Abstract
Context
Androgen deprivation therapy (ADT) for prostate cancer (PCa) is associated with increased cardiovascular mortality and sudden cardiac death, with some events occurring early after initiation of ADT. Testosterone levels are inversely associated with corrected QT (QTc) interval duration; therefore, prolongation of QTc duration could be responsible for some of these events during ADT.
Objective
To evaluate changes in QTc duration during ADT.
Design and Interventions
A 6-month prospective cohort study that enrolled men with PCa about to undergo ADT (ADT group) and a control group of men who previously underwent prostatectomy for PCa and never received ADT (non-ADT group).
Patients
At study entry, all participants were eugonadal and had no history of cardiac arrhythmias or complete bundle branch block.
Outcomes
Difference in change in QTc duration from baseline on a 12-lead electrocardiogram at 6, 12, and 24 weeks after initiation of ADT compared with electrocardiograms performed at the same intervals in the non-ADT group. PR, QRS, and QT interval durations were also evaluated.
Results
Seventy-one participants formed the analytical sample (33 ADT and 38 non-ADT). ADT was associated with prolongation of the QTc by 7.4 ms compared with the non-ADT group [95% confidence interval (CI) 0.08 to 14.7 ms; P = 0.048]. ADT was also associated with shortening of the QRS interval by 2.4 ms (95% CI −4.64 to −0.23; P = 0.031). Electrolytes did not change.
Conclusions
Men undergoing ADT for PCa experienced prolongation of the QTc. These findings might explain the increased risk of sudden cardiac death seen in these patients.
Keywords: arrhythmia, cardiovascular risk, ECG, GnRH agonist, QT interval, testosterone
Androgen deprivation therapy in men with prostate cancer is associated with a significant prolongation in QTc interval duration.
Prostate cancer (PCa) is the most common nondermatological cancer in men [1, 2]. As prostate is an androgen-dependent tissue [3], androgen deprivation therapy (ADT) has been the cornerstone of treatment in men with locally invasive and metastatic PCa [4, 5]. The goal of ADT is to lower serum testosterone levels into the castrate range (<50 ng/dL; to convert to nanomoles per liter, multiply by 0.0347) [6], which can be achieved surgically (bilateral orchiectomy) or medically [gonadotropin-releasing hormone (GnRH) agonists or antagonists]. Although ADT has beneficial effects in a subset of patients, the profound androgen deficiency that results from ADT is associated with a number of adverse effects including sexual dysfunction, osteoporosis, hot flashes, and changes in body composition [7, 8]. Additionally, ADT is also associated with insulin resistance, diabetes, and metabolic syndrome [9–11], as well as cardiovascular morbidity and mortality [12, 13]. Some studies have reported that cardiovascular events, including sudden cardiac death, occur early after initiation of ADT [13]. As metabolic changes and progression of atherosclerosis occur gradually over years, the mechanisms that predispose patients to these early events remain unclear.
Although much has been written about cardiovascular complications of ADT, one aspect that has not been investigated in a controlled setting is the potential effect of ADT on the cardiac conduction system. In an electrocardiogram (ECG), ventricular depolarization and repolarization are represented by the QRS complex and T wave, respectively. The QT interval duration corresponds to the total time from ventricular depolarization to repolarization. The QT interval corrected for heart rate (QTc) has been widely used in clinical settings to assess the risk of arrhythmias; indeed, prolongation in QTc is considered a risk factor for tachyarrhythmias and sudden cardiac death [14–16]. Previous studies have shown that gonadal steroids, testosterone in particular, modulate QTc. Indeed, the QTc duration is similar in newborn male and female babies [17], and no sex-related differences are observed before 10 years of age [18]. However, after puberty, the QTc significantly shortens in boys compared with girls, suggesting a direct effect of testosterone production of cardiac conduction system [19]. Studies in young men have shown that endogenous serum testosterone levels are inversely associated with QTc duration [20]. However, as men age, their QTc durations also increase, which is associated with age-related decline in serum testosterone levels [21, 22]. Clinical trials in community-dwelling men with low serum testosterone levels have also shown that testosterone administration is associated with shortening of QTc [23]. Hence, it is conceivable that profound androgen deficiency that occurs because of ADT slows ventricular repolarization and consequently leads to QTc prolongation, which, in turn, might predispose these patients to cardiac arrhythmias [24]. Indeed, a previous small, uncontrolled study had also observed prolongation of QTc with ADT [25].
The Androgen Deprivation Therapy and Pain Perception (ADT and Pain) Study was a prospective observational study that evaluated the impact of ADT on clinical and experimental pain in men with nonmetastatic PCa and compared them to men with localized PCa who had previously undergone prostatectomy and were in remission [26]. The present substudy was designed to assess the impact of ADT-induced androgen deficiency on ECG parameters, in particular QTc. The prospective study design allowed us to follow participants prospectively in a systematic way by performing serial ECGs at the same time intervals in both cohorts.
1. Methods
A. Study Design and Participants
The ADT and Pain Study was a prospective cohort study designed to evaluate changes in pain perception and tolerance in men undergoing ADT for PCa. Thirty-seven men about to undergo medical ADT with GnRH agonist [22.5 mg leuprolide acetate (Lupron Depot; TAP Pharmaceuticals)] every 3 months with a planned intervention of at least 6 months were enrolled from the Dana-Farber Cancer Institute (ADT group). All men received an androgen receptor antagonist (bicalutamide) during the first month of treatment to prevent tumor flare, and half of them also received concurrent radiation therapy. Additionally, 40 men with PCa who had undergone prostatectomy and/or radiation therapy for organ-confined PCa at least 6 months prior to enrollment and were in remission were also enrolled and served as the control group; they were recruited from the Brigham and Women’s Hospital (non-ADT group). All men had normal serum total testosterone concentrations at the time of enrollment and were free of any chronic pain condition. Other exclusion criteria included orchiectomy, skeletal metastasis, use of opioid analgesics, peripheral neuropathy, painful inflammatory conditions, use of glucocorticoids, diabetes, and moderate to severe depression as assessed by the depression module of the Patient Health Questionnaire [27].
For the substudy described in this manuscript, participants with a history of cardiac arrhythmias or complete bundle branch block at baseline were also excluded from the analysis conforming to the American Heart Association recommendations regarding interpretation of ECG [28]. Seventy-one men (33 in the ADT group and 38 in the non-ADT group) formed the analytical sample for this substudy.
The ADT and Pain Study protocol was approved by the institutional review board at the Dana-Farber Cancer Institute (Boston, MA). Enrollment took place between July 2013 and April 2016; the last participant completed the study in November 2016. All participants provided written informed consent.
B. Laboratory Measurement
Fasting serum samples were collected in the morning at baseline and each of the follow-up visits for measurement of serum total testosterone levels using liquid chromatography-tandem mass spectrometry (the gold-standard method) that was performed in a Centers for Disease Control and Prevention–certified laboratory. The lowest detection limit with this method was 2 ng/dL [29]. Because sodium, potassium, and calcium play an important role in cardiac conduction system [30], these electrolytes were also measured at baseline and at all subsequent visits in all participants. Laboratory measurements and ECGs were performed on the same day (within the time frame of an hour) at all study visits.
C. ECG
ECGs were performed at baseline and at 6, 12, and 24 weeks into treatment in the ADT group. In the non-ADT group, ECGs were performed at similar intervals after enrollment. Standard 12-lead ECGs were performed using the same equipment (GE MAC 800; GE Healthcare, Marlborough, MA) and by the same technician at a speed of 25 mm/s and amplification of 0.1 mV/mm. The PR, QRS, and QT interval durations were measured electronically. The QTc was calculated using the Fridericia QT correction formula [31], which provides the best prediction of short- and long-term mortality [32].
D. Statistical Analysis
The study was designed to insure, conservatively, 80% power to detect a 24-week standardized difference in outcomes between ADT and non-ADT arms of at least 0.5, corresponding to a difference in QTc duration of ~12 ms, provided the baseline and 24-week measurements had intraindividual Pearson correlations of at least 0.5. This threshold was well exceeded in practice; for instance, QTc durations at baseline and 24 weeks had Pearson correlations of 0.67, and use of all three follow-up measurements (at 6, 12, and 24 weeks postrandomization) increased the information available for analysis. Tabular and graphical summaries were used to assess empirical evidence in favor of differences in ECG measurements between ADT and non-ADT arms at baseline and over time. Outcome trends with time were estimated using group- and time-specific means and 95% confidence intervals (CIs). Mixed-effects regression was used to estimate group differences at baseline and follow-up (the latter was the average effect over the 24 weeks of intervention) using terms for group (ADT vs non-ADT), time, and the interaction between the two and incorporating a random intercept at the participant level to acknowledge serial correlation of repeated measurements. Statistical significance was evaluated using Wald-type tests of the hypothesis that the true interaction between treatment and time—that is, the influence of treatment on the trajectory of mean change in outcomes with time—is zero.
2. Results
Seventy-one men (33 in the ADT group and 38 in the non-ADT group) met all eligibility criteria for the present work and formed the analytical sample. The median age of the participants was 66 years (range 53 to 89 years), and mean body mass index was 28.1 kg/m2 (range 21.9 to 37.7 kg/m2). Baseline characteristics of the participants are detailed in Table 1.
Table 1.
Baseline Characteristics of Participants in Each Group
| Non-ADT (n = 38) | ADT (n = 33) | |
|---|---|---|
| Demographics | ||
| Age, mean ± SD, y | 66 ± 7 | 67 ± 8 |
| Weight, mean ± SD, kg | 85.2 ± 12.4 | 87.1 ± 12.5 |
| Height, mean ± SD, cm | 174 ± 7 | 175 ± 6 |
| BMI, mean ± SD, kg/m2 | 27.9 ± 3.1 | 28.4 ± 3.7 |
| Medical history | ||
| Hypertension, % | 39.5 | 45.5 |
| CAD, % | 15.8 | 3.0 |
| Stroke, % | 5.3 | 3.0 |
| Myocardial infarction, % | 5.3 | 0 |
| Congestive heart failure, % | 2.6 | 0 |
| Obesity, % | 26.3 | 30.3 |
| Hyperlipidemia, % | 39.5 | 51.5 |
| PCa history | ||
| Prostatectomy, % | 95 | 73 |
| Radiation, % | 5 | 52 |
| Gleason score, mean ± SD | 6.5 ± 1.1 | 7.4 ± 1.0 |
| ECG | ||
| PR, mean ± SD, ms | 171 ± 16 | 175 ± 32 |
| QRS, mean ± SD, ms | 89 ± 10 | 88 ± 10 |
| QT, mean ± SD, ms | 415 ± 32 | 431 ± 32 |
| QTc, mean ± SD, ms | 412 ± 20 | 422 ± 17 |
| Laboratory parameters | ||
| Total testosterone, mean ± SD, ng/dL | 486 ± 196 | 545 ± 196 |
| Sodium, mean ± SD, mEq/L | 140 ± 2 | 140 ± 2 |
| Potassium, mean ± SD, mEq/L | 4.3 ± 0.3 | 4.3 ± 0.3 |
| Calcium, mean ± SD, mg/dL | 9.5 ± 0.3 | 9.3 ± 0.4 |
| Creatinine, mean ± SD, mg/dL | 1.04 ± 0.17 | 1.04 ± 0.15 |
There were no substantial between-group differences. To convert total testosterone to nmol/L, multiply by 0.0347. To convert calcium to mmol/L, multiply by 0.25. To convert creatinine to µmol/L, multiply by 88.4.
Abbreviations: BMI, body mass index; CAD, coronary artery disease.
A. Serum Testosterone and Electrolytes
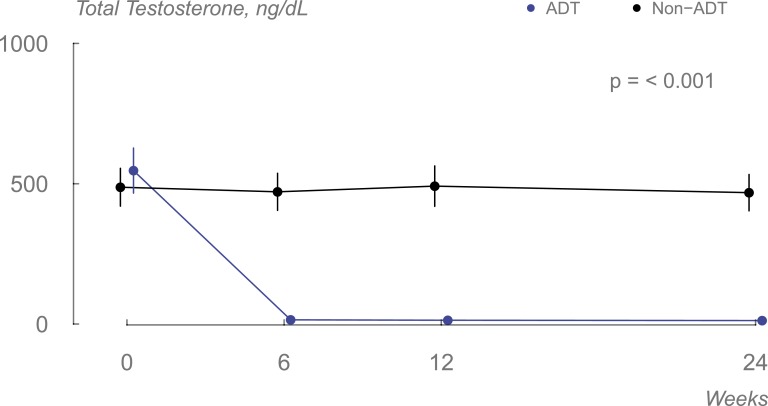
ADT successfully suppressed testosterone production [mean (standard deviation [SD]) 13 (8) ng/dL], whereas testosterone levels did not change in the non-ADT group [mean (SD) 473 (188) ng/dL] (Fig. 1).
Figure 1.
Changes in serum total testosterone levels in the two groups during the course of the study (data displayed as means, and error bars are 95% CI).
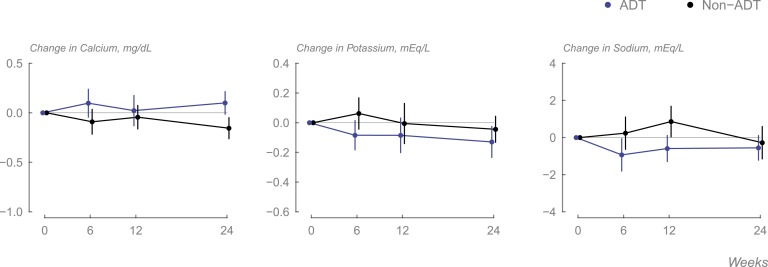
No meaningful changes were seen in any of the electrolytes in either group (Fig. 2).
Figure 2.
Changes in (A) serum calcium, (B) serum potassium, and (C) serum sodium levels in each group during the course of the study (data displayed as means, and error bars are 95% CI).
B. ECG Parameters
B-1. QT and QTc intervals
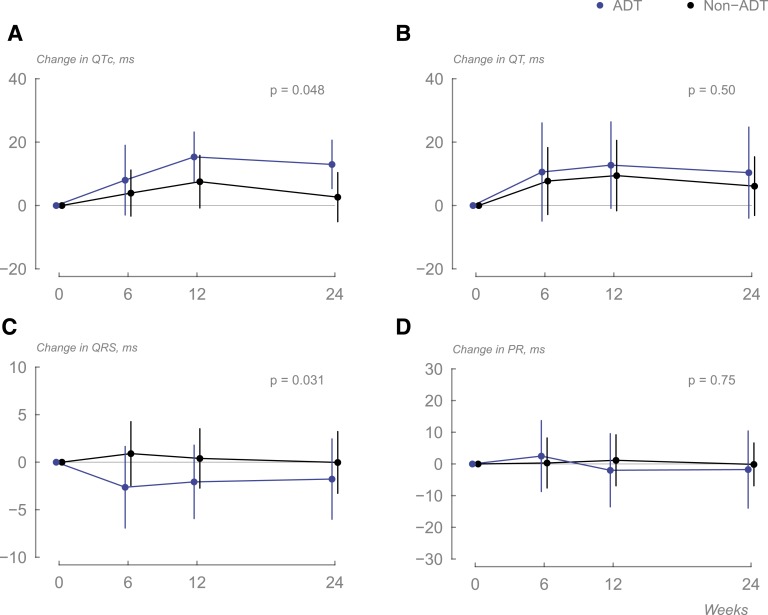
Significant prolongation in the QTc was seen in men receiving ADT (estimated change 12 ms; 95% CI 6.4 to 17.5 ms; P < 0.001). This effect was still significant when compared with the non-ADT group (treatment difference of 7.4 ms; 95% CI 0.08 to 14.7; P = 0.048; Table 2; Fig. 3).
Table 2.
Estimated Changes from Baseline and 95% CIs for ECG Interval Times
| Variables | Non-ADT | ADT | Difference | P value |
|---|---|---|---|---|
| PR | −1.5 (−4.8 to 1.9) | −0.6 (−4.5 to 3.3) | 0.8 (−4.3 to 6.0) | 0.75 |
| QRS | 0.3 (−1.1 to 1.7) | −2.1 (−3.8 to −0.5) | −2.4 (−4.6 to −0.2) | 0.031 |
| QT | 7.6 (0.8–14.4) | 11.2 (3.3 to 19.1) | 3.6 (−6.8 to 14.0) | 0.50 |
| QTc | 4.6 (−0.2 to 9.3) | 12.0 (6.4–17.5) | 7.4 (0.1–14.6) | 0.048 |
Data are expressed as estimated interval time change from baseline in milliseconds (95% CIs).
Figure 3.
Changes in (A) QTc, (B) QT interval, (C) QRS complex, and (D) PR interval duration in the two groups during the study (data displayed as means, and error bars are 95% CI).
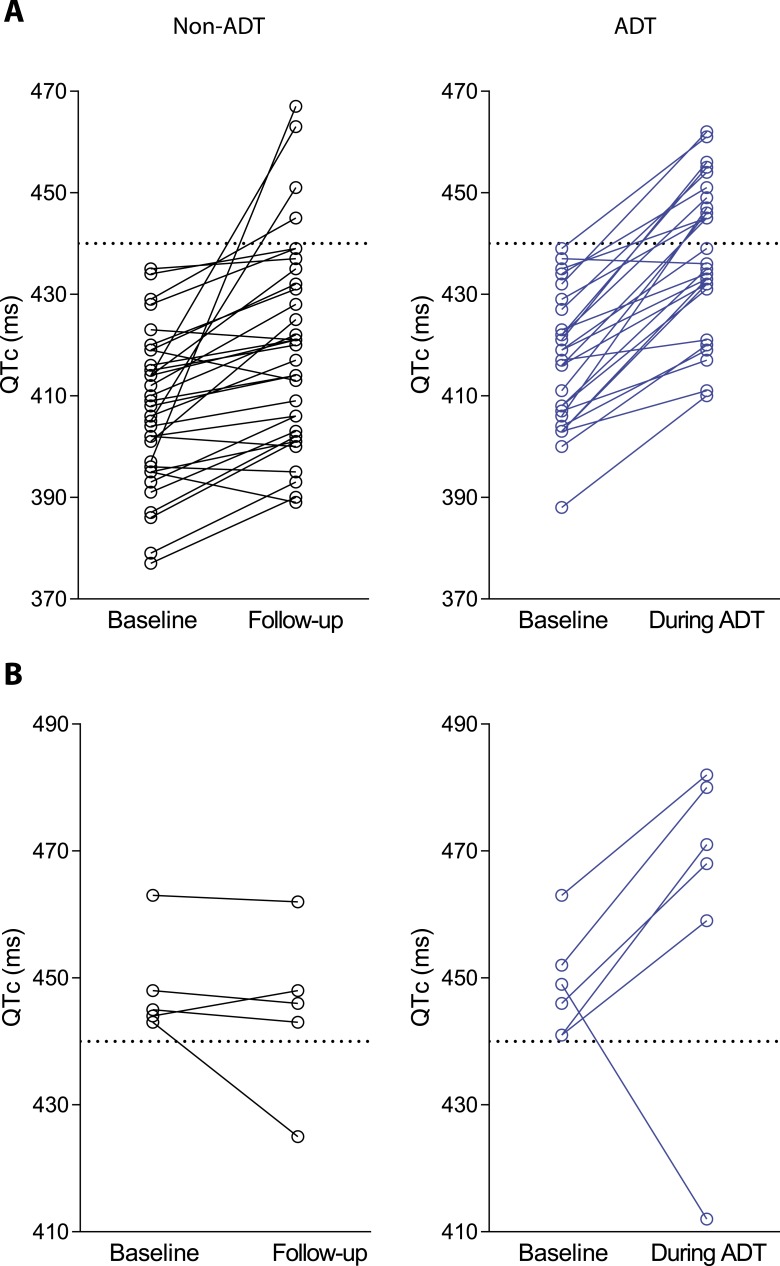
As a QTc of >440 ms is considered abnormal in men [33], we analyzed those participants who had normal QTc duration at baseline but exceeded this threshold during follow-up. Only 4 participants (12%) in the non-ADT group exceeded this threshold, whereas 12 subjects receiving ADT (44%) exceeded a QTc of 440 ms during the course of the study (Fig. 4). Furthermore, among subjects with a QTc >440 ms at baseline, there was a continued increase in QTc in five out of six subjects in the ADT group, whereas no further increase was seen in the non-ADT group (Fig. 4).
Figure 4.
(A) Increase in QTc >440 ms among participants who had normal QTc (<440 ms) at baseline. (B) Increase in QTc among participants who had QTc >440 ms at baseline.
ADT did not affect QT interval duration (Table 2; Fig. 3), with average time remaining unchanged in both groups (treatment difference 3.6 ms; 95% CI −6.9 to 14.0 ms; P = 0.5).
B-2. QRS complex interval
Men undergoing ADT experienced significant shortening of the QRS complex interval (treatment difference −2.4 ms; 95% CI −4.6 to −0.23 ms; P = 0.031; Table 2; Fig. 3) compared with the participants not receiving ADT.
B-3. PR interval
ADT did not affect PR interval duration (Table 2; Fig. 3), with average time remaining unchanged in both groups (treatment difference 0.84 ms; 95% CI −4.3 to 6.0 ms; P = 0.75).
C. Adverse Events
Few adverse events were observed during the 24-week duration of the study. Three cardiovascular events were observed in participants in the non-ADT group (one exacerbation of hypertension, one syncope, and one atrial fibrillation), whereas two events were seen in the ADT group (one ventricular tachycardia and one exacerbation of hypertension). No deaths occurred in either group. Vasomotor symptoms were seen in men undergoing ADT.
3. Discussion
ADT is increasingly being used in the management of PCa [1–4]. Although ADT improves survival in a subset of patients, it has been associated with cardiovascular morbidity and mortality in some studies [34]. Some reports have suggested that cardiovascular events, including sudden death, occur soon after initiation of ADT [13, 35]. As atherosclerosis is a slow process [7, 9], these early events suggest effects of ADT on the cardiac conduction system. Population studies have shown that endogenous serum testosterone levels are inversely associated with QTc duration [36–38]. Indeed, clinical trials have shown that testosterone administration is associated with shortening of QTc [23, 39]. Therefore, withdrawal of testosterone could lead to prolongation of QTc, which in turn could predispose these patients to arrhythmias. We found that ADT with GnRH agonists leads to marked prolongation of QTc (mean increase of 7 ms), and the US Food and Drug Administration considers this increase in QTc clinically relevant [40]. Indeed, prolongation of QTc is independently associated with cardiovascular mortality and sudden death [15, 41–44]. More participants in the ADT group experienced increases in QTc that led to durations >440 ms, the threshold beyond which the risk of arrhythmias increases [16, 40, 45, 46] compared with men in the non-ADT group. Furthermore, the QTc duration continued to increase in those androgen-deprived participants who already had baseline QTc of ≥440 ms, suggesting that ADT leads to prolongation of QTc irrespective of baseline value. Another important finding of the study was the shortening of QRS complex duration, suggesting rapid ventricular depolarization. These findings are important and provide insight regarding the potential mechanisms by which ADT might predispose patients to cardiac arrhythmias and sudden death. These findings, however, require further investigation.
As serum testosterone levels are inversely associated with QTc, it is likely that prolongation of QTc duration in men undergoing ADT is a direct consequence of testosterone suppression. Studies have shown that testosterone shortens ventricular cardiomyocyte repolarization time by two mechanisms: it increases potassium currents derived from the human ether-a-go-go–related gene via the androgen receptor [47], and it inhibits the depolarizing delayed calcium current via a nonandrogen receptor–mediated pathway [48]. Therefore, in men undergoing ADT, profound androgen deficiency favors ventricular depolarization, as seen by a shortened QRS complex, and prolongation of QTc. In a previous trial of testosterone replacement in community-dwelling older men [23], we had observed a reduction in QTc and a trend toward prolongation of QRS complex duration; this supports our current finding that withdrawal of testosterone during ADT leads to prolongation of the QTc and shortening of the QRS complex. Previous cross-sectional studies that have evaluated QRS complex duration in men and women have found that men have longer QRS complex duration compared with women, which they attributed to sex differences in cardiac size [49, 50]. Our observation of QTc prolongation because of ADT confirms findings from a previous uncontrolled prospective study [25]. Unlike QTc prolongation, which has consistently been associated with risk of cardiac arrhythmias, the clinical implications of QRS complex shortening remain unclear and deserve further investigation.
Although suppressed serum testosterone levels are likely responsible for the alterations seen in the cardiac conduction system, one cannot exclude a direct effect of GnRH agonists on cardiac electrophysiology. Indeed, some previous reports have suggested that use of GnRH agonists is associated with a greater risk of cardiovascular disease compared with bilateral orchiectomy, whereas other reports have not confirmed these findings [12, 13, 51, 52]. A possible explanation for these discrepant observations might be the small number of men who underwent bilateral orchiectomy in these compared with those receiving GnRH agonists. However, GnRH receptors are expressed in the cardiac tissue [53, 54], and stimulation of GnRH receptors by GnRH agonists in vitro has shown an increase in intracellular Ca2+ and prolongation of intracellular Ca2+ decay in mouse cardiomyocytes [55]. Mechanistic studies are needed to further understand the drug effect of both GnRH agonists and antagonists on cardiac conduction.
Serum electrolytes sodium, potassium, and calcium, in particular, have an effect on cardiac conduction [30]. Hypokalemia and hypocalcemia can result in QTc prolongation, whereas hypercalcemia leads to shortening of QTc duration [56]. We measured these electrolytes at the same time intervals (and on the same day) that the ECG was performed to exclude any potential effect on ECG parameters. We found no meaningful changes in these electrolytes, suggesting that QTc prolongation is likely related to profound androgen deficiency as a result of ADT.
The current study has several strengths: (1) the study had a prospective design that allowed us to carefully follow changes in ECG parameters; (2) all ECGs were performed by the same technician using the same equipment; (3) enrollment of eugonadal men provided an opportunity to determine the direct effect of testosterone suppression on cardiac electrophysiology; (4) serum testosterone was measured using liquid chromatography-tandem mass spectrometry (the gold-standard method); (5) none of the participants had history of cardiac arrhythmias or bundle branch block, allowing us to accurately measure QTc; (6) all men received medical ADT with GnRH agonists, and no one underwent orchiectomy, thus providing a homogenous sample; (7) enrollment of a non-ADT group as controls who were well matched with the ADT group; and (8) QTc was calculated using the Fridericia QT correction formula, which is known to provide the best prediction of short- and long-term mortality [32]. The present work also has some limitations. This work was a secondary analysis of the ADT and Pain Study. Furthermore, we only enrolled men undergoing ADT with GnRH agonists (this was by design to have a homogenous sample of participants); future mechanistic studies should evaluate the various modalities of ADT (GnRH agonists, GnRH antagonists, and orchiectomy) on ECG parameters.
In conclusion, ADT with GnRH agonists results in considerable prolongation in QTc in men with PCa. These changes in cardiac electrophysiology might explain cardiovascular events, including sudden cardiac death, that are seen soon after initiation of ADT. These findings should guide both physicians and patients to make informed decisions regarding their treatment.
Acknowledgments
Financial Support: This study was supported by Grant R21CA171316 (to S.B. and R.R.E.) from the National Cancer Institute.
Disclosure Summary: P.L.N. has consulted for Ferring Pharmaceuticals, Medivation, Bayer, and Astellas Pharma and receives research funding from Astellas Pharma and Janssen. A.S.K. consults for Janssen, MDxHealth, and Profound Pharma. S.B. has previously received grant support from Abbott Laboratories for investigator-initiated studies unrelated to this study and previously consulted for Eli Lilly and Company and Regeneron Pharmaceuticals. The remaining authors have nothing to disclose.
Glossary
Abbreviations:
- ADT
androgen deprivation therapy
- ADT and Pain
Androgen Deprivation Therapy and Pain Perception
- CI
confidence interval
- ECG
electrocardiogram
- GnRH
gonadotropin-releasing hormone
- PCa
prostate cancer
- QTc
corrected QT
- SD
standard deviation
References and Notes
- 1. Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57(1):43–66. [DOI] [PubMed] [Google Scholar]
- 2. Dy GW, Gore JL, Forouzanfar MH, Naghavi M, Fitzmaurice C. Global burden of urologic cancers, 1990-2013. Eur Urol. 2017;71(3):437–446. [DOI] [PubMed] [Google Scholar]
- 3. Huggins C, Hodges CV. Studies on prostatic cancer. I. The effect of castration, of estrogen and androgen injection on serum phosphatases in metastatic carcinoma of the prostate. CA Cancer J Clin. 1972;22(4):232–240. [DOI] [PubMed] [Google Scholar]
- 4. Jones CU, Hunt D, McGowan DG, Amin MB, Chetner MP, Bruner DW, Leibenhaut MH, Husain SM, Rotman M, Souhami L, Sandler HM, Shipley WU. Radiotherapy and short-term androgen deprivation for localized prostate cancer. N Engl J Med. 2011;365(2):107–118. [DOI] [PubMed] [Google Scholar]
- 5. Bolla M, Collette L, Blank L, Warde P, Dubois JB, Mirimanoff RO, Storme G, Bernier J, Kuten A, Sternberg C, Mattelaer J, Lopez Torecilla J, Pfeffer JR, Lino Cutajar C, Zurlo A, Pierart M. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomised trial. Lancet. 2002;360(9327):103–106. [DOI] [PubMed] [Google Scholar]
- 6. Bubley GJ, Carducci M, Dahut W, Dawson N, Daliani D, Eisenberger M, Figg WD, Freidlin B, Halabi S, Hudes G, Hussain M, Kaplan R, Myers C, Oh W, Petrylak DP, Reed E, Roth B, Sartor O, Scher H, Simons J, Sinibaldi V, Small EJ, Smith MR, Trump DL, Wilding G. Eligibility and response guidelines for phase II clinical trials in androgen-independent prostate cancer: recommendations from the Prostate-Specific Antigen Working Group. J Clin Oncol. 1999;17(11):3461–3467. [DOI] [PubMed] [Google Scholar]
- 7. Basaria S. Androgen deprivation therapy, insulin resistance, and cardiovascular mortality: an inconvenient truth. J Androl. 2008;29(5):534–539. [DOI] [PubMed] [Google Scholar]
- 8. Harle LK, Maggio M, Shahani S, Braga-Basaria M, Basaria S. Endocrine complications of androgen-deprivation therapy in men with prostate cancer. Clin Adv Hematol Oncol. 2006;4(9):687–696. [PubMed] [Google Scholar]
- 9. Shahani S, Braga-Basaria M, Basaria S. Androgen deprivation therapy in prostate cancer and metabolic risk for atherosclerosis. J Clin Endocrinol Metab. 2008;93(6):2042–2049. [DOI] [PubMed] [Google Scholar]
- 10. Braga-Basaria M, Dobs AS, Muller DC, Carducci MA, John M, Egan J, Basaria S. Metabolic syndrome in men with prostate cancer undergoing long-term androgen-deprivation therapy. J Clin Oncol. 2006;24(24):3979–3983. [DOI] [PubMed] [Google Scholar]
- 11. Basaria S, Muller DC, Carducci MA, Egan J, Dobs AS. Hyperglycemia and insulin resistance in men with prostate carcinoma who receive androgen-deprivation therapy. Cancer. 2006;106(3):581–588. [DOI] [PubMed] [Google Scholar]
- 12. Keating NL, O’Malley AJ, Freedland SJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy: observational study of veterans with prostate cancer. J Natl Cancer Inst. 2010;102(1):39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Keating NL, O’Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol. 2006;24(27):4448–4456. [DOI] [PubMed] [Google Scholar]
- 14. Zhang Y, Post WS, Blasco-Colmenares E, Dalal D, Tomaselli GF, Guallar E. Electrocardiographic QT interval and mortality: a meta-analysis. Epidemiology. 2011;22(5):660–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Noseworthy PA, Peloso GM, Hwang SJ, Larson MG, Levy D, O’Donnell CJ, Newton-Cheh C. QT interval and long-term mortality risk in the Framingham Heart Study. Ann Noninvasive Electrocardiol. 2012;17(4):340–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nielsen JB, Graff C, Rasmussen PV, Pietersen A, Lind B, Olesen MS, Struijk JJ, Haunsø S, Svendsen JH, Køber L, Gerds TA, Holst AG. Risk prediction of cardiovascular death based on the QTc interval: evaluating age and gender differences in a large primary care population. Eur Heart J. 2014;35(20):1335–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stramba-Badiale M, Spagnolo D, Bosi G, Schwartz PJ. Are gender differences in QTc present at birth? MISNES Investigators. Multicenter Italian Study on Neonatal Electrocardiography and Sudden Infant Death Syndrome. Am J Cardiol. 1995;75(17):1277–1278. [PubMed] [Google Scholar]
- 18. Alimurung MM, Joseph LG, Craige E, Massell BF. The Q-T interval in normal infants and children. Circulation. 1950;1(6):1329–1337. [DOI] [PubMed] [Google Scholar]
- 19. Rautaharju PM, Zhou SH, Wong S, Calhoun HP, Berenson GS, Prineas R, Davignon A. Sex differences in the evolution of the electrocardiographic QT interval with age. Can J Cardiol. 1992;8(7):690–695. [PubMed] [Google Scholar]
- 20. Salem JE, Alexandre J, Bachelot A, Funck-Brentano C. Influence of steroid hormones on ventricular repolarization. Pharmacol Ther. 2016;167:38–47. [DOI] [PubMed] [Google Scholar]
- 21. Bidoggia H, Maciel JP, Capalozza N, Mosca S, Blaksley EJ, Valverde E, Bertran G, Arini P, Biagetti MO, Quinteiro RA. Sex differences on the electrocardiographic pattern of cardiac repolarization: possible role of testosterone. Am Heart J. 2000;140(4):678–683. [DOI] [PubMed] [Google Scholar]
- 22. Jonsson MK, Vos MA, Duker G, Demolombe S, van Veen TA. Gender disparity in cardiac electrophysiology: implications for cardiac safety pharmacology. Pharmacol Ther. 2010;127(1):9–18. [DOI] [PubMed] [Google Scholar]
- 23. Gagliano-Jucá T, Içli TB, Pencina KM, Li Z, Tapper J, Huang G, Travison TG, Tsitouras P, Harman SM, Storer TW, Bhasin S, Basaria S. Effects of testosterone replacement on electrocardiographic parameters in men: findings from two randomized trials. J Clin Endocrinol Metab. 2017;102(5):1478–1485. [DOI] [PubMed] [Google Scholar]
- 24. Wallis CJ, Mahar AL, Satkunasivam R, Herschorn S, Kodama RT, Lee Y, Kulkarni GS, Narod SA, Nam RK. Cardiovascular and skeletal-related events following localized prostate cancer treatment: role of surgery, radiotherapy, and androgen deprivation. Urology. 2016;97:145–152. [DOI] [PubMed] [Google Scholar]
- 25. Sağlam H, Çakar A, Köse O, Kumsar Ş, Budak S, Gökhan Beyaz S, Adsan Ö. Changes in electrocardiogram findings during treatment with gonadotropin-releasing hormone agonist and surgical castration for prostate carcinoma. Open J Urology. 2012;2(3A):153–156. [Google Scholar]
- 26. Gagliano-Jucá T, Travison TG, Nguyen PL, Kantoff PW, Taplin ME, Kibel AS, Manley R, Hally K, Bearup R, Beleva YM, Huang G, Edwards RR, Basaria S. Effects of androgen deprivation therapy on pain perception, quality of life, and depression in men with prostate cancer. J Pain Symptom Manage. 2018;55(2):307–317.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Surawicz B, Childers R, Deal BJ, Gettes LS, Bailey JJ, Gorgels A, Hancock EW, Josephson M, Kligfield P, Kors JA, Macfarlane P, Mason JW, Mirvis DM, Okin P, Pahlm O, Rautaharju PM, van Herpen G, Wagner GS, Wellens H; American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; American College of Cardiology Foundation; Heart Rhythm Society . AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part III: intraventricular conduction disturbances: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. J Am Coll Cardiol. 2009;53(11):976–981. [DOI] [PubMed] [Google Scholar]
- 29. Bhasin S, Pencina M, Jasuja GK, Travison TG, Coviello A, Orwoll E, Wang PY, Nielson C, Wu F, Tajar A, Labrie F, Vesper H, Zhang A, Ulloor J, Singh R, D’Agostino R, Vasan RS. Reference ranges for testosterone in men generated using liquid chromatography tandem mass spectrometry in a community-based sample of healthy nonobese young men in the Framingham Heart Study and applied to three geographically distinct cohorts. J Clin Endocrinol Metab. 2011;96(8):2430–2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. El-Sherif N, Turitto G. Electrolyte disorders and arrhythmogenesis. Cardiol J. 2011;18(3):233–245. [PubMed] [Google Scholar]
- 31. Fridericia LS. Die systolendauer im elektrokardiogramm bei normalen menschen und bei herzkranken. Acta Med Scand. 1920;53(1):469–486. [Google Scholar]
- 32. Vandenberk B, Vandael E, Robyns T, Vandenberghe J, Garweg C, Foulon V, Ector J, Willems R, Which QT. Which QT correction formulae to use for QT monitoring? J Am Heart Assoc. 2016;5(6):e003264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Johnson JN, Ackerman MJ. QTc: how long is too long? Br J Sports Med. 2009;43(9):657–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Basaria S. Cardiovascular disease associated with androgen-deprivation therapy: time to give it due respect. J Clin Oncol. 2015;33(11):1232–1234. [DOI] [PubMed] [Google Scholar]
- 35. D’Amico AV, Denham JW, Crook J, Chen MH, Goldhaber SZ, Lamb DS, Joseph D, Tai KH, Malone S, Ludgate C, Steigler A, Kantoff PW. Influence of androgen suppression therapy for prostate cancer on the frequency and timing of fatal myocardial infarctions. J Clin Oncol. 2007;25(17):2420–2425. [DOI] [PubMed] [Google Scholar]
- 36. Zhang Y, Ouyang P, Post WS, Dalal D, Vaidya D, Blasco-Colmenares E, Soliman EZ, Tomaselli GF, Guallar E. Sex-steroid hormones and electrocardiographic QT-interval duration: findings from the third National Health and Nutrition Examination Survey and the Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol. 2011;174(4):403–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Junttila MJ, Tikkanen JT, Porthan K, Oikarinen L, Jula A, Kenttä T, Salomaa V, Huikuri HV. Relationship between testosterone level and early repolarization on 12-lead electrocardiograms in men. J Am Coll Cardiol. 2013;62(17):1633–1634. [DOI] [PubMed] [Google Scholar]
- 38. van Noord C, Dörr M, Sturkenboom MC, Straus SM, Reffelmann T, Felix SB, Hofman A, Kors JA, Haring R, de Jong FH, Nauck M, Uitterlinden AG, Wallaschofski H, Witteman JC, Völzke H, Stricker BH. The association of serum testosterone levels and ventricular repolarization. Eur J Epidemiol. 2010;25(1):21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schwartz JB, Volterrani M, Caminiti G, Marazzi G, Fini M, Rosano GM, Iellamo F. Effects of testosterone on the Q-T interval in older men and older women with chronic heart failure. Int J Androl. 2011;34(5 Pt 2):e415–e421. [DOI] [PubMed] [Google Scholar]
- 40.US Department of Health and Human Services, International Conference on Harmonization. Guidance for Industry: E14 Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-Antiarrhythmic Drugs. Available at: http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm073153.pdf. Accessed 26 September 2017.
- 41. Okin PM, Devereux RB, Howard BV, Fabsitz RR, Lee ET, Welty TK. Assessment of QT interval and QT dispersion for prediction of all-cause and cardiovascular mortality in American Indians: The Strong Heart Study. Circulation. 2000;101(1):61–66. [DOI] [PubMed] [Google Scholar]
- 42. Schouten EG, Dekker JM, Meppelink P, Kok FJ, Vandenbroucke JP, Pool J. QT interval prolongation predicts cardiovascular mortality in an apparently healthy population. Circulation. 1991;84(4):1516–1523. [DOI] [PubMed] [Google Scholar]
- 43. Robbins J, Nelson JC, Rautaharju PM, Gottdiener JS. The association between the length of the QT interval and mortality in the Cardiovascular Health Study. Am J Med. 2003;115(9):689–694. [DOI] [PubMed] [Google Scholar]
- 44. de Bruyne MC, Hoes AW, Kors JA, Hofman A, van Bemmel JH, Grobbee DE. Prolonged QT interval predicts cardiac and all-cause mortality in the elderly. The Rotterdam Study. Eur Heart J. 1999;20(4):278–284. [DOI] [PubMed] [Google Scholar]
- 45. Algra A, Tijssen JG, Roelandt JR, Pool J, Lubsen J. QTc prolongation measured by standard 12-lead electrocardiography is an independent risk factor for sudden death due to cardiac arrest. Circulation. 1991;83(6):1888–1894. [DOI] [PubMed] [Google Scholar]
- 46. Drew BJ, Ackerman MJ, Funk M, Gibler WB, Kligfield P, Menon V, Philippides GJ, Roden DM, Zareba W; American Heart Association Acute Cardiac Care Committee of the Council on Clinical Cardiology; Council on Cardiovascular Nursing; American College of Cardiology Foundation . Prevention of torsade de pointes in hospital settings: a scientific statement from the American Heart Association and the American College of Cardiology Foundation. J Am Coll Cardiol. 2010;55(9):934–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ridley JM, Shuba YM, James AF, Hancox JC. Modulation by testosterone of an endogenous hERG potassium channel current. J Physiol Pharmacol. 2008;59(3):395–407. [PubMed] [Google Scholar]
- 48. Er F, Michels G, Brandt MC, Khan I, Haase H, Eicks M, Lindner M, Hoppe UC. Impact of testosterone on cardiac L-type calcium channels and Ca2+ sparks: acute actions antagonize chronic effects. Cell Calcium. 2007;41(5):467–477. [DOI] [PubMed] [Google Scholar]
- 49. Mason JW, Ramseth DJ, Chanter DO, Moon TE, Goodman DB, Mendzelevski B. Electrocardiographic reference ranges derived from 79,743 ambulatory subjects. J Electrocardiol. 2007;40(3):228–234. [DOI] [PubMed] [Google Scholar]
- 50. Vicente J, Johannesen L, Galeotti L, Strauss DG. Mechanisms of sex and age differences in ventricular repolarization in humans. Am Heart J. 2014;168(5):749–756. [DOI] [PubMed] [Google Scholar]
- 51. Gandaglia G, Sun M, Popa I, Schiffmann J, Abdollah F, Trinh QD, Saad F, Graefen M, Briganti A, Montorsi F, Karakiewicz PI. The impact of androgen-deprivation therapy (ADT) on the risk of cardiovascular (CV) events in patients with non-metastatic prostate cancer: a population-based study. BJU Int. 2014;114(6b):E82–E89. [DOI] [PubMed] [Google Scholar]
- 52. Albertsen PC, Klotz L, Tombal B, Grady J, Olesen TK, Nilsson J. Cardiovascular morbidity associated with gonadotropin releasing hormone agonists and an antagonist. Eur Urol. 2014;65(3):565–573. [DOI] [PubMed] [Google Scholar]
- 53. Kakar SS, Jennes L. Expression of gonadotropin-releasing hormone and gonadotropin-releasing hormone receptor mRNAs in various non-reproductive human tissues. Cancer Lett. 1995;98(1):57–62. [PubMed] [Google Scholar]
- 54. Lee CY, Ho J, Chow SN, Yasojima K, Schwab C, McGeer PL. Immunoidentification of gonadotropin releasing hormone receptor in human sperm, pituitary and cancer cells. Am J Reprod Immunol. 2000;44(3):170–177. [DOI] [PubMed] [Google Scholar]
- 55. Dong F, Skinner DC, Wu TJ, Ren J. The heart: a novel gonadotrophin-releasing hormone target. J Neuroendocrinol. 2011;23(5):456–463. [DOI] [PubMed] [Google Scholar]
- 56. Vandael E, Vandenberk B, Vandenberghe J, Willems R, Foulon V. Risk factors for QTc-prolongation: systematic review of the evidence. Int J Clin Pharm. 2017;39(1):16–25. [DOI] [PubMed] [Google Scholar]