Abstract
Broilers that have early access to feed have been shown to have enhanced immune system and gut development and heightened resilience against necrotic enteritis (NE). This study examined the effect of early feeding a high amino acid density diet on performance of broilers under a sub-clinical NE challenge model. Ross 308 broilers (n = 576) were assigned to a 2 × 2 × 2 factorial design with 2 feeding regimes (feed access either within 6 h post-hatch or after 48 h post-hatch), 2 diets (control diet or the control diet with an additional 10% digestible amino acids [HAA]) and either presence or absence of NE challenge. Oral administrations of Eimeria species (d 9) and a field strain of Clostridium perfringens (d 14) were used to induce NE. Broiler performance was analysed at d 13, 23, 30 and 35. Intestinal lesion score and bacterial count were analysed on d 16. The NE challenge reduced overall bird performance and induced severe intestinal lesions, without causing notable mortality. At d 23 bird weight was significantly lower (P < 0.001) in the challenged birds compared with the unchallenged birds, but by d 30 the challenged birds had recovered and challenge no longer had an impact on bird performance. Birds fed the HAA diet had greater body weight by d 35 and heightened Lactobacillus content in the ileum at d 16 (P < 0.05). Birds that were fed the HAA diet after a period of fasting performed better in terms of feed conversion ratio (FCR) under challenge. The findings from this study suggest there are beneficial effects of feeding high amino acid diets to birds in response to external stresses, such as post-hatch fasting and subclinical NE.
Keywords: Early feeding, Amino acid, Necrotic enteritis, Broiler chicken
1. Introduction
Necrotic enteritis (NE) is a multifactorial, bacterial borne enteric disease that causes devastating losses to poultry flocks (up to 30% mortality in an infected flock), costing approximately US$2 billion per annum worldwide (Dahiya, 2006, Van Immerseel et al., 2009, Wade and Keyburn, 2015). The causative agent is Clostridium perfringens; a Gram-positive, anaerobic, rod spore-forming bacterium that is ubiquitous and is capable of producing a variety of extracellular toxins and invasive enzymes (Gibert et al., 1997, Keyburn et al., 2010). Its pathogenic state can be triggered from predisposing factors that disturb the integrity of the gut mucosa; such as increased viscosity of intestinal digesta, high level of protein in the diet and coccidiosis infection (Collier et al., 2008, Rodgers et al., 2014). The clinical form of NE results in significant necrosis of the small intestine and hence catastrophic levels of mortality (Kaldhusdal and Løvland, 2000, Van Immerseel et al., 2009). The subclinical form exhibits less obvious signs, with initial symptoms of just slightly reduced growth due to impaired nutrient utilisation from damaged intestinal mucosa. As a result there is often delayed onset of effective treatment and spreading amongst the flock, resulting in a substantial loss to production (Skinner et al., 2010, Van der Sluis, 2000).
C. perfringens strains have been shown to be susceptible to various anti-microbial drugs in both in vitro and in vivo studies (Devriese et al., 1993, Geier et al., 2010). However, worldwide concerns about the emergence of antimicrobial resistant bacteria strains mean that alternatives to in-feed antibiotics in animal production, that can both combat bacterial infections and replace antibiotic growth promoters, are desperately being sought after (Bedford, 2000, Choct, 2001, Dibner and Richards, 2005, Ferket, 2004, Huyghebaert, 2011). Replacements for antibiotics that can reduce the prevalence of C. perfringens induced NE must therefore be established.
Bacterial pathogens in the gut thrive in the presence of imbalanced feed compositions. For example, a diet with high crude protein or soluble non-starch polysaccharides causes increased gastrointestinal transit time, which induces mass proliferation of opportunistic C. perfringens in the microflora (Annett et al., 2002, Choct and Annison, 1992, Moore, 2016, Riddell and Kong, 1992, Tech, 1999, Wu et al., 2014). A refined nutritional formula that makes the broiler gut environment less susceptible to C. perfringens domination is therefore critical for maintaining optimal health and performance in broiler chickens and reducing production costs (Croom et al., 2000, Dahiya, 2006, Moore, 2016, Van Immerseel et al., 2009).
The amount of time between hatch and access to feed has been shown to greatly influence survivability and performance in young birds (Geyra et al., 2001). Due to the nature of the broiler industry, birds often do not get access to water and feed for up to 48 h post-hatch (Dibner et al., 1998). Early nutrient availability, especially access to essential amino acids, could play a significant role in immune development (Ao et al., 2002, Kidd, 2004, Li et al., 2007). Ao et al. (2002) reported that birds that had early access to feed and water post-hatch had improved immune development and better performance in the presence of NE than birds that had been fasted for 48 h post-hatch. Essential amino acids have been shown to be important components in development of the immune system in birds and amino acid utilisation is prioritised towards tissues involved in immune response and inflammation (Li et al., 2007, Le Floc'h et al., 2004). Kidd et al. (2004) reported that healthy broilers responded positively to high dietary amino acid inclusion, and the positive effect on performance was more evident in birds exposed to the dietary treatment immediately post-hatch. The impact of amino acid supplementation in young broilers is still however under debate and the relationship between amino acids and NE is poorly understood. The aim of this study was to examine the benefit of early access to feed and of feeding starter diets with a high amino acid density on the response of birds to NE challenge.
2. Materials and methods
2.1. Experimental design and feeding treatments
A total of 576 day-old male broiler chickens (Ross 308) were procured from Baiada Country Road Hatchery (Tamworth, NSW, Australia) at the day of hatch. Chicks were randomised by weight and placed in floor pens (approx. area 120 cm × 75 cm per pen), bedded on clean wood shavings. Birds were vaccinated against Marek's disease, infectious bronchitis, and Newcastle disease at the hatchery before arrival. The lighting regimen used was 23, 18 and 23 h of light during days 0 to 24, 25 to 30 and 31 to 35, respectively. The floor bedding temperature was maintained at 34 to 35 °C from d 0 to 3 and was then gradually decreased by 3 °C per week onward until 22 to 24 °C was reached by d 21. The rearing facility at the University of New England meets the Australian standards, and the area was sterilised before bird arrival. All experimental procedures involved in this study were approved by the Animal Ethics Committee of the University of New England. The study had a 2 × 2 × 2 factorial design, resulting in 8 treatments; 2 feeding regimes of either immediate access to feed (FED) or access to feed delayed by 48 h post-hatch (HELD), 2 starter diets with (control) or without amino acid density 10% above the recommended level (HAA) and either exposed to NE challenge or not (Challenged or Unchallenged). Birds were allocated to 48 pens with 12 birds per pen and 6 replicates per treatment (72 birds/treatment). Birds were evenly distributed to ensure that there were no statistical differences between initial starting pen weights.
Birds in the FED treatment group had ad libitum access to feed on arrival (within 6 h post-hatch), whereas the birds in the HELD treatment group had access to only water on arrival and feed was then introduced after 48 h post-hatch. The starter diets were fed as crumble until d 7 and then as pellet (⌀2 to 3 mm). The diets were wheat, sorghum, meat meal and soybean based and were formulated based on Aviagen (2012) nutritional specification guidelines. The HAA starter diet was the same as the control diet but with an additional 10% digestible amino acids (Table 1). From d 13 all birds were fed the same grower diet until d 24 and then the same finisher diet from d 24 to 35, both fed as pellets (⌀3 to 3.5 mm).
Table 1.
Starter diet formulation and nutrient composition.
| Item | Control | HAA |
|---|---|---|
| Ingredient, % | ||
| Wheat | 30 | 30 |
| Sorghum | 26.6 | 21 |
| Soy bean meal | 30.1 | 36.1 |
| Canola meal solvent extracted | 3 | 1 |
| Meat and bone meal | 2 | 3.8 |
| Canola oil | 4.3 | 4.7 |
| Limestone | 1.2 | 1 |
| Dical Phos 18P/21Ca | 1,476 | 1,089 |
| NaCl | 0.128 | 0.101 |
| Na bicarb | 0.2 | 0.2 |
| UNE vitamin premix1 | 0.05 | 0.05 |
| UNE trace mineral premix2 | 0.075 | 0.075 |
| Choline Cl 70% | 0.038 | 0.045 |
| Analysed composition, % | ||
| ME, kcal/kg | 3,025 | 3,027 |
| Crude protein | 22.94 | 25.34 |
| Crude fat | 6.21 | 6.65 |
| Crude fibre | 3.00 | 3.03 |
| Digestible amino acids | ||
| Isoleucine | 1.01 | 1.11 |
| Arginine | 1.34 | 1.52 |
| L-lysine | 1.27 | 1.40 |
| DL-methionine | 0.60 | 0.67 |
| Methionine + cystine | 0.94 | 1.03 |
| Tryptophan | 0.23 | 0.26 |
| L-threonine | 0.83 | 0.91 |
| Valine | 0.94 | 1.03 |
HAA = high amino acid starter diet (10% more essential amino acid over the Aviagen recommendations); UNE = University of New England.
Vitamin premix per kg contains: vitamin A, 12 MIU; vitamin D, 5 MIU; vitamin E, 75 mg; vitamin K, 3 mg; nicotinic acid, 55 mg; pantothenic acid, 13 mg; folic acid, 2 mg; riboflavin, 8 mg; cyanocobalamin, 0.016 mg; biotin, 0.25 mg; pyridoxine, 5 mg; thiamine, 3 mg; antioxidant, 50 mg.
Mineral premix per kg contains: Cu, 16 mg as copper sulphate; Mn, 60.
2.2. Necrotic enteritis challenge
On day 9, each bird in the NE-challenge group was given 1 mL per os vaccine strain of Eimeria (Bioproperties Pty Ltd., Sydney, Australia). Each 1 mL gavage included phosphate buffered saline (PBS) suspension of approximately 5,000 oocysts each of Eimeria acervulina and Eimeria maxima, and 2,500 oocysts of Eimeria brunetti. To the unchallenged group, 1 mL of sterile PBS was administered as a stress treatment. On d 14 and 15, a field strain of C. perfringens EHE-NE18 producing a netB toxin (approx. 108 cfu/mL) (CSIRO Livestock Industries, Geelong, Victoria, Australia) in 2 mL thioglycolate broth was administered per os per bird. A sterile thioglycolate broth was administered to the non-challenged group. The C. perfringens count in the inoculant had been quantified on perfringens tryptose-sulfite-cy-closerine (TSC) selective agar (Oxoid) following serial dilutions.
2.3. Parameters analysed
The parameters analysed in the present study were bird weight, feed intake, feed conversion ratio, intestinal lesion score and intestinal bacteria count.
2.3.1. Bird weight, feed intake and feed conversion ratio
Pen weight and cumulative pen feed intake were recorded on days 0, 13, 23, 30 and 35 and used to calculate mean bird weight, feed intake and FCR (corrected for mortality).
2.3.2. Post-mortem and lesion scoring
On day 16, two birds were randomly selected from each pen and euthanised by cervical dislocation. The duodenal loop, the jejunum (from the end of the duodenal loop to the Meckel's diverticulum) and the ileum (from the Meckel's diverticulum to the ileo-caeco-colonic junction) were excised. Caecal and ileal digesta were collected into sterile 30 mL sample tubes, one tube per bird per section of gastrointestinal tract. The entire length of the section of small intestine underwent a lesion scoring process, based on a previously reported lesion scoring system that ranges from 0 to 4 (Broussard et al., 1986, Prescott et al., 1978). Score 0 referred to intestine of healthy appearance, 1 referred to gas-filled intestine with evidence of at least 2 necrotic lesions, 2 referred to ballooned, friable, foul-smelling intestine with evidence of necrotic lesions, 3 referred to intestines that displayed all the above along with a yellow pseudomembrane (often described as having a “Turkish towel” or flannelette blanket-like appearance) and 4 referred to prevalence of above description with ruptures of the intestinal epithelial layer, blood filled intestine or multiple petechial haemorrhages. Three experienced personnel, with no knowledge of the trial design, were involved in the scoring process.
2.3.3. Enumeration of bacteria using quantitative PCR
To quantitatively measure intestinal bacterial population, a section of the ileum (approximately 3 cm long), directly adjacent to the Meckel's diverticulum, was excised. And 1 g of digesta from this section of ileum was aseptically transferred into a 2 mL Eppendorf safe-lock tube, snap-frozen in liquid nitrogen and stored at −20 °C until DNA extraction was performed. PCR amplification of 16S ribosomal DNA was used to determine the chromosomal DNA counts of the subject bacterium. Total gut bacterium, Lactobacillus spp., C. perfringens and Enterobacteria spp. were quantified. Template DNA samples were prepared from the ileal digesta using Bio line Isolate II Plant DNA Kit. Approximately 200 mg of ileal digesta was accurately weighed and vigorously shaken with 0.2 g of ⌀0.1 mm glass beads prior to the extraction step. For DNA preparation of the caecal digesta, 60 mg of caecal digesta was processed by a Qiaxtractor automated DNA extractor robot (Qiagen, Australia). A NanoDrop ND-8000 UV spectrophotometer was used to assess the DNA purity (Thermo Fisher Scientific, Waltham, USA). Only DNA elutions emitting ratios of between 1.6 and 1.8 in 260/280 nm wavelength were used in following analysis. The quantitative PCR analysis was performed on a Rotorgene-6500 real-time PCR machine (Corbett, Sydney, Australia). A total volume of 10 μL was used in each PCR reaction, with duplicate reactions of each sample. SensiMix SYBR No-ROX (Bioline, Meridian Life Science, Memphis, USA) was used to amplify the 16S ribosomal DNA for analysis of the total bacteria, Lactobacillus spp. and Enterobacteria spp. The annealing primers involved were maintained at a concentration of 300 nm (total bacteria: 5′-CGG YCC AGA CTC CTA CGG G-3′ and 5′-TTA CCG CGG CTG CTG GCA C-3′; Lactobacillus: 5′-CAC CGC TAC ACA TGG AG-3′ and 5′-AGC AGT AGG GAA TCT TCC A-3′; Enterobacteria: 5′-CAT TGA CGT TAC CCG CAG AAG-3′ and 5′-CTC TAC GAG ACT CAA G-3′) (Bartosch et al., 2004, Lee et al., 1996). SensiFAST SYBR NO-ROX (Bioline, Meridian Life Science, Memphis, USA) and TaqMan probe were used in the qPCR assay to quantify 16S ribosomal DNA of C. perfringens (5′-GCA TAA CGT TGA AAG ATG G-3′ and 5′-CCT TGG TAG GCC GTT ACC C-3′). Serial dilutions of linearised plasmid DNA (pCR4-TOPO Vector, Life Technologies, Carlsbad, USA) inserted with respective amplicons were used to construct a standard curve. A threshold cycle average from the replicate samples was assigned for quantification analysis. The number of target DNA copies was calculated from the mass of the DNA, taking into account the size of the amplicon insert in the plasmid. Mean value of replicates were calculated. While the bacterial numbers were expressed as log10 genomic DNA copy number per gram of digesta (wet weight).
2.3.4. Statistical analysis
All data were analysed using the JMP 11 statistical programme (SAS Institute Inc. Cary, NC). FCR calculations took into account the weights of sample birds and mortalities. Data were analysed by ANOVA with treatment as the individual variance. When differences among variances were significant, the Duncan's multiple range test was then used to compare the means. Statistical significance was declared at P ≤ 0.05.
3. Results
3.1. Bird performance
Total mortality in this trial was 3.5%, but none of the observed mortalities were induced by the presence of the NE challenge, based on necropsy analysis. As illustrated in Table 2, birds fed the HAA diet had significantly improved BW at d 13 (P < 0.001), d 30 (P = 0.029) and d 35 (P = 0.005) compared with birds fed the control diet. An interaction between challenge and regime was observed at d 13 (P < 0.001), showing that the challenged birds fed immediately post-hatch had the heaviest body weights, followed by the unchallenged birds that were fasted post-hatch. At d 30, birds offered the diets immediately post-hatch had lower body weight (P = 0.007) compared with those that were not offered feed until 48 h post-hatch. Birds from the unchallenged group were well feathered, whereas birds that were subjected to the NE challenge had ruffled feathers. A regime × challenge interaction suggested that FED and challenged combination effect produced the heaviest BW while the FED and non-challenge combination produced the lightest BW (data not shown). Significant interactions between regime and diet (P = 0.014) and challenge and diet (P = 0.045) were observed at d 23. Birds fed the HAA diet after fasting or the control diet immediately post-hatch had heavier body weights compared with the other treatments, and birds fed the control diet after fasting had the lowest average body weight. By d 30, the effect of NE challenge on BW was diminished. At d 35, main effect of HAA diet yielded higher body weights compared with those of the control diet. Also a significant (P = 0.020) full factorial interaction between challenge, regime and diet was observed at d 35. This interaction showed that body weight was lower in the unchallenged birds fed the control diet immediately post-hatch compared with the unchallenged birds fed either diet after fasting, the challenged birds fed the HAA diet after fasting or control diet immediately post-hatch.
Table 2.
Interaction of necrotic enteritis challenge, feeding regime and diet on broiler body weight.1
| Treatments | Day 13 | Day 23 | Day 30 | Day 35 | ||
|---|---|---|---|---|---|---|
| Unchallenged | HELD | Control | 489.0 ± 8.2 | 1,301.0 ± 18.4 | 2,022.3 ± 31.2 | 2,586.2 ± 44.0 abc |
| HAA | 518.8 ± 15.4 | 1,375.2 ± 15.3 | 2,103.8 ± 29.8 | 2,667.7 ± 39.0 abc | ||
| FED | Control | 464.2 ± 16.6 | 1,221.5 ± 49.3 | 1,883.8 ± 74.8 | 2,434.8 ± 75.0d | |
| HAA | 492.0 ± 12.4 | 1,282.5 ± 35.6 | 1,989.2 ± 57.1 | 2,624.0 ± 54.1 abc | ||
| Challenged | HELD | Control | 476.8 ± 8.7 | 1,192.8 ± 16.4 | 1,961.2 ± 23.5 | 2,494.7 ± 45.8 cd |
| HAA | 513.2 ± 2.8 | 1,183.7 ± 15.5 | 2,110.2 ± 31.2 | 2,685.2 ± 29.4 abc | ||
| FED | Control | 563.5 ± 16.6 | 1,218.0 ± 24.3 | 2,012.5 ± 28.3 | 2,585.5 ± 42.3 abc | |
| HAA | 594.5 ± 12.5 | 1,194.3 ± 34.9 | 1,958.7 ± 49.2 | 2,540.3 ± 56.1 bcd | ||
| Mean of main effects | ||||||
| Unchallenged | 490.2 ± 6.2b | 1,293.1 ± 15.0a | 2,000.9 ± 23.7 | 2,578.1 ± 26.4 | ||
| Challenged | 542.9 ± 6.7a | 1,196.8 ± 16.1b | 2,011.0 ± 25.4 | 2,560.0 ± 28.3 | ||
| HELD | 501.2 ± 6.2b | 1,270.0 ± 15.0 | 2,062.7 ± 23.7a | 2,613.6 ± 26.4 | ||
| FED | 531.9 ± 6.7a | 1,219.9 ± 16.1 | 1,949.3 ± 25.4b | 2,524.5 ± 28.3 | ||
| CONTROL | 504.9 ± 6.9b | 1,239.1 ± 16.5 | 1,983.4 ± 26.1b | 2,524.3 ± 29.1b | ||
| HAA | 528.2 ± 6.0a | 1,250.8 ± 14.5 | 2,028.6 ± 23.0a | 2,613.9 ± 25.6a | ||
| P-value | ||||||
| Challenge | <0.001 | <0.001 | 0.729 | 0.961 | ||
| Regime | 0.002 | 0.101 | 0.007 | 0.085 | ||
| Diet | 0.001 | 0.215 | 0.029 | 0.005 | ||
| Challenge × regime | <0.001 | 0.014 | 0.226 | 0.324 | ||
| Challenge × diet | 0.786 | 0.045 | 0.466 | 0.380 | ||
| Regime × diet | 0.837 | 0.735 | 0.158 | 0.370 | ||
| Challenge × regime × diet | 0.925 | 0.987 | 0.076 | 0.020 | ||
HAA = high amino acid starter diet (10% more essential amino acid over the Aviagen recommendations); FED = access to feed with 6 h post-hatch; HELD = access to feed after 48 h post-hatch; Challenge = necrotic enteritis induced by oral administrations of Eimeria species and a field strain of Clostridium perfringens.
a, b, c, d Means within the same column with no common superscript differ significantly (P ≤ 0.05).
Means represent the average individual body weight of 12 birds per pen, 6 pens per treatment (72 birds/treatment).
Table 3 illustrates that challenge had a significant impact on feed intake at d 13 (P = 0.028), d 23 (P < 0.001) and d 30 (P < 0.001). There was also a full factorial interaction between challenge, diet and regime on feed intake at d 35, showing that feed intake was highest in the challenged birds fed the HAA diet immediately post-hatch and the control diet after fasting, and was lowest in the unchallenged birds fed the control diet. Birds that were fed immediately post-hatch had higher feed intake at d 13 compared with birds that were fasted 48 h (P < 0.001). At d 23, there was an interaction between regime and diet (P = 0.044) which showed that feed intake was greater in birds fed the control diet compared with the HAA diet when birds were fed immediately post-hatch, but when birds were fasted feed intake was comparatively greater in birds fed the HAA diet (data not shown) (see Table 4).
Table 3.
Interaction of necrotic enteritis challenge, feeding regime and diet on broiler feed intake.1
| Treatments | Day 13 | Day 23 | Day 30 | Day 35 | ||
|---|---|---|---|---|---|---|
| Unchallenged | HELD | Control | 512.0 ± 8.1 | 1,800.2 ± 37.7 | 2,851.8 ± 38.2 | 3,741.3 ± 31.4cd |
| HAA | 519.0 ± 6.9 | 1,932.8 ± 32.5 | 3,020.5 ± 37.7 | 3,960.2 ± 51.1c | ||
| FED | Control | 539.3 ± 22.7 | 1,815.2 ± 71.8 | 2,785.7 ± 116.9 | 3,570.7 ± 123.8d | |
| HAA | 551.2 ± 14.1 | 1,903.2 ± 38.2 | 2,968.3 ± 46.0 | 3,893.0 ± 86.5c | ||
| Challenged | HELD | Control | 519.2 ± 9.2 | 2,008.8 ± 18.6 | 3,048.8 ± 33.4 | 4,216.7 ± 58.1b |
| HAA | 530.7 ± 1.5 | 2,137.8 ± 42.5 | 3,228.7 ± 50.3 | 4,476.7 ± 64.5a | ||
| FED | Control | 581.8 ± 13.6 | 2,226.7 ± 108.4 | 3,294.7 ± 105.3 | 4,529.2 ± 156.7a | |
| HAA | 569.8 ± 11.6 | 2,079.7 ± 28.8 | 3,135.7 ± 53.4 | 4,328.2 ± 58.1ab | ||
| Main effects | ||||||
| Unchallenged | 531.5 ± 6.6b | 1,868.7 ± 22.3b | 2,910.9 ± 32.4b | 3,783.5 ± 37.5b | ||
| Challenged | 545.9 ± 7.0a | 2,084.2 ± 23.9a | 3,148.6 ± 34.7a | 4,349.8 ± 40.2a | ||
| HELD | 521.9 ± 6.6b | 1,982.3 ± 22.3 | 3,053.1 ± 32.4 | 4,117.3 ± 37.5 | ||
| FED | 555.5 ± 7.0a | 1,970.6 ± 23.9 | 3,006.4 ± 34.7 | 4,016.1 ± 40.2 | ||
| Control | 534.6 ± 7.2 | 1,944.1 ± 24.5 | 2,981.3 ± 35.6 | 3,989.6 ± 41.3b | ||
| HAA | 542.9 ± 6.4 | 2,008.8 ± 21.5 | 3,078.1 ± 31.3 | 4,143.8 ± 36.3a | ||
| P-value | ||||||
| Challenge | 0.028 | <0.001 | <0.001 | <0.001 | ||
| Regime | <0.001 | 0.353 | 0.857 | 0.768 | ||
| Diet | 0.605 | 0.197 | 0.058 | 0.021 | ||
| Challenge × regime | 0.235 | 0.265 | 0.162 | 0.114 | ||
| Challenge × diet | 0.585 | 0.130 | 0.090 | 0.060 | ||
| Regime × diet | 0.598 | 0.044 | 0.096 | 0.159 | ||
| Challenge × regime × diet | 0.425 | 0.142 | 0.071 | 0.029 | ||
HAA = high amino acid starter diet (10% more essential amino acid over the Aviagen recommendations); FED = access to feed with 6 h post-hatch; HELD = access to feed after 48 h post-hatch; Challenge = necrotic enteritis induced by oral administrations of Eimeria species and a field strain of Clostridium perfringens.
a, b, c, d Means within the same column with no common superscript differ significantly (P ≤ 0.05).
Means represent the average individual body weight of 12 birds per pen, 6 pens per treatment (72 birds/treatment).
Table 4.
Interaction of necrotic enteritis challenge, feeding regime and diet on broiler feed conversion.1
| Treatments | Day 13 | Day 23 | Day 30 | Day 35 | ||
|---|---|---|---|---|---|---|
| Unchallenged | HELD | Control | 1,052 ± 0.027 | 1,392 ± 0.038 | 1,406 ± 0.019 | 1,448 ± 0.017c |
| HAA | 1,006 ± 0.022 | 1,405 ± 0.031 | 1,436 ± 0.016 | 1,485 ± 0.014c | ||
| FED | Control | 1,161 ± 0.022 | 1,488 ± 0.031 | 1,478 ± 0.016 | 1,466 ± 0.014c | |
| HAA | 1,135 ± 0.024 | 1,507 ± 0.034 | 1,507 ± 0.017 | 1,473 ± 0.015c | ||
| Challenged | HELD | Control | 1,085 ± 0.024 | 1,685 ± 0.034 | 1,553 ± 0,017 | 1,709 ± 0.015a |
| HAA | 1,035 ± 0.022 | 1,808 ± 0.031 | 1,530 ± 0.016 | 1,667 ± 0.014b | ||
| FED | Control | 0.963 ± 0.031 | 1,732 ± 0.043 | 1,578 ± 0.022 | 1,702 ± 0.019ab | |
| HAA | 0.961 ± 0.024 | 1,750 ± 0.034 | 1,607 ± 0.017 | 1,724 ± 0.015a | ||
| Main effects | ||||||
| Unchallenged | 1,088 ± 0.012a | 1,448 ± 0.017b | 1,457 ± 0.008b | 1,468 ± 0.007b | ||
| Challenged | 1,011 ± 0.013b | 1,744 ± 0.018a | 1,567 ± 0.009a | 1,701 ± 0.008a | ||
| HELD | 1,044 ± 0.012 | 1,573 ± 0.017 | 1,481 ± 0.008b | 1,577 ± 0.007 | ||
| FED | 1,055 ± 0.013 | 1,619 ± 0.018 | 1,543 ± 0.009a | 1,591 ± 0.008 | ||
| Control | 1,065 ± 0.013 | 1,574 ± 0.018 | 1,504 ± 0.009 | 1,581 ± 0.008 | ||
| HAA | 1,034 ± 0.011 | 1,618 ± 0.016 | 1,520 ± 0.008 | 1,587 ± 0.007 | ||
| P-value | ||||||
| Challenge | <0.001 | <0.001 | <0.001 | <0.001 | ||
| Regime | 0.522 | 0.0635 | <0.001 | 0.212 | ||
| Diet | 0.080 | 0.085 | 0.206 | 0.598 | ||
| Challenge × regime | <0.001 | 0.042 | 0.421 | 0.323 | ||
| Challenge × diet | 0.774 | 0.276 | 0.299 | 0.145 | ||
| Regime × diet | 0.328 | 0.322 | 0.323 | 0.435 | ||
| Challenge × regime × diet | 0.690 | 0.263 | 0.303 | 0.037 | ||
HAA = high amino acid starter diet (10% more essential amino acid over the Aviagen recommendations); FED = access to feed with 6 h post-hatch; HELD = access to feed after 48 h post-hatch; Challenge = necrotic enteritis induced by oral administrations of Eimeria species and a field strain of Clostridium perfringens.
a, b, c Means within the same column with no common superscript differ significantly (P ≤ 0.05).
Means represent the average individual body weight of 12 birds per pen, 6 pens per treatment (72 birds/treatment).
Interactions between challenge and regime on feed conversion were observed at d 13 (P < 0.001) which showed that when birds were fed immediately post-hatch, FCR was significantly higher in the unchallenged birds compared with the challenged birds, but there was no significant difference between the challenged and unchallenged birds when they were fasted post-hatch. At d 23 there was also an interaction between challenge and regime on feed conversion (P = 0.042), illustrating that FCR was lowest in the unchallenged birds that were fasted and was significantly higher in the challenged birds compared with the unchallenged birds. At d 30 challenge (P < 0.001) and regime (P < 0.001) had a significant effect on FCR, showing that FCR was higher in the challenged birds compared with the unchallenged birds, and was higher in birds that were fed immediately post-hatch compared with those that were fasted. A full factorial interaction (P = 0.037) was detected at d 35, showing that FCR was higher in the challenged birds compared with the unchallenged birds, and that the challenged birds fed the HAA diet after fasting had lower FCR than the challenged birds fed the control diet after fasting or HAA diet immediately post-hatch.
3.2. Lesion score and intestinal bacterial enumeration
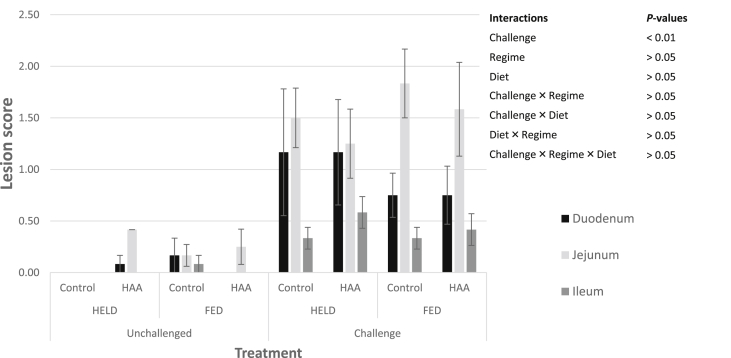
Necropsy of the small intestine revealed healthy intestines in birds from the unchallenged group, in contrast to birds from the challenged group which had enlarged gas-filled small intestines and prevalence of gross lesions. As expected, the necrotic lesions in the proximal duodenum, jejunum, and ileum were significantly (P < 0.001) more severe in the birds subjected to the NE challenge compared with those that were not challenged (Fig. 1, Fig. 2). In this study feeding regime and diet had no significant impact on intestinal lesion scores.
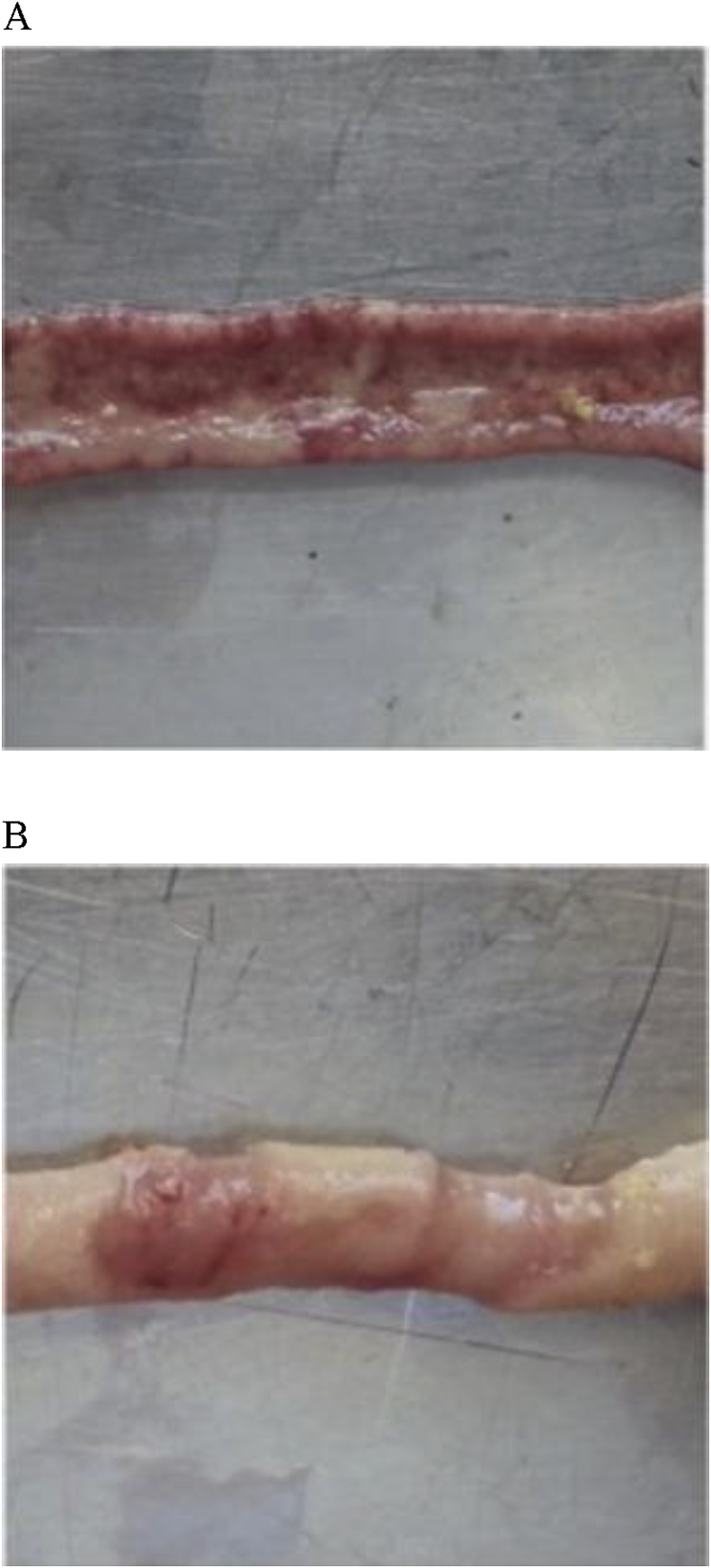
Fig. 1.
Examples of necropsy of gross lesions observed in the small intestine of 16-day-old birds exposed to necrotic enteritis challenge. (A) Ileum section (score 3) and (B) jejunum section (score 2).
Fig. 2.
Interaction of necrotic enteritis challenge, feeding regime and diet on lesion score in 16-day-old broilers in the duodenum, jejunum and ileum. HAA = High amino acid starter diet; HELD = access to feed after 48 h post-hatch; FED = access to feed with 6 h post-hatch; Challenge = necrotic enteritis induced by oral administrations of Eimeria species and a field strain of Clostridium perfringens.
Table 5 shows there were interactions between challenge and diet on measured Lactobacillus (P = 0.047) and Enterobacter (P = 0.028) populations in the ileal digesta. In the unchallenged birds, the number of Lactobacillus was lower in birds fed the HAA diet compared with those fed the control diet. The Enterobacter population was greater in the challenged birds fed the control diet compared with the unchallenged birds fed the HAA diet. The number of Lactobacillus observed in the ileum was significantly lower (P = 0.008) in digesta from the challenged birds compared with the unchallenged birds, but the number of C. perfringens in the ileum was markedly higher (P < 0.001) in the challenged birds. In the challenged birds, feeding the HAA diet resulted in a higher Lactobacillus population compared with feeding the control diet. Enterobacter population in the caeca was significantly higher (P = 0.006) in the challenged birds compared with the unchallenged birds. C. perfringens colonisation in the caecum of the unchallenged birds was below the threshold of the detectable level, but was detectable in the caeca of the challenged birds.
Table 5.
Interaction of necrotic enteritis challenge, feeding regime and diet on Log10 DNA enumeration of gut bacteria in ileal and caecal digesta from d 16 broilers, using 16S-rDNA qPCR quantification.1
| Treatments | Ileal digesta |
Caecal digesta |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Lac2 | Ent2 | Tot2 | Cp2 | Lac2 | Ent2 | Tot2 | Cp2 | |||
| Unchallenged | HELD | Control | 8.7 ± 0.2 | 6.6 ± 0.2 | 8.2 ± 0.2 | 7.2 ± 0.4 | 9.1 ± 0.2 | 7.5 ± 0.4 | 10.3 ± 0.1 | – |
| HAA | 8.7 ± 0.4 | 6.8 ± 0.1 | 8.3 ± 0.4 | 7.3 ± 0.5 | 9.4 ± 0.1 | 7.5 ± 0.3 | 10.4 ± 0.1 | – | ||
| FED | Control | 9.0 ± 0.3 | 6.6 ± 0.2 | 8.6 ± 0.4 | 7.7 ± 0.1 | 9.3 ± 0.1 | 6.9 ± 0.3 | 10.4 ± 0.0 | – | |
| HAA | 8.5 ± 0.2 | 6.9 ± 0.2 | 8.0 ± 0.2 | 8.4 ± 0.1 | 9.2 ± 0.2 | 7.3 ± 0.3 | 10.4 ± 0.0 | – | ||
| Challenged | HELD | Control | 8.0 ± 0.2 | 7.0 ± 0.2 | 8.3 ± 0.2 | 10.3 ± 0.3 | 9.2 ± 0.1 | 8.0 ± 0.3 | 10.2 ± 0.1 | 11.6 ± 0.2 |
| HAA | 8.8 ± 0.4 | 6.6 ± 0.1 | 8.7 ± 0.1 | 10.2 ± 0.4 | 9.2 ± 0.1 | 8.0 ± 0.2 | 10.3 ± 0.0 | 11.0 ± 0.6 | ||
| FED | Control | 7.6 ± 0.2 | 6.9 ± 0.1 | 7.8 ± 0.1 | 9.9 ± 0.2 | 9.4 ± 0.1 | 7.5 ± 0.3 | 10.2 ± 0.1 | 11.5 ± 0.2 | |
| HAA | 8.2 ± 0.3 | 6.6 ± 0.2 | 8.4 ± 0.2 | 10.5 ± 0.1 | 9.5 ± 0.1 | 8.0 ± 0.2 | 10.3 ± 0.0 | 11.8 ± 0.1 | ||
| P-value | ||||||||||
| Challenge | 0.008 | 0.848 | 0.837 | <0.001 | 0.452 | 0.006 | 0.254 | – | ||
| Regime | 0.274 | 0.918 | 0.384 | 0.155 | 0.181 | 0.164 | 0.567 | 0.362 | ||
| Diet | 0.302 | 0.653 | 0.360 | 0.151 | 0.206 | 0.325 | 0.135 | 0.700 | ||
| Challenge × regime | 0.201 | 0.767 | 0.295 | 0.120 | 0.184 | 0.678 | 0.682 | – | ||
| Challenge × diet | 0.047 | 0.028 | 0.056 | 0.776 | 0.601 | 0.759 | 0.559 | – | ||
| Regime × diet | 0.399 | 0.642 | 0.540 | 0.241 | 0.231 | 0.307 | 0.569 | 0.207 | ||
| Challenge × regime × diet | 0.696 | 0.883 | 0.242 | 0.917 | 0.273 | 0.916 | 0.488 | – | ||
HAA = high amino acid starter diet (10% more essential amino acid over the Aviagen recommendations); FED = access to feed with 6 h post-hatch; HELD = access to feed after 48 h post-hatch; Challenge = necrotic enteritis induced by oral administrations of Eimeria species and a field strain of Clostridium perfringens.
“–” means below detection level.
Means represent the average individual feed intake of 12 birds per pen, 6 pens per treatment (72 birds/treatment).
Lac, Ent, Tot and Cp refer to bacteria genus Lactobacillus, Enterobacter, total bacteria enumeration, and Clostridium perfringens species, respectively.
4. Discussion
The NE challenge model (Rodgers et al., 2014, Wu et al., 2010) employed in the current study successfully induced subclinical NE, which was characterised by a significant deterioration of bird performance without affecting mortality. It was surprising that the challenge led to an average lesion score of more than 1.5 in the jejunum, but the affected birds gradually recovered by d 30, as illustrated by the mean weight of the birds in the challenge treatment being similar to that of the unchallenged birds. A significant loss of feed efficiency and severe gross lesions, but without observable mortality, all indicate that subclinical NE outbreaks can indeed be economically devastating to a broiler flock (Skinner et al., 2010).
It is well documented that an unbalanced diet that contains excessive amounts of dietary ingredients, such as barley, wheat, oat, rye or fishmeal, can predispose the intestinal environment to C. perfringens proliferation, possibly leading to NE outbreak in broilers (Kaldhusdal and Løvland, 2000). However, to the best of our knowledge, the current study was the first to examine the potential beneficial implications of feeding a high density of digestible amino acids to NE challenged birds. Indeed, the current study used a high-quality diet that met or exceeded the nutrient requirements recommended for Ross 308 broilers (Aviagen, 2012). Throughout the study, the body weight of the flock was higher than the Ross 308 broiler standard. For instance, at d 13, the body weights of all the groups ranged from 476 to 600 g (Ross 308 male bird standard is approximately 428 g) and at d 35 it ranged from 2,435 to 2,685 g (Ross 308 male standard is approximately 2,250 g). Previously, the same challenge model with the same organism was used and resulted in up to 30% NE-related mortalities (Mikkelsen et al., 2009, Wu et al., 2010), whereas in the current study, despite the presence of severe NE lesions, no NE-related mortalities was recorded. The only observed difference between the current study and previous studies using the same study design is the diet. Supplementing 10% extra digestible amino acids over the recommended level proved to have long-lasting benefits to a bird that was subjected to multiple stress conditions. Although bird weight in the HAA and control diets did not differentiate in the full factorial setup, the mean weight of birds fed the HAA diet was greater by the end of the trial. Moreover, when the main effects of challenge and non-challenge were separately analysed, post-hatch fasted, i.e., the HELD birds fed the diet with HAA performed better in terms of the FCR under challenge. This finding may constitute the beneficial effect of high levels of amino acids to birds in response to external stresses, for instance post-hatch fasting and subclinical NE. Traditionally, the majority of essential amino acids, such as arginine, histidine, glycine and lysine, are perceived as a critical resource for immune function and cytokine production (Kidd, 2004, Li et al., 2007). Therefore, it is possible that demand for essential amino acids would increase in the presence of inflammation or immune stress (Le Floc'h et al., 2004). However, studies on the mere characterisation of nutrient utilisation on carcass weight suggest that additional digestible amino acid supplementation should not exceed the level of recommendation in the healthy flock if the crude protein had been adequately provided, since the consumed nitrogen composition in excess would not be utilised and would hence be excreted (Aletor et al., 2000). Abiding to the hypothesis of the present study, the role of excess amounts of essential amino acids, such as lysine and arginine, may have induced improvements in birds that are recovering from disease stress, although the excess likely did not instigate improvements in the healthy flock (Aletor et al., 2000, Li et al., 2007). However, due to limited evidence provided in the present study, further investigation might be required to confirm the potential benefit of excess amino acid density.
Any negative change to dietary constituents may be accompanied by drastic changes in the gut microflora, which may potentially predispose the gut to pathogenic infection (Mikkelsen et al., 2009, Wu et al., 2014; Stanley et al., 2014). C. perfringens is considered highly opportunistic, and its population rapidly changes depending on alterations to the host gut environment (Lee et al., 2011, Timbermont, 2011). C. perfringens strains also possess the capability to secrete proteinaceous enzymes, which act as invasive toxic compounds to closely related species and competitively exclude bacterial strains (Bannam et al., 2011, Jack et al., 1995). Pathogenic C. perfringens strains can take advantage of a high viscosity and high protein environment in the digestive tract to further proliferate, which can result in an NE outbreak (Timbermont, 2011, Van Immerseel et al., 2009). It is not surprising that in this study a significantly larger number of C. perfringens was present in the gut in the challenged birds compared with the unchallenged birds, which resulted in a decrease in Lactobacilli present in the ileum. Lactobacillus is a beneficial gut commensal; it lowers the pH of the gastrointestinal environment and balances gut microflora in the lower intestine (Jin et al., 1998, Waititu et al., 2014). Cao et al. (2012) reported a successful example of a decreased number of C. perfringens colonies as a result of dietary induction of Lactobacillus in chickens. In this study, the HAA starter diet heightened the presence of Lactobacilli in the ileum of the challenged birds but it did not affect the Lactobacillus population in the unchallenged birds. The effect of NE challenge on the gut microbiota is extremely complex. Stanley et al. (2014) reported vastly different gut flora in broilers with or without NE challenge. It is thus difficult to explain the interaction detected in the current study. One interesting finding was that higher numbers of Enterobacter were found in the caecal digesta of the challenged birds. It is difficult to determine the exact cause of such an effect. Enterobacter is a facultative anaerobe and hence may be able to retain growth over other strict anaerobes, after the environmental change caused by an increased population of C. perfringens in the caecum (Gabriel et al., 2006).
It is reported that delayed access to diet post-hatch generates production loss when the bird is older (Dibner, 1999, Gonzales et al., 2003). The yolk is capable of sustaining the bird for the first few days post-hatch, and it contains maternal antibodies that strengthen immune development (Dibner, 1999, Dibner et al., 1998). During a nutrient scarcity, it is possible that vital antibodies may be degraded to meet the demand of amino acids for physical development of rapidly growing chickens, rather than functioning as a component of passive immunity (Dibner, 1999). Parental generated antibodies that induce passive immunity against NE challenge have been shown to pass on to the offspring (Lovland et al., 2004). This hypothesis indicates the important role of early feed access in broiler chickens in preserving the vital role of immune function of the yolk (Gonzales et al., 2003). Thus, fasting in the early post-hatch period may not only impede immune development, but it also possibly suppresses the physiological development of the gut, thereby further impairing development and performance as the bird ages (Geyra et al., 2001, Kidd et al., 2004). Geyra et al. (2001) reported that early feed restriction resulted in negative impacts on intestinal and critical organ development in broilers. A similar observation was found by Corless and Sell (1999) in fasted young turkey poults. However, their findings indicate that it is the period of fasting as opposed to the act of fasting itself that causes depressed growth in the older bird (Corless and Sell, 1999), and the impact of the period of fasting is largely dependent on the nutrient quality of the yolk (Moran and Reinhart, 1980).
5. Conclusion
The subclinical form of NE induced in this study resulted in a significant loss of feed efficiency and severe gross lesions, but without an observable level of mortality. This indicates that subclinical NE outbreaks can indeed be economically devastating to a broiler flock. Supplementing 10% extra digestible amino acids over the recommended level proved to have long-lasting benefits to birds that were subjected to multiple stress conditions, namely post-hatch fasting and NE. This was illustrated in this study by the fact the mean weight of birds fed the HAA diet was greater by the end of the trial compared with those fed the control diet, and post-hatch fasted birds fed the HAA diet performed better in terms of FCR under challenge. This was likely because demand for essential amino acids is increased in the presence of inflammation or immune stress. The HAA diet also appeared to promote intestinal Lactobacillus population in the lower small intestine. Further investigation is required to assess the potential benefit of feeding broilers diets with excess amino acid density in different husbandry and dietary conditions. Further investigation is however warranted for determining the threshold period of post-hatch fasting that affects the susceptibility of birds to NE. In this study a limited number of bacterial species were enumerated, so it may be advantageous to characterise a more comprehensive range of bacterial species in NE challenged birds in order to fully understand its impact on the gut microbial community.
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- Aletor V.A., Hamid I.I., Niess E., Pfeffer E. Low-protein amino acid-supplemented diets in broiler chickens: effects on performance, carcass characteristics, whole-body composition and efficiencies of nutrient utilisation. J Sci Food Agric. 2000;80:547–554. [Google Scholar]
- Annett C., Viste J., Chirino-Trejo M., Classen H.L., Middleton D., Simko E. Necrotic enteritis: effect of barley, wheat and corn diets on proliferation of Clostridium perfringens type A. Avian Path. 2002;31:598–601. doi: 10.1080/0307945021000024544. [DOI] [PubMed] [Google Scholar]
- Ao Z., Kocher A., Choct M. Effects of dietary additives and early feeding on the performance, gut development and immune status of broiler chickens challenged with Clostridium perfringens. Asian-Aust J Anim Sci. 2002;25:541–551. doi: 10.5713/ajas.2011.11378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviagen . 2012. Ross 308 Broiler: nutrition specification.http://www.aviagen.com [Google Scholar]
- Bannam T.L., Yan X.X., Harrison P.F., Seemann T., Keyburn A.L., Stubenrauch C. Necrotic enteritis-derived Clostridium perfringens strain with three closely related independently conjugative toxin and antibiotic resistance plasmids. MBio. 2011;2 doi: 10.1128/mBio.00190-11. e00190–00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartosch S., Fite A., Macfarlane G.T., McMurdo M.E. Characterization of bacterial communities in feces from healthy elderly volunteers and hospitalized elderly patients by using real-time PCR and effects of antibiotic treatment on the fecal microbiota. AEM. 2004;70:3575–3581. doi: 10.1128/AEM.70.6.3575-3581.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedford M. Removal of antibiotic growth promoters from poultry diets: implications and strategies to minimise subsequent problems. Worlds Poult Sci J. 2000;56:347–365. [Google Scholar]
- Broussard C., Hofacre C., Page R., Fletcher O. Necrotic enteritis in cage-reared commercial layer pullets. Avian Dis. 1986:617–619. [PubMed] [Google Scholar]
- Cao L., Yang X., Li Z., Sun F., Wu X., Yao J. Reduced lesions in chickens with Clostridium perfringens-induced necrotic enteritis by Lactobacillus fermentum 1.2029. Poult Sci. 2012;91:3065–3071. doi: 10.3382/ps.2012-02548. [DOI] [PubMed] [Google Scholar]
- Choct M. ASA Technical Bulletin; 2001. Alternatives to in-feed antibiotics in monogastric animal industry. AN. 30. [Google Scholar]
- Choct M., Annison G. Anti-nutritive effect of wheat pentosans in broiler chickens: roles of viscosity and gut microflora. Br Poult Sci. 1992;33:821–834. doi: 10.1080/00071669208417524. [DOI] [PubMed] [Google Scholar]
- Collier C., Hofacre C., Payne A., Anderson D., Kaiser P., Mackie R. Coccidia-induced mucogenesis promotes the onset of necrotic enteritis by supporting Clostridium perfringens growth. Vet Immunol Immunopathol. 2008;122:104–115. doi: 10.1016/j.vetimm.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Corless A.B., Sell J. The effects of delayed access to feed and water on the physical and functional development of the digestive system of young turkeys. Poult Sci. 1999;78:1158–1169. doi: 10.1093/ps/78.8.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croom J., Edens F., Ferket P. Proc. 27th Ann. Carolina poultry nutrition conference. Vol. 16. Carolina Feed Industry Association, Research Triangle Park; November 2000. The impact of nutrient digestion and absorption on poultry performance and health; pp. 65–73. [Google Scholar]
- Dahiya J.P. Potential strategies for controlling necrotic enteritis in broiler chickens in post-antibiotic era. Anim Feed Sci Tech. 2006;129:60–88. [Google Scholar]
- Devriese L., Daube G., Hommez J., Haesebrouck F. In vitro susceptibility of Clostridium perfringens isolated from farm animals to growth-enhancing antibiotics. J Appl Microbiol. 1993;75:55–57. doi: 10.1111/j.1365-2672.1993.tb03407.x. [DOI] [PubMed] [Google Scholar]
- Dibner J. Feeding hatchling poultry. Avoid any delay. Feed Int. 1999;20:30–34. [Google Scholar]
- Dibner J., Knight C., Kitchell M., Atwell C., Downs A., Ivey F. Early feeding and development of the immune system in neonatal poultry. J Appl Poult Res. 1998;7:425–436. [Google Scholar]
- Dibner J., Richards J. Antibiotic growth promoters in agriculture: history and mode of action. Poult Sci. 2005;84:634–643. doi: 10.1093/ps/84.4.634. [DOI] [PubMed] [Google Scholar]
- Ferket P.R. vol. 20. Nottingham Univ Press; 2004. Alternatives to antibiotics in poultry production: responses, practical experience and recommendations; pp. 54–67. (Nutritional biotechnology in the feed and foods Industries). [Google Scholar]
- Gabriel I., Lessire M., Mallet S., Guillot J.F. Microflora of the digestive tract: critical factors and consequences for poultry. Worlds Poult Sci. 2006;62:499–512. [Google Scholar]
- Geier M.S., Mikkelsen L.L., Torok V.A., Allison G.E., Olnood C., Boulianne M. Comparison of alternatives to in-feed antimicrobials for the prevention of clinical necrotic enteritis. J Appl Microbiol. 2010;109:1329–1338. doi: 10.1111/j.1365-2672.2010.04758.x. [DOI] [PubMed] [Google Scholar]
- Geyra A., Uni Z., Sklan D. The effect of fasting at different ages on growth and tissue dynamics in the small intestine of the young chick. Br J Nutr. 2001;86:53–61. doi: 10.1079/bjn2001368. [DOI] [PubMed] [Google Scholar]
- Gibert M., Jolivet-Renaud C., Popoff M.R. Beta2 toxin, a novel toxin produced by Clostridium perfringens. Gene. 1997;203:65–73. doi: 10.1016/s0378-1119(97)00493-9. [DOI] [PubMed] [Google Scholar]
- Gonzales E., Kondo N., Saldanha E., Loddy M., Careghi C., Decuypere E. Performance and physiological parameters of broiler chickens subjected to fasting on the neonatal period. Poult Sci. 2003;82:1250–1256. doi: 10.1093/ps/82.8.1250. [DOI] [PubMed] [Google Scholar]
- Huyghebaert G. An update on alternatives to antimicrobial growth promoters for broilers. Vet J. 2011;187:182–188. doi: 10.1016/j.tvjl.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Jack R.W., Tagg J.R., Ray B. Bacteriocins of gram-positive bacteria. Microbiol Rev. 1995;59:171–200. doi: 10.1128/mr.59.2.171-200.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin L.Z., Ho Y.W., Abdullah N., Jalaludin S. Growth performance, intestinal microbial populations, and serum cholesterol of broilers fed diets containing Lactobacillus cultures. Poult Sci. 1998;77:1259–1265. doi: 10.1093/ps/77.9.1259. [DOI] [PubMed] [Google Scholar]
- Kaldhusdal M., Løvland A. The economical impact of Clostridium perfringens is greater than anticipated. World Poult. 2000;16:50–51. [Google Scholar]
- Keyburn A.L., Bannam T.L., Moore R.J., Rood J.I. NetB, a pore-forming toxin from necrotic enteritis strains of Clostridium perfringens. Toxins. 2010;2:1913–1927. doi: 10.3390/toxins2071913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd M. Nutritional modulation of immune function in broilers. Poult Sci. 2004;83:650–657. doi: 10.1093/ps/83.4.650. [DOI] [PubMed] [Google Scholar]
- Kidd M., McDaniel C., Branton S., Miller E., Boren B., Fancher B. Increasing amino acid density improves live performance and carcass yields of commercial broilers. J Appl Poult Res. 2004;13:593–604. [Google Scholar]
- Le Floc'h N., Melchior D., Obled C. Modifications of protein and amino acid metabolism during inflammation and immune system activation. Livest Prod Sci. 2004;87:37–45. [Google Scholar]
- Lee D.-H., Zo Y.-G., Kim S.-J. Nonradioactive method to study genetic profiles of natural bacterial communities by PCR-single-strand-conformation polymorphism. Appl Environ Microbiol. 1996;62:3112–3120. doi: 10.1128/aem.62.9.3112-3120.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K., Lillehoj H., Jeong W., Jeoung H., An D. Avian necrotic enteritis: experimental models, host immunity, pathogenesis, risk factors, and vaccine development. Poult Sci. 2011;90:1381–1390. doi: 10.3382/ps.2010-01319. [DOI] [PubMed] [Google Scholar]
- Li P., Yin Y.-L., Li D., Kim S.W., Wu G. Amino acids and immune function. Br J Nutr. 2007;98:237–252. doi: 10.1017/S000711450769936X. [DOI] [PubMed] [Google Scholar]
- Lovland A., Kaldhusdal M., Redhead K., Skjerve E., Lillehaug A. Maternal vaccination against subclinical necrotic enteritis in broilers. Avian Path. 2004;33:81–90. doi: 10.1080/0379450310001636255. [DOI] [PubMed] [Google Scholar]
- Mikkelsen L.L., Vidanarachchi J.K., Olnood G.G., Bao Y.M., Selle P.H., Choct M. Effect of potassium diformate on growth performance and gut microbiota in broiler chickens challenged with necrotic enteritis. Br Poult Sci. 2009;50:66–75. doi: 10.1080/00071660802613252. [DOI] [PubMed] [Google Scholar]
- Moore R.J. Necrotic enteritis predisposing factors in broiler chickens. Avian Path. 2016:1–22. doi: 10.1080/03079457.2016.1150587. [DOI] [PubMed] [Google Scholar]
- Moran E., Reinhart B. Poult yolk sac amount and composition upon placement: effect of breeder age, egg weight, sex, and subsequent change with feeding or fasting. Poult Sci. 1980;59:1521–1528. doi: 10.3382/ps.0591521. [DOI] [PubMed] [Google Scholar]
- Prescott J., Sivendra R., Barnum D. The use of bacitracin in the prevention and treatment of experimentally-induced necrotic enteritis in the chicken. Can Vet J. 1978;19:181. [PMC free article] [PubMed] [Google Scholar]
- Riddell C., Kong X.-M. The influence of diet on necrotic enteritis in broiler chickens. Avian Dis. 1992:499–503. [PubMed] [Google Scholar]
- Rodgers N.J., Swick R.A., Geier M.S., Moore R.J., Choct M., Wu S.-B. A multifactorial analysis of the extent to which eimeria and fishmeal predispose broiler chickens to necrotic enteritis. Avian Dis. 2014;59:38–45. doi: 10.1637/10774-011614-reg.1. [DOI] [PubMed] [Google Scholar]
- Skinner J.T., Bauer S., Young V., Pauling G., Wilson J. An economic analysis of the impact of subclinical (mild) necrotic enteritis in broiler chickens. Avian Dis. 2010;54:1237–1240. doi: 10.1637/9399-052110-Reg.1. [DOI] [PubMed] [Google Scholar]
- Stanley D., Wu S.B., Rodgers N., Swick R., Moore R.J. Differential responses of cecal microbiota to fishmeal, Eimeria and Clostridium perfringens in a necrotic enteritis challenge model in chickens. PLoS One. 2014;9:104739. doi: 10.1371/journal.pone.0104739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tech R. Necrotic enteritis and associated conditions in broiler chickens. World Poult. 1999;15:44–47. [Google Scholar]
- Timbermont E.A. Necrotic enteritis in broilers: an updated review on the pathogenesis. Avian Path. 2011;40:341–347. doi: 10.1080/03079457.2011.590967. [DOI] [PubMed] [Google Scholar]
- Van der Sluis W. Clostridial enteritis is an often underestimated problem. World Poult. 2000;16:42–43. [Google Scholar]
- Van Immerseel F., Rood J.I., Moore R.J., Titball R.W. Rethinking our understanding of the pathogenesis of necrotic enteritis in chickens. Trend Microbiol. 2009;17:32–36. doi: 10.1016/j.tim.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Wade B., Keyburn A. The true cost of necrotic enteritis. World Poult. 2015;31:16–17. [Google Scholar]
- Waititu S.M., Yitbarek A., Matini E., Echeverry H., Kiarie E., Rodriguez-Lecompte J.C. Effect of supplementing direct-fed microbials on broiler performance, nutrient digestibilities, and immune responses. Poult Sci. 2014;93:625–635. doi: 10.3382/ps.2013-03575. [DOI] [PubMed] [Google Scholar]
- Wu S.-B., Rodgers N., Choct M. Optimized necrotic enteritis model producing clinical and subclinical infection of Clostridium perfringens in broiler chickens. Avian Dis. 2010;54:1058–1065. doi: 10.1637/9338-032910-Reg.1. [DOI] [PubMed] [Google Scholar]
- Wu S.-B., Stanley D., Rodgers N., Swick R.A., Moore R.J. Two necrotic enteritis predisposing factors, dietary fishmeal and Eimeria infection, induce large changes in the caecal microbiota of broiler chickens. Vet Microbiol. 2014;169:188–197. doi: 10.1016/j.vetmic.2014.01.007. [DOI] [PubMed] [Google Scholar]