Abstract
The effect of graded levels of phytase on performance, bone characteristics, excreta/litter quality and odorant emissions was examined using 720 Ross 308 male d-old broilers. A 2 × 4 factorial arrangement of treatments was employed with 6 replicates of 15 birds per pen. Factors were: diets-positive and negative control (PC, NC); phytase – 0, 500, 1,000, 1,500 FTU/kg. The PC was formulated to meet the 2014 Ross 308 nutrient specifications, whereas the NC was formulated with lower Ca (−1.4 g/kg), available P (−1.5 g/kg), Na (−0.3 g/kg), dLys (−0.2 g/kg) and MEn (−0.28 MJ/kg) equivalent to nutrient matrix values for 500 FTU/kg phytase in the starter, grower and finisher periods (i.e., downspec diet). On d 24, phytase decreased FCR by 1.6, 4.3 and 4.6 points at inclusion levels of 500, 1,000 and 1,500 FTU/kg, respectively (P < 0.01) across all diets. Phytase by diet interactions on BW gain were observed on d 24 and 35 (P < 0.01). The effect of phytase was much more pronounced in the NC diet as compared with the PC diet. On d 24, phytase increased BW gain by 37, 55 and 68 g in the PC and 127, 233 and 173 g in the NC at 500, 1,000 and 1,500 FTU/kg, respectively. Diet by phytase interactions were also observed for tibia ash, litter quality and water to feed intake ratio (P < 0.01) with higher phytase effect in NC as compared with PC. Neither diet nor phytase impacted excreta moisture content on d 18 or 21 (P > 0.05). Solid phase micro-extraction gas chromatography-mass spectrometry (SPME-GC-MS) analysis of gaseous emissions on d 39 indicated no difference in the emission of alcohols, aldehydes, ketones, volatile fatty acids and phenols between treatments (P > 0.05). The results indicate that phytase has greater benefits when formulated using nutrient matrix values as compared with adding it over the top in an already nutrient sufficient diet. The later method would be expected to increase feed costs without concomitant performance benefits.
Keywords: Broiler, Litter quality, Meat chickens, Odour, Performance, Phytase
1. Introduction
Phytase is commonly used in broiler diets to degrade the phytate content, thus eliminating its anti-nutritional effects, which include reduced nutrient digestion, increased endogenous losses of nutrients and decreased performance (Woyengo and Nyachoti, 2013). The degradation of phytate at ileal or total tract level has been shown to be incomplete at the standard 500 FTU/kg inclusion rate of phytase (Selle and Ravindran, 2007). Phytase at higher levels produces extra-phosphoric effects in broilers, such as improved energy, amino acid and P digestibility (Amerah et al., 2014, Cowieson et al., 2011), and enhanced growth and feed efficiency (Walk et al., 2014). A recent study showed improvements in litter quality with higher doses of phytase (Bedford and Walk, 2015), although some reports suggest that phytase is associated with wet litter (Debicki-Garnier and Hruby, 2003, Bedford et al., 2012). Phytase may increase water consumption and contribute to wet droppings when formulated into diets without using nutrient matrix values. Phytase is normally assigned nutrient matrix values for amino acids/protein and energy in addition to Ca and P to incorporate it into least cost formulations (Shelton et al., 2004). The level of Na in basal diets and Na matrix for phytase are often overlooked during formulation. High dietary Na and/or Ca levels may trigger increased water consumption and hence diuresis and wet litter (Collett, 2012). Phytase added at higher rates without lowering dietary Na and/or Ca may consequently affect litter quality.
Wet litter and odour issues from poultry farms have become prominent worldwide. It is important to maintain litter quality to promote bird health and welfare and to minimize odour emissions. Litter quality may deteriorate due to increased excretion of minerals by birds (van der Hoeven-Hangoor et al., 2013). Phytate can bind with minerals and protein in the diet making them unavailable for digestion (Cowieson et al., 2006, Selle et al., 2012). Phytase may therefore potentially improve litter quality by increasing the bioavailability of minerals and protein. Excreta/litter quality is generally measured by water activity, moisture and free water contents (van der Hoeven-Hangoor et al., 2014). The amount of water in the litter has been found to affect microbial activity (Himathongkham et al., 1999, Wadud et al., 2012), ammonia emissions (Miles et al., 2011) and odorant emissions (Sharma et al., 2016). It is, therefore, important to study the effect of phytase on litter quality and odour emissions together with performance, especially when used at higher levels. The aim of this study was to investigate the effects of graded levels of phytase in nutritionally adequate and downspec wheat based broiler diets on performance, excreta/litter quality and odour emissions.
2. Material and methods
2.1. Animal ethics
All the experimental procedures were approved by the University of New England, Australia animal ethics committee (AEC15-011).
2.2. Bird husbandry, experimental design and diets
A total of 720 d-old Ross 308 male broiler chicks were assigned to 8 dietary treatments with 6 replicates of 15 birds/pen in a 2 × 4 factorial arrangement with 2 diet types, positive and negative controls (PC, NC), and 4 doses of phytase (0, 500, 1,000, 1,500 FTU/kg) (Buttiauxella phytase, Axtra PHY10000 TPT, Dupont Animal Nutrition). This phytase is thermostable up to 95 °C during pelleting according to the supplier's information (Feedworks, Australia). The replications were allocated in a completely randomized design. The chicks (average weight of 37 ± 1 g) were weighed before placement to ensure no significant difference between treatments. Each pen measured 1.2 m × 0.76 m and consisted of a feeder and a double outlet cup drinker. Fresh pine shavings (Hysorb wood shavings, ECW, Australia) were used as bedding material and were added at 10.35 kg/pen. The PC diet was formulated to meet the 2014 Ross 308 nutrient specifications, whereas the NC diet was formulated to give the same nutrient specifications of the PC diet when using nutrient matrix values for 500 FTU phytase as recommended by the phytase supplier. The lower specifications applied to the NC diet was as follows: Ca (−1.4 g/kg), available P (av. P – 1.5 g/kg), Na (−0.3 g/kg), amino acids (dLys − 0.2 g/kg, dMet + Cys − 0.17 g/kg, dThr − 0.17 g/kg, dArg − 0.16 g/kg, dIle − 0.16 g/kg, dTrp − 0.05 g/kg, dVal − 0.15 g/kg) and energy (ME − 0.28 MJ/kg). These down-specifications were applied across the starter (0 to 10 d), grower (11 to 24 d) and finisher (25 to 35 d) periods. The composition of experimental diets and their calculated and analysed nutrients are presented in Table 1, Table 2. Zinc bacitracin (ALBAC 150, Zoetis) was included at the rate of 330 g/t of feed resulting in an active dose of 50 mg/kg during the starter period only. Salinomycin (SACOX 120) was included as a coccidiostat at 500 g/t of feed at all stages of growth resulting in an active dose of 60 mg/kg feed. Phytase enzyme was added to both the PC and NC diets at 4 levels: 0, 50 (500 FTU/kg), 100 (1,000 FTU/kg) and 150 g/t (1,500 FTU/kg). Feed was mixed and pelleted at 65 °C at the University of New England, Australia. All feeds were fed in crumble form to 10 d and as pellets thereafter until finishing the 35 d study period. Feed and water were provided ad libitum throughout the study. Lighting program was provided according to the Ross 308 breed management manual (Aviagen, 2014). In short, a continuous lighting was provided during the first 48 h of chicks arrival followed by a 1-h darkness each day up to d 7 and gradually increased to 6 h darkness from d 10.
Table 1.
Ingredient composition and calculated nutrients of the experimental diets (as fed basis).
| Item | Positive control |
Negative control |
||||
|---|---|---|---|---|---|---|
| Starter | Grower | Finisher | Starter | Grower | Finisher | |
| Ingredients, g/kg | ||||||
| Wheat | 572 | 587 | 645 | 607 | 621 | 675 |
| Soybean meal | 279 | 223 | 175 | 269 | 213 | 173 |
| Meat meal | 45 | 30 | 30 | 45 | 30 | 22 |
| Canola meal | 40 | 80 | 80 | 40 | 80 | 80 |
| Canola oil | 33 | 45 | 44 | 16 | 29 | 29 |
| Dicalcium phosphate | 8.5 | 8.7 | 6.8 | 0.3 | 0.6 | 0.3 |
| Limestone | 6.3 | 7.1 | 6.3 | 7.2 | 7.9 | 8.2 |
| D,L-methionine | 3.41 | 2.74 | 2.31 | 3.20 | 2.53 | 2.10 |
| L-lysine HCl | 3.03 | 2.70 | 2.50 | 2.95 | 2.62 | 2.40 |
| NaCl | 2.72 | 1.86 | 1.86 | 2.05 | 1.20 | 1.30 |
| Premix1 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 |
| L-threonine | 1.96 | 1.47 | 1.17 | 1.83 | 1.33 | 1.02 |
| Na bicarbonate | 1.50 | 2.00 | 2.00 | 1.50 | 2.00 | 2.00 |
| Choline Cl, 60% | 0.69 | 0.69 | 0.57 | 0.65 | 0.65 | 0.54 |
| Xylanase powder2 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| Salinomycin3 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| Zn bacitracin4 | 0.33 | – | – | 0.33 | – | – |
| Titanium dioxide | – | 5.0 | – | – | 5.0 | – |
| Calculated nutrients, g/kg | ||||||
| ME, MJ/kg | 12.55 | 12.97 | 13.18 | 12.27 | 12.69 | 12.90 |
| Crude protein | 233 | 213 | 197 | 232 | 212 | 194 |
| Crude fibre | 28.1 | 31.3 | 31.1 | 28.6 | 31.8 | 31.7 |
| dLysine | 12.8 | 11.5 | 10.3 | 12.6 | 11.3 | 10.1 |
| dMet + Cys | 9.5 | 8.7 | 8.0 | 9.3 | 8.5 | 7.8 |
| dLeucine | 14.6 | 13.4 | 12.4 | 14.6 | 13.4 | 12.3 |
| dArginine | 13.7 | 12.3 | 11.0 | 13.5 | 12.1 | 10.8 |
| dIsoleucine | 8.9 | 8.2 | 7.5 | 8.9 | 8.1 | 7.5 |
| dThreonine | 8.6 | 7.7 | 6.9 | 8.4 | 7.5 | 6.7 |
| dValine | 9.9 | 9.1 | 8.3 | 9.8 | 9.0 | 8.2 |
| Ca | 9.6 | 8.7 | 7.9 | 8.2 | 7.3 | 6.5 |
| Total P | 7.7 | 7.1 | 6.6 | 6.2 | 5.6 | 5.0 |
| Av. P | 4.8 | 4.3 | 3.9 | 3.3 | 2.8 | 2.4 |
| Na | 2.1 | 1.8 | 1.8 | 1.8 | 1.5 | 1.5 |
| K | 9.4 | 8.6 | 7.8 | 9.3 | 8.5 | 7.8 |
| Cl | 3.0 | 2.3 | 2.3 | 2.6 | 1.9 | 1.9 |
| Mg | 2.0 | 2.0 | 1.8 | 1.9 | 1.9 | 1.9 |
| S | 2.3 | 2.3 | 2.1 | 2.3 | 2.3 | 2.1 |
| dEB5, mEq | 267 | 251 | 229 | 263 | 247 | 227 |
| dEB6, mEq | 287 | 274 | 252 | 280 | 267 | 249 |
Vitamin-Mineral concentrate supplied per kilogram of diet: retinol, 1,2000 IU; cholecalciferol, 5,000 IU; tocopheryl acetate, 75 mg; menadione, 3 mg; thiamine, 3 mg; riboflavin, 8 mg; niacin, 55 mg; pantothenate, 13 mg; pyridoxine, 5 mg; folate, 2 mg; cyanocobalamine, 16 μg; biotin, 200 μg; cereal-based carrier, 149 mg; mineral oil, 2.5 mg; Cu (sulphate), 16 mg; Fe (sulphate), 40 mg; I (iodide), 1.25 mg; Se (selenate), 0.3 mg; Mn (sulphate and oxide), 120 mg; Zn (sulphate and oxide), 100 mg; cereal-based carrier, 128 mg; mineral oil, 3.75 mg.
Feedzyme XBC 1000 (Feedworks, Australia).
Sacox 120 (coccidiostat).
Albac 150 (Zoetis).
dEB = Na+ + K+−Cl−.
dEB = Na+ + K+ + Mg2+−Cl−−S2−.
Table 2.
Analysed nutrient composition (g/kg) of the experimental diets.
| Nutrients | Positive control |
Negative control |
||||
|---|---|---|---|---|---|---|
| Starter | Grower | Finisher | Starter | Grower | Finisher | |
| Dry matter | 90.1 | 90.4 | 91.1 | 90.1 | 90.3 | 90.9 |
| Gross energy, MJ/kg | 17.36 | 17.53 | 17.71 | 17.15 | 17.24 | 17.52 |
| Crude protein | 242.8 | 220.9 | 210.6 | 237.9 | 217.9 | 210.4 |
| Crude fat | 64.7 | 57.4 | 71.6 | 37.4 | 89.7 | 102.2 |
| Crude fibre | 24.4 | 25.6 | 25.6 | 26.3 | 24.9 | 24.9 |
| Ca | 10.8 | 10.4 | 10.0 | 8.7 | 8.1 | 7.8 |
| Total P | 7.2 | 7.1 | 6.2 | 5.8 | 5.4 | 5.2 |
| Na | 2.2 | 1.7 | 1.7 | 1.5 | 1.3 | 1.3 |
| K | 9.6 | 9.1 | 8.0 | 9.6 | 8.6 | 8.5 |
| Cl | 2.2 | 2.0 | 1.9 | 2.0 | 1.9 | 1.9 |
| Mg | 1.8 | 1.9 | 1.8 | 1.8 | 1.9 | 1.8 |
| S | 3.0 | 3.0 | 2.8 | 3.1 | 2.9 | 2.8 |
2.3. Performance of birds
Birds, feed and water were weighed in all the pens on d 0. They were again weighed on d 10, 24 and 35 to calculate feed intake, water to feed intake ratio, weight gain and FCR (corrected for mortality). Water intake was measured by subtracting the final and initial weight of water in the 20 L water tank in each pen. Two birds from each pen were euthanized by cervical dislocation on d 24 and the left tibias were removed to measure tibia weight, length, diameter and ash content. The tibia was defleshed according to the autoclave method as described by Hall et al. (2003) and was oven dried overnight at 105 °C. Dried tibia samples were weighed and the length and diameter measured using a micrometer (Mitutoyo No. 2050-08, Japan). Tibia was ashed at 600 °C to constant weight to calculate percentage tibia ash. Liver, spleen, bursa and small intestine were collected from the same birds to measure their weights. On d 35, 2 birds were sampled from each pen to measure the weights of breast meat, abdominal fat and empty small intestine (duodenum, jejunum and ileum).
2.4. Excreta and litter characteristics
Litter quality was measured on d 35. Each pen was divided into 2 halves and litter quality was scored for each half of the pen and averaged. Three independent observers were involved in the litter scoring process to obtain the average value. Litter quality scores were taken visually, which ranged from 1 to 5, with 1 being extremely dry and no caked litter and 5 being total pen coverage of caked litter. On d 10, a total of 96 birds (2 birds/pen) were selected and transferred to metabolic cages in a climate controlled room with 6 replications of 2 birds per cage for each of the 8 treatments. The birds were adapted in the cages for 8 d and on d 18 fresh excreta samples were collected four times at 2-h intervals to measure moisture and free water content. The moisture content from fresh excreta was measured by subjecting 10 g of samples to forced air at 105 °C for 48 h and measuring the weight difference before and after drying. Excreta free water content was measured according to the procedures described by van der Hoeven-Hangoor et al. (2013). In short, approximately 22 ± 0.3 g of pooled excreta was weighed in a pre-weighed centrifuge tube and centrifuged at 2,230 × g at 5 °C for 24 min. The supernatant was manually removed using a pipette and the tube was re-weighed. Excreta free water was calculated as the difference between the initial weight and weight post removal of the supernatant and expressed as a percentage of the original excreta weight. Excreta was also collected daily from d 19 to 21 to measure the moisture content by following the same procedure as described above. In both cases, excreta was refrigerated at 4 °C immediately upon collection. Excreta pH was determined by mixing excreta and de-ionised water in the ratio of 1:5 and measuring the pH with a pH meter (EcoScan 5/6 pH meter, Eutech Instrument Pte Ltd; Singapore).
2.5. Solid phase micro-extraction (SPME) measurement of odorants
After the studies were finished with the cages, the same birds were sampled to study odorant emissions. At the age of 22 d, 45 birds of uniform body weight were selected out of 96 birds from the cages and adapted for 3 d to metabolic chambers to measure odour emissions (Sharma et al., 2015) and fed their respective test diets. The experiment was conducted as a completely randomized design with 5 treatments and 3 replications of 3 birds/replicate. The treatments were: PC, NC, NC + 500 FTU/kg, NC + 1,000 FTU/kg and NC + 1,500 FTU/kg phytase. The experiment started at d 25 and emissions were measured on d 39. Headspace samples from birds and litter were collected with 85 μm carboxen/polydimethylsiloxane SPME fibres (Supelco, Sigma–Aldrich, Australia) and analysed using an Agilent HP 6890 series GC system/5973 MSD, gas chromatography-mass spectrometry (GC–MS, California, USA) for identification of odorants. New fibres were conditioned according to the manufacturer's instructions. Before each sampling, the SPME fibre was desorbed in a GC injector for 5 min at 260 °C, and then SPME collections were performed by direct fibre exposure in the dynamic headspace of the chambers for 20 min. This sampling time was selected based on preliminary tests of control headspaces with varying SPME sampling times; the 20 min sampling time consistently resulted in detectable amounts of odorants that are associated with broiler litter. Immediately after the sampling, the odorants were analysed using the GC–MS equipped with a 30 m polar capillary column, 0.25 mm internal diameter and 0.25 μm film thickness (Zebron capillary GC column, Phenomenex, Australia). The injector was set at 260 °C and the column was set at an initial temperature of 40 °C and final temperature of 240 °C. The carrier gas was helium. Mass/charge (m/z) ratio range was set between 40 and 300 amu (atomic mass unit). Spectra were collected at 5.36 scans/s and electron multiplier voltage was 1,224 V. The detector was auto-tuned daily. The volatile organic compounds (VOC) were identified by matching mass spectra of unknown compounds with the spectra from the National Institute of Standards and Technology (NIST, USA) commercial MS library. Only the important odorous VOC in broiler chicken house that were reported by Murphy et al. (2014) were targeted.
2.6. Chemical analyses
Proximate analyses were conducted using AOAC methods (AOAC, 1990). Dry matter content of the diets and litter were determined by placing duplicate samples in a drying oven at 105 °C for 24 h (AOAC, 1990). Gross energy content of the diets was determined on a 0.5 g sample using an adiabatic bomb calorimeter (IKA Werke, C7000, GMBH and Co., Staufen, Germany) with benzoic acid as a standard. Nitrogen content of the diets and excreta was determined on a 0.15 g sample with a combustion analyzer (Leco model FP-2000N analyzer, Leco Corp., St. Joseph, MI) using EDTA as a calibration standard. Crude protein was calculated by multiplying percentage N by 6.25. Minerals in the feed were analysed using inductively coupled plasma optical emission spectrometer (ICP-OES, Model-725 radial viewed) using perchloric acid and hydrogen peroxide for digestion of the samples (Anderson and Henderson, 1986). Phytase activity in the feed was measured according to the AOAC method 2000.12 (AOAC, 2005).
2.7. Statistical analysis
Performance data and excreta/litter parameters were analysed following a 2 × 4 factorial arrangement using JMP statistical software version 8 (SAS Institute Inc, Cary, NC) to test the main effects of diets, phytase and their interaction. Data were subjected to two-way ANOVA and means were separated by Tukey's HSD test at a probability level of 0.05. Odorant concentration measurements were analysed by one-way ANOVA and means were separated by Tukey's HSD test at a probability level of 0.05. The P-values above 0.10 were shown in short form as NS in the tables.
3. Results
3.1. Feed composition and phytase activity
The NC diet contained more limestone and much less dicalcium phosphate than the PC diet. The analysed values for Ca and total P in the diets were slightly higher than the calculated ones but the trend was similar across the treatments (Table 2). The analysed values for phytase activity in the PC and NC diets were close to the expected values when the background phytase levels (with added phytase) were taken into account (Table 3).
Table 3.
Analysed phytase activity (FTU/kg)1 in the experimental diets.
| Experimental diets | Starter (d 0 to 10) | Grower (d 11 to 24) | Finisher (d 25 to 35) |
|---|---|---|---|
| Positive control (PC) | 400 | 379 | 456 |
| PC + 500 FTU/kg of phytase | 1,005 | 1,041 | 1,063 |
| PC + 1,000 FTU/kg of phytase | 1,524 | 1,585 | 1,538 |
| PC + 1,500 FTU/kg of phytase | 2,050 | 2,063 | 2,145 |
| Negative control (NC) | 434 | 314 | 425 |
| NC + 500 FTU/kg of phytase | 1,016 | 1,007 | 1,011 |
| NC + 1,000 FTU/kg of phytase | 1,552 | 1,540 | 1,573 |
| NC + 1,500 FTU/kg of phytase | 2,063 | 2,193 | 2,006 |
One unit of phytase activity (FTU) is defined as the quantity of enzyme that liberates 1 μmol of inorganic P per minute from sodium phytate at pH 5.5 and 37 °C.
3.2. Growth performance and water to feed intake ratio
The overall mortality during the entire study period was less than 4% and there was no diet related mortality (P > 0.05, data not shown). The effect of supplementing diets with increasing levels of phytase on bird performance is shown in Tables 4 to 6. During the starter period (Table 4), the birds fed the PC diet had higher FI, higher BW gain (P < 0.001) and 3 points better FCR (P < 0.01) than those fed the NC diet. The diets supplemented with phytase improved BW gain and FCR in the birds at all the levels compared with the diets without phytase (P < 0.001). There was no diet × phytase interaction during the starter period.
Table 4.
Performance and water to feed intake ratio of broilers fed nutritionally adequate (PC) and downspec (NC) wheat based diets supplemented with increasing levels of phytase (0 to 10 d).
| Treatments | FI, g | BWG, g | Corrected FCR | WI, g | WI:FI |
|---|---|---|---|---|---|
| Diets | |||||
| NC | 296b | 246b | 1.207a | 639 | 2.16 |
| PC | 303a | 258a | 1.173b | 649 | 2.15 |
| SEM | 2.14 | 2.07 | 0.008 | 9.87 | 0.03 |
| Phytase, FTU/kg | |||||
| 0 | 294 | 238b | 1.239a | 645 | 2.20 |
| 500 | 298 | 253a | 1.181b | 637 | 2.14 |
| 1,000 | 304 | 261a | 1.167b | 665 | 2.19 |
| 1,500 | 301 | 258a | 1.173b | 630 | 2.09 |
| SEM | 3.03 | 2.93 | 0.01 | 13.9 | 0.04 |
| Dietary treatments | |||||
| NC + 0 FTU/kg of phytase | 286 | 232 | 1.233 | 660abc | 2.31a |
| NC + 500 FTU/kg of phytase | 295 | 245 | 1.204 | 607cd | 2.06cd |
| NC + 1,000 FTU/kg of phytase | 307 | 261 | 1.176 | 696a | 2.27ab |
| NC + 1,500 FTU/kg of phytase | 297 | 246 | 1.207 | 594d | 2.00d |
| PC + 0 FTU/kg of phytase | 303 | 244 | 1.242 | 630bcd | 2.09cd |
| PC + 500 FTU/kg of phytase | 301 | 261 | 1.153 | 666ab | 2.22abc |
| PC + 1,000 FTU/kg of phytase | 302 | 260 | 1.162 | 634bcd | 2.10bcd |
| PC + 1,500 FTU/kg of phytase | 305 | 269 | 1.134 | 665ab | 2.18abc |
| SEM | 2.13 | 4.15 | 0.02 | 19.7 | 0.06 |
| P-value | |||||
| Diets | <0.05 | <0.001 | <0.01 | NS | NS |
| Phytase | NS | <0.001 | <0.001 | NS | NS |
| Diet × phytase | NS | NS | NS | <0.01 | <0.01 |
FI = feed intake; BWG = body weight gain; FCR = feed conversion ratio; WI = water intake.
a,b,c,d Means in the same column with different superscripts differ significantly (P < 0.01).
Table 6.
Performance and water to feed intake ratio of broilers fed nutritionally adequate (PC) and downspec (NC) wheat based diets supplemented with increasing levels of phytase (0 to 35 d).
| Treatments | FI, g | BWG, g | Corrected FCR | WI, g | WI:FI |
|---|---|---|---|---|---|
| Diets | |||||
| NC | 3,481 | 2,460b | 1.388a | 7,580 | 2.18a |
| PC | 3,548 | 2,538a | 1.367b | 7,530 | 2.12b |
| SEM | 36.69 | 26.18 | 0.005 | 116.1 | 0.02 |
| Phytase, FTU/kg | |||||
| 0 | 3,367 | 2,364 | 1.397a | 7,446 | 2.21a |
| 500 | 3,560 | 2,527 | 1.383ab | 7,700 | 2.16ab |
| 1,000 | 3,573 | 2,555 | 1.365b | 7,543 | 2.11b |
| 1,500 | 3,559 | 2,549 | 1.364b | 7,531 | 2.12b |
| SEM | 51.89 | 37.03 | 0.007 | 164.2 | 0.03 |
| Dietary treatments | |||||
| NC + 0 FTU/kg of phytase | 3,061b | 2,125b | 1.440 | 7,071b | 2.31a |
| NC + 500 FTU/kg of phytase | 3,625a | 2,555a | 1.419 | 7,927a | 2.19b |
| NC + 1,000 FTU/kg of phytase | 3,674a | 2,590a | 1.419 | 7,736a | 2.11b |
| NC + 1,500 FTU/kg of phytase | 3,565a | 2,569a | 1.388 | 7,585ab | 2.13b |
| PC + 0 FTU/kg of phytase | 3,474a | 2,460a | 1.412 | 7,400a | 2.13b |
| PC + 500 FTU/kg of phytase | 3,494a | 2,499a | 1.398 | 7,472ab | 2.14b |
| PC + 1,000 FTU/kg of phytase | 3,492a | 2,520a | 1.386 | 7,351ab | 2.11b |
| PC + 1,500 FTU/kg of phytase | 3,553a | 2,529a | 1.405 | 7,477ab | 2.10b |
| SEM | 73.38 | 52.36 | 0.01 | 232.2 | 0.04 |
| P-value | |||||
| Diets | NS | <0.05 | <0.01 | NS | <0.05 |
| Phytase | <0.05 | <0.01 | <0.01 | NS | <0.05 |
| Diet × phytase | <0.01 | <0.01 | NS | 0.05 | 0.057 |
FI = feed intake; BWG = body weight gain; FCR = feed conversion ratio; WI = water intake.
a,b,c,d Means in the same column with different superscripts differ significantly (P < 0.01).
During the period of 0 to 24 d (Table 5) and 0 to 35 d (Table 6), diet by phytase interactions were observed for FI and BW gain (P < 0.01). The birds fed the NC diet without phytase had lower FI and BW gain compared with those fed the PC diet without phytase. The addition of phytase to the NC diet increased FI and BW gain in a dose-dependent manner up to the level of 1,000 FTU/kg but the increment was lower at 1,500 FTU/kg compared with 1,000 FTU/kg on d 24 (P < 0.01). On d 24, when phytase was added to the PC diet, there was no effect on FI at any levels but BW gain was higher by 4.2% at inclusion rates of 1,000 or 1,500 FTU/kg (P < 0.01). The birds fed the NC diet had 2 points higher FCR at d 24 compared with those fed the PC diet (P < 0.01). As the main effect, phytase improved FCR by 2 and 5 points at inclusion levels of 500 and 1,000 and 1,500 FTU/kg, respectively (P < 0.01). On d 35, results followed a similar pattern in that phytase had a greater impact in birds fed the NC as compared with PC diet (Table 6).
Table 5.
Performance and water to feed intake ratio of broilers fed nutritionally adequate (PC) and downspec (NC) wheat based diets supplemented with increasing levels of phytase (0 to 24 d).
| Treatments | FI, g | BWG, g | Corrected FCR | WI, g | WI:FI |
|---|---|---|---|---|---|
| Diets | |||||
| NC | 1,620 | 1,262 | 1.281a | 3,555 | 2.20 |
| PC | 1,690 | 1,336 | 1.260b | 3,593 | 2.13 |
| SEM | 9.92 | 7.70 | 0.003 | 38.74 | 0.02 |
| Phytase, FTU/kg | |||||
| 0 | 1,575 | 1,212 | 1.297a | 3,573 | 2.27 |
| 500 | 1,664 | 1,295 | 1.281b | 3,560 | 2.14 |
| 1,000 | 1,704 | 1,357 | 1.254c | 3,638 | 2.14 |
| 1,500 | 1,677 | 1,333 | 1.251c | 3,524 | 2.10 |
| SEM | 14.02 | 10.89 | 0.004 | 54.78 | 0.03 |
| Dietary treatments | |||||
| NC + 0 FTU/kg of phytase | 1,485d | 1,129d | 1.315 | 3,601 | 2.42a |
| NC + 500 FTU/kg of phytase | 1,632c | 1,256c | 1.299 | 3,495 | 2.14b |
| NC + 1,000 FTU/kg of phytase | 1,712a | 1,362a | 1.257 | 3,650 | 2.14b |
| NC + 1,500 FTU/kg of phytase | 1,650bc | 1,302b | 1.267 | 3,473 | 2.10b |
| PC + 0 FTU/kg of phytase | 1,665abc | 1,296bc | 1.285 | 3,545 | 2.13b |
| PC + 500 FTU/kg of phytase | 1,696ab | 1,333ab | 1.272 | 3,626 | 2.14b |
| PC + 1,000 FTU/kg of phytase | 1,695ab | 1,351a | 1.255 | 3,626 | 2.14b |
| PC + 1,500 FTU/kg of phytase | 1,703ab | 1,364a | 1.249 | 3,575 | 2.10b |
| SEM | 19.83 | 15.39 | 0.005 | 77.48 | 0.04 |
| P-value | |||||
| Diets | <0.01 | <0.01 | <0.01 | NS | <0.05 |
| Phytase | <0.01 | <0.01 | <0.01 | NS | <0.01 |
| Diet × phytase | <0.01 | <0.01 | NS | NS | <0.01 |
FI = feed intake; BWG = body weight gain; FCR = feed conversion ratio; WI = water intake.
a,b,c,d Means in the same column with different superscripts differ significantly (P < 0.01).
The effect of graded levels of phytase on water to feed intake ratio (WI:FI) is shown in Table 4, Table 5, Table 6. There was an interaction between diet and phytase on the WI:FI at 0 to 10 d, and 0 to 24 d (P < 0.01). On d 35, there was a tendency for this interaction to persist (P = 0.06). The birds fed the NC diet without phytase had a higher WI:FI at all stages of growth compared with those fed the PC diet without phytase (P < 0.01). At 0 to 24 d, the addition of phytase to the NC diet at all the levels reduced WI:FI of birds by 11.5% to 13% (P < 0.01), but WI:FI was not affected when phytase was added at any level to the PC diet.
3.3. Carcass yield and tibia bone characteristics
There was no effect of diet on the relative weight of the liver, spleen, bursa, small intestine, breast meat or abdominal fat (Table 7). On d 24, the addition of phytase to the diets increased relative weight of the spleen at 500 FTU/kg (P < 0.05) but there was no further increase in weight at the higher levels of phytase. The addition of phytase to the diets also decreased the relative weight of the small intestine at 1,000 and 1,500 FTU/kg by 8% on d 24 (P < 0.05) but there was no effect on d 35 (P > 0.05). Phytase tended to increase (P = 0.08) breast meat yield on d 35 across the diets and the increment was 4.94% at a level of 1,000 FTU/kg. Phytase had no effect on the relative weight of liver, bursa or abdominal fat. There was no interaction between diet and phytase on any carcass or visceral organ parameters measured (P > 0.05).
Table 7.
Carcass yield and relative weights of visceral organs (g/kg) of broilers fed nutritionally adequate (PC) and downspec (NC) wheat based diets supplemented with increasing levels of phytase.
| Treatments | Liver |
Spleen |
Bursa |
Small intestine1 |
Breast meat |
Abdominal fat |
|
|---|---|---|---|---|---|---|---|
| d 24 | d 24 | d 35 | d 35 | d 35 | |||
| Diets | |||||||
| NC | 26.2 | 0.79 | 1.77 | 31.3 | 21.3 | 201.2 | 9.65 |
| PC | 26.0 | 0.81 | 1.75 | 31.0 | 21.6 | 199.8 | 10.30 |
| SEM | 0.31 | 0.03 | 0.07 | 0.51 | 0.54 | 2.39 | 0.292 |
| Phytase, FTU/kg | |||||||
| 0 | 26.1 | 0.70b | 1.72 | 32.3a | 22.9 | 194.5 | 9.70 |
| 500 | 26.3 | 0.84a | 1.75 | 32.7a | 21.0 | 198.5 | 9.90 |
| 1,000 | 26.1 | 0.87a | 1.85 | 29.9b | 20.7 | 204.1 | 10.1 |
| 1,500 | 25.7 | 0.79ab | 1.74 | 29.9b | 21.2 | 205.0 | 10.2 |
| SEM | 0.44 | 0.04 | 0.10 | 0.75 | 0.76 | 3.68 | 0.41 |
| P-value | |||||||
| Diet | NS | NS | NS | NS | NS | NS | NS |
| Phytase | NS | <0.05 | NS | <0.05 | NS | 0.08 | NS |
| Diet × phytase | NS | NS | NS | NS | NS | NS | NS |
a,b Means in the same column with different superscripts differ significantly (P < 0.01).
Empty weight of duodenum, jejunum and ileum relative to live body weight.
The effect of graded levels of phytase on the length, weight, diameter and ash content of tibia bones of broilers are shown in Table 8. The birds fed the PC diet had an average of 1.4 mm longer tibias than those fed the NC diet (P < 0.01). Phytase increased tibia length by 2.7% and 3.4% when included in the diets at the levels of 500 and 1,000 FTU/kg, respectively (P < 0.01). There was a tendency of diet × phytase interaction (P = 0.06) on tibia length with a bigger response when phytase was added to the NC diet than to the PC diet. Neither diet nor phytase affected the diameter of the tibia bone (P > 0.05). There was an interaction between diet and phytase on percentage tibia ash (P < 0.001) and tibia ash weight (P < 0.05). The birds fed the NC diet without phytase had lower tibia ash weight (P < 0.05) and percentage tibia ash (P < 0.001) compared with those fed the PC diet without phytase (P < 0.001). The addition of phytase to the NC diet increased percentage tibia ash at all levels equivalent to that of birds fed the PC diet (P < 0.001) but there was no effect of phytase on percentage tibia ash when it was added to the PC diet. Phytase also increased tibia ash weight of birds when it was added to the NC diet at the levels of 1,000 or 1,500 FTU/kg (P < 0.05) but no such effect was observed when phytase was added to the PC diet.
Table 8.
Tibia bone characteristics of broilers fed nutritionally adequate (PC) and downspec (NC) wheat based diets supplemented with increasing levels of phytase (d 24).
| Treatments | Tibia bone characteristics |
|||
|---|---|---|---|---|
| Ash, % | Length, mm | Ash weight, g | Diameter, mm | |
| Diets | ||||
| NC | 49.4 | 71.4b | 1.36 | 6.1 |
| PC | 50.6 | 72.8a | 1.48 | 6.2 |
| SEM | 0.23 | 0.31 | 0.02 | 0.07 |
| Phytase, FTU/kg | ||||
| 0 | 48.3 | 70.6b | 1.30 | 6.2 |
| 500 | 50.0 | 72.5a | 1.37 | 6.1 |
| 1,000 | 50.5 | 73.0a | 1.52 | 6.3 |
| 1,500 | 51.0 | 72.2ab | 1.47 | 6.1 |
| SEM | 0.33 | 0.45 | 0.04 | 0.10 |
| Dietary treatments | ||||
| NC + 0 FTU/kg of phytase | 46.3b | 69.0 | 1.15b | 6.2 |
| NC + 500 FTU/kg of phytase | 49.5a | 71.5 | 1.33ab | 5.9 |
| NC + 1,000 FTU/kg of phytase | 50.6a | 72.9 | 1.52a | 6.3 |
| NC + 1,500 FTU/kg of phytase | 51.0a | 72.1 | 1.42a | 6.0 |
| PC + 0 FTU/kg of phytase | 50.2a | 72.2 | 1.45a | 6.2 |
| PC + 500 FTU/kg of phytase | 50.5a | 73.5 | 1.42a | 6.3 |
| PC + 1,000 FTU/kg of phytase | 50.5a | 73.1 | 1.53a | 6.3 |
| PC + 1,500 FTU/kg of phytase | 51.1a | 72.3 | 1.51a | 6.2 |
| SEM | 0.47 | 0.63 | 0.05 | 0.14 |
| P-value | ||||
| Diets | <0.001 | <0.01 | <0.001 | NS |
| Phytase | <0.001 | <0.01 | <0.001 | NS |
| Diet × phytase | <0.001 | NS | <0.05 | NS |
a,b Means in the same column with different superscripts differ significantly (P < 0.01).
3.4. Excreta and litter characteristics
The effect of diet or phytase on excreta moisture, pH, excreta free water and litter score is presented in Table 9. The moisture content of fresh excreta at d 18 was 11.3% higher than the excreta collected for 3 d from d 19 to 21 (P < 0.001). Neither diet nor phytase had an effect on excreta moisture content on d 18 or 21. There was a tendency for a phytase × diet interaction (P = 0.07) with the lowest value for excreta moisture found with the inclusion of 1,500 FTU/kg in the NC diet. Phytase at the level of 500 FTU/kg decreased excreta free water content by 32.5% (P < 0.05). The addition of phytase to the diets had no effect on excreta pH (P > 0.05) but the birds fed the NC diet produced 0.22 points lower excreta pH than those fed the PC diet (P < 0.01). There was an interaction between diet type and phytase on litter score at d 35 (P < 0.05). Phytase improved litter quality in the birds fed the NC diet but not those fed the PC diet, but NC diet fed birds produced poorer litter quality compared with the PC diet fed birds when phytase was not added.
Table 9.
Excreta characteristics of broilers fed nutritionally adequate (PC) and downspec (NC) wheat based diets supplemented with increasing levels of phytase on d 18 and d 19 to 21.
| Treatments | Excreta moisture1, % | Excreta pH2 | Excreta free water3, % | Litter score4 d 35 |
|---|---|---|---|---|
| Diet | ||||
| NC | 76.6 | 5.75b | 26.8 | 2.2 |
| PC | 76.7 | 5.97a | 27.3 | 1.9 |
| SEM | 0.38 | 0.05 | 1.08 | 0.1 |
| Phytase, FTU/kg | ||||
| 0 | 77.5 | 5.84 | 30.2a | 2.5 |
| 500 | 76.6 | 5.90 | 22.8b | 2.0 |
| 1,000 | 76.8 | 5.82 | 28.0ab | 1.6 |
| 1,500 | 75.8 | 5.88 | 27.2ab | 2.0 |
| SEM | 0.54 | 0.08 | 1.52 | 0.16 |
| Days | ||||
| 18 | 80.8a | |||
| 19 to 21 | 72.6b | |||
| SEM | 0.38 | |||
| Dietary treatments | ||||
| NC + 0 FTU/kg of phytase | 77.6 | 5.69 | 30.6 | 3.0a |
| NC + 500 FTU/kg of phytase | 77.6 | 5.76 | 24.4 | 2.0b |
| NC + 1,000 FTU/kg of phytase | 76.4 | 5.75 | 27.3 | 1.6b |
| NC + 1,500 FTU/kg of phytase | 74.8 | 5.80 | 24.9 | 2.0b |
| PC + 0 FTU/kg of phytase | 77.5 | 5.99 | 29.7 | 1.9b |
| PC + 500 FTU/kg of phytase | 75.5 | 6.05 | 21.2 | 2.1b |
| PC + 1,000 FTU/kg of phytase | 77.2 | 5.88 | 28.8 | 1.7b |
| PC + 1,500 FTU/kg of phytase | 76.8 | 5.95 | 29.6 | 2.1b |
| SEM | 0.75 | 0.10 | 1.27 | 0.22 |
| P-value | ||||
| Diet | NS | <0.01 | NS | 0.05 |
| Phytase | NS | NS | <0.05 | <0.01 |
| Days | <0.001 | |||
| Diet × phytase | 0.07 | NS | NS | <0.05 |
| Diet × days | NS | |||
| Phytase × days | NS | |||
| Diet × phytase × days | NS | |||
a,b Means in the same column with different superscripts differ significantly (P < 0.05).
Excreta collected over 3 d (d 19 to 21).
pH of excreta collected over 3 d (d 19 to 21).
Excreta collected every 2 h for 4 times on d 18.
Litter score from floor pens at d 35 (score 0 to 5). The lower score represents drier litter.
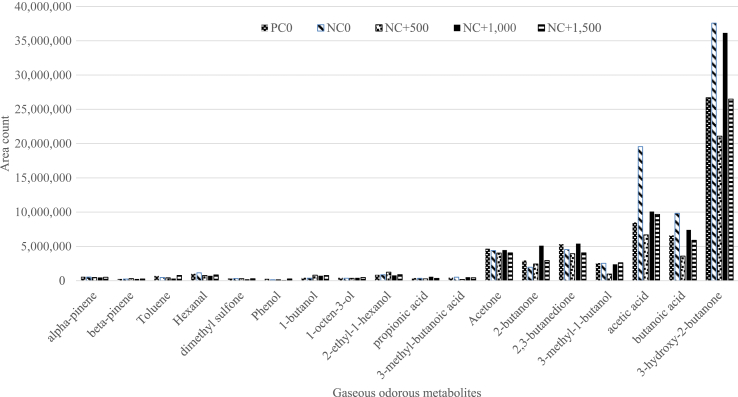
3.5. Volatile organic compound emissions measured by SPME-GC-MS
More than 50 VOC were detected with SPME-GC-MS measurements from the chamber but only 18 were consistently measured from all the treatments and are presented in Fig. 1. There was no effect of diet or phytase on the concentration of these VOC (P > 0.05). With the exception of dimethyl sulfone, no other sulfur compounds were detected by SPME-GC-MS technique. There was no effect of diet or phytase on the concentration of odorants such as dimethyl sulfone, 2,3-butanedione, 3-methyl-1-butanol, 3-hydroxy-2-butanone and 2-butanone (P > 0.05).
Fig. 1.
Effects of graded levels of phytase (0, 500, 1,000, 1,500 FTU/kg) on the downspec negative control (NC) compared with the positive control (PC) on gaseous odour emissions measured from the chamber with SPME-GC-MS on d 39.
4. Discussion
Although higher levels of phytase in diets have generally resulted in better performance in broilers (Walk et al., 2014), phytase has sometimes been associated with increased litter moisture and reduced litter quality. According to Debicki-Garnier and Hruby (2003), fungal phytase at 1,000 FTU/kg increased litter moisture at both 7 and 21 d post-hatch. The evidence of wet litter with the use of phytase is thought to be due to the inappropriate dietary formulation (Dunlop et al., 2016) that do not use appropriate matrix values for Ca, P and Na in the formulation of phytase supplemented diets. This will result in a higher concentration of these minerals in the gut lumen that makes the intestinal contents more hypertonic to blood and reduces water absorption, thereby resulting in the wet litter (van der Klis and Lensing, 2007). This experiment was conducted to study the effect of graded levels of phytase on performance, water to feed intake ratio, litter quality and odour emissions from broilers when it was added over the top of the diet or into the formulation with nutrient matrix values.
Increased Ca: Av. P has been shown to reduce phytate degradation and P digestibility (Amerah et al., 2014) due to the formation of Ca-phytate complex and increase in pH of the proximal digestive tract (Selle et al., 2009). In the current study, the NC diet with no phytase had wide Ca:av. P and the birds fed NC diet had highest WI: FI compared with those fed other diets. This is likely due to birds drinking more water in an attempt to dilute the higher solute concentration in the gut lumen or perhaps to remove the Ca-phytate complex that may have irritating characteristics. Phytate can also bind with protein in the diet (Cowieson et al., 2006, Selle et al., 2012) making amino acids unavailable for digestion. Both a mineral imbalance and reduction in protein digestion may lead to compromised gut integrity and reduced net water absorption from the GI tract. This increased water intake and output thus affects litter quality (van der Klis and de Lange, 2013). The addition of phytase to the NC diet released Av. P thereby restoring Ca:av. P balance and removing Ca-phytate complex. In addition, it is possible that the cell wall architecture around the aleurone layer is broken down by phytase resulting in improved digesta flow and digestibility of nutrients. This would allow more efficient recycling of water in the large intestine and caeca causing birds to consume less water per unit of feed intake.
The highest WI:FI in birds fed the NC diet without phytase in this study also produced poor litter quality with higher litter score compared with all other treatments. The NC diet contained more Ca from limestone than dicalcium phosphate as compared with the PC diet. There is a debate to whether limestone and dicalcium phosphate as Ca sources have any relation with wet litter (Dunlop et al., 2016) but so far there is no valid conclusion on this. High Ca in broiler diets has been reported to deteriorate litter quality in the previous study (Enting et al., 2009). It has been suggested that litter quality can be improved by lowering Ca (Pos et al., 2003); Ca, available P and Na (Bedford and Walk, 2015) in diets when phytase is used. The improved litter score with the addition of phytase in the NC diet (with low Ca, available P and Na) in the current study is in agreement with the above studies.
The current study shows that the effect of phytase is much greater in low av. P diets using a nutrient matrix to formulate feed as compared with adding over the top of an already av. P sufficient diet. While small performance benefits may be observed when adding phytase to av. P sufficient diets, the benefits are not economically sound. This is evident by the results on d 24 and again on d 35 where phytase increased BW gain by a small margin when added over the top of the PC diet but markedly when added to the NC diet at 500, 1,000 or 1,500 FTU/kg, respectively. These results show that phytase is more effective with a lower than higher av. P level in diets to improve FI and BW gain of broilers. Similar to our findings, Walk et al. (2013) reported the extra-phosphoric effects of using higher levels of phytase to a downspec diet and this was thought to be due to the mitigation of anti-nutritional effects of phytate rather than improved P utilization. Shirley and Edwards (2003) also reported performance benefits of using higher levels of phytase to nutritionally marginal diets. The lower tibia ash weight and tibia ash as a percent of body weight in the birds fed the NC diet compared with those fed the PC diet and the subsequent improvements after addition of phytase to the NC diet but not to the PC diet observed in this study were similar to the previous findings (Dilger et al., 2004, Walk et al., 2013). This suggests that any amount of P released in the gut by degradation of phytate in the birds fed nutritionally adequate diet with phytase would not deposit in the bone but may get excreted.
The lower weight of small intestine on d 24 with the addition of 1,000 or 1,500 FTU/kg phytase in diet observed in this study is similar to the finding by Wu et al. (2015). It represents a more efficient intestine that uses less energy for maintenance and thus the surplus energy may be utilized for growth and absorption of nutrients. This finding may be correlated with the low FCR observed in the birds on d 24 when they were fed diets with 1,000 or 1,500 FTU/kg phytase. A recent report suggests that higher levels of phytase may modulate ileal microbiota in broiler chickens (Ptak et al., 2015). It may be possible that the addition of higher levels of phytase decrease the undesirable microbial load and thus the amount of inflammation in the gut and its size.
It has been reported that high litter moisture favors the emission of odorous metabolites such as methyl mercaptan, hydrogen sulfide, dimethyl sulfide, ammonia, trimethyl amine, phenol, indole, and 3-methyl-indole (Sharma et al., 2016). In the current study, dietary phytase did not reduce excreta moisture, however, litter score was improved by phytase. This was expected to reduce emissions of these odorous metabolites from the litter. Further, the possible increased amino acid digestibility by the application of higher levels of phytase (Amerah et al., 2014) was expected to reduce the amino acid fermentation products in the litter. Most of the sulfurous odorants such as methyl mercaptan, dimethyl sulfide and hydrogen sulfide mostly originate from sulfur-containing amino acids (methionine and cysteine) in excreta (Mackie et al., 1998). Similarly, phenol and cresol originate from microbial degradation of L-tyrosine; indole and skatole originate from microbial fermentation of L-tryptophan; amines and ammonia are produced by decarboxylation and deamination of amino acids (Mackie et al., 1998). A range of VOC was measured in this study that belonged to the group of volatile fatty acids, alcohols, aldehydes, ketones and phenols. Among these VOC, the important odorous ones included diacetyl, 3-methyl-1-butanol, 3-hydroxy-2-butanone, 2-butanone, acetic acid, propionic acid, butyric acid and phenol. These compounds are carbohydrate fermentation products and can affect the environment surrounding the broiler chicken sheds (Mackie et al., 1998, Wadud, 2011). However, with the SPME-GC-MS method used in this study, neither diet nor phytase affected the concentration of these odorants. This perhaps indicated that phytase did not have a profound effect on the digestion of carbohydrate rich substrates. However, the SPME-GC-MS technique may not be as sensitive as SIFT-MS to measure the odorants in broilers. Recently, differences in headspace concentrations of odorants between litters varying in moisture content were found to be significant using SIFT-MS (Sharma et al., 2016). The SPME-GC-MS technique used in this study was also unable to measure sulfurous compounds such as mercaptans that are considered significant contributors of odour.
5. Conclusion
The results indicate that phytase has greater performance benefits when formulated using nutrient matrix values as compared with adding it over the top in an already nutrient sufficient diet. The later method would be expected to increase feed costs without concomitant performance benefits. While litter quality was improved by phytase in the downspec diet, a reduction in odour from birds and litter could not be detected using the SPME-GC-MS technique. Further work is warranted to study the effect of phytase enzyme on odour emissions using instrumentation designed to detect sulfurous compounds more highly associated with smells from broiler farms.
Acknowledgements
This research was conducted within the Poultry CRC, established and supported under the Australian Government's Cooperative Research Centres Program and is a part of Poultry CRC sub project grant 2.2.8. The authors would like to thank Dr. Tim Walker from Poultry CRC for providing valuable suggestions and staff of Animal House at the University of New England, Australia for their helping hands during this project.
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- Amerah AM, Plumstead P.W., Barnard L.P., Kumar A. Effect of calcium level and phytase addition on ileal phytate degradation and amino acid digestibility of broilers fed corn-based diets. Poult Sci. 2014;93:906–915. doi: 10.3382/ps.2013-03465. [DOI] [PubMed] [Google Scholar]
- Anderson D.L., Henderson L.J. Sealed chamber digestion for plant nutrient analysis. Agron J. 1986;78:937–938. [Google Scholar]
- AOAC . 15th ed. Assoc Off Anal Chem; Arlington, VA: 1990. Official methods of analysis. [Google Scholar]
- AOAC . 18th ed. Assoc Off Anal Chem; Arlington, VA: 2005. Official methods of analysis. [Google Scholar]
- Aviagen . 2014. Ross broiler management manual.http://pt.aviagen.com/assets/Tech_Center/Ross_Broiler/Ross_Broiler_Manual [Google Scholar]
- Bedford M.R., Walk C.L., Kuhn I. Is phytase responsible for increasing the water:feed intake ratio? Poult Sci. 2012;91(Suppl. 1):18. [Google Scholar]
- Bedford M.R., Walk C.L. Superdosing phytase in wheat-based diets improves litter and foot pad score whilst simultaneously improving performance. Proc Aust Poult Sci Symp. 2015;26:193. [Google Scholar]
- Collett S.R. Nutrition and wet litter problems in poultry. Anim Feed Sci Tech. 2012;173:65–75. [Google Scholar]
- Cowieson A.J., Acamovic T., Bedford M.R. Phytic acid and phytase: implications for protein utilization by poultry. Poult Sci. 2006;85:878–885. doi: 10.1093/ps/85.5.878. [DOI] [PubMed] [Google Scholar]
- Cowieson A.J., Wilcock P., Bedford M.R. Super-dosing effects of phytase in poultry and other monogastrics. W Poult Sci J. 2011;67:225–236. [Google Scholar]
- Debicki-Garnier A.M., Hruby M. Proc 14th Eur Symp Poult Nutr WPSA, Norway. 2003. The effect of phytase and betaine on broiler performance and excreta characteristics; pp. 14–15. [Google Scholar]
- Dilger R.N., Onyango E.M., Sands J.S., Adeola O. Evaluation of microbial phytase in broiler diets. Poult Sci. 2004;83:962–970. doi: 10.1093/ps/83.6.962. [DOI] [PubMed] [Google Scholar]
- Dunlop M.W., Moss A.F., Groves P.J., Wilkinson S.J., Stuetz R.M., Selle P.H. The multidimensional casual factors of 'wet litter' in chicken-meat production. Sci Tot Environ. 2016;562:766–776. doi: 10.1016/j.scitotenv.2016.03.147. [DOI] [PubMed] [Google Scholar]
- Enting H., del los Mozos J., Gutiérrez del Álamo A., Pérez de Ayala P. 17th Eur Symp Poult Nutr WPSA, Edinburgh, UK. 2009. Influence of minerals on litter moisture; pp. 47–52. [Google Scholar]
- Hall L.E., Shirley R.B., Bakalli R., Aggrey S., Pesti G., Edwards H. Power of two methods for the estimation of bone ash of broilers. Poult Sci. 2003;82:414–418. doi: 10.1093/ps/82.3.414. [DOI] [PubMed] [Google Scholar]
- Himathongkham S., Nuanualsuwan S., Riemann H. Survival of Salmonella enteritidis and Salmonella typhimurium in chicken manure at different levels of water activity. FEMS Micro Lett. 1999;172:159–163. doi: 10.1111/j.1574-6968.1999.tb13464.x. [DOI] [PubMed] [Google Scholar]
- Mackie R.I., Stroot P.G., Varel V.H. Biochemical identification and biological origin of key odour components in livestock waste. J Anim Sci. 1998;76:1331–1342. doi: 10.2527/1998.7651331x. [DOI] [PubMed] [Google Scholar]
- Miles D.M., Rowe D.E., Cathcart T.C. Litter ammonia generation: moisture content and organic versus inorganic bedding materials. Poult Sci. 2011;90:1162–1169. doi: 10.3382/ps.2010-01113. [DOI] [PubMed] [Google Scholar]
- Murphy K.R., Parcsi G., Stuetz R.M. Non-methane volatile organic compounds predict odour emitted from five tunnel ventilated broiler sheds. Chemosphere. 2014;95:423–432. doi: 10.1016/j.chemosphere.2013.09.076. [DOI] [PubMed] [Google Scholar]
- Pos J., Enting H., Veldman A. Proc 14th Eur Symp Poult Nutr WPSA Norway. 2003. Effect of phytase and dietary calcium level on litter quality and broiler performance; pp. 17–18. [Google Scholar]
- Ptak A., Bedford M.R., Swiatkiewicz S., Zyla K., Jozefiak D. Phytase modulates ileal microbiota and enhances growth performance of the broiler chickens. PLoS ONE. 2015;10(3):e0119770. doi: 10.1371/journal.pone.0119770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selle P.H., Cowieson A.J., Cowieson N.P., Ravindran V. Protein–phytate interactions in pig and poultry nutrition: a reappraisal. Nutr Res Rev. 2012;25:1–17. doi: 10.1017/S0954422411000151. [DOI] [PubMed] [Google Scholar]
- Selle P.H., Cowieson A.J., Ravindran V. Consequences of calcium interactions with phytate and phytase for poultry and pigs. Liv Sci. 2009;124:126–141. [Google Scholar]
- Selle P.H., Ravindran V. Microbial phytase in poultry nutrition. Anim Feed Sci Technol. 2007;135:1–41. [Google Scholar]
- Sharma N.K., Choct M., Wu S., Smillie R., Swick R.A. Dietary composition affects odour emissions from meat chickens. Anim Nutr. 2015;1:24–29. doi: 10.1016/j.aninu.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma N.K., Choct M., Dunlop M.W., Wu S., Castada H.Z., Swick R.A. Characterisation and quantification of changes in odorants from litter headspace of meat chickens fed diets varying in protein levels and additives. Poult Sci. 2016 doi: 10.3382/ps/pew309. [in press] [DOI] [PubMed] [Google Scholar]
- Shelton J.L., Southern L.L., Gaston L.A., Foster A. Evaluation of the nutrient matrix values for Phytase in Broilers. J Appl Poult Res. 2004;13:213–221. [Google Scholar]
- Shirley R., Edwards H. Graded levels of phytase past industry standards improves broiler performance. Poult Sci. 2003;82:671–680. doi: 10.1093/ps/82.4.671. [DOI] [PubMed] [Google Scholar]
- van der Hoeven-Hangoor E., Paton N.D., van de Linde I.B., Verstegen M.W.A., Hendriks W.H. Moisture content in broiler excreta is influenced by excreta nutrient contents. J Anim Sci. 2013;91(12):5705–5713. doi: 10.2527/jas.2013-6573. [DOI] [PubMed] [Google Scholar]
- van der Hoeven-Hangoor E., Rademaker C.J., Paton N.D., Verstegen M.W.A., Hendriks W.H. Evaluation of free water and water activity measurements as functional alternatives to total moisture content in broiler excreta and litter samples. Poult Sci. 2014;93:1782–1792. doi: 10.3382/ps.2013-03776. [DOI] [PubMed] [Google Scholar]
- van der Klis J.D., de Lange L.O.E.K. 19th Eur Symp Poult Nutr. 2013. Water intake of poultry.http://www.psa.com/proceedings/ESPN_2013/assets/html/318.htm [Google Scholar]
- van der Klis J.D., Lensing M. Wet litter problems relate to host-microbiota interactions. World Poult. 2007;23(8):20–22. [Google Scholar]
- Wadud S., Michaelsen A., Gallagher E., Parcsi G., Zemb O., Stuetz R.M. Bacterial and fungal community composition over time in chicken litter with high or low moisture content. Br Poult Sci. 2012;53:561–569. doi: 10.1080/00071668.2012.723802. [DOI] [PubMed] [Google Scholar]
- Wadud S. University of New South Wales; Australia: 2011. Understanding the microbial ecology of chicken litter in the context of odour production. (PhD thesis) [Google Scholar]
- Walk C.L., Bedford M.R., Santos T.S., Paiva D., Bradley J.R., Wladecki H. Extra-phosphoric effects of superdoses of a novel microbial phytase. Poult Sci. 2013;92:719–725. doi: 10.3382/ps.2012-02727. [DOI] [PubMed] [Google Scholar]
- Walk C.L., Santos T.T., Bedford M.R. Influence of superdoses of a novel microbial phytase on growth performance, tibia ash, and gizzard phytate and inositol in young broilers. Poult Sci. 2014;93:1172–1177. doi: 10.3382/ps.2013-03571. [DOI] [PubMed] [Google Scholar]
- Woyengo T.A., Nyachoti C.M. Review: anti-nutritional effects of phytic acid in diets for pigs and poultry – current knowledge and directions for future research. Can J Anim Sci. 2013;93:9–21. [Google Scholar]
- Wu D., Wu S.B., Choct M., Swick R.A. Comparison of 3 phytases on energy utilization of a nutritionally marginal wheat-soybean meal broiler diet. Poult Sci. 2015;94:2670–2676. doi: 10.3382/ps/pev222. [DOI] [PubMed] [Google Scholar]