Abstract
Exposure to ionizing radiation (IR) induces various types of DNA damage, of which DNA double-strand breaks are the most severe, leading to genomic instability, tumorigenesis, and cell death. Hence, cells have developed DNA damage responses and repair mechanisms. IR also causes the accumulation of endogenous reactive oxidative species (ROS) in the irradiated cells. Upon exposure to low-dose irradiation, the IR-induced biological effects mediated by ROS were relatively more significant than those mediated by DNA damage. Accumulating evidence suggests that such increase in endogenous ROS is related with mitochondria change in irradiated cells. Thus, in this review we focused on the mechanism of mitochondrial ROS production and its relationship to the biological effects of IR. Exposure of mammalian cells to IR stimulates an increase in the production of endogenous ROS by mitochondria, which potentially leads to mitochondrial dysfunction. Since the remains of damaged mitochondria could generate or leak more ROS inside the cell, the damaged mitochondria are removed by mitophagy. The disruption of this pathway, involved in maintaining mitochondrial integrity, could lead to several disorders (such as neurodegeneration) and aging. Thus, further investigation needs to be performed in order to understand the relationship between the biological effects of low-dose IR and mitochondrial integrity.
Keywords: mitochondria, ROS, low-dose irradiation, oxidative damage, mitophagy
INTRODUCTION
Exposure to ionizing radiation (IR) causes various biological effects on organ-forming cells. Most of these biological effects are stimulated by nuclear DNA (nucDNA) damage. Among the various types of DNA damage, the DNA double-strand breaks (DSBs) are the most severe. Exposure of mammalian cells to 1 gray (Gy) of acute γ-irradiation is estimated to generate ~50 nucDNA DSBs [1]. However, exposure to <10 mGy of γ-rays generates <1 DSB per nucleus. Thus, the contribution of DNA damage (by low-dose IR) towards these biological effects is expected to be negligible. IR can also induce the production of reactive oxygen species (ROS). Commonly, production of ROS upon exposure to IR is caused by water radiolysis, which in turn is mediated by the indirect effects of low linear energy transfer (LET) IRs such as γ-rays and X-rays [1, 2] (Fig. 1). Absorption of high-energy γ-ray and X-ray photons induces excitation and ionization of water molecules. This leads to the production of free radicals and free electrons, which attack important biomolecules such as DNA. These free radicals and electrons can also react with other water and oxygen molecules and generate the highly reactive secondary free radicals, such as the superoxide anion radical (O2*−). ROS can also attack critical biomolecules. These events resulting in ROS production, which are indirectly mediated by γ-rays or X-rays, are completed in a very short span of time (less than 10−6 sec). The ROS life-time is very short because of their high reactivity with the surrounding molecules [1, 2]. Accumulating evidence indicates that exposure to a high dose of acute IR causes a sustained increase in the production of endogenous ROS over a few hours. On the contrary, several other reports suggest a delay in the induction of endogenous ROS production following exposure to IR [2–5]. These observations suggest that the amount of ROS produced due to water radiolysis forms a considerably small proportion of the total ROS produced following exposure to IR.
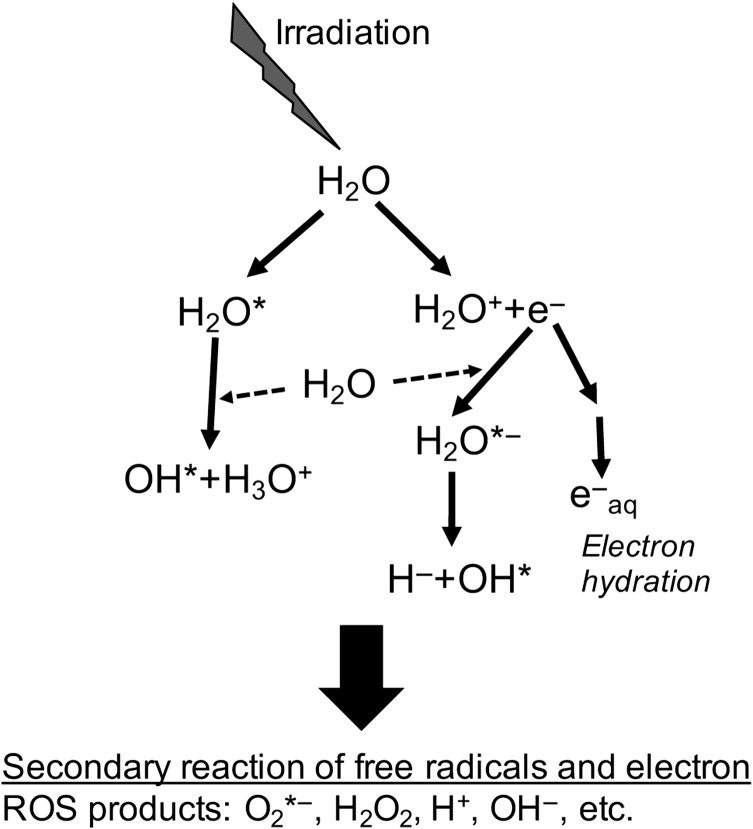
Fig. 1.
Water radiolysis by the indirect effects of low-LET IRs (γ-rays and X-rays). Absorption of high-energy γ-ray and X-ray photons induces excitation and ionization of water molecules, leading to the production of free radicals and free electrons. These free radicals and electrons can also react with other water and oxygen molecules and generate the highly reactive secondary free radicals, such as the superoxide anion radical (O2*−).
Without exposure to IR, ROS are routinely produced in organelles such as mitochondria and endoplasmic reticulum (ER) [6]. Mitochondria are the organelles that produce ATP, a high-energy biomolecule, through the electron transport chain (ETC) located on their inner membranes. In the ETC, electrons react with the O2 molecules and produce a small amount of O2*− radical, which is a type of ROS. These superoxide radicals are transformed to hydrogen peroxide (H2O2) by the mitochondrial manganese superoxide dismutase (SOD2). Subsequently, H2O2 is converted to H2O and O2 by the action of catalase. Although it rarely happens, O2*− and H2O2 may leak into the cell cytoplasm. These leaked ROS can react with important biomolecules, leading to the activation of oxidative stress responses to counteract the ROS. The disturbance of mitochondrial homeostasis due to defective ETC-related proteins causes leakage of ROS into the cytoplasm and genomic instability, which may subsequently result in tumorigenesis or neurodegeneration [7–9].
The ER is another organelle that produces ROS during the unfolded protein response (UPR) [10, 11]. ER is the site for folding and post-translational modifications of newly synthesized proteins. When protein folding in ER is disturbed, aggregates of misfolded proteins accumulate, which stimulates the UPR in order to facilitate correct protein folding. During the UPR, the ER oxidoreductases [the protein disulfide isomerase (PDI) family] catalyze thiol oxidation of cysteines and subsequent disulfide bond formation in the misfolded proteins, thereby folding them correctly. Next, the reduced PDI is oxidized by thiol oxidoreductase (ER oxidoreductin 1, ERO1). This oxidation reaction generates H2O2. Since the UPR is activated in response to protein misfolding, 25% of total cellular ROS production takes place during this process [10]. Recently, it was reported that the misfolded proteins present in cytoplasm were imported to mitochondria for their degradation [mediated via the mitochondria-associated degradation (MAD) pathway or the mitochondrial unfolded protein response (UPRmt)] [12]. However, the impairment of mitochondrial function of protein degradation could result in the accumulation of ROS via unknown mechanisms [12, 13]. Thus, mitochondria and ER contribute to the production of endogenous ROS under normal conditions in cells. Several studies have also reported the role of mitochondria in continuous endogenous ROS production following exposure to IR. Therefore, in this review, we have mainly focused on the role of mitochondria in the production of ROS after exposure to IR.
Mitochondria-dependent ROS production in irradiated cells
So far, several studies have reported that exposure of mammalian cells to IR increased the production of endogenous ROS in the irradiated cells [2–5]. A remarkable increase in the production of ROS was observed in the U937 lymphoma cell line, 12 h after exposing the cells to acute γ-irradiation (7 Gy) [3]. DCFDA (2’,7’-dichlorofluorescin diacetate) was used to measure the activity of hydroxyl (OH*), peroxyl (ROO*), and other ROS produced in the cells. It was also observed that the mitochondrial transmembrane potential also decreased after irradiation [3]. The detection reagent DCFDA was used to detect a similar increase in the production of ROS in MCF-7, a human breast cancer cell line, exposed to >2 Gy of X-ray irradiation [4]. Using the aminophenyl fluorescein (APF) reagent, which specifically measures the hydroxyl and peroxyl radicals, a rapid increase in ROS production was observed in the hTERT-immortalized chondrocyte fibroblast cell line exposed to γ-rays (>2 Gy) [5]. In the same study, the increase in mitochondrial ROS production was detected using the MitoSOX reagent, which detects the superoxide radicals present in mitochondria. A similar induction of the superoxide radical in mitochondria upon exposure to IR was reported by several other studies [14, 15]. Irradiation with α-particles also resulted in an increase in the mitochondrial superoxide production 2 h post-IR in the hTERT-immortalized chondrocyte fibroblast cell line [16]. In some cases, changes in mitochondrial characteristics (such as a decrease in mitochondrial membrane potential or an increase in mitochondrial mass) were observed in the irradiated cells [3, 4, 17]. This evidence suggests that the increase in the level of ROS in irradiated cells was due to the production of mitochondrial superoxide radical associated with mitochondrial dysfunction. Using DCFDA, it has been observed that overexpression of SOD2 in HeLa cells resulted in the repression of IR-induced production of mitochondrial superoxide radical, as well as that of total ROS [14]. Furthermore, exposure to IR resulted in persistent accumulation of ROS [3–5, 15]. Several studies demonstrated that the accumulation of total ROS or mitochondrial superoxide radicals in the irradiated cells persisted or was enhanced over a period of 24 h (post-IR). Additionally, some mitochondrial changes also persisted post-IR [4, 5, 18]. This suggested that after exposure to IR, the irreversible changes in mitochondria (or mitochondrial dysfunctions) may result in continuous production of the mitochondrial superoxide radical, which can then subsequently leak into the cytoplasm.
Effects of IR on mitochondrial DNA
Human mitochondrial DNA (mtDNA) is circular and contains 16 659 base pairs. Mammalian cells possess several copies of mtDNA in mitochondria. Human mtDNA codes for rRNA, tRNA and 13 proteins that are necessary for maintaining mitochondrial structure and function [19]. Other necessary proteins for mitochondria are encoded by the nucDNA. Several reports have suggested that mutations in mtDNA could influence ATP production. Disturbances in mtDNA integrity are associated with neurodegeneration, premature aging, and several other diseases [20–23]. Exposure to IR causes severe DNA damage, including DSBs and oxidative damage to nucDNA; however, mtDNA is affected more by oxidative damage due to IR than nucDNA is. When mitochondria and nuclei isolated from rat liver were directly irradiated with 150 Gy of γ-rays, a 6-fold increase in the level of 8-hydroxy-2’-deoxyguanosine (8-OHdG) was observed in the mtDNA compared with its level in the nucDNA [24]. Exposure of mammalian cells to γ-rays also induced the production of more 8-OHdG in mtDNA compared with that produced in nucDNA. This induction seemed to be correlated with the expression level of DNA polymerase γ(POLG), which was essential for the replication of mtDNA [25]. Furthermore, it was reported that irradiating the A7r5 cells (rat smooth muscle cells) with γ-rays (5 Gy) caused a delayed (from 24- to 72-h post-irradiation) increase in the 8-OHdG lesions in mtDNA, but not in the nucDNA [26]. The presence of 8-OHdG in mtDNA leads to G-to-T transversions [2]. Although the integrity of mtDNA seems to be maintained by similar repair systems to the systems for nucDNA [27], the DNA repair efficiency for oxidative damage in mtDNA is less than that in nucDNA [28]. Transgenic mice expressing the proofreading-deficient POLG accumulated somatic mtDNA mutations, displayed mitochondrial dysfunction, and showed premature aging [29]. Moreover, the POLG-deficient autosomal dominant or recessive progressive external ophthalmoplegia patients showed aging-dependent accumulation of mtDNA mutations, mitochondrial dysfunction–associated neurodegeneration, and muscle weakness [30]. Thus, the accumulation of mutations in mtDNA reduces mitochondrial functions such as ATP production, which leads to several diseases, including premature aging, muscle abnormalities, and neurodegeneration. Hence, accumulation of mtDNA mutations induced by oxidative damage upon exposure to IR may cause the pathogenesis of several diseases associated with the reduction of mitochondrial activity.
IR also induces DSBs in mtDNA. Exposure of the colon cancer cell lines to 560 Gy of γ-rays induced strand breaks in both nucDNA and mtDNA; however, the repair efficiency of mtDNA was less than that of the nucDNA [31]. It is known that the common deletion (CD) between nucleotide positions 8470 and 13 446 in mtDNA occurs frequently. The CD region contains the genes for mitochondrial ATPase, NADH dehydrogenase (Complex I), and cytochrome c oxidase [2, 15], suggesting that deletion of the CD region could result in the reduction of mitochondrial function. Several studies have reported the association between CD and the pathogenesis of various diseases (as well as aging) [32–34]. Exposure to IR resulted in accumulation of CDs in a dose-dependent manner [2, 15]. When hTERT-immortalized chondrocyte fibroblast cell lines were exposed to γ-rays, a dose-dependent (0.1–10 Gy) increase in CD accumulation was observed 72 h post-IR using quantitative PCR analysis. Additionally, CD was also observed after exposure to low-dose (0.05 Gy) γ-rays [35]. Similarly, induction of CD upon exposure to IR was reported in primary human hepatocytes, HepG2 cells, and primary human fibroblasts [36, 37]; however, whether mtDNA with CDs persists in the mitochondria of irradiated cells remains unclear [15, 35, 36]. Thus, exposure to IR can induce DSB damage in mtDNA. It was observed that irradiating mice with 5 Gy of γ-rays resulted in the appearance of mtDNA fragments in the cytosolic fractions of the brain cells, 1 h post-irradiation [37]. Moreover, experiments with budding yeast demonstrated the migration of mtDNA from mitochondria to nucleus [38]. In humans, the nuclei contain partial sequences of mtDNA integrated at more than 20 sites in the chromosomal DNA [39]. This suggests the possibility that mtDNA fragments (containing the CD region) generated after exposure to IR, may migrate to the nucleus and integrate into the chromosomal DNA by inducing DSB damage [38–40].
The copy number of nucDNA is two per cell; however, that of mtDNA is more than 1000 [2]. The copy number of mtDNA is also influenced by exposure to IR. Exposure of mammalian cells to X-rays or γ-rays stimulated an increase in the copy number of their mtDNA within a few days post-IR, followed by a secondary increase at a prolonged time-point (more than a month) [4, 35, 41]. Mouse whole-body irradiation with γ-rays also increased the copy number of mtDNA in the brain, spleen, bone marrow, and other tissues; however, the original copy number (before irradiation) was restored after a few days post-IR [42–44]. Patients with Alpers syndrome show a remarkable decrease in the copy number of mtDNA induced by mutations in POLG, and display signs of refractory seizures, neurodegeneration, and liver diseases [45]. Currently, it is unclear whether an increase in the copy number of mtDNA is associated with any other diseases. Hence, the significance of an increase in mtDNA post-IR is debatable.
We conclude that exposure of mammalian cells to IR induces oxidative damage to mtDNA, CDs associated with DSBs, and an increase in the copy number of mtDNA. Of these, oxidative damage and CDs can result in the dysfunction of the genes present in mtDNA, subsequently resulting in a decrease in mitochondrial function. It is unclear whether there exists a direct relationship between the fate of the cell and changes in its mtDNA. However, since both CDs and increase in copy number of mtDNA are induced by exposure to low doses of IR (such as 10 or 50 mGy), these changes may act as useful biomarkers for detecting DNA damage due to IR [35, 41].
Potential fate of mitochondria damaged by IR
Exposure of mammalian cells to IR triggers changes in the mtDNA (oxidative damage, CD, and other types of damage) and also induces an increase in the production of mitochondrial ROS in the irradiated cells. This happens potentially due to mitochondrial dysfunction. Hence, it is suggested that the IR-induced changes in mtDNA may be directly associated with mitochondrial dysfunction and the subsequent increase in endogenous ROS production. This direct association has been investigated using Rho zero (ρ°) cells, which lack mtDNA and were generated using treatment with a low dose of ethidium bromide. The induction of ROS in ρ° cells by exposure to 4 Gy of IR was significantly less than that in the control cells (with intact mtDNA) [46]. The radioresistance of the ρ° cells to radon irradiation was higher than that of the control cells. Additionally, the ρ° cells showed lower ROS production after exposure to radon irradiation [47]. Furthermore, increasing the protein expression of the mitochondrial ETC complex II subunit B stabilized the mitochondrial membrane potential and reduced the production of ROS post-IR [48]. Enhanced mtDNA integrity was achieved by increasing the expression of POLG, which subsequently reduced the oxidative damage in mtDNA post-IR [24]. These observations suggest that mtDNA could be a key molecule involved in IR-induced mitochondrial ROS production. Damage to mtDNA due to IR-induced ROS production may provoke higher accumulation of oxidative and other types of damages in the cell.
Although IR causes mtDNA damage, the fate of the mitochondria harboring such damages remains unknown. IR-induced ROS may attack proteins, as well as DNA present in the mitochondria and other organelles. Protein carbonylation, a type of protein oxidation, was observed to increase at 3 h post-irradiation (0.8 Gy) [2, 49]. Oxidation of proteins can result in their functional dysregulation [50]. This suggests that the production of oxidized proteins in mitochondria due to IR-induced ROS may cause mitochondrial dysfunction and result in further accumulation of mitochondrial ROS. The presence of mitochondria with damaged mtDNA and oxidized proteins raises a critical question about whether such damaged mitochondria can be eliminated from the irradiated cells.
Autophagy is a cellular catabolic system for removing damaged or superfluous organelles through degradation by lysosomes, and then their degraded components are recycled [51]. The autophagy pathway usually includes the following steps. The first step is initiation of autophagosome formation by membrane distension from the ER or Golgi complex. The second step is marking of the damaged organelles (to make them recognizable) and enclosing them within the precursor of the autophagosome. This marking step is regulated by ATG family proteins and LC3 [51]. Autophagosome formation is completed with entire enclosing of the damaged organelles, following fusion with lysosomes for degrading them.
Damaged mitochondria can also be eliminated by mitochondria-specific autophagy systems (‘mitophagy’) [51]. The mtDNA integrity is maintained during the fission and fusion cycles, and Drp1 is a critical factor required for mitochondrial fission [52]. Several reports have demonstrated that mitochondrial fission is stimulated in mammalian cells by IR, and that this is accompanied by an increase in DRP1 [5, 16]. DRP1 is also important for the activation of mitophagy [52]. In humans, IR also increases the expression of Parkin (a regulator of mitophagy), suggesting the activation of mitophagy in irradiated cells [53]. Moreover, Parkin-overexpressing cells seem to facilitate the removal of damaged mitochondria and to repress mitochondrial ROS production [54]. These observations suggest that dysfunctional mitochondria (those containing damaged mtDNA and oxidized proteins) can be removed (via mitophagy) in order to repress the effect of leakage of ROS from the damaged mitochondria into the whole cell (and subsequently into the whole body) [51]. This hypothesis may be supported by the fact that CD in mtDNA post-IR is a transient event [36].
Perspective
Accumulating evidence has indicated that the exposure of cells to IR can induce mitochondrial dyisfunction, leading to a sustained increase in the production of endogenous ROS and causing extensive oxidative damage to the whole cell (including damage to the nucDNA and mitochondria) (Fig. 2). When mammalian cells are exposed to IR, water radiolysis generates free radicals and induces DNA damage in both the nucDNA and the mtDNA, as an indirect effect of IR. Such free radicals also attack proteins, including mitochondrial proteins. The accumulation of such damaged mtDNAs and mitochondrial proteins represses mitochondrial function, including the maintenance of a stable mitochondrial membrane potential. This results in continuous leaking of the mitochondrial ROS, such as superoxide radicals, into the whole cell. Furthermore, such sustained increase in the level of ROS inside the whole cell further damages the DNA and proteins. However, in order to avoid the accumulation of mitochondrial ROS and subsequent damage to biomolecules present in the cell, the damaged mitochondria are eliminated via mitophagy. Mitophagy acts as a mitochondrial quality control measure and prevents excess mitochondrial ROS accumulation in cells post-IR.
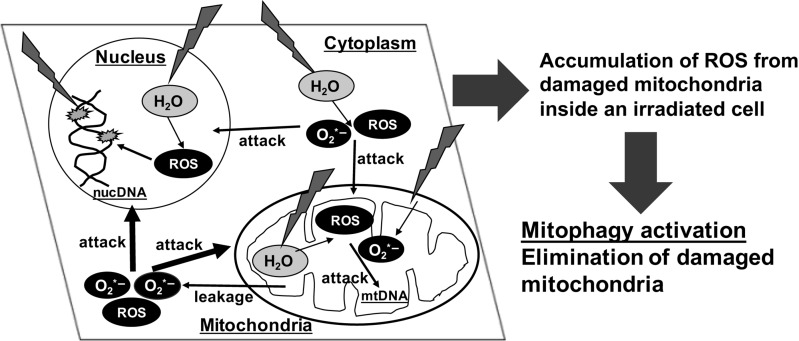
Fig. 2.
IR-induced mitochondrial damages cause ROS production. When mammalian cells are exposed to IR, water radiolysis generates free radicals. Such free radicals attack both nucDNA and mtDNA, and proteins. The accumulation of damaged mtDNAs and mitochondrial proteins represses mitochondrial function, leading to continuous leakage of the mitochondrial ROS inside the whole cell and then amplification of damages to nucDNA and mitochondria. However, in order to avoid the accumulation of mitochondrial ROS and the subsequent damage to biomolecules in the cell, the damaged mitochondria are eliminated via mitophagy. Mitophagy acts as a mitochondrial quality control measure and prevents excess mitochondrial ROS accumulation in cells post-IR.
Excess leakage of ROS from mitochondria into the cytoplasm can damage nucDNA. Irradiation of cell cytoplasm with α-particles induces the nuclear 53BP1 foci after 3 h, while irradiation of the nucleus induces 53BP1 foci appearance earlier (after 1 h) [55]. Formation of the 53BP1 foci by irradiation of the cytoplasm was repressed upon DMSO (a free radical scavenger) treatment, and was remarkably decreased in the ρ° cells. This suggests that irradiation of the cytoplasm resulted in mitochondrial ROS production, and that this was responsible for the appearance of nuclear 53BP1 foci. It is known that IR induces micronuclei formation, probably due to DSB-induced damage. It has been observed that repression of mitochondrial ROS production by an ETC inhibitor reduced micronuclei formation [56]. Thus, IR-induced mitochondrial ROS can migrate into the nucleus and damage nucDNA. Mitochondrial ROS can also damage proteins by oxidatively denaturing them. We reported that patients with ataxia with oculomotor apraxia type 3 (AOA3, an ataxia-telangiectasia–like syndrome) showed significant mitochondrial ROS accumulation [57]. They also displayed a marked decrease in ATM activation and in homologous recombination repair activity. Thus, excess accumulation of mitochondrial ROS can cause protein oxidation, and subsequently hamper the DNA damage responses. Furthermore, oxidation of histones (the essential components of eukaryotic chromatin) can alter chromatin structure, which can influence the regulation of gene expression and DNA repair [2, 6, 58]. IR-induced mitochondrial ROS can also invade the adjacent unirradiated cells and induce oxidative damage. Irradiation with α-particles induces 53BP1 foci formation in adjacent unirradiated cells, as a bystander effect [55]. Several IR experiments have revealed similar bystander effects with respect to gene mutation, micronucleus formation, and ATM-dependent phosphorylation [59].
Chronic low-dose IRs result in less DNA damage. Thus, in this case, the contribution of mitochondrial ROS towards the biological effects of IR was relatively greater. Mitochondrial ROS invade the cell nucleus, as well as the adjacent unirradiated cells, and alters the DNA damage responses (such as DNA repair or ATM-dependent cell cycle checkpoints), leading to tumorigenesis or tissue dysfunction. Excess accumulation of mitochondrial ROS could also cause neurodegenerative disorders, such as cerebellar ataxia [7–9, 57]. Therefore, further investigation needs to be performed in order to estimate the contribution of mitochondrial ROS towards the biological effects of low-dose IR.
ACKNOWLEDGEMENTS
We thank Dr Kenshi Komatsu for his critical analysis of the manuscript. We also thank Yukiko Hayuka and Kae Yanagida for their technical support, and Michi Tanizaki for preparing the manuscript.
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest.
FUNDING
This work was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI (Grant Numbers JP24310041, JP25550026, and JP15H02819), and supported in part by the National Institute for Fusion Science (NIFS) Collaborative Research Program (NIFS17KOCA002).
REFERENCES
- 1. Hall EJ, Giaccia AJ. Radiobiology for the Radiologist. Philadelphia: Lippincott Williams & Wilkins, 2012. [Google Scholar]
- 2. Azzam EI, Jay-Gerin JP, Pain D. Ionizing radiation–induced metabolic oxidative stress and prolonged cell injury. Cancer Lett 2012;327:48–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kim EM, Yang HS, Kang SW et al. . Amplification of the gamma-irradiation–induced cell death pathway by reactive oxygen species in human U937 cells. Cell Signal 2008;20:916–24. [DOI] [PubMed] [Google Scholar]
- 4. Zhou X, Li N, Wang Y et al. . Effects of X-irradiation on mitochondrial DNA damage and its supercoiling formation change. Mitochondrion 2011;11:886–92. [DOI] [PubMed] [Google Scholar]
- 5. Kobashigawa S, Suzuki K, Yamashita S. Ionizing radiation accelerates Drp1-dependent mitochondrial fission, which involves delayed mitochondrial reactive oxygen species production in normal human fibroblast-like cells. Biochem Biophys Res Commun 2011;414:795–800. [DOI] [PubMed] [Google Scholar]
- 6. Szumiel I. Ionizing radiation–induced oxidative stress, epigenetic changes and genomic instability: the pivotal role of mitochondria. Int J Radiat Biol 2015;91:1–12. [DOI] [PubMed] [Google Scholar]
- 7. Kowaltowski AJ, Castilho RF, Vercesi AE. Mitochondrial permeability transition and oxidative stress. FEBS Lett 2001;495:12–15. [DOI] [PubMed] [Google Scholar]
- 8. Brady NR, Hamacher-Brady A, Westerho HV et al. . A wave of reactive oxygen species (ROS)-induced ROS release in a sea of excitable mitochondria. Antioxid Redox Signal 2006;8:1651–65. [DOI] [PubMed] [Google Scholar]
- 9. Drose S, Brandt U. Molecular mechanisms of superoxide production by the mitochondrial respiratory chain. Adv Exp Med Biol 2012;748:145–69. [DOI] [PubMed] [Google Scholar]
- 10. Malhotra JD, Kaufman RJ. The endoplasmic reticulum and the unfolded protein response. Semin Cell Dev Biol 2007;18:716–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Luo B, Lee AS. The critical roles of endoplasmic reticulum chaperones and unfolded protein response in tumorigenesis and anticancer therapies. Oncogene 2013;32:805–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ruan L, Zhou C, Jin E et al. . Cytosolic proteostasis through importing of misfolded proteins into mitochondria. Nature 2017;543:443–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. D’Amico D, Sorrentino V, Auwerx J. Cytosolic proteostasis networks of the mitochondrial stress response. Trends Biochem Sci 2017;42:712–25. [DOI] [PubMed] [Google Scholar]
- 14. Hosoki A, Yonekura S, Zhao QL et al. . Mitochondria-targeted superoxide dismutase (SOD2) regulates radiation resistance and radiation stress response in HeLa cells. J Radiat Res 2012;53:58–71. [DOI] [PubMed] [Google Scholar]
- 15. Kam WW, Banati RB. Effects of ionizing radiation on mitochondria. Free Radic Biol Med 2013;65:607–19. [DOI] [PubMed] [Google Scholar]
- 16. Zhang B, Davidson MM, Zhou H et al. . Cytoplasmic irradiation results in mitochondrial dysfunction and DRP1-dependent mitochondrial fission. Cancer Res 2013;73:6700–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nugent SM, Mothersill CE, Seymour C et al. . Increased mitochondrial mass in cells with functionally compromised mitochondria after exposure to both direct gamma radiation and bystander factors. Radiat Res 2007;168:134–42. [DOI] [PubMed] [Google Scholar]
- 18. Morales A, Miranda M, Sánchez-Reyes A et al. . Oxidative damage of mitochondrial and nuclear DNA induced by ionizing radiation in human hepatoblastoma cells. Int J Radiat Oncol Biol Phys 1998;42:191–203. [DOI] [PubMed] [Google Scholar]
- 19. Harman D. The biologic clock: the mitochondria? J Am Geriatr Soc 1972;20:145–7. [DOI] [PubMed] [Google Scholar]
- 20. DiMauro S, Hirano M. Pathogenesis and treatment of mitochondrial disorders. Adv Exp Med Biol 2009;652:139–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wallace DC, Singh G, Lott MT et al. . Mitochondrial DNA mutation associated with Leber’s hereditary optic neuropathy. Science 1988;242:1427–30. [DOI] [PubMed] [Google Scholar]
- 22. Wallace DC, Zheng XX, Lott MT et al. . Familial mitochondrial encephalomyopathy (MERRF): genetic, pathophysiological, and biochemical characterization of a mitochondrial DNA disease. Cell 1988;55:601–10. [DOI] [PubMed] [Google Scholar]
- 23. Beckman KB, Ames BN. The free radical theory of aging matures. Physiol Rev 1998;78:547–81. [DOI] [PubMed] [Google Scholar]
- 24. Richter C, Park JW, Ames BN. Normal oxidative damage to mitochondrial and nuclear DNA is extensive. Proc Natl Acad Sci U S A 1988;85:6465–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ueta E, Sasabe E, Yang Z et al. . Enhancement of apoptotic damage of squamous cell carcinoma cells by inhibition of the mitochondrial DNA repairing system. Cancer Sci 2008;99:2230–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yoshida T, Goto S, Kawakatsu M et al. . Mitochondrial dysfunction, a probable cause of persistent oxidative stress after exposure to ionizing radiation. Free Radic Res 2012;46:147–53. [DOI] [PubMed] [Google Scholar]
- 27. Alexeyev M, Shokolenko I, Wilson G et al. . The maintenance of mitochondrial DNA integrity—critical analysis and update. Cold Spring Harb Perspect Biol 2013;5:a012641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yakes FM, Van Houten B. Mitochondrial DNA damage is more extensive and persists longer than nuclear DNA damage in human cells following oxidative stress. Proc Natl Acad Sci U S A 1997;94:514–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Trifunovic A, Wredenberg A, Falkenberg M et al. . Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature 2004;429:417–23. [DOI] [PubMed] [Google Scholar]
- 30. Wanrooij S, Luoma P, van Goethem G et al. . Twinkle and POLG defects enhance age-dependent accumulation of mutations in the control region of mtDNA. Nucleic Acids Res 2004;32:3053–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. May A, Bohr VA. Gene-specific repair of gamma-ray-induced DNA strand breaks in colon cancer cells: no coupling to transcription and no removal from the mitochondrial genome. Biochem Biophys Res Commun 2000;269:433–7. [DOI] [PubMed] [Google Scholar]
- 32. Holt IJ, Harding AE, Morgan-Hughes JA. Deletions of muscle mitochondrial DNA in patients with mitochondrial myopathies. Nature 1998;331:717–9. [DOI] [PubMed] [Google Scholar]
- 33. Johns DR, Rutledge SL, Stine OC et al. . Directly repeated sequences associated with pathogenic mitochondrial DNA deletions. Proc Natl Acad Sci U S A 1989;86:8059–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cortopassi GA, Arnheim N. Detection of a specific mitochondrial DNA deletion in tissues of older humans. Nucleic Acids Res 1990;18:6927–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schilling-Toth B, Sandor N, Kis E et al. . Analysis of the common deletions in the mitochondrial DNA is a sensitive biomarker detecting direct and non-targeted cellular effects of low dose ionizing radiation. Mutat Res 2011;716:33–9. [DOI] [PubMed] [Google Scholar]
- 36. Wang L, Kuwahara Y, Li L et al. . Analysis of common deletion (CD) and a novel deletion of mitochondrial DNA induced by ionizing radiation. Int J Radiat Biol 2007;83:433–42. [DOI] [PubMed] [Google Scholar]
- 37. Prithivirajsingh S, Story MD, Bergh SA et al. . Accumulation of the common mitochondrial DNA deletion induced by ionizing radiation. FEBS Lett 2004;571:227–32. [DOI] [PubMed] [Google Scholar]
- 38. Thorsness PE, Fox TD. Escape of DNA from mitochondria to the nucleus in Saccharomyces cerevisiae. Nature 1990;346:376–9. [DOI] [PubMed] [Google Scholar]
- 39. Ricchetti M, Tekaia F, Dujon B. Continued colonization of the human genome by mitochondrial DNA. PLoS Biol 2004;2:E273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ricchetti M, Fairhead C, Dujon B. Mitochondrial DNA repairs double-strand breaks in yeast chromosomes, Nature 1999;402:96–100. [DOI] [PubMed] [Google Scholar]
- 41. Murphy JEJ, Nugent S, Seymour C et al. . Mitochondrial, DNA point mutations and a novel deletion induced by direct low-LET radiation and by medium from irradiated cells. Mutat Res 2005;585:127–36. [DOI] [PubMed] [Google Scholar]
- 42. Zhang H, Maguire D, Swarts S et al. . Replication of murine mitochondrial DNA following irradiation. Adv Exp Med Biol 2009;645:43–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gubina NE, Merekina OS, Ushakova TE. Mitochondrial, DNA transcription in mouse liver, skeletal muscle, and brain following lethal X-ray irradiation. Biochemistry 2010;75:777–83. [DOI] [PubMed] [Google Scholar]
- 44. Gubina NE, Evdokimovskii EV, Ushakova TE. Mitochondrial genetic apparatus functioning in mice spleen cells under radiation-induced apoptosis. Mol Biol 2010;44:1027–35. [PubMed] [Google Scholar]
- 45. Naviaux RK, Nguyen KV. POLG mutations associated with Alpers’ syndrome and mitochondrial DNA depletion. Ann Neurol 2004;55:706–12. [DOI] [PubMed] [Google Scholar]
- 46. Leach JK, Van Tuyle G, Lin PS et al. . Ionizing radiation–induced, mitochondria-dependent generation of reactive oxygen/nitrogen. Cancer Res 2001;61:3894–901. [PubMed] [Google Scholar]
- 47. Li BY, Sun J, Wei H et al. . Radon-induced reduced apoptosis in human bronchial epithelial cells with knockdown of mitochondria DNA. J Toxicol Environ Health A 2012;75:1111–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dayal D, Martin SM, Owens KM et al. . Mitochondrial complex II dysfunction can contribute significantly to genomic instability after exposure to ionizing radiation. Radiat Res 2009;172:737–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Autsavapromporn N, de Toledo SM, Little JB et al. . The role of gap junction communication and oxidative stress in the propagation of toxic effects among high-dose α-particle–irradiated human cells. Radiat Res 2011;175:347–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fang J, Holmgren A. Inhibition of thioredoxin and thioredoxin reductase by 4-hydroxy-2-nonenal in vitro and in vivo. J Am Chem Soc 2006;128:1879–85. [DOI] [PubMed] [Google Scholar]
- 51. Hu L, Wang H, Huang L et al. . Crosstalk between autophagy and intracellular radiation response (Review). Int J Oncol 2016;49:2217–26. [DOI] [PubMed] [Google Scholar]
- 52. Twig G, Elorza A, Molina AJ et al. . Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J 2008;27:433–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Shimura T, Kobayashi J, Komatsu K et al. . Severe mitochondrial damage associated with low-dose radiation sensitivity in ATM- and NBS1-deficient cells. Cell Cycle 2016;15:1099–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Correia-Melo C, Marques FD, Anderson R et al. . Mitochondria are required for pro-ageing features of the senescent phenotype. EMBO J 2016;35:724–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tartier L, Gilchrist S, Burdak-Rothkamm S et al. . Cytoplasmic irradiation induces mitochondrial-dependent 53BP1 protein relocalization in irradiated and bystander cells. Cancer Res 2007;67:5872–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Choi KM, Kang CM, Cho ES et al. . Ionizing radiation–induced micronucleus formation is mediated by reactive oxygen species that are produced in a manner dependent on mitochondria, Nox1, and JNK. Oncol Rep 2007;17:1183–8. [PubMed] [Google Scholar]
- 57. Kobayashi J, Saito Y, Okui M et al. . Increased oxidative stress in AOA3 cells disturbs ATM-dependent DNA damage responses. Mutat Res Genet Toxicol Environ Mutagen 2015;782:42–50. [DOI] [PubMed] [Google Scholar]
- 58. Wondrak GT, Cervantes-Laurean D, Jacobson EL et al. . Histone carbonylation in vivo and in vitro. Biochem J 2000;351:769–77. [PMC free article] [PubMed] [Google Scholar]
- 59. Wang H, Yu KN, Hou J et al. . Radiation-induced bystander effect: early process and rapid assessment. Cancer Lett 2015;356:137–44. [DOI] [PubMed] [Google Scholar]