Abstract
The Fukushima Daiichi accident highlighted the difficulty in making good decisions regarding post-accident actions for the protection of members of the public. Discussions are continuing between the authorities and the residents about ‘how safe is safe’. Although governmental officials have argued that 20 mSv per year is a safe level of exposure, many residents have expressed strong doubts, and one of their major concerns is the greater health risk of radiation exposure for children. For settling this controversy, the author has demonstrated risk projections for cancer mortality of female children (0 to 18 years old) resulting from four different levels of radiocaesium deposits on the ground. The results showed that, for female children, the cumulative lifetime attributable risk of cancer mortality due to 18-years external radiation exposure from radiocaesium in soil would be 0.9% for 134Cs and 2.4% for 137Cs for an initial annual dose of 20 mGy/year; when the initial dose was 5 mGy/year, the cumulative lifetime cancer risk would be 0.2% and 0.6% for 134Cs and 137Cs, respectively. These results indicate the critical importance of accurate information about the composition and behavior of major radionuclides released to the environment, as well as precise dose monitoring and risk coefficients, for proper decision-making regarding protective actions for members of the public.
Keywords: radiation, cancer risk, female children, evacuation, radiocaesium, post accident
INTRODUCTION
The severe accident at the Fukushima Daiichi Nuclear Power Station in 2011 led to >160 000 evacuees from the 20 km zone and from other areas having relatively high radiation levels [1]. Though the evacuation saved the residents from excessive radiation exposure [2], it has raised a controversial question—‘how safe is safe?’—with respect to return of the evacuees. The authorities have argued that 20 mSv per year is acceptable according to the International Standard [3], but many of the residents have expressed strong concerns about possible radiation-induced damage to their health, particularly for children, if they return.
Settlement of this controversy required a clear answer about the projected health risk of residents returning to the affected area. It is known that an equivalent dose of radiation exposure can cause different health effects, depending on the age and sex of the person; as a general rule, children are more susceptible to radiation than adults in regard to both cancer incidence and mortality [3–7].
Whereas, effective dose—an index widely used in discussion about protection of the public from the stochastic health effects of radiation exposure—is defined as a quantity for a nominal population having typical age and sex distributions, represented by a ‘Reference Person’, not a particular individual. Also, nominal risk coefficients are provided for just two groups: a whole population (the public) and adults (workers), not for a specific age-group such as children. As a result, a subsequent risk estimate using effective dose would be the same for all the members of a respective group, regardless of their individual properties. Though this was intentional for avoiding unnecessarily discriminatory actions [3], a member of the group can thus feel anxiety if there are reasons that the consequences of his/her radiation exposure might differ from that of the ‘Reference Person’.
In view of this, the author has sought to demonstrate here procedures for estimating the cancer mortality risks of the most radiation-sensitive group returning to an evacuated area at a given time after a radioactive contamination event.
METHODS FOR RISK PROJECTION
In the present study, the radiation exposure for returning residents was assumed to be due to external exposure in the form of γ-rays emitted from radiocaesium (134Cs or 137Cs) that has been deposited onto the ground; other short-lived radionuclides (131I, 132Te, etc.) were assumed to have decayed because they were below detection levels. Internal exposure due to the radiocaesium in the soil was also assumed to be negligibly small, based on whole-body monitoring data for the Fukushima residents [2, 8].
Assuming that the whole-body absorbed dose of a targeted resident in the initial year, D(0), was well assessed from in situ measurements and other relevant information, we can calculate the chronological change in the absorbed dose from radiocaesium in the soil as follows:
| (1) |
where r(t) is the ratio of the γ-ray dose rate above undisturbed open ground to that for a reference depth distribution of a radionuclide in soil at time t, which accounts for attenuation mainly due to the migration of radionuclides in the soil; and λ is the decay constant of the radionuclide. The attenuation function r(t) can be given from the sum of the short- and long-term decay components as follows:
| (2) |
where p1 and p2 are empirically-derived constants of which the sum is 1; and T1 and T2 are environmental half-lives for short- and long-term decay components, respectively. Here, the environmental decays were given as T1 = 1.5 years and T2 = 50 years, according Attachment C-12 of the UNSCEAR 2013 report [2]; it was assumed that those numbers could be uniformly applied to any locations. The ratios of the two components were given here as p1 = 0.4 and p2 = 0.6 under the assumption that the decision would be made at one year after the major deposition, as the initial value of 0.5 was employed by UNSCEAR for both parameters [2].
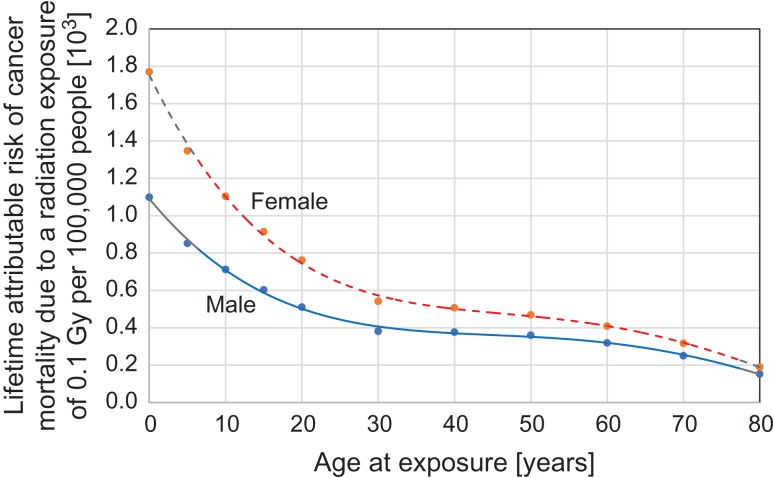
In cancer risk projections, the author focused on female children (0 to 18 years old), because this group is known to be the most radiation sensitive regarding all-cancer risk [5, 7]. Figure 1 shows the plots of the lifetime attributable risk (LAR) of cancer mortality attributable to a single whole-body exposure of 0.1 Gy as a function of age at exposure; they were taken from the BEIR VII report [USNRC, 2006]. The original LAR values were expressed as the numbers of cases per 100 000. As seen in this figure, the radiation-induced cancer risk is greater by two to three times in children than in adults, and females are more susceptible to radiation than males.
Fig. 1.
Lifetime attributable risk of radiation-induced cancer mortality as a function of age at acute γ-ray exposure for males (solid line) and females (dotted line) (after the BEIR VII report [5]).
In the following risk estimation, the female children from 0 to 18 years old (till graduation from a high school) were targeted as a critical group. Integrating the number of cancer mortality, L(y), was calculated by integrating the annual absorbed dose multiplied by the time-dependent LAR for the period in respect. Here, the elapsed time is the same as the age of the female children, because their age in the initial year was 0. That is, L(y) is calculated by:
| (3) |
where L(0) is 0.
RESULTS AND DISCUSSION
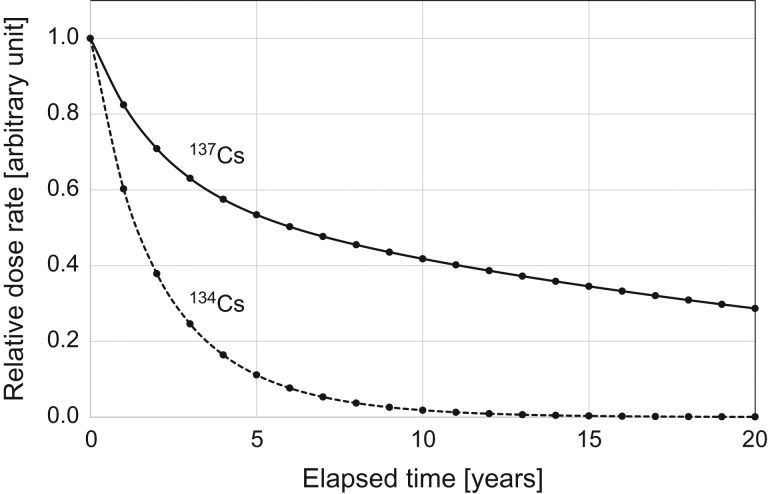
Predicted changes in the relative annual doses from 134Cs and 137Cs deposited onto the ground are plotted in Fig. 2. It is seen that the dose level of 134Cs decreased more sharply than 137Cs, reflecting the difference in the physical half lives (134Cs: 2.06 year and 137Cs: 30.1 year).
Fig. 2.
Predicted change of relative external dose rate from 137Cs (solid line) and 134Cs (dotted line) that deposited onto the ground.
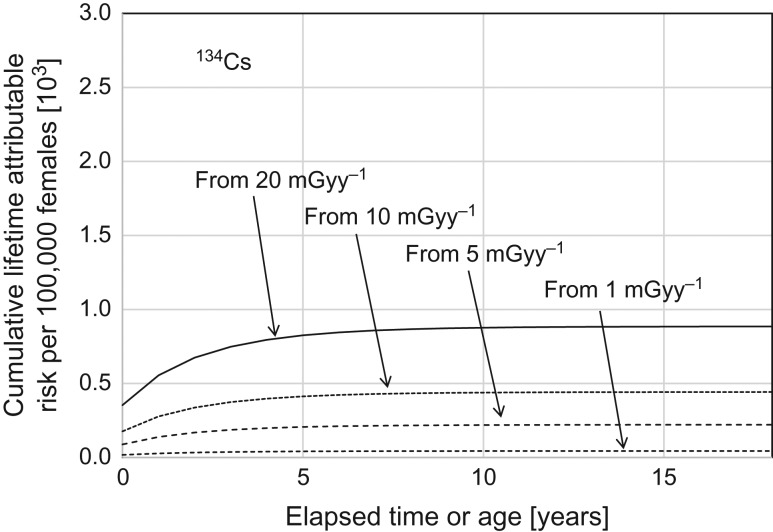
Predictions of the integrated number of cancer mortalities as cumulative products of the annual dose rate from 134Cs in soil (see Fig. 2) and the age-dependent lifetime attributable risk for females (see Fig. 1) are shown in Table 1 and also plotted in Fig. 3 as a function of age up to 18 years. Calculations were made for four cases with different initial levels of annual external radiation exposure: 1, 5, 10 and 20 mGy in the first year; those levels were assumed to have been well substantiated from comprehensive monitoring data and typical behavior of the targeted residents.
Table 1.
Cumulative risks of cancer mortality [%] from 134Cs deposited onto the ground, as predicted for a one-year-old child till the age of 18 years for four different initial levels of external radiation exposure
| Age [years] | External dose at the initial year from 134Cs [mGy] | |||
|---|---|---|---|---|
| 1 | 5 | 10 | 20 | |
| 0 | 0.018 | 0.089 | 0.18 | 0.35 |
| 1 | 0.028 | 0.14 | 0.29 | 0.56 |
| 2 | 0.034 | 0.17 | 0.34 | 0.68 |
| 3 | 0.037 | 0.19 | 0.37 | 0.75 |
| 4 | 0.040 | 0.20 | 0.40 | 0.80 |
| 5 | 0.041 | 0.21 | 0.41 | 0.83 |
| 6 | 0.042 | 0.21 | 0.42 | 0.85 |
| 7 | 0.043 | 0.22 | 0.43 | 0.86 |
| 8 | 0.043 | 0.22 | 0.43 | 0.87 |
| 9 | 0.044 | 0.22 | 0.44 | 0.87 |
| 10 | 0.044 | 0.22 | 0.44 | 0.88 |
| 11 | 0.044 | 0.22 | 0.44 | 0.88 |
| 12 | 0.044 | 0.22 | 0.44 | 0.88 |
| 13 | 0.044 | 0.22 | 0.44 | 0.88 |
| 14 | 0.044 | 0.22 | 0.44 | 0.88 |
| 15 | 0.044 | 0.22 | 0.44 | 0.88 |
| 16 | 0.044 | 0.22 | 0.44 | 0.88 |
| 17 | 0.044 | 0.22 | 0.44 | 0.88 |
| 18 | 0.044 | 0.22 | 0.44 | 0.88 |
Fig. 3.
Cumulative risk of radiation-induced cancer mortality from 0 to 18 years old for different levels of initial external radiation doses from 134Cs in the surface soil.
As seen in Fig. 3, the curves of the cumulative cancer risks reached a plateau at ~10 years in all cases. The cumulative risk of radiation-induced cancer mortality during childhood was estimated to be 0.9% in the case with the highest initial dose of 20 mGy year−1; it would not exceed 1% for any longer period. The cumulative risk decreased in proportion to the initial dose level. For example, the cumulative risk for 18 years was 0.22% in the case of a 5 mGy year−1 initial dose.
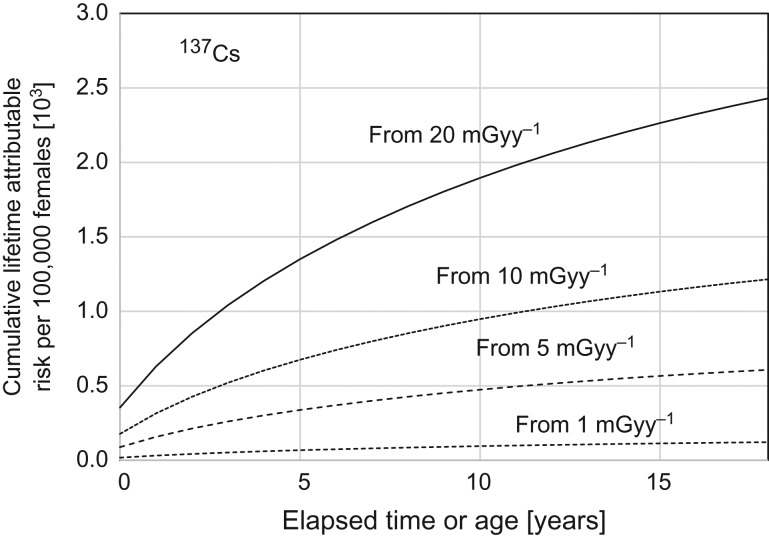
In the same way, cumulative risks from 137Cs deposited onto the ground were calculated to be as shown in Table 2 and Fig. 4. In contrast to those with 134Cs (Fig. 3) exposure, the risk levels were quite high and they were still increasing at the age of 18 years in all cases. The estimated cumulative risks of female children from 0 to 18 years old were 2.4 % for the initial dose of 20 mGy year−1 and 0.6 % for 5 mGy year−1.
Table 2.
Cumulative risk of cancer mortality [%] from 137Cs deposited onto the ground, as predicted for a one-year-old child till the age of 18 years for four different initial levels of external radiation exposure
| Age [years] | External dose at the initial year from 137Cs [mGy] | |||
|---|---|---|---|---|
| 1 | 5 | 10 | 20 | |
| 0 | 0.018 | 0.089 | 0.18 | 0.35 |
| 1 | 0.031 | 0.16 | 0.32 | 0.63 |
| 2 | 0.043 | 0.21 | 0.43 | 0.85 |
| 3 | 0.052 | 0.26 | 0.52 | 1.0 |
| 4 | 0.060 | 0.30 | 0.60 | 1.2 |
| 5 | 0.068 | 0.34 | 0.68 | 1.4 |
| 6 | 0.074 | 0.37 | 0.74 | 1.5 |
| 7 | 0.080 | 0.40 | 0.80 | 1.6 |
| 8 | 0.085 | 0.43 | 0.85 | 1.7 |
| 9 | 0.090 | 0.45 | 0.90 | 1.8 |
| 10 | 0.095 | 0.47 | 0.95 | 1.9 |
| 11 | 0.099 | 0.50 | 0.99 | 2.0 |
| 12 | 0.10 | 0.52 | 1.0 | 2.1 |
| 13 | 0.11 | 0.53 | 1.1 | 2.1 |
| 14 | 0.11 | 0.55 | 1.1 | 2.2 |
| 15 | 0.11 | 0.57 | 1.1 | 2.3 |
| 16 | 0.12 | 0.58 | 1.2 | 2.3 |
| 17 | 0.12 | 0.60 | 1.2 | 2.4 |
| 18 | 0.12 | 0.61 | 1.2 | 2.4 |
Fig. 4.
Cumulative risk of radiation-induced cancer mortality from 0 to 18 years old for different levels of initial external radiation doses from 137Cs in the surface soil.
It is not simple to judge what risk level should be deemed acceptable or unacceptable, because all individuals have different perceptions about risks. The final decision about returning to the evacuation area should be made through comprehensive discussions between interested parties, with careful attention to social, economic and psychological factors, following the concept for optimization in radiological protection, i.e. ‘As Low As Reasonably Achievable (ALARA)’ [4].
Nevertheless, we could say that, if the dominant radionuclide is 134Cs and the initial annual dose is 10 mGy y−1 or lower, the additional risk of cancer mortality would not exceed 0.5%, even for the most radiation-sensitive group, which is quite low and would be indiscernible because female Japanese people have a risk of 16% of cancer death until 80 years of age, and the age-adjusted cancer mortality by prefecture for female Japanese shows a large geographical variation by a factor of up to 1.4 [9]. On the other hand, in the case that 137Cs is dominant and the initial dose level is 20 mGy y−1, the risk of cancer mortality could be more than 2% for the critical group, which should be carefully discussed in comparison with cumulative health risks in other areas they might choose for living; it should be noted that moving from the affected area to another region would not necessarily reduce the cancer risk, because other causes (food, weather, etc.) could have a more significant impact on cancer development, as suggested from the cancer statistics [9].
In the scenario in which we employed the nominal risk coefficient (5.7% for whole population) as provided in the ICRP recommendation [3], the nominal risk for the former case (i.e. 10 mGy year−1 initial dose due to 134Cs) was calculated to be 0.16% and that for the latter case (i.e. 20 mGy y−1 initial dose due to 137Cs) was 1.1%. These values were two to three times higher than those calculated exclusively for the female children, which confirms that the nominal risk is given for sex-averaged and age-at-exposure-averaged lifetime risk estimates for a representative population and thus is unsuitable for discussing the health risk of a certain group, such as female children.
CONCLUSION
When a severe radiation disaster occurs, prompt decisions and actions by the authorities for the protection of the public are clearly important. However, decision-making is often difficult because of the trade-offs between the various detrimental factors, such as health risks, costs, inconvenience, mental stress, etc., as highlighted in the Fukushima Daiichi accident. To enable good decision-making regarding the most appropriate action, it is desirable to prepare various risk projections in advance for typical scenarios.
With this thought, the author has presented here an approach for estimating the cancer mortality risks of female children (the most radiation-sensitive sector), assuming they were forced to evacuate because of a significant radiocaesium deposition onto the ground and are now thinking of returning to the evacuated area. The data required for such estimations highlights the importance of having accurate information on the composition and behavior of radionuclides in the environment, in addition to radiation monitoring data to indicate current radiation levels. The author hopes that the procedures demonstrated in this paper will contribute to improvement in decision-making in ongoing and future protective actions for members of the public affected by a radiation disaster.
ACKNOWLEDGEMENTS
The work in this paper was continuously supported by Research Institute for Radiation Biology and Medicine (RIRBM), Hiroshima University.
CONFLICT OF INTEREST
The author (HY) has no actual or potential conflict of interest related to this paper.
FUNDING
This work was supported in part by the Program of the network-type joint Usage/Research Center for Radiation Disaster Medical Science funded by Ministry of Education, Culture, Sports, Science and Technology of Japanese Government.
REFERENCES
- 1. Fukushima Prefectural Government Fukushima Revitalization Station http://www.pref.fukushima.lg.jp/site/portal/list271.html (24 February 2018, date last accessed), in Japanese.
- 2. United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) UNSCEAR 2013 Report to the General Assembly with scientific annexes, Annex A: Levels and effects of radiation exposure due to the nuclear accident after the 2011 Great East-Japan Earthquake and Tsunami. New York: United Nations, 2014.
- 3. International Commission on Radiological Protection (ICRP) 2007 Recommendations of the International Commission on Radiological Protection. ICRP Publication 103. Ann ICRP2007;37(2–4). [DOI] [PubMed]
- 4. International Commission on Radiological Protection (ICRP) 1990 Recommendations of the International Commission on Radiological Protection. ICRP Publication 60. Ann ICRP1991;21(1–3). [PubMed]
- 5. U.S. National Research Council Health Risks From Exposure to Low Levels of Ionizing Radiation: BEIR VII Phase 2. Washington, DC: National Academies Press, 2006. [PubMed] [Google Scholar]
- 6. United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) UNSCEAR 2006 Report to the General Assembly with scientific annexes, Annex A: Epidemiological studies of radiation and cancer. New York: United Nations, 2008.
- 7. United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) UNSCEAR 2013 Report to the General Assembly with scientific annexes, Annex B: Effects of radiation exposure of children. New York: UNSCEAR, 2013.
- 8. International Atomic Energy Agency (IAEA) The Fukushima Daiichi Accident. Report by the Director General IAEA, 2015.
- 9. National Cancer Center Japan Cancer Registration and Statistics: Cancer Information Service https://ganjoho.jp/reg_stat/statistics/stat/summary.html (24 February 2018, date last accessed), in Japanese.