Abstract
Background:
The incidence of esophageal squamous cell carcinoma (ESCC) is increasing, causing catastrophic health burdens on communities. Curcumin has shown promise as a therapeutic agent in the treatment of colon, colorectal, pancreatic, and esophageal cancers but it has very poor bioavailability. The application of nano-carriers as drug delivery systems increases curcumin's bioavailability. Cyclin D1 is overexpressed in ESCC and curcumin may change its expression.
Methods:
In this study, the effect of SinaCurcumin®, a novel nano-micelle product containing 80 mg curcumin, on the growth of KYSE-30 cells and expression of cyclin D1, was investigated. Paclitaxel and Carboplatin served as reference drugs.
Results:
Nano-curcumin increased cell cytotoxicity, decreased IC50, and down-regulated of cyclin D1. However, treatment of cells with nano-curcumin might result in multidrug resistance.
Conclusion:
Nano-curcumin suppressed proliferation of KYSE-30 cells and expression of cyclin D1 although its use in combination with other chemotherapeutic agents requires further testing.
Key Words: Curcumin, Cyclin D1 gene, Drug resistance, KYSE-30 cells, Nano-Micelle
Introduction
Esophageal cancer is the eighth-most common cancer, and sixth in terms of mortality, worldwide (1). The annual mortality rate due to esophageal cancer in the United States is 4–10 per 100,000 individuals, with men over 50 having the highest overall incidence (2). This cancer occurs most often in the non-Western world, with mortality rates of 100-180100,000 in northern Iran and northern China (3). The two most-common types of esophageal cancer are squamous cell carcinoma and adenocarcinoma (4). Esophageal squamous cell carcinoma (ESCC) is the predominant subtype in Asia (5). It is the most common cancer in men and the second-most common cancer in women in India's North Eastern states (6). Despite breakthroughs in surgery and adjuvant chemoradiotherapy, patients afflicted with ESCC have poor prognoses. Despite differences in ESCC development in individuals, characterization of gene expression in response to different therapeutic agents could improve the design of targeted ESCC therapy (7). Because cyclin D1 is frequently amplified and over-expressed in ESCC, it is believed to play a pivotal role in the disease. Cyclin D1 amplification leads to tumorigenesis and tumor growth (8).
Curcumin, the primary active agent derived from the root of Curcuma longa L. (English: turmeric, Persian: Zardchobeh) has antioxidative, anti-inflammatory, chemopreventive, and chemotherapeutic potential (9-12). Curcumin has shown promise as a therapeutic agent in the treatment of colon, colorectal, pancreatic, and esophageal cancers (13-17). Synergistic delivery of curcumin and doxorubicin in nanoparticles improved outcomes in diethylnitrosamine-induced hepatocellular carcinoma in mice (18). Oral curcumin potentiated the anti-tumor activity of gemcitabine by enhancing its ability to inhibit tumor proliferation and angiogenesis (19). The safety of curcumin has been reported in many animal studies as well as human trials (20-24). Curcuma aromatica oil preserved the function of manganese superoxide dismutase in rat models, which prevented the transition of esophageal epithelium to Barrett’s esophagus and then to esophageal adenocarcinoma (25).
Recently Sina Curcumin®, a novel product based on curcumin (has been developed using nanotechnology (26). Its efficiency and safety was investigated in diabetic patients. Body Mass Indexes (BMI) and lipid profiles, as main risk factors in diabetics and cardiovascular diseases, were reduced in subjects in this randomized clinical trial (26). In the present study, KYSE-30 cells were utilized as an in vitro model to evaluate the potential effect of nano-curcumin on cyclin D1 expression.
Materials and Methods
Chemicals
Nano-curcumin was graciously provided by the Exir Nano Sina Company (Tehran, Iran). Each nano-curcumin soft gel contained 80 mg of curcumin (26). Paclitaxel (30 mg/5mL) and Carboplatin (50 mg/15 mL) which served as the reference drugs were purchased from Actavis Pharma, Inc. (USA).
Cell Lines
The human esophageal squamous cell carcinoma cells (KYSE-30; Cat No: 94072011) were purchased from the Cell Bank, Pasteur Institute of Iran (Tehran, Iran). All the reagents and medium used in this study were prepared immediately before use. Normal cells (Cat No: 85120602) were used as healthy control cells.
Cell Culture
The cells were cultured in DMEM (Gibco, Germany) supplemented with 10% FBS (GIBCO, Germany), penicillin (1% v/v), and streptomycin (1% v/v) at 37 °C in 5% CO2 until confluent.
MTT Assay
The effects of nano-curcumin, paclitaxel, and carboplatin on the viability of KYSE-30 and normal cells were determined by the MTT assay (Sigma, USA). Briefly, an initial population of 5000 cells/mL was plated into each well of a 96-well culture plate containing 100 µL aliquots of growth medium, and incubated for 48 hours. Nano-curcumin was added to each well at concentrations of 0, 0.23, 0.46, 0.93, 1.87, 3.75, 7.5, 15, 30, and 60 mg/mL in triplicate. Paclitaxel at concentrations of 0, 0.11, 0.23, 0.46, 0.93, 1.87, 3.75, 7.5, 15, and 30 mg/mL, and carboplatin at concentrations of 0, 0.312, 0.625, 1.25, 2.5, 5, 10, 20, 40, and 80 mg/mL, were added in triplicate to other plates of KYSE-30 or normal cells. Following 24 h of incubation, 5 mg/mL MTT solution added to culture plates to a final concentration of 0.5 mg/mL. The plates were incubated for 3.5 h at 37 °C and the formazan precipitate was then dissolved with 200 µL of dimethyl sulfoxide. The absorbance was read on an ELISA plate reader (Anthos, Australia) at 490 nm. The IC50 was measured as mg/mL.
Gene Expression
To calculate gene expression in this study, samples were loaded with nano-curcumin and the drugs at IC50. RNAs from the esophageal and normal cell lines were isolated using an RNA extraction kit (Roche, Cat No: 11828665001). Nine samples were prepared; these were: KYSE-30 + carboplatin (A); KYSE-30 + carboplatin + nano-curcumin (B); KYSE-30 + paclitaxel (C); KYSE-30 + paclitaxel + nano-curcumin (D); KYSE-30 + paclitaxel + carboplatin (Sample E); KYSE-30 + nano-curcumin + paclitaxel + carboplatin (Sample F); KYSE-30 + nano-curcumin (Sample G); normal cells + nano-curcumin (Sample H); KYSE-30 (negative control). All procedures were carried out in an RNase/DNase free environment. The quality of the RNA was ascertained by gel electrophoresis and quantitated using NanoDrop® (Thermo Scientific, Wilmington, USA). Ten ngof RNA (10 ng) were reverse transcribed using a cDNA kit (Parstous Co., Tehran, Iran) according to the manufacturer’s instructions. Primers for quantitative real-time polymerase chain reaction are showen in Table 1. Quantitative analysis was performed by real time RT-PCR using the SYBR Green PCR Master Mix (Parstous Co, Tehran, Iran) and Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). Each reaction mixture contained 10 µl ofmaster mix, 1 µl of cDNA, and 10 µl of primer). The quantitative RT-PCR conditions were: 95 °C for 30 seconds (1 cycle), 95 °C for 4 seconds (45 cycles), then 60 °C for 32 seconds (45 cycles), for melting curve: 95 °C for 10 seconds, and 60 °C for 60 seconds. The 2-ΔΔCt method was used to quantify gene expression with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) used as a housekeeping gene. Results were expressed as relative fold changes in gene expression and then normalized to the corresponding reference gene (GAPDH) levels (primers in Table 1).
Table 1.
Primers used in this study
| Primers | Forward | Reverse |
|---|---|---|
| Cyclin D1 | TGCACCACCAACTGCTTAGC | GGCATGGACTGTGGTCATGAG |
| GAPDH | GGATGCTGGAGGTCTGCGAGGAAC | GAGAGGAAGCGTGTGAGGCGGTAG |
Statistical Analysis
All measurements were performed in triplicate. Data was described as mean ± standard deviation (SD). Considering normal distribution, one-way ANOVA and Tukey multiple range tests were initially conducted to determine significant difference at P-values < 0.05 microbiological counts (SPSS 19.0 software Package, IBM Inc., Chicago IL, USA).
Results
Effect of Nano-curcumin on Cell Viability
The functional effect of nano-curcumin on KYSE-30 and normal squamous cell proliferation was investigated by MTT assay. Figure 1 shows that treatment of the cells with nano-curcumin for 24 h, decreased cell proliferation in the KYSE-30 cell line by 71.09%, which was larger than Paclitaxel (61.30) and Carboplatin (62.32%), but not in the normal cell line, indicating that nano-curcumin effectively and selectively affects the proliferation of cancer cells leaving the normal cells unaffected. Interestingly, Carboplatin presented this reduction at higher doses as compared with nano-curcumin. When it comes to Paclitaxel, the count of the KYSE-30 cells was approximately higher at 1.87 mg/mL, meaning that nano-curcumin was of greater potential to affect cancerous cells. Moreover, the cell viability of KYSE-30 was in sharp decline as the concentration of nano-curcumin increased however it was associated with a non-considerable adverse effect on normal cells, revealing the cell cytotoxicity under the test concentrations. IC50 of nano-curcumin (1.87 mg/mL) in KYSE-30 was significantly lower than those of the free drugs (Paclitaxel 7.5 mg/mL and Carboplatin 40 mg/mL) in KYSE-30 as well as that of nano-curcumin (10 mg/mL) in the normal cells, confirming the improved cytotoxicity of curcumin in nanomicelles.
Figure 1.
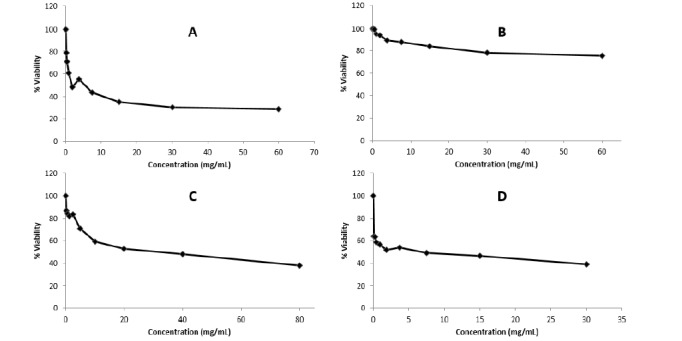
Effects of nano-curcumin, paclitaxel, and carboplatin of KYSE-30 and normal cells: (A) cytotoxicity of nano-curcumin in KYSE-30 cells; (B) cytotoxicity of nano-curcumin in normal cells; (C) cytotoxicity of Paclitaxel in KYSE-30 cells; (D) cytotoxicity of Carboplatin in KYSE-30 cells.
Expression of cyclin D1 in KYSE-30 cells
The expression level of cyclin D1 (Figure 2) notably diminished in cancerous cells treated with nano-curcumin (Sample G) as compared with those exposed to the drugs (Samples A, C, E; p<0.05) or their combination with nano-curcumin (Samples B, D, F; p<0.05), suggesting an increased activity against cyclin D1 by an individual treatment with nano-curcumin. Moreover, cyclin D1 in KYSE-30 cells in the presence of the drugs (Samples A, C, E) was (3.21-fold of) higher than that in KYSE-30 cells treated with nano-curcumin (Sample G). KYSE-30 in samples A, C, and E which received an individual or combined treatment with Carboplatin, Carboplatin presented a comparable reduction in the expression level of cyclin D1 akin to cells in the corresponding samples in the presence of nano-curcumin (Samples B, D, E) (p>0.05). Considering Sample G and H, there were significantly inhibitory impacts of nano-curcumin only on the expression of cyclin D1 in cancerous cells (p<0.05). In normal sample, the expression level of cyclin D1 was 4.91- fold of those in cancerous samples treated with nano-curcumin.
Figure 2.
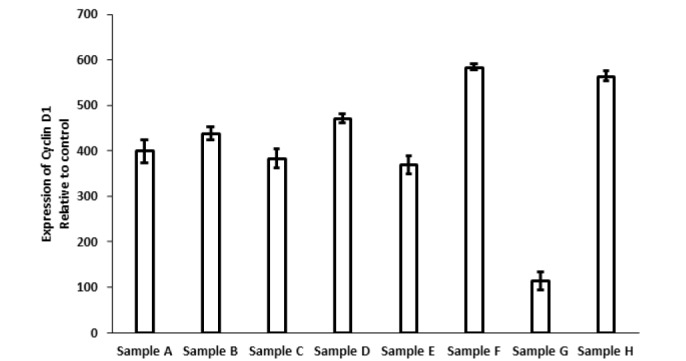
Expression of cyclin D1 in different samples of A (Carboplatin), B (Carboplatin and nano-curcumin), C (Paclitaxel), D (Paclitaxel and nano-curcumin), E (Carboplatin and Paclitaxel), F (Carboplatin, Paclitaxel and nano-curcumin), G (nano-curcumin) in tumor cells, and H (nanocurcumin) in normal cells.
Discussion
Curcumin has been demonstrated to affect many cellular components involved in cancer progression. As an example, it targets NF-ĸB which modulates the expression of various genes which play a pivotal role in anti-apoptosis, proliferation, angiogenesis, and invasion (27, 28). Nevertheless, this natural substance is of high hydrophobicity and then seems poorly absorbed when administered orally (29). Accordingly, considerable doses of free curcumin are required to achieve a high response rate (30). To overcome such limitations, distinct curcumin formulations have been created by using nanotechnology, which are efficient in either cell-based tests or clinical trials. THERACURMIN is new colloidal nano-particles, which exerts significant effects on bioavailability of curcumin in an animal design (31). Although, its safety has been reported frequently, its biological activity in interaction with other biomolecules has yet to be investigated. In this study, we evaluated an innovative nano-micelle containing curcumin and showed its improved anti-proliferation potential in KYSE-30 cells, which might be modulated via declined expression level of cyclin D1 gene. We also observed increased cytotoxicity and decreased IC50 in KYSE-30 cells treated with nano-curcumin. These findings are corroborated by previous studies which have exhibited the anti-proliferative function of curcumin against a great range of cancerous cells (27,32, 33). How curcumin can inhibit the growth of this type of cells is complex and not adequately understood. The results of the present study showed that nano-curcumin can beneficially suppress the viability of KYSE-30 cells, which might be associated with the down-regulation of cyclin D1 gene. On the other hand, real-time analysis revealed that multi-drug resistance may contribute to the reduced activity of nano-curcumin when administered along with an individual or combined treatment Paclitaxel and Carboplatin. Some scholars, however, demonstrated the therapeutic improvements as curcumin is used in combination with other cytotoxic agents. Synergic effects between curcumin and 5-fluorouracil on the proliferation of HT-29 cell lines were shown after the combination treatment (34). It was found that curcumin can augment the antitumor activity of gemcitabine for therapeutic application in pancreatic cancer while suppressing NF-ĸB-targeted gene products (19).
It was concluded that nano-curcumin did inhibit cyclin D1 expression. The down-regulation of cyclin D1 as a result of nano-curcumin in esophageal tumors that show overexpression of this proto-oncogene may be effective in the suppression of this cancer progression. Although the effectiveness of nano-curcumin in a cell-based model has been established, the possible multi-drug resistance provides a rationale for the design of more in vitro and in vivo investigations.
Acknowledgements
Mashhad University of Medical Sciences approved this study and had no role in the design and conduct of the study.
The authors declare that there is no conflict of financial interest.
References
- 1.Dawsey SP, Tonui S, Parker RK, Fitzwater JW, Dawsey SM, White RE, et al. Esophageal cancer in young people: a case series of 109 cases and review of the literature. PloS one. 2010;5(11):e14080. doi: 10.1371/journal.pone.0014080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bosetti C, Levi F, Ferlay J, Garavello W, Lucchini F, Bertuccio P, et al. Trends in oesophageal cancer incidence and mortality in Europe. International journal of cancer. 2008;122(5):1118–29. doi: 10.1002/ijc.23232. [DOI] [PubMed] [Google Scholar]
- 3.Rafiemanesh H, Maleki F, Mohammadian-Hafshejani A, Salemi M, Salehiniya H. The trend in histological changes and the incidence of esophagus cancer in Iran (2003-2008) Int J Prev Med. 2016;7(1):31. doi: 10.4103/2008-7802.175990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Enzinger PC, Mayer RJ. Esophageal cancer. The New England journal of medicine. 2003;349(23):2241–52. doi: 10.1056/NEJMra035010. [DOI] [PubMed] [Google Scholar]
- 5.Lehrbach DM, Nita ME, Cecconello I. Molecular aspects of esophageal squamous cell carcinoma carcinogenesi. Arq Gastroenterol. 2003;40:256–61. doi: 10.1590/s0004-28032003000400011. Clinical Genomics of Esophageal Cancer Group. [DOI] [PubMed] [Google Scholar]
- 6.Phukan RK, Ali MS, Chetia CK, Mahanta J. Betel nut and tobacco chewing; potential risk factors of cancer of oesophagus in Assam, India. British journal of cancer. 2001;85(5):661–7. doi: 10.1054/bjoc.2001.1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin DC, Du XL, Wang MR. Protein alterations in ESCC and clinical implications: a review. Diseases of the esophagus: official journal of the International Society for Diseases of the Esophagus. 2009;22(1):9–20. doi: 10.1111/j.1442-2050.2008.00845.x. [DOI] [PubMed] [Google Scholar]
- 8.Dey B, Raphael V, Khonglah Y, GiriLynrah K. Expression of Cyclin D1 and P16 in Esophageal Squamous Cell Carcinoma. Middle East journal of digestive diseases. 2015;7(4):220–5. [PMC free article] [PubMed] [Google Scholar]
- 9.Chuengsamarn S, Rattanamongkolgul S, Phonrat B, Tungtrongchitr R, Jirawatnotai S. Reduction of atherogenic risk in patients with type 2 diabetes by curcuminoid extract: a randomized controlled trial. The Journal of nutritional biochemistry. 2014;25(2):144–50. doi: 10.1016/j.jnutbio.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 10.Rahimi HR, Kazemi Oskuee R. Curcumin From Traditional Iranian Medicine to Molecular Medicine. Razavi Int J Med. 2014;2(2):e19982. [Google Scholar]
- 11.Sandur SK, Pandey MK, Sung B, Ahn KS, Murakami A, Sethi G, et al. Curcumin, demethoxycurcumin, bisdemethoxycurcumin, tetrahydrocurcumin and turmerones differentially regulate anti-inflammatory and anti-proliferative responses through a ROS-independent mechanism. Carcinogenesis. 2007;28(8):1765–73. doi: 10.1093/carcin/bgm123. [DOI] [PubMed] [Google Scholar]
- 12.Menon VP, Sudheer AR. Antioxidant and anti-inflammatory properties of curcumin. Advances in experimental medicine and biology. 2007;595:105–25. doi: 10.1007/978-0-387-46401-5_3. [DOI] [PubMed] [Google Scholar]
- 13.Kunnumakkara AB, Diagaradjane P, Anand P, Harikumar KB, Deorukhkar A, Gelovani J, et al. Curcumin sensitizes human colorectal cancer to capecitabine by modulation of cyclin D1, COX-2, MMP-9, VEGF and CXCR4 expression in an orthotopic mouse model. International journal of cancer. 2009;125(9):2187–97. doi: 10.1002/ijc.24593. [DOI] [PubMed] [Google Scholar]
- 14.O'Sullivan-Coyne G, O'Sullivan GC, O'Donovan TR, Piwocka K, McKenna SL. Curcumin induces apoptosis-independent death in oesophageal cancer cells. British journal of cancer. 2009;101(9):1585–95. doi: 10.1038/sj.bjc.6605308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sahu RP, Batra S, Srivastava SK. Activation of ATM/Chk1 by curcumin causes cell cycle arrest and apoptosis in human pancreatic cancer cells. British journal of cancer. 2009;100(9):1425–33. doi: 10.1038/sj.bjc.6605039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sandur SK, Deorukhkar A, Pandey MK, Pabon AM, Shentu S, Guha S, et al. Curcumin modulates the radiosensitivity of colorectal cancer cells by suppressing constitutive and inducible NF-kappaB activity. International journal of radiation oncology, biology, physics. 2009;75(2):534–42. doi: 10.1016/j.ijrobp.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jutooru I, Chadalapaka G, Lei P, Safe S. Inhibition of NFkappaB and pancreatic cancer cell and tumor growth by curcumin is dependent on specificity protein down-regulation. The Journal of biological chemistry. 2010;285(33):25332–44. doi: 10.1074/jbc.M109.095240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao X, Chen Q, Li Y, Tang H, Liu W, Yang X. Doxorubicin and curcumin co-delivery by lipid nanoparticles for enhanced treatment of diethylnitrosamine-induced hepatocellular carcinoma in mice. European journal of pharmaceutics and biopharmaceutics: official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik eV. 2015;93:27–36. doi: 10.1016/j.ejpb.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 19.Kunnumakkara AB, Guha S, Krishnan S, Diagaradjane P, Gelovani J, Aggarwal BB, et al. Curcumin potentiates antitumor activity of gemcitabine in an orthotopic model of pancreatic cancer through suppression of proliferation, angiogenesis, and inhibition of nuclear factor-kappaB-regulated gene products. Cancer research. 2007;67(8):3853–61. doi: 10.1158/0008-5472.CAN-06-4257. [DOI] [PubMed] [Google Scholar]
- 20.Shankar TN, Shantha NV, Ramesh HP, Murthy IA, Murthy VS. Toxicity studies on turmeric (Curcuma longa): acute toxicity studies in rats, guineapigs & monkeys. Indian journal of experimental biology. 1980;18(1):73–5. [PubMed] [Google Scholar]
- 21.Sharma RA, Euden SA, Platton SL, Cooke DN, Shafayat A, Hewitt HR, et al. Phase I clinical trial of oral curcumin: biomarkers of systemic activity and compliance. Clinical cancer research : an official journal of the American Association for Cancer Research. 2004;10(20):6847–54. doi: 10.1158/1078-0432.CCR-04-0744. [DOI] [PubMed] [Google Scholar]
- 22.Lao CD, Ruffin MTt, Normolle D, Heath DD, Murray SI, Bailey JM, et al. Dose escalation of a curcuminoid formulation. BMC complementary and alternative medicine. 2006;6:10. doi: 10.1186/1472-6882-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dhillon N, Aggarwal BB, Newman RA, Wolff RA, Kunnumakkara AB, Abbruzzese JL, et al. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2008;14(14):4491–9. doi: 10.1158/1078-0432.CCR-08-0024. [DOI] [PubMed] [Google Scholar]
- 24.Huang MT, Lou YR, Ma W, Newmark HL, Reuhl KR, Conney AH. Inhibitory effects of dietary curcumin on forestomach, duodenal, and colon carcinogenesis in mice. Cancer research. 1994;54(22):5841–7. [PubMed] [Google Scholar]
- 25.Li Y, Wo JM, Liu Q, Li X, Martin RC. Chemoprotective effects of Curcuma aromatica on esophageal carcinogenesis. Annals of surgical oncology. 2009;16(2):515–23. doi: 10.1245/s10434-008-0228-0. [DOI] [PubMed] [Google Scholar]
- 26.Rahimi HR, Mohammadpour AH, Dastani M, Jaafari MR, Abnous K, Ghayour Mobarhan M, et al. The effect of nano-curcumin on HbA1c, fasting blood glucose, and lipid profile in diabetic subjects: a randomized clinical trial. Avicenna journal of phytomedicine. 2016;6(5):567–77. [PMC free article] [PubMed] [Google Scholar]
- 27.Mukhopadhyay A, Banerjee S, Stafford LJ, Xia C, Liu M, Aggarwal BB. Curcumin-induced suppression of cell proliferation correlates with down-regulation of cyclin D1 expression and CDK4-mediated retinoblastoma protein phosphorylation. Oncogene. 2002;21(57):8852–61. doi: 10.1038/sj.onc.1206048. [DOI] [PubMed] [Google Scholar]
- 28.Aggarwal S, Ichikawa H, Takada Y, Sandur SK, Shishodia S, Aggarwal BB. Curcumin (diferuloylmethane) down-regulates expression of cell proliferation and antiapoptotic and metastatic gene products through suppression of IkappaBalpha kinase and Akt activation. Molecular pharmacology. 2006;69(1):195–206. doi: 10.1124/mol.105.017400. [DOI] [PubMed] [Google Scholar]
- 29.Li L, Braiteh FS, Kurzrock R. Liposome-encapsulated curcumin: in vitro and in vivo effects on proliferation, apoptosis, signaling, and angiogenesis. Cancer. 2005;104(6):1322–31. doi: 10.1002/cncr.21300. [DOI] [PubMed] [Google Scholar]
- 30.Bhawana, Basniwal RK, Buttar HS, Jain VK, Jain N. Curcumin Nanoparticles: Preparation, Characterization, and Antimicrobial Study. J Agric Food Chem. 2011;59(5):2056–61. doi: 10.1021/jf104402t. [DOI] [PubMed] [Google Scholar]
- 31.Sasaki H, Sunagawa Y, Takahashi K, Imaizumi A, Fukuda H, Hashimoto T, et al. Innovative preparation of curcumin for improved oral bioavailability. Biological & pharmaceutical bulletin. 2011;34(5):660–5. doi: 10.1248/bpb.34.660. [DOI] [PubMed] [Google Scholar]
- 32.Milano F, Mari L, van de Luijtgaarden W, Parikh K, Calpe S, Krishnadath KK. Nano-curcumin inhibits proliferation of esophageal adenocarcinoma cells and enhances the T cell mediated immune response. Frontiers in oncology. 2013;3:137. doi: 10.3389/fonc.2013.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Subramaniam D, Ponnurangam S, Ramamoorthy P, Standing D, Battafarano RJ, Anant S, et al. Curcumin induces cell death in esophageal cancer cells through modulating Notch signaling. PloS one. 2012;7(2):e30590. doi: 10.1371/journal.pone.0030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Du B, Jiang L, Xia Q, Zhong L. Synergistic inhibitory effects of curcumin and 5-fluorouracil on the growth of the human colon cancer cell line HT-29. Chemotherapy. 2006;52(1):23–8. doi: 10.1159/000090238. [DOI] [PubMed] [Google Scholar]


