1,000 | 11.93 | 0.705 | 61.47 | 10.35 | |
| Sorghum | 0 | 0 | 12.28 | 0.750 | 62.76 | 11.10 |
| 0 | 1,000 | 12.31 | 0.754 | 65.06 | 11.06 | |
| 2.75 | 0 | 12.48 | 0.763 | 65.95 | 11.19 | |
| 2.75 | 1,000 | 12.46 | 0.760 | 65.64 | 11.15 | |
| SEM | 0.1431 | 0.0086 | 1.1036 | 0.1357 | ||
| Main effects grain | ||||||
| Wheat | 12.09 | 0.727 | 63.08 | 10.73 | ||
| Sorghum | 12.38 | 0.756 | 64.85 | 11.13 | ||
| SMBS, g/kg | ||||||
| 0 | 12.30 | 0.746 | 64.16 | 11.01 | ||
| 2.75 | 12.17 | 0.737 | 63.77 | 10.85 | ||
| Protease, units/kg | ||||||
| 0 | 12.32 | 0.746 | 64.06 | 11.01 | ||
| 1,000 | 12.16 | 0.737 | 63.87 | 10.84 | ||
| Significance (P-value) | ||||||
| Grain | 0.008 | <0.001 | 0.030 | <0.001 | ||
| SMBS | 0.205 | 0.189 | 0.630 | 0.112 | ||
| Protease | 0.130 | 0.138 | 0.814 | 0.083 | ||
| Grain × SMBS | 0.005 | 0.006 | 0.006 | 0.015 | ||
| Grain × Protease | 0.104 | 0.120 | 0.141 | 0.179 | ||
| SMBS × Protease | 0.901 | 0.856 | 0.745 | 0.761 | ||
| Grain × SMBS × Protease | 0.889 | 0.681 | 0.188 | 0.790 | ||
| Grain × SMBS interactions | ||||||
| Wheat | 12.31 | 0.740 | 64.40 | 10.93 | ||
| Wheat plus 2.75 g/kg SMBS | 11.88 | 0.713 | 61.75 | 10.53 | ||
| Sorghum | 12.29 | 0.752 | 63.91 | 11.08 | ||
| Sorghum plus 2.75 g/kg SMBS | 12.47 | 0.761 | 65.80 | 11.17 | ||
The effects of dietary treatments on apparent protein (N) digestibility coefficients in four small intestinal segments at 28 days post–hatch are shown in Table 4, no significant treatment effects were observed. However, protease increased proximal jejunal protein (N) digestibility coefficients by 11.0% (0.537 versus 0.491; P = 0.052), which closely approached significance.
Table 4.
The effects of grain type and dietary inclusions of sodium metabisulphite (SMBS) and protease on protein (N) digestibility coefficients in four small intestinal segments at 28 days post–hatch.
| Treatments |
Protein (N) digestibility coefficients |
|||||
|---|---|---|---|---|---|---|
| Grain | SMBS, g/kg | Protease, units/kg | Proximal jejunum | Distal jejunum | Proximal ileum | Distal ileum |
| Wheat | 0 | 0 | 0.464 | 0.741 | 0.773 | 0.780 |
| 0 | 1,000 | 0.515 | 0.706 | 0.759 | 0.790 | |
| 2.75 | 0 | 0.490 | 0.692 | 0.753 | 0.786 | |
| 2.75 | 1,000 | 0.601 | 0.695 | 0.743 | 0.761 | |
| Sorghum | 0 | 0 | 0.499 | 0.690 | 0.749 | 0.790 |
| 0 | 1,000 | 0.513 | 0.712 | 0.780 | 0.760 | |
| 2.75 | 0 | 0.511 | 0.700 | 0.767 | 0.777 | |
| 2.75 | 1,000 | 0.520 | 0.721 | 0.743 | 0.783 | |
| SEM | 0.0334 | 0.0193 | 0.0136 | 0.0146 | ||
| Main effects grain | ||||||
| Wheat | 0.517 | 0.708 | 0.757 | 0.779 | ||
| Sorghum | 0.511 | 0.705 | 0.760 | 0.777 | ||
| SMBS, g/kg | ||||||
| 0 | 0.498 | 0.712 | 0.765 | 0.780 | ||
| 2.75 | 0.530 | 0.701 | 0.752 | 0.777 | ||
| Protease, units/kg | ||||||
| 0 | 0.491 | 0.705 | 0.761 | 0.783 | ||
| 1,000 | 0.537 | 0.708 | 0.756 | 0.773 | ||
| Significance (P-value) | ||||||
| Grain | 0.769 | 0.794 | 0.801 | 0.880 | ||
| SMBS | 0.166 | 0.401 | 0.211 | 0.765 | ||
| Protease | 0.052 | 0.785 | 0.674 | 0.373 | ||
| Grain × SMBS | 0.329 | 0.157 | 0.706 | 0.428 | ||
| Grain × Protease | 0.144 | 0.147 | 0.457 | 0.854 | ||
| SMBS × Protease | 0.551 | 0.470 | 0.246 | 0.983 | ||
| Grain × SMBS × Protease | 0.491 | 0.498 | 0.177 | 0.092 | ||
The effects of dietary treatments on protein (N) disappearance rates in four small intestinal segments are shown in Table 5. There was a significant interaction (P < 0.025) between grain type and Na metabisulphite in the distal jejunum. Sodium metabisulphite increased protein disappearance rates by 8.64% [23.9 versus 22.0 g/(bird·day)] in sorghum-based diets; but decreased protein disappearance rates by 7.08% [(22.3 versus 24.0 g/(bird·day)] in wheat-based diets. As a main effect, wheat-based diets supported a higher distal ileal protein disappearance rate by 13.9% [27.0 versus 23.7 g/(bird·day); P < 0.001] than sorghum-based diets.
Table 5.
The effects of grain type and dietary inclusions of sodium metabisulphite (SMBS) and protease on protein (N) disappearance rates (g/bird/day) in four small intestinal segments at 28 days post–hatch.
| Treatments |
Protein (N) disappearance rates, g/(bird·day) |
|||||
|---|---|---|---|---|---|---|
| Grain | SMBS, g/kg | Protease, units/kg | Proximal jejunum | Distal jejunum | Proximal ileum | Distal ileum |
| Wheat | 0 | 0 | 18.9 | 24.7 | 25.9 | 26.8 |
| 0 | 1,000 | 17.4 | 23.4 | 25.1 | 26.9 | |
| 2.75 | 0 | 17.5 | 23.2 | 25.2 | 26.8 | |
| 2.75 | 1,000 | 16.1 | 21.5 | 23.0 | 27.3 | |
| Sorghum | 0 | 0 | 15.8 | 22.6 | 24.5 | 22.1 |
| 0 | 1,000 | 15.7 | 21.4 | 23.5 | 25.4 | |
| 2.75 | 0 | 18.2 | 23.2 | 25.7 | 23.5 | |
| 2.75 | 1,000 | 14.0 | 24.5 | 25.4 | 24.0 | |
| SEM | 1.2541 | 1.0362 | 1.2330 | 1.0415 | ||
| Main effects grain | ||||||
| Wheat | 17.5 | 23.2 | 24.8 | 27.0b | ||
| Sorghum | 15.9 | 22.9 | 24.8 | 23.7a | ||
| SMBS, g/kg | ||||||
| 0 | 16.9 | 23.0 | 24.7 | 25.3 | ||
| 2.75 | 16.5 | 23.1 | 24.8 | 25.4 | ||
| Protease, units/kg | ||||||
| 0 | 17.6 | 23.4 | 25.3 | 24.8 | ||
| 1,000 | 15.8 | 22.7 | 24.2 | 25.9 | ||
| Significance (P-value) | ||||||
| Grain | 0.083 | 0.728 | 0.995 | <0.001 | ||
| SMBS | 0.613 | 0.905 | 0.993 | 0.902 | ||
| Protease | 0.051 | 0.335 | 0.229 | 0.151 | ||
| Grain × SMBS | 0.361 | 0.021 | 0.110 | 0.918 | ||
| Grain × Protease | 0.686 | 0.296 | 0.612 | 0.289 | ||
| SMBS × Protease | 0.261 | 0.518 | 0.825 | 0.421 | ||
| Grain × SMBS × Protease | 0.239 | 0.355 | 0.530 | 0.268 | ||
a,b Means within columns not sharing a common suffix are significantly different at the 5% level of probability.
The effects of dietary treatments on starch digestibility coefficients and starch disappearance rates in the proximal jejunum and distal ileum are shown in Table 6. Sorghum-based diets supported higher distal ileal starch digestibility coefficients by 4.64% (0.880 versus 0.841; P < 0.05) in comparison to wheat-based diets. Also, sorghum-based diets generated more rapid starch disappearance rates by 22.7% [52.4 versus 42.7 g/(bird·day); P < 0.001] in the proximal jejunum and by 7.74% [69.6 versus 64.6 g/(bird·day); P < 0.02] in the distal ileum when compared to wheat-based diets. There was a significant sodium metabisulphite by protease interaction (P < 0.01) for starch disappearance rates in the distal ileum. Protease alone retarded starch disappearance rates from 68.8 to 65.7 g/(bird·day); whereas, in combination with sodium metabisulphite, protease accelerated rates from 62.8 to 71.0 g/(bird·day).
Table 6.
The effects of grain type and dietary inclusions of sodium metabisulphite (SMBS) and protease on starch digestibility coefficients and starch disappearance rates [g/(bird·day)] in proximal jejunum and distal ileum at 28 days post–hatch.
| Treatments |
Starch digestibility coefficients |
Starch disappearance rates |
||||
|---|---|---|---|---|---|---|
| Grain | SMBS, g/kg | Protease, units/kg | Proximal jejunum | Distal ileum | Proximal jejunum | Distal ileum |
| Wheat | 0 | 0 | 0.404 | 0.887 | 42.2 | 69.0 |
| 0 | 1,000 | 0.437 | 0.805 | 43.2 | 60.7 | |
| 2.75 | 0 | 0.416 | 0.821 | 42.2 | 61.2 | |
| 2.75 | 1,000 | 0.467 | 0.853 | 43.0 | 67.5 | |
| Sorghum | 0 | 0 | 0.358 | 0.875 | 51.4 | 68.5 |
| 0 | 1,000 | 0.358 | 0.868 | 51.1 | 70.7 | |
| 2.75 | 0 | 0.407 | 0.873 | 52.2 | 64.6 | |
| 2.75 | 1,000 | 0.367 | 0.904 | 54.9 | 74.6 | |
| SEM | 0.0492 | 0.0273 | 2.459 | 2.736 | ||
| Main effects grain | ||||||
| Wheat | 0.431 | 0.841a | 42.7a | 64.6a | ||
| Sorghum | 0.373 | 0.880b | 52.4b | 69.6b | ||
| SMBS, g/kg | ||||||
| 0 | 0.389 | 0.859 | 47.0 | 67.2 | ||
| 2.75 | 0.414 | 0.863 | 48.1 | 67.0 | ||
| Protease, units/kg | ||||||
| 0 | 0.396 | 0.864 | 47.0 | 65.8 | ||
| 1,000 | 0.407 | 0.858 | 48.1 | 68.4 | ||
| Significance (P-value) | ||||||
| Grain | 0.100 | 0.046 | <0.001 | 0.015 | ||
| SMBS | 0.475 | 0.825 | 0.534 | 0.888 | ||
| Protease | 0.746 | 0.740 | 0.548 | 0.199 | ||
| Grain × SMBS | 0.904 | 0.494 | 0.490 | 0.901 | ||
| Grain × Protease | 0.375 | 0.323 | 0.919 | 0.082 | ||
| SMBS × Protease | 0.875 | 0.052 | 0.715 | 0.007 | ||
| Grain × SMBS× Protease | 0.674 | 0.318 | 0.648 | 0.387 | ||
a,b Means within columns not sharing a common suffix are significantly different at the 5% level of probability.
The effects of dietary treatments on starch:protein disappearance rate ratios in proximal jejunum and distal ileum are shown in Table 7. Wheat-based diets supported more condensed disappearance rate ratios in the proximal jejunum (3.48 versus 2.50; P < 0.001) and distal ileum (2.41 versus 2.96; P < 0.001) than sorghum-based diets. There was a significant sodium metabisulphite by protease interaction (P < 0.01) in the distal ileum. Individually, sodium metabisulphite and protease condensed ratios from 2.84 to 2.53 and 2.55, respectively. In contrast, the two feed additives in combination had little impact on disappearance rate ratios from (2.82 versus 2.84).
Table 7.
The effects of grain type and dietary inclusions of sodium metabisulphite (SMBS) and protease on starch:protein disappearance rate ratios in proximal jejunum and distal ileum at 28 days post–hatch.
| Treatments |
Starch:protein disappearance rate ratios |
|||
|---|---|---|---|---|
| Grain | SMBS, g/kg | Protease, units/kg | Proximal jejunum | Distal ileum |
| Wheat | 0 | 0 | 2.33 | 2.56 |
| 0 | 1,000 | 2.52 | 2.26 | |
| 2.75 | 0 | 2.45 | 2.30 | |
| 2.75 | 1,000 | 2.69 | 2.50 | |
| Sorghum | 0 | 0 | 3.29 | 3.12 |
| 0 | 1,000 | 3.46 | 2.79 | |
| 2.75 | 0 | 2.90 | 2.79 | |
| 2.75 | 1,000 | 4.26 | 3.13 | |
| SEM | 0.2832 | 0.1414 | ||
| Main effects grain | ||||
| Wheat | 2.50a | 2.41a | ||
| Sorghum | 3.48b | 2.96b | ||
| SMBS, g/kg | ||||
| 0 | 2.90 | 2.68 | ||
| 2.75 | 3.08 | 2.68 | ||
| Protease, units/kg | ||||
| 0 | 2.74a | 2.69 | ||
| 1,000 | 3.23b | 2.70 | ||
| Significance (P-value) | ||||
| Grain | <0.001 | <0.001 | ||
| SMBS | 0.381 | 0.971 | ||
| Protease | 0.021 | 0.825 | ||
| Grain × SMBS | 0.876 | 0.921 | ||
| Grain × Protease | 0.181 | 0.804 | ||
| SMBS × Protease | 0.131 | 0.007 | ||
| Grain × SMBS × Protease | 0.169 | 0.683 | ||
a,b Means within columns not sharing a common suffix are significantly different at the 5% level of probability.
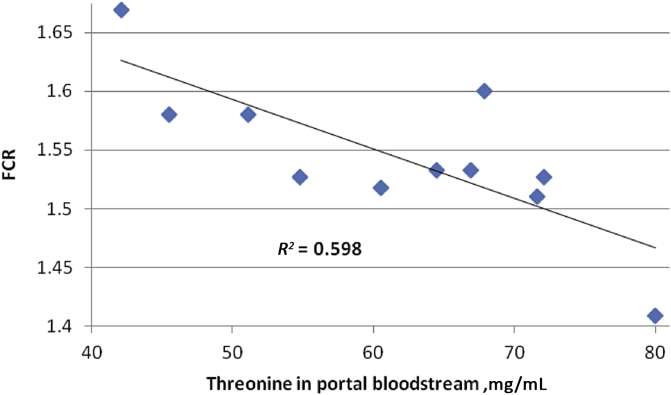
The effects of sodium metabisulphite plus protease inclusions in wheat-based diets on concentrations of free amino acids and glucose in the portal circulation are shown in Table 8. The combined inclusion of these feed additives significantly depressed (P < 0.05) the “gross portal transfer” (concentrations of amino acids in plasma taken from the anterior mesenteric vein) of 10 from 20 amino acids which included histidine, isoleucine, methionine, valine, alanine, cystine, glutamine, glycine, proline and serine. Instructively, concentrations of specific amino acids in plasma taken from the anterior mesenteric vein were significantly correlated with FCR (Table 9). Of the essential amino acids 6 from 10 were significantly negatively correlated (P < 0.05) with FCR (isoleucine, leucine, methionine, phenylalanine, threonine, valine) and a further 3 (arginine, histidine, lysine) tended to be negatively correlated (P < 0.10). Threonine had the most prominent relationship with FCR (r = −0.773; P = 0.005) and this is illustrated in Fig. 1. Across the non-essential amino acids, 3 from 10 (asparagine, glycine proline) were significantly, negatively correlated with FCR.
Table 8.
The effects of feed additives (sodium metabisulphite plus protease) (mg/mL) in wheat-based diets on concentrations of free amino acids and glucose in the portal circulation of broiler chickens at 28 days post–hatch.
| Item | Control | Feed additives | SEM | Significance (P-value) |
|---|---|---|---|---|
| Arginine | 108.9 | 85.4 | 12.117 | 0.200 |
| Histidine | 12.2 | 2.3 | 0.923 | 0.023 |
| Isoleucine | 18.6 | 15.5 | 0.976 | 0.048 |
| Leucine | 26.1 | 23.7 | 1.153 | 0.180 |
| Lysine | 47.0 | 39.3 | 3.321 | 0.133 |
| Methionine | 15.8 | 12.5 | 0.829 | 0.017 |
| Phenylalanine | 21.0 | 17.9 | 1.116 | 0.075 |
| Threonine | 63.8 | 50.8 | 7.446 | 0.247 |
| Tryptophan | 6.2 | 6.0 | 0.477 | 0.791 |
| Valine | 27.8 | 23.3 | 1.305 | 0.037 |
| Alanine | 88.7 | 66.7 | 5.911 | 0.025 |
| Aspartic acid | 13.1 | 23.5 | 3.830 | 0.084 |
| Asparagine | 30.0 | 21.4 | 4.474 | 0.202 |
| Cystine | 13.6 | 9.9 | 0.852 | 0.001 |
| Glutamic acid | 47.9 | 52.2 | 2.680 | 0.280 |
| Glutamine | 196.6 | 143.8 | 10.734 | 0.006 |
| Glycine | 51.9 | 37.7 | 2.463 | 0.002 |
| Proline | 69.2 | 48.3 | 5.099 | 0.016 |
| Serine | 59.2 | 48.1 | 3.299 | 0.039 |
| Tyrosine | 27.6 | 27.2 | 2.400 | 0.909 |
| Glucose, mmol/L | 17.4 | 19.3 | 1.427 | 0.365 |
Table 9.
Correlations between concentrations of free amino acids in the portal circulation and feed conversion ratios of broilers offered wheat-based diets without and with sodium metabisulphite plus protease from 7 to 28 days post–hatch.
| Essential amino acids | Correlation coefficient (r) | Significance (P-value) | Non-essential amino acids | Correlation coefficient (r) | Significance (P-value) |
|---|---|---|---|---|---|
| Arginine | −0.563 | 0.072 | Alanine | −0.157 | 0.644 |
| Histidine | −0.568 | 0.068 | Aspartic acid | −0.019 | 0.956 |
| Isoleucine | −0.643 | 0.033 | Asparagine | −0.606 | 0.048 |
| Leucine | −0.621 | 0.042 | Cystine | −0.329 | 0.322 |
| Lysine | −0.575 | 0.064 | Glutamic acid | +0.295 | 0.379 |
| Methionine | −0.660 | 0.027 | Glutamine | −0.501 | 0.116 |
| Phenylalanine | −0.683 | 0.021 | Glycine | −0.692 | 0.018 |
| Threonine | −0.773 | 0.005 | Proline | −0.802 | 0.003 |
| Tryptophan | −0.247 | 0.464 | Serine | −0.499 | 0.118 |
| Valine | −0.657 | 0.028 | Tyrosine | +0.085 | 0.804 |
Fig. 1.
Linear relationship (r = −0.773; P = 0.005) between free threonine concentrations in blood samples taken from the anterior mesenteric vein with FCR in broiler chickens from 7 to 28 days post–hatch.
4. Discussion
The tandem inclusions of sodium metabisulphite and protease in sorghum-based diets numerically advantaged weight gain by 3.09% (1,533 versus 1,487 g/bird), FCR by 1.09% (1.538 versus 1.555), AME by 0.18 MJ (12.46 versus 12.28 MJ/kg) and ME:GE ratios by 1.33% (0.760 versus 0.750). In contrast, tandem inclusions in wheat-based diets disadvantaged weight gain by 0.77% (1,541 versus 1,553 g/bird), FCR by 4.56% (1.582 versus 1.513), AME by 0.55 MJ (11.93 versus 12.48 MJ/kg) and ME:GE ratios by 6.00% (0.705 versus 0.750). These outcomes may be reflective of the fact that both feed additives increased distal ileal starch digestibility by 3.31% (0.904 versus 0.875) in sorghum-based diets but depressed starch digestibility by 3.83% (0.853 versus 0.887) in wheat-based diets. Therefore, while there is a cautious case to be made for sodium metabisulphite and protease inclusions in sorghum-based diets, this does not appear to apply to wheat-based diets.
The individual inclusion of 2.75 g/kg sodium metabisulphite in sorghum-based diets fractionally advantaged weight gain (1,493 versus 1,487 g/bird), FCR (1.545 versus 1.555), AME by 0.20 MJ (12.48 versus 12.28 MJ/kg), ME:GE ratios by 1.73% (0.763 versus 0.750) and N retention by 5.08% (65.95% versus 62.76%). Again in contrast, sodium metabisulphite alone in wheat-based diets disadvantaged weight gain by 1.55% (1,529 versus 1,553 g/bird), FCR by 3.97% (1.573 versus 1.513), AME by 0.44 MJ (12.04 versus 12.48 MJ/kg), ME:GE ratios by 3.73% (0.722 versus 0.750) and N retention by 5.25% (62.04% versus 65.48%). Again, especially on the basis of N retention, there is a case to be made for sodium metabisulphite inclusions in sorghum-based diets but not in wheat-based diets.
Sulphite reducing agents, including sodium metabisulphite, have the capacity to depolymerise starch via oxidative–reductive reactions (Paterson et al., 1996, Paterson et al., 1997). However, starch digestibility coefficients and disappearance rates were not influenced by sodium metabisulphite in the present study (Table 6). This suggests that any starch depolymerisation generated by reducing agents was of limited importance in the present study. Sulphite reducing agents have the capacity to reduce disulphide cross-linkages which are ubiquitous in protein components of all relevant feedstuffs. However, in this context, the in vitro data of Hamaker et al. (1987) and Zhang and Hamaker (1998) are instructive. These researchers demonstrated that pepsin digestibility of sorghum is responsive to reducing agents but this was not the case with maize and wheat (Hamaker et al., 1987). Similarly, starch digestibility of four sorghum varieties responded to sodium metabisulphite which was not the case for maize (Zhang and Hamaker, 1998). The genesis of this pivotal difference could be the presence of disulphide cross-linkages in the β- and γ-fractions of kafirin protein bodies. These spherical protein bodies are located in sorghum endosperm where the β- and γ-fractions encapsulate the central core of α-kafirin. Starch granules and kafirin protein bodies are both embedded in the glutelin protein matrix of sorghum endosperm (Selle et al., 2010, Liu et al., 2015). It is generally accepted that kafirin impedes starch utilisation in sorghum via biophysical and biochemical starch–protein interactions probably involving disulphide cross-linkages in β- and γ-kafirin (Taylor and Emmambux, 2010). Thus the benefits of sulphite reducing agents in poultry diets may be “sorghum-specific” because of these unique structural factors in grain sorghum endosperm.
Truong et al. (2015) reported on the individual and combined additions of sodium metabisulphite and exogenous phytase to sorghum-based broiler diets. However, in this study the effects of sodium metabisulphite and phytase in wheat-based diets were also determined but this data has not been published. Nevertheless, in short, there were not any indications that the inclusion of sodium metabisulphite in wheat-based diets, either alone or in tandem with phytase, was advantageous. Therefore, the tentative conclusion from this study, supported by the unpublished data, is that the established benefits of sodium metabisulphite in sorghum-based diets do not extend to wheat-based broiler diets. The advantages from sodium metabisulphite inclusions in sorghum-based diets may quite specifically stem from the reduction of disulphide cross-linkages of β- and γ-kafirins located in the periphery of protein bodies.
The results of this study indicate that the inclusion of sodium metabisulphite in wheat-based diets may have negative impacts. This may be related to the sulphur content of the reducing agent; a 2.75 g/kg sodium metabisulphite inclusion translates to an additional 0.93 g/kg sulphur in the diet. This, in turn, may negatively influence the dietary electrolyte balance to a greater extent in wheat-based diets or, more specifically, the dietary cation-anion difference [DCAD (mEq/kg) = (Na + K) – (Cl + S)] (Khajali and Slominski, 2012). In one survey the sulphur content of wheat ranged from 1.3 to 2.1 g/kg (Byers et al., 1987) and Summers et al. (1990) proposed that in the order of 2.0 g/kg sulphur was the maximum dietary level that could be tolerated by broiler chickens. Thus attention should be paid to the sulphur contents of relevant feedstuffs in the context of formulating broiler diets with sodium metabisulphite.
In the present study, the significant impacts of protease as a main effect were limited to a 2.91% increase in feed intake and a widening of the starch:protein disappearance rate ratio from 2.74 to 3.23 in the proximal jejunum. However, our findings have generally indicated that condensed starch:protein disappearance rate ratios are more likely to advantage broiler performance. Protease increased proximal jejunal protein (N) digestibility coefficients by 9.37% (0.537 versus 0.491) which closely approached significance. It should be noted that protease would have been almost certainly disadvantaged by quite high inclusions of synthetic amino acids. Synthetic amino acids represented approximately 27% of total lysine, 50% of methionine, 21% of threonine and 32% of arginine across the two diets in the present study and, notionally, these amino acid proportions are completely digestible.
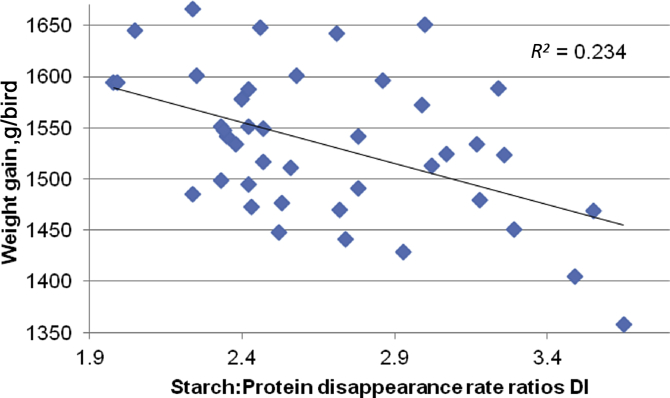
Digestive dynamics, or the bilateral bioavailability of starch and protein, impact on the performance of broiler chickens (Liu and Selle, 2015). Therefore, it is noteworthy that in the present study starch:protein disappearance rate ratios in the distal ileum were negatively correlated with weight gain as illustrated in Fig. 2. This indicates that the condensation of the ratio or the disappearance of more protein relative to starch is advantageous to growth performance. In turn, this infers that if starch is too rapidly, and protein too slowly, digested and absorbed from the gut lumen as glucose and amino acids and that this imbalance is compromising growth performance.
Fig. 2.
Linear relationship (r = −0.484; P = 0.0012) between distal ileal starch:protein disappearance rate ratios and weight gain from 7 to 28 days post–hatch.
However, both amino acids and glucose may be catabolised in the avian gut mucosa as substrates for energy provision to drive gut function (Watford et al., 1979) such that catabolism in the gut mucosa will modulate their post-enteral availability. Thus, as a preliminary investigation, blood samples were taken from the anterior mesenteric vein of birds in two treatment groups to determine plasma concentrations of amino acids and glucose in the portal circulation. The treatments selected proved serendipitous as the control wheat-based diet numerically supported the best FCR but the poorest following the inclusion of both feed additives. Feed conversion ratios were compromised by 4.56% (1.582 versus 1.513; P = 0.084) following the inclusions of sodium metabisulphite and protease and this difference approached significance on the basis of a pair-wise comparison. Therefore, that the gross portal transfer or post-enteral availability of certain amino acids, including methionine, phenylalanine, threonine and the branched-chain amino acids, were significantly related to FCR is an encouraging outcome from this preliminary investigation.
In conclusion, this study indicates that sodium metabisulphite inclusions in wheat-based broiler do not generate the ‘energy sparing’ responses that have been observed in sorghum-based diets in this and more so in other experiments. This may suggest that reductions of disulphide cross-linkages in the periphery of kafirin bodies in the endosperm of sorghum are key to these responses by indirectly enhancing starch utilisation. It also appears that tandem inclusion of sodium metabisulphite and protease in sorghum-based diets may hold some promise but this does not seem to be the case in wheat-based diets. The relationship detected between starch and protein digestive dynamics and weight gain is instructive and it would appear that further investigations into free amino acid concentrations in the portal circulation are certainly justified.
Acknowledgements
This research was [partly] conducted within the Poultry CRC, established and supported under the Australian Government's Cooperative Research Centres Program. We would like to acknowledge the Poultry CRC for funding the project and support of Ms Ha Truong's PhD candidature. We would like to thank Ms Moreen Ali for formulating the diets and Ms Judy O'Keefe for providing the protease feed enzyme. As always, we must thank Ms Joy Gill and her Poultry Research Foundation team for their technical assistance.
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- Byers M., McGrath S.P., Webster R. A survey of the sulphur content of wheat grown in Britain. J Sci Food Agric. 1987;38:151–166. [Google Scholar]
- Cohen S.A. Amino acid analysis using precolumn derivatisation with 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate. Methods Mol Biol. 2001;159:39–47. doi: 10.1385/1-59259-047-0:039. [DOI] [PubMed] [Google Scholar]
- Hamaker B.R., Kirleis A.W., Butler L.G., Axtell J.D., Mertz E.T. Improving the in vitro protein digestibility of sorghum with reducing agents. Proc Natl Acad Sci U S A. 1987;84:626–628. doi: 10.1073/pnas.84.3.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill F.W., Anderson D.L. Comparison of metabolisable energy and productive energy determinations with growing chicks. J Nutr. 1958;64:587–603. doi: 10.1093/jn/64.4.587. [DOI] [PubMed] [Google Scholar]
- Khajali F., Slominski B.A. Factors that affect the nutritive value of canola meal for poultry. Poult Sci. 2012;91:2564–2575. doi: 10.3382/ps.2012-02332. [DOI] [PubMed] [Google Scholar]
- Liu S.Y., Selle P.H., Cowieson A.J. Protease supplementation of sorghum-based broiler diets enhances amino acid digestibility coefficients in four small intestinal sites and accelerates their rates of digestion. Anim Feed Sci Technol. 2013;183:175–183. [Google Scholar]
- Liu S.Y., Selle P.H., Khoddami A., Roberts T.H., Cowieson A.J. Graded inclusions of sodium metabisulphite in sorghum-based diets: II. Modification of starch pasting properties in vitro and beneficial impacts on starch digestion dynamics in broiler chickens. Anim Feed Sci Technol. 2014;190:68–78. [Google Scholar]
- Liu S.Y., Fox G., Khoddami A., Neilson K.A., Truong H.H., Moss A.F. Grain sorghum: a conundrum for chicken-meat production. Agric. 2015;5:1224–1251. [Google Scholar]
- Liu S.Y., Selle P.H. A consideration of starch and protein digestive dynamics in chicken-meat production. World's Poult Sci J. 2015;71:297–310. [Google Scholar]
- Mahasukhonthachat K., Sopade P.A., Gidley M.J. Kinetics of starch digestion and functional properties of twin-screw extruded sorghum. J Cereal Sci. 2010;51:392–401. [Google Scholar]
- Paterson L.A., Mitchell J.R., Hill S.E., Blanshard J. Evidence for sulfite induced oxidative reductive depolymerisation of starch polysaccharides. Carbohydr Res. 1996;292:143–151. [Google Scholar]
- Paterson L.A., Hill S.E., Mitchell J.R., Blanshard J. Sulphite and oxidative—reductive depolymerization reactions. Food Chem. 1997;60:143–147. [Google Scholar]
- Selle P.H., Cadogan D.J., Li X., Bryden W.L. Implications of sorghum in broiler chicken nutrition. Anim Feed Sci Technol. 2010;156:57–74. [Google Scholar]
- Selle P.H., Liu S.Y., Cai J., Caldwell R.A., Cowieson A.J. Preliminary assessment of including a reducing agent (sodium metabisulphite) in ‘all-sorghum’ diets for broiler chickens. Anim Feed Sci Technol. 2013;186:81–90. [Google Scholar]
- Selle P.H., Liu S.Y., Cai J., Cowieson A.J. Steam-pelleting temperatures, grain variety, feed form and protease supplementation of mediumly ground, sorghum-based broiler diets: influences on growth performance, relative gizzard weights, nutrient utilisation, starch and nitrogen digestibility. Anim Prod Sci. 2013;53:378–387. [Google Scholar]
- Selle P.H., Liu S.Y., Cai J., Caldwell R.A., Cowieson A.J. Graded inclusions of sodium metabisulphite in sorghum-based diets: I. Reduction of disulphide cross-linkages in vitro and enhancement of energy utilisation and feed conversion efficiency in broiler chickens. Anim Feed Sci Technol. 2014;190:59–67. [Google Scholar]
- Siriwan P., Bryden W.L., Mollah Y., Annison E.F. Measurement of endogenous amino-acid losses in poultry. Br Poult Sci. 1993;34:939–949. doi: 10.1080/00071669308417654. [DOI] [PubMed] [Google Scholar]
- Summers J.D., Bedford M., Spratt D. Interaction of calcium and sulphur in canola and soybean meal diets fed to broiler chickens. Canad J Anim Sci. 1990;70:685–694. [Google Scholar]
- Taylor J.R.N., Emmambux M.N. Developments in our understanding of sorghum polysaccharides and their health benefits. Cereal Chem. 2010;87:263–271. [Google Scholar]
- Truong H.H., Cadogan D.J., Liu S.Y., Selle P.H. Addition of sodium metabisulfite and microbial phytase, individually and in combination, to a sorghum-based diet for broiler chickens from 7 to 28 days post-hatch. Anim Prod Sci. 2015;198:248–256. [Google Scholar]
- Truong H.H., Neilson K.A., McInerney B.V., Khoddami A., Roberts T.H., Liu S.Y. Sodium metabisulphite enhances energy utilisation in broiler chickens offered sorghum-based diets with five different grain varieties. Anim Sci Tech. 2016;219:159–174. [Google Scholar]
- Watford M., Lund P., Krebs H.A. Isolation and metabolic characteristics of rat and chicken enterocytes. Biochem J. 1979;178:589–596. doi: 10.1042/bj1780589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G., Hamaker B.R. Low α-amylase starch digestibility of cooked sorghum flours and the effect of protein. Cereal Chem. 1998;75:710–713. [Google Scholar]