Abstract
Polyamines such as putrescine, spermidine, spermine and agmatine are aliphatic polycationic compounds present in all living cells, and are derived from amino acids, intestinal bacteria, exfoliated enterocytes and supported from diet. Polyamines as the key compounds play essential role in cell proliferation, growth and differentiation. They also exert significant effects on embryonic development, implantation, embryonic diapause, placentation, angiogensis and fetal development. This review paper summarizes the functions of polyamines and embryo/fetus development and its regulatory mechanism which should help to provide some evidences for clinic.
Keywords: Polyamines, Polyamine biosynthesis, Embryo/fetus development
1. Introduction
Polyamines are low molecular weight organic compounds, and ubiquitously present in all living cells and exert important impacts on growth and development of animals. Polyamines metabolism is regulated by ornithine decarboxylase (ODC) (Xing et al., 2014). In mammals, polyamines are synthesized from 3 sources: 1) de novo synthesis by amino acids, such as arginine, proline, methionine and l-ornithine by decarboxylated S-adenosylmethionine, 2) import from the diet and 3) produced by intestinal microflora (Bardocz, 1993; Hessels et al., 1989; Larqué et al., 2007, Tabor and Tabor, 1984, Wu and Morris, 1998, Wu et al., 2008). Moreover, another source of polyamines derived from exfoliated enterocytes known as luminal sources comparatively rich in cellular contents of polyamines (McCormack and Johnson, 1991). Free polyamines are regulated in a disciplined manner and based on 4 steps such as de novo synthesis, interconversion, terminal degradation and transport (Larqué et al., 2007). Polyamine deficiency might have adverse effects on reproduction (Lefèvre et al., 2011), and the low concentration of polyamines have been associated with neurodegenerative diseases and aging (Minois, 2014). However, an excessive level of polyamines has been observed in cancer diseases which showed hazard effects (Ramani et al., 2014). Remarkably, production of polyamines via endogenous enzymes decreases as age advances (Lefèvre et al., 2011; Miller-Fleming et al., 2015). Therefore, it is suggested that polyamine-rich diet should be implicated during reproduction, aging, memory loss, and in different model species including humans (Kalac, 2014, Minois, 2014, Gupta et al., 2013).
Polyamines are recognized to be essential for various reproductive processes and play significant roles in harvesting embryo, implantation and in placentation. Polyamines especially putrescine, spermine and spermidine play pivotal roles in regulating DNA and protein synthesis, proliferation and differentiation (Igarashi and Kashiwagi, 2000). Polyamines were exhibited to exert antioxidant effect via scavenging reactive oxygen species thus protecting DNA, proteins and lipids from oxidative damage (Chattopadhyay et al., 2003). Research evidences reported that polyamines regulate angiogenesis, early embryogenesis, placental trophoblastic growth and embryonic development (Wu and Morris, 1998). This review paper highlights the significant role of polyamines in embryo/fetal development and its regulatory mechanism which might give some new directions on future research.
2. Synthesis of polyamines
Polyamines such as putrescine, spermidine, spermine and agmatine were first reported in human semen. Putrescine is formed by decarboxylation of ornithine by a catalyzed ODC in mammalian cells. Ornithine synthesized from arginase to form arginine. Synthesis of spermidine and spermine required 2 enzymes, S-adenosyl-methionine decarboxylase and a transferase enzyme (spermidine synthase or spermine synthase). Agmatine is derived by decarboxylation of l-arginine (Arg) by arginine decarboxylase (ADC) (Tabor and Tabor, 1984, Dudkowska et al., 2003). The three polyamines are synthesized from l-Arg (via l-Ornithine) and l-Methionine by series of interdependent enzyme reactions.
3. Bioavailability of polyamines
Polyamines reach into the gut via consumption of food and are promptly absorbed into duodenum and jejenum lumen by passive diffusion mechanism. The absorption process consists of carriers and cellular absorption. High amounts of polyamines are degraded in the gut before the part of systemic circulation (Milovic, 2001, Chang and Rao, 1994). Luminal polyamines are absorbed and distributed throughout the body and in various organs. Uptake of polyamines is necessary for cellular proliferation, and its activation occurs by mitogen and peptide growth factors (Milovic, 2001). Furthermore, deamination of polyamines leads to toxic metabolic products (Agostinelli et al., 2010). Moreover, polyamine concentration is regulated by synthesis and catabolism via transport of polyamines within the internal and external cellular environment which consists of several mechanisms (Persson, 2009). In addition, polyamine concentrations for development of Escherichia coli may transport polyamine into the cells via specific transporters or synthesize them in the lack of polyamines cellular uptake (Kurihara et al., 2010). Alteration in polyamine pool has been reported due to the amino acid or growth factors (Chabanon et al., 2000) showing that environmental factors may alter polyamine absorption in response to intestinal bacteria.
4. Regulation of polyamine biosynthesis in uterus
Exposure of steroid hormones during the pregnancy induces the formation of polyamines at various phases of gestation. Higher level of intracellular polyamine in reproductive system has shown to modulate with exogenous steroid hormones. A study conducted in ovariectomized mouse indicates that estrogen hormone is responsible for activation of ODC1 and AZI genes in uterus (Zhao et al., 2008). At the sites of implantation, ODC1 expression gene is overactivated in mouse uterus after induction of estrogen (Zhao et al., 2008). In addition, induction of estrogen antagonists decrease the ODC1 gene expression in mouse and rat uterus respectively (Zhao et al., 2008, Dwivedi et al., 1999). However, this effect has not been observed in rodents due to the ODC1 activity which is considerably exceeding in humans endometrium under the follicular phase rather than luteal phase (Holinka and Gurpide, 1985). Therefore, it is proposed that estrogen is the crucial regulator for uterine ODC1 activity and has different response among different species and gestation timings. For example, porcine uterine SAT1 gene expression is more susceptible to progesterone during elongation of pregnancy than estrogen (Green et al., 1996). And it also has shown that uterine polyamine concentration at genes levels can be modulated with steroid hormones.
5. Polyamines and embryonic development
Polyamines in early embryonic development have shown higher transcription of ODC1 in 2 cell embryos of mouse and their expressions increase throughout blastocyst stages (Domashenko et al., 1997). The ODC1 activity increases as the cell progress into blastocyst stage in the mouse. Late morula/early blastocyst stage cannot survive in vitro due to apoptosis in the inner cell mass because of the ODC-1 genotype. Early stages of implantation in mouse embryo model can be rescued by putrescine in drinking water but it could not survive beyond this stage (Pendeville et al., 2001). Incubation of 8 cell embryos with inhibitor of S-adenosylmethionine decarboxylase proenzyme (SAMDC) which prevents blastocyst formation in vitro, spermine or spermidine in culture media may antagonize this effect (Zwierzchowski et al., 1986).
Polyamines, being the viral source of cell survival which initiates signaling, starts from transcription to translations (Pegg, 2009). Polyamines can regulate translation via eukaryotic translation initiation factor 5A (eIF5A), highly essential protein (Chatterjee et al., 2006) and it consists of unusual spermidine residues, i.e., hypusine (Wolff et al., 2007) that expresses key roles in the cellular activity (Zanelli and Valentini, 2007). The requirement of eIF5A protein for cell growth may increase the possibility of polyamines involved in cellular proliferation (Hyvönen et al., 2007).
Arginine is a precursor of nitric oxide (NO) and polyamine synthesis by nitric synthase and ODC (Flynn et al., 2002). Nitric oxide is a key factor of derivation of endothelium relaxing factor, and is crucial for regulating placenta and fetal blood flows for transferring nutrients and oxygen between mothers to fetus (Bird et al., 2003). Similarly, polyamine is required for DNA and protein synthesis for cellular proliferation and differentiation (Flynn et al., 2002, Ishida et al., 2002). Therefore, NO and polyamines are essential for regulation of angiogenesis and embryogenesis (Reynolds and Redmer, 2001).
6. Polyamines and embryonic diapause
Polyamines related genes such as ODC1, SAT1, and AZI and uterine polyamine contents were markedly up-regulated in early embryonic activation (Lefèvre and Murphy, 2009, Lefèvre et al., 2011). Embryonic activation in the mink blastocyst to a diapause-like state by suppressing cell proliferation with inhibition of polyamine biosynthesis by 2-(difluoromethyl) ornithine (DFMO), an inhibitor of polyamine biosynthesis. In addition, further delayed implantation with this treatment has no any harmful effects on pregnancy (Lefèvre et al., 2011). In mouse facultative diapause the polyamine related genes such as ODC1, SAT1, SAMDC, AZI, Sms and Smox were up-regulated in the uterine stroma when estrogen induced reactivation of embryo from facultative diapause (Zhao et al., 2008). Delayed implantation or embryonic diapause is an irreversible process in embryo development before implantation at blastocyst stage (Dev et al., 2004).
In uterine epithelial cells, supplementation of putrescine in embryonic diapause resulted in normalize embryo development. Moreover, it was shown a potent evidence that in vivo diapause occured due to the lack of polyamines which was restored following prolactin induction (Fenelon et al., 2016).
7. Polyamine synthesis and placental development
The results in the deferent days of gestation gilts reported that proline markedly increased polyamine synthesis and concentration between 20 and 40 days of gestation and declined within 40 to 90 days of gestation. Moreover, it was denoted that proline is responsible for polyamine synthesis in porcine placenta and higher activity was observed during early pregnancy which leads to rapid placental growth (Wu et al., 2005).
8. Polyamines and intrauterine growth restriction
Intrauterine growth restriction (IUGR) causes abnormal growth and development in mammalian embryo-fetus which was considered as a main problem with human pregnancy (Murphy et al., 2006) and animal productions (Wu et al., 2006). It has shown that maternal arginine deficiency proved IUGR which had higher fetal absorption, and outcomes of perinatal mortality in rats and supplementation of dietary arginine restored fetal growth restriction in rat models of IUGR induced by NOS (Vosatka et al., 1998). Nitric oxide and polyamine via NO synthase and ODC were derived from arginine (Lefèvre and Murphy, 2009). Placental polyamine synthesis which is responsible for placental size and fetal growth in mouse and impairment in polyamine synthesis inhibited embryogenesis (Ishida et al., 2002).
Notably, arginine stimulates the mammalian target of rapamycin (mTOR) signaling pathways to activate protein synthesis in the placenta, uterus, and fetus. l-Arginine dietary supplementations of 0.83% to gilts during gestation period increases fecundity and birth weight in rats and ewes. Collectively, enhancement of uterine as well as placental growth and function through dietary arginine supplementation provides an effective solution to improving embryonic and fetal survival and growth (Wu et al., 2013).
8.1. Role of agmatine in concepts development
Agmatine, a polyamine which is derived from arginine, also performs multiple functions to increase reproductive performance in woman (Di Renzo et al., 2005, Xiao and Li, 2005), rats (Zeng et al., 2008) and gilts (Mateo et al., 2007), and it promotes cell migration, proliferation and gene expression in ovine trophoectoderm (oTr1) cells (Bazer et al., 2015a, Bazer et al., 2015b). Agmatine, a precursor of putrescine production via, agmatinase (AGMAT) (Piletz et al., 2013), stimulates embryo development through a novel ADC/AGMAT pathway in ovine trophoectoderm cells (Wang et al., 2014). Thus, it indicates that agmatine is an essential component for progression of uterine environment (Wang et al., 2014, Bazer et al., 2015a). Considering the functions of agmatine, it was reported that it activates insulin secretion and production of luteinizing hormone in dose specific manner and vasodilation of blood vessels (Reis and Regunathan, 1999).
In addition, it has indicated that both arginine and agmatine regulated basic amino acid transport such as (arginine) polyamine synthesis and secretion of catecholamines by oTr1 cells. Agmatine, upregulates mRNAs expressions of SLC7A1, agmatinase and OAZ2, whereas the arginine and agmatine collectively reduced the mRNAs expressions of ODC1, SLC7A1, OAZ1 and OAZ3 by oTr1 cells. Moreover, agmatine does not involve in proliferation, migration or adhesion of oTr1 cells or their catecholamine's secretion, but promotes the transcription of SLC7A1, agmatinase and OAZ2 genes which would enhance the ability of oTr1 cells to produce polyamines (Lenis et al., 2016).
9. Conclusion
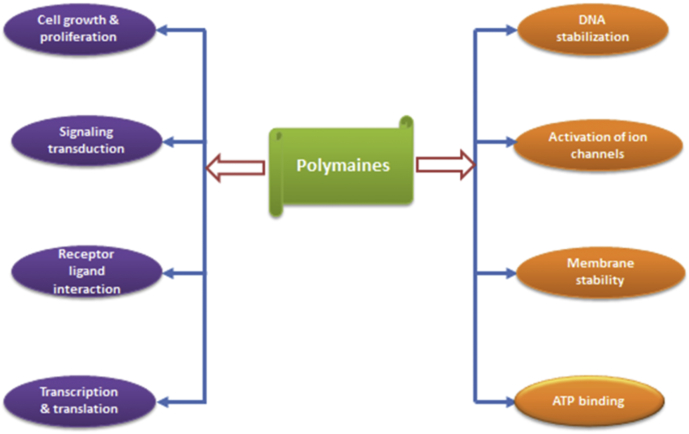
Polyamine regulates various cellular functions from gene expression to protein synthesis which performs embryo/fetus proliferation, growth and differentiation (Fig. 1). Supplementation of polyamines such as spermine, spermidine, putrescine and agmatine has been actively involved in embryo survival which ultimately results in improvements in fertility. More evidences should be collected to explore polyamine role in gametogenesis, placental development, and interaction of fetus with mothers, clinical studies and its underlying molecular mechanism. In addition, polyamines also regulate endocrine functions, such as estrogen and prolactin, which enhances the ODC1 expressions in uterus and shows an essential role in polyamine regulation. Meanwhile, manipulation of polyamine biosynthetic pathway and ADC/AGMAT pathway might give some new directions on concepts development which ultimately enhance livestock productions.
Fig. 1.
Multiple functions of polyamine in reproduction.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgement
This project was supported by the National Science and Technology Ministry (2014BAD08B11), the National Natural Science Foundation of China (No. 31330075, 31560640, 31372326, and 31301989) and the Science and Technology Department of Hunan Province (2015JC3126). We are also grateful to CAS-TWAS President's Fellowship and UCAS financial and infrastructure support, as well as Changsha Lvye Biotechnology Limited Company Academician Expert Workstation.
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- Agostinelli E., Marques M.P., Calheiros R., Gil F.P., Tempera G., Viceconte N. Polyamines: fundamental characters in chemistry and biology. Amino Acids. 2010;38:393–403. doi: 10.1007/s00726-009-0396-7. [DOI] [PubMed] [Google Scholar]
- Bardocz S. The role of dietary polyamines. Eur J Clin Nutr. 1993;47(10):683–690. [PubMed] [Google Scholar]
- Bazer F.W., Johnson G.A., Wu G. Amino acids and conceptus development during the peri-implantation period of pregnancy. Adv Exp Med Biol. 2015;843:23–52. doi: 10.1007/978-1-4939-2480-6_2. [DOI] [PubMed] [Google Scholar]
- Bazer F.W., Ying W., Wang X., Dunlap K.A., Zhou B., Johnson G.A. The many faces of interferon tau. Amino Acids. 2015;47:449–460. doi: 10.1007/s00726-014-1905-x. [DOI] [PubMed] [Google Scholar]
- Bird I.M., Zhang L.B., Magness R.R. Possible mechanisms underlying pregnancy-induced changes in uterine artery endothelial function. Am J Physiol. 2003;284(2):R245–R258. doi: 10.1152/ajpregu.00108.2002. [DOI] [PubMed] [Google Scholar]
- Chabanon H., Persson L., Wallace H.M., Ferrara M., Brachet P. Increased translation efficiency and antizyme-dependent stabilization of ornithine decarboxylase in amino acid-supplemented human colon adenocarcinoma cells. Caco-2 Biochem J. 2000;348(Pt 2):401–408. [PMC free article] [PubMed] [Google Scholar]
- Chang E.B., Rao M.C. Intestinal water and electrolyte transport. In: Johnson L.R., editor. Physiology of the gastrointestinal tract. 3rd ed. Raven Press; New York: 1994. pp. 2027–2081. [Google Scholar]
- Chatterjee I., Gross S.R., Kinzy T.G., Chen K.Y. Rapid depletion of mutant eukaryotic initiation factor 5A at restrictive temperature reveals connections to actin cytoskeleton and cell cycle progression. Mol Genet Genomics. 2006;275(3):264–276. doi: 10.1007/s00438-005-0086-4. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay M.K., Tabor C.W., Tabor H. Polyamines protect Escherichia coli cells from the toxic effect of oxygen. Proc Natl Acad Sci USA. 2003;100(5):2261–2265. doi: 10.1073/pnas.2627990100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dev S.K., Lim H., Das S.K., Reese J., Bc Paria, Daikoku T. Molecular cues to implantation. Endocr Rev. 2004;25:341–373. doi: 10.1210/er.2003-0020. [DOI] [PubMed] [Google Scholar]
- Di Renzo G.C., Clerici G., Neri I., Facchinetti F., Caserta G., Alberti A. Potential effects of nutrients on placental function and fetal growth. Nestle Nutr Workshop Ser Pediatr Program. 2005;55:73–77. doi: 10.1159/000082594. [DOI] [PubMed] [Google Scholar]
- Domashenko A.D., Latham K.E., Hatton K.S. Expression of myc-family, myc-interacting, and myc-target genes during preimplantation mouse development. Mol Reprod Dev. 1997;47(1):57–65. doi: 10.1002/(SICI)1098-2795(199705)47:1<57::AID-MRD8>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Dudkowska M., Lai J., Gardini G., Stachurska A., Grzelakowska-Sztabert B., Colombatto S. Agmatine modulates the in vivo biosynthesis and interconversion of polyamines and cell proliferation. Biochim Biophys Acta. 2003;1619:159–166. doi: 10.1016/s0304-4165(02)00476-2. [DOI] [PubMed] [Google Scholar]
- Dwivedi A., Gupta G., Keshri G., Dhar J.D. Changes in uterine ornithine decarboxylase activity and steroid receptor levels during decidualization in the rat induced by CDRI-85/287. Eur J Endocrinol. 1999;141:426–430. doi: 10.1530/eje.0.1410426. [DOI] [PubMed] [Google Scholar]
- Fenelon J.C., Banerjee A., Lefèvre P., Gratian F., Murphy B.D. Polyamine-mediated effects of prolactin dictate emergence from mink obligate embryonic diapause. Biol Reprod. 2016;95(1):6. doi: 10.1095/biolreprod.116.139204. [DOI] [PubMed] [Google Scholar]
- Flynn N.E., Meininger C.J., Haynes T.E., Wu G. The metabolic basis of arginine nutrition and pharmacotherapy. Biomed Pharmacother. 2002;56(9):427–438. doi: 10.1016/s0753-3322(02)00273-1. [DOI] [PubMed] [Google Scholar]
- Green M.L., Blaeser L.L., Simmen F.A., Simmen R.C. Molecular cloning of spermidine/spermine N1-acetyltransferase from the periimplantation porcine uterus by messenger ribonucleic acid differential display: temporal and conceptus-modulated gene expression. Endocrinology. 1996;137:5447–5455. doi: 10.1210/endo.137.12.8940370. [DOI] [PubMed] [Google Scholar]
- Gupta V.K., Scheunemann L., Eisenberg T., Mertel S., Bhukel A., Koemans T.S. Restoring polyamines protects from age-induced memory impairment in an autophagy-dependent manner. Nat Neurosci. 2013;16(10):1453–1460. doi: 10.1038/nn.3512. [DOI] [PubMed] [Google Scholar]
- Hessels J., Kingma A.W., Ferwerda H., Keij J., van den Berg G.A., Muskiet F.A. Microbial flora in the gastrointestinal tract abolishes cytostatic effects of -difluoromethylornithine in vivo. Int J Cancer. 1989;43:1155–1164. doi: 10.1002/ijc.2910430632. [DOI] [PubMed] [Google Scholar]
- Holinka C.F., Gurpide E. Ornithine decarboxylase activity in human endometrium and endometrial cancer cells. In Vitro Cell Dev Biol. 1985;21:697–706. doi: 10.1007/BF02620925. [DOI] [PubMed] [Google Scholar]
- Hyvönen M.T., Keinänen T.A., Cerrada-Gimenez M., Sinervirta R., Grigorenko N., Khomutov A.R. Role of hypusinated eukaryotic translation initiation factor 5A in polyamine depletion-induced cytostasis. J Biol Chem. 2007;282(48):34700–34706. doi: 10.1074/jbc.M704282200. [DOI] [PubMed] [Google Scholar]
- Igarashi K., Kashiwagi K. Polyamines: mysterious modulators of cellular functions. Biochem Biophys Res Commun. 2000;271(3):559–564. doi: 10.1006/bbrc.2000.2601. [DOI] [PubMed] [Google Scholar]
- Ishida M., Hiramatsu Y., Masuyama H., Mizutani Y., Kudo T. Inhibition of placental ornithine decarboxylase by DL-α- difluoro-methyl ornithine causes fetal growth restriction in rat. Life Sci. 2002;70(12):1395–1405. doi: 10.1016/s0024-3205(01)01510-7. [DOI] [PubMed] [Google Scholar]
- Kalac P. Health effects and occurrence of dietary polyamines: a review for the period 2005-mid 2013. Food Chem. 2014;161:27–39. doi: 10.1016/j.foodchem.2014.03.102. [DOI] [PubMed] [Google Scholar]
- Kurihara S., Kato K., Asada K., Kumagai H., Suzuki H. A putrescine-inducible pathway comprising PuuE-YneI in which gamma-aminobutyrate is degraded into succinate in Escherichia coli K-12. J Bacteriol. 2010;192:4582–4591. doi: 10.1128/JB.00308-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larqué E., Sabater-Molina M., Zamora S. Biological significance of dietary polyamines. Nutrition. 2007;23(1):87–95. doi: 10.1016/j.nut.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Lefèvre P.L., Murphy B.D. Differential gene expression in the uterus and blastocyst during the reactivation of embryo development in a model of delayed implantation. Methods Mol Biol. 2009;550:11–61. doi: 10.1007/978-1-60327-009-0_2. [DOI] [PubMed] [Google Scholar]
- Lefèvre P.L., Palin M.F., Chen G., Turecki G., Murphy B.D. Polyamines are implicated in the emergence of the embryofrom obligate diapause. Endocrinology. 2011;152(4):1627–1639. doi: 10.1210/en.2010-0955. [DOI] [PubMed] [Google Scholar]
- Lenis Y.Y., Wang X., Tang W., Wu G., Bazer F.W. Effects of agmatine on secretion of interferon tau and catecholamines and expression of genes related to production of polyamines by ovine trophectoderm cells. Amino Acids. 2016;48(10):2389–2399. doi: 10.1007/s00726-016-2216-1. [DOI] [PubMed] [Google Scholar]
- Mateo R.D., Wu G., Bazer F.W., Park J.C., Shinzato I., Kim S.W. Dietary l-arginine supplementation enhances the reproductive performance of gilts. J Nutr. 2007;137:652–656. doi: 10.1093/jn/137.3.652. [DOI] [PubMed] [Google Scholar]
- McCormack S.A., Johnson L.R. Role of polyamines in gastrointestinal mucosal growth. Am J Physiol. 1991;260:G795–G806. doi: 10.1152/ajpgi.1991.260.6.G795. [DOI] [PubMed] [Google Scholar]
- Miller-Fleming L., Olin-Sandoval V., Campbell K., Ralser M. Remaining mysteries of molecular biology: the role of polyamines in the cell. J Mol Biol. 2015;427(21):3389–3406. doi: 10.1016/j.jmb.2015.06.020. [DOI] [PubMed] [Google Scholar]
- Milovic V.I. Polyamines in the gut lumen: bioavailability and biodistribution. Eur J Gastroenterol Hepatol. 2001;13(9):1021–1025. doi: 10.1097/00042737-200109000-00004. [DOI] [PubMed] [Google Scholar]
- Minois N. Molecular basis of the 'anti-aging' effect of spermidine and other natural polyamines—a mini-review. Gerontology. 2014;60(4):319–326. doi: 10.1159/000356748. [DOI] [PubMed] [Google Scholar]
- Murphy V.E., Smith R., Giles W.B., Clifton V.L. Endocrine regulation of human fetal growth: the role of the mother, placenta, and the fetus. Endocr Rev. 2006;27(2):141–169. doi: 10.1210/er.2005-0011. [DOI] [PubMed] [Google Scholar]
- Pegg A.E. Mammalian polyamine metabolism and function. IUBMB Life. 2009;61(9):880–894. doi: 10.1002/iub.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendeville H., Carpino N., Marine J.C., Takahashi Y., Muller M., Martial J.A. The ornithine decarboxylase gene is essential for cell survival during early murine development. Mol Cell Biol. 2001;21(19):6549–6558. doi: 10.1128/MCB.21.19.6549-6558.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson L. Polyamine homeostasis. Essays Biochem. 2009;46:11–24. doi: 10.1042/bse0460002. [DOI] [PubMed] [Google Scholar]
- Piletz J.E., Aricioglu F., Cheng J.T., Fairbanks C.A., Gilad V.H., Haenisch B. Agmatine: clinical applications after 100 years in translation. Drug Discov Today. 2013;18:880–893. doi: 10.1016/j.drudis.2013.05.017. [DOI] [PubMed] [Google Scholar]
- Ramani D., De Bandt J.P., Cynober L. Aliphatic polyamines in physiology and diseases. Clin Nutr. 2014;33(1):14–22. doi: 10.1016/j.clnu.2013.09.019. [DOI] [PubMed] [Google Scholar]
- Reis D.J., Regunathan S. Agmatine: an endogenous ligand at imidazoline receptors is a novel neurotransmitter. Ann N Y Acad Sci. 1999;881:65–80. doi: 10.1111/j.1749-6632.1999.tb09343.x. [DOI] [PubMed] [Google Scholar]
- Reynolds L.P., Redmer D.A. Angiogenesis in the placenta. Biol Reprod. 2001;64(4):1033–1040. doi: 10.1095/biolreprod64.4.1033. [DOI] [PubMed] [Google Scholar]
- Tabor C.W., Tabor H. Methionine adenosyltransferase (S-adenosylmethionine synthetase) and S-adenosylmethionine decarboxylase. Adv Enzymol Relat Areas Mol Biol. 1984;56:251–282. doi: 10.1002/9780470123027.ch4. [DOI] [PubMed] [Google Scholar]
- Vosatka R.J., Hassoun P.M., Harvey-Wilkes K.B. Dietary L-arginine prevents fetal growth restriction in rats. Am J Obstet Gynecol. 1998;178(2):242–246. doi: 10.1016/s0002-9378(98)80007-0. [DOI] [PubMed] [Google Scholar]
- Wang X., Ying W., Dunlap K.A., Lin G., Satterfield M.C., Burghardt R.C. Arginine decarboxylase and agmatinase: an alternative pathway for de novo biosynthesis of polyamines for development of mammalian conceptuses. Biol Reprod. 2014;90:84. doi: 10.1095/biolreprod.113.114637. [DOI] [PubMed] [Google Scholar]
- Wolff E.C., Kang K.R., Kim Y.S., Park M.H. Posttranslational synthesis of hypusine: evolutionary progression and specificity of the hypusine modification. Amino Acids. 2007;33(2):341–350. doi: 10.1007/s00726-007-0525-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G., Morris S.M., Jr. Arginine metabolism: nitric oxide and beyond. Biochem J. 1998;336(1):1–17. doi: 10.1042/bj3360001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G., Bazer F.W., Hu J., Johnson G.A., Spencer T.E. Polyamine synthesis from proline in the developing porcine placenta. Biol Reproduction. 2005;72(4):842–850. doi: 10.1095/biolreprod.104.036293. [DOI] [PubMed] [Google Scholar]
- Wu G., Bazer F.W., Wallace J.M., Spencer T.E. Intrauterine growth retardation: implications for the animal sciences. J Anim Sci. 2006;84(9):2316–2337. doi: 10.2527/jas.2006-156. [DOI] [PubMed] [Google Scholar]
- Wu G., Bazer F.W., Datta S., Johnson G.A., Li P., Satterfield M.C. Proline metabolism in the conceptus: implications for fetal growth and development. Amino Acids. 2008;35(4):691–702. doi: 10.1007/s00726-008-0052-7. [DOI] [PubMed] [Google Scholar]
- Wu G., Bazer F.W., Satterfield M.C., Li X., Wang X., Johnson G.A. Impacts of arginine nutrition on embryonic and fetal development in mammals. Amino acids. 2013;45(2):241–256. doi: 10.1007/s00726-013-1515-z. [DOI] [PubMed] [Google Scholar]
- Xiao X.M., Li L.P. L-Arginine treatment for asymmetric fetal growth restriction. Int J Gynaecol Obstet. 2005;88:15–18. doi: 10.1016/j.ijgo.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Xing Yi, Yuanliang M.O., Dongmei J., Bo K., Hui H.E., Rong M.A. Biological functions of polyamine and its regulatory mechanisms. Chin J Anim Nutr. 2014;2 [Google Scholar]
- Zanelli C.F., Valentini S.R. Is there a role for eIF5A in translation? Amino Acids. 2007;33(2):351–358. doi: 10.1007/s00726-007-0533-0. [DOI] [PubMed] [Google Scholar]
- Zeng X., Wang F., Fan X., Yang W., Zhou B., Li P. Dietary arginine supplementation during early pregnancy enhances embryonic survival in rats. J Nutr. 2008;138:1421–1425. doi: 10.1093/jn/138.8.1421. [DOI] [PubMed] [Google Scholar]
- Zhao Y.C., Chi Y.J., Yu Y.S., Liu J.L., Su R.W., Ma X.H. Polyamines are essential in embryo implantation: expression and function of polyamine-related genes in mouse uterus during peri-implantation period. Endocrinology. 2008;149:2325–2332. doi: 10.1210/en.2007-1420. [DOI] [PubMed] [Google Scholar]
- Zwierzchowski L., Członkowska M., Guszkiewicz A. Effect of polyamine limitation on DNA synthesis and development of mouse preimplantation embryos in vitro. J Reprod Fertil. 1986;76(1):115–121. doi: 10.1530/jrf.0.0760115. [DOI] [PubMed] [Google Scholar]