Abstract
Although the vast majority of DNA damage induced by radiation exposure disappears rapidly, some lesions remain in the cell nucleus in very small quantities for days to months. These lesions may cause a considerable threat to an organism and include certain types of DNA double-strand breaks (DSBs) called ‘unrepairable DSBs’. Unrepairable DSBs are thought to cause persistent malfunctioning of cells and tissues or cause late effects of radiation, especially the induction of delayed cell death, mutation, senescence, or carcinogenesis. Moreover, the measurement of unrepairable DSBs could potentially be used for retrospective biodosimetry or for identifying individuals at greater risk for developing the adverse effects associated with radiotherapy or chemotherapy. This review summarizes the concept of unrepairable DSBs in the context of persistent repair foci formed at DSBs.
Keywords: radiation, unrepairable DSBs, gamma H2AX, senescence, biodosimetry
INTRODUCTION
Among the genome damage induced by ionizing radiation, DNA double-strand breaks (DSBs) represent the most biologically deleterious type of lesion. To tackle these potentially lethally damaging lesions, cells have evolved orchestrated and conserved mechanisms known collectively as the DNA damage response (DDR) [1–4]; the DDR coordinates cellular DSB repair activities immediately after the damage is detected [4]. Indeed, within seconds, DSB repair proteins start to accumulate at the site of DSBs, and the DDR directs the cells to repair the breaks, undergo apoptosis, or become senescent [3]. DSB repair is a quick and efficient process whereby broken ends are rejoined. Using traditional and biochemical DNA size analyses [5–7] coupled with immunocytochemical staining [8–10], the kinetics of DSB repair have been shown to occur in two phases: a fast phase lasting up to a few hours, with a half-life of 30 min to 1 h, followed by a slower phase that may persist for a long time. A few persistent DSBs remain into the next day, and some of these DSBs persist for days, months, or even years [10–13]. These DSBs are more difficult to repair or remain unrepaired DSBs. Alternatively, these persistent DSBs are termed residual DSBs and are retained in the damaged cells unless the cells die and slip away [10, 14]. The number of unrepairable DSBs in a cell is measured by detecting and counting the number of persistent repair foci, which are composed of multiple proteins that accumulate at DSBs. Specifically, it is suggested that individual γH2AX or 53BP1 foci represent a single DSB with a ratio of 1:1 [8, 15, 16]; hence, very few repair foci out of the many that form immediately after radiation exposure remain and become persistent in the cell nucleus. In this review, I summarize the observations obtained from the exposure of quiescent normal human cells to radiation, incorporate these observations into a discussion of the literature, and then discuss the biological implications of unrepairable DSBs with respect to the effects of radiation on cells and tissues. I also discuss the possible application of unrepairable DSB measurement for retrospective biodosimetry.
GENERATION OF UNREPAIRABLE DSBS AND PERSISTENT REPAIR FOCI
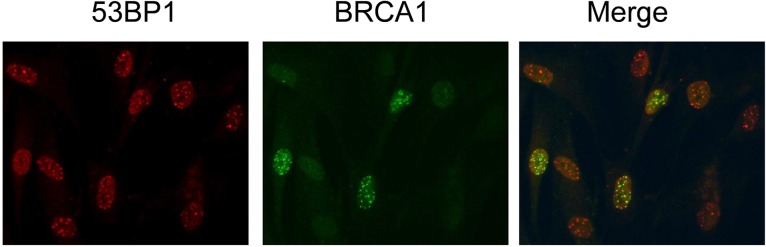
Radiation-induced DSBs are first detected by the MRE11/Rad50/NBS1 (MRN) complex and the Ku70/Ku80 heterodimer [1, 3]. Accordingly, the DDR initiates many possible mediators and effectors to potentiate distinct signals within a cell. The MRN complex promotes the binding of a phosphorylated form of ATM and the phosphorylation of H2AX. The MRN complex also promotes the binding of MDC1 and a group of ubiquitination proteins (RNF8 and RNF168) and their effectors at the DSBs, thereby forming the fundamental structures of repair foci. Repair foci are a cluster of many kinds of proteins. They have different components depending on whether the cells are undergoing the NHEJ pathway (including microhomology-mediated NHEJ) or entering S phase, during which the cells undergo HR-mediated repair. In fact, in exponentially growing normal human fibroblasts, 100% of cell nuclei have many γH2AX/53BP1 foci after radiation exposure, whereas only 30% of those exhibit BRCA1-positive foci. The repair foci containing BRCA1 are specifically formed for recombination repair in S phase (Fig. 1). This observation indicates that the accumulation of BRCA1 protein depends on cell progression through S phase. In somatic cells in living organisms, most of the cells are in quiescent (Go) phase; they are not dividing and are instead terminally differentiated, functioning to preserve their specified phenotype and exhibiting very long lifespans, up to years or decades. To create a model system for this in vivo cell status, we have adopted quiescent cultures of normal human diploid fibroblasts (NHDFs) in which the cells are maintained in MEM + 0.1% FCS. In this condition, the cells are permanently growth arrested (Go state) and can survive as long as weekly medium changes are maintained (any time after quiescence, cells can reenter the cell cycle in the presence of medium containing 10% FCS, which provides growth stimulation).
Fig. 1.
Staining of exponentially growing NHDFs immediately after radiation exposure (1 Gy/1 h) with antibodies against 53BP1 and BRCA1.
Analysis of the DSB repair kinetics of quiescent NHDFs showed that 95% of the initial DSBs disappeared within a couple of days after radiation exposure, followed by a very slow decline to ~1% DSBs remaining after 2 weeks. These DSBs persisted in the cell nucleus as long as the culture continued (up to 1 year in our study) [10], thus we designated them ‘unrepaired DSBs’. The formation and resolution of γH2AX/53BP1 foci have been extensively studied in vitro [8, 17–20], and the kinetics have been shown to be biphasic [10–12, 21], which matches well with the kinetics of DSB repair [5]. Although the persistence of repair foci could arguably reflect insufficient H2AX dephosphorylation [22], it has been readily accepted that the foci represent unrepaired DSBs because the foci also include 53BP1 and all other previously reported DDR proteins. Moreover, the phosphorylated form of DNA-PKcs (ser-2056), which marks the initial recognition of a DSB, was also detected in the foci [10]. Treatment of cells with the ATM inhibitor KU55933 completely abolished the persistent foci, and the foci recovered at the DSB sites when the inhibitor was chased off. This effect was observed in cultures even 6 months post exposure to radiation. Treatment with the polyubiquitination inhibitor MG132 also produced the same effects. These results indicate that there is a continuous turnover of foci components at the unrepaired DSBs, which persists for a long time after the exposure.
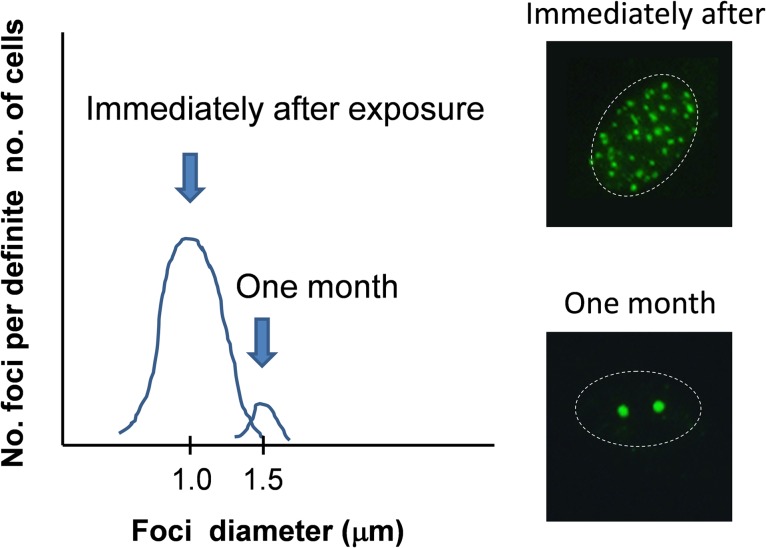
Therefore, what is the function of the slow to very slow type of DSB repair that follows the fast phase? Is it truly a process of very slow repair, does this process establish unrepaired DSBs, or is it a mixture of both processes? At present, we do not know the biochemical difference between repairable and unrepairable DSB foci; however, it is almost certain that the size of the foci grows larger over time [10, 23–26]. We measured the foci enlargement over time and found that the unrepaired foci could be sorted from the small foci of the fast phase that would eventually disappear upon the completion of repair. The repairable foci displayed a normal size distribution immediately after radiation exposure, whereas after 1 or 2 months the unrepaired DSB foci formed a distinct distribution of foci larger in diameter and could be separately counted (Fig. 2).
Fig. 2.
Images of the size distribution of repair foci immediately after or a long time after radiation exposure. One month after exposure, transient/repairable DSB foci disappeared, and persistent DSB foci remained in the nucleus.
PROSPECTIVE APPLICATIONS OF UNREPAIRABLE DSBS TO BIODOSIMETRY
We know that the effects of radiation stem from unrepairable damage and not from the repair process of repairable damage. It has been argued that the radiosensitivity of an individual cell can be attributed to the presence of unrepairable DSBs [27–29]. These unrepairable DSBs are responsible for the late effects of radiation, i.e. changes in the aging process, late onset mutations, and cancer. It is also argued that the measurement of unrepairable DSBs could be applied to radiation dosimetry on previously irradiated cells and tissues [30–33].
By counting the number of large-sized foci remaining 1 month after irradiation, we found that there was a linear relationship between the radiation dose and the number of unrepaired DSBs. Assuming that one unrepairable DSB is one lethal hit, then the actual average one-hit dose is ‘Do’, which is the dose required for 37% cell survival in classic ‘radiation hit theory’. A similar idea enables the calculation of the average two-hit dose required for 13.5% cell survival. Then, from the dose–response curves of the formation of unrepairable DSBs we made hypothetical survival curves, which agreed well with actual cell survival phenotypes. The concept that unrepairable DSBs limit the radiosensitivity of cells has been validated by other studies [28, 29]. In fact, Menegakis et al. have also attempted to calculate hypothetical cell survival [34].
Based on observations that the number of unrepairable DSBs accumulates with repeated exposure [10, 20], retrospective radiation biodosimetry has been proposed [10, 20, 35, 36]. By measuring the number of unrepaired DSB foci per unit area of mouse skin, Bhogal et al. reported the possibility of biodosimetry [28]. In minipig, skin biopsies taken 70 days after a 50 Gy exposure showed typical unrepaired DSB foci patterns. Accordingly, minipig [37] and macaque models [38] for persistent DSB-mediated biodosimetry have been reported. Cells from plucked hair appeared to be especially useful [32]. In mouse spinal cord, the persistent foci were detectable 1 year after the exposure [39]. Overall, these reports may offer a new avenue for the use of unrepairable DSBs in radiation biodosimetry. We also observed the appearance of large repair foci one month after mouse pancreas was irradiated with 6 Gy. Similar observations have been reported in human buccal cells [40] and mouse germ cells [39, 41]. Moreover, increases in the number of unrepairable DSBs have been associated with aging in many studies [20, 36, 42–44].
Some pediatric cancer patients display increased unrepairable DSB production levels in their peripheral blood lymphocytes. These patients might represent groups at higher risk during radiation exposure [45]. A group of patients who demonstrated an excessive response to radiotherapy, which was assessed by normal tissue toxicity, exhibited increased levels of persistent repair foci [46]. The levels of persistent repair foci per cell in lymphocytes were examined after radiotherapy in breast cancer patients [29, 47]. This calculation will enable the fine-tuning of radiation doses for improved cancer treatments. In head and neck cancer cases, prescreening the levels of persistent foci in 2 Gy irradiated lymphocytes was applied to reduce the side effects of radiotherapy [48]. Thus, persistent repair foci are a useful measure for the detection and evaluation of previously irradiated cells.
However, it has been reported that repair kinetics and the formation of persistent repair foci vary extensively among mouse tissues [49, 50]. Therefore, tissue differences should be considered when evaluating unrepairable DSBs for biodosimetry.
THE REAL IDENTITY OF UNREPAIRABLE DSBS: ARE THEY TELOMERE DSBS OR ARE THEY LOCATED INSIDE THE CHROMOSOME?
Although the chemical structures of unrepairable DSBs have not yet been precisely determined, two types of ‘complex’ lesions can be postulated: those with non-ligatable termini due to crosslinks between bases and sugars (dirty DSBs) and those with damaged sites in which multiple DSBs/SSBs and/or base damage arise in close proximity (clustered damage) [51]. The complexity of the damage structure is evident by the slow rejoining kinetics of DSBs induced by high-LET radiation [52]. It is apparent that the damage induced by radiation is extremely heterogeneous and that damage occurs randomly throughout the chromosome.
Unrepaired DSBs induce permanent cell growth arrest or death. This fact is especially true in non-apoptotic terminally differentiated cells, which in general have very long lifespans. In these cells, cellular senescence is induced (radiation-induced senescence). It has been shown that many adult survivors of juvenile cancer develop symptoms of premature aging later in life [53], which may be related to the unrepaired DSBs that were induced after radiotherapy in normal tissues adjacent to the tumor. Their symptoms include advanced frailty with increased risk for heart failure, severe cognitive decline, coronary heart disease, and secondary neoplasms [53–55]. Meanwhile, neuronal stem cells bearing unrepairable DSBs undergo premature senescence or terminal differentiation to become astroglial cells [56]. This process also reduces stem cell number, leading to a premature aging of neuronal systems. Likewise, after radiation exposure, melanocyte stem cells in hair bulge undergo terminal differentiation in their niches, producing gray hairs [57].
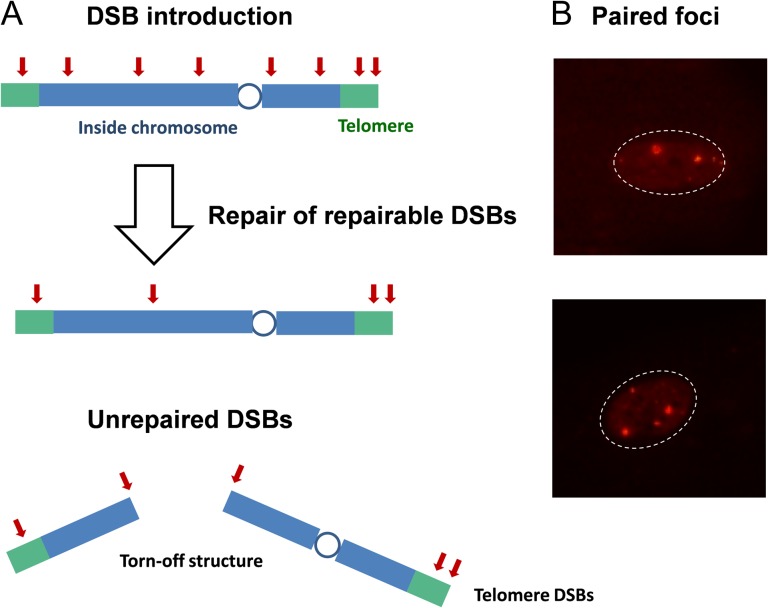
Telomere-driven replicative senescence has been widely accepted as a primary mechanism of organismal aging and cellular senescence. Under this theory, eroded chromosome ends continuously activate the DDR, thereby permanently inducing growth arrest and senescence. Similarly, it has been hypothesized that radiation-induced unrepairable DSBs eventually accumulate in chromosome ends, which establishes senescence independent of the cell’s telomere length [14, 35, 36, 58]. This idea is based on the specific structure of chromosome ends, known as Shelterin, which protect chromosomes from improper recombination and degradation. Indeed, TRF2 and RAP1, the components of Shelterin, were shown to inhibit DSB repair throughout the entire telomere [35, 59–61], not only at the very ends. Therefore, radiation-induced DSBs near the chromosome ends would be preferentially protected from repair systems, even though radiation exposure induces DSBs randomly throughout the chromosome. Such a mechanism may eventually provoke continuous DDRs, leading to cellular senescence in cells bearing radiation-induced unrepairable DSBs (Fig. 3A). This idea has been confirmed via confocal microscope observations and telomere ChIP experiments [36]. Thus, the telomere hypothesis suggests that the location, not the chemical structure, is important for the establishment of unrepairable DSBs. This hypothesis may explain the radiation-induced senescence of young cells in vitro and non-dividing quiescent cells in vivo. Both of these cell types have long telomeres and no chance of undergoing replicative senescence. Assuming that animals carrying longer telomere sequences have a wider target for the creation of unrepairable DSBs, telomere length may have a negative effect on lifespan. Indeed, inverse correlations between telomere length and lifespan have been reported in different animal species [62].
Fig. 3.
(A) Possible mechanisms for the generation of unrepaired DSBs. Although radiation exposure induces DSBs randomly in the chromosome, those occurring proximal to the telomere or unrepairable DSBs inside the chromosome eventually become persistent. (B) Persistent repair foci tend to make pairs. In the lower photo, two pairs of foci of different sizes can be seen.
However, other mechanisms of radiation-induced senescence may exist. Most nuclei of 6 Gy-irradiated and 1-month-cultured young quiescent cells carried a few typical, large γH2AX/53BP1 foci, as mentioned above. Careful observations have revealed that these foci often appear in pairs (i.e. even numbers per nucleus), as if they had originated from a single DSB and then distantly separated. Taking a closer look, each partner of the foci pairs can be identified because each foci had a distinct size (Fig. 3B and reference photos provided in the supplemental data) [10]. In this case, the DSBs should have originated from the inside of the chromosome and not from the very end. Data in reports from Hewitt [36] and Fumagalli [35] indicates that, at most, 50% of unrepaired DSBs are located in telomeres; therefore, it is reasonable to assume that the causative mechanisms of unrepairable DSBs can be attributed to both their locations and chemical structures [63]. Collectively, while the fast phase of DSB repair constitutes rejoining of easy-to-repair ‘clean DSBs’, the slower phase might be composed of two distinct components, namely, telomere-silenced DSBs and unrepairable DSBs.
In yeast, several studies have indicated that persistent DNA lesions relocate to either the nuclear pore complex (NPC) or the nuclear envelope (NE) [64–66]. In such cases, specific membrane structures, including the components of nuclear pore proteins, appear to be crucial for the repair of persistent DSBs and eroded telomeres. In mammals, mutated forms of nuclear lamin A, referred to as progerin, induce deformation of the NE and impair certain processes of DSB repair. This process has been argued to contribute to the decrease in repair efficiency [67, 68] or to the enhanced production of unrepairable DSBs [69, 70]. In either case the expression of progerin has an adverse effect on DSB repair and induces premature senescence in cells. Likewise, NHDFs (normal cells) bearing radiation-induced unrepairable DSBs, which undergo premature senescence, show dysfunctional nuclear membrane structures [70]. These results indicate a possible link between unrepairable DSBs and premature senescence, which are both mediated by a dysfunctional nuclear membrane. The nuclear membrane and its periphery has been shown to associate with heterochromatin, where ‘gene deserts’ have also been shown to localize [71, 72]. The DSB repair of such regions is slow and dependent on both ATM and Artemis [51]. Overall, these data suggest that the final destination of unrepairable DSBs is the nuclear membrane. However, the nuclear periphery is also a place where telomeres locate [73] and is where telomere silencing occurs. Therefore, it is reasonable to speculate that both types of unrepairable DSBs, which primarily originated in either the telomere or heterochromatin, eventually colocalize in the nuclear membrane. New technologies [63, 74] and biomarkers will be necessary to distinguish radiation-induced unrepairable DSBs from those found at telomere ends. A further application of unrepaired DSBs measurement would be its use as an early indicator for radiation risk.
New methods that artificially introduce unrepairable DSBs at specific chromatin sites have been developed. White et al. [75] delivered Sac I restriction enzyme to mouse liver using adenovirus. They observed a liver-specific pathology of aging and inflammation. However, lipofuscin accumulation was not observed. Kim et al. [76] generated a conditional I-PpoI restriction enzyme expression system in mouse. In this system, 19 persistent DSBs could be formed in each cell, and the mice appeared to exhibit premature aging phenotypes. However, the introduction of such unrepairable DSBs could not fully explain all of the normal aging phenomena. The application of recent gene editing technologies will enable the introduction of unrepairable DSBs at specific sites in the chromosome. Such systems will help us to understand the risks of unrepairable DSBs in specific cells and tissues in living organisms.
CONCLUSION: A VIEW OF RADIATION BIOLOGY USING A NEW BIOMARKER
Although considerable efforts have been made to analyze the repair of repairable damage, studies that measure and elucidate the biology of unrepairable DSBs have not come to the forefront until recently. To better measure unrepairable DSBs, especially in vivo, we need definitive criteria for distinguishing unrepairable DSBs from transient and repairable DSBs. These criteria may include characterizing the precise sequences and structures where these breaks originated or new biomarkers that can specifically detect and measure these lesions. Such new technologies may enable the further application of radiation-induced unrepairable DSB quantification, which could lead to a better understanding of the risks of radiation to the processes of organismal development, growth, and aging.
Supplementary Material
ACKNOWLEDGEMENTS
This research was presented at the 1st International Symposium on the network-type Joint Usage/Research Center for Radiation Disaster Medical Science.
SUPPLEMENTARY DATA
Supplementary data are available at the Journal of Radiation Research online.
CONFLICT OF INTEREST
The author has no conflict of interest to disclose.
FUNDING
The Radiation Effects Research Foundation (RERF), Hiroshima and Nagasaki, Japan is a public interest incorporated foundation funded by the Japanese Ministry of Health, Labour and Welfare (MHLW) and the US Department of Energy (DOE). This publication was supported by the Program of the Network-type (Joint Usage/Research Center for Radiation Disaster Medical Science of Hiroshima University, Nagasaki University, and Fukushima Medical University), by the Radiation Effects Research Foundation (RERF) [Research Protocol A4-09] and by the Japan Society for the Promotion of Science (JSPS) KAKENHI [Grant Numbers 25550038 and 25220102]. The views of the authors do not necessarily reflect those of the two governments.
REFERENCES
- 1. Jackson SP, Bartek J. The DNA-damage response in human biology and disease. Nature 2009;461:1071–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ciccia A, Elledge SJ. The DNA damage response: making it safe to play with knives. Mol Cell 2010;40:179–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Polo SE, Jackson SP. Dynamics of DNA damage response proteins at DNA breaks: a focus on protein modifications. Genes Dev 2011;25:409–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ceccaldi R, Rondinelli B, D’Andrea AD. Repair pathway choices and consequences at the double-strand break. Trends Cell Biol 2016;26:52–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bradley MO, Kohn KW. X-ray induced DNA double strand break production and repair in mammalian cells as measured by neutral filter elution. Nucleic Acids Res 1979;7:793–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Metzger L, Iliakis G. Kinetics of DNA double-strand break repair throughout the cell cycle as assayed by pulsed field gel electrophoresis in CHO cells. Int J Radiat Biol 1991;59:1325–39. [DOI] [PubMed] [Google Scholar]
- 7. Frankenberg-Schwager M, Frankenberg D. Survival curves with shoulders: damage interaction, unsaturated but dose-dependent rejoining kinetics or inducible repair of DNA double-strand breaks? Radiat Res 1994;138:S97–100. [PubMed] [Google Scholar]
- 8. Rogakou EP, Pilch DR, Orr AH et al. . DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem 1998;273:5858–68. [DOI] [PubMed] [Google Scholar]
- 9. Nazarov IB, Smirnova AN, Krutilina RI et al. . Dephosphorylation of histone gamma-H2AX during repair of DNA double-strand breaks in mammalian cells and its inhibition by calyculin A. Radiat Res 2003;160:309–17. [DOI] [PubMed] [Google Scholar]
- 10. Noda A, Hirai Y, Hamasaki K et al. . Unrepairable DNA double-strand breaks that are generated by ionising radiation determine the fate of normal human cells. J Cell Sci 2012;125:5280–7. [DOI] [PubMed] [Google Scholar]
- 11. Riballo E, Kuhne M, Rief N et al. . A pathway of double-strand break rejoining dependent upon ATM, Artemis, and proteins locating to gamma-H2AX foci. Mol Cell 2004;16:715–24. [DOI] [PubMed] [Google Scholar]
- 12. Lobrich M, Shibata A, Beucher A et al. . γH2AX foci analysis for monitoring DNA double-strand break repair: strengths, limitations and optimization. Cell Cycle 2010;9:662–9. [DOI] [PubMed] [Google Scholar]
- 13. Sharma PM, Ponnaiya B, Taveras M et al. . High throughput measurement of γH2AX DSB repair kinetics in a healthy human population. PLoS One 2015;10:e0121083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rossiello F, Herbig U, Longhese MP et al. . Irreparable telomeric DNA damage and persistent DDR signalling as a shared causative mechanism of cellular senescence and ageing. Curr Opin Genet Dev 2014;26:89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rogakou EP, Boon C, Redon C et al. . Megabase chromatin domains involved in DNA double-strand breaks in vivo. J Cell Biol 1999;146:905–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sedelnikova OA, Rogakou EP, Panyutin IG et al. . Quantitative detection of 125IdU-induced DNA double-strand breaks with gamma-H2AX antibody. Radiat Res 2002;158:486–92. [DOI] [PubMed] [Google Scholar]
- 17. Stiff T, O’Driscoll M, Rief N et al. . ATM and DNA-PK function redundantly to phosphorylate H2AX after exposure to ionizing radiation. Cancer Res 2004;64:2390–6. [DOI] [PubMed] [Google Scholar]
- 18. Madigan JP, Chotkowski HL, Glaser RL. DNA double-strand break–induced phosphorylation of Drosophila histone variant H2Av helps prevent radiation-induced apoptosis. Nucleic Acids Res 2002;30:3698–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Roch-Lefevre S, Mandina T, Voisin P et al. . Quantification of gamma-H2AX foci in human lymphocytes: a method for biological dosimetry after ionizing radiation exposure. Radiat Res 2010;174:185–94. [DOI] [PubMed] [Google Scholar]
- 20. Sedelnikova OA, Horikawa I, Zimonjic DB et al. . Senescing human cells and ageing mice accumulate DNA lesions with unrepairable double-strand breaks. Nat Cell Biol 2004;6:168–70. [DOI] [PubMed] [Google Scholar]
- 21. Jeggo PA, Geuting V, Lobrich M. The role of homologous recombination in radiation-induced double-strand break repair. Radiother Oncol 2011;101:7–12. [DOI] [PubMed] [Google Scholar]
- 22. Mamouni K, Cristini A, Guirouilh-Barbat J et al. . RhoB promotes gammaH2AX dephosphorylation and DNA double-strand break repair. Mol Cell Biol 2014;34:3144–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yamauchi M, Oka Y, Yamamoto M et al. . Growth of persistent foci of DNA damage checkpoint factors is essential for amplification of G1 checkpoint signaling. DNA Repair 2008;7:405–17. [DOI] [PubMed] [Google Scholar]
- 24. Paris L, Cordelli E, Eleuteri P et al. . Kinetics of gamma-H2AX induction and removal in bone marrow and testicular cells of mice after X-ray irradiation. Mutagenesis 2011;26:563–72. [DOI] [PubMed] [Google Scholar]
- 25. Markova E, Schultz N, Belyaev IY. Kinetics and dose-response of residual 53BP1/gamma-H2AX foci: co-localization, relationship with DSB repair and clonogenic survival. Int J Radiat Biol 2007;83:319–29. [DOI] [PubMed] [Google Scholar]
- 26. Bracalente C, Ibanez IL, Molinari B et al. . Induction and persistence of large gammaH2AX foci by high linear energy transfer radiation in DNA-dependent protein kinase-deficient cells. Int J Radiat Oncol Biol Phys 2013;87:785–94. [DOI] [PubMed] [Google Scholar]
- 27. Banath JP, Klokov D, MacPhail SH et al. . Residual gammaH2AX foci as an indication of lethal DNA lesions. BMC Cancer 2010;10:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bhogal N, Kaspler P, Jalali F et al. . Late residual gamma-H2AX foci in murine skin are dose responsive and predict radiosensitivity in vivo. Radiat Res 2010;173:1–9. [DOI] [PubMed] [Google Scholar]
- 29. Djuzenova CS, Elsner I, Katzer A et al. . Radiosensitivity in breast cancer assessed by the histone gamma-H2AX and 53BP1 foci. Radiat Oncol 2013;8:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Taneja N, Davis M, Choy JS et al. . Histone H2AX phosphorylation as a predictor of radiosensitivity and target for radiotherapy. J Biol Chem 2004;279:2273–80. [DOI] [PubMed] [Google Scholar]
- 31. Dikomey E, Dahm-Daphi J, Brammer I et al. . Correlation between cellular radiosensitivity and non-repaired double-strand breaks studied in nine mammalian cell lines. Int J Radiat Biol 1998;73:269–78. [DOI] [PubMed] [Google Scholar]
- 32. Redon CE, Nakamura AJ, Gouliaeva K et al. . The use of gamma-H2AX as a biodosimeter for total-body radiation exposure in non-human primates. PLoS One 2010;5:e15544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Qvarnstrom OF, Simonsson M, Johansson KA et al. . DNA double strand break quantification in skin biopsies. Radiother Oncol 2004;72:311–7. [DOI] [PubMed] [Google Scholar]
- 34. Menegakis A, Yaromina A, Eicheler W et al. . Prediction of clonogenic cell survival curves based on the number of residual DNA double strand breaks measured by gammaH2AX staining. Int J Radiat Biol 2009;85:1032–41. [DOI] [PubMed] [Google Scholar]
- 35. Fumagalli M, Rossiello F, Clerici M et al. . Telomeric DNA damage is irreparable and causes persistent DNA-damage-response activation. Nat Cell Biol 2012;14:355–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hewitt G, Jurk D, Marques FD et al. . Telomeres are favoured targets of a persistent DNA damage response in ageing and stress-induced senescence. Nat Commun 2012;3:708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ahmed EA, Agay D, Schrock G et al. . Persistent DNA damage after high dose in vivo gamma exposure of minipig skin. PLoS One 2012;7:e39521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Moroni M, Maeda D, Whitnall MH et al. . Redon CE, Evaluation of the gamma-H2AX assay for radiation biodosimetry in a swine model. Int J Mol Sci 2013;14:14119–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Andratschke N, Blau T, Schill S et al. . Late residual gamma-H2AX foci in murine spinal cord might facilitate development of response-modifying strategies: a research hypothesis. Anticancer Res 2011;31:561–4. [PubMed] [Google Scholar]
- 40. Siddiqui MS, Francois M, Fenech MF et al. . gammaH2AX responses in human buccal cells exposed to ionizing radiation. Cytometry A 2015;87:296–308. [DOI] [PubMed] [Google Scholar]
- 41. Ahmed EA, van der Vaart A, Barten A et al. . Differences in DNA double strand breaks repair in male germ cell types: lessons learned from a differential expression of Mdc1 and 53BP1. DNA Repair 2007;6:1243–54. [DOI] [PubMed] [Google Scholar]
- 42. Rube CE, Fricke A, Widmann TA et al. . Accumulation of DNA damage in hematopoietic stem and progenitor cells during human aging. PLoS One 2011;6:e17487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Titus S, Li F, Stobezki R et al. . Impairment of BRCA1-related DNA double-strand break repair leads to ovarian aging in mice and humans. Sci Transl Med 2013;5:172ra21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wang C, Jurk D, Maddick M et al. . DNA damage response and cellular senescence in tissues of aging mice. Aging Cell 2009;8:311–23. [DOI] [PubMed] [Google Scholar]
- 45. Rube CE, Fricke A, Schneider R et al. . DNA repair alterations in children with pediatric malignancies: novel opportunities to identify patients at risk for high-grade toxicities. Int J Radiat Oncol Biol Phys 2010;78:359–69. [DOI] [PubMed] [Google Scholar]
- 46. Bourton EC, Plowman PN, Smith D et al. . Prolonged expression of the gamma-H2AX DNA repair biomarker correlates with excess acute and chronic toxicity from radiotherapy treatment. Int J Cancer 2011;129:2928–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chua ML, Somaiah N, A’Hern R et al. . Residual DNA and chromosomal damage in ex vivo irradiated blood lymphocytes correlated with late normal tissue response to breast radiotherapy. Radiother Oncol 2011;99:362–6. [DOI] [PubMed] [Google Scholar]
- 48. Goutham HV, Mumbrekar KD, Vadhiraja BM et al. . DNA double-strand break analysis by gamma-H2AX foci: a useful method for determining the overreactors to radiation-induced acute reactions among head-and-neck cancer patients. Int J Radiat Oncol Biol Phys 2012;84:e607–12. [DOI] [PubMed] [Google Scholar]
- 49. Hudson D, Kovalchuk I, Koturbash I et al. . Induction and persistence of radiation-induced DNA damage is more pronounced in young animals than in old animals. Aging 2011;3:609–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Firsanov D, Vasilishina A, Kropotov A et al. . Dynamics of gammaH2AX formation and elimination in mammalian cells after X-irradiation. Biochimie 2012;94:2416–22. [DOI] [PubMed] [Google Scholar]
- 51. Woodbine L, Brunton H, Goodarzi AA et al. . Endogenously induced DNA double strand breaks arise in heterochromatic DNA regions and require ataxia telangiectasia mutated and Artemis for their repair. Nucleic Acids Res 2011;39:6986–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Schmid TE, Dollinger G, Beisker W et al. . Differences in the kinetics of gamma-H2AX fluorescence decay after exposure to low and high LET radiation. Int J Radiat Biol 2010;86:682–91. [DOI] [PubMed] [Google Scholar]
- 53. Oeffinger KC, Mertens AC, Sklar CA et al. . Chronic health conditions in adult survivors of childhood cancer. N Engl J Med 2006;355:1572–82. [DOI] [PubMed] [Google Scholar]
- 54. Inskip PD, Robison LL, Stovall M et al. . Radiation dose and breast cancer risk in the childhood cancer survivor study. J Clin Oncol 2009;27:3901–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ness KK, Krull KR, Jones KE et al. . Physiologic frailty as a sign of accelerated aging among adult survivors of childhood cancer: a report from the St Jude Lifetime cohort study. J Clin Oncol 2013;31:4496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Schneider L, Pellegatta S, Favaro R et al. . DNA damage in mammalian neural stem cells leads to astrocytic differentiation mediated by BMP2 signaling through JAK-STAT. Stem Cell Rep 2013;1:123–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Inomata K, Aoto T, Binh NT et al. . Genotoxic stress abrogates renewal of melanocyte stem cells by triggering their differentiation. Cell 2009;137:1088–99. [DOI] [PubMed] [Google Scholar]
- 58. Siddiqui MS, Francois M, Fenech MF et al. . Persistent gammaH2AX: a promising molecular marker of DNA damage and aging. Mutat Res Rev Mutat Res 2015;766:1–19. [DOI] [PubMed] [Google Scholar]
- 59. Bae NS, Baumann P. A RAP1/TRF2 complex inhibits nonhomologous end-joining at human telomeric DNA ends. Mol Cell 2007;26:323–34. [DOI] [PubMed] [Google Scholar]
- 60. Sarthy J, Bae NS, Scrafford J et al. . Human RAP1 inhibits non-homologous end joining at telomeres. EMBO J 2009;28:3390–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Bombarde O, Boby C, Gomez D et al. . TRF2/RAP1 and DNA-PK mediate a double protection against joining at telomeric ends. EMBO J 2010;29:1573–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Gomes NM, Ryder OA, Houck ML et al. . Comparative biology of mammalian telomeres: hypotheses on ancestral states and the roles of telomeres in longevity determination. Aging Cell 2011;10:761–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Crosetto N, Mitra A, Silva MJ et al. . Nucleotide-resolution DNA double-strand break mapping by next-generation sequencing. Nat Methods 2013;10:361–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Therizols P, Fairhead C, Cabal GG et al. . Telomere tethering at the nuclear periphery is essential for efficient DNA double strand break repair in subtelomeric region. J Cell Biol 2006;172:189–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Nagai S, Dubrana K, Tsai-Pflugfelder M et al. . Functional targeting of DNA damage to a nuclear pore-associated SUMO-dependent ubiquitin ligase. Science 2008;322:597–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Freudenreich CH, Su XA (1 December 2016) Relocalization of DNA lesions to the nuclear pore complex. FEMS Yeast Res, 10.1093/femsyr/fox095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Liu B, Wang J, Chan KM et al. . Genomic instability in laminopathy-based premature aging. Nat Med 2005;11:780–5. [DOI] [PubMed] [Google Scholar]
- 68. Constantinescu D, Csoka AB, Navara CS et al. . Defective DSB repair correlates with abnormal nuclear morphology and is improved with FTI treatment in Hutchinson-Gilford progeria syndrome fibroblasts. Exp Cell Res 2010;316:2747–59. [DOI] [PubMed] [Google Scholar]
- 69. Richards SA, Muter J, Ritchie P et al. . The accumulation of un-repairable DNA damage in laminopathy progeria fibroblasts is caused by ROS generation and is prevented by treatment with N-acetyl cysteine. Hum Mol Genet 2011;20:3997–4004. [DOI] [PubMed] [Google Scholar]
- 70. Noda A, Mishima S, Hirai Y et al. . Progerin, the protein responsible for the Hutchinson-Gilford progeria syndrome, increases the unrepaired DNA damages following exposure to ionizing radiation. Genes Environ 2015;37:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Redon CE, Bonner WM. High salt and DNA double-strand breaks. Proc Nat Acad Sci U S A 2011;108:20281–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Dmitrieva NI, Cui K, Kitchaev DA et al. . DNA double-strand breaks induced by high NaCl occur predominantly in gene deserts. Proc Nat Acad Sci U S A 2011;108:20796–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Burla R, La Torre M, Saggio I. Mammalian telomeres and their partnership with lamins. Nucleus 2016;7:187–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Canela A, Sridharan S, Sciascia N et al. . DNA breaks and end resection measured genome-wide by end sequencing. Mol Cell 2016;63:898–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. White RR, Milholland B, de Bruin A et al. . Controlled induction of DNA double-strand breaks in the mouse liver induces features of tissue ageing. Nat Commun 2015;6:6790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Kim J, Sturgill D, Tran AD et al. . Controlled DNA double-strand break induction in mice reveals post-damage transcriptome stability. Nucleic Acids Res 2016;44:e64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.