Abstract
Practical diets for monogastric livestock must be supplemented with zinc (Zn) due to their high contents of antagonistic substances like phytates. Current feeding recommendations include quite generous safety margins because of uncertainties regarding the gross Zn requirements under varying rearing conditions. Furthermore, the use of pharmacological Zn doses to stabilise animal performance and wellbeing is widespread. Taken together, modern diets for pigs and poultry contain considerably more Zn than necessary to meet animal requirements, which is associated with concerns related to the environment as well as animal and consumer safety. Therefore, European authorities most recently reduced the allowed upper limits for Zn in complete feed. To maintain animal productivity and wellbeing while reducing the Zn load in complete feed, all measures that stabilize feed Zn bioavailability must be applied. Most importantly, reliable information on the gross Zn requirement under practical conditions must be provided, considering the bioavailability of native or supplemented feed Zn, antagonisms with dietary factors as well as the physiological status of the animal.
Keywords: Zinc, Requirement, Recommendation, Pharmacological, Pig, Poultry
1. Introduction
Higher organisms are depending on zinc (Zn) as a structural or catalytic cofactor of peptide biochemistry (Frassinetti et al., 2006). It has been estimated that around 10% of the human genome code for Zn metalloproteins. Taking into account transcript variants of genes, ∼30% of the human proteome appears to be Zn dependent (Andreini et al., 2006). This magnitude may be extrapolated on eukaryotic species in general. Therefore, Zn must be present in adequate amounts and in a bioavailable form within the diet. In case of modern monogastric livestock, this is only the case if Zn is supplemented in significant amounts (NRC, 1994, NRC, 2012). Zinc emissions per unit of animal product to the environment can be quite high, depending on the extent of dietary fortification (Brugger and Windisch, 2015).
This review highlights the challenges and strategies to increase the precision in Zn feeding to monogastric species considering current feeding practices and environmental concerns arising from the usage of Zn supplements. It will focus on the example of the pig as representative of conventional monogastric production systems.
2. Current zinc feeding practices in livestock rearing
In 2001, the European Union banned most sources of animal protein from livestock diets in response to the BSE (bovine spongiform encephalopathy) crisis (European Parliament and the Council of the European Union, 2001). In terms of mineral availability, replacement of animal protein by plant based products put a lot of pressure on monogastric livestock. In nature, pigs and poultry are omnivorous species that depend on the consumption of significant amounts of animal protein (Cheeke and Dierenfeld, 2010). Such feedstuffs contain highly bioavailable (trace) mineral pools due to the low abundance of antagonists (Smith et al., 1962). On the contrary, plant biomass and especially seeds and kernels, which represent the basis of modern monogastric diets, are rich in substances that impair mineral utilization. The most prominent example is phytic acid. This ring-shaped molecule represents the major storage form of phosphorus in plant seeds. Under neutral (pH 7) aqueous conditions, it develops sustainable bonds to divalent cations at their phosphorus residues yielding insoluble complexes, so-called phytates (Humer et al., 2015). In strong contrast to ruminants, pigs and poultry harbour only negligible activities of native phytase (phytate degrading enzymes) within their digestive systems. This renders them almost unable to utilize the mineral pools of plant biomass. Feeding a conventional corn-soybean meal based diet without Zn fortification significantly reduces the Zn status of growing piglets after only 8 days (Brugger et al., 2014). Therefore, phytic acid represents an efficient dietary stimulus for research approaches that aim in promoting Zn deficiency in monogastric species. For example, Windisch and Kirchgessner (1999) have demonstrated that true Zn absorption in 65Zn-labelled adult rats declines dose-dependently in response to increasing dietary contents of phytic acid. They recognized a drop in true feed Zn absorption from ∼70% down to 0 when feeding ZnSO4 supplemented semi-synthetic diets expressing finely-graded differences in phytic acid concentration between 1 and 9 g/kg. As modern pig diets may contain phytic acid at up to 9 g/kg diet (e.g., Brugger et al., 2014), piglets fed practical diets without Zn supplementation express first signs of severe Zn deficiency already within 2 weeks (Windisch, 2003).
Current feeding recommendations for piglets are based on experiments that used factorial approaches to estimate the Zn requirement of pigs in the course of the production cycle (NRC, 2012). This is done by dose–response studies which monitor the change of suitable parameters (e.g., performance parameters, certain Zn status parameters) to a stepwise increase in Zn supplementation to Zn deficient animals. The breakpoint in response marks the point of satisfied gross Zn requirement. It is defined as the net Zn requirement (amount of Zn that must be present behind the gut barrier to maintain physiological function) plus the amount of surplus Zn necessary to compensate the activity of antagonists in the diet as well as incomplete feed Zn absorption. Present Zn feeding recommendations use such thresholds and expand them by generous safety margins. Depending on the source of feeding requirements, the extent of the safety margins may vary significantly. For example, the current recommendations for piglets (7 to 11 kg life weight) by the US-American National Research Council (NRC) and the German Society of Nutrition Physiology (GfE) are 100 and 90 mg/kg diet (90% dietary dry matter), respectively (NRC, 2012, GfE, 2006). In light of an estimated gross Zn requirement of growing piglets under practical feeding conditions of 50 to 60 mg/kg diet (Smith et al., 1958, Smith et al., 1962, Brugger et al., 2014, Lewis et al., 1956, Lewis et al., 1957a, Lewis et al., 1957b, Luecke et al., 1956, Stevenson and Earle, 1956, Miller et al., 1970) this equals safety margins of ∼50% and higher. Such margins reflect great uncertainty regarding the gross Zn requirement under varying rearing conditions as well as fluctuations in dietary contents of native Zn and antinutritive substances.
Apart from Zn supplementation for Zn demand coverage, the use of “pharmacological doses” to early weaned piglets has gained much attention. These are surplus quantities of Zn, which may produce positive effects in the animal that go beyond the coverage of the actual Zn requirement. Since the ban of antibiotic growth promoters, this practice has gained much attention. Indeed, it has been shown that Zn doses from ZnO of ≥2,500 mg/kg diet may enhance daily weight gain and stabilise gut health in early weaned piglets (Poulsen, 1995, Højberg et al., 2005, Hahn and Baker, 1993, Windisch et al., 1998). There are many hypothesis on the basic mode-of-action, like effects on the development of gut microbiota, improvement of intestinal barrier function, modulation of the inflammatory response or the prevention of temporal post-weaning Zn deficiency (Højberg et al., 2005, Vahjen et al., 2010, Vahjen et al., 2011, Pieper et al., 2012, Starke et al., 2013, Starke et al., 2014, Sturniolo et al., 2002, Canani et al., 2005, Carlson et al., 2008, Li et al., 2001, Sargeant et al., 2011, Davin et al., 2013). Yet, there is no clear answer, which indicates that this is a multifactorial event.
Taken together, irrespective of the feeding strategy (following published recommendations, usage of pharmacological doses) state-of-the-art diets for monogastric livestock contain considerable surplus amounts of dietary Zn. This is associated with concerns related to environmental safety as well as animal and consumer health.
3. Concerns arising from surplus zinc in livestock feed
To understand the effects of excessive Zn dosing on the animal organism as well as the environment, it is necessary to understand the basic principles of Zn metabolism. Apart from its essential nature, Zn also exhibits a significant toxic potential if the levels within a biological system exceed a certain threshold. In both cases, the mode-of-action is based on the binding of Zn to peptides. However, under the terms of Zn overload, this interaction may appear to be unspecific and uncontrolled which has been associated with toxic side-effects (Goldhaber, 2003, Valko et al., 2005). Therefore, evolution has evolved a complex network of regulative mechanisms that tightly control Zn uptake from the diet as well as Zn excretion to the environment (Holt et al., 2012, Lichten and Cousins, 2009).
The most important parameters of Zn homeostatic regulation seem to be the absorption of Zn from and the Zn excretion into the gastrointestinal lumen. Earlier studies on 65Zn-labelled rats clearly demonstrated how the relative absorption efficiency increases in times of deficient supply. At the same time, the excretion of endogenous Zn into the gastrointestinal tract decreased to an inevitable amount. The truly and apparently digested amounts of alimentary Zn as well as the Zn losses to the gastrointestinal tract (GIT) exhibited a direct non-linear response to increasing dietary Zn (Weigand and Kirchgessner, 1980, Windisch and Kirchgessner, 1994, Windisch and Kirchgessner, 1999). All parameters showed a significant change in behaviour at a certain Zn dose, which marks the point of satisfied gross Zn requirement. Zinc supplementation above the gross Zn requirement promotes a drastic decline in the relative efficiency of Zn absorption and, at the same time, an increase in the efficiency of Zn excretion. Hence, any quantity of Zn ingested in excess to the metabolic requirement will be inevitably excreted and accumulate in manure on a mid-term scale (Brugger et al., 2014, Windisch et al., 1998, Weigand and Kirchgessner, 1980). The finely orchestrated opposite response of absorptive and excretive pathways equilibrates the basal Zn load behind the gut barrier at a fairly constant level. Even though active transport mechanisms (mainly the apical solute carrier family member A4 [ZIP4]) are downregulated, there is still some passive influx due to the interaction of Zn with transport mechanisms for other divalent cations and maybe even leakage of luminal Zn ions through loosely connected mucosal cells (Martin et al., 2013a, Martin et al., 2013b). Therefore, excessive dosing over several weeks is always associated with Zn accumulation in certain tissue fractions (Pieper et al., 2015, Martin et al., 2013a, Martin et al., 2013b, Schell and Kornegay, 1996, Carlson et al., 1999).
In an earlier study, feeding only ∼30 mg Zn/kg diet above the gross Zn requirement threshold (88.0 vs. 58.0 mg Zn/kg diet under given experimental conditions) promoted a doubling of Zn contents in faeces dry matter (Brugger et al., 2014). Hence, there is an increased risk of Zn accumulation in the environment, namely soil and ground water (Wuana and Okieimen, 2011, German Environment Agency (UBA), 2004, Asada et al., 2010). As mentioned above, Zn has a strong toxic potential. This does not only apply to animal organisms but biological systems in general, including flora and fauna of soils. In fact, increasing Zn load has been associated with a disruption of the soil microbiome, which has negative consequences for plant development (Rout and Das, 2003). Furthermore, the mobility of Zn in soil may be high. Therefore, it is transferred quite quickly to ground water where it represents a potential threat to the drinking water supply chain (UBA, 2004).
As already mentioned, dietary Zn amounts exceeding the actual requirements may accumulate in animal tissue through uncontrolled passive influx. Experiments in piglets receiving pharmacological Zn doses promoted concentrations of ∼1,100 and ∼125 mg Zn/kg dry weight in liver and pancreas, respectively (Pieper et al., 2015, Schell and Kornegay, 1996). This clearly exceeds the basal levels recognised in piglets fed according to the current feeding recommendations (NRC, 2012), e.g., ∼127 and ∼91 mg/kg dry weight of liver and pancreas (Brugger et al., 2014, Brugger and Windisch, 2016). It is not surprising that especially these tissue fractions are affected, as they are involved in the redistribution and excretion (detoxification) of body Zn (Holt et al., 2012). Apart from a potential risk for consumer safety (liver is a potential food of animal origin), tissue accumulation of Zn is accompanied by increased cellular stress (Valko et al., 2005). The functional background of these observations may be a higher synthesis of Zn binding peptides that are associated with intracellular Zn storage and excretion. Pieper et al. (2015) demonstrated that the pancreas of piglets receiving pharmacological doses for several weeks exhibited an increased tissue Zn content (∼4 fold compared with control) and simultaneously higher abundances of metallothioneins, digestive enzymes as well as stress-responsive peptides. Therefore, long-term feeding of excessive amounts of dietary Zn may impair animal wellbeing.
Increasing evidence suggests a connection between high Zn contents within the intestinal chymus and manure, respectively, and an increased abundance of antibiotic-resistant bacteria within (Hölzel et al., 2012, Vahjen et al., 2015). This seems to be an adaptive resistance, which disappears when Zn contents decline to basal levels. Several mechanisms are discussed to underly this co-selection of antibiotic and metal-resistance, representing events of co-resistance (linkage desequilibrium of responsible genes) and cross-resistance (the same gene is responsible for antibiotic as well as metal resistance) (Baker-Austin et al., 2006). Therefore, using pharmacological Zn doses as a health-promoting measure might under certain conditions interfere with veterinary intervention.
Within the European Union, the legislative authorities already recognised the problems associated with high dietary Zn loads. The European Commission most recently implemented regulation (EU) 2016/1095 which reduces the allowed upper limits of Zn in complete feed for pigs to 150 and 120 mg/kg diet (for piglets/sows and fattening pigs/boars, respectively) (European Commission, 2016). This decision was based on an earlier opinion published by the European Food Safety Authority (EFSA) (2014). Within the same document the EFSA recommended even lower contents (−30%) in the presence of 500 FTU phytase activity/kg diet. This might be also implemented into legal regulations on a mid-term scale. Hence, the permissible range for Zn safety margins in complete feed is shrinking. This results in the urgent necessity to increase the precision of Zn feeding.
4. Strategies to increase the precision of feeding zinc to monogastric livestock
Investigations upon Zn loads within manure of pig and cattle producing farms in Central Europe indicated obvious differences. The Zn contents of pig farms exceeded that of cattle farms by 5 orders of magnitude, with peak levels of ∼1,500 mg/kg dry matter (vs. ∼300 mg/kg dry matter on cattle farms) (German Environment Agency (UBA), 2004, Hölzel et al., 2012, Hackenberg et al., 1996, Müller, 1997, Bannick et al., 2001, Müller and Ebert, 2002, Kühnen and Goldbach, 2002, Kickinger et al., 2008). Feeding pigs according to published feeding recommendations (NRC, 2012, Society of Nutrition Physiology (GfE), 2008) yields Zn loads within manure dry matter between 500 and 700 mg/kg (Brugger et al., 2014, Kickinger et al., 2010). Hence, the pig production sector seems to be the predominant user of excessive dietary Zn doses that drastically exceed the current feeding recommendations. New upper limits of 200–450 mg Zn/kg DM are discussed for manure, which are currently only reached by cattle farms.
To reduce the feed Zn content without jeopardising animal productivity and wellbeing, 2 points must be addressed. Apart from increasing the precision in Zn feeding, feed Zn bioavailability from plant biomass must be increased and stabilised by appropriate dietary intervention.
It has been initially discussed that the most important bottleneck of the Zn utilisation from plant biomass is its high content of antinutritive components, especially phytates. Feeding diets free of phytate to growing piglets results in a gross Zn requirement of ∼15 mg/kg diet (Smith et al., 1962, Shanklin et al., 1968). In contrast, under practical feeding conditions (feeding on base of cereals and soybean meal), this threshold rises by ∼3 to 4 orders of magnitude (∼50–60 mg Zn/kg diet) (Smith et al., 1958, Smith et al., 1962, Brugger et al., 2014, Lewis et al., 1956, Lewis et al., 1957a, Lewis et al., 1957a, Luecke et al., 1956, Stevenson and Earle, 1956, Miller et al., 1970). Therefore, dietary interventions to optimise Zn utilisation under the current feeding conditions must compensate the antinutritive potential of plant biomass.
In conventional monogastric production systems, the usages of phytase supplements to break down the phytate complex are already state-of-the-art. For example, Windisch and Kirchgessner (1995) showed that adding 600 FU/kg to diets for pigs and broilers increased the amounts of apparently digested feed Zn by 1.6 and 1.8 orders of magnitude, respectively. Further studies came to comparable conclusions (Ettle et al., 2005, Gebert et al., 1999). Hence, faecal Zn excretion of pigs and poultry at given dietary Zn intake decreases in response to exogenous phytase activity. Anyway, addition of other exogenous enzyme supplements (e.g., proteases, amylases, xylanases, etc.) may also increase the amounts of available minerals at the gut barrier. This may be due to a more efficient breakdown of the feed matrix (Cowieson, 2005). In the overall context of enzyme supplements to monogastric diets, transgenic modification of livestock organisms represents a promising approach. A well-known example is the so-called Enviropig, which expresses a significant phytase activity within saliva (Forsberg et al., 2013). Thereby it is able to better utilize the native mineral pools of plant biomass compared with wildtype pigs (Golovan et al., 2001).
The chemical form (species) in which Zn is supplemented to the diet directly affects the bioavailability of feed Zn from complete feed. Zinc is transported in its ionic form (Zn2+) through biological membranes (Holt et al., 2012, Lichten and Cousins, 2009). The amount of available Zn (free and loosely bound Zn ions) at the gut barrier is a result of the efficiency of digestive processes as well as the interaction of chemical Zn species between each other and with further dietary components (Windisch, 2002). There are still many studies published each year that aim in evaluating the efficacy of certain Zn supplements under practical feeding conditions. However, due to the complex chemical interactions within the GIT as well as differences in the experimental setups, the available data are quite contradictory and in most cases just reflects semi-quantitative comparisons. Furthermore, the in vitro solubility of a Zn supplement has been shown to be not necessarily predictive for its bioavailability (Windisch et al., 2003). A further review article would be necessary to present the available literature in a comprehensive manner. Nevertheless, the conclusion of such a manuscript would be that there exists no reliable data on the gross Zn requirements in response to the usage of certain Zn species in practical diets. One major reason for this is the lack of competitive dose–response studies. Furthermore, the available datasets do not provide information on the chemical species and physical properties (e.g. particle size distribution) of Zn along the GIT. Therefore, we still have just a rudimentary understanding on the functional basis of potential differences between Zn feeding interventions. Hence, the claim to use only Zn supplements with the highest bioavailability to reduce total feed Zn has no solid foundation. Closing these gaps in the datasets would also be interesting regarding the usage of pharmacological doses. For example, it has been shown that a technological processing of ZnO drastically reduces the necessary dose to induce a growth promoting effect compared with regular ZnO (150 vs. 3,000 mg/kg diet for processed vs. regular ZnO) (Morales et al., 2012).
Indeed, the most obvious measure to increase mineral utilization from practical diets would be a reduction in the total amounts of antagonistic substances. For example, a replacement of plant protein sources (mainly soybean and rapeseed meal) by such of animal origin drastically declines the total phytate contents within practical diets. However, current legal regulations forbid a usage of animal protein in practical feeding (European Parliament and the Council of the European Union, 2001). In this context, the search for alternative protein sources with reduced or no contents of antagonists of mineral utilization should be intensified. For example, it is possible to collect protein-rich concentrates from plant juice, which protein values equal that of soybean meal (Brugger et al., 2016, Johns, 1986, Szymczyk et al., 1995). As phytate is predominantly present in seeds and kernels (Humer et al., 2015), the pools of Zn and other minerals within such concentrates may exhibit a high bioavailability. However, more research is necessary to investigate the feed value of such products and especially their impact on the mineral utilization of complete feed.
Taken together, there are promising approaches that can increase and stabilise the availability of feed Zn and, at the same time, reduce the necessary amounts of total Zn in the diet. However, to enable practical pig and poultry feeders to increase the precision of Zn feeding, they need reliable information on the gross Zn requirement, considering differences in the availability of native and supplemented Zn, antagonisms between Zn and other dietary factors as well as the physiological status of the animal. Furthermore, unpredictable events like infection, inflammation, stress, etc., which presumably cause a temporary increase of the daily Zn demand must also be considered.
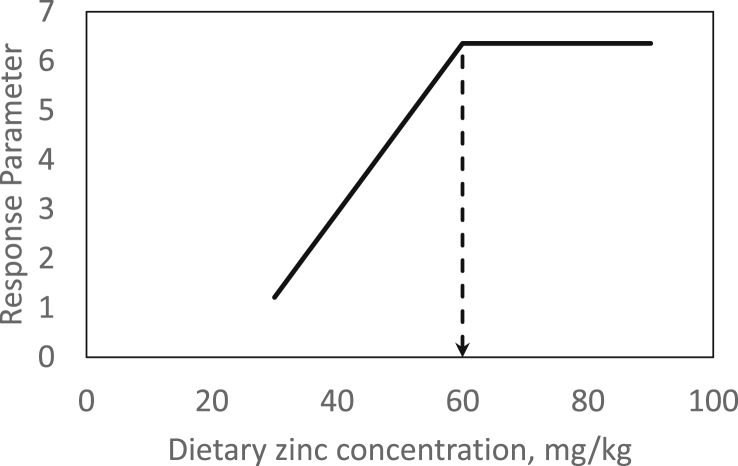
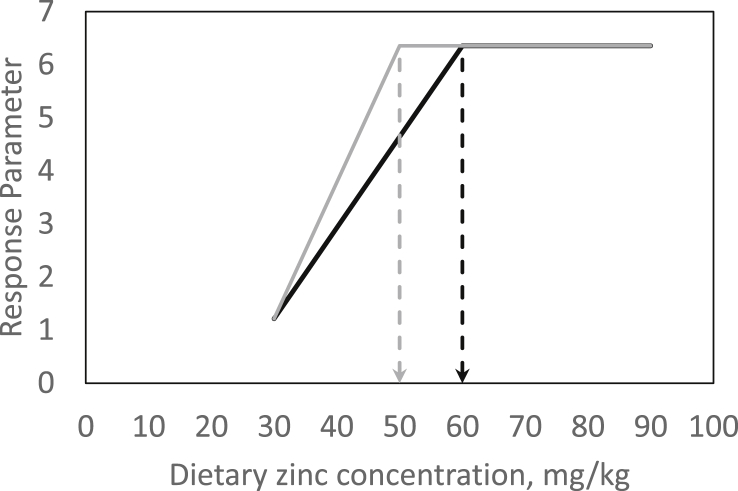
An experimental approach for the estimation of gross Zn requirements must ensure the differentiation between sufficiently and inadequately supplied individuals. Therefore, a dose–response study with finely-graded differences in alimentary Zn supply, spanning the range from potentially deficient levels to mild oversupply, must be applied. Such a model was recently published for piglets (Brugger et al., 2014). In this study, the amount of apparently digested feed Zn was used as response parameter for the estimation of the gross Zn requirement. It has been proven earlier in 65Zn-labelled rats, that this measure directly correlates to the truly absorbed amounts of Zn from the GIT (Weigand and Kirchgessner, 1980). The intensity in response of both parameters to increasing dietary Zn supply significantly changes at the point of satisfied Zn demand. This threshold can be estimated by broken-line regression models (Robbins et al., 2006) (Fig. 1). Such an experiment could also be performed in a competitive manner to compare the availability from certain Zn supplements. Therefore, by doubling the sample size, i.e., 96 instead of 48 animals in case of Brugger et al. (2014), both supplements of interest can be evaluated parallel to each other. Differences in efficacy would be evident by comparing the breakpoints in response to increasing dietary Zn as well as the slopes in response over the deficiently supplied groups to the point of gross Zn requirement, respectively. A higher slope or lower dietary threshold, respectively, would indicate a higher percentage utilization of feed Zn under given dietary conditions (Fig. 2).
Fig. 1.
Theoretical broken-line response of a random zinc (Zn) status parameter to changes in dietary zinc concentration. In this example, the parameter (e.g., apparently digested feed Zn) exhibits a plateau in response above a dietary threshold of 60 mg Zn/kg diet, below which it decreases or increases by a slope of 0.17/mg reduction or rise in dietary Zn, respectively. The dietary threshold (breakpoint) represents the gross Zn requirement at given experimental conditions.
Fig. 2.
Theoretical competitive broken-line response of a random zinc status parameter to changes in dietary zinc (Zn) concentration from 2 different supplemental Zn species. In this example, the parameter (e.g., apparently digested feed Zn) exhibits a plateau in response above dietary thresholds of 60 and 50 mg Zn/kg diet when feeding Zn species A (black) and B (grey), respectively. Below the respective threshold, parameter response to changes in dietary Zn concentration from species A and B decreases or increases by 0.17 and 0.26/mg reduction or rise in dietary Zn, respectively. In conclusion, feeding Zn species B results in a decreased gross Zn requirement (−10 mg Zn/kg diet). Based on the slope comparison, feed Zn species B provided a 1.5-fold higher feed Zn utilization compared with species A based on the relative differences of the respective slopes in response.
Most trials, which aimed at investigating the efficacy of certain Zn feeding strategies, used animal cohorts with more or less pronounced states of clinical (severe) Zn deficiency. However, under the conditions of a clinical Zn deficiency a multitude of severe secondary metabolic events occur (Holt et al., 2012). Such events correlate only indirectly to Zn homeostatic regulation. This drastically increases the background noise when assessing the true effects of Zn feeding on the metabolic response. The occurrence of clinical Zn deficiency in practical feeding is rather unlikely, as complete feed of conventional herds is generously supplemented with Zn. In contrast, subclinical events of insufficient Zn supply are supposed to happen more frequently (Holt et al., 2012). A subclinical Zn deficiency is defined by a reduction in the animals' Zn status and changes in associated compensatory metabolic pathways but, simultaneously, an absence of visible symptoms (growth depression, anorexia, skin necrosis etc.). The already mentioned approach by Brugger et al. (2014) induces such a subclinical Zn deficiency, which presumably happens in practical piglet rearing. This has been associated with impaired biochemical functions, e.g., digestion or redox metabolism (Brugger and Windisch, 2016, Brugger and Windisch, 2017). Under such conditions, the gross Zn utilisation and the bioavailability of feed Zn can be estimated much more realistically because the response of Zn absorptive/excretive mechanisms occurs within basal ranges. In contrast, individuals that exhibit a clinical Zn deficiency must not only satisfy their basal Zn demand but also replenish their depleted body Zn stores and compensate for degenerative processes. This may lead to an unusual high expression of active Zn transporters at the gut mucosa, which causes an overestimation of feed Zn utilization.
5. Conclusion
Modern diets for monogastric livestock usually contain more Zn than the organism could utilise. This surplus can accumulate within the organism itself as well as the environment and expresses a strong toxic potential. As the legal dose-range for safety margins is shrinking in light of stricter legal regulations within the European Union, practical animal feeders must supply their herds more closely to the actual gross Zn requirements. This is only possible, if all measures that increase and stabilise feed Zn bioavailability are applied. Finally, reliable information must be provided on the gross Zn requirement under varying practical conditions, considering differences in the availability of native and supplemented Zn pools, antagonisms with dietary factors as well as the animals' physiological status.
Conflict of interest
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Acknowledgements
The authors thank their colleagues from the DÖF for valuable discussion and advice.
Footnotes
Presented at the 4th international forum on micronutrient and feed safety, Changsha, China, September 24th, 2016. Both authors contributed equally to this work.
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
Contributor Information
Daniel Brugger, Email: daniel.brugger@wzw.tum.de.
Wilhelm M. Windisch, Email: wilhelm.windisch@wzw.tum.de.
References
- Andreini C., Banci L., Bertini I., Rosato A. Counting the zinc-proteins encoded in the human genome. J Proteome Res. 2006;5:196–201. doi: 10.1021/pr050361j. [DOI] [PubMed] [Google Scholar]
- Asada K., Toyota K., Nishimura T., Ikeda J., Hori K. Accumulation and mobility of zinc in soil amended with different levels of pig-manure compost. J Environ Sci Health B. 2010;45:285–292. doi: 10.1080/03601231003704580. [DOI] [PubMed] [Google Scholar]
- Baker-Austin C., Wright M.S., Stepanauskas R., McArthur J.V. Co-selection of antibiotic and metal resistance. Trends Microbiol. 2006;14:176–182. doi: 10.1016/j.tim.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Bannick CG, Bieber E, Böken H, Brach M, Brackemann H, Ehrmann H, et al. Grundätze und Maßnahmen für eine vorsorgeorientierte Begrenzung von Schadstoffeinträgen in landbaulich genutzten Böden. UBA Texte 59/2001, ISSN: 0722-186x; 2001.
- Brugger D., Windisch W. Environmental responsibilities of livestock feeding using trace mineral supplements. Anim Nutr. 2015;1:113–118. doi: 10.1016/j.aninu.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugger D., Windisch W. Subclinical zinc deficiency impairs pancreatic digestive enzyme activity and digestive capacity of weaned piglets. Br J Nutr. 2016;116:425–433. doi: 10.1017/S0007114516002105. [DOI] [PubMed] [Google Scholar]
- Brugger D., Windisch W. Short-term subclinical zinc deficiency in weaned piglets affects cardiac redox metabolism and zinc concentration. J Nutr. 2017 doi: 10.3945/jn.116.240804. [DOI] [PubMed] [Google Scholar]
- Brugger D., Buffler M., Windisch W. Development of an experimental model to assess the bioavailability of zinc in practical piglet diets. Arch Anim Nutr. 2014;68:73–92. doi: 10.1080/1745039X.2014.898392. [DOI] [PubMed] [Google Scholar]
- Brugger D., Nadler C., Windisch W.M., Bolduan C. Feed protein value of acidic precipitates obtained from press juices of three types of green forage leaves. Anim Feed Sci Technol. 2016;222:236–241. [Google Scholar]
- Canani R.B., Cirillo P., Buccigrossi V., Ruotolo S., Passariello A., De Luca P. Zinc inhibits cholera toxin-induced, but not Escherichia coli heat-stable enterotoxin-induced, ion secretion in human enterocytes. J Infect Dis. 2005;191:1072–1077. doi: 10.1086/428504. [DOI] [PubMed] [Google Scholar]
- Carlson M.S., Hill G.M., Link J.E. Early and traditional weaned nursery pigs benefit from phase-feeding pharmacological concentrations of zinc oxide: effect on metallothionein and mineral concentrations. J Anim Sci. 1999;77:1199–1207. doi: 10.2527/1999.7751199x. [DOI] [PubMed] [Google Scholar]
- Carlson D., Sehested J., Feng Z., Poulsen H.D. Serosal zinc attenuate serotonin and vasoactive intestinal peptide induced secretion in piglet small intestinal epithelium in vitro. Comp Biochem Physiol A. 2008;149:51–58. doi: 10.1016/j.cbpa.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Cheeke P.R., Dierenfeld E.S. CABI; Boston (USA): 2010. Comparative animal nutrition and metabolism. [Google Scholar]
- Cowieson A.J. Factors that affect the nutritional value of maize for broilers. Anim Feed Sci Technol. 2005;119:293–305. [Google Scholar]
- Davin R., Manzanilla E.G., Klasing K.C., Pérez J.F. Effect of weaning and in-feed high doses of zinc oxide on zinc levels in different body compartments of piglets. J Anim Physiol Anim Nutr. 2013;97(Suppl. 1):6–12. doi: 10.1111/jpn.12046. [DOI] [PubMed] [Google Scholar]
- Ettle T., Windisch W., Roth F.X. In: TEMA 12: 12th international symposium on trace elements in man and animals. Strain J.J., et al, editors. University of Ulster; Coleraine, Northern Ireland, United Kingdom: 2005. p. 55. [Google Scholar]
- European Commission Commission implementing regulation (EU) 2016/1095 of 6 July 2016 concerning the authorisation of zinc acetate dihydrate, zinc chloride anhydrous, zinc oxide, zinc sulphate heptahydrate, zinc sulphate monohydrate, zinc chelate of amino acids hydrate, zinc chelate of protein hydrolysates, zinc chelate of glycine hydrate (solid) and zinc chelate of glycine hydrate (liquid) as feed additives for all animal species and amending regulations (EC) No 1334/2003, (EC) No 479/2006, (EU) No 335/2010 and implementing regulations (EU) No 991/2012 and (EU) No 636/2013. OJEU. 2016;182:7–27. [Google Scholar]
- European Food Safety Authority (EFSA) Scientific opinion on the potential reduction of the currently authorised maximum zinc content in complete feed. EFSA J. 2014;12:3668. [Google Scholar]
- European Parliament and the Council of the European Union Regulation (EC) No 999/2001 of the European parliament and the council of 22 May 2001 laying down rules for the prevention, control and eradication of certain transmissible spongiform encephalopathies. OJEU. 2001;32:289–328. [Google Scholar]
- Forsberg C.W., Meidinger R.G., Liu M., Cottrill M., Golovan S., Phillips J.P. Integration, stability and expression of the E. coli phytase transgene in the Cassie line of Yorkshire Enviropig™. Transgenic Res. 2013;22:379–389. doi: 10.1007/s11248-012-9646-7. [DOI] [PubMed] [Google Scholar]
- Frassinetti S., Bronzetti G., Caltavuturo L., Cini M., Croce C.D. The role of zinc in life: a review. J Environ Pathol Toxicol Oncol. 2006;25:597–610. doi: 10.1615/jenvironpatholtoxicoloncol.v25.i3.40. [DOI] [PubMed] [Google Scholar]
- Gebert S., Bee G., Pfirther H.P., Wenk C. Growth performance and nutrient utilisation as influenced in pigs by microbial phytase and vitamin E supplementation to a diet of high oxidative capacity. Ann Zootech. 1999;48:105–115. [Google Scholar]
- German Environment Agency (UBA) UBA Berling; Berlin (Germany): 2004. Erfassung von Schwermetallströmen in landwirtschaftlichen Tierproduktionsbetrieben (engl. Mapping heavy metal fluxes in agricultural animal production systems) [Google Scholar]
- GfE . In: Energie und Nährstoffbedarf landwirtschaftlicher Nutztiere. GfE, editor. DLG-Verlag; Frankfurt am Main: 2006. [Google Scholar]
- Goldhaber S.B. Trace element risk assessment: essentiality vs. toxicity. Reg Tox Pharmacol. 2003;38:232–242. doi: 10.1016/s0273-2300(02)00020-x. [DOI] [PubMed] [Google Scholar]
- Golovan S.P., Meidinger R.G., Ajakaiye A., Cottrill M., Wiederkehr M.Z., Barney D.J. Pigs expressing salivary phytase produce low-phosphorus manure. Nat Biotechnol. 2001;19:741–745. doi: 10.1038/90788. [DOI] [PubMed] [Google Scholar]
- Hackenberg S., Wegener H.-R., Eurich-Menden B. Verlag Institut für Bodenkunde und Bodenerhaltung; 1996. Herkunft der Schadstoffe in Komposten: Schadstoffgehalte in Komposten und anderen Dünge- und Bodenverbesserungsmitteln; Vor- und Nachteile beim Einsatz von Komposten in der Land- und Forstwirtschaft sowie im Landschafts- und Weinbau; (Literaturstudie) [Google Scholar]
- Hahn J.D., Baker D.H. Growth and plasma zinc responses of young pigs fed pharmacologic levels of zinc. J Anim Sci. 1993;71:3020–3024. doi: 10.2527/1993.71113020x. [DOI] [PubMed] [Google Scholar]
- Højberg O., Canibe N., Poulsen H., Hedemann M.S., Jensen B.B. Influence of dietary zinc oxide and copper sulfate on the gastrointestinal ecosystem in newly weaned piglets. Appl Environ Microbiol. 2005;71:2267–2277. doi: 10.1128/AEM.71.5.2267-2277.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt R.R., Uiu-Adams J.Y., Keen C.L. In: Present knowledge in nutrition. Erdman J.W., Macdonald I.A., Zeisel S.H., editors. Wiley-Blackwell; Hoboken, New Jersey: 2012. pp. 521–539. [Google Scholar]
- Hölzel C.C., Müller C., Harms K.S., Mikolajewski S., Schäfer S., Schwaiger K. Heavy metals in liquid pig manure in light of bacterial antimicrobial resistance. Environ Res. 2012 doi: 10.1016/j.envres.2012.01.002. [DOI] [PubMed] [Google Scholar]
- Humer E., Schwarz C., Schedle K. Phytate in pig and poultry nutrition. Anim Physiol Anim Nutr. 2015 doi: 10.1111/jpn.12258. [DOI] [PubMed] [Google Scholar]
- Johns D.C. Evaluation of the nutritional value of ryegrass-white clover leaf protein concentrate. NZ J Agric Res. 1986;29:249–256. [Google Scholar]
- Kickinger T., Humer J., Aichberger K., Würzner H., Windisch W. Survey on zinc and copper contents in dung from Austrian livestock production. Bodenkultur. 2008;59:101–110. [Google Scholar]
- Kickinger T., Würzner H., Windisch W. Zinc and copper in feeds, slurry and soils from Austrian pig fattening farms feeding commercial complete feed or feed mixtures produced on-farm. Bodenkultur. 2010;60:47–58. [Google Scholar]
- Kühnen V., Goldbach H.E. Institut für Pflanzenernährung, Rheinische Friedrichs-Wilhelms-Universität Bonn; Germany: 2002. Schwermetallbilanzen verschiedener Betriebstypen: Eintragswege, Flüsse, Minderungspotential. Forschungsbericht Nr. 118. [Google Scholar]
- Lewis P.K., Hoekstra W.C., Grummer R.H., Phillips P.H. The effects of certain nutritional factors including calcium, phosphorus and zinc on parakeratosis. J Anim Sci. 1956;15:741–751. [Google Scholar]
- Lewis P.K., Grummer R.H., Hoekstra W.C. The effect of method of feeding upon the susceptibility of the pig to parakeratosis. J Anim Sci. 1957;16:927–936. [Google Scholar]
- Lewis P.K., Hoekstra W.C., Grummer R.H. Restricted calcium feeding versus zinc supplementation for the control of parakeratosis in swine. J Anim Sci. 1957;16:578–588. [Google Scholar]
- Li B.T., Van Kessel A.G., Caine W.R., Huang S.X., Kirkwood R.N. Small intestinal morphology and bacterial populations in ileal digesta and feces of newly weaned pigs receiving a high dietary level of zinc oxide. Can J Anim Sci. 2001;81:511–516. [Google Scholar]
- Lichten L.A., Cousins R.J. Mammalian zinc transporters: nutritional and physiologic regulation. Annu Rev Nutr. 2009;29:153–176. doi: 10.1146/annurev-nutr-033009-083312. [DOI] [PubMed] [Google Scholar]
- Luecke R.W., Hoefer J.A., Brammell W.G., Thorp F. Mineral interrelationships in parakeratosis of swine. J Anim Sci. 1956;15 [Google Scholar]
- Martin L., Lodemann U., Bondzio A., Gefeller E.M., Vahjen W., Aschenbach J.R. A high amount of dietary zinc changes the expression of zinc transporters and metallothionein in jejunal epithelial cells in vitro and in vivo but does not prevent zinc accumulation in jejunal tissue of piglets. J Nutr. 2013 doi: 10.3945/jn.113.177881. [DOI] [PubMed] [Google Scholar]
- Martin l., Pieper R., Schunter N., Vahjen W., Zentek J. Performance, organ zinc concentration, jejunal brush border membrane enzyme activities and mRNA expression in piglets fed with different levels of dietary zinc. Arch Anim Nutr. 2013;67:248–261. doi: 10.1080/1745039X.2013.801138. [DOI] [PubMed] [Google Scholar]
- Miller E.R., Liptrap H.D., Ullrey D.E. In: Trace element metabolism in animals. Mills C.F., editor. E. & S. Livingstone; Edinburgh (UK): 1970. [Google Scholar]
- Morales J., Cordero G., Pineiro C., Durosoy S. Zinc oxide at low supplementation level improves productive performance and health status of piglets. J Anim Sci. 2012;90:436–438. doi: 10.2527/jas.53833. [DOI] [PubMed] [Google Scholar]
- Müller G. Nur noch geringer Eintrag anthropogener Schwermetalle in den Bodensee – neue Daten zur Entwicklung der Belastung der Sedimente. Naturwissenschaften. 1997;84:37–38. [Google Scholar]
- Müller C., Ebert T. Schwermetall-Einträge durch Wirtschaftsdünger von 1996 bis heute. Ergebnisse aus dem bayerischen Bodenbeobachtungsprogramm. VDLUFA-Schriftenreihe. 2002;58:635–639. [Google Scholar]
- NRC . Nat. Acad. Press; Washington, D.C., USA: 1994. Nutrient requirements of poultry. [Google Scholar]
- NRC . Nat. Acad. Press; Washington, D.C., USA: 2012. Nutrient requirements of swine. 11th. [Google Scholar]
- Pieper R., Vahjen W., Neumann K., Van Kessel A.G., Zentek J. Dose-dependent effects of dietary zinc oxide on bacterial communities and metabolic profiles in the ileum of weaned piglets. J Anim Physiol Anim Nutr. 2012 doi: 10.1111/j.1439-0396.2011.01231.x. [DOI] [PubMed] [Google Scholar]
- Pieper R., Martin L., Schunter N., Tudela C.V., Weise C., Klopfleisch R. Impact of high dietary zinc on zinc accumulation, enzyme activity and proteomic profiles in the pancreas of piglets. J Trace Elem Med Biol. 2015;30:30–36. doi: 10.1016/j.jtemb.2015.01.008. [DOI] [PubMed] [Google Scholar]
- Poulsen H. Zinc oxide for weanling piglets. Act Agric Scand A Anim Sci. 1995;45:159–167. [Google Scholar]
- Robbins K.R., Saxton A.M., Southern L.L. Estimation of nutrient requirements using broken-line regression analysis. J Anim Sci. 2006;84:E155–E165. doi: 10.2527/2006.8413_supple155x. [DOI] [PubMed] [Google Scholar]
- Rout G.R., Das P. Effect of metal toxicity on plant growth and metabolism: I. Zinc. Agronomie. 2003;23:3–11. [Google Scholar]
- Sargeant H.R., MIller H.M., Shaw M.A. Inflammatory response of porcine epithelial IPEC J2 cells to enterotoxigenic E. coli infection is modulated by zinc supplementation. Mol Immunol. 2011;48:2113–2121. doi: 10.1016/j.molimm.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Schell T.C., Kornegay E.T. Zinc concentration in tissues and performance of weanling pigs fed pharmacological levels of zinc from ZnO, Zn-methionine, Zn-lysine, or ZnSO4. J Anim Sci. 1996;74:1584–1593. doi: 10.2527/1996.7471584x. [DOI] [PubMed] [Google Scholar]
- Shanklin S.H., Miller E.R., Ullrey D.E., Hoefer J.A., Luecke R.W. Zinc requirement of baby pigs on casein diets. J Nutr. 1968;96:101–108. [Google Scholar]
- Smith W.H., Plumlee M.P., Beeson W.M. Zinc requirement for growing swine. Science. 1958;128:1280–1281. doi: 10.1126/science.128.3334.1280. [DOI] [PubMed] [Google Scholar]
- Smith W.H., Plumlee M.P., Beeson W.M. Effect of source of protein on zinc requirement of the growing pig. J Anim Sci. 1962;21:399–405. [Google Scholar]
- Society of Nutrition Physiology (GfE) DLG-Verlag; Frankfurt (Germany): 2008. Recommendations for the supply of energy and nutrients to pigs. [Google Scholar]
- Starke I.C., Zentek J., Vahjen W. Ex vivo-growth response of porcine small intestinal bacterial communities to pharmacological doses of dietary zinc oxide. PLoS ONE. 2013 doi: 10.1371/journal.pone.0056405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starke I.C., Pieper R., Neumann K., Zentek J., Vahjen W. The impact of high dietary zinc oxide on the development of the intestinal microbiota in weaned piglets. FEMS Microbiol Ecol. 2014;87:416–427. doi: 10.1111/1574-6941.12233. [DOI] [PubMed] [Google Scholar]
- Stevenson J.W., Earle I.P. Studies on parakeratosis in swine. J Anim Sci. 1956;15:1036–1045. [Google Scholar]
- Sturniolo G.C., Fries W., Mazzon E., Di Leo V., Barollo M., D′inca R. Effect of zinc supplementation on intestinal permeability in experimental colitis. J Lab Clin Med. 2002;139:311–315. doi: 10.1067/mlc.2002.123624. [DOI] [PubMed] [Google Scholar]
- Szymczyk B., Gwiazda S., Hanczakowski P. Nutritive value for rats of unextracted and defatted green fractions of leaf protein concentrate from rec clover. Anim Feed Sci Technol. 1995;56:169–175. [Google Scholar]
- Vahjen W., Pieper R., Zentek J. Bar-coded pyrosequencing of 16S rRNA gene amplicons reveals changes in ileal porcine bacterial communities due to high dietary zinc intake. Appl Environ Microbiol. 2010;76:6689–6691. doi: 10.1128/AEM.03075-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahjen W., Pieper R., Zentek J. Increased dietary zinc oxide changes the bacterial core and enterobacterial composition in the ileum of piglets. J Anim Sci. 2011;89:2430–2439. doi: 10.2527/jas.2010-3270. [DOI] [PubMed] [Google Scholar]
- Vahjen W., Pietruszynska D., Starke I.C., Zentek J. High dietary zinc supplementation increases the occurrence of tetracycline and sulfonamide resistance genes in the intestine of weaned pigs. Gut Pathog. 2015;7:23. doi: 10.1186/s13099-015-0071-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valko M., Morris H., Cronin M.T.D. Metals, toxicity and oxidative stress. Curr Med Chem. 2005;12:1161–1208. doi: 10.2174/0929867053764635. [DOI] [PubMed] [Google Scholar]
- Weigand E., Kirchgessner M. Total true efficiency of zinc utilization: determination and homeostatic dependence upon the zinc supply status in young rats. J Nutr. 1980;110:469–480. doi: 10.1093/jn/110.3.469. [DOI] [PubMed] [Google Scholar]
- Windisch W. Interaction of chemical species with biological regulation of the metabolism of essential trace elements. Anal Bioanal Chem. 2002;372:421–425. doi: 10.1007/s00216-001-1117-6. [DOI] [PubMed] [Google Scholar]
- Windisch W. Effect of microbial phytase on the bioavailability of zinc in piglet diets. Proc Soc Nutr Physiol. 2003;12:33. [Google Scholar]
- Windisch W., Kirchgessner M. Measurement of homeostatic adaption of Zn metabolism to deficient and high zinc supply after an alimentary 65Zn labeling procedure. 1. Effect of different zinc supply on the quantitative zinc exchange in the metabolism of adult rats. J Anim Physiol Anim Nutr. 1994;71:98–107. [Google Scholar]
- Windisch W., Kirchgessner M. Zum Effekt von Phytase auf die scheinbare Verdaulichkeit und Gesamtverwertung von Eisen, Kupfer, Zink und Mangan bei abgestufter Ca-Versorgung in der Ferkelaufzcht und Broilermast. The effects microbial phytase on the apparent digestibility and total utilization of iron, copper, zinc and manganese at varying Ca supply to piglets and broilersAgribiol Res. 1995;49:23–29. [Google Scholar]
- Windisch W., Kirchgessner M. Zinc absorption and excretion in adult rats at zinc deficiency induced by dietary phytate additions: I. Quantitative zinc metabolism of 65Zn-labelled adult rats at zinc deficiency. J Anim Physiol Anim Nutr. 1999;82:106–115. [Google Scholar]
- Windisch W., Schwarz F.J., Gruber K., Kirchgessner M. Effect of pharmacological dietary doses of zinc oxide on performance and fecal characteristics of weanling piglets. Agribiol Res. 1998;51:277–285. [Google Scholar]
- Windisch W., Vikari A., Hilz C. Homeostatic response of Zn metabolism to dietary Zn supplements from sulfate, gluconate, orotate, aspartate or histidine in 65Zn labeled non-growing rats as a model to adult individuals. Trace Elem Electrolytes. 2003;20:125–133. [Google Scholar]
- Wuana Raymond A., Okieimen Felix E. Heavy metals in contaminated soils: a review of sources, chemistry, risks and best available strategies for remediation. ISRN Ecol. 2011;2011:20. 402647. [Google Scholar]