Abstract
Neurosteroids are powerful modulators of γ-aminobutyric acid (GABA)-A receptors. Ganaxolone (3α-hydroxy-3β-methyl-5α-pregnan-20-one, GX) and synthetic analogs of the neurosteroid allopregnanolone (AP) are designed to treat epilepsy and related conditions. However, their precise mechanism of action in native neurons remains unclear. Here, we sought to determine the mode of action of GX and its analogs at GABA-A receptors in native hippocampal neurons by analyzing extrasynaptic receptor-mediated tonic currents and synaptic receptor-mediated phasic currents. Concentration-response profiles of GX were determined in two cell types: δ-containing dentate gyrus granule cells (DGGCs) and γ2-containing CA1 pyramidal cells (CA1PCs). GX produced significantly greater potentiation of the GABA-A receptor-activated chloride currents in DGGCs (500%) than CA1PCs (200%). In the absence of GABA, GX evoked 2-fold greater inward currents in DGGCs than CA1PCs, which were 2-fold greater than AP within DGGCs. In hippocampus slices, GX potentiated and directly activated tonic currents in DGGCs. These responses were significantly diminished in DGGCs from δ-subunit knockout (δKO) mice, confirming GX’s selectivity for δGABA-A receptors. Like AP, GX potentiation of tonic currents was prevented by protein kinase C inhibition. Furthermore, GX’s protection against hippocampus-kindled seizures was significantly diminished in δKO mice. GX analogs exhibited greater potency and efficacy than GX on δGABA-A receptor-mediated tonic inhibition. In summary, these results provide strong evidence that GX and its analogs are preferential allosteric modulators and direct activators of extrasynaptic δGABA-A receptors regulating network inhibition and seizures in the dentate gyrus. Therefore, these findings provide a mechanistic rationale for the clinical use of synthetic neurosteroids in epilepsy and seizure disorders.
Introduction
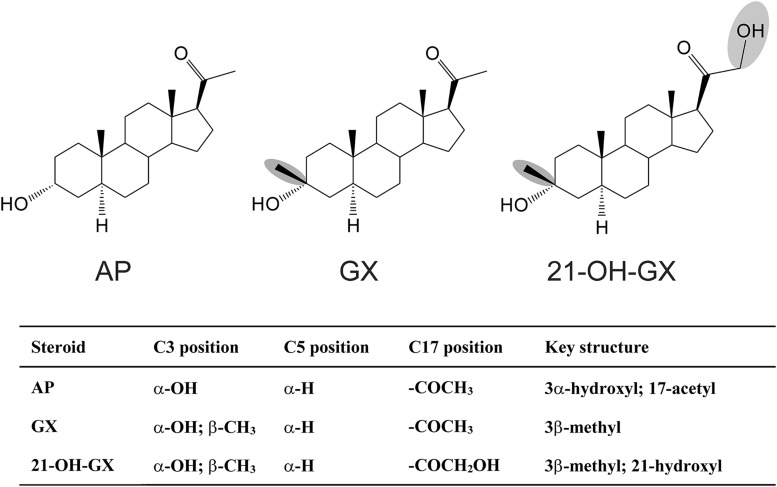
The γ-aminobutyric acid (GABA)-A receptors regulate fast inhibitory transmission in the brain. These chloride channels are composed of a pentamer of subunits, of which there are 19 subtypes (α1–6, β1–3, γ1–3, δ, ε, θ, π, ρ1–3). There are two classes of GABA-A receptors in the hippocampus, categorized on the basis of their localization (Chuang and Reddy, 2018). Synaptic receptors (γ-containing) are responsible for phasic inhibition via synaptic neurotransmission, whereas extrasynaptic receptors (α4/δ-containing in dentate gyrus, α5/γ2-containing in CA1) mediate tonic inhibition through high affinity and low efficacy binding of ambient GABA. Endogenous neurosteroids are potent allosteric agonists of GABA-A receptors. Allopregnanolone (3α-hydroxy-5α-pregnan-20-one, AP) is an endogenous neurosteroid with potent antiseizure effects mediated by GABA-A receptors (Fig.1) (Reddy et al., 2004; Carver et al., 2016; Clossen and Reddy, 2017a). Neurosteroids have a greater sensitivity for δGABA-A receptors, which are highly expressed in the dentate gyrus (Brown et al., 2002; Bianchi and Macdonald, 2003; Carver and Reddy, 2013; Reddy, 2018). Natural neurosteroids, such as AP (also known as brexanolone), have several limitations for therapeutic use, including low bioavailability, ultra-short t1/2, and hormonal side effects owing to their steroid metabolites (Rupprecht et al., 1993). Therefore, synthetic analogs have been prepared to surpass these limitations (Reddy and Estes, 2016). Ganaxolone (3α-hydroxy-3β-methyl-5α-pregnan-20-one, GX) is the 3β-methylated analog of AP, first characterized in 1997 with recombinant α1β1γ2L receptors expressed in Xenopus oocytes (Carter et al., 1997). GX was shown to have comparable modulatory activity to that of AP (Carver and Reddy, 2016). The synthetic 3β-substitution provides a more favorable pharmacokinetic profile as an anticonvulsant drug, overcoming the limitations of natural neurosteroids by preventing the oxidation of the 3α-hydroxyl group (Carter et al., 1997). GX has broad-spectrum anticonvulsant activity in animal seizure models, and is currently being evaluated in clinical trials for the treatment of epilepsy including partial-onset seizures, infantile spasms, catamenial epilepsy, genetic seizure conditions, and chemical neurotoxicity (Kerrigan et al., 2000; Laxer et al., 2000; Nohria and Giller, 2007; Pieribone et al., 2007; Reddy and Rogawski, 2012; Bialer et al., 2015; Braat et al., 2015; Reddy, 2016a; Clossen and Reddy, 2017b; Sperling et al., 2017; Younus and Reddy, 2018). Surprisingly, there has been limited published investigation of the actual mechanism of action of GX in the brain.
Fig. 1.
Chemical structures of the neurosteroid AP and its 3β-methyl analogs. (A) Allopregnanolone (AP). (B) Ganaxolone (GX). (C) 21-OH-GX.
Phosphorylation status of GABA-A receptors influences surface expression, chloride conductance, and sensitivity to neurosteroids. Several GABA-A receptor subunits contain residues that can be phosphorylated by protein kinases (Moss and Smart, 1996; Brandon et al., 2000; Chuang and Reddy, 2018). Specific β2/3-subunit isoforms play a role in neuronal excitability (Reddy et al., 2018). Conserved serine residues (Ser-409 or Ser-410) of the β-subunits are phosphorylated by protein kinase C (PKC), protein kinase A (PKA), Ca2+/calmodulin-dependent protein kinase II (CaMKII), and cGMP-dependent protein kinase. Two additional serine residues of the β-subunits (Ser-408 and Ser-383) are phosphorylated by PKA and CaMKII, respectively (McDonald et al., 1998; Saliba et al., 2012). Additionally, the serine residues (Ser-443) within the intracellular domain of the α4-subunit, and the Ser-408 and Ser-409 in β3-subunits are phosphorylated by PKC (Leidenheimer and Chapell, 1997; Fancsik et al., 2000; Harney et al., 2003; Abramian et al., 2010, 2014; Adams et al., 2015). However, the extent of functional impact of PKC activity on the neurosteroid potentiation of extrasynaptic GABA-A receptor-mediated tonic inhibition remains unclear.
In this study, we demonstrate that GX and its analogs are preferential positive allosteric modulators of extrasynaptic GABA-A receptors in δ-subunit-rich native dentate gyrus granule cells (DGGCs) that regulate tonic inhibition and seizure protection. Since the expression of δ-subunit is higher in DGGCs than in CA1 pyramidal neurons, we used DG neurons for detailed characterization of GX and its analogs on whole-cell and extrasynaptic tonic currents. Our results on the phosphorylation-dependent enhancement of tonic inhibition by GX provide a molecular mechanistic rationale for potential clinical use of synthetic neurosteroids in seizure disorders.
Materials and Methods
Animals.
Two- to three-month-old, male C57BL/6 mice were used in the study. Experiments were conducted in wild-type (WT) and GABA-A receptor δ-subunit knockout (Gabrd−/−, δKO) mice (Mihalek et al., 1999; Carver and Reddy, 2016). Four mice were housed per cage under standard laboratory conditions with a 12-hour light/dark cycle. The animals were cared for in compliance with the guidelines in the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Animal procedures were conducted in compliance with a protocol approved by the Texas A&M Institutional Animal Care and Use Committee.
Hippocampal Slice Preparation.
Patch-clamp electrophysiological studies were conducted in acutely dissociated neurons and slices using established methods as described previously (Reddy and Jian, 2010; Carver and Reddy, 2016). An adult male mouse was anesthetized with isoflurane and the brain was rapidly removed and placed in ice-cold artificial cerebrospinal fluid (aCSF) buffer. Transverse slices (300 μm thickness) of the hippocampus were obtained with a Vibratome in 3.5°C aCSF (model 1500 with 900 Refrigeration System; Leica Microsystems, Inc., Bannockburn, IL). The aCSF buffer for slice cutting was composed of (in millimolars): 0.3 kynurenic acid (Tocris Bioscience, Bristol, UK), 126 NaCl, 3 KCl, 0.5 CaCl2, 5 MgCl2, 26 NaHCO3, 1.25 NaH2PO4, 11 glucose (pH adjusted to 7.35–7.40 with 95% O2–5% CO2, 305–315 mOsm/kg).
Whole-Cell GABA-Gated Currents in Dissociation of Neurons.
Hippocampus CA1 and DG neurons were acutely dissociated by the standard enzymatic technique as described previously (Kay and Wong, 1986; Reddy and Jian, 2010). The hippocampal CA1 or DG region was microdissected under a microscope (model SMZ 647; Nikon, Tokyo, Japan) and incubated in aCSF for 1 hour at 28°C. The isolated CA1 and DG slices were transferred into an enzymatic solution containing aCSF with protease XXIII (3 mg/ml; MilliporeSigma, St. Louis, MO). Next, the slices were incubated for precisely 23–25 minutes at 28°C, rinsed twice with aCSF, and gently triturated through three fire-polished Pasteur pipettes to yield single cells. For each batch, slices were triturated six to eight times with each pipette in approximately 1 ml of aCSF. The solution was then allowed to settle for 1 minute, and the suspension of freshly isolated cells was plated onto the recording chamber (Warner Instruments, Hamden, CT). Electrophysiological recordings in dissociated cells were conducted in the whole-cell mode as described previously (Reddy and Jian, 2010). Recordings were acquired with an Axopatch 200B amplifier (Molecular Devices, Sunnyvale, CA) with a holding potential of −70 mV. The current values were normalized to cell capacitance and expressed as current density (pA/pF). A multichannel perfusion system (Automate Scientific, Berkeley, CA) was used for fast perfusion of drug solutions covering the recording neurons. The perfusion pipette was positioned <200 μm away from the cell. A 2-minute wash with bath solution after each drug trial prevented receptor desensitization.
Tonic and Phasic Currents in Hippocampus Slices.
Extrasynaptic GABA-A receptor-mediated tonic currents were recorded by standard patch-clamp electrophysiology per the standard protocols described previously (Wu et al., 2013; Carver et al., 2014; Carver and Reddy, 2016). Hippocampal neurons were identified and imaged with an Olympus BX51 microscope with a 40× water-immersion objective, infrared-differential interference contrast optics, and video camera. Electrophysiological recordings in hippocampal slices were conducted at room temperature (22–24°C) in the whole-cell mode with a holding potential of −65 mV. Currents were acquired with an Axopatch 200B amplifier (Molecular Devices, San Jose, CA). The membrane capacitance and series and input resistance of the recordings were monitored by applying a 5-mV (100-milliseconds) depolarizing voltage. Signals were low-pass filtered at 2 kHz and digitized at 10 kHz with Digidata 1440A system (Molecular Devices). Currents from each cell were normalized to membrane capacitance as current density (pA/pF). The miniature inhibitory postsynaptic currents (mIPSCs) were recorded for at least 2 minutes for each drug response and condition. The amplitude and decay time constants of mIPSCs were determined using MiniAnalysis software (Synaptosoft, Decatur, GA). Nonoverlapping events with single peaks were used to generate an ensemble average mIPSC. A mean weighted decay time constant was calculated from bi-exponential fitting function I(t) = A1 × e(−t/τ1) + A2 × e(−t/τ2) as τw = (A1 × τ1 + A2 × τ2)/(A1 + A2). Four to six mice were used to obtain adequate sample size for recording of tonic currents.
Hippocampus Kindling Seizures.
The mouse kindling model of epilepsy was used for the evaluation of protective effect of GX and its analogs. Surgical procedures and drug testing protocols in the hippocampus kindling model were conducted as described previously (Reddy and Mohan, 2011; Reddy et al., 2015, 2018). Mice were used for drug testing when they exhibited consistent generalized (stage 5) seizures. The electrographic afterdischarge (AD) was acquired using the Grass CP511 preamplifier system (Astro-Med, West Warwick, RI). The behavioral seizures were scored according to the Racine’s scale (Racine, 1972): stage 0 = no response or behavior arrest; stage 1 = chewing or facial twitches; stage 2 = chewing and head nodding; stage 3 = forelimb clonus; stage 4 = bilateral forelimb clonus and rearing; stage 5 = bilateral forelimb clonus/rearing and falling.
Drugs and Reagents.
All chemicals used in electrophysiology experiments were acquired from Sigma-Aldrich unless otherwise specified. Allopregnanolone (3α-hydroxy-5α-pregnan-20-one, AP) and ganaxolone (3α-hydroxy-3β-methyl-5α-pregnan-20-one, GX) were prepared as 2 mM stock solutions in dimethyl sulfoxide for electrophysiology experiments. Stock solutions were diluted in the external perfusion solution to the desired concentration for electrophysiological use. The concentration of dimethyl sulfoxide in final solution was less than 1%. AP was purchased from Steraloids (Newport, RI), and GX and GF 109203X were purchased from Tocris. 21-OH-GX was synthesized in the laboratory. Tetrodotoxin (TTX) was acquired from Calbiochem (Billerica, MA). GX was made in 15% β-cyclodextrin solution for in vivo study. Drugs were given subcutaneously in a volume equivalent to 1% of the body weight of the animals.
Statistical Analysis.
Data were expressed as the mean ± S.E.M. For whole-cell GABA current recordings, fractional potentiation generated by allosteric modulator was expressed as IA/IGABA, where IGABA is the GABA peak current amplitude and IA is the peak current response of the coapplication of GABA and the allosteric drug (0.05–1 μM). GABA at 3 μM produced 10% of the maximal current (EC10), as described previously in dissociated murine CA1 pyramidal cells (CA1PCs) (Reddy and Jian, 2010) and DGGCs (Wu et al., 2013; Carver and Reddy, 2016). The concentration-response relationship was fitted by the nonlinear Hill function to derive the median effective concentration (EC50), which is the concentration of test drug required to generate 50% of maximal efficacy. In slice electrophysiology studies, concentration-response curves were subjected to nonlinear, logistic fitting. A curve fitting was applied for concentration-responses that achieved a plateau at maximal levels. Comparisons of statistical significance of data were made using a Student’s t test. Comparison of the differences in seizure stage between groups was made with the nonparametric Kruskal-Wallis test followed by the Mann-Whitney U test. The differences in means of the AD duration and the percentage inhibition of seizure stage between groups were compared with one-way analysis of variance, followed by Student’s t test and Wilcoxon signed ranks test, respectively. The criterion for statistical difference was P < 0.05.
Results
Allosteric Activation of GABA-Gated Currents by GX in Acutely Dissociated Hippocampal Neurons.
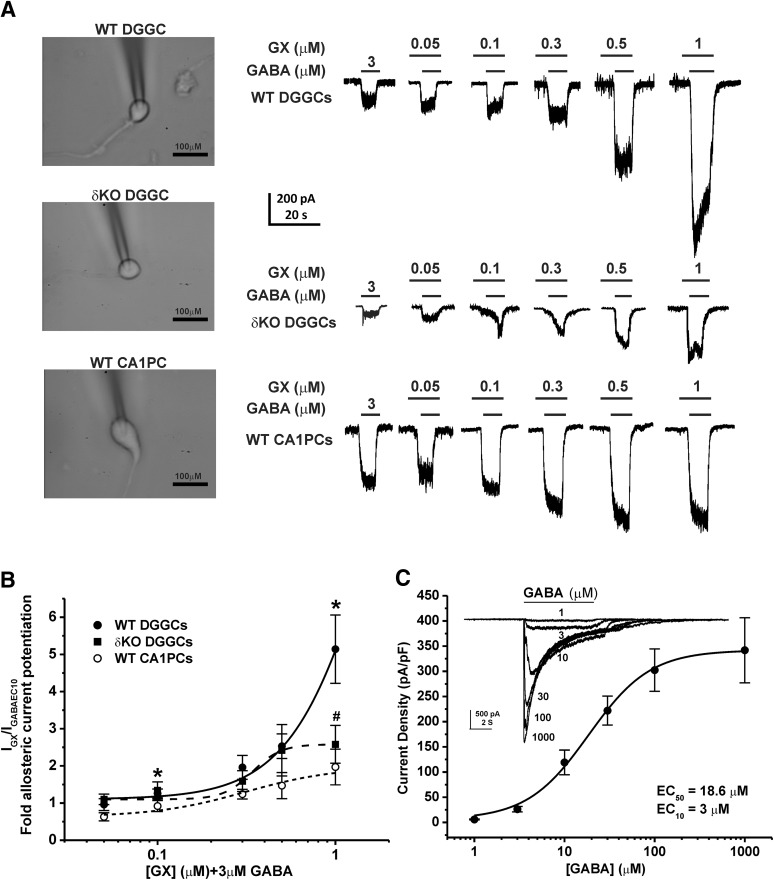
To determine the modulatory effects of GX on whole-cell GABA-gated currents, we studied native neurons using patch-clamp electrophysiology. To examine the effects of subunit composition on GABA-A receptor function, concentration-response profiles of GX were compiled in two cell types: δ-containing dentate gyrus granule cells and γ-containing CA1PCs. GABA-A receptor currents were recorded from acutely dissociated, voltage-clamped DGGCs or CA1PCs from adult male mice in whole-cell mode. We used 3 μM GABA, which was within the range of EC10 response for both DGGCs (Wu et al., 2013; Carver and Reddy, 2016) and CA1PCs (Reddy and Jian, 2010), to determine a baseline response and allosteric activation by tested compounds. We confirmed the EC10 value of GABA in DGGCs (Fig. 2C). Increasing concentrations of GX were coapplied with 3 μM GABA to obtain the fractional potentiation of GABAergic currents mediated by GX. Both cell types responded to GX in a concentration-dependent manner (Fig. 2A). Concentration-response plots were generated for DGGCs and CA1PCs to determine allosteric potentiation by GX (0.05–1 μM) (Fig. 2B). Owing to the lack of a response plateau, a nonlinear curve could not be fit to the data for either DGGCs or CA1PCs. On the basis of previous structure-activity data, 1 μM GX-mediated current was denoted as the constrained maximum efficacy response for allosterically modulated activity. Higher micromolar concentrations of neurosteroids directly activate receptors and exhibit a biphasic modality at a separate neurosteroid binding site (Puia et al., 1990; Carver and Reddy, 2013). As shown in Fig. 2B, GX (0.1 μM) potentiated GABA-gated currents significantly higher in DGGCs than CA1PCs (n = 8–9 cells per group, P = 0.038). GX at 0.3 and 0.5 μM concentrations also showed markedly higher GABA-gated currents in DGGCs than CA1PCs (n = 6–10 cells per group, P = 0.05). In maximal efficacy estimation for allosteric potentiation, GX (1 μM) displayed significantly greater GABA-gated currents in DGGCs (5-fold potentiation) than CA1PCs (2-fold potentiation; n = 7 cells per group, P < 0.05). To confirm the contributory role of the δ-subunit for enhanced allosteric potentiation of GX in DGGCs, we studied GX allosteric activation of GABA-gated currents in DGGCs from δKO mice, which lack δ-containing receptors (Mihalek et al., 1999; Carver and Reddy, 2016). GX-potentiated GABAergic currents (1 μM) were significantly reduced in δKO DGGCs than WT DGGCs (Fig. 2, A and B; n = 5–6 cells per group, P = 0.038). These findings suggest that GX has higher sensitivity at neurons that have a high expression of δ-containing GABA-A receptors, possibly driving the allosteric selectivity.
Fig. 2.
GX allosteric activation of GABA-gated currents in acutely dissociated neurons. GX (1 μM) displayed significantly greater GABA-gated chloride currents in WT DGGCs (5.1 ± 0.9-fold potentiation) than CA1PCs (2.0 ± 0.5-fold potentiation). However, GX-potentiated GABAergic currents were significantly reduced in δKO DGGCs. (A) Representative whole-cell current recordings of DGGCs and CA1PCs. Neurons displayed concentration-dependent responses to GX potentiation of 3 μM GABA (EC10). (B) Concentration-response of GX-modulated allosteric potentiation of chloride currents in DGGCs and CA1PCs from wild-type or δ-subunit knockout (Gabrd−/−, δΚΟ) mice. (C) Concentration-response profile of GABA-potentiated current density. GABA EC10 = 3 μM. Each point represents mean ± S.E.M. of data from 5–10 cells. *P < 0.05 vs. CA1PCs; #P < 0.05 vs. WT DGGCs.
GX Modulation of Inhibition Via GABA-A Receptors.
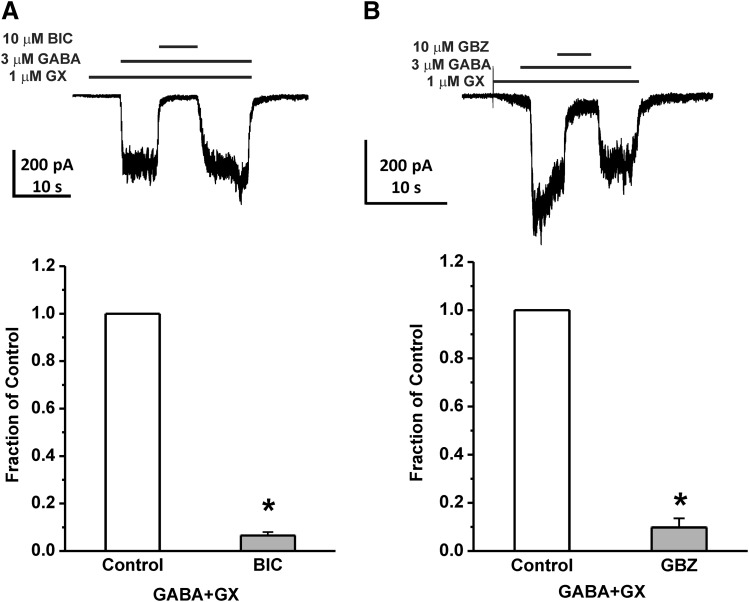
To verify the target specificity of GX inhibitory activity in native DGGCs, we studied the blockade of GABAergic currents with specific GABA-A receptor antagonists. At 10 μM, the competitive antagonists bicuculline or gabazine (GBZ) completely blocked whole-cell GABA-gated current potentiation by GX (n = 4–5 cells per group, P < 0.05; Fig. 3). When the antagonists were removed by washing, the GX-potentiated GABA-gated currents returned to the same level as before the application of antagonists. These results indicate that GX modulation of GABA-gated currents is GABA-A receptor-mediated.
Fig. 3.
GX-activated chloride currents are blocked by GABA-A receptor antagonists. (A) Representative recording and fractional block of currents by bicuculline (BIC, 10 μM) in DGGCs. (B) Representative recording and fractional block of currents by gabazine (GBZ, 10 μM) in DGGCs. Each bar represents mean ± S.E.M. of data from four to five cells. *P < 0.05 vs. control.
Direct Activation of GABA-Gated Currents by GX in Acutely Dissociated Hippocampal Neurons.
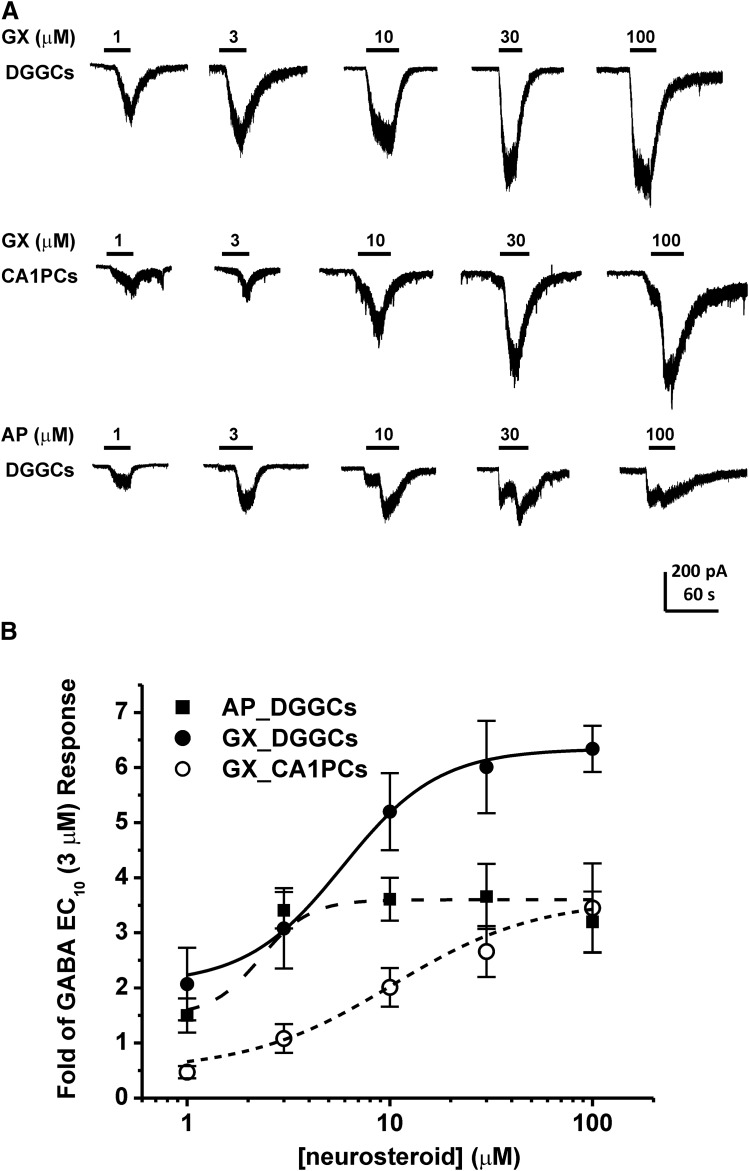
Neurosteroids have been shown to directly activate GABA-A receptor chloride channels at concentrations exceeding 1 μM (Puia et al., 1990; Reddy and Rogawski, 2002). This direct activation may occur in the absence of GABA, but when GABA is present, the potentiating effects of neurosteroids are enhanced. Therefore, we examined direct activation of whole-cell GABAergic currents in acutely dissociated DGGCs and CA1PCs. Cells were voltage-clamped in the same preparation denoted in Fig. 2. Increasing concentrations of GX (1–100 μM, no GABA) were applied by rapid perfusion to the cell until saturation of the inward current response was observed (Fig. 4A). Concentration-response curves were derived for DGGCs and CA1PCs (Fig. 4B). GX evoked significantly greater inward currents in DGGCs than CA1PCs. At each concentration tested, GX potentiated-GABA currents were significantly higher in DGGCs (2.07 ± 0.66-fold of 3 μM GABA response at 1 μM; 3.08 ± 0.37-fold at 3 μM; 5.20 ± 0.70-fold at 10 μM; 6.01 ± 0.84-fold at 30 μM; and 6.34 ± 0. 24-fold at 100 μM) than CA1PCs (0.36 ± 0.09-fold of 3 μM GABA response at 1 μM; 0.83 ± 0.20-fold at 3 μM; 1.55 ± 0.27-fold at 10 μM; 2.05 ± 0.36-fold at 30 μM; and 2.67 ± 0. 62-fold at 100 μM). Such greater currents in response to high GX (1–100 μM) are mostly owing to abundance of δGABA-A receptors in DGGCs. Next, we compared the efficacy and potency of GX with AP for direct activation of GABAergic inward currents (Fig. 4). Table 1 shows the summary data on the efficacy and potency of GX and AP for allosteric and direct-gating effects at GABA-A receptors in DGGCs. In allosteric activation, GX appears to be similar in potency and efficacy to AP. AP at 1 μM caused 4.3-fold potentiation (EF(2-fold GABA) = 474 nM), which is not significantly different from GX-potentiated currents (5.1-fold; EF(2-fold GABA) = 389 nM). In direct-gating effects, GX exhibited 1.5- to 2-fold higher efficacy than AP in DGGCs. Other parameters could not be derived because concentration responses were largely saturated at concentrations ≥10 μM. Overall, GX has greater efficacy than AP for direct activation of inward currents at micromolar levels (Fig. 4B).
Fig. 4.
GX direct activation of GABA-A receptors in acutely dissociated neurons. GX and AP directly activated chloride currents in a concentration-dependent manner in hippocampal neurons. GX evoked significantly greater inward currents in DGGCs than CA1PCs. (A) Representative whole-cell current recordings of GX and AP in DGGCs and GX in CA1PCs. (B) Concentration-response curves for direct activation of inward currents by GX or AP alone in DGGCs and CA1PCs. Each point represents mean ± S.E.M. of data from 4 to 13 cells. *P < 0.05 vs. CA1PCs. Other derivative parameters are listed in Table 1.
TABLE 1.
Comparative efficacy of GX and AP for allosteric and direct-gating effect at GABA-A receptors in DGGCs
| Compound | GABA-Gated Whole-Cell Current |
Tonic Current |
||
|---|---|---|---|---|
| Allosteric Effect: EF(2-fold GABA)a | Direct Effect: E30 pA | |||
| E1 pA | EF(2-fold GABA)a | |||
| nM | μMb | μMc | nM | |
| AP | 474 | 273.5 | 100.6 | 80 |
| GX | 389 | 336.1 | 64.0 | 290 |
| GABAd | — | 1426.3 | 19.6 | — |
EF values represent the effective functional concentration of drug (nanomolars) required to double the 3 μM GABA (EC10) response.
E30 μM values represent the mean tonic current responses of drug at 30 μM concentration.
E1 μM values represent the mean tonic current responses of drug at 1 μM concentration co-applied with 1 μM GABA. GABA 1 μM tonic current response: 0.66 ± 0.22 pA/pF, 19.6 pA.
GABA median effective concentration (EC50): 18.6 μM. EC50 values represent the concentration required to produce half of its own maximal effect.
Allosteric Potentiation of Extrasynaptic δGABA-A Receptor-Mediated Tonic Currents by GX in Hippocampus Slices.
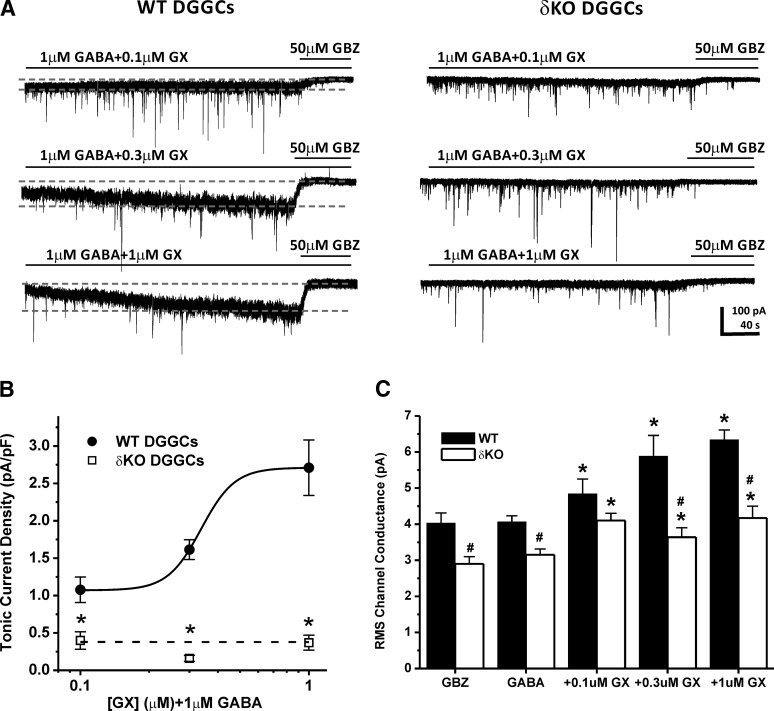
To ascertain the functional role of GX in potentiating extrasynaptic δ-containing GABA-A receptors within the hippocampus, we explored the tonic current levels in WT and δΚΟ DGGCs in slice recordings. We used voltage-clamp electrophysiology to record enhancement of GABAergic tonic currents by GX in the presence of the NMDA receptor antagonist APV (40 μM), the AMPA receptor antagonist DNQX (10 μM), and the sodium channel blocker TTX (0.5 μM). Baseline tonic currents were derived in a bath perfusion with 1 μM GABA. GX (0.1–1 μM) was coapplied with 1 μM GABA in tonic current recordings (Fig. 5, A and B). At the end of each recording, 50 μM GBZ was perfused to determine the total tonic current shift. Tonic current of each cell was normalized to the cell capacitance as a measure of current density (pA/pF). RMS (root mean square) noise was determined for each recording as previously specified (see Materials and Methods) (Fig. 5C). WT DGGCs displayed concentration-dependent sensitivity to GX-mediated enhancement of tonic currents (1.08 ± 0.17 pA/pF at 0.1 μM; 1.61 ± 0.18 pA/pF at 0.3 μM; and 2.48 ± 0.49 pA/pF at 1 μM). However, the δΚΟ DGGCs exhibited completely attenuated tonic current response at all concentrations of GX tested (0.40 ± 0.12 pA/pF at 0.1 μM; 0.16 ± 0.04 pA/pF at 0.3 μM; and 0.37 ± 0.10 pA/pF at 1 μM). WT DGGCs also showed greater concentration-dependent potentiation of GX-mediated RMS conductance than δΚΟ neurons (Fig. 5C). At 0.3 and 1 μM GX, RMS channel conductance was significantly higher in WT DGGCs compared with δΚΟ neurons, indicating greater enhancement in chloride conductance by GX in WT DGGCs. Overall, these results indicate that the δ-subunit plays an obligatory role in GX potentiation of extrasynaptic receptor-mediated tonic inhibition.
Fig. 5.
GX allosteric potentiation of GABA-A receptor-mediated tonic currents and RMS channel conductance were attenuated in DGGCs from δΚΟ mice. (A) Representative whole-cell current recordings of DGGCs from WT or δΚΟ mice. Qualification of tonic current shift (between first and second gray dashed lines) was achieved relative to complete block by gabazine (GBZ) at 50 μM. (B) Concentration-response curves for allosteric activation of normalized tonic current density (pA/pF) by GX in DGGCs from WT or δΚΟ mice. *P < 0.05 vs. WT. (C) IRMS channel conductance (pA) of GX modulation in DGGCs from WT or δΚΟ mice. Each point or bar represents mean ± S.E.M. *P < 0.05 vs. GABA; #P < 0.05 vs. WT (n = 6–16 cells).
Direct Activation of Extrasynaptic δGABA-A Receptor-Mediated Tonic Currents by GX in Hippocampus Slices.
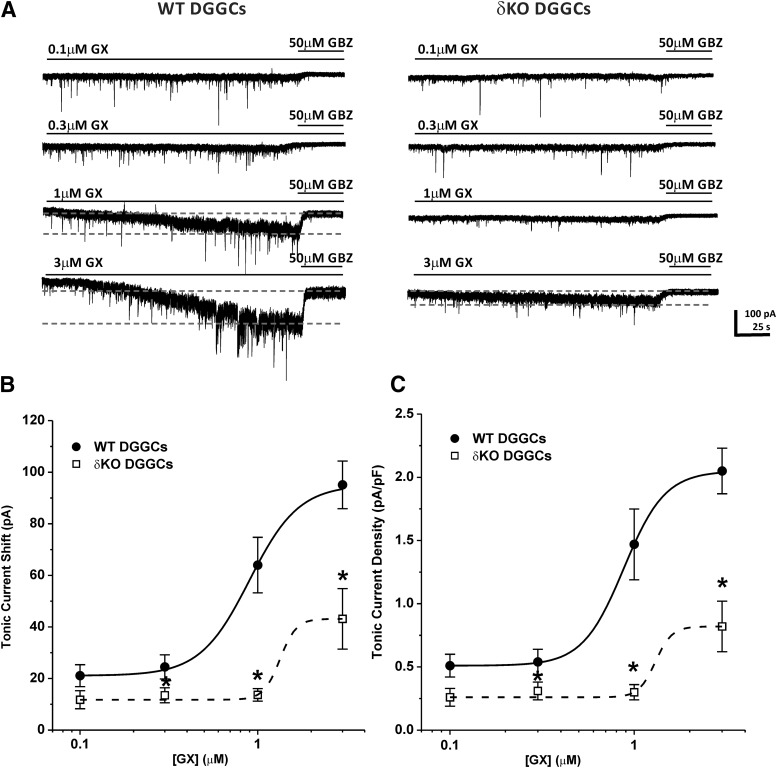
To further explore the functional role of the δ-subunit in GX activation of GABA-A receptors, we examined direct activation of extrasynaptic tonic currents in DGGCs from WT and δΚΟ slices. Voltage-clamp electrophysiology was used to record GABA-A receptor-mediated potentiation of tonic currents by GX in the absence of GABA application. GX (0.1–3 μM) was applied in slice recordings (Fig. 6, A and B). Fifty micromolar GBZ was added at the end of each recording to confirm the total tonic current shift. Current density (pA/pF) was obtained by normalizing the tonic current shift with the cell capacitance of each cell. GX (0.1–3 μM) directly activated GABA-A receptor-mediated tonic currents without exogenous GABA application in WT DGGCs in a concentration-dependent manner (0.51 ± 0.09 pA/pF at 0.1 μM; 0.54 ± 0.10 pA/pF at 0.3 μM; 1.50 ± 0.28 pA/pF at 1 μM; and 2.05 ± 0.18 pA/pF at 3 μM). However, in δΚΟ DGGCs, GX tonic current responses were completely diminished at 0.1, 0.3, and 1 μM (0.26 ± 0.07 pA/pF at 0.1 μM; 0.31 ± 0.07 pA/pF at 0.3 μM; and 0.30 ± 0.06 pA/pF at 1 μM). At 3 μM, GX (0.82 ± 0.20 pA/pF) slightly potentiated tonic current response in δΚΟ DGGCs, possibly eliciting a non-δ-mediated response. These results demonstrate that GX potentiation of tonic currents is highly selective for δ-containing extrasynaptic GABA-A receptors.
Fig. 6.
GX direct activation of GABA-A receptor-mediated tonic currents was attenuated in DGGCs from δΚΟ mice. (A) Representative whole-cell current recordings of DGGCs from WT or δΚΟ mice. Qualification of tonic current shift (between first and second gray dashed lines) was achieved relative to complete block by gabazine (GBZ) at 50 μM. Concentration-response curves for direct activation of tonic currents [pA, (B)] and normalized tonic current density [pA/pF, (C)] by GX in DGGCs from WT or δΚΟ mice. Each point represents mean ± S.E.M. *P < 0.05 vs. WT (n = 5–8 cells).
GX Modulation of Phasic Currents in DGGCs from WT and δΚΟ Mice.
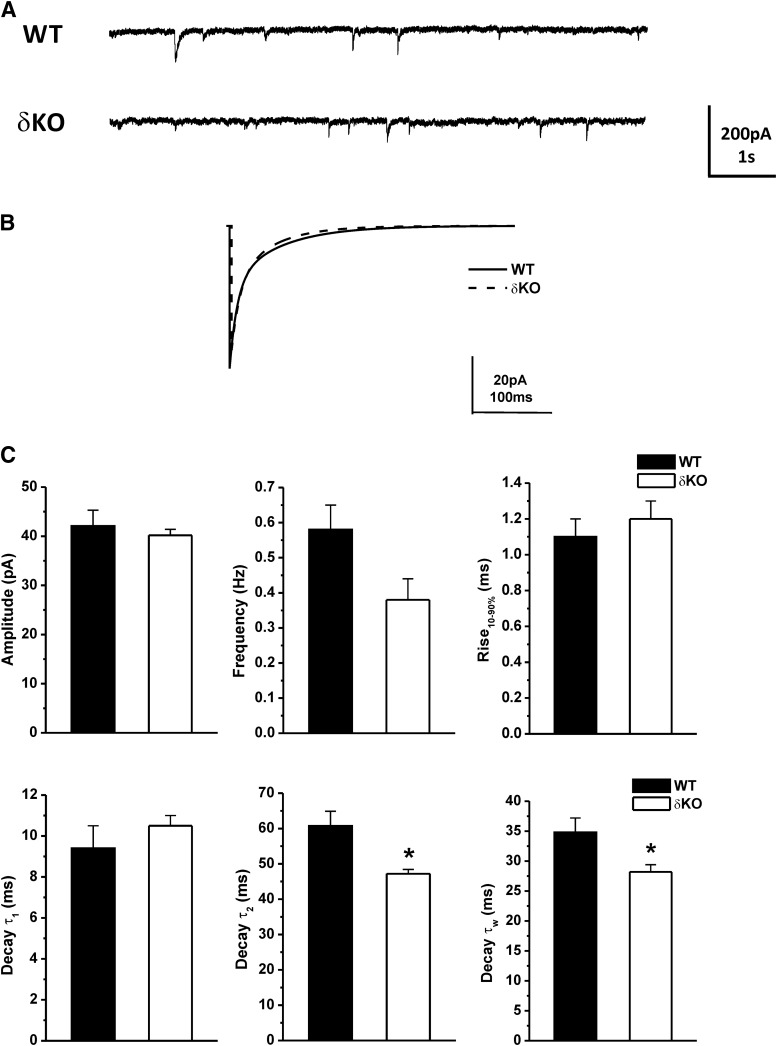
The miniature inhibitory postsynaptic currents (mIPSCs) predominantly reflect the activation of synaptic GABA-A receptors. To determine the effects of GX on synaptic GABA-A receptor-mediated phasic currents, the mIPSCs were isolated in the presence of the NMDA receptor antagonist APV (40 μM), the AMPA receptor antagonist DNQX (10 μM), and the sodium channel blocker TTX (0.5 μM). First, we recorded the endogenous mIPSCs of WT and δΚΟ DGGCs. The average amplitude, frequency, 10%–90% rise time, and decay time-constant of mIPSCs from WT and δΚΟ DGGCs are displayed in Fig. 7. Representative traces and ensemble average mIPSCs from WT and δΚΟ DGGCs are shown in Fig. 7, A and B. The average mIPSC from each cell was best fit with a double-exponential decay curve, depicted as τ1 and τ2. A mean weighted decay constant τw was also derived from τ1 and τ2 (see Materials and Methods). The average amplitude and rise time of mIPSCs were not different between WT and δΚΟ mice (Fig. 7C). However, the mean weighted decay time (τw) of mIPSCs in δΚΟ neurons (n = 10, 28.2 ± 1.2 milliseconds) was significantly faster than WT neurons (n = 9, 34.8 ± 2.4 milliseconds), signifying that changes in receptor subunit composition can alter the channel kinetics. Furthermore, the frequency of mIPSCs in δΚΟ DGGCs was modestly smaller than WT DGGCs (n = 10–12 cells per group, P = 0.06), showing a small reduction in the functional number of GABAergic synapse release sites and the rate of presynaptic release in δΚΟ neurons (Cherubini and Conti, 2001). Overall, these findings are similar and consistent with the results reported previously in δΚΟ mice(Spigelman et al., 2003).
Fig. 7.
Alterations in mIPSCs’ kinetics in δΚΟ mice. (A) Representative traces of endogenous phasic currents by patch-clamp recording from WT or δΚΟ DGGCs. (B) Averaged mIPSC events in the WT (solid line) or δΚΟ (dashed line) DGGCs. (C) The bar graphs represent: amplitude, frequency, rise time (10%–90%), decay τ1, decay τ2, and mean weighted decay (τw) of mIPSCs in DGGCs from WT or δΚΟ mice. Each bar represents mean ± S.E.M. *P < 0.05 vs. WT (n = 9–10 cells).
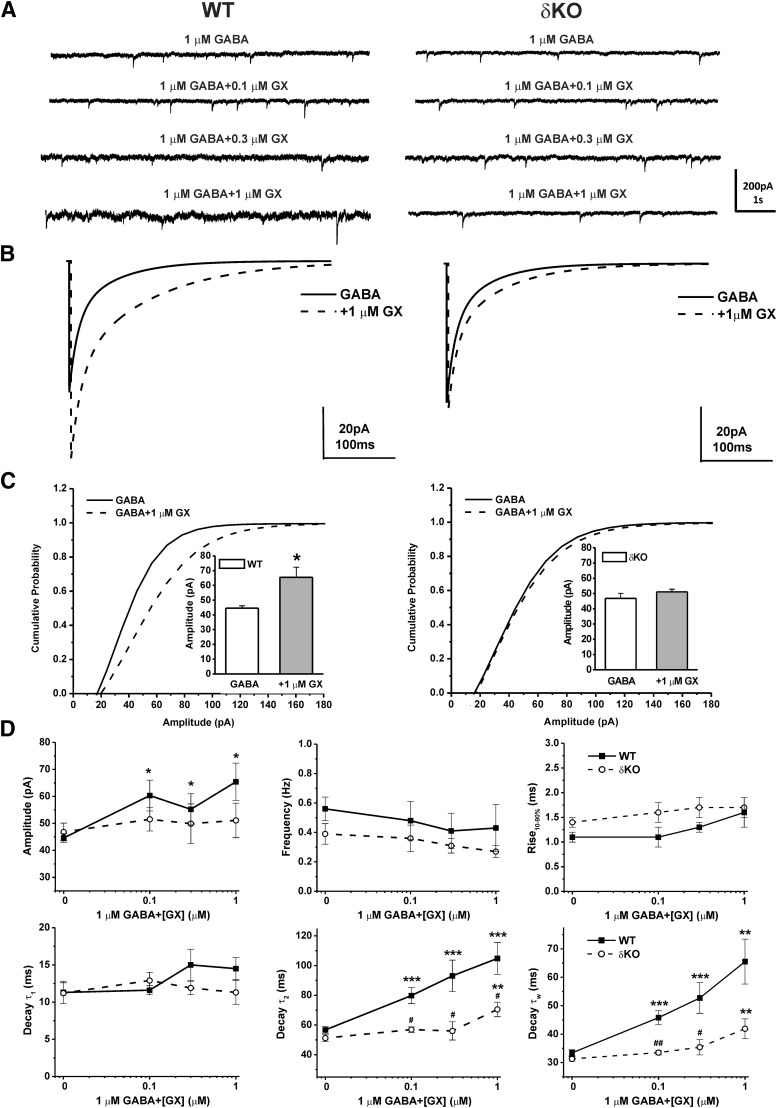
Next, we analyzed the potentiation of synaptic GABA-A receptor-mediated mIPSCs by bath application of GX (0.1–1 μM) with GABA (1 μM) in WT DGGCs. To determine whether the GX-mediated changes to synaptic activity are related to δ-subunit expression, we also recorded mIPSCs in DGGCs from δΚΟ mice (Fig. 8). Representative traces and ensemble average mIPSCs for each drug concentration and condition are shown in Fig. 8, A and B. GX has no significant effect on frequency or rise time of mIPSCs (Fig. 8D). However, the cumulative probability of amplitude and the mean peak amplitude were strongly enhanced by GX (1 μM) in WT DGGCs but not in δΚΟ DGGCs (Fig. 8C). Concentration-dependent potentiation of amplitude of mIPSCs by GX was evident in WT neurons but not in δΚΟ DGGCs. In addition, GX prolonged the decay time constant τ2 and the mean weighted decay time τw of mIPSCs in a concentration-dependent manner in both WT and δΚΟ neurons. However, GX prolonged τ2 and τw decay time constants of mIPSCs to a significantly greater degree in WT DGGCs compared with δKO DGGCs (τ2: P = 0.014 at 0.1 μM; P = 0.015 at 0.3 μM; and P = 0.049 at 1 μM; τw: P = 0.007 at 0.1 μM; P = 0.030 at 0.3 μM; and P = 0.066 at 1 μM, WT vs. δΚΟ). These results suggest that δ-containing GABA-A receptors highly contribute to GX-potentiated amplitude of mIPSCs, which reflects its influence on postsynaptic receptor density and dendritic property, and play a pivotal role in the GX-potentiated kinetics of mIPSCs and thereby to the net inhibition.
Fig. 8.
GX concentration-dependent potentiation of mIPSCs was attenuated in DGGCs from δΚΟ mice. (A) Representative traces of phasic currents by patch-clamp recording from WT or δΚΟ DGGCs in the presence of 1 μM GABA, 1 μM GABA + 0.1 μM GX, 1 μM GABA + 0.3 μM GX, and 1 μM GABA + 1 μM GX. (B) Averaged mIPSC events recorded from WT [(B), left] or δΚΟ [(B), right] DGGCs in the presence of 1 μM GABA (solid line) or 1 μM GABA coapplied with 1 μM GX (dashed line). Cumulative probability curves for WT (C, left) or δΚΟ [(C), right] mIPSC amplitude, plotted from all events. The Kolmogorov-Smirnov test was used to compare mIPSCs before and after application of GX in DGGCs. [(C), insets] Mean peak amplitude of mIPSCs in WT (left) or δΚΟ (right) DGGCs in the presence of 1 μM GABA or 1 μM GABA coapplied with 1 μM GX. *P < 0.05 vs. GABA alone (n = 14–19 cells per drug concentration). (D) Summary graphs of concentration-response relationship for amplitude, frequency, rise time (10%–90%), decay τ1, decay τ2, and mean weighted decay time (τw) of GX modulation in DGGCs from WT or δΚΟ mice. Each point represents mean ± S.E.M. *P < 0.05; **P < 0.01; ***P < 0.001 vs. GABA; #p < 0.05; ##p < 0.01 vs. WT (n = 7-10 cells).
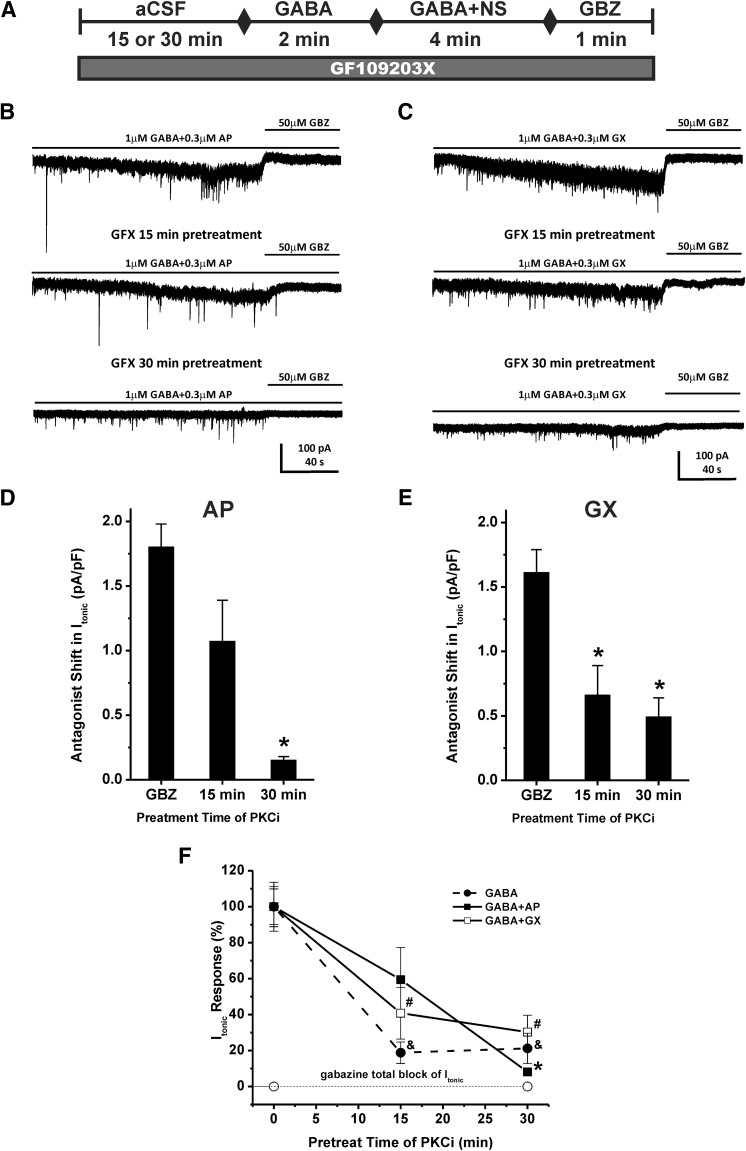
Inhibition of Protein Kinase C Prevents Allosteric Potentiation of Tonic Currents by GX and AP in Hippocampus Slices.
Protein kinase C activity may affect neurosteroid modulation of GABA-A receptor function (Fancsik et al., 2000; Harney et al., 2003; Adams et al., 2015). We hypothesize that the PKC inhibitor (PKCi) may also influence the extent of neurosteroid-potentiated tonic inhibition. To explore the effects of PKC activity on GX allosteric potentiation of tonic currents, the PKC inhibitor GF109203X (1 μM) was applied to the bath solution for 15 or 30 minutes before the perfusion of GABA, and the application was continued during GABA or neurosteroid perfusion. To compare the mechanism of action of GX with AP, we also examined the influence of PKC inhibitor on AP-potentiated tonic currents. The experimental protocol is shown in Fig. 9A. Pretreatment of PKC inhibitor for 15 or 30 minutes prior the application of GABA reduced GABA-evoked tonic current density from 0.62 ± 0.08 to 0.11 ± 0.04 pA/pF and 0.13 ± 0.05, respectively (n = 5 cells per group; Fig. 9F). The PKC inhibitor attenuated the allosteric potentiation of AP and GX in a time-dependent manner (Fig. 9, B–F). Pretreatment of PKC inhibitor for 15 or 30 minutes prior to the application of GABA reduced the tonic current density potentiated by AP from 1.80 ± 0.18 to 1.07 ± 0.32 pA/pF and 0.15 ± 0.03, respectively (n = 6–9 cells per group, 40.6% ± 17.8% decrease, P = 0.082 and 91.9% ± 1.8% decrease, P = 0.00001, respectively; Fig. 9, D and F). Pretreatment of PKC inhibitor for 15 or 30 minutes prior to the application of GABA also significantly attenuated the tonic current density potentiated by GX from 1.61 ± 0.18 to 0.66 ± 0.23 pA/pF and 0.49 ± 0.15 pA/pF, respectively (n = 6–7 cells per group, 59.2% ± 14.4% decrease, P = 0.009 and 69.8% ± 9.4% decrease, P = 0.001, respectively; Fig. 9, E and F). These results demonstrate that both AP and GX potentiation of tonic current are regulated by the extent of PKC activity in the neurons.
Fig. 9.
PKC inhibitor (PKCi) prevents neurosteroid potentiation of tonic currents in DGGCs. (A) Scheme demonstrating experimental protocol. PKCi GX109203X (GFX, 1 μM) was applied to the bath solution for 15 or 30 minutes before the GABA application, and the PKCi application was continued during GABA, neurosteroid, and gabazine perfusion. Representative whole-cell current recordings of DGGCs in the presence of GABA + AP (B) or GABA + GX (C) and GBZ with or without PKCi application. Qualification of tonic current shift was achieved relative to complete block by GBZ at 50 μM. (D and E) PKCi attenuation of AP- or GX- potentiated tonic current response. (F) Fractional response of GABA-, GABA + AP-, or GABA + GX-modulated tonic current response owing to PKCi perfusion. Each point or bar represents mean ± S.E.M. of data from five to nine cells. *P < 0.05 vs. GBZ or GABA + AP without PKCi group; #P < 0.05 vs. GABA + GX without PKCi group; &P < 0.05 vs. GABA without PKCi group. NS, neurosteroid.
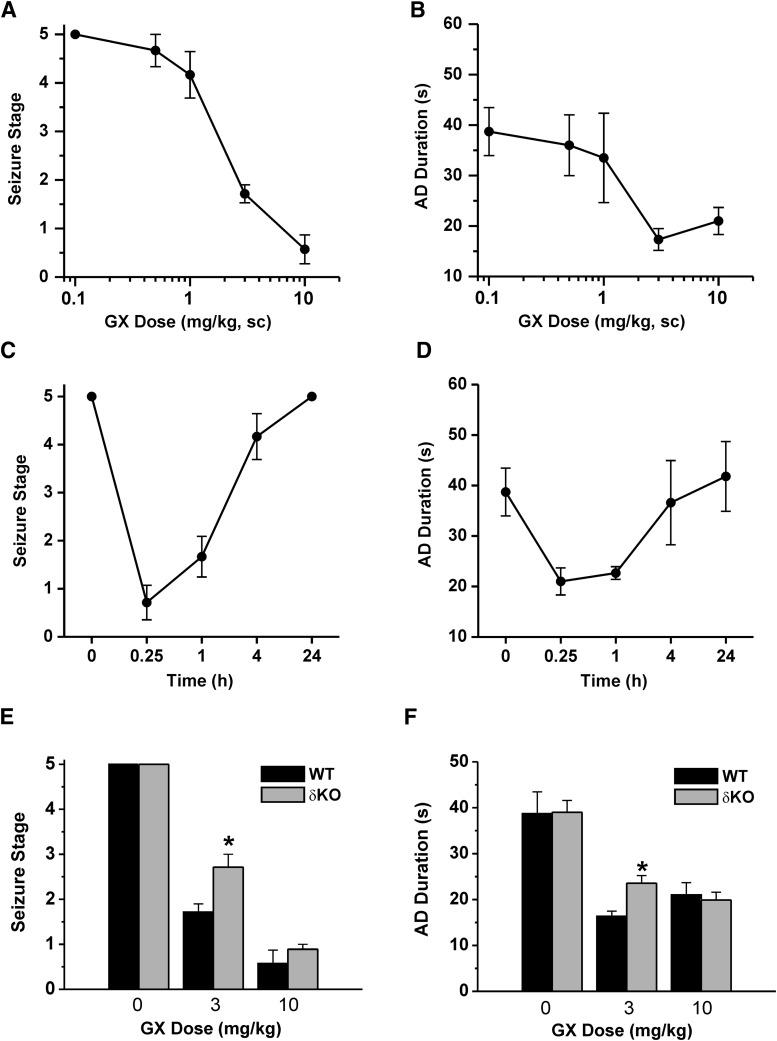
Antiseizure Activity of GX in the Hippocampus Kindling Model.
To evaluate the activity of GX in protecting against hippocampus-kindled seizures, fully kindled mice were treated with various doses of GX 15 minutes prior to stimulation. GX produced a dose-dependent reduction of behavioral seizure activity with significant effects on both at 5 and 10 mg/kg (Fig. 10A). At the highest dose tested, behavioral seizures were almost entirely inhibited. GX pretreatment markedly reduced the AD duration in a dose-dependent manner (Fig. 10B). Furthermore, the overall amplitude was decreased by about 50% after 5 and 10 mg/kg GX. The estimated ED50 value for suppression of seizure stage and AD duration is 3.2 ± 0.7 and 3.0 ± 0.8 mg/kg, respectively. The time courses for behavioral seizure stage and AD duration after a 10 mg/kg dose of GX are shown in Fig. 10, C and D, respectively. The seizure-protective effect of GX (10 mg/kg s.c.) occurred rapidly. The protection was maximal at 15 minutes and but reduced during the 240-minute period after the administration, as evident by its time-dependent decrease in seizure protection (Fig. 10C) and AD duration (Fig. 10D). On the day after GX treatment, all mice exhibited stage 5 seizures with AD duration (35 ± 6 second) not significantly different from the control duration (38 ± 5 second), indicating that GX suppresses the expression of behavioral seizures but does not influence the kindled state. To examine the role of δ-subunit in GX protection against kindled seizures, the behavioral seizure stage and AD duration at various doses of GX in WT and δΚΟ mice were compared. GX at 3 mg/kg displayed significantly stronger reduction in seizure stage (Fig. 10E) and AD duration (Fig. 10F) in WT mice compared with δΚΟ mice, indicating that GX suppression of seizure activity is affected by the δ-subunit. These results are compatible with the hypothesis that GX protection is possibly the result of potentiation of GABA-A receptor-mediated inhibition that occurs rapidly within a few minutes, an effect selective for δ-containing GABA-A receptors.
Fig. 10.
Antiseizure activity of GX in fully kindled WT and δΚΟ mice. (A) GX dose-dependent curves of behavioral seizure activity and (B) afterdischarge (AD) duration. (C) Time courses for behavioral seizure stage and (D) AD duration with GX at 10 mg/kg s.c. in fully kindled mice. (E) GX dose-dependent responses of behavioral seizure activity and (F) AD duration in WT and δΚΟ mice. Each point or bar represents mean ± S.E.M. *P < 0.05 vs. WT (n = 6–9 animals per group).
GX Analogs as More Selective δGABA-A Receptor Modulators.
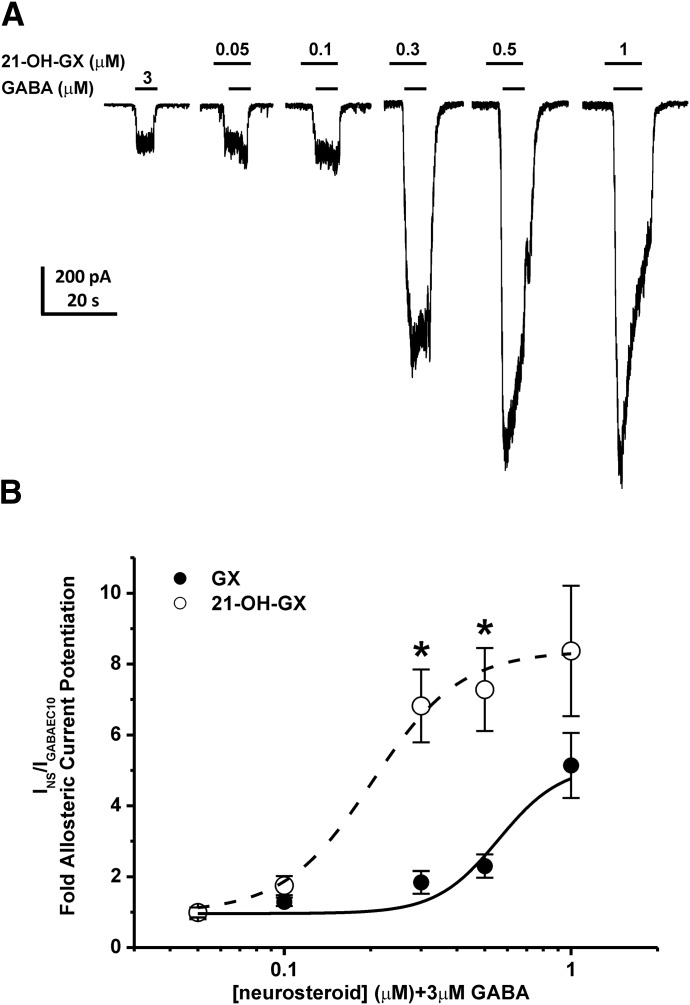
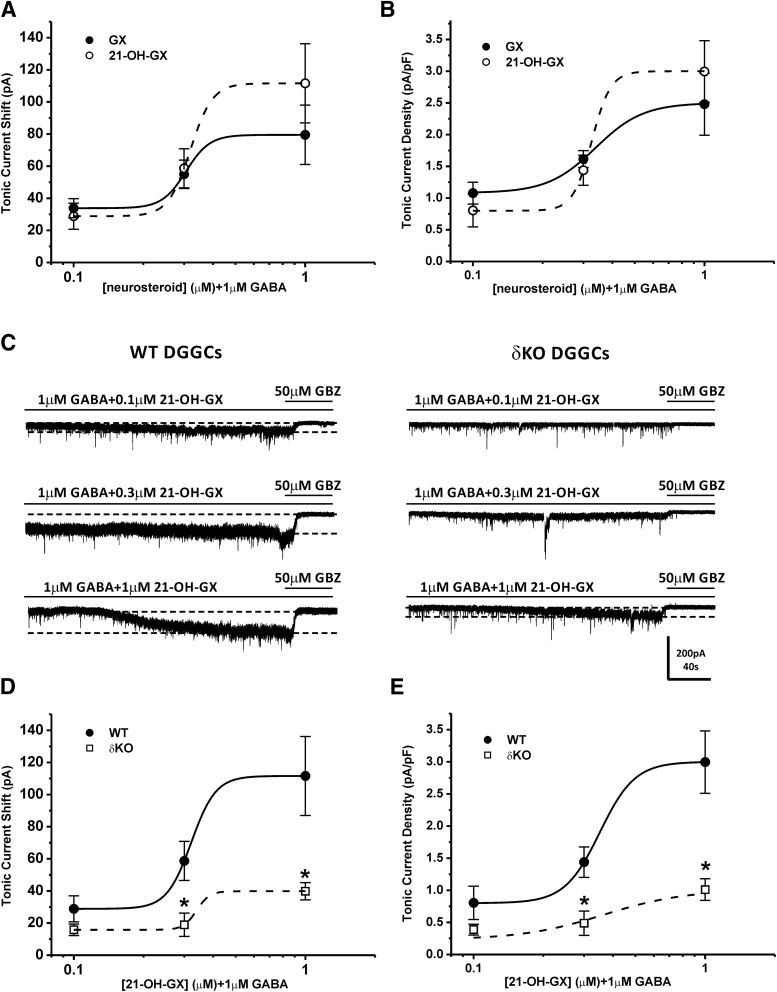
Structure-activity relationship (SAR) studies have shown the importance of the α-OH group at the C3 position in the binding affinity and potentiation of synaptic γGABA-A receptors by neurosteroids (Harrison et al., 1987; Mitchell et al., 2008). Additionally, the ketone group at the C17 and C20 position and the lipophilic properties of neurosteroids are critical for the potency and efficacy of receptor modulation (Kokate et al., 1994; Upasani et al., 1997; Covey et al., 2000; Chisari et al., 2009; Reddy and Jian, 2010; Qian et al., 2014). Our SAR studies at extrasynaptic δGABA-A receptors also demonstrate the requirement of the α-OH group at the C3 position for the functional activation of extrasynaptic receptors and the influence of alternations at the C17 and C20 position in the modulation efficacy and receptor binding affinity (Carver and Reddy, 2016). To further identify key structural features of neurosteroids that are important for the functional activation of extrasynaptic δGABA-A receptors, we compared the modulatory effects of GX and its analog 21-OH-GX, which has an additional OH group at C21 position, on whole-cell GABA-gated chloride currents and extrasynaptic δGABA-A receptor-mediated tonic currents. We also investigated the role of δ-subunit in 21-OH-GX potentiation of tonic inhibition. In dissociated DGGCs, 21-OH-GX allosterically potentiated GABA-gated currents in a concentration-dependent manner (Fig. 11A). 21-OH-GX displayed significantly greater potentiation of GABA-gated chloride currents (EF2-fold GABA = 0.20 μM) than GX (EF2-fold GABA = 0.43 μM; Fig. 11B). In hippocampus slices, WT DGGCs displayed concentration-dependent sensitivity to 21-OH-GX-mediated enhancement of tonic currents (0.80 ± 0.26 pA/pF at 0.1 μM; 1.44 ± 0.24 pA/pF at 0.3 μM; and 3.00 ± 0.49 pA/pF at 1 μM). The tonic current responses were not significantly different between GX and 21-OH-GX at 0.1 and 0.3 μM but were potentiated slightly more by 21-OH-GX at 1 μM (GX, 2.48 ± 0.49 pA/pF at 1 μM, EF2-fold GABA = 0. 29 μM; 21-OH-GX, 3.00 ± 0.49 pA/pF at 1 μM, EF2-fold GABA = 0. 23 μM; Fig. 12, A and B). However, 21-OH-GX potentiation of tonic inhibition at 0.3 and 1 μM was significantly attenuated in DGGCs from δΚΟ mice (0.39 ± 0.08 pA/pF at 0.1 μM; 0.49 ± 0.19 pA/pF at 0.3 μM; and 0.90 ± 0.15 pA/pF at 1 μM, *P < 0.05 vs. WT; Fig. 12, C–E), indicating its selectivity for extrasynaptic δGABA-A receptors.
Fig. 11.
Comparison of allosteric modulation of GABA-gated currents by GX and 21-OH-GX in acutely dissociated DGGCs. 21-OH-GX displayed significantly greater potentiation of GABA-gated chloride currents (EF2-fold GABA = 0.20 μM) than GX (EF2-fold GABA = 0.43 μM). (A) Representative whole-cell current recordings in DGGCs. (B) Concentration-response curves of neurosteroid-modulated allosteric potentiation of chloride currents in DGGCs. Neurons displayed concentration-dependent responses to neurosteroid potentiation of 3 μM GABA (EC10). Each point represents mean ± S.E.M. *P < 0.05 vs. GX (n = 6–10 cells).
Fig. 12.
Comparison of allosteric modulation of tonic currents by GX and 21-OH-GX in hippocampus slices from WT and δΚΟ mice. 21-OH-GX displayed slightly greater potentiation of tonic currents (EF2-fold GABA = 0. 23 μM) than GX (EF2-fold GABA = 0. 29 μM). 21-OH-GX potentiation of tonic inhibition at 0.3 and 1 μM was significantly diminished in DGGCs from δΚΟ mice. Concentration-response curves for allosteric activation of tonic current [pA, (A) and (D)] and normalized tonic current density [pA/pF, (B) and (E)] by GX and 21-OH-GX in DGGCs from WT or δΚΟ mice. (C) Representative whole-cell current recordings of DGGCs from WT or δΚΟ mice. Qualification of tonic current response (between first and second gray dashed lines) was achieved relative to complete block by gabazine (GBZ) at 50 μM. Each point represents mean ± S.E.M. *P < 0.05 vs. WT (n = 7–16 cells per drug concentration).
Antiseizure Activity of GX Analogs in the Kindling Model.
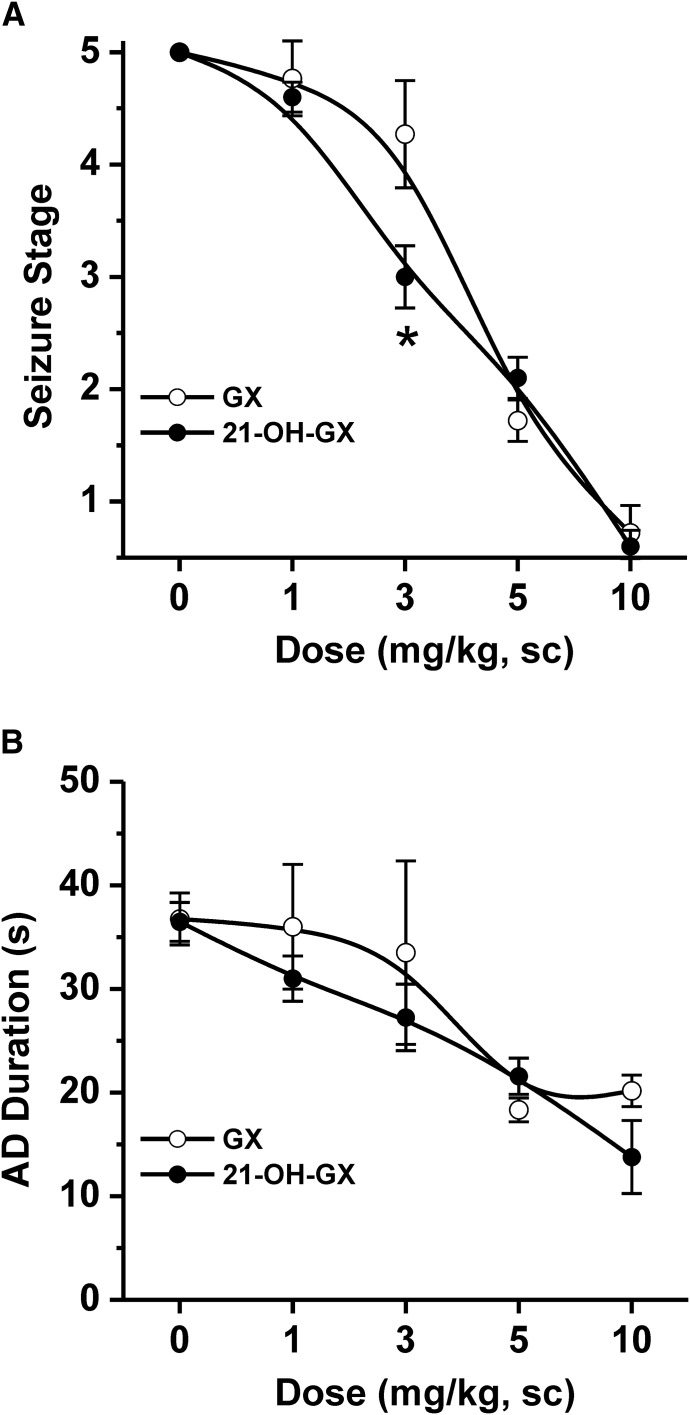
We compared the activity of GX and 21-OH-GX in protecting against hippocampus-kindled seizures. Fully kindled mice were treated with various doses of GX and 21-OH-GX 15 minutes prior to stimulation. As shown in Fig. 13, both GX and 21-OH-GX produced a dose-dependent reduction of behavioral seizure activity (Fig. 13A) and AD duration (Fig. 13B). However, 21-OH-GX displayed greater antiseizure activity compared with GX although such effect was significant at 3 mg/kg dose (Fig. 13A). Overall, the GX analog 21-OH-GX displayed stronger potentiation in GABAergic chloride currents and extrasynaptic δGABA-A receptor-mediated tonic inhibition; possibly conveying a stronger antiseizure effect than GX.
Fig. 13.
Comparative antiseizure effects of GX and 21-OH-GX in the hippocampus kindling model in mice. (A) Dose-response curves of behavioral seizure activity and (B) afterdischarge (AD) duration in fully kindled WT mice. Each point represents mean ± S.E.M. *P < 0.05 vs. GX (n = 6–9 animals per group).
Discussion
The principal finding of this study is the demonstration of GX and its analogs as preferential positive allosteric modulators and direct activators of extrasynaptic δGABA-A receptors in the dentate gyrus that regulate network tonic inhibition and seizures. GX and its analogs are highly selective for PKC-phosphorylated extrasynaptic δGABA-A receptor-mediated tonic inhibition in native hippocampal neurons. In addition, GX enhancement of tonic inhibition is diminished in mice lacking δGABA-A receptors. These results suggest that GX controls seizure susceptibility probably by potentiating GABA-A receptor-medicated synaptic and tonic inhibition though allosteric and/or direct action. Overall, these findings provide a mechanistic rationale for the clinical use of GX and its analog in seizure disorders (Reddy and Estes, 2016).
GX Activation of Extrasynaptic δGABA-A Receptors and Tonic Inhibition.
Neurosteroids are endogenous modulators of GABA-A receptors acting through an allosteric binding site at submicromolar concentrations and a direct activation site at micromolar concentrations (Hosie et al., 2007). GX is a synthetic neurosteroid with robust antiseizure properties (Reddy and Woodward, 2004). Unlike AP, its natural prototype, GX, with its additional methyl group at the 3β position, is not readily metabolized to the hormonally active 3-keto derivative (Gee et al., 1995). Therefore, GX possesses higher bioavailability and provides a more favorable pharmacokinetic profile as a promising antiepileptic drug. Although GX was previously tested in recombinant α1β1γ2L GABA-A receptors (Carter et al., 1997), its precise mode of action on native hippocampal neurons remained largely unclear. Here we demonstrated that GX is a potent allosteric modulator of both synaptic and extrasynaptic GABA-A receptors in native hippocampal neurons. We compared the modulatory effects of GX with AP. GX and AP at 0.3 μM allosterically potentiated tonic current density to 1.61 ± 0.18 and 1.80 ± 0.18 pA/pF, respectively (Figs. 5 and 9). In seizure models including the 6-Hz test, GX exhibited improved potency compared with AP (Kaminski et al., 2004; Carver and Reddy, 2016). This may be attributed to its enhanced pharmacokinetic profile preventing its metabolism, thereby offering increased bioavailability.
GX Analogs as Selective Modulators of Extrasynaptic δGABA-A Receptors.
Like our previous SAR study (Carver and Reddy, 2016), we compared the potency and efficacy of GX with its analog 21-OH-GX. Our results demonstrate that, with the addition of a hydroxyl group in the C21 position, 21-OH-GX displayed higher efficacy than GX in the augmentation of GABA currents and tonic inhibition. These could be related to the plausible comparable bioavailability and higher binding affinity to GX. In addition, both GX and 21-OH-GX displayed dose-dependent reduction of seizure stage and AD duration. However, 21-OH-GX exhibited significantly greater antiseizure potency, an effect highly selective for extrasynaptic δGABA-A receptors. Our studies confirm that 21-OH-GX possesses higher efficacy in potentiation of GABA currents and extrasynaptic δGABA-A receptor-mediated tonic inhibition, possibly contributing to its better antiseizure activity.
Enhanced Neurosteroid Sensitivity at Extrasynaptic δGABA-A Receptors.
The δ-subunit and its expressional plasticity remain key factors in understanding neurosteroid sensitivity, network excitability, and seizure susceptibility (Mihalek et al., 1999; Carver and Reddy, 2013; Whissell et al., 2015; Reddy et al., 2017; Chuang and Reddy, 2018). Mice lacking the δ-subunit display significantly decreased sleep time in response to neurosteroid alphaxolone and reduced sensitivity to GX-exerted anxiolytic response (Mihalek et al., 1999; Spigelman et al., 2002, 2003; Porcello et al., 2003). The δ-selective effect for neurosteroid modulation is also established by electrophysiological studies. THDOC-potentiated tonic currents and spontaneous IPSCs are absent in the δΚΟ mice (Vicini et al., 2002; Wohlfarth et al., 2002; Stell et al., 2003). AP-enhanced GABAergic currents and tonic inhibition in WT DGGCs are also attenuated in δΚΟ mice (Carver et al., 2014; Carver and Reddy, 2016; Carver et al., 2016), signifying the obligatory role of the δ-subunit in neurosteroid sensitivity. In the present study, we demonstrate that δ-subunit plays an essential role in GX’s allosteric modulation and direct augmentation of tonic inhibition in native neurons. These findings are consistent with the emerging importance of δ-containing GABA-A receptors to tonic inhibition and neurosteroid sensitivity.
Role of δGABA-A Receptors in Phasic Currents in the Hippocampus.
The IPSCs primarily represent the activation of synaptic GABA-A receptors. To elucidate the extent of GX’s modulation of synaptic GABA-A receptors and the role of δ-subunit in such modulation, we recorded the mIPSCs from WT and δΚΟ DGGCs. GX has a greater effect on the amplitude and the decay time of mIPSCs in WT DGGCs, an effect prevented by the deletion of δ-subunit, which indicates that δGABA-A receptors mediate this effect. Previous studies have shown the effect of δ-subunit on the neurosteroid-mediated phasic currents (Carver and Reddy, 2016). In cerebellar granule neurons, which have the highest expression of the δ-subunit, THDOC exerts strong potentiation of spontaneous IPSCs. However, this is significantly diminished in δΚΟ neurons (Vicini et al., 2002). In WT DGGCs, which have the second highest expression of the δ-subunit, alphaxolone strongly prolongs the decay time of mIPSCs, but not in those from δΚΟ mice (Spigelman et al., 2003). δKO mice were observed to have a significant reduction in the expression of the α4-subunit and an increase in the γ2-subunit (Peng et al., 2002; Spigelman et al., 2003). This notion is consistent with previous reports that α4δ-containing GABA-A receptors are more sensitive to neurosteroid modulation (Brown et al., 2002; Wohlfarth et al., 2002). It is also possible that a population of perisynaptic GABA-A receptors could respond to synaptic spillover of GABA, and this effect probably would be prevented by the deletion of δ-subunit (Bianchi and Macdonald, 2002; Carver et al., 2014). However, another study reports that δ-containing receptors are less sensitive to GABA that could be present through synaptic spillover (Bright et al., 2011). Overall, these results demonstrate that δΚΟ mice exhibit significantly attenuated responses to neurosteroid modulation of synaptic GABA-A receptor-mediated phasic currents, suggesting the significance of δ-subunit in the neurosteroid sensitivity.
Influence of PKC Activity on Neurosteroid Potentiation of Tonic Inhibition.
Protein kinase activity influences the surface expression of GABA-A receptors and the neurosteroid sensitivity. Several GABA-A receptor subunits are substrates of PKC, including the α4- and β-subunits (Abramian et al., 2010; Adams et al., 2015). PKC activator potentiates THDOC-enhanced GABA-gated currents in recombinant receptors (Leidenheimer and Chapell, 1997), whereas inhibition of PKC reduces the THDOC and AP-prolonged decay time of mIPSCs in murine neurons (Fancsik et al., 2000; Harney et al., 2003). THDOC also promotes the phosphorylation of the S443 within the α4-subunit and the upregulation of α4-containing GABA-A receptors in the cell membrane, leading to increased tonic inhibition (Abramian et al., 2010, 2014). The δ-subunit is predominately assembled with the α4- and α6-subunit (Jones et al., 1997; Peng et al., 2002; Spigelman et al., 2003). In the present study, we demonstrate that PKC activity mediates the potentiation of tonic currents by AP and GX in DGGCs. The inhibition of PKC may cause the downregulation of α4δ-containing GABA-A receptors in the cell membrane through reduced phosphorylation of α4-subunits and subsequent attenuated tonic current potentiation by neurosteroids. A recent study indicates that sustained application of AP, but not GX, leads to increased phosphorylation and surface expression of the β3-containing GABA-A receptors, and tonic current potentiation. These effects are prevented by the PKC inhibitor (Modgil et al., 2017). Although GX does not have a metabotropic effect on GABA-A receptor phosphorylation and trafficking, inhibition of PKC still dampens the potentiation of tonic inhibition by neurosteroids. This is probable because sustained application of the PKC inhibitor causes decreased phosphorylation and subsequent internalization of receptors and, therefore, reduced tonic current potentiation (Comenencia-Ortiz et al., 2014).
Therapeutic Implications of GX and Its Analogs as Anticonvulsants.
We confirmed that GX has potent antiseizure effect in the hippocampus kindling model of epilepsy, which is a well accepted model of complex partial seizures (Albertson et al., 1980; Sutula, 1990; Reddy et al., 2010). The comparative protective profile of GX and AP is outlined in Table 2. Like other neurosteroids such as AP, GX is highly effective against seizures induced by a variety of triggers, including chemoconvulsants, electrical kindling, and chemical kindling (Gasior et al., 2000; Kaminski et al., 2003; Reddy and Woodward, 2004; Reddy and Rogawski, 2012). GX is active in the 6-Hz model, which has been shown to be very responsive to positive modulators of GABA-A receptors (Kaminski et al., 2004; Reddy et al., 2015). GX is developed as a rational analog of AP, which is a potent positive allosteric agonist of GABA-A receptors (Carter et al., 1997). Since neurosteroids exhibit greater selectivity toward extrasynaptic receptors, the results from this study confirm that the antiseizure effect of GX is mostly a result of its preferential interaction with δGABA-A receptors besides its actions on synaptic receptors (Carver et al., 2014; Carver and Reddy, 2016). The pharmacokinetic/pharmacodynamic correlation of key outcomes from pharmacological and electrophysiological supports this conclusion. In animal seizure models, the ED50 value of GX ranges from 2 to 6 mg/kg (Table 2). On the basis of the GX plasma levels, the estimated threshold plasma concentration for 50% seizure protection is in the range of 510−750 ng/ml (1.5−2.3 μM) (Reddy and Rogawski, 2000). These concentrations exceed the range for GX allosteric potentiation in DGGCs (0.1–0.3 μM), and apparently near the range for producing direct activation (1−2 μM), indicating that GX potentiation of GABA-A receptor-mediated inhibition primarily contributes to its antiseizure activity. However, there is limited pharmacokinetic/pharmacodynamic data from clinical studies. Single oral doses of 50–500 mg in healthy subjects resulted in plasma concentrations of 32−376 ng/ml (0.01−1.1 μM) (Monaghan et al., 1997). Thus, GX and its analogs are powerful anticonvulsants with utility in the treatment of epilepsy and seizures in conditions with intact δGABA-A receptors, including catamenial epilepsy, status epilepticus, and chemical neurotoxicity (Reddy, 2016a,b).
TABLE 2.
Comparative antiseizure ED50 values of GX and AP in mouse models of epilepsy
Values in parentheses are 95% confidence limits.
| AP | GX | References | |
|---|---|---|---|
| mg/kg | mg/kg | ||
| Kindling models | |||
| Hippocampus kindling | 3.5 | 3.5 | Reddy et al. (2012), Carver et al. (2014) |
| Amygdala kindling | 14 (8–23) | 6.6 (5.1–9.7) | Reddy and Rogawski (2010) |
| Cocaine kindling | 17.0 (ND) | 17.0 (ND) | Kaminski et al. (2003) |
| Pentylenetetrazol kindling | ND | 3.5 (2.4–5.1) | Gasior et al. (2000) |
| Corneal kindling | ND | 4.5 (4.0–5.1) | Carter et al. (1997) |
| Chemoconvulsant models | |||
| Pentylenetetrazol (mice) | 13.7 (10.1–18.7) | 3.5 (2.1–5.8) | Kokate et al. (1994), Carter et al. (1997) |
| Pentylenetetrazol (rats) | 2.14 (1.10–4.15) | 4.3 (2.8–6.9) | Reddy and Rogawski (2000, 2001) |
| Bicuculline | 12 (10–15) | 4.6 (3.2–6.8) | Carter et al. (1997) |
| Picrotoxin | 10 (5–19) | ND | Belelli et al. (1989) |
| t-Butylbicycloorthobenzoate | ND | 11.7 (8.8–15.7) | Carter et al. (1997) |
| Flurothyl (rats) | ND | 5.0 (ND) | Liptakova et al. (2000) |
| N-Methyl-d-aspartate | >40 | >30 | Carter et al. (1997) |
| Kainic acid | >40 | >30 | Carter et al. (1997) |
| 4-Aminopyridine | >40 | 11.5 (8.1–16.3) | Carter et al. (1997) |
| Strychnine | >40 | >40 | Carter et al. (1997) |
| Electroshock models | |||
| Maximal electroshock | 29 (19–44) | 29.7 (25.3–34.8) | Carter et al. (1997) |
| 6-Hz stimulation | 4.2 (2.7–5.8) | 1.5 (1.3–1.7) | Carver and Reddy (2016) |
| Status epilepticus models | |||
| Pilocarpine | 7 (4–11) | ∼6 | Kokate et al. (1996), Briyal and Reddy (2008) |
| Kainic acid | ∼20 | ND | Rogawski et al. (2013) |
ND, not determined.
In conclusion, these results demonstrate that GX and its analogs are preferential allosteric modulators of both synaptic and extrasynaptic GABA-A receptors in native hippocampal neurons. GX potentiation of tonic inhibition is δ-subunit-dependent and greatly influenced by PKC activity. These outcomes provide a strong mechanistic basis for the therapeutic use of GX and synthetic neurosteroids in epilepsy and related brain disorders (Younus and Reddy, 2018).
Acknowledgments
The authors thank Chase Carver and Kushal Bakshi for their help with patch-clamp.
Abbreviations
- aCSF
Artificial cerebrospinal fluid
- AD
afterdischarge
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- AP
allopregnanolone (3α-hydroxy-5α-pregnan-20-one)
- APV
2-amino-5-phosphonopentanoic acid
- CA1PCs
CA1 pyramidal cells
- DGGCs
dentate gyrus granule cells
- DNXQ
6,7-dinitroquinoxaline-2,3(1H,4H)-dione
- EC10
concentration required to achieve 10% of the maximal currents
- GABA
γ-aminobutyric acid
- GBZ
gabazine
- GX
ganaxolone (3α-hydroxy-3β-methyl-5α-pregnan-20-one)
- δKO
GABA-A receptor δ-subunit knockout
- 21-OH-GX
21-hydroxy-ganaxolone
- mIPSC
miniature inhibitory postsynaptic current
- NMDA
N-methyl-d-aspartic acid
- pA/pF
current density
- PKC
protein kinase C
- RMS
root mean square
- SAR
structure-activity relationship
- THDOC
allotetrahydrodeoxycorticosterone (3α,21-dihydroxy-5α-pregnan-20-one)
- TTX
tetrodotoxin (4R,4a R,5 R,6S,7S,8S,8a R,10S,12S)-2-azaniumylidene-4,6,8,12-tetrahydroxy-6-(hydroxymethyl)-2,3,4,4a,5,6,7,8-octahydro-1H-8a,10-methano-5,7-(epoxymethanooxy)quinazolin-10-olate
- WT
wild type
Authorship Contributions
Participated in research design: Reddy.
Conducted experiments: Chuang, Reddy.
Performed data analysis: Chuang, Reddy.
Wrote or contributed to the writing of the manuscript: Chuang, Reddy.
Footnotes
This research was supported by the CounterACT Program, Office of the Director and the National Institute of Neurologic Disorders and Stroke, National Institutes of Health, [Grant U01 NS083460 (to D.S.R.)].
References
- Abramian AM, Comenencia-Ortiz E, Modgil A, Vien TN, Nakamura Y, Moore YE, Maguire JL, Terunuma M, Davies PA, Moss SJ. (2014) Neurosteroids promote phosphorylation and membrane insertion of extrasynaptic GABAA receptors. Proc Natl Acad Sci USA 111:7132–7137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abramian AM, Comenencia-Ortiz E, Vithlani M, Tretter EV, Sieghart W, Davies PA, Moss SJ. (2010) Protein kinase C phosphorylation regulates membrane insertion of GABAA receptor subtypes that mediate tonic inhibition. J Biol Chem 285:41795–41805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams JM, Thomas P, Smart TG. (2015) Modulation of neurosteroid potentiation by protein kinases at synaptic- and extrasynaptic-type GABAA receptors. Neuropharmacology 88:63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albertson TE, Peterson SL, Stark LG. (1980) Anticonvulsant drugs and their antagonism of kindled amygdaloid seizures in rats. Neuropharmacology 19:643–652. [DOI] [PubMed] [Google Scholar]
- Belelli D, Bolger MB, Gee KW. (1989) Anticonvulsant profile of the progesterone metabolite 5 alpha-pregnan-3 alpha-ol-20-one. Eur J Pharmacol 166:325–329. [DOI] [PubMed] [Google Scholar]
- Bialer M, Johannessen SI, Levy RH, Perucca E, Tomson T, White HS. (2015) Progress report on new antiepileptic drugs: a summary of the twelfth eilat conference (EILAT XII). Epilepsy Res 111:85–141. [DOI] [PubMed] [Google Scholar]
- Bianchi MT, Macdonald RL. (2002) Slow phases of GABA(A) receptor desensitization: structural determinants and possible relevance for synaptic function. J Physiol 544:3–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi MT, Macdonald RL. (2003) Neurosteroids shift partial agonist activation of GABA(A) receptor channels from low- to high-efficacy gating patterns. J Neurosci 23:10934–10943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braat S, D’Hulst C, Heulens I, De Rubeis S, Mientjes E, Nelson DL, Willemsen R, Bagni C, Van Dam D, De Deyn PP, et al. (2015) The GABAA receptor is an FMRP target with therapeutic potential in fragile X syndrome. Cell Cycle 14:2985–2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon NJ, Delmas P, Kittler JT, McDonald BJ, Sieghart W, Brown DA, Smart TG, Moss SJ. (2000) GABAA receptor phosphorylation and functional modulation in cortical neurons by a protein kinase C-dependent pathway. J Biol Chem 275:38856–38862. [DOI] [PubMed] [Google Scholar]
- Bright DP, Renzi M, Bartram J, McGee TP, MacKenzie G, Hosie AM, Farrant M, Brickley SG. (2011) Profound desensitization by ambient GABA limits activation of δ-containing GABAA receptors during spillover. J Neurosci 31:753–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briyal S, Reddy DS. (2008) Neuroactive steroid therapy of status epilepticus in epilepsy rats. Epilepsia 49 (Suppl 7):3055. [Google Scholar]
- Brown N, Kerby J, Bonnert TP, Whiting PJ, Wafford KA. (2002) Pharmacological characterization of a novel cell line expressing human α(4)β(3)δ GABA(A) receptors. Br J Pharmacol 136:965–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter RB, Wood PL, Wieland S, Hawkinson JE, Belelli D, Lambert JJ, White HS, Wolf HH, Mirsadeghi S, Tahir SH, et al. (1997) Characterization of the anticonvulsant properties of ganaxolone (CCD 1042; 3α-hydroxy-3β-methyl-5α-pregnan-20-one), a selective, high-affinity, steroid modulator of the gamma-aminobutyric acid(A) receptor. J Pharmacol Exp Ther 280:1284–1295. [PubMed] [Google Scholar]
- Carver CM, Chuang SH, Reddy DS. (2016) Zinc selectively blocks neurosteroid-sensitive extrasynaptic δGABAA receptors in the hippocampus. J Neurosci 36:8070–8077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver CM, Reddy DS. (2013) Neurosteroid interactions with synaptic and extrasynaptic GABA(A) receptors: regulation of subunit plasticity, phasic and tonic inhibition, and neuronal network excitability. Psychopharmacology (Berl) 230:151–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver CM, Reddy DS. (2016) Neurosteroid structure-activity relationships for functional activation of extrasynaptic δGABA(A) receptors. J Pharmacol Exp Ther 357:188–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver CM, Wu X, Gangisetty O, Reddy DS. (2014) Perimenstrual-like hormonal regulation of extrasynaptic δ-containing GABAA receptors mediating tonic inhibition and neurosteroid sensitivity. J Neurosci 34:14181–14197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherubini E, Conti F. (2001) Generating diversity at GABAergic synapses. Trends Neurosci 24:155–162. [DOI] [PubMed] [Google Scholar]
- Chisari M, Eisenman LN, Krishnan K, Bandyopadhyaya AK, Wang C, Taylor A, Benz A, Covey DF, Zorumski CF, Mennerick S. (2009) The influence of neuroactive steroid lipophilicity on GABAA receptor modulation: evidence for a low-affinity interaction. J Neurophysiol 102:1254–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang SH, Reddy DS. (2018) Genetic and molecular regulation of extrasynaptic GABA-A receptors in the brain: therapeutic insights for epilepsy. J Pharmacol Exp Ther 364:180–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clossen BL, Reddy DS. (2017a) Catamenial-like seizure exacerbation in mice with targeted ablation of extrasynaptic δGABA-a receptors in the brain. J Neurosci Res 95:1906–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clossen BL, Reddy DS. (2017b) Novel therapeutic approaches for disease-modification of epileptogenesis for curing epilepsy. Biochim Biophys Acta 1863:1519–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comenencia-Ortiz E, Moss SJ, Davies PA. (2014) Phosphorylation of GABAA receptors influences receptor trafficking and neurosteroid actions. Psychopharmacology (Berl) 231:3453–3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covey DF, Nathan D, Kalkbrenner M, Nilsson KR, Hu Y, Zorumski CF, Evers AS. (2000) Enantioselectivity of pregnanolone-induced gamma-aminobutyric acid(A) receptor modulation and anesthesia. J Pharmacol Exp Ther 293:1009–1016. [PubMed] [Google Scholar]
- Fáncsik A, Linn DM, Tasker JG. (2000) Neurosteroid modulation of GABA IPSCs is phosphorylation dependent. J Neurosci 20:3067–3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasior M, Ungard JT, Beekman M, Carter RB, Witkin JM. (2000) Acute and chronic effects of the synthetic neuroactive steroid, ganaxolone, against the convulsive and lethal effects of pentylenetetrazol in seizure-kindled mice: comparison with diazepam and valproate. Neuropharmacology 39:1184–1196. [DOI] [PubMed] [Google Scholar]
- Gee KW, McCauley LD, Lan NC. (1995) A putative receptor for neurosteroids on the GABAA receptor complex: the pharmacological properties and therapeutic potential of epalons. Crit Rev Neurobiol 9:207–227. [PubMed] [Google Scholar]
- Harney SC, Frenguelli BG, Lambert JJ. (2003) Phosphorylation influences neurosteroid modulation of synaptic GABAA receptors in rat CA1 and dentate gyrus neurones. Neuropharmacology 45:873–883. [DOI] [PubMed] [Google Scholar]
- Harrison NL, Majewska MD, Harrington JW, Barker JL. (1987) Structure-activity relationships for steroid interaction with the gamma-aminobutyric acidA receptor complex. J Pharmacol Exp Ther 241:346–353. [PubMed] [Google Scholar]
- Hosie AM, Wilkins ME, Smart TG. (2007) Neurosteroid binding sites on GABA(A) receptors. Pharmacol Ther 116:7–19. [DOI] [PubMed] [Google Scholar]
- Jones A, Korpi ER, McKernan RM, Pelz R, Nusser Z, Mäkelä R, Mellor JR, Pollard S, Bahn S, Stephenson FA, et al. (1997) Ligand-gated ion channel subunit partnerships: GABAA receptor α6 subunit gene inactivation inhibits delta subunit expression. J Neurosci 17:1350–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski RM, Gasior M, Carter RB, Witkin JM. (2003) Protective efficacy of neuroactive steroids against cocaine kindled-seizures in mice. Eur J Pharmacol 474:217–222. [DOI] [PubMed] [Google Scholar]
- Kaminski RM, Livingood MR, Rogawski MA. (2004) Allopregnanolone analogs that positively modulate GABA receptors protect against partial seizures induced by 6-Hz electrical stimulation in mice. Epilepsia 45:864–867. [DOI] [PubMed] [Google Scholar]
- Kay AR, Wong RK. (1986) Isolation of neurons suitable for patch-clamping from adult mammalian central nervous systems. J Neurosci Methods 16:227–238. [DOI] [PubMed] [Google Scholar]
- Kerrigan JF, Shields WD, Nelson TY, Bluestone DL, Dodson WE, Bourgeois BF, Pellock JM, Morton LD, Monaghan EP. (2000) Ganaxolone for treating intractable infantile spasms: a multicenter, open-label, add-on trial. Epilepsy Res 42:133–139. [DOI] [PubMed] [Google Scholar]
- Kokate TG, Cohen AL, Karp E, Rogawski MA. (1996) Neuroactive steroids protect against pilocarpine- and kainic acid-induced limbic seizures and status epilepticus in mice. Neuropharmacology 35:1049–1056. [DOI] [PubMed] [Google Scholar]
- Kokate TG, Svensson BE, Rogawski MA. (1994) Anticonvulsant activity of neurosteroids: correlation with γ-aminobutyric acid-evoked chloride current potentiation. J Pharmacol Exp Ther 270:1223–1229. [PubMed] [Google Scholar]
- Laxer K, Blum D, Abou-Khalil BW, Morrell MJ, Lee DA, Data JL, Monaghan EP, Ganaxolone Presurgical Study Group (2000) Assessment of ganaxolone’s anticonvulsant activity using a randomized, double-blind, presurgical trial design. Epilepsia 41:1187–1194. [DOI] [PubMed] [Google Scholar]
- Leidenheimer NJ, Chapell R. (1997) Effects of PKC activation and receptor desensitization on neurosteroid modulation of GABA(A) receptors. Brain Res Mol Brain Res 52:173–181. [DOI] [PubMed] [Google Scholar]
- Liptáková S, Velísek L, Velísková J, Moshé SL. (2000) Effect of ganaxolone on flurothyl seizures in developing rats. Epilepsia 41:788–793. [DOI] [PubMed] [Google Scholar]
- McDonald BJ, Amato A, Connolly CN, Benke D, Moss SJ, Smart TG. (1998) Adjacent phosphorylation sites on GABAA receptor beta subunits determine regulation by cAMP-dependent protein kinase. Nat Neurosci 1:23–28. [DOI] [PubMed] [Google Scholar]
- Mihalek RM, Banerjee PK, Korpi ER, Quinlan JJ, Firestone LL, Mi ZP, Lagenaur C, Tretter V, Sieghart W, Anagnostaras SG, et al. (1999) Attenuated sensitivity to neuroactive steroids in gamma-aminobutyrate type A receptor delta subunit knockout mice. Proc Natl Acad Sci USA 96:12905–12910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell EA, Herd MB, Gunn BG, Lambert JJ, Belelli D. (2008) Neurosteroid modulation of GABAA receptors: molecular determinants and significance in health and disease. Neurochem Int 52:588–595. [DOI] [PubMed] [Google Scholar]
- Modgil A, Parakala ML, Ackley MA, Doherty JJ, Moss SJ, Davies PA. (2017) Endogenous and synthetic neuroactive steroids evoke sustained increases in the efficacy of GABAergic inhibition via a protein kinase C-dependent mechanism. Neuropharmacology 113 (Pt A):314–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan EP, Navalta LA, Shum L, Ashbrook DW, Lee DA. (1997) Initial human experience with ganaxolone, a neuroactive steroid with antiepileptic activity. Epilepsia 38:1026–1031. [DOI] [PubMed] [Google Scholar]
- Moss SJ, Smart TG. (1996) Modulation of amino acid-gated ion channels by protein phosphorylation. Int Rev Neurobiol 39:1–52. [DOI] [PubMed] [Google Scholar]
- Nohria V, Giller E. (2007) Ganaxolone. Neurotherapeutics 4:102–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Z, Hauer B, Mihalek RM, Homanics GE, Sieghart W, Olsen RW, Houser CR. (2002) GABA(A) receptor changes in delta subunit-deficient mice: altered expression of alpha4 and gamma2 subunits in the forebrain. J Comp Neurol 446:179–197. [DOI] [PubMed] [Google Scholar]
- Pieribone VA, Tsai J, Soufflet C, Rey E, Shaw K, Giller E, Dulac O. (2007) Clinical evaluation of ganaxolone in pediatric and adolescent patients with refractory epilepsy. Epilepsia 48:1870–1874. [DOI] [PubMed] [Google Scholar]
- Porcello DM, Huntsman MM, Mihalek RM, Homanics GE, Huguenard JR. (2003) Intact synaptic GABAergic inhibition and altered neurosteroid modulation of thalamic relay neurons in mice lacking delta subunit. J Neurophysiol 89:1378–1386. [DOI] [PubMed] [Google Scholar]
- Puia G, Santi MR, Vicini S, Pritchett DB, Purdy RH, Paul SM, Seeburg PH, Costa E. (1990) Neurosteroids act on recombinant human GABAA receptors. Neuron 4:759–765. [DOI] [PubMed] [Google Scholar]
- Qian M, Krishnan K, Kudova E, Li P, Manion BD, Taylor A, Elias G, Akk G, Evers AS, Zorumski CF, et al. (2014) Neurosteroid analogues. 18. Structure-activity studies of ent-steroid potentiators of γ-aminobutyric acid type A receptors and comparison of their activities with those of alphaxalone and allopregnanolone. J Med Chem 57:171–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racine RJ. (1972) Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol 32:281–294. [DOI] [PubMed] [Google Scholar]
- Reddy DS. (2016a) Neurosteroids for the potential protection of humans against organophosphate toxicity. Ann N Y Acad Sci 1378:25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS. (2016b) Catamenial epilepsy: discovery of an extrasynaptic molecular mechanism for targeted therapy. Front Cell Neurosci 10:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS. (2018) GABA-A receptors mediate tonic inhibition and neurosteroid sensitivity in the brain. Vitam Horm 107:177–191. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Castaneda DC, O’Malley BW, Rogawski MA. (2004) Anticonvulsant activity of progesterone and neurosteroids in progesterone receptor knockout mice. J Pharmacol Exp Ther 310:230–239. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Estes WA. (2016) Clinical potential of neurosteroids for CNS disorders. Trends Pharmacol Sci 37:543–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Gangisetty O, Briyal S. (2010) Disease-modifying activity of progesterone in the hippocampus kindling model of epileptogenesis. Neuropharmacology 59:573–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Gangisetty O, Wu X. (2017) PR-independent neurosteroid regulation of α2-GABA-A receptors in the hippocampus subfields. Brain Res 1659:142–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Gould J, Gangisetty O. (2012) A mouse kindling model of perimenstrual catamenial epilepsy. J Pharmacol Exp Ther 341:784–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Jian K. (2010) The testosterone-derived neurosteroid androstanediol is a positive allosteric modulator of GABAA receptors. J Pharmacol Exp Ther 334:1031–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Mohan A. (2011) Development and persistence of limbic epileptogenesis are impaired in mice lacking progesterone receptors. J Neurosci 31:650–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA. (2000) Chronic treatment with the neuroactive steroid ganaxolone in the rat induces anticonvulsant tolerance to diazepam but not to itself. J Pharmacol Exp Ther 295:1241–1248. [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA. (2001) Enhanced anticonvulsant activity of neuroactive steroids in a rat model of catamenial epilepsy. Epilepsia 42:337–344. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA. (2002) Stress-induced deoxycorticosterone-derived neurosteroids modulate GABA(A) receptor function and seizure susceptibility. J Neurosci 22:3795–3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA. (2010) Ganaxolone suppression of behavioral and electrographic seizures in the mouse amygdala kindling model. Epilepsy Res 89:254–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA. (2012) Neurosteroids - endogenous regulators of seizure susceptibility and role in the treatment of epilepsy, in Jasper’s Basic Mechanisms of the Epilepsies (Noebels JL, Avoli M, Rogawski MA, Olsen RW, and Delgado-Escueta AV, eds) pp 984–1002, National Center for Biotechnology Information, Bethesda, MD. [PubMed] [Google Scholar]
- Reddy DS, Woodward R. (2004) Ganaxolone: a prospective overview. Drugs Future 29:227–242. [Google Scholar]
- Reddy DS, Yoshimura RF, Ramanathan G, Carver C, Johnstone TB, Hogenkamp DJ, Gee KW. (2018) Role of β2/3-specific GABA-A receptor isoforms in the development of hippocampus kindling epileptogenesis. Epilepsy Behav 82:57–63. [DOI] [PubMed] [Google Scholar]
- Reddy SD, Younus I, Clossen BL, Reddy DS. (2015) Antiseizure activity of midazolam in mice lacking δ-subunit extrasynaptic GABA(A) receptors. J Pharmacol Exp Ther 353:517–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogawski MA, Loya CM, Reddy K, Zolkowska D, Lossin C. (2013) Neuroactive steroids for the treatment of status epilepticus. Epilepsia 54 (Suppl 6):93–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupprecht R, Reul JM, Trapp T, van Steensel B, Wetzel C, Damm K, Zieglgänsberger W, Holsboer F. (1993) Progesterone receptor-mediated effects of neuroactive steroids. Neuron 11:523–530. [DOI] [PubMed] [Google Scholar]
- Saliba RS, Kretschmannova K, Moss SJ. (2012) Activity-dependent phosphorylation of GABAA receptors regulates receptor insertion and tonic current. EMBO J 31:2937–2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling MR, Klein P, Tsai J. (2017) Randomized, double-blind, placebo-controlled phase 2 study of ganaxolone as add-on therapy in adults with uncontrolled partial-onset seizures. Epilepsia 58:558–564. [DOI] [PubMed] [Google Scholar]
- Spigelman I, Li Z, Banerjee PK, Mihalek RM, Homanics GE, Olsen RW. (2002) Behavior and physiology of mice lacking the GABAA-receptor δ subunit. Epilepsia 43 (Suppl 5):3–8. [DOI] [PubMed] [Google Scholar]
- Spigelman I, Li Z, Liang J, Cagetti E, Samzadeh S, Mihalek RM, Homanics GE, Olsen RW. (2003) Reduced inhibition and sensitivity to neurosteroids in hippocampus of mice lacking the GABA(A) receptor δ subunit. J Neurophysiol 90:903–910. [DOI] [PubMed] [Google Scholar]
- Stell BM, Brickley SG, Tang CY, Farrant M, Mody I. (2003) Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by δ subunit-containing GABAA receptors. Proc Natl Acad Sci USA 100:14439–14444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutula TP. (1990) Experimental models of temporal lobe epilepsy: new insights from the study of kindling and synaptic reorganization. Epilepsia 31 (Suppl 3):S45–S54. [DOI] [PubMed] [Google Scholar]
- Upasani RB, Yang KC, Acosta-Burruel M, Konkoy CS, McLellan JA, Woodward RM, Lan NC, Carter RB, Hawkinson JE. (1997) 3 α-Hydroxy-3 β-(phenylethynyl)-5 β-pregnan-20-ones: synthesis and pharmacological activity of neuroactive steroids with high affinity for GABAA receptors. J Med Chem 40:73–84. [DOI] [PubMed] [Google Scholar]
- Vicini S, Losi G, Homanics GE. (2002) GABA(A) receptor δ subunit deletion prevents neurosteroid modulation of inhibitory synaptic currents in cerebellar neurons. Neuropharmacology 43:646–650. [DOI] [PubMed] [Google Scholar]
- Whissell PD, Lecker I, Wang DS, Yu J, Orser BA. (2015) Altered expression of δGABAA receptors in health and disease. Neuropharmacology 88:24–35. [DOI] [PubMed] [Google Scholar]
- Wohlfarth KM, Bianchi MT, Macdonald RL. (2002) Enhanced neurosteroid potentiation of ternary GABA(A) receptors containing the delta subunit. J Neurosci 22:1541–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Gangisetty O, Carver CM, Reddy DS. (2013) Estrous cycle regulation of extrasynaptic δ-containing GABA(A) receptor-mediated tonic inhibition and limbic epileptogenesis. J Pharmacol Exp Ther 346:146–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younus I, Reddy DS. (2018) A resurging boom in new drugs for epilepsy and brain disorders. Expert Rev Clin Pharmacol 11:27–45. [DOI] [PubMed] [Google Scholar]