Abstract
A high-casualty incident may result in a significant human toll due to the inability of a community to meet the health care demands of the population. A successful medical response requires health care facilities to not only communicate and integrate medical services, meet surge capacity, protect health care workers and implement triage and treatment protocols, but also to provide the venue for clinical management of acute radiation injuries and their associated infections. Today, clinical management is primarily guided by the recommendations of a Consultancy that were made at the World Health Organization (WHO). This international consensus was reached on evidence-based, clinical management of each of the four sub-syndromes that compose acute radiation syndrome (ARS), including the hematopoietic subsyndrome (HS), gastrointestinal subsyndrome (GIS), neurovascular subsyndrome (NVS) and cutaneous subsyndrome (CS). Major findings in studies meeting inclusion criteria for management strategies for HS were that (i) no randomized controlled studies of medical countermeasures have been (or will likely ever be) performed for ARS cases, (ii) the data for management of HS are restricted by the lack of comparator groups, and (iii) reports of countermeasures for management of injury to non-hematopoietic organs are often incompletely described. Here, (i) recommendations made in Geneva are summarized; (ii) the analysis of countermeasures for HS is updated by review of two additional cases and extended to published reports not meeting inclusion criteria; and (iii) guidelines are provided for management of microbial infections based upon patient risk for prolonged immunosuppression.
Keywords: acute radiation syndrome, management of infections in ARS, clinical guidelines for treating infections in ARS, medical management of ARS, evidence-based management of ARS, hospital emergency response to a R/N incident
INTRODUCTION
Detonation of a nuclear weapon or improvised nuclear device (IND) in a major city will impact the urban health care system and cause numerous casualties [1]. A sudden and massive inundation of health care facilities will occur, as patients seek medical care for disorders ranging from combined injuries (radiation injury plus thermal burns and/or mechanical trauma) to psychiatric and psychosocial trauma [2, 3]. Public service announcements will advise individuals about when and where to shelter and/or prepare for evacuation [4]. Hospitals, urgent care centers and ambulatory clinics that withstand the initial blast of a nuclear detonation and suffer no or inconsequential physical damage may not be accessible due to destruction of surrounding infrastructure (i.e. roadways and railways) or contamination with fallout. Health care resources will be limited and surge capacity will be taxed at facilities located outside the immediate zones of destruction and contamination. Ethical principles will be applied to the allocation of scarce resources [5].
Hospitals will implement an incident command system and assume new functions. A successful medical response will require health care facilities to communicate with one another and to integrate their medical services to meet a surge in demand for clinical care. New functions will need to be addressed, including protection of health care workers from contamination with radioactive materials, and implementation of unfamiliar triage and treatment protocols for individuals with radiation injuries, trauma and/or burns [2]. Above all, hospitals and other public health facilities will function as first receivers or secondary referral centers for the injured, as they strive to provide an environment for efficient, state-of-the-art clinical management of radiation injuries [3].
Here, I briefly summarize the current state of the recommendations that were made at a World Health Organization (WHO) Consultancy that was convened in Geneva 16–18 March 2009, in which a panel of 34 experts from 12 countries on four continents met to discuss clinical management of acute radiation syndrome (ARS) within the context of a hypothetical scenario involving the hospitalization of 100–200 victims [6, 7]. Evidence-based recommendations were made for the use of cytokines to manage hematopoietic subsyndrome (HS) based on the then-current published reports of cases that met specific inclusion criteria. Next, I update cytokine use in two cases reported after March 2009, one of which met inclusion criteria. I then analyze the results in the case not meeting these criteria and in 27 additional cases that did not meet the inclusion criteria. Finally, I discuss the occurrence and clinical management of infectious diseases that lead to high morbidity and mortality among individuals exposed to a moderate (3–5 Gy) or high (≥5 Gy) radiation dose.
RECOMMENDATIONS OF THE WHO CONSULTANCY
Prior to 2009, recommendations for the clinical management of radiation injuries were based on expert opinion [1, 8], the weakest form of scientific evidence. To strengthen the scientific basis for clinical management guidelines, an international Consultancy was convened in Geneva, Switzerland by the WHO to consider the optimal management of ARS in a hypothetical scenario involving the hospitalization of 100–200 individuals. The goal was to apply the Grading of Recommendations Assessment Development and Evaluation (GRADE) system for evaluating the quality of the evidence supporting a guideline [6] to review and assess the quality of the literature. A numerical system was used to assess the strength of the recommendation based on net benefit for evidence reported in studies of high (A), moderate (B), low (C) or very low (D) quality. For treatment of the hematopoietic sub-syndrome (HS) with cytokines and/or hematopoietic stem cell transplantation (HSCT), data were extracted from eight publications [6]. Owing to publication of incomplete information, a narrative review was made of evidence supporting the use of 14 additional treatment strategies in the management of ARS affecting non-hematopoietic organ systems [7]. Quality of evidence and strength of recommendation were discussed and voted upon individually, and mean scores were determined. The results of ranking each treatment strategy are presented in Tables 1 and 2.
Table 1.
Summary of recommendations for HS and GIS by subject matter experts, WHO Consultancy
| Syndrome | Recommendation | Strength of recommendation |
|---|---|---|
| Hematopoietic | Administer G-CSF or GM-CSF when ANC <0.500 × 109 cells/l | Strong (B-1a) |
| Administer ESAs when prolonged anemia is present in order to avoid need for red blood cell infusion | Weak (C-1b) | |
| Administer hematopoietic stem cells after failure of 2–3 weeks of cytokine treatment to induce recovery from marrow aplasia in the absence of non-hematopoietic organ failure | Weak (D-1b) | |
| Gastrointestinal | Administer fluoroquinolone or similar antibiotic from 2–4 days after radiation exposure | Weak (B-1b) |
| Provide bowel decontamination and parenteral antibiotics when indicated, if resources permit | Weak (C-1b) | |
| Administer a serotonin receptor antagonist prophylactically when suspected exposure is >2 Gy | Weak (B-1b) | |
| Administer loperamide, as needed for control of diarrhea | Weak (B-1b) |
HS = hematopoietic subsyndrome, GIS = gastrointestinal subsyndrome. Strength of recommendation was determined by assignment of quality of the evidence (A = high, B = moderate, C = low or D = very low) and strong (1a) or weak (1b) recommendation in favor of the practice. The scale used for strength of recommendation is summarized in legend to Table 1. Modified from Dainiak et al. (Literature review and global consensus on management of acute radiation syndrome affecting nonhematopoietic organ systems. Disaster Med Public Health Prep 2011;5:183–201) and Dainiak et al. (First global consensus for evidence-based management of the hematopoietic syndrome resulting from exposure to ionizing radiation. Disaster Med Public Health Prep 2011;5:202–12).
Table 2.
Summary of recommendations for CS, NVS and critical care medicine by subject matter experts, WHO Consultancy
| Syndrome | Recommendation | Strength of recommendation |
|---|---|---|
| Cutaneous | Administer topical class II–III steroids, topical antibiotics and topical antihistamines to radiation burns, ulcer or blisters | Strong (A-1a) |
| Administer systemic steroids for radiation burns, ulcers or necrosis in the absence of a specific indication for systemic steroid use | Strong against (D-2a) | |
| Surgically excise and graft radiation ulcers or localized necrosis with intractable pain | Strong (B-1a) | |
| Neurovascular | Provide supportive care with a serotonin receptor antagonist, mannitol, furosemide and analgesics | Strong (A-1a) |
| Critical care | Administer fluid and electrolyte replacement therapy and sedatives when significant burns, hypovolemia and/or shock occur | Strong (A-1a) |
| Administer mechanical ventilation with a lung-protective strategy for acute respiratory failure | Strong (A-1a) | |
| Administer SOD or SDD to decontaminate the digestive tract | Weak (B-1b) | |
| Maintain average blood glucose of 140–180 mg/dl for majority of critical care patients | Weak (B-1b) | |
| Administer H2 blocker or PPI | Weak (B-1b) |
CS = cutaneous subsyndrome, NVS = neurovascular subsyndrome. Strength of recommendation was determined by assignment of quality of the evidence (A = high, B = moderate, C = low or D = very low) and strong (1a) or weak (1b) recommendation in favor of the practice. The scale used for strength of recommendation is summarized in the legend to Table 1. Modified from Dainiak et al. (Literature review and global consensus on management of acute radiation syndrome affecting nonhematopoietic organ systems. Disaster Med Public Health Prep 2011;5:183–201).
Based upon their mechanism of action on hematopoietic stem/progenitor cells and utility in improving survival in febrile neutropenia and myelodysplasia, and accelerating neutrophil recovery after chemotherapy for malignancies, a strong rationale exists for using cytokines to treat radiation-induced damage to the bone marrow [9]. For the purpose of establishing the presence/absence of efficacy in humans, criteria for inclusion in the analysis of cytokine outcomes were determined to be: (i) establishment of bone marrow failure, (ii) documentation of cytokine use, and (iii) demonstration of an effect (or lack of an effect) on hematopoiesis.
Individuals receiving cytokines after an exposure resulting in a whole-body, absorbed dose of ≤5 Gy survived longer (14/15 patients were long-term survivors, living >1 year) than those receiving a dose of >5 Gy (1/3 long-term survivors). Based on this finding, a strong recommendation was made for the use of granulocyte colony-stimulating factor (G-CSF) or granulocyte macrophage colony-stimulating factor (GM-CSF) for treating HS. Owing to a limited number of studies, a weak recommendation was made to use erythropoiesis-stimulating agents (i.e. erythropoietin) to avoid red blood cell transfusions.
The above analysis and subsequent publication [7] were reviewed by the author in support of approval by the US Federal Drug Administration (FDA) for the use of myeloid cytokines [G-CSF: filgrastim (Neupogen®, Amgen, Inc., Thousand Oaks, CA, USA) and pegylated G-CSF: pegfilgrastim (Neulasta®, Amgen, Inc., Thousand Oaks, CA, USA]) in patients who have been acutely exposed to myelosuppressive doses of radiation [10]. Based on its ‘animal rule’ and subsequent guidance document [11], the FDA approved these myeloid cytokines after efficacy was demonstrated in landmark studies in non-human primates by MacVittie and co-workers [12, 13]. In addition, under its Emergency Use Authorization (EUA) program, the FDA approved the use of sargramostim (Leukine®/Sargramostim, Sanofi-Aventis U.S. LLC, Bridgewater, NJ, USA) in an emergency when neither G-CSF nor pegylated G-CSF is available (reviewed in [14]). Leukine®/Sargramostim is a formulation of granulocyte–macrophage colony-stimulating factor (GM-CSF) that is expressed in yeast.
Bleeding due to thrombocytopenia is a significant cause of death among patients with ARS. Unfortunately, aside from platelet transfusions, no specific medical countermeasures are available currently for mitigating thrombocytopenia. Several agents are being assessed, including interleukin-11, recombinant human thrombopoietin (TPO) and the TPO mimetics Romiplostim (a peptide that binds distally to and activates the TPO receptor) and Eltrombopag (a non-peptide that binds to the transmembrane domain of the TPO receptor). Unfortunately, neutralizing antibodies develop after TPO administration, and it is no longer manufactured. However, alloimmunization has not been observed after the administration of the TPO mimetics. TPO mimetics have been shown to improve the platelet count in early phase (I and II) studies of patients with aplastic anemia or autoimmune thrombocytopenia [15, 16]. Studies are underway to assess the effects of TPO mimetics in irradiated ‘humanized’ mice [17].
The rationale for HSCT is driven by the Do values for stem/progenitor cells in the marrow and circulation, which is remarkably similar to the threshold dose of ~1 Gy at which HS is initiated, and the morphological appearance of an ‘empty’ bone marrow after high-dose irradiation, mimicking that seen in aplastic anemia where HCST may be curative. Despite initial enthusiasm for HSCT, overall outcomes have been poor among irradiated patients who have received this therapy [18–20]. In a review of three relevant case series, it was evident to the WHO subject matter experts that survival was more likely among individuals with HS who had received <9 Gy and no HSCT, than in those who received <9 Gy and who were treated with HSCT [7]. Nevertheless, important caveats include: (i) the data are too restrictive for definitive statistical analysis; and (ii) the majority of transplant recipients (and controls) developed non-hematopoietic organ failure prior to or after transplantation. Based on the reported outcomes, the published literature was judged to be of very low quality, and a weak recommendation was made for the use of HSCT in individuals whose only organ failure is limited to the hematopoietic system, and among these patients, only after failure of a 2–3 week course of cytokine therapy.
Compared with reports of HS management, studies reporting clinical management of individuals with GIS, CS and/or NVS are even less complete [7]. Owing to the lack of detailed evidence in the published literature, all recommendations for the management of the GIS were weak, including the use of fluoroquinolones, bowel decontamination, serotonin receptor antagonists (SRAs), loperamide and enteral nutritional support (see Table 1) [7]. For the cutaneous syndrome (CS), strong recommendations were made for administration of topical steroids, topical antihistamines and topical antibiotics to patients with radiation ulcers, blisters and burns, and for surgical excision and grafting of radiation ulcers and localized necrosis with intractable pain (see Table 2) [7]. Strong recommendations were also made against the use of systemic steroids for radiation-induced skin lesions in the absence of another specific indication for their use; and in favor of providing supportive care with SRAs, mannitol, furosemide and analgesics for individuals with neurovascular sub-syndrome (NVS) (see Table 2). Finally, therapeutic and prophylactic strategies for critical care management of patients with unstable hemodynamic measurements, respiratory insufficiency or diabetes mellitus were recommended (see Table 2) [7].
UPDATE OF RECOMMENDATIONS OF WHO CONSULTANCY
Since the WHO consultancy met in 2009, two additional cases were reported wherein an individual received cytokines after exposure to ionizing radiation [21, 22]. The first incident involved an operator who entered the irradiation room of a sterilization facility in Fleurus, Belgium on 11 March 2006. The operator was unaware that a 60Co source was partly out of its secure position due to a failure of the hydraulic control system that raises and lowers the source from a safe storage pool [23]. As he reset a gamma radiation monitor alarm system, he was exposed for ~22 s to the source, whose activity was 2.96 × 1016 Bq (800 000 Ci) at a dose rate of 5000 Gy/h. A heterogeneous, whole-body dose of 4.2–4.8 Gy was received. The operator experienced vomiting within a few hours after exposure and over the next 18 days, he developed diarrhea, headache and widespread epilation. On Day 20 post exposure, the patient was admitted to Percy Military Hospital, Paris, France for further evaluation and medical therapy that included cytokines and antibiotics [21].
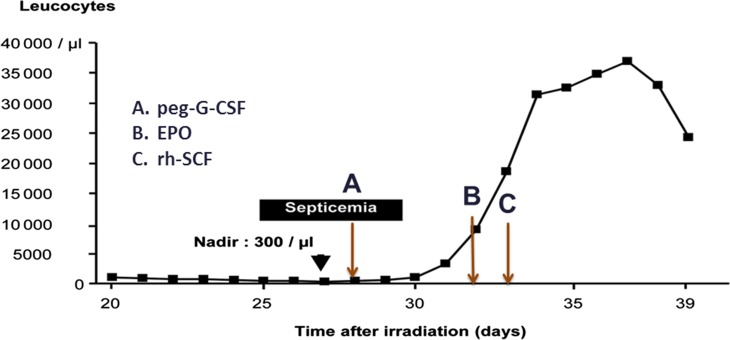
The diagnosis of Grade 4 HS [ARS Response Category (RC]) 4], as defined by Fliedner and colleagues [24], was confirmed by documentation of pancytopenia on a complete blood cell count (CBC) with calculation of the differential of the leukocytes, and platelet count. Treatment with pegylated G-CSF was initiated on Day 28 post exposure. On Days 32 and 33 post exposure, the patient was administered pegylated erythropoietin and recombinant human stem cell factor (Stemgen®, Swedish Orphan Biovitrum AB, Stockholm, Sweden), also known as Ancestim, c-kit ligand, steel factor and mast cell growth factor [21]. Stem cell factor when used together with G-CSF is believed to show a synergistic effect in mobilizing HSCs in the circulation. The total leucocyte count was measured daily and is graphed in Fig. 1. Although an incremental response to cytokines is evident from inspection of the graph, it is uncertain which cytokine or combination of cytokines was responsible for this favorable effect. If the results of this case are combined with those of the cases previously analyzed by the WHO Consultancy experts, the number of long-term survivors among those receiving a dose of ≤5 Gy is increased from 14/15 patients (93.3%) analyzed initially to 15/16 patients (93.8%) analyzed here, thereby having no effect on the recommendations made at the WHO Consultancy [6].
Fig. 1.
Cytokine response in individual from Fleurus, Belgium Accident (2006). Shown are total leukocyte counts over time before and after therapy with pegylated granulocyte colony-stimulating factor (G-CSF), erythropoietin (EPO) and recombinant human Stem Cell Factor (rh-SCF). Modified from Gourmelon P et al. (European consensus on the medical management of acute radiation syndrome and analysis of the radiation accidents in Belgium and Senegal. Health Phys 2010;98:825–32).
The second incident involved a worker who on 1 April 2008 was exposed to a 60Co source (activity of 18 000 Ci) in its working position at a distance of 80–150 cm as he worked at a sterilization plant in Taiyuan, Shanix Province, China, that is used to irradiate traditional Chinese medicines [22]. The operator developed vomiting within 30 min after exposure, together with diffuse erythema and fever (temperature of 38.9°C), and was transported to the Affiliated Hospital of the Academy of Military Medical Sciences, Beijing, China at 14 h after exposure. The whole-body dose of gamma radiation was estimated to be 14.5 Gy, using dicentric chromosome analysis. The patient developed Grade 4 HS (RC 4) and was infused with HLA-mismatched peripheral blood stem cells and mesenchymal stem cells. Although the patient also received G-CSF therapy, the published report did not indicate start and end times of this therapy, dose of G-CSF or a statement regarding its efficacy or lack thereof [22]. Therefore, this case would not have met inclusion criteria and consequently would have been excluded from the WHO Consultancy analysis. The patient developed cutaneous ulcerations, gastrointestinal syndrome (GIS) with sloughing of the bowel that required a bowel resection, hypotension and widespread fungal sepsis with diffuse intravascular coagulation. He expired with multi-organ failure on Day 62 after exposure.
To further test the strength of the recommendations made by the WHO consultants, an analysis was made of all other known cases where cytokines were used but where not all inclusion criteria were met (which therefore were excluded from analysis at the Consultancy). Table 3 provides a summary of the observed outcomes (i.e. recovery or expired) that are presumably due to a cytokine effect. Interestingly, 88.9% of the patients (16/18 patients) whose dose was ≤6 Gy recovered, while none of the 8 patients whose dose was >6 Gy survived. These results are remarkably similar to the findings of the Consultancy group, in which survival was 93.3% and 33.0%, respectively, for those meeting inclusion criteria with a cut-off of ≤5 Gy for a favorable response. Together with the Fleurus, Belgium case, the results of this additional analysis of cases not meeting inclusion criteria are consistent with and support the conclusions of the Consultancy subject matter experts.
Table 3.
Responses to cytokines in reports not meeting WHO inclusion criteria
| Incident | Cytokine | Number | Dose | Effect |
|---|---|---|---|---|
| Chernobyl, Ukraine, 1986 [20] | GM-CSF | 3 | 5.0, 5.0 and 5.0 Gy | 2/3 recovered |
| San Salvador, El Salvador, 1989 [25] | GM-CSF | 3 | 3.0 and 3.8 Gy | Recovered |
| 8.2 Gy | Expired | |||
| Soreq, Israel, 1990 [26] | GM-CSF + IL-3 | 1 | 10–20 Gy | Expired |
| Nesvizh, Belarus, 1991 [27] | GM-CSF | 1 | 11.0 Gy | Expired |
| Yanango, Peru, 1999 [28] | GM-CSF | 1 | 1.2–1.3 Gy | Recovered |
| Samut Prakarn, Thailand, 2000 [29] | GM + G-CSF | 9 | 2.0, 2.0 and 2.0 Gy | Recovered |
| <6.0 and <6.0 Gy | Recovered | |||
| >6.0, >6.0, >6.0 and >6.0 Gy | Expired | |||
| Meet Haifa, Egypt, 2000 [30] | G-CSF | 5 | 3.5–4.0 Gy | 4/5 recovered |
| Nueva Aldea, Chile, 2005 [31] | G-CSF | 1 | 1.3 Gy | Recovered |
| Dakar, Senegal, 2006 [21] | Peg-G-CSF + EPO + SCF | 1 | 3.1–3.3 Gy | Recovered |
| Taiyuan, China, 2008 [22] | G-CSF | 1 | 14.5 Gy | Expired |
MANAGEMENT OF INFECTIONS IN IRRADIATED INDIVIDUALS
Timely administration of antimicrobial agents reduces mortality in small and large animals receiving radiation in the LD 50/30 range [32, 33]. The pathophysiology of radiation risk for and antimicrobial treatment of infections associated with accidental exposure to radiation is reviewed below.
Pathophysiology and risk
In addition to bleeding, sepsis secondary to microbial infections contributes significantly to mortality among individuals receiving a medically significant dose of radiation. Widely disseminated bacterial, fungal and viral infections develop as a consequence of immunosuppression that is well described, with declines in circulating neutrophil and lymphocyte (in particular, helper T-cell) counts, impaired and augmented expression of the various genes encoding lymphokines, and breeches in the skin and mucosa of the gastrointestinal tract [34–36]. Not only acute but also late effects from IR exposure, including chronic inflammation and fibrosis of the lung, are mediated by alterations in lymphocyte number and function [37]. In the setting of radiation necrosis, the release of tumor necrosis factor, nitric oxide and prostaglandins occurs as part of the systemic inflammatory response syndrome, leading to arterial vasodilation, increased cardiac output, maldistribution of blood flow and tissue hypoxia, resulting in death from multi-organ failure [38–42].
These changes in innate immunity, hemodynamics and tissue oxygenation are complicated further by radiation-induced impaired stem cell responses, affecting stem cells located in the bone marrow, intestinal crypts and basal layer of the skin. The loss of stem cell number and function is a fundamental pathophysiologic process that underlies ARS. Stem cell damage prevents recapitulation of lympho-hematopoiesis, replacement of the tips of the villi (from which mucosal cells are routinely sloughed), and replacement of desquamated epidermal cells [35, 43]. At the same time, alterations in naturally occurring barriers to microbes in the integument and gastrointestinal mucosa result in translocation of infectious organisms into the circulation, setting the stage for invasion by and unimpeded growth of bacteria, fungi and viruses, as well as reactivation of prior infections with viruses and mycobacteria whose pathogenicity correlates with their adoption of immune evading strategies.
Clinical practice guidelines for febrile neutropenia
Patients with an absolute neutrophil count (ANC) of <0.5 × 109 cells/l are at high risk for infection, and may benefit from both cytokine therapy and prophylaxis with oral antimicrobial agents [7]. Antibiotic prophylaxis has been found to improve survival among neutropenic patients undergoing cytotoxic therapy for cancer [44]. The Infectious Diseases Society of America (IDSA) has developed clinical practice guidelines for the administration of antimicrobial agents to patients with cancer who develop febrile neutropenia after chemotherapy [45, 46]. In 2008, these guidelines were modified for management of patients with radiation-induced neutropenia and fever, based on their risk for developing prolonged (≥7 days) immunosuppression [47]. These modified guidelines were adopted by the Radiation Emergency Assistance Center/Training Site (REAC/TS) and presented twice annually thereafter in its Advanced Radiation Medicine (ARM) course. The REAC/TS recommendations were last updated after revision of the IDSA guidelines in 2010 [46], and continue to be presented as part of the ARM course.
An important caveat when comparing infections occurring after chemotherapy with those occurring after accidental exposure to IR is that infectious organisms are actually identified in only 20–30% of febrile episodes after chemotherapy [43], compared with documentation in nearly all febrile episodes after accidental irradiation [20, 22, 28, 48, 49]. This difference in identification rate may possibly be due to widespread radiation injury to organs such as the skin and gastrointestinal tract that have a protective barrier function of keeping bacteria outside the body, as well as to the relatively short duration (typically days to weeks) of absolute neutropenia after chemotherapy, compared with the frequently prolonged immunosuppression (typically several weeks to months) after irradiation at a high radiation dose (>5 Gy). Nevertheless, the sites of infection are similar in both settings: primarily the skin, lung and gastrointestinal tract. Furthermore, management strategies in both settings include (i) early intervention of prophylaxis with antimicrobial agents; (ii) aggressive diagnostic work-up with pancultures (including two sets of blood cultures, and cultures from suspected sites of infection), imaging testing (including a chest X-ray for those with respiratory signs and symptoms), serial CBCs with differential leukocyte and platelet counts, and serum creatinine and urea nitrogen to monitor for possible antibiotic toxicity; (iii) close monitoring of clinical signs and symptoms that can be used to document the source of infection; and (iv) prompt adjustment of antimicrobial therapy based on patterns of antimicrobial sensitivity and resistance of documented infectious organisms.
Selection of antimicrobial therapy
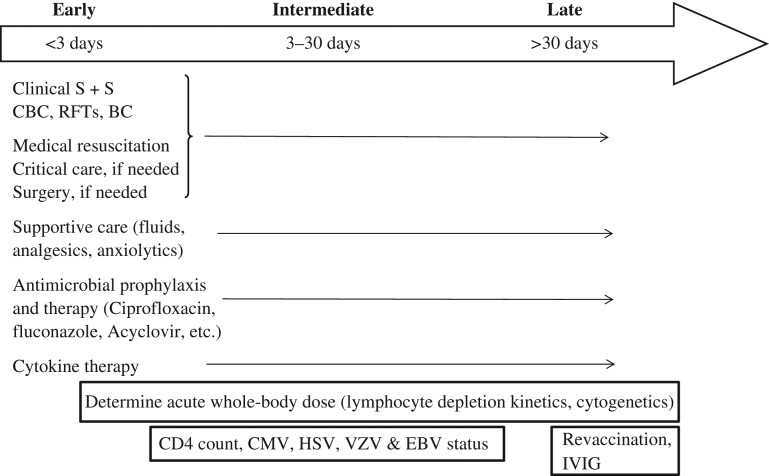
The overall approach to treatment of infections in the irradiated patient may be thought of in terms of time after exposure. As summarized in Fig. 2, an arbitrary time-line is divided up into <3 days (the ‘early’ period), 3–30 days (the ‘intermediate’ period) and >30 days (the ‘late’ period) to emphasize the importance of providing therapy for likely infectious organisms and therapies that are dictated by the time-course for impaired immunity to develop after an exposure to a medically significant radiation dose. During the early period after exposure, the ANC is likely to be >0.5 × 109/l, and attention should be given to medical resuscitation, fluid replacement and surgery, if needed. Lymphocyte depletion kinetics should be determined [50, 51], and prophylaxis with antibiotics and cytokines should be strongly considered for those whose projected radiation dose predicts an absolute neutropenia. During the intermediate period after exposure, the results of the individual biodosimetry should be confirmed and assessment of the CD4 count and virus status should be made. Depending on the clinical course, consideration should be given to adding anti-viral agents and anti-fungal agents. During the late phase, immune reconstitution should be considered, with special attention paid to (i) administering intravenous immunoglobulin to those with recurrent sinopulmonary infections and a low immunoglobulin level, (ii) obtaining convalescent serology tests at 1 year, (iii) performing revaccination according to IDSA/Centers for Disease Control and Prevention (CDC) guidelines, and (iv) vaccination with polysaccharide for encapsulated organisms.
Fig. 2.
Summary of clinical management of infections based on time after exposure. Shown are treatment strategies for three arbitrarily designated periods (‘early’, ‘intermediate’ and ‘late’) following exposure to ionizing radiation.
Candidates for prophylactic antimicrobial therapy include those with (i) fever [defined as a single oral temperature of ≥38.3°C (101°F) or a temperature of ≥38.0°C (100.4°F)] plus neutropenia (defined as an ANC of <0.5 × 109/l), (ii) afebrile neutropenia with clinical signs and symptoms of infection (often presenting as subtle pain), and (iii) clinical signs and symptoms of infection without neutropenia. Subtle pain may occur in the periodontium, pharynx, esophagus, lung, perineum, eye, skin and nails. The ‘low-risk’ irradiated patient is defined as one who has an anticipated absolute neutropenia of ≤7 days, is hemodynamically stable and has no medical comorbidities.
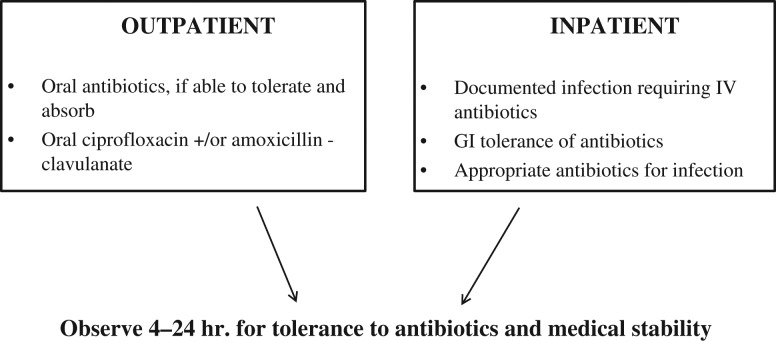
The selection of prophylaxis for low-risk patients is determined by local community susceptibility patterns of the most likely infectious organisms. It is generally recommended to provide broad-spectrum coverage for gram-positive streptococci and staphylococci, and gram-negative Psuedomonas and Enterobacteriaceae. An empirical regimen of a fluoroquinolone and amoxicillin/clavulanate provides this coverage. Fig. 3 presents an approach to antibiotic management of irradiated individuals in the ambulatory and hospitalized settings. Among patients with febrile neutropenia, gram-positive bacteria (including methicillin-resistant staphylococci and vancomycin-resistant enterococci) account for the majority of microbiologically documented infections, with gram-negative bacilli (including P. aeruginosa, Escherichia coli and Klebsiella organisms) accounting for a substantial proportion of the remaining infections [45]. In randomized, controlled studies in the USA and Europe, outcomes with oral ciprofloxacin and amoxicillin–clavulanate were equivalent to those with parenteral antibiotics [46]. Hospitalization is indicated for those who require intravenous antibiotics for documented infections and those who cannot tolerate oral therapy due to persistent vomiting. For documented infections, antibiotics should be continued as indicated by the organism and site of infection, and at least until the ANC exceeds 0.5 × 109/l [46].
Fig. 3.
Antibiotics recommended for low-risk patients in the ambulatory (outpatient) and hospitalized (inpatient) settings, based upon Freifield AG et al. (Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis 2011;52:e56-e93).
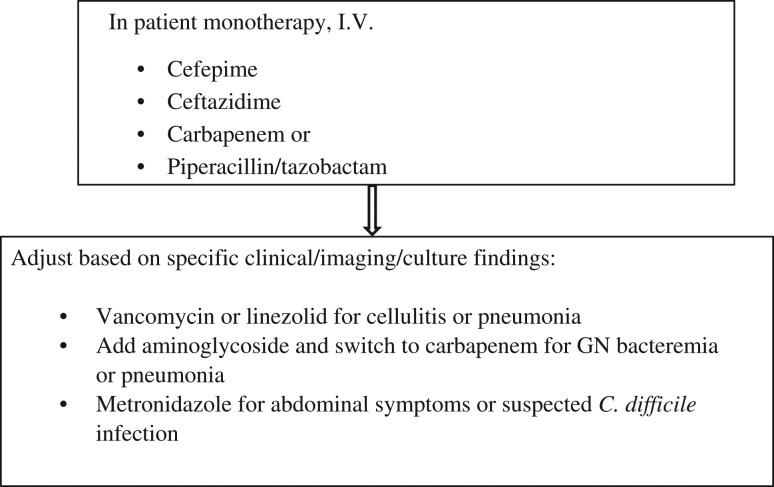
Those with profound neutropenia (i.e. an ANC of < 0.1 × 109/l), major comorbid medical conditions (i.e. hemodynamic instability, severe mucositis, gastrointestinal symptoms, mental status changes, intravenous catheter infections, new pulmonary infiltrates or underlying chronic lung disease), hepatic insufficiency or renal insufficiency require hospitalization for administration of parenteral antibiotics. These ‘high-risk’ patients should be treated with a single intravenous antibiotic (i.e. a fourth-generation cephalosporin such as cefepine or ceftazidime, carbapenem or piperacillin/tazobactam), until the results of cultures, imaging tests and clinical sequelae (persistent fever, change in blood pressure and pulse, and evolving physical signs and symptoms suggestive of a source of infection) are known (see Fig. 4). Adjustment of the antibiotic regimen is dictated by specific clinical, imaging and culture findings, and includes the addition of: (i) vancomycin for cellulitis or pneumonia, (ii) an aminoglycoside for gram-negative bacteremia or pneumonia, and (iii) metronidazole for abdominal signs and symptoms [46].
Fig. 4.
Antibiotics recommended for high-risk patients, based upon Freifield AG et al. (Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis 2011;52:e56-e93).
Whereas fungal infections typically occur as superinfections in individuals with an intact immune system, they often appear as primary infections in individuals with radiation-induced immunosuppression [48, 49]. Candida species are responsible for most fungus infections in neutropenic patients [45, 46]. They range in toxicity from local overgrowth on mucous membranes to multisystem failure due to widespread metastatic infection of the kidneys, heart, liver, spleen and meninges of the brain. In immunosuppressed hosts, widespread dissemination occurs after Candida gains access to the blood (i.e. candidemia).
Fluconazole prophylaxis should be administered to immunosuppressed patients whenever a fungal infection is suspected by the presence of characteristic clinical signs and symptoms (such as change in taste, oral thrush, difficulty swallowing, odynophagia and retrosternal chest discomfort), or when persistent fever lasting 4–7 days occurs in the face of negative bacterial cultures and imaging studies of the chest and sinuses of the face. Fluconazole lessens the duration of mucosal and invasive Candida infections, and reduces mortality after allogeneic bone marrow transplantation [48]. However, it has no effect on other fungi such as Aspergillus and resistant species of Candida. Voriconazole and Amphotericin B should be considered for patients who are unresponsive to fluconazole. An Infectious Diseases specialist should be consulted whenever disseminated candidiasis develops.
Unlike viral infections in immunocompetent hosts, these infections in patients with IR-induced immunosuppression may be invasive, resulting in sepsis with widespread dissemination and a high mortality rate [49, 52]. Empirical acyclovir or valacyclovir should be considered in all patients with a past medical history of a herpes simplex virus (HSV) infection and in those who are found to be seropositive for HSV (Type I or II) to prevent virus reactivation from its latent state in nerve cell bodies. The development of meningitis, encephalitis, pneumonitis and hepatitis is associated with a high mortality rate. Patients who are seropositive for cytomegalovirus (CMV) should be treated with gangcyclovir or valacyclovir, and should receive only CMV-negative or leukoreduced blood products. An Infectious Diseases specialist should be consulted whenever HSV dissemination or CMV disease (i.e. CMV pneumonia, hepatitis, enteritis or esophagitis) develops.
Illustrative case histories of multiple infections following local accidental irradiation
To illustrate that the above infections do, in fact, occur after high-dose irradiation, I refer to the Tokai-mura, Japan accident in September 1999, in which two fatal cases of profound pancytopenia and subsequent pathologically confirmed, multi-organ failure resulted from local injury to the upper body and whole-body exposure [53, 54]. This criticality accident occurred at the JCO Co. Ltd processing plant, as three workers prepared an excess of uranyl nitrate in a precipitation tank while processing uranium [54]. Worker A held a funnel, while Worker B poured the uranyl nitrate from a bucket into the tank, resulting in a critical reaction with average exposure doses of 17–24 Gy Eq and 8–12 Gy Eq for Workers A and B, respectively. Worker C, who sat at a desk that was separated from the tank room by a wall, received a much lower dose (estimated total dose of ~2–3 Gy) and survived the accident [55]. The workers were transported to the National Radiation Research Center (NIRS), Tokyo, Japan to initiate therapy, and then to the Institute of Medical Sciences, University of Tokyo for further evaluation and clinical management. Table 4 summarizes estimated doses of neutrons and gamma rays, infections and treatment for each of the workers.
Table 4.
Doses, Treatment and Infections in Individuals from Tokai-mura, Japan (1999)
| Worker | Role | Neutrons | γ-Rays | Transplant | Infections |
|---|---|---|---|---|---|
| A | Held Funnel | 5.4 Gy | 8.5 Gy | PB |
|
| B | Poured fuel | 2.9 Gy | 4.5 Gy | CB (GVHD) |
|
| C | Sat at desk | 0.8 Gy | 1.3 Gy | None |
|
Worker A received a peripheral blood stem cell transplantation at the University of Tokyo Hospital from an HLA-identical sibling for Grade 4 HS (RC 4) with a hypoplastic bone marrow. Unfortunately, he developed a rapidly fatal fungal infection of the chest wall, from which Candida albicans was subsequently cultured [54], and expired on Day 82. Autopsy showed extensive erosions of the entire gastrointestinal tract, liver necrosis, and pulmonary hemorrhage with edema and congestion of the lungs [54]. Worker B developed Grade 4 HS (RC 4) that was managed by administration of placental cord blood stem cells, cyclosporine A, antithymocyte globulin, G-CSF, TPO, GM-CSF, erythropoietin, and repeated platelet infusions at the Hospital of the Institute of Medical Sciences. He developed transient reactivation of CMV that was successfully treated with gangcyclovir. The blood counts improved at ~50 days post transplant and then declined, as the patient developed graft-versus-host disease (GVHD). An infection of an allogeneic cadaver skin graft with methicillin-resistant Staphylococcus aureus (MRSA) developed that was unresponsive to therapy with vancomycin and arbekacin. The patient expired with pneumonia on Day 210 [53]. The surviving worker (Worker C) developed Grade 2 HS (RC 2) that was managed at the NIRS with prophylaxis for fungal and viral infections, G-CSF and reverse isolation [55]. At ~1 month after exposure, he developed conjunctivitis and gingivitis that resolved with good oral and eye hygiene. At ~2 months, he developed retinal hemorrhages, and at 33 months, cataracts were documented in the posterior subcapsular area. During the course of evaluation, diabetes mellitus and subclinical hypothyroidism were also documented [55].
In another radiation accident, numerous infectious complications developed in a welder in Yanango, Peru, who in February 1999 picked up an 192Ir source (activity of 36.75 Ci) that had been dislodged from its container. The worker placed the source into his pants pocket for 6–7 h [28]. He sustained a skin dose of 9966 Gy at 1 cm depth that resulted in a blister with erythema over his right posterior thigh on Day 2 after exposure (see Fig. 5). Grade 4 HS (RC 4) developed during Days 5–38 after exposure, with nadirs of 0.3 × 109/l and 1.44 × 109/l for the absolute lymphocyte and neutrophil counts, respectively. The HS status fluctuated between Grades 2 and 4 based on the absolute lymphocyte count, until transfer to Paris, France on Day 91 after exposure.
Fig. 5.
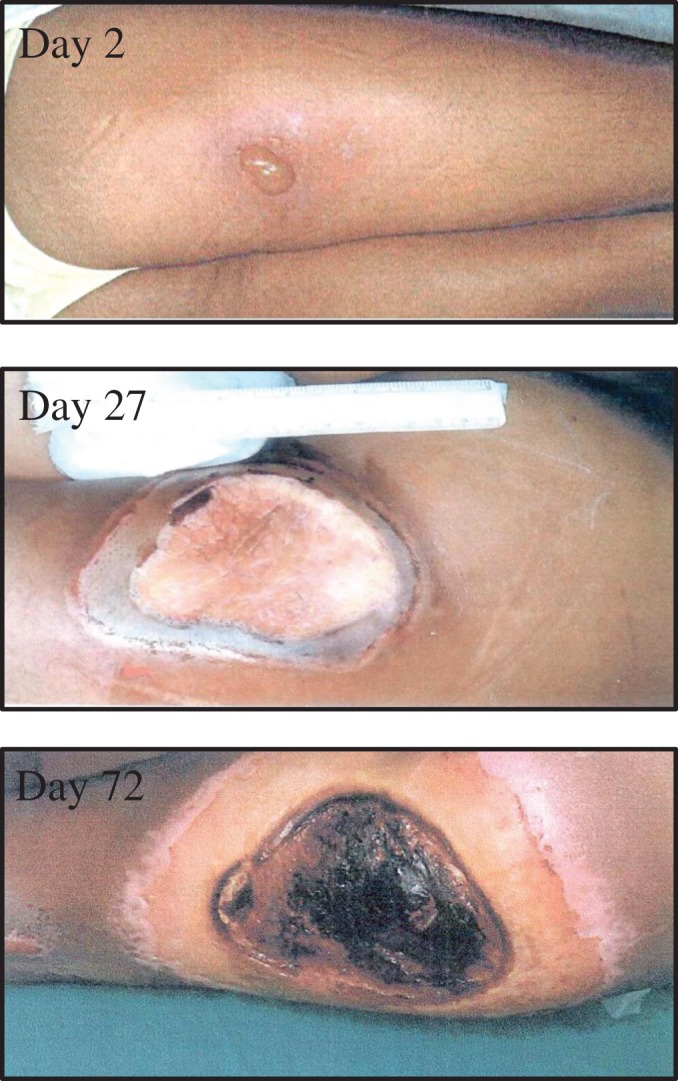
Evolution of skin lesion in welder from Yanango, Peru Accident (1999). The individual developed a blister with erythema (Day 2), which evolved into an ulcer of the right thigh, which in turn became infected with an exudative response (Day 27). Purulent necrosis developed (Day 72), requiring surgical management, including amputation of the right lower extremity.
The skin lesion evolved into a superinfected ulcer that became covered with a fibrin crust on Day 27 that in turn, enlarged and became necrotic by Day 72 (see Fig. 5). The patient was transferred to the Burn Treatment Center at the Percy Military Hospital, Paris, France on Day 97, where he developed new infections and required radical surgery that included amputation of the right lower extremity [28]. The culture results from the evolving wound, together with the development of infections elsewhere in the body, and the prescribed antimicrobial therapy are summarized in Table 5.
Table 5.
Summary of infections and their treatment in individual from Yanango, Peru (1999)
| Day | Site | Organism | Treatment | |
|---|---|---|---|---|
| Admitted to Natl. Cancer Hospital, Lima, Peru→ | 1 | - | Prophylaxis: Ciprofloxacin | |
| 2 | Blister | +Clindamycin | ||
| 14 | Genital herpes | Acyclovir, Zinnat | ||
| 38 | Skin ulcer | Klebsiella, Streptococcus | Clindamycin, Cefoterazone | |
| 43 | Gastric ulcer | Cytomegalovirus | Ulceran | |
| 45 | Ulcer | Streptococcus | Vancomycin | |
| 57 | Ulcer (necrotic) | Enterobacter, Staphylococcus aureus | + Amikacin | |
| 65 | Abscess drained in OR | Escherica coli | Same | |
| 72 | Necrotic ulcer (2nd lesion) | Klebsiella, Streptococcus | Same + Hyberbaric Oxygen | |
| Transferred to Percy Medical Hospital, France→ | 101 | Necrotic tissue in OR | Methicillin-Resistant Staphylococcal aureus, Escherichia coli, Enterobacter, Pseudomonas aruginosa | Imipenam |
| 121 | Necrotic ulcer (2nd lesion) | Candida albicans | Topical Sulphonamide |
The patient received prophylaxis with Ciprofloxacin on admission (Day 1) to the National Cancer Hospital, Lima, Peru. Clindamycin was added on Day 2, when local erythema and a blister developed. On Day 14, the patient developed genital lesions that were treated with Acyclovir. Cultures from the ulcer on the thigh were positive at various times for Kebsiella, Streptococcus, Staphylococcus aureus, Escherichia coli, MRSA, Pseudomonas aruginosa and Candida albicans. The infections were treated with Clindamycin, vancomycin, imipenem and topical sulphonamide [28].
On Day 176, the patient was found to have tracks of purulent necrosis that extended toward the right hip. The internal rectus and major and minor gluteus muscles were severed and the hip was disarticulated. An iliac colostomy was performed to avoid future contamination of the area. The patient developed extensive radionecrosis of the surgical site that extended to the perineum. He was transferred back to Peru where he continued to have infections that responded to parenteral antibiotics. The aggressive use of antimicrobial agents and meticulous local care to avoid infections resulted in a good outcome, as the patient fully recovered and is alive without active infection at this time.
SUMMARY
The management of health consequences from a high-casualty radiological incident requires that hospitals and the hospital networks apply their resources to integrative activities within the emergency response. Clinical management of patients having one or more subsyndromes of ARS is guided by recommendations from subject matter experts who, at a WHO Consultancy, conducted an evidence-based review of the exposed cases published in the literature. The recommendations of this expert group do not change as a result of an analysis of the cases that have been published since the consultants met in Geneva. Individuals receiving a radiation dose that causes immunosuppression are at an increased risk of developing infections, some of which may be widespread and life threatening. Individuals exposed to radiation may be divided into ‘low-risk’ and ‘high-risk’ susceptibility groups, based upon degree of neutropenia and the presence of comorbidities. Recommendations are provided for the administration of antimicrobial agents for the management of bacterial, fungal and viral infections that have complicated the clinical course of individuals who have been accidently exposed to ionizing radiation. These recommendations from REAC/TS have been adapted from the clinical practice guidelines that were developed by the IDSA for neutropenic patients with cancer, and take into account projected duration of immunosuppression based upon exposure dose. Illustrative cases from accidents in Tokai-mura, Japan and Yanango, Peru are summarized from an infectious diseases perspective, wherein multiple infections requiring aggressive use of antimicrobials was essential to prolonged survival.
ACKNOWLEDGEMENTS
The subject material of this manuscript was presented at The 1st International Symposium of the Network-type Joint Usage/Research Center for Radiation Disaster Medical Science—Scientific Underpinning for Restoration from a Radiation Disaster held 22 February 2017 at Hiroshima University, Hiroshima, Japan.
CONFLICT OF INTEREST
There are no potential conflicts of interest regarding any aspect of the content of this manuscript.
REFERENCES
- 1. Waselenko JK, MacVittie TJ, Blakeley WF et al. Medical management of the acute radiation syndrome: recommendations of the Strategic National Stockpile Radiation Working Group. Ann Intern Med 2004;140:1037–51. [DOI] [PubMed] [Google Scholar]
- 2. Coleman CN, Weinstock DM, Casagrande R et al. Triage and treatment tools for use in a scarce resources-crisis standards of care setting after a nuclear detonation. Disaster Med Public Health Prep 2011;5:S111–21. [DOI] [PubMed] [Google Scholar]
- 3. Albanese J, Skudlarska B, Smith D et al. Impact of mass casualties resulting from radiation exposure on healthcare systems In: Nriagu JO. (Ed). Encyclopedia Environmental Health, Vol. 3 New York: Elsevier, 2011, 165–77. [Google Scholar]
- 4.Rad Resilient City. A local planning tool to save lives following a nuclear detonation. Center for Biosecurity of UPMC, Baltimore, Maryland, 2011, www.radresilientcity.org (July 20 2017, date last accessed).
- 5. White DB, Katz MH, Luce JM et al. Who should receive life support during a public health emergency? Using ethical principles to improve allocation decisions. Ann Intern Med 2009;150:132–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dainiak N, Gent RN, Carr Z et al. Literature review and global consensus on management of acute radiation syndrome affecting nonhematopoietic organ systems. Disaster Med Public Health Prep 2011;5:183–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dainiak N, Gent RN, Carr Z et al. First global consensus for evidence-based management of the hematopoietic syndrome resulting from exposure to ionizing radiation. Disaster Med Public Health Prep 2011;5:202–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gorin N-C, Fliedner TM, Gourmelon P et al. Consensus conference on European preparedness for haematological and other medical management of mass radiation accidents. Ann Hematol 2006; 85: 671–9. [DOI] [PubMed] [Google Scholar]
- 9. Dainiak N. Rationale and recommendations for treatment of radiation injury with cytokines. Health Phys 2010;98:838–42. [DOI] [PubMed] [Google Scholar]
- 10.U.S. Food & Drug Administration. Guidances (Drugs).https://www.fda.gov/emergencypreparedness/counterterrorism/.../ucm443245.htm (15 August, 2017, date last accessed).
- 11.U.S. Food & Drug Administration. https://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/default.htm (30 November 2017, date last accessed).
- 12. Farese AM, Cohen MV, Katz BP et al. Filgrastim improves survival in lethally irradiated nonhuman primates. Radiat Res 2013;179:89–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hankey KG, Farese AM, Blaauw EC et al. Pegfilgrastim improves survival of lethally irradiated nonhuman primates. Radiat Res 2015;183:643–55. [DOI] [PubMed] [Google Scholar]
- 14. Smith TJ, Bohlke K, Armitage JO. Recommendations for the use of white blood cell growth factors: American Society of Clinical Oncology practice guideline update. J Oncol Pract 2015;11:511–4. [DOI] [PubMed] [Google Scholar]
- 15. Zaja F, Barcellini W, Cantoni S et al. Thrombopoietin receptor agonists for preparing adult patients with immune thrombocytopenia to splenectomy: results of a retrospective, observational GIMEMA study. Am J Hematol 2016;91:E293–5. [DOI] [PubMed] [Google Scholar]
- 16. Townsley DM, Scheinberg P, Winkler T et al. Eltrombopag added to standard immunosuppression for aplastic anemia. N Engl J Med 2017;376:1540–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen Y, Nowak I, Tsai Y et al. Eltrombopag, an alternative to thrombopoietin for the treatment of acute radiation bone marrow injury. In: Global Conference on Radiation Topics, ConRad 2017. Final Program, p. 69a.
- 18. Densow D, Kindler H, Baranov AE et al. Criteria for selection of radiation accident victims for stem cell transplantation. Stem Cells 1997;15:287–97. [DOI] [PubMed] [Google Scholar]
- 19. Dainiak N, Ricks RC The evolving role of haematopoietic cell transplantation in radiation injury: potentials and limitations. In: Fliedner TM, Meineke V, Akashi M et al. (eds). Advanced Radiation Workshop on radiation-induced multi-organ involvement and failure: a challenge for pathogenetic, diagnostic and therapeutic approaches and research. Brit J Radiol 2005;(Suppl.) 27:169–74.
- 20. Baranov A, Gale RP, Guskova A et al. Bone marrow transplantation after the Chernobyl nuclear accident. N Engl J Med 1989;321:205–12. [DOI] [PubMed] [Google Scholar]
- 21. Gourmelon P, Benderitter M, Bertho JM et al. European consensus on the medical management of acute radiation syndrome and analysis of the radiation accidents in Belgium and Senegal. Health Phys 2010;98:825–32. [DOI] [PubMed] [Google Scholar]
- 22. Guo M, Dong Z, Qiao J et al. Severe acute radiation syndrome: treatment of a lethally Co-60-source irradiated accident victim in China with HLA-mismatched peripheral blood stem cell transplantation and mesenchymal stem cells. J Radiat Res 2014;55:205–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Johnston WR. Fleurus Irradiator Accident, 2006 http://www.johnstonesarchive.net/nuclear/radevents/2006BELG1.html (20 July 2017, date last accessed).
- 24. Fliedner TM, Friesecke I, Beyrer K. Medical Management of Radiation Accidents: Manual on Acute Radiation Syndrome. Oxford, UK: British Institute of Radiobiology, 2001. [Google Scholar]
- 25. International Atomic Energy Agency The Radiological Accident in San Salvador Vienna: IAEA, 1990. [Google Scholar]
- 26. International Atomic Energy Agency The Radiological Accident in Soreq Vienna: IAEA, 1993. [Google Scholar]
- 27. International Atomic Energy Agency The Radiological Accident at the Irradiation Facility in Nesvizh Vienna: IAEA, 1996. [Google Scholar]
- 28. International Atomic Energy Agency The Radiological Accident in Yanango Vienna: IAEA, 2000. [Google Scholar]
- 29. International Atomic Energy Agency The Radiological Accident in Samut Prakarn Vienna: IAEA, 2002. [Google Scholar]
- 30. Naggar AM, Mahmoud MH. The Radiological Accident at Meet Halfa, Qaloubiya Cairo, Egypt: Egyptian Atomic Energy Authority, 2000. [Google Scholar]
- 31. International Atomic Energy Agency The Radiological Accident in Nueva Aldea Vienna: IAEA, 2009. [Google Scholar]
- 32. Fliedner TM, Dorr HD, Meinke V Multi-organ involvement as a pathogenetic principle of the radiation syndromes: a study involving 110 case histories documented in SEARCH and classified as the bases of haematopoietic indicators of effect. In: Fliedner TM, Meineke, Akashi M et al. (eds). Advanced Radiation Workshop on radiation-induced multi-organ involvement and failure: a challenge for pathogenetic, diagnostic and therapeutic approaches and research. Brit J Radiol Suppl 2005;(Suppl.) 27:1–8.
- 33. Akashi M. Role of infection and bleeding in multiple organ involvement and failure. Br J Radiol 2005;27:69–74. [Google Scholar]
- 34. Brook I, Walker RI, MacVittie TJ. Effect of antimicrobial therapy on the bowel flora and bacterial infection in irradiated mice. Int J Radiat Biol Relat Stud Phys Chem Med 1988;53:709–16. [DOI] [PubMed] [Google Scholar]
- 35. Kumar KS, Srinivasan V, Toles RE et al. High-dose antibiotic therapy is superior to a 3-drug combination of prostanoids and lipid A derivative in protecting irradiated canines. J Radiat Res 2002;43:361–70. [DOI] [PubMed] [Google Scholar]
- 36. Dainiak N, Waselenko JK, Armitage JO et al. The hematologist and radiation casualties. Hematology Am Soc Hematol Educ Program 2003:473–96. [DOI] [PubMed] [Google Scholar]
- 37. Hauer-Jensen M, Wang J, Boerma M et al. Radiation damage to the gastrointestinal tract: mechanisms, diagnosis and management. Curr Opin Support Palliat Care 2007;1:23–9. [DOI] [PubMed] [Google Scholar]
- 38. Schaue D, McBride WH. T lymphocytes and normal tissue responses to radiation. Front Oncol 2012;2:119–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wirsdorfer F, Jendrossek V. The role of lymphocytes in radiotherapy-induced adverse late effects in the lung. Front Immunol 2016;7:591–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Goans RE, Wald Radiation accidents with multi-organ failure—selected historical experience in the United States. In: Fliedner TM, Meineke V, Akashi M et al. (eds). Advanced Radiation Workshop on radiation-induced multi-organ involvement and failure: a challenge for pathogenetic, diagnostic and therapeutic approaches and research. Brit J Radiol 2005;(Suppl.) 27:41–6.
- 41. Gourmelon P, Marquette C, Agay D et al. Involvement of the central nervous system in radiation-induced multi-organ dysfunction and/or failure. In: Fliedner TM, Meineke V, Akashi M et al. (eds), Advanced Radiation Workshop on radiation-induced multi-organ failure involvement and failure: a challenge for pathogenetic, diagnostic and therapeutic approaches and research. Brit J Radiol 2005:(Suppl.) 27:62–8.
- 42. Dainiak N. Biology and Clinical Features of Radiation Injury in Adults. UpToDate.www.uptodate.com (1 August 2017, date last accessed).
- 43. Wingard JR, Dainiak N. Treatment of Radiation Injury in the Adult. UpToDate.www.uptodate.com (1 August 2017, date last accessed).
- 44. Gafter-Gvili A, Fraser A, Paul M et al. Meta-analysis: antibiotic prophylaxis reduces mortality in neutropenic patients. Ann Intern Med 2005;142:979–95. [DOI] [PubMed] [Google Scholar]
- 45. Hughes WT, Armstrong D, Body GP et al. 2002 guidelines for the use of antimicrobial agents in neutropenic patients with cancer. Clin Infect Dis 2002;34:730–51. [DOI] [PubMed] [Google Scholar]
- 46. Freifeld AG, Bow EJ, Sepkowitz KA et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis 2011;52:e56–93. [DOI] [PubMed] [Google Scholar]
- 47. Dainiak N. Clinical management of infections after exposure to ionizing radiation. REAC/TS Courses in Medical Management of Radiation Emergencies, REAC/TS, US Department of Energy/National Nuclear Security Administration, 2008.
- 48. Slavin MA, Osborne B, Adams R et al. Efficacy and safety of flucanozole prophylaxis for fungal infections after marrow transplantation—a prospective, randomized double-blind study. J Infect Dis 1995;171:1545–52. [DOI] [PubMed] [Google Scholar]
- 49. Asano S. Multi-organ involvement: lessons from the experience of one victim of the Tokai-mura criticality accident. In: Fliedner TM, Meineke V, Akashi M et al. (eds). Advanced Radiation Workshop on radiation-induced multi-organ involvement and failure: a challenge for pathogenetic, diagnostic and therapeutic approaches and research. Brit J Radiol 2005;(Suppl.) 27:9–12.
- 50. Goans RE, Holloway EC, Berger ME et al. Early dose assessment following severe radiation accidents. Health Phys 1996;72:513–8. [DOI] [PubMed] [Google Scholar]
- 51. Goans RE, Holloway RE, Berger ME et al. Early dose assessment in criticality accidents. Health Phys 2001;81:446–9. [DOI] [PubMed] [Google Scholar]
- 52. International Atomic Energy Agency The Radiological Accident in Lia, Georgia Vienna: IAEA, 2014. [Google Scholar]
- 53. Asano S. Multi-organ involvement: lessons from the experience of one victim of the Tokai-mura criticality accident. Br J Radiol 2005;27:9–12. [Google Scholar]
- 54. Uozaki H, Fukayama M, Nakagawa K et al. The pathology of multi-organ involvement: two autopsy cases from the Tokai-mura criticality accident. Br J Radiol 2005;27:13–6. [Google Scholar]
- 55. Hirama T, Akashi M. Multi-organ involvement in the patient who survived the Tokai-mura criticality accident. Br J Radiol 2005;27:17–20. [Google Scholar]