Abstract
Background:
Pasteurella multocida continues to pose a danger to prone farm and wild animals all over the world. Chemotherapeutic treatments are progressively losing their effectiveness, last for long time, and cost a lot of money, as well as being toxic to human consumers. Therefore, clearing the way for immunization as a big-wheel alternative against the economic grain. Yet, the vaccines available in the market do not confer the necessary protection against the pathogen. The integration of the well adjuvanted killed vaccine with the attenuated vaccines proved to offer an effective protection to the host animals. However, the bare use of the killed bacterin to provide protection from the possible harm of the live attenuated vaccine was doubtful.
Methods:
In the present study, propolis extracts were used to ameliorate the immunogenicity of the Pasteurella bacterin. The cellular and humoral activities were assessed for the different bacterin formulations.
Results:
Propolis extracts adjuvants proved to broaden and extend the IgG potency, as well as to induce a unique mucosal protection against the bacterium. Simultaneously it offered an anti-inflammatory effect that increased the tolerability to the bacterin. While the cellular activity was relatively reduced with propolis extracts.
Conclusion:
These results confirm the effectiveness of the formulation of the bacterin with propolis to offer a potent homologous primary protection to the animals against the long-life use of the attenuated Pasteurella vaccines
Key Words: Bacterin, Pasteurella multocida, Pasteurellosis, Propolis, Vaccine
Introduction
Pasteurella multocida is the etiologic agent of the virulent respiratory disease pasteurellosis. The disease induces several types of illnesses including respiratory infections, otitis, meningitides, genital infection, abscesses, soft tissue infection, and septicaemia (1). It causes an increasing economic lose in the farm animals husbandry with a mortality rate that trespassed 50 % especially in poultry, buffaloes, goats, sheep and swine (2). It was recorded as well in farmed fishes (3), wild animals (4) and immune-compromised human patients (5). P. multocida became one of the most vicious worldwide veterinary pathogens due to the ease of infection spread especially in crowded farming systems (6), the increased susceptibility to infection among crowded stressed animals’ transportation (7), and the increasing antibiotics resistance (8). This danger increases as a result for the presence of symptomless carriers such as cats (9), the presence of subclinical cases (10), the variability of microbial strains (11, 12), the increase of the risk of outbreaks with environmental factors (13), and the lack of fully potent vaccines to eradicate it (14).
Our previous investigations (14) highlighted an optimum regimen (15) to profit from the benefit of both the safety and efficiency of available vaccines. The proposed formulation applies a well-adjuvanted killed preparation from Pasteurella to confer safety followed by a life-time application of orally administered attenuated vaccine to provide a heterologous extended therapeutic potency (14). Our point of view was consolidated with other researchers that proposed to develop a potent regimen against the pathogen by using the oil adjuvanted killed vaccine and the attenuated vaccine to combat buffaloes pasteurellosis (16). Some researchers still follow classical ways to prepare limited-potency homologous vaccines against the pathogen (17-22), despite it not being recommended with some of them already confirmed as ineffectiveness through their trials (17, 21). Although being unsafe, the research to develop new attenuated Pasteurella vaccines progressed using virulent genes deletion (23, 24) or went through the dose evaluation of live attenuated intranasal aerosols (25) and proved their efficacy.
The aim of this research article is to develop and to evaluate the formalin-killed Pasteurella bacterin formulated with different propolis extracts as adjuvants. This was consolidated by the facts that the whole propolis extract enhances the immunogenicity of the incorporated antigens, as well as being a safe anti-inflammatory natural product, unlike the oil-adjuvants (26).
Materials and Methods
Ethics approval and consent to participate
The ethical approval and standards for this study regarding the use of rabbits as animal models was granted by the Medical Research Institute, Alexandria University.
Propolis’ flavonoids preparation and quantification
Propolis flavonoids (PF) was extracted according to the method described by Shouqin et al., (27) with further modifications. All processes were performed in dark. In brief, dry ground powder of propolis (Egyptian market) was added to acetone (1:10) and agitated overnight on 150 rpm at room temperature (RT). The extract was filtered, and the acetone extract was vacuum evaporated at 40 °C. The aqueous solution was extracted by excess hexane. The aqueous layer was extracted with equal amount of ethyl acetate. The non-aqueous layer was concentrated on at 40 °C. The final extract was kept in aliquots at -20 °C. The flavonoids content was determined by the aluminium trichloride method using quercetin as a reference compound (28).
Propolis’ polysaccharides preparation and quantification
The propolis’ polysaccharides (PPS) were extracted according to the method of Sun et al. (29) with some modifications. In brief, dry ground powder of propolis was mixed with distilled water (10 gm/dL), and heated at 85 °C for 8 hours with continuous stirring. The extract was left to decant overnight at 4 °C. The wax toping was removed mechanically, and the homogenous supernatant was concentrated at 40 °C. Then, the concentrate was centrifuged at 12000 for 15 minutes at 14 °C. The supernatant was adjusted on pH 4.4, and added to 4 times its volume freezed 95 % ethanol while stirring. The mixture was kept at -20 °C overnight, then centrifuged at 4000 g for 15 min at 4 °C. The pellets were washed by cold 95 % ethanol and centrifuged at 4000 g for 15 min at 4 °C. The pellets were washed by acetone, and centrifuged at the same conditions. The pellet was air dried, suspended in minimal amount of pyrogen-free water and kept in aliquots at -20 °C. PPS was quantified based on the polysaccharide content against glucose standard, by the phenol-sulphuric acid method (30).
Propolis’ extract preparation and quantification
Fine ground propolis was extracted in dark with 70 % ethanol (7 g/dL) at RT for 7 days in a shaker incubator at 150 rpm. Then the suspension was filtered, concentrated, sealed, divided and stored at -20 °C (31, 32). The PF and PPS content of propolis extract (PE) were determined by the aluminium trichloride method (28) and by the phenol-sulphuric acid method (30); respectively.
Isolation and identification of the P. multocida bacterium
Oral specimens were taken by swabbing the anterior surface of the gums and the interior of the cheek, from two White New Zealand rabbits (Dr. Tarek Adnan rabbit’s collection) manifesting infection by Pasteurella (33). The swabs were streaked on Pasteurella selective medium plates (34), and incubated at 37 °C for 24 hours. The identity of the pure colonies was tested by API-20E strips (bioMerieux, France) according to the producer recommendations (35). The positive samples were confirmed by the VITEK®2 automated system (bioMerieux, USA).
Pasteurella’s bacterin preparation
The pure bacterial cells of Pasteurella were cultured in brain-heart infusion (BHI) broth at 37 °C for 18 hours at 150 rpm. The broth culture was deactivated by 0.8 % formalin (v/v) 3 hours at RT. The pellet was collected aseptically by centrifugation at 4000 g X for 15 min at 4 °C, then washed twice by sterile pyrogen-free water (36). The sterility of the preparation was confirmed on BHI agar plates for 5 days at 37 °C. Then the bacterial suspension was adjusted to be 2x109 cfu/mL using McFarland gradient solutions (37).
Experimental rabbits care
Healthy white New Zealand rabbits with an age of 6 weeks and an average weight of 1450 g ± 50 g, were purchased from the experimental farm of the Faculty of Agriculture in Alexandria University. The rabbits were allowed to accommodate for two weeks in the new animal house at the Medical Research Institute of Alexandria University, where they were kept under the experimental animal ethics rules of Alexandria University. Throughout that period the behavioural parameters, body weight and temperature were recorded. Nasal and oral samples were weekly cultured on Pasteurella’s selective medium (34) as previously described, to ensure that the rabbits are free from any possible infection from Pasteurella (38).
The inspection of the rabbits started at zero time, 4 hours after injection, then after 2 and 6 days after each injection. The recorded parameters included body weight variation, body temperature (rectal) variation, tachycardia, tachypnoea, hyperactivity, changes in the eyes brightness, oliguria, haematuria, diarrhoea, constipation, hair loss, appetite loss, abnormal sexual behaviour, and manifestation of disease (39-41).
Injection schedule and blood samples collection
The rabbits were grouped into 5 groups, consisting of 3 rabbits each. They were injected subcutaneously. The booster dose was after 28 days, week 4, after the first one (W0). Blood samples were collected on 2 weeks’ intervals. Heparinised samples were used for cellular proliferation assessment, while serum was separated and kept at -80 °C for further humoral assessment ( Fig. 1). The first dose consisted of 1 X 109 cfu/mL bacterin formulated with the safe dose (42) of propolis flavonoids equivalent to 0.65 mg/Kg in PF and PE, and 0.7 mg/Kg for the PPS. While the second dose consisted of 2 X 109cfu/mL bacterin formulated with the same safe dose of the adjuvant.
Fig. 1.
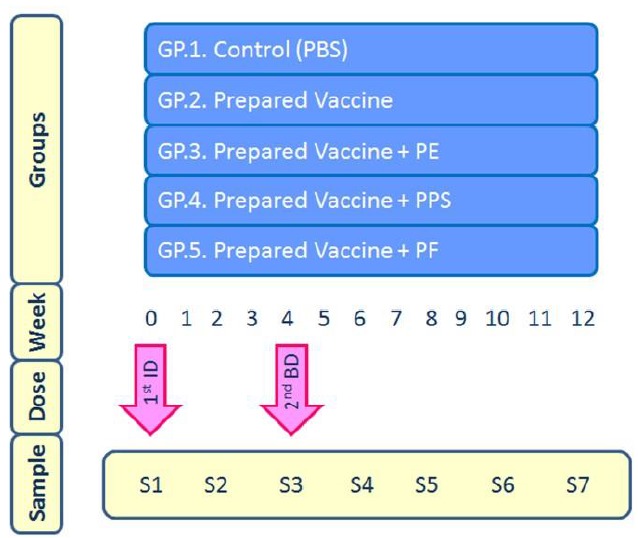
Injection schedule of the 5 rabbits' groups with an illustration of the immunization intervals. 1st ID = first injection dose; 2ndBD = second injection or booster dose; GP = group; PBS = Phosphate buffer saline; PE = propolis extract; PF = propolis flavonoids; PPS = propolis polysaccharides; S = Sample.
The detection of the specific antibodies to Pasteurella
The in-house prepared ELISA plates were coated with the previously optimized concentration (105 for IgG and 107 for IgA) of the bacterial cells with a replica. The IgA and IgG content in the serum samples were estimated using horse radish peroxidise (HRP)-labelled polyvalent bovine anti-rabbit IgG or IgA and substrate at 450 nm (43, 44) using micro-plate reader (Infinite F50 Tecan®, Switzerland) supported by Magellan® statistical software. The accepted data had a probability (P) value ≤ 0.05.
Cellular immunity evaluation
The cellular response of the vaccine preparations was estimated by the lympho-proliferation assay using Neutral Red (NR) dye (45, 42) using Ficoll- Histopaque 1.083 (Sigma- Alrdich, USA). The cell count was adjusted to 2x106 according to Castro-Concha et al. (47) and Hoffman (48). The readings were performed on micro-plate reader (Infinite F50 Tecan®, Switzerland).
Statistical analysis of the data
Statistical data analysis was performed using IBM SPSS version 20 to obtain the mean and standard deviation. The same software was used to do the one-way ANOVA for the data. The significance value was set on a value ≤ 0.05.
Results
The safe dose of propolis extracts injected to each rabbit was calculated according to the previous research of Türkez et al. (42). It was estimated that the propolis extract (PE) extracted by the ethanol method yielded a weight of 20.98 % from the crude propolis. While the propolis polysaccharide (PPS) extracted by the hot-water method yielded 7 % from crude propolis. However, the propolis flavonoids (PF) extracted by the acetone method yielded 6.49 % from crude propolis. Therefore according to Türkez et al. (42) the safe dose of PF was 0.65 mg/Kg and the safe dose of PPS was 0.7 mg/Kg. These were formulated with the prepared vaccine according to the rabbits’ weight.
The inspections did not show any remarkable abnormal change in the natural behaviour or weight of the injected rabbits after both injection doses. The fold increase in temperature of the groups after the two injections ( Figs. 2A and B) confirmed the significant relative increase of the body temperature of all groups after 4 hours from the vaccination. The PPS and vaccine groups showed the highest increase in body temperature when compared to the other groups. While the PF, showed the lowest increase. The statistics confirmed the significant reduction in temperature for groups (3 and 5) after 4 hours with both injections, when compared to the vaccine group 2. This correlation was not remarkable with group 4.
Figs. 2.
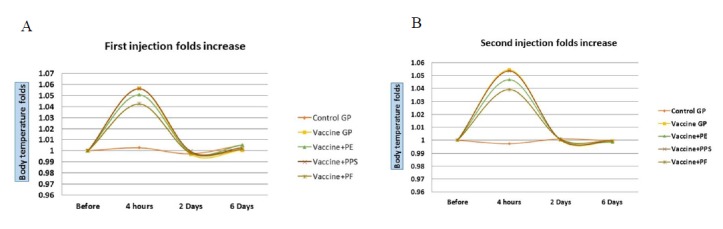
(A) The first injection fold increase of the body temperature on week 0. (B) The second injection fold increase of the body temperature on week 4. The vaccine was formulated with Propolis extract (PE), Propolis polysaccharides (PPS) or Propolis flavones (PF).
Figure 3 illustrates the IgG response of the rabbits injected by the different bacterin preparations. The rabbits’ groups were injected at week 0 and week 4, as indicated by the red arrow in Figure 3. The control group still keeps its basal increase as being a non-vaccinated one. The propolis extracts formulated groups manifested a statistically significant obvious higher IgG response than the crude vaccine, with a remarked superiority of the PF formulation in term of folds and longevity. It is
Figure 3.
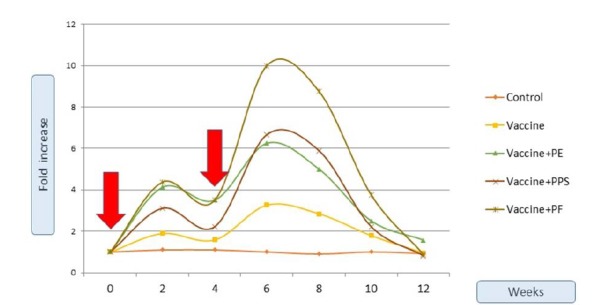
Fold increase of IgG over 12 weeks. The vaccine was formulated with Propolis extract (PE), Propolis polysaccharides (PPS) or Propolis flavones (PF). The red arrows indicate the injection doses at week (0) and week (4).
noted as well that the IgG level of all the vaccinated groups IgG reverted to the initial basal value after 6-8 weeks after the booster dose. The cut-off value (31) which denotes the fold-increase that enables the vaccinated animal to combat the microbial infection due to Pasteurella, was calculated to be equal to 1.85-folds. The crude vaccine reached the cut-off value a week after the booster dose and just retained its value for 5 weeks, while all other dose containing propolis components reached the cut-off value a week after the first dose and remained with a significant increase for 10 weeks. Statistics confirmed that groups 3 and 5; but not 4 retained significant high IgG fold-increase from that of the vaccine group 2. Concurrently, figure 4 shows the relative fold-increase of IgA of the different groups in proportional of the initiative IgA concentration at the rabbits’ sera. The control group keeps its basal increase as being a non-vaccinated one. The cut-off value (31) was calculated to be equal to 1.9-folds. The PE-formulated vaccine was the only preparation that enabled the IgA fold-increase to reach the cut-off value. This was achieved after 10 days from the booster dose and only for a period of less than two weeks, this was confirmed statistically.
figure 4.
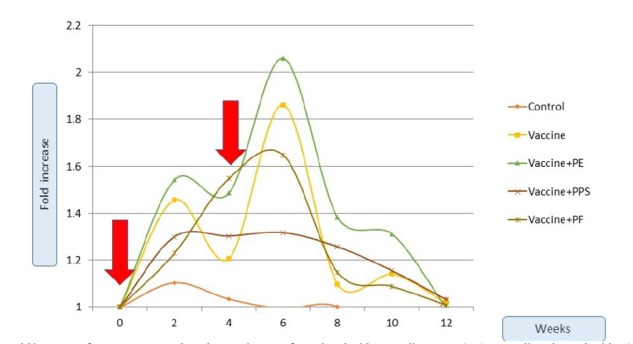
Fold increase of IgA over 12 weeks. The vaccine was formulated with Propolis extract (PE), Propolis polysaccharides (PPS) or Propolis flavones (PF). The red arrows indicate the injection doses at week (0) and week (4).
Figure 5 shows the stimulation index that denotes the cellular proliferation of the different groups of tested rabbits. All groups showed an induction in cellular response after the different injections. However, the statistical analysis of the data confirmed the non-significant increase values with all propolis preparations. The non-formulated vaccine group showed the significant obviously highest cellular proliferation in comparison to all other groups of rabbits. After the first injection dose, PPS showed the highest negative effect on the cellular proliferation, followed by PE, then PF. While after the second dose, the negative effect of PF increased while that of the PPS decreased.
Figure 5.
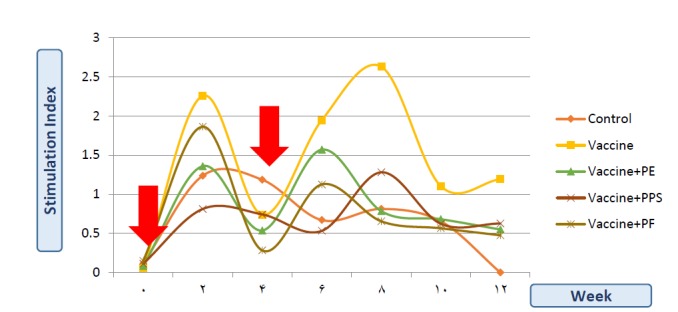
Stimulation Index (SI) of the neutral red dye, as an indication of proliferation activity of the mononuclear cells of the different rabbits’ groups’ along 12 weeks. The vaccine was formulated with Propolis extract (PE), Propolis polysaccharides (PPS) or Propolis flavones (PF). The red arrows indicate the injection doses at week (0) and week (4).
Discussion
Pasteurella multocida proved to be an emerging bacterial pathogen that threatens the animal husbandry and human health all over the world since biosecurity measures, chemotherapeutics and available vaccination regimen were not sufficient to control that bug. Although vaccination emerged as the most promising solution, the majority of the available vaccines were homologous, non-therapeutic, expensive, or were not proven safe. Therefore, our previous investigations proposed a vaccination regimen that applies a dose of well-adjuvanted killed vaccine to act itself as a vaccine against the possible harm of the live-attenuated vaccine. This dose will confer a homologous protection against the same strains used to produce the attenuated vaccine. This should be followed by the attenuated vaccine that offers heterologous cellular long protection to attain the desired protection (14). Our aim was to develop the protection of Pasteurella vaccination regimen through the production of that well-adjuvanted bacterin that confers a primary protection against the attenuated vaccine.
The use of propolis as a safe cheap vaccine adjuvant was proposed to enhance the immunization term and reduce local inflammation due to its safety (26). It also has no effect on epitopes modifications, especially since the main epitopes of Pasteurella showed to be proteins (14, 26). Previous trials assessed the 5 X 109 single-dose (31) or double equal-doses of 2 X 109 (49) formulated with the crude extract (with 70 % ethanol-PE) of propolis over seven weeks. They confirmed that propolis has no adverse effect on the metabolic activities of the animal, enhances the immune response and reduce the mortality rate (31, 49). Our study developed those previous ones by imitating the same conditions applied while using the commercial vaccine in terms of dose and times of vaccination, rabbits’ age and weight, and the vaccination regimen. Moreover, we evaluated the specific circulating humoral antibodies, and assessed the absolute adjuvanticity of the major groups of propolis components. Simultaneously, experimental rabbits were assessed for a possible spread of subclinical infection by continuous bacterial cultures. In order to provide a universally accepted research, our doses were calculated according to the flavonoid and polysaccharide contents, and not as weight proportions of the raw propolis, since propolis components differs from region to region and season to season (26).
The body temperatures of the rabbits were elevated after 4 hours from the first and second injection. This was referred to the immune response activation and the production of inflammatory cytokines against the injection, regardless to the vaccine formulation as previously noted (25). The vaccine formulated with PF and PE showed a relative reduction in the animals’ post-injection temperature. This was the referred to the anti-inflammatory effect of the propolis (50, 51). The anti-inflammatory effect was the highest with PF, moderate with PE and absent with PPS. This confirms that the anti-inflammatory components are non-polar ones present in the flavonoids extract. Therefore, propolis flavones may be used as vaccine additive to reduce the inflammatory effect and vaccine side effects. Especially since rabbits are very sensitive to physiological changes and usually respond to that by a loss of appetite and, subsequently, weight. Unlike with the first dose and after the booster dose, the PPS caused a non-significant mild reduction in temperature. Together with the previous pathological and physiological assessments performed for the bacterin formulation with PE (26, 31, 49, 50), our estimation for the behavioural characters and body weight variation confirms the safety of the different preparations on the animals’ health and growth.
The IgG raised in all rabbits after 2-weeks from the first dose and after 3-weeks from the booster dose. It started to decrease after 7-weeks of injection, which confirm that the evaluation of Pasteurella bacterin necessitates a period of at least 12-weeks for experimentation. Moreover, the protection of the crude vaccine measured by the cut-off value estimation lasted only from week-5 to week-10, after the booster dose. This confirms that the protective potency of the crude commercial vaccine is less than what is announced to be 8-weeks after the last dose injection. In addition, the protective capacity only initiates after one-week from the booster dose. These facts confirm that no wonder that the commercial vaccine available on the market is unable to protect vulnerable animals, especially young ones. For instance, the bacterin is injected in 2 month-old rabbits and then boosted at the age of 3-months. This means that young rabbits do not profit from the bacterin protection before the age of one week after the third month, especially that bacterin do not confer maternal protection like the attenuated preparations (14).
The PF formulation showed an obviously higher IgG response and relatively longer persistence throughout the injection schedule. Statistics confirmed the significant increase in IgG-folds for the PF containing injections, groups 3 and 5. This confirms its compatibility as an adjuvant for the bacterin, and its positive effect on extending the humoral protection as previously noted (52). The protective potency of the PE and PPS were from week 1 to 10, and that of the PF extends for an extra week over the other propolis extracts. This confirms that the PF, and to a lesser extent propolis extracts, are able to prolong the protective capacity of the vaccine. Moreover, it proves that the IgG is boosted mainly by the non-polar constituents of the propolis. Therefore, they may be used to develop the potency of the bacterin.
Not to mention that Pasteurella is a respiratory pathogen, and hence the mucosal immune activity plays an important role to control it. Although the IgA titre was affected by the injection schedule, the non-formulated, PPS and even PF vaccines were not able to boost the mucosal immunity to the cut-off protective value (2-folds). The only preparation that conferred a mucosal protection was that of the PE, that offered a protective capacity at the 6th week after the first dose, and just for a period of 1-week. This may propose the use of the whole PE to increase the mucosal immunity against Pasteurella. Moreover, it worth mentioning that the importance of assessing the IgA response for a vaccine that will be used to protect from a possible harm of an orally administered vaccine lies in that, animals while drinking the attenuated vaccine will most likely wet their nose and eyes. Simultaneously, some research proved the superiority of the nasal attenuated vaccines over the subcutaneous ones (15). Therefore, the specific local mucosal immunity for those organs is concurrently necessary. Our hereby previous instigation on the anti-inflammatory response and the IgG showed the correlation in the influence of PF and PPS together with PE, since PE's effect is empirically the synergic effect of the PF and PPS. However, in the case of IgA assessment the PE showed behaviour that was independent from its two main individual components. This points to the fact that the component that induced the IgA increase is not extractable by the methods used to extract the PPS or the PF. Therefore, formulating a mucosal vaccine adjuvant should contain the PE.
All the propolis preparations had a relative negative effect on the cellular proliferation when compared with the crude bacterin response. Although some studies has shown the significant increase in cellularity in rabbits injected with water soluble derivatives of propolis (53), the PPS had the highest negative effect. Moreover, the PF formulation showed the lowest one, indicating the presence of toxic substances in the propolis extracts, especially the polar ones, as previously noted. Ansorag et al. (54) confirmed that T-cells were suppressed by the different ethanol/chloroform as well as the aqueous extracts of propolis. These inhibitory effects were due to the presence of specific propolis components such as caffeic acid phenethyl ester (CAPE), quercetin, and hesperidin (55). The immune system response to the propolis preparations did not follow the injection schedule; this was attributed to the different tolerance capacities to the toxins throughout the injection schedule.
Although propolis proved to be a safe natural product on animals’ body, it did not prove to be remarkably efficiency at increase the cellular activity of the Pasteurella bacterin. However, its ability to induce a unique protective level of mucosal immunity and a longer protection by IgG favours its use as a protective vaccine additive that may be used to protect the animals from the possible harm induced by the live-attenuated Pasteurella vaccine. Moreover, it showed an obvious anti-inflammatory effect against the undesired irritation induced by the bacterin.
It is recommended that the rabbits get vaccinated at weeks 0 and 4 by the developed Pasteurella bacterin formulated with a tolerable content of PE/PF. Then the in-water life-time attenuated vaccination program can begin safely at the middle of the third week after the booster dose.
Acknowledgements
The authors express their deep appreciation to Ms. Dina Tawfik, from the SeptivaK Research Group, Immunology and Allergy Department, Medical Research Institute, Alexandria University for her technical assistance. A similar appreciation would be dedicated to Mrs. Aishah Hassan, chief-editor at PETT services (VA, USA), for editing the manuscript. The authors declare no financial and non-financial conflict of interests.
References
- 1.Gyles CL, Prescott JF, Songer JG, Thoen CO. Pathogenesis of bacterial infections in animals. 2004 [Google Scholar]
- 2.Mohamed RA, Abdelsalam EB. A review on pneumonic pasteurellosis (respiratory mannheimiosis) with emphasis on pathogenesis, virulence mechanisms and predisposing factors. Bulguarian Journal of Veterinay Medecine. 2008;11:139–60. [Google Scholar]
- 3.Alarcon M, Gulla S, Rosaeg MV, Ronneseth A, Wergeland H, Poppe TT, et al. Pasteurellosis in lumpsucker Cyclopterus lumpus, farmed in Norway. Journal of Fish Diseases. 2016;39:489–95. doi: 10.1111/jfd.12366. [DOI] [PubMed] [Google Scholar]
- 4.Kondgen S, Leider M, Lankester F, Bethe A, Lubke-Becker A, Leendertz FH, et al. Pasteurella multocida involved in respiratory disease of wild chimpanzees. PloS one. 2011;6:e24236. doi: 10.1371/journal.pone.0024236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christenson ES, Ahmed HM, Durand CM. Pasteurella multocida infection in solid organ transplantation. Lancet Infectious Diseases. 2015;15:235–40. doi: 10.1016/S1473-3099(14)70895-3. [DOI] [PubMed] [Google Scholar]
- 6.Cantas L, Suer K. The important bacterial zoonoses in “One Health” concept. Frontier Public Health. 2014;2:144–50. doi: 10.3389/fpubh.2014.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moustafa AM, Ali SN, Bennett MD, Hyndman TH, Robertson ID, Edwards J. A case-control study of haemorrhagic septicaemia in buffaloes and cattle in Karachi, Pakistan, in 2012. Transbound Emerging Diseases. 2015 doi: 10.1111/tbed.12393. [DOI] [PubMed] [Google Scholar]
- 8.De Alwis MCL. Hemorrhagic septicemia. Canberra, Australia: Australian Centre for International Agriculture Research. 1999 [Google Scholar]
- 9.Klein NC, Cunha BA. Pasteurella multocida pneumonia. Seminar of Respiratory Infections. 1997;12:54–6. [PubMed] [Google Scholar]
- 10.Snyder SB, Fox JG, Soave OA. Subclinical otitis media associated with Pasteurella multocida infections in New Zealand white rabbits (Oryctolagus cuniculus). Laboratory Animals Science. 1973;23:270–2. [PubMed] [Google Scholar]
- 11.Wilson BA, Ho MF. Pasteurella multocida: from Zoonosis to Cellular Microbiology. Clinical Microbioly Reviews. 2013;26:631–55. doi: 10.1128/CMR.00024-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cardoso-Toset F, Gomez-Laguna J, Callejo M, Vela AI, Carrasco L, Fernandez-Garayzabal JF, et al. Septicaemic pasteurellosis in free-range pigs associated with an unusual biovar 13 of Pasteurella multocidas. Veterinary Microbiology. 2013;167(690):4. doi: 10.1016/j.vetmic.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 13.Gao X, Xiao JH, Qin HY, Cao Z, Wang HB. Impact of meteorological factors on the prevalence of porcine pasteurellosis in the southcentral of Mainland China. Preventive Veterinary Medecine. 2016;125:75–81. doi: 10.1016/j.prevetmed.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 14.Ahmad TA, Rammah SS, Sheweita SA, Haroun M, El-Sayed LH. Development of immunization trials against Pasteurella multocida. Vaccine. 2014;32:909–17. doi: 10.1016/j.vaccine.2013.11.068. [DOI] [PubMed] [Google Scholar]
- 15.Kharb S, Charan S. Mucosal immunization provides better protection than subcutaneous immunization against Pasteurella multocida (B:2) in mice preimmunized with the outer membrane proteins. Veterinary Research Communications. 2011;35:457–61. doi: 10.1007/s11259-011-9484-8. [DOI] [PubMed] [Google Scholar]
- 16.Zamri-Saad M, Annas S. Vaccination against hemorrhagic septicemia of bovines: A review. Pakistan Veterinary Journal. 2016;36:1–5. [Google Scholar]
- 17.Gong Q, Qu N, Niu MF, Qin CL. Evaluation of immunogenicity and protective efficacy of recombinant ptfA of avian Pasteurella multocidae. Iran Journal of Veterinary Research. 2016;17:84–8. [PMC free article] [PubMed] [Google Scholar]
- 18.Shivachandra SB, Yogisharadhya R, Kumar A, Mohanty NN, Nagaleekar VK, editors. Recombinant transferrin binding protein A (rTbpA) fragments of Pasteurella multocida serogroup B:2 provide variable protection following homologous challenge in mouse model. Research in Veterinary Science. 2015;98:1–6. doi: 10.1016/j.rvsc.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 19.Thanasarasakulpong A, Poolperm P, Tankaew P, Sawada T, Sthitmatee N. Protectivity conferred by immunization with intranasal recombinant outer membrane protein H from Pasteurella multocida serovar A:1 in chickens. Journal of Veterinary Medical Science. 2015;77:321–6. doi: 10.1292/jvms.14-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar A, Mohanty NN, Chacko N, Yogisharadhya R, Shivachandra SB. Structural features of a highly conserved Omp16 protein of Pasteurella multocida strains and comparison with related peptidoglycan-associated lipoproteins (PAL). Indian Journal of Microbiology. 2015;55:50–6. [Google Scholar]
- 21.Çirçir A. Development of recombinant vaccine candidates composed of LtkA from Mannheimia haemolytica A1 and PlpECc-OmpH from Pasteurella multocida A:3 against bovine respiratory disease. Ankara: Middle East Technical University. 2014 [Google Scholar]
- 22.Ghani RA, Khattak NA, Saqlain M, Asad MJ, Khanum A, Naqvi SMS, et al. Comparison of the common immunogenic protein components of Pasteurella multocida serotypes B:2 and B: 3,4. Kafkas Universitesi Veteriner Fakultesi Dergisi. 2016;22:485–91. [Google Scholar]
- 23.Zhao X, Liu Q, Xiao K, Hu Y, Liu X, Li Y, et al. Identification of the crp gene in avian Pasteurella multocida and evaluation of the effects of crp deletion on its phenotype, virulence and immunogenicity. BMC Microbiology. 2016;16:125. doi: 10.1186/s12866-016-0739-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiao K, Liu Q, Liu X, Hu Y, Zhao X, Kong Q. Identification of the avian Pasteurella multocida phoP gene and evaluation of the effects of phoP deletion on virulence and immunogenicity. International Journal of Molecular Science. 2015;17 doi: 10.3390/ijms17010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saleem L, Munir R, Ferrari G, Afzal M, Chaudhary FR. Efficacy and cross-protection of live intranasal aerosol hemorrhagic septicemia vaccine in buffalo calves. International Journal of Current Microbiology and Applied Sciences. 2014;3:300–7. [Google Scholar]
- 26.El Ashry ESH, Ahmad TA. The use of propolis as vaccine's adjuvant. Vaccine. 2012;31:31–9. doi: 10.1016/j.vaccine.2012.10.095. [DOI] [PubMed] [Google Scholar]
- 27.Shouqin Z, Jun X, Changzheng W. Note: Effect of high hydrostatic pressure on extraction of flavonoids in propolis. Food Science and Technology International. 2005;11:213–6. [Google Scholar]
- 28.Chang CC, Yang MH, Wen HM, Chern JC. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. Journal of Food and Drug Analysis. 2002;10:178–82. [Google Scholar]
- 29.Sun ZH, Wei K, Yan ZG, Zhu XL, Wang XJ, Wang H, et al. Effect of immunological enhancement of aloe polysaccharide on chickens immunized with Bordetella avium inactivated vaccine. Carbohydrates Polymers. 2011;86:684–90. [Google Scholar]
- 30.Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Analytical Chememistry. 1956;28:350–6. [Google Scholar]
- 31.Nassar SA, Mohamed AH, Soufy H, Nasr SM, Mahran KM. Immunostimulant effect of Egyptian propolis in rabbits. ScientificWorldJournal. 2012;901516 doi: 10.1100/2012/901516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coneac G, Gafitanu E, Hadaruga DI, Pinzaru IA, Rusu G, Ursica L, et al. Propolis extract/β-cyclodextrin nanoparticles: Synthesis, physico-chemical and multivariate analyses. Journal of Agroaliment Processes Technology. 2008;14:58–70. [Google Scholar]
- 33.Quinn PJ, Carter ME, Markey B, Carter GR, Shivachandra SB. In: Clinical Veterinary Microbiology. Quinn PJ CM, Markey B, Carter GR, editors. London: Wolfe Publishing, Mosby-Year Book Europe; 1994. 686 pp. [Google Scholar]
- 34.Knight DP, Paine JE, Speller DCE. A selective medium for Pasteurella multocida and its use with animal and human specimens. Journal of Clinical Pathology. 1983;36:591–4. doi: 10.1136/jcp.36.5.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holmes B, Willcox WR, Lapage SP. Identification of Enterobacteriaceae by Api 20E system. Journal of Clinical Pathology. 1978;31:22–30. doi: 10.1136/jcp.31.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith RH, Babiuk LA, Stockdale PHG. Intranasal immunization of mice against Pasteurella multocida. Infection and Immunity. 1981;31:129–35. doi: 10.1128/iai.31.1.129-135.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McFarland J. The nephelometer - An instrument for estimating the number of bacteria in suspensions used for calculating the opsonic index and for vaccines. Journal of American Medical Association. 1907;49:1176–8. [Google Scholar]
- 38.Digiacomo RF, Xu YM, Allen V, Hinton MH, Pearson GR. Naturally acquired Pasteurella multocida infection in rabbits - Clinicopathological aspects. Canadian Journal of Veterinary Research. 1991;55:234–8. [PMC free article] [PubMed] [Google Scholar]
- 39.Myint A, Jones TO, Nyunt HH. Safety, efficacy and cross-protectivity of a live intranasal aerosol haemorrhagic septicaemia vaccine. Veterinary Records. 2005;156:41–5. doi: 10.1136/vr.156.2.41. [DOI] [PubMed] [Google Scholar]
- 40.Delong D, Manning PJ. In: The biology of the laboratory rabbits. 2nd ed. Manning PJ, Ringler DH, CE N, et al., editors. San Diego, California: Academic Press; 1994. [Google Scholar]
- 41.Ahmad TA. Construction and evaluation of a conjugate vaccine for the prevention of septicemia. Egypt: Alexandria: Alexandria University; 2012. [Google Scholar]
- 42.Turkez H, Yousef MI, Geyikoglu F. Propolis prevents aluminium-induced genetic and hepatic damages in rat liver. Food and Chemical Toxicology. 2010;48:2741–6. doi: 10.1016/j.fct.2010.06.049. [DOI] [PubMed] [Google Scholar]
- 43.Huang WQ, Yao YF, Long Q, Yang X, Sun WJ, Liu CB, et al. Immunization against multidrug-resistant Acinetobacter baumannii effectively protects mice in both pneumonia and sepsis models. Plos One. 2014;9 doi: 10.1371/journal.pone.0100727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vandermeer NM, Appelmelk BJ, Verweijvanvught AMJJ, Nimmich W, Kosma P, Thijs LG, et al. Binding-studies of a monoclonal-antibody specific for 3-deoxy-D-manno-octulosonic acid with a panel of Klebsiella pneumoniae lipopolysaccharides representing all of the O-serotypes. Infection and Immunity. 1994;62:1052–7. doi: 10.1128/iai.62.3.1052-1057.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bieback K, Breer C, Nanan R, ter Meulen V, Schneider-Schaulies S. Expansion of human gamma/delta T cells in vitro is differentially regulated by the measles virus glycoproteins. Journal of General Virology. 2003;84:1179–88. doi: 10.1099/vir.0.19027-0. [DOI] [PubMed] [Google Scholar]
- 46.Repetto G, del Peso A, Zurita JL. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nature Protocols. 2008;3:1125–31. doi: 10.1038/nprot.2008.75. [DOI] [PubMed] [Google Scholar]
- 47.Castro-Concha LA, Escobedo RM, Miranda-Ham Mde L. Measurement of cell viability in in vitro cultures. Methods in Molecular Biology. 2006;318:71–6. doi: 10.1385/1-59259-959-1:071. [DOI] [PubMed] [Google Scholar]
- 48.Hoffman TL. In: Cell biology: a laboratory handbook. 3rd ed. Celis JE, editor. New York: Academic Press; 2006. pp. 21–4. [Google Scholar]
- 49.Nassar SA, Mohamed AH, Soufy H, Nasr SM. Protective effect of Egyptian propolis against rabbit pasteurellosis. Biomed Reseacrh International. 2013;2013:1–9. doi: 10.1155/2013/163724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sforcin JM, Bankova V. Propolis: Is there a potential for the development of new drugs? Journal of Ethnopharmacology. 2011;133:253–60. doi: 10.1016/j.jep.2010.10.032. [DOI] [PubMed] [Google Scholar]
- 51.Khayyal MT, Elghazaly MA, Elkhatib AS. Mechanisms involved in the anti-inflammatory effect of propolis extract. Drug under Experimental and Clinical Reseacrch. 1993;19:197–203. [PubMed] [Google Scholar]
- 52.Sforcin JM, Orsi RO, Bankova V. Effect of propolis, some isolated compounds and its source plant on antibody production. Journal of Ethnopharmacology. 2005;98:301–5. doi: 10.1016/j.jep.2005.01.042. [DOI] [PubMed] [Google Scholar]
- 53.Ivanovska ND, Dimov VB, Pavlova S, Bankova VS, Popov SS. Immunomodulatory action of propolis .5. Anti-complementary activity of a water-soluble derivative. Journal of Ethnopharmacology. 1995;47:135–43. doi: 10.1016/0378-8741(95)01273-g. [DOI] [PubMed] [Google Scholar]
- 54.Ansorag S, Reinhold D, Lendeckel U. Propolis and some of its constituents down-regulate DNA synthesis and inflammatory cytokine production but induce TGF-β1 production of human cells. Zeitschrift fur Naturforschung. 2003;58:580–9. doi: 10.1515/znc-2003-7-823. [DOI] [PubMed] [Google Scholar]
- 55.Márquez N, Sancho R, Macho A, Calzado MA, Fiebich BL, Muñoz E. Caffeic acid phenethyl ester inhibits T-cells activation by targeting both nuclear factor of activated T-cells and NF-κB tanscription factors. The Journal of Pharmacology and Experimental Therapeutics. 2004;308:993–1001. doi: 10.1124/jpet.103.060673. [DOI] [PubMed] [Google Scholar]


