Abstract
The effects of ultraviolet-B (UV-B) radiation on stomatal conductance (gs) in pea (Pisum sativum L.), commelina (Commelina communis L.), and oilseed rape (Brassica napus L.) plants were investigated. Plants were grown in a greenhouse either with three different high ratios of UV-B to photosynthetically active radiation or with no UV-B radiation. Pea plants grown in the highest UV-B radiation (0.63 W m−2) exhibited a substantial decrease of adaxial and abaxial gs (approximately 80% and 40%, respectively). With growth in 0.30 W m−2 of UV-B adaxial gs was decreased by 23%, with no effect on abaxial gs, and lower UV-B irradiance of 0.21 W m−2 had no effect on either surface. Although abaxial gs increased when leaves were turned over in control plants, it did not in plants grown with the highest UV-B. Adaxial gs in commelina and oilseed rape also decreased on exposure to high UV-B (0.63 W m−2). For previously unexposed pea plants the time course of the effect of UV-B on gs was slow, with a lag of approximately 4 h, and a time constant of approximately 3 h. We conclude that there is a direct effect of UV-B on stomata in addition to that caused by changes in mesophyll photosynthesis.
On exposure to increased levels of UV-B radiation, many plant species exhibit reductions in their net photosynthetic rate and productivity (Teramura and Ziska, 1996). High UV-B irradiance has been shown to inhibit photosynthesis in pea (Nogués and Baker, 1995), oilseed rape (Allen et al., 1997), soybean (Middleton and Teramura, 1993), rice (Ziska and Teramura, 1992), and algae (Lesser, 1996). Such inhibition of photosynthetic competence primarily involves the loss of both Rubisco activity and content (Allen et al., 1997), but is also associated with the loss of activity of sedoheptulose 1,7-biphosphatase (Allen et al., 1998), and probably that of other Calvin cycle enzymes, and is sometimes associated with damage to PSII photochemistry (Nogués and Baker, 1995; Baker et al., 1997; Allen et al., 1998).
It is not clear whether changes in stomatal function play a major role in the UV-B-induced inhibition of CO2 assimilation. An increase in stomatal limitation observed in oilseed rape (Allen et al., 1997) and soybean (Middleton and Teramura, 1993), together with a reduction in the intercellular CO2 concentration (ci) in pea (Day and Vogelmann, 1995), suggests that there may be a direct UV-B effect on stomatal function. However, it is widely reported that any UV-B effects on stomata do not affect CO2 assimilation (Murali and Teramura, 1986; Sullivan and Teramura, 1989; Teramura et al., 1991; Ziska and Teramura, 1992). Recent studies on pea leaves developed under high UV-B irradiance showed that there were no changes in any photosynthetic parameter measured: light-saturated net CO2 assimilation rate (Asat), maximum carboxylation velocity of Rubisco (Vcmax), maximum potential rate of electron transport contributing to RuBP regeneration (Jmax), ratio of variable to maximal chlorophyll fluorescence yield (Fv/Fm), and the relative quantum efficiency of PSII photochemistry (φPSII) although there were reductions of adaxial stomatal conductance (gs), but not abaxial gs (Nogués et al., 1998). The effects on adaxial gs were mediated by changes in aperture, as there was no reduction in stomatal density in these pea leaves (Nogués et al., 1998). This demonstrated direct effects of high UV-B on gs in the long term (days). In contrast, small (30%) increases in the natural dose had no measurable effects on the gs of pea plants grown in the field (Allen et al., 1999).
The objective of this study was to further characterize the effect of UV-B radiation on gs. We studied the effect of growth under three different ratios of UV-B to PAR or with no UV-B radiation on adaxial and abaxial gs in leaves of pea (Pisum sativum). Only at the higher UV-B irradiances (>3× maximum midsummer UK values) was gs reduced, and the adaxial surface was more affected. This effect of high UV-B was confirmed in two other species, commelina (Commelina communis) and oilseed rape (Brassica napus). Clearly, abaxial stomata are exposed to a lower UV-B irradiance than those on the adaxial surface, and therefore the possibility that the abaxial stomata were similarly sensitive was investigated by inverting leaves in pea plants. The effect of sudden exposure on plants grown without UV-B on gs was also examined over several days, together with recovery of gs in those grown in UV-B when the UV-B was removed. Finally, the detailed time course of the UV-B effect on gs and the net CO2 assimilation rate (A) was characterized. The results strongly suggest that there is a direct UV-B effect on stomata, together with additional effects caused by changes in mesophyll photosynthetic activity.
MATERIALS AND METHODS
Plant Material
Pea (Pisum sativum L. cv Meteor), commelina (Commelina communis L.), and oilseed rape (Brassica napus L. cv Apex) plants were grown from seed in pots in a greenhouse as described by Nogués et al. (1998). Minimum PPFD during a 14-h photoperiod was maintained at approximately 500 μmol m−2 s−1 by supplementary lighting from high-pressure sodium lamps (SON-T DLS 400 W, Thorn, G.E. Lighting, Kingston-upon-Thames, UK). Temperature and the leaf-to-air vapor pressure difference (VPD) were maintained at approximately 23°C/19°C and 1.7/1.3 kPa day/night, respectively.
Exposure to Different UV-B Irradiance and Leaf Inversion Experiments
After the pea seeds were sown, pots were placed in a transparent UV-exposure cabinet within the greenhouse, as described by Allen et al. (1997). The UV-C radiation was screened out by cellulose diacetate film, and the control treatments were under the same configuration of lamps as the UV-B treatments, but the UV-B was screened out with Mylar-D film. The UV spectrum at the top of the plants was measured with a scanning spectroradiometer (SR 991-PC, Macam Photometrics, Livingston, UK) and was the same as that previously described (Allen et al., 1997). Greenhouse and cabinet transmission of UV-A radiation, supplemented by the UV fluorescent lamps, ensured that UV-A exposure was maintained for photorepair and flavonoid biosynthesis (Teramura and Ziska, 1996). Plants were grown throughout their development without UV-B or with three different UV-B doses. The biologically weighted UV-B dosages over the 14-h exposure period according to the generalized plant action spectrum (normalized to 300 nm; Caldwell, 1971) for the high-, medium-, and low-UV-B and control treatments were 0.63 W m−2 (32 kJ m−2 d−1), 0.30 W m−2 (15 kJ m−2 d−1), 0.21 W m−2 (11 kJ m−2 d−1), and 0.001 W m−2, respectively. The UV-exposure cabinet was divided into four independent sections, and plants and treatments were regularly exchanged between these sections to minimize any between-section differences other than UV-B treatments. Individual plants were considered as replicates in all statistical analyses. The experiment started with 18 plants in each section. After 21 d of growth from sowing under control or different UV-B treatments, the sixth leaf pair (numbered from the base, i.e. chronologically) of six plants was turned over in situ, leaves were held in an inverted position using fine nylon line for 9 d, and these were compared with six plants with normally positioned leaves.
The adaxial and abaxial gs were measured in situ between midday and early afternoon on both normal and inverted leaves every day using a transit-time porometer (AP4, Delta-T Devices, Cambridge, UK), taking measurements from six leaves per treatment according to the method of Nogués et al. (1998). The sixth leaf pair (fully expanded on d 21) was used for all measurements.
In a second experiment, plants of oilseed rape were also grown in the same experimental system, but only at the highest UV-B dose (0.63 W m−2). Measurements of gs were taken after full expansion of both first and second true leaves. In a third experiment, commelina plants were grown in the greenhouse and after 21 d they were placed in the control and high UV-B sections of the UV-exposure cabinet described above for 5 d. The adaxial and abaxial gs were measured in situ around midday as above after 5 d of high-UV-B or control treatments.
Sudden Exposure and Recovery Experiments in Pea
After 5 d of the above experiment with pea, the remaining six plants were transferred from high UV-B (0.63 W m−2) to the control treatment to determine recovery, and simultaneously six control plants were transferred to the high-UV-B treatment to determine the kinetics of the effect on stomata. Measurements of gs were taken on the seventh leaf (fully expanded on d 26).
Kinetics of Stomatal Closure in Response to UV-B
Pea plants were grown in the greenhouse described above for 21 d without UV-B. Attached mature leaves were then enclosed for 14 h in a temperature-controlled leaf cuvette connected to a programmable gas-exchange system (model MPH-1000, Campbell Scientific, Logan, UT) incorporating an IR gas analyzer (model LI-6262, LI-COR, Lincoln, NE). The glass top of the cuvette was replaced by 2-mm-thick quartz glass (Optiglass, Essex, UK), allowing UV-B radiation to reach the leaf tissue. UV-B radiation was provided by two UV-B tubes (model TL40W, Philips, Hamburg, Germany) mounted above the chamber. The UV spectrum reaching the leaves was measured with the scanning spectroradiometer with the sensor placed below the quartz glass and was the same as in the UV-exposure cabinet. The biologically weighted UV-B dosages were the same as the high UV-B and control treatments used in the cabinet (i.e. 0.63 and 0.001 W m−2, respectively). Leaf temperature was maintained at 25°C ± 0.5°C, with 800 μmol m−2 s−1 of incident PPFD and a VPD of 1.5 kPa.
At the beginning and end of the 14-h measurement period, analyses of the response of net carbon assimilation to intercellular CO2 concentration at 1200 μmol m−2 s−1 of incident PPFD were carried out to separate possible limitations imposed by stomata, the carboxylation velocity, and the capacity for regeneration of RuBP on leaf photosynthesis (Allen et al., 1997).
RESULTS
Exposure to Different UV-B Irradiances
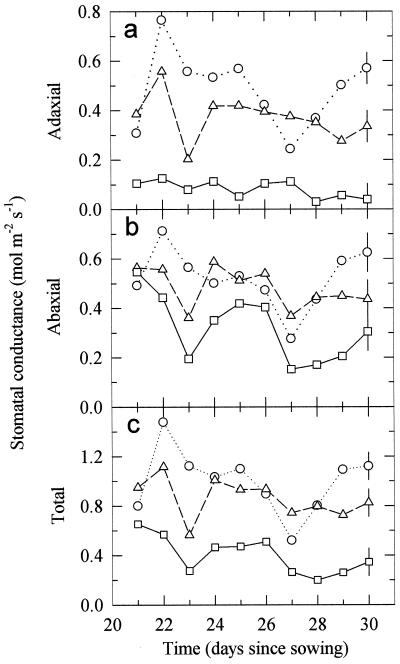
To evaluate the UV-B dose that affects gs, pea plants were grown throughout their development without UV-B or with three different UV-B doses (0.21, 0.30, and 0.63 W m−2; Fig. 1). For clarity, results from the low-UV-B dose, 0.21 W m−2, are not shown since they were indistinguishable from the controls. Growth of pea plants under the high dose of UV-B radiation (0.63 W m−2) reduced adaxial gs by 83% (Fig. 1a; Table I) compared with the control (no UV-B) plants, and abaxial gs by 39% (Fig. 1b; Table I). Therefore, the total gs (Fig. 1c) decreased. The medium UV-B dose (0.30 W m−2) reduced adaxial gs slightly (23%, Table I), but had no significant effect on abaxial gs. There were no significant effects on either adaxial or abaxial gs when pea plants were grown under the low-UV-B dose (0.21 W m−2). It should be noted that while artificial lighting was used in the exposure cabinets and the greenhouse was approximately temperature controlled, the environmental conditions were not constant, and some of the day-to-day variation was caused by varying environmental conditions and by leaf aging.
Figure 1.
Changes in the adaxial (a), abaxial (b), and total (adaxial plus abaxial) (c) gs for mature pea leaves during 10 d of UV-B treatment. Plants were grown from seed for 21 d prior to these measurements either without UV-B radiation (○) or with 0.30 (▵) or 0.63 (□) W m−2 of UV-B radiation. Another treatment of 0.21 W m−2 of UV-B had no detectable effect, and is not shown. Data are the means of six replicates ± 1 pooled se derived from ANOVA shown on the last day.
Table I.
The effects of UV-B exposure during growth on gs of pea leaves
| UVB treatment | Normal Leaf Position
|
||
|---|---|---|---|
| Adaxial | Abaxial | Total | |
| mol m−2 s−1 | |||
| Control | 0.484 a | 0.520 a | 1.000 a |
| Low | 0.435 ab | 0.496 a | 0.930 ab |
| Medium | 0.371 b | 0.482 a | 0.859 b |
| High | 0.082 c | 0.319 b | 0.401 c |
| se | 0.020 | 0.024 | 0.036 |
| Leaves Inverted
|
|||
|---|---|---|---|
| Adaxial inverted | Abaxial inverted | Total | |
| Control | 0.081 a | 0.770 a | 0.851 a |
| Low | 0.120 ab | 0.666 ab | 0.786 ab |
| Medium | 0.130 b | 0.577 b | 0.709 b |
| High | 0.036 c | 0.340 c | 0.376 c |
| se | 0.012 | 0.029 | 0.034 |
Results averaged over 9 or 10 d after growth from seed for 21 d either without UV-B radiation (control), or with 0.21 (low), 0.30 (medium), or 0.63 (high) W m−2 UV-B. Total gs is the sum of abaxial and adaxial gs. Means and pooled se for each UVB treatment were calculated from separate ANOVA for each parameter with data from single leaves on six plants in each treatment, with leaves in the normal position (average over 10 d) or leaves inverted after the 1st d of measurement (average over 9 d). Means within the same part of a column (either normal or inverted) followed by same letter are not significantly different (P > 0.05).
Leaf Inversion Experiments
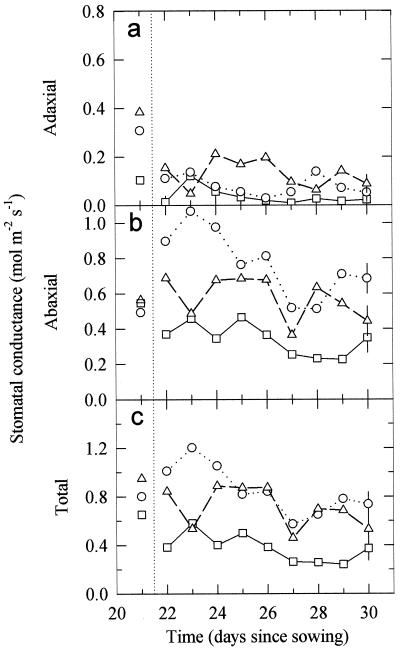
To investigate whether the inhibition of adaxial gs is a direct result of higher UV-B irradiances on this surface, pea leaves were turned over for 9 d in the different UV-B treatments (Fig. 2; for clarity, data from the low-UV-B dose, 0.21 W m−2, are not shown as they were indistinguishable from the controls). In all treatments leaf inversion resulted in a reduction in adaxial gs, as this surface now received less PPFD (Fig. 2a; Table I), although the effect was not large for the highest UV-B treatment, where adaxial gs was very low prior to inversion (see also Fig. 1a). In the control (no UV-B), low-, and medium-UV-B treatments, inversion caused a substantial increase in abaxial gs, as this surface was now illuminated directly with PPFD (compare Fig. 2b with Fig. 1b; Table I). In the highest UV-B-irradiated plants there was no increase in abaxial gs when the leaves were turned over due to the simultaneous increase in UV-B irradiation, despite the increase in PPFD (Fig. 2b; Table I). However, the direct exposure to higher irradiance of UV-B did not cause any appreciable decrease in abaxial gs (Table I). Therefore, for the high-UV-B treatment the total gs (Fig. 2c) was approximately the same in inverted leaves as normal leaves, but lower for the control, low-, and medium-UV-B treatments.
Figure 2.
Changes in the adaxial (a), abaxial (b), and total (adaxial plus abaxial) (c) gs for mature pea leaves during 9 d of UV-B treatment after leaves were turned over (indicated by the dotted line). The plants were grown from seed for 21 d prior to these measurements either without UV-B radiation (○) or with 0.30 (▵) or 0.63 W m−2 (□) of UV-B radiation. Another treatment of 0.21 W m−2 of UV-B radiation had no detectable effect, and is not shown. Data are the means of six replicates ± 1 pooled se derived from ANOVA shown on the last day.
Sudden Exposure and Recovery Experiments
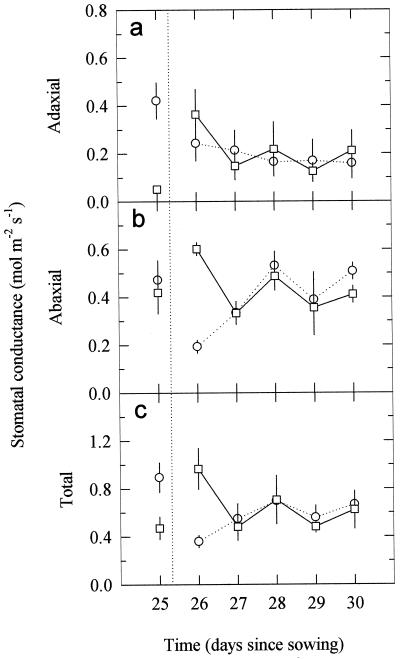
A reciprocal transfer of pea plants from control (no UV-B) and high-UV-B growth treatments (0.63 W m−2) for 5 d showed large effects on the 1st d (Fig. 3). Initial gs values were similar to those of plants shown in Figure 1. After the 1st d of exposure of control plants to UV-B radiation, adaxial gs decreased by approximately 42% (Fig. 3a), with further decreases subsequently. The abaxial gs (Fig. 3b) also decreased sharply on the 1st d, but subsequently recovered to a level similar to that in the beginning of the experiment. When UV-B-irradiated pea plants were transferred to the control (no UV-B) treatment (Fig. 3a), there was an initial large increase in adaxial gs, followed by a decline to very similar values to those of plants moved into UV-B and comparable to those of plants continually exposed to high UV-B (compare with Fig. 1a). Abaxial and total gs increased on the 1st d after transfer out of UV-B (Fig. 3, b and c), but thereafter in both transferred groups of plants gs values on either side of the leaf were indistinguishable, and were similar to those in plants continually exposed to high UV-B (Fig. 1, but note these were leaf 6, not leaf 7). Adaxial gs of leaf 6 for no UV-B and high UV-B plants not transferred over this same period was 0.422 ± 0.075 and 0.128 ± 0.052 mol m−2 s−1, respectively, and for the abaxial surface, gs was 0.472 ± 0.082 and 0.419 ± 0.088 mol m−2 s−1, respectively.
Figure 3.
Changes in the adaxial (a), abaxial (b), and total (adaxial plus abaxial) (c) gs over 5 d for previously unexposed 21-d-old pea plants moved to 0.63 W m−2 of UV-B (○) or for previously exposed plants moved to no UV-B (□). The vertical dotted line indicates the time of transfer. Data are the means ± se of six replicates (se values are shown when larger than the symbols).
When mature commelina leaves previously unexposed to UV-B were irradiated with high UV-B for 5 d, the adaxial gs was reduced by approximately 40% (Table II), somewhat less than that shown for the previously unexposed pea in Figure 3. Oilseed rape plants grown in high UV-B also showed similar reductions in adaxial gs. For all of this material the reductions in adaxial gs led to reductions in total gs, as the abaxial gs was either unchanged or reduced.
Table II.
Effect of UV-B irradiation (0.63 W m−2) on gs
| Species | Leaf Position | Control | UV-B | Percentage Reduction | P |
|---|---|---|---|---|---|
| mol m−2 s−1 | |||||
| C. communis (n = 5) | Adaxial | 0.09 ± 0.02 | 0.06 ± 0.01 | 39 | 0.099 |
| Abaxial | 0.33 ± 0.04 | 0.27 ± 0.03 | 17 | NS | |
| Total | 0.43 ± 0.05 | 0.33 ± 0.03 | 22 | 0.041 | |
| B. napus (leaf 1) (n = 6) | Adaxial | 0.33 ± 0.03 | 0.18 ± 0.03 | 46 | 0.002 |
| Abaxial | 0.63 ± 0.06 | 0.49 ± 0.06 | 22 | 0.073 | |
| Total | 0.96 ± 0.07 | 0.67 ± 0.07 | 31 | 0.007 | |
| B. napus (leaf 2) (n = 6) | Adaxial | 0.39 ± 0.03 | 0.14 ± 0.01 | 65 | <0.001 |
| Abaxial | 0.57 ± 0.07 | 0.58 ± 0.11 | — | NS | |
| Total | 0.96 ± 0.08 | 0.71 ± 0.11 | 26 | 0.045 | |
Measurements on (a) leaves of mature C. communis previously unexposed after 5 d of irradiation (b & c) 1st and 2nd leaves of B. napus cv Apex after growth with 0.63 W m−2 of UV-B radiation. Total gs is the sum of abaxial and adaxial gs. Means ± se are given. P, The probability of difference between treatments from one-tailed t test; NS, P > 0.10.
Kinetics of Stomatal Closure in Response to UV-B
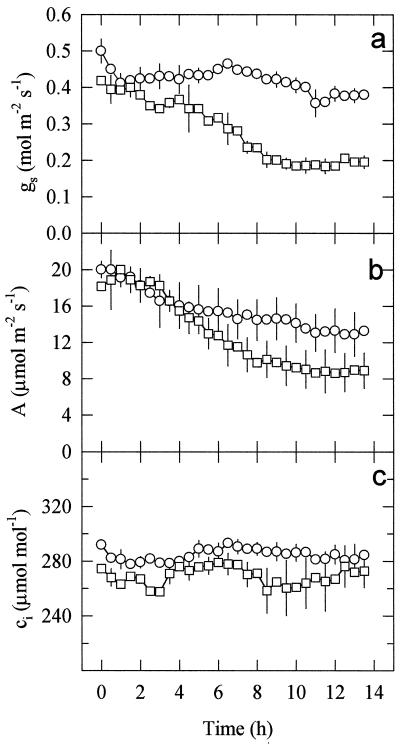
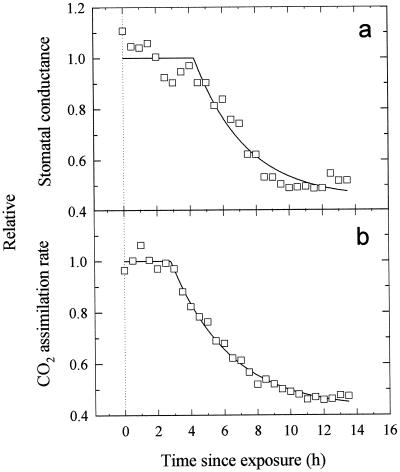
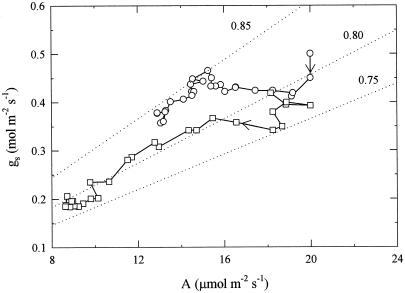
To evaluate with higher temporal resolution the time course of UV-B-induced stomatal closure, attached, mature pea leaves (grown without UV-B exposure) were enclosed in a leaf cuvette connected to a gas-exchange system, and illuminated with 800 μmol m−2 s−1 of PPFD for 14 h either without UV-B or with high UV-B (Fig. 4). While VPD, PPFD, and chamber CO2 concentration were closely controlled, the gas exchange system estimated total gs only, as the whole leaf was enclosed. In control leaves total gs and A had decreased by approximately 20% by the end of the 14-h measurement period and ci had not significantly changed. In leaves irradiated with 0.63 W m−2 of UV-B, both gs and A started to drop within 3 h of the start of irradiation, and after 14 h of treatment they had both decreased by approximately 50% (Fig. 4, a and b). However, ci remained almost constant at first, only decreasing 20 to 25 μmol mol−1 after about 7 h (Fig. 4c). The time courses of A and gs after exposure to UV-B-fitted exponential declines well (Fig. 5, r2 = 0.991 and 0.948, respectively, using nonlinear regression), although there was some uncertainty over the early part of the time course for gs. The exponential model gave lag times of 2.8 h (±0.12 se) and 4.3 h (±0.30 se) for A and gs, respectively, but similar time constants (3.51 and 3.11 h, respectively, not significantly different), and final estimated reductions of 57.6% and 55.2%, respectively (not significantly different). This suggests a close coupling of A and gs (Fig. 6). In the leaves that were not exposed to UV-B, the small decline of gs and the larger decline in A during the experiment resulted in the slope of the gs/A relationship (Fig. 6) increasing slightly from that equivalent to a ratio of intercellular to ambient CO2 (ci/ca) of about 0.80 to that equivalent to 0.85. In the UV-B-irradiated leaves the more closely matching declines in A and gs over a wider range than in the control plants resulted in a more constant gs/A, with a value equivalent to a ci/ca ratio between 0.80 and 0.75.
Figure 4.
Changes in gs, A, and ci in illuminated mature pea leaves throughout 14 h of no UV-B (○) or 0.63 W m−2 of UV-B (□) irradiation treatments in a leaf chamber. Incident PPFD was 800 μmol m−2 s−1 and leaf temperature was maintained at 25°C ± 0.5°C, with a VPD of 1.5 kPa. Data are the means ± se of three replicates (se values are shown when larger than the symbols).
Figure 5.
Exponential decline time courses of total gs (a) and A (b) in illuminated mature pea leaves after exposure at t = 0 to 0.63 W m−2 of UV-B irradiation. Lines shown were fitted by nonlinear regression using the model y = yf + ae−b(t−to), where yf is the estimated final value, b is (time constant)−1, to is the lag time, and a the overall change in y. Symbols indicate means of three leaves replotted from Figure 4.
Figure 6.
Relationship between total gs and A in illuminated mature pea leaves throughout 14 h of no UV-B (○) and 0.63 W m−2 of UV-B (□) treatments. Points were joined in the time course in the order indicated by the arrows. Dotted lines indicate the relationship between gs and A if the ratio ci/ca was constant at the value indicated. Data are the means of three replicates, and are replotted from Figure 4.
Analyses of the response of A to ci were carried out at the beginning and at the end of the 14-h measurement period to characterize the effect of the UV-B on photosynthesis. After 14 h of constant illumination in the leaf cuvette without UV-B the CO2-saturated net CO2 assimilation rate (Amax), the Asat, Vcmax, and the Jmax were decreased by approximately 20% to 30% compared with the values obtained at the beginning of the measurement period (Table III). There was little change in stomatal limitation, indicating close coupling between mesophyll assimilation and stomatal aperture as leaf activity changed. In comparison, irradiation for 14 h with high UV-B caused larger reductions of Asat (55% compared with 28%) and increased the limitation imposed by stomata to CO2 uptake (l) from approximately 12% to 20% (Table III).
Table III.
Analysis of the response of A to ci in pea leaves before or after exposure to high-UV B (0.63 W m−2) irradiation for 14 h
| Parameter | Control
|
+UV-B
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 h | 14 h | P | Percentage change | 0 h | 14 h | P | Percentage change | Pdiff | |
| Amax (μmol m−2 s−1) | 35.8 ± 1.4 | 27.8 ± 2.5 | 0.033 | −22 | 25.6 ± 5.6 | 16.3 ± 3.0 | 0.179 | −28 | NS |
| Asat (μmol m−2 s−1) | 23.3 ± 1.0 | 16.9 ± 1.5 | 0.004 | −28 | 18.2 ± 2.4 | 7.7 ± 1.4 | 0.054 | −55 | 0.050 |
| Vc,max (μmol m−2 s−1) | 99.5 ± 7.1 | 66.1 ± 5.8 | 0.011 | −34 | 70.1 ± 10.6 | 42.2 ± 11.1 | 0.160 | −33 | NS |
| Jmax (μmol m−2 s−1) | 238 ± 11 | 159 ± 19 | 0.002 | −33 | 173 ± 37 | 99 ± 28 | 0.169 | −34 | NS |
| l (%) | 11.1 ± 1.4 | 12.4 ± 1.2 | 0.050 | +1 | 12.1 ± 2.5 | 19.8 ± 1.9 | 0.108 | +8 | 0.110 |
Parameters estimated from analysis were: Amax, Asat, Vc,max, Jmax, and l. During measurement PPFD was 1200 μmol m−2 s−1, leaf temperature was 25°C ± 0.5°C, and VPD = 1.5 kPa. Values shown are means ± 1 se of three replicates. P, Probability for observed differences between 0- and 14-h measurements for each treatment, calculated from paired, one-tailed t test; Pdiff, probability for difference between treatments in percentage change, calculated from one-tailed t test. NS, Pdiff > 0.20.
DISCUSSION
Growth of pea plants in high (0.63 W m−2) and medium (0.30 W m−2) UV-B radiation doses resulted in a substantial decrease of gs (Fig. 1; Table I), with a much larger effect on adaxial than on abaxial gs. However, the lowest doses observed to exert significant effects (0.30 W m−2) were approximately three times the current maximum midsummer UK exposure. There were similar reductions in gs in commelina and oilseed rape plants (Table II), indicating that this is a general effect. In our previous work with pea (Nogués et al., 1998), although the decline in total gs was very similar to that reported here, only adaxial gs was affected by high UV-B, and that change was mediated by changes in aperture, as there was no reduction in stomatal density (number of stomata per millimeter). The results from the leaf inversion experiments with pea (Fig. 2) and the transfer experiments for pea (Fig. 3) and commelina (Table II) also show that UV-B affected stomatal aperture, as changes in cell development and stomatal density cannot be involved over the short time scales of these gs changes in fully developed leaves.
We conclude that the UV-B affects guard cells directly, independently of changes in the mesophyll photosynthetic activity for three reasons. First, our previous work with pea (Nogués et al., 1988) in an identical experimental arrangement to that used here showed that there were no changes in any photosynthetic parameter measured (Asat, Vcmax, Jmax, Fv/Fm, or φPSII) in plants developed under high UV-B (0.63 W m−2). Second, the effects of UV-B was largest on the exposed adaxial leaf surface. If UV-B was affecting mesophyll photosynthesis it presumably would have affected both leaf surfaces equally. Lastly, on leaf inversion, the light level on the different epidermes is changed by 10- to 50-fold, but photosynthesis should not be affected, as the same total photon flux density is incident on the mesophyll, (see early examples of this technique by Turner, 1970; Pemadasa, 1979). Therefore, in the control plants (Fig. 2), the so-called “direct” response of guard cells to light, which acts independently of the response to ci or to some mesophyll photosynthesis-related signal, resulted in abaxial stomata opening and adaxial stomata closing. However, in the UV-B treatments the opening response of abaxial stomata on inversion was either reduced or eliminated at the highest dose, while the adaxial stomata (with a lower sensitivity to light) closed, demonstrating a direct effect of UV-B on the guard cells. It is interesting to speculate that the larger closing effect of UV-B on adaxial compared with abaxial stomata when equally exposed is related to their well-established lower sensitivity to light (e.g. Pemadasa, 1979; Lu et al., 1993).
Adaxial guard cells receive much higher UV-B irradiation than the mesophyll cells and abaxial guard cells due to attenuation through the leaf by UV-B-adsorbing pigments such as flavonoids, particularly in the epidermis (Bilger et al., 1997; Allen et al., 1998). It is tempting to think of the UV-B induced reduction in gs as “damage” to the stomatal mechanism. However, it should be noted that the stomata most affected in the adaxial surface did still close in response to shading when inverted (Fig. 2a; Table I). In addition, the stomata in the normal adaxial surface (Fig. 1a) and in the inverted abaxial surface still responded to environmental stimuli, as the day-to-day variations closely followed that of control plants (compare Figs. 1 and 2). Even so, upon inversion, stomata in the abaxial surface of the high-UV-B treatment did not open in response to greater illumination (Fig. 2b; Table I).
The results of the sudden exposure and removal of UV-B experiments are intriguing (Fig. 3). For the long-term irradiated plants there was a brief “recovery” on the 1st d after removal of UV-B, followed by a return to previous reduced gs values, suggesting that the effects of UV-B irradiation on gs were persistent. We can offer no explanation for the brief recovery. Plants newly exposed to high UV-B showed a large decline in gs that took 2 to 3 d to reach a new steady value, but which was already marked within 1 d. Further, kinetic analyses (Figs. 4 and 5) showed that the inhibitory effect of UV-B started within 4 to 5 h of the onset of irradiation. However, while there may be a more rapid short-term effect on adaxial gs (perhaps responsible for the drop in total gs evident after approximately 2 h in Fig. 5), the major effect seemed to be associated with the decline in A. Indeed, there was a close but not complete correlation of gs with A (Fig. 6), as was first noted by Wong et al. (1979) and as is often observed with a wide range of environmental conditions.
It should be noted that there is a substantial difference in the effect of UV-B on photosynthesis depending on whether plants have developed under it (as in the plant material used for Figs. 1 and 2, and part of Fig. 3) or whether they are suddenly exposed (plant material in part of Fig. 3 and Figs. 4–6). In previous work with peas we found no effect of high-UV-B dose on Asat, Vc,max, and Jmax when plants were grown under high UV-B (Nogués et al., 1998), but declines in Asat after 12 h of approximately 50% (Nogués and Baker, 1995) for newly exposed plant material, which is consistent with the observed reductions of A shown in Figures 4 and 5, and the declines in Asat shown in Table III. In newly exposed leaves of oilseed rape, the effects on Asat were smaller and took longer, but were still of the order of 50% after 5 d (Allen et al., 1997), and were accompanied by decreases in carboxylation velocity and Rubisco activity and content.
The approximately constant ci value as A changed by 50% might suggest that stomata act to maintain ci constant, but the analyses of Farquhar and colleagues and others (e.g. Farquhar et al., 1978; Wong et al., 1978; Morison and Jarvis, 1983) have shown that usually the sensitivity of stomata to ci is not sufficient to result in a constant ci. Instead, it appears that there is some other mechanism that results in the close coupling of gs and A. Recently, Jarvis and Davies (1998) have revived the proposal of Farquhar and Wong (1984) that stomata respond to a carbon-fixing substrate pool that Jarvis and Davies term the “residual photosynthetic capacity.” The decline in gs observed in Figures 4 and 5 during exposure to high UV-B may be an example of such a finely tuned response of gs to the photosynthetic activity being reduced by UV-B, on which the direct effect of UV-B on stomata, particularly those on the exposed adaxial surface, is superimposed.
The observed increase in stomatal limitation after irradiation with high UV-B (Table III) was similar to that found in oilseed rape plants newly exposed to UV-B (Allen et al., 1997). However, the effect observed here was not large, because photosynthetic capacity declined (indicated by Amax) in addition to the direct effect of UV-B on gs. In contrast, in pea plants grown under high UV-B, the small increase in stomatal limitation was entirely due to reductions in gs (Nogués et al., 1998) as photosynthesis was not affected. It is clear that the high UV-B doses that affect stomata can lead to effects on CO2 assimilation (see introduction).
The mechanism for the UV-B effect on stomata is not known. Stomatal opening follows a K+ influx along a electrochemical gradient formed by ATPase outward proton pumps situated in the guard cell plasmalemma (Zeiger, 1983). Wright and Murphy (1982) have shown that UV-B radiation can induce stomatal closure directly by inhibiting K+ accumulation, and Negash et al. (1987) demonstrated the leakage of 86Rb+ from guard cells in response to UV-B irradiation. By extrapolation from the numerous studies on mesophyll photosynthesis (Allen et al., 1998), these effects could be due to damage to PSII in the guard cells, affecting photophosphorylation and hence ion transport. A second mechanism may involve a direct inhibition by UV-B of the plasmalemma ATPase proton pump (Allen et al., 1998). Alternatively, UV-B may not directly affect the generation of the guard cell turgor pressure, but rather may modify the effect of this turgor on pore size through UV-B-induced changes in the elasticity of the cell walls or the cytoskeleton of guard cells and the neighboring epidermal cells (Allen et al., 1998).
CONCLUSIONS
This study has shown that growth of pea plants in high-UV-B radiation resulted in a decrease of gs, with direct effects on the exposed guard cells (usually the adaxial surface). Leaf inversion experiments showed that both adaxial and abaxial stomata could be directly affected by this UV-B, although it appeared there was different sensitivity of the stomata on the two surfaces, with adaxial stomata being more affected. There was no long-term recovery in gs after cessation of long-term UV-B exposure, indicating that the effect is permanent. The time course of the effect of high-UV-B irradiance on stomata of previously unexposed plants was rapid (a time constant of approximately 3 h after a lag of approximately 4 h), and this was closely correlated with changes in A. We conclude that high-UV-B irradiances affect stomata both directly, by acting on the guard cell aperture control mechanisms, and indirectly, through changes in the mesophyll photosynthesis.
ACKNOWLEDGMENTS
We are grateful to Ian F. McKee for assistance with the Campbell gas-exchange system, and we thank Prof. W.J. Davies for providing commelina seeds.
Footnotes
This research was supported partly by a research grant from Generalitat de Catalunya (grant no. 1996BEAI300222 to S.N.). D.J.A. received a research studentship from the UK Biotechnology and Biological Sciences Research Council.
LITERATURE CITED
- Allen DJ, McKee IF, Farage PK, Baker NR. Analysis of the limitation to CO2 assimilation on exposure of leaves of two Brassica napus cultivars to UV-B. Plant Cell Environ. 1997;20:633–640. [Google Scholar]
- Allen DJ, Nogués S, Baker NR. Ozone depletion and increased UV-B radiation: is there a real threat to photosynthesis? J Exp Bot. 1998;49:1775–1788. [Google Scholar]
- Allen DJ, Nogués S, Morison JIL, Greenslade PD, McLeod AR, Baker NR. A thirty percent increase in UV-B has no impact on photosynthesis in well-watered and droughted pea plants in the field. Global Change Biol. 1999;5:235–244. [Google Scholar]
- Baker NR, Nogués S, Allen DJ. Photosynthesis and photoinhibition. In: Lumsden P, editor. Plants and UV-B: Responses to Environmental Change. Cambridge, UK: Cambridge University Press; 1997. pp. 95–111. [Google Scholar]
- Bilger W, Veit M, Schreiber L, Schreiber U. Measurement of leaf epidermal transmittance of UV radiation by chlorophyll fluorescence. Physiol Plant. 1997;101:754–763. [Google Scholar]
- Caldwell MM. Solar UV irradiation and the growth and development of higher plants. In: Giese AC, editor. Photophysiology. Vol. 6. New York: Academic Press; 1971. pp. 131–177. [Google Scholar]
- Day TA, Vogelmann TC. Alterations in photosynthesis and pigment distribution in pea leaves following UV-B exposure. Physiol Plant. 1995;94:433–440. [Google Scholar]
- Farquhar GD, Dubbe DR, Raschke K. Gain of the feedback loop involving carbon dioxide and stomata. Plant Physiol. 1978;62:406–412. doi: 10.1104/pp.62.3.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar GD, Wong SC. An empirical model of stomatal conductance. Aust J Plant Physiol. 1984;11:191–210. [Google Scholar]
- Jarvis AJ, Davies WJ. The coupled response of stomatal conductance to photosynthesis and transpiration. J Exp Bot. 1998;49:399–406. [Google Scholar]
- Lesser MP. Acclimation of phytoplankton to UV-B radiation: oxidative stress and photoinhibition of photosynthesis are not prevented by UV-absorbing compounds in the dinoflagellate Prorocentrum micans. Mar Ecol Prog Ser. 1996;132:287–297. [Google Scholar]
- Lu Z, Quiñones MA, Zeiger E. Abaxial and adaxial stomata from Pima cotton (Gossypium barbadense L.) differ in their pigment content and sensitivity to light quality. Plant Cell Environ. 1993;16:851–858. [Google Scholar]
- Middleton EM, Teramura AH. The role of flavonol glycosides and carotenoids in protecting soybean from ultraviolet-B damage. Plant Physiol. 1993;103:741–752. doi: 10.1104/pp.103.3.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morison JIL, Jarvis PG. Direct and indirect effects of light on stomata. II. In Commelina communis. Plant Cell Environ. 1983;6:103–109. [Google Scholar]
- Murali NS, Teramura AH. Effectiveness of UV-B radiation on the growth and physiology of field-grown soybean modified by water-stress. Photochem Photobiol. 1986;44:215–219. [Google Scholar]
- Negash L, Jensén P, Björn LO. Effects of ultraviolet radiation on accumulation and leakage of 86Rb+ in guard cells of Vicia faba. Physiol Plant. 1987;69:200–204. [Google Scholar]
- Nogués S, Allen DJ, Morison JIL, Baker NR. Ultraviolet-B radiation effects on water relations, leaf development and photosynthesis in droughted pea plants. Plant Physiol. 1998;117:173–181. doi: 10.1104/pp.117.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogués S, Baker NR. Evaluation of the role of damage to photosystem II in the inhibition of CO2 assimilation in pea leaves on exposure to UV-B. Plant Cell Environ. 1995;18:781–787. [Google Scholar]
- Pemadasa MA. Movements of abaxial and adaxial stomata. New Phytol. 1979;82:69–80. [Google Scholar]
- Sullivan JH, Teramura AH. The effects of ultraviolet-B radiation on loblolly pine: 1. Growth, photosynthesis and pigment production in greenhouse-grown seedlings. Physiol Plant. 1989;77:202–207. [Google Scholar]
- Teramura AH, Ziska LH. Ultraviolet-B radiation and photosynthesis. In: Baker NR, editor. Photosynthesis and the Environment. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1996. pp. 435–450. [Google Scholar]
- Teramura AH, Ziska LH, Sztein AE. Changes in growth and photosynthetic capacity of rice with increased UV-B radiation. Physiol Plant. 1991;83:373–380. [Google Scholar]
- Turner NC. Responses of abaxial and adaxial stomata to light. New Phytol. 1970;69:647–653. [Google Scholar]
- Wong SC, Cowan IR, Farquhar GD. Leaf conductance in relation to rate of assimilation in Eucalyptus pauciflora Sieb. ex Spreng. Plant Physiol. 1978;62:670–674. doi: 10.1104/pp.62.4.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong SC, Cowan IR, Farquhar GD. Stomatal conductance correlates with photosynthetic capacity. Nature. 1979;282:424–426. [Google Scholar]
- Wright LA, Murphy TM. Short-wave ultraviolet light closes leaf stomata. Am J Bot. 1982;69:1196–1199. [Google Scholar]
- Zeiger E. Biology of stomatal guard cells. Annu Rev Plant Physiol. 1983;34:441–475. [Google Scholar]
- Ziska LH, Teramura AH. CO2 enhancement of growth and photosynthesis in rice (Oryza sativa): modification by increased ultraviolet-B radiation. Plant Physiol. 1992;99:473–481. doi: 10.1104/pp.99.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]