Abstract
Background
Onyx® is a liquid embolic agent, which is approved for the treatment of cerebral vascular lesions but still rarely used in peripheral interventional radiology. The goal of this study is to report the feasibility and safety of embolization with Onyx® for peripheral hemostatic and non-hemostatic endovascular procedures.
Methods
Retrospective study of all consecutive patients who underwent visceral or peripheral embolization with Onyx® for hemostatic or non-hemostatic purpose in our department between May 2014 and November 2016. Demographic data, clinical presentation, underlying etiology, culprit vessel, endovascular procedure, pain during embolization, outcomes, and follow-up data were collected.
Results
Fifty patients (males, 34; females, 16; mean age, 56±18 years; range, 15–89 years) were included. Twenty-nine (58%) of patients underwent hemostatic embolization for arterial (n=22, 44%) or venous (n=7, 14%) bleeding lesions, whereas 21 (42%) of patients underwent non-hemostatic embolization for arterial aneurysms (n=8, 16%), preoperative portal vein deprivation (n=6, 12%) or other indications (n=7, 14%). Onyx-18 was used in 37 (74%) patients, Onyx-34 in 9 (18%) patients, and a combination of both in 4 (8%) patients. Onyx was used alone in 25 (50%) patients and in combination with other agent in 25 (50%) patients. Mean number of Onyx® vials used was 3.7 (range, 1–17). Immediate technical success rate was 100%. Primary clinical success was achieved in all patients. Recurrent bleeding occurred in two patients. Significant pain (pain score ≥3) was noted during injection in 10 (20%) patients. No major complication or side effects were noted within 1 month.
Conclusions
Transcatheter embolization with Onyx® is feasible and safe in the peripheral arterial or venous vasculature for both bleeding and non-bleeding patients whatever the anatomic site.
Keywords: Transcatheter embolization, ethylene vinyl alcohol (EVOH) copolymer, peripheral vascular lesions, hemorrhage, portal vein system
Introduction
First described in 1990, Onyx® is an elastic polymer composed of ethylene vinyl alcohol (EVOH) copolymer dissolved in dimethyl sulfoxide (DMSO) and mixed with micronized tantalum powder. The name Onyx® is a reference to the onyx stone, a dark-colored gem mainly found in Brazil. Onyx® is a liquid embolic agent that was granted approval by the US Food and Drug Administration in the treatment of cerebral arteriovenous malformations (AVMs) in 2005 (1). It is categorized as a non-absorbable, non-adhesive, permanent liquid embolic agent that may be used off-label for both small and large vessels (2). These features making EVOH copolymer effective in treating cerebrovascular lesions may benefit peripheral applications, which explain why its use is becoming more widespread among interventional radiologists in the treatment of peripheral lesions (3-13). However, Onyx® is still perceived by some interventional radiologists as an expensive and complex embolic agent. Nowadays, it is still one of the least popular embolic agents used. Although relatively straightforward to use, it is important to observe strictly the related technical details and particular recommendations. In France, Onyx® is currently approved and reimbursed in some peripheral applications only for the treatment of AVMs, type 2 endoleak after endovascular abdominal aneurysm repair (EVAR) and massive or recurrent hemoptysis after failure of particulate embolization. The goal of this study is to assess the feasibility and safety of embolization using Onyx® in some other off-label peripheral hemostatic and non-hemostatic applications for which this promising embolic agent may be used.
Methods
Patient population
We retrospectively included a total of 50 patients who underwent Onyx® peripheral and visceral hemostatic or non-hemostatic embolization procedures in our Interventional Radiology Department at Dijon University Hospital between May 2014 and November 2016. When necessary, additional embolic agents such as N-butyl-cyanoacrylate (NBCA) glue, coils, stents or gelatin sponge particles were used. Cerebral, ear, nose and throat lesions were excluded from the study.
Pre-operative imaging
All patients were assessed prior to endovascular treatment. Patients suffering from peripheral hemorrhagic lesions underwent a pre-embolization computed tomography (CT) scan in order to confirm and locate the bleeding. After an unenhanced acquisition, arterial phase and venous phase images were obtained following the administration of iodinated contrast. Hemorrhage was defined as a contrast extravasation or false aneurysm as a spherical or ovoid cavity communicating with a breached vessel wall. Patients suffering from peripheral artery aneurysm also underwent a CT scan with arterial phase that enabled to visualize the aneurysm and its afferent and efferent branches, consider treatment possibilities and evaluate the ischemic risk of downstream. The analysis of the portal venous anatomy prior to preoperative portal vein embolization was also refined by a contrast-enhanced abdominal CT. Patients with renal angiomyolipoma and type II endoleaks underwent a contrast-enhanced abdominal CT prior to embolization. The varicocele diagnoses were confirmed via Doppler ultrasound whereas cutaneous vascular malformations were assessed using magnetic resonance imaging (MRI).
Endovascular procedure
All patients underwent diagnostic angiography performed either under local or general anesthesia. As for catheterization, it was achieved using standard coaxial techniques. A DMSO-compatible microcatheter was used to perform selective and super-selective catheterization until the embolization site was reached. The most commonly used microcatheters were the 2.7-, 2.4- or 2.0-Fr Progreat® microcatheter (Terumo, Tokyo, Japan) as well as the 2.4-F Maestro® microcatheter (Merit Medical, South Jordan, UT, USA).
Onyx® (Onyx® LES, Covidien, Plymouth, MN, USA) was used in all cases, alone or in combination with other embolic agents. It is a non-adhesive liquid embolic agent composed of EVOH copolymer dissolved in DMSO with micronized tantalum powder enabling visualization under fluoroscopy. The Onyx® liquid embolic system consists of a 1.5 mL vial of Onyx®, a 1.5 mL vial of DMSO and three 1 mL syringes and is available in two product formulations: Onyx®-18 (6% EVOH) and Onyx®-34 (8% EVOH). Due to its lower viscosity, Onyx®-18 travels more distally and penetrates deeper than Onyx®-34. Before use, the product temperature must be held between 19 and 24 °C and the vials must be shaken on a mixer for at least 20 minutes.
Once the microcatheter is positioned correctly, it has to be flushed with saline solution and then with DMSO to fill the microcatheter’s “dead space”. Onyx is then drawn up into a 1 mL syringe before being injected slowly to fill the microcatheter and replace the DMSO in said dead space. Finally, the product is injected using fluoroscopic guidance to make sure both the volume and speed are low enough in such a way as to prevent Onyx® reflux around the microcatheter. We observed that copolymer precipitation is initiated by diffusion of DMSO upon contact between the mixture and aqueous solution. This procedure starts on the external surface while the core remains liquid and results in a soft, non-adherent, lava-like mass, which does not adhere to either the endothelium or the tip of the catheter, thus ensuring a successful procedure without any fragmentation during injection.
The injection was maintained and repeated until total occlusion of the targeted bleeding site or vascular anomaly was achieved. It should also be noted that final solidification occurred within 5 minutes for both product formulations. Due to its non-adhesive feature, multiple Onyx® injections could be performed. Post-embolization angiography through the standard catheter after removal of the microcatheter later confirmed the success of the different procedures.
Data collection
In the framework of this study, we retrospectively collected each patient’s demographic data, clinical presentation, underlying etiology and interventional data. Demographic data included age and gender; whereas interventional data included endovascular treatment, the emergency nature of the procedure, the type of anesthesia (local or general), the culprit embolized vessel (artery or vein), the number of Onyx® vials used, the concentration of Onyx® [Onyx-18 (6% EVOH) or Onyx-34 (8% EVOH)], the use of other embolic agents (coils, glue, stents or gelatin sponge), the angiographic success, the level of pain experienced during embolization as well as the complications and recurrence within 1 month following the procedure.
Technical success was defined as a complete occlusion of the targeted bleeding site or vascular anomaly on final angiography. All complications were recorded and classified as major or minor complications according to the Society of Interventional Radiology classification system (Table 1).
Table 1. SIR classification system for complications by outcome.
| Minor complications |
| No therapy, no consequence |
| Nominal therapy, no consequence; includes overnight admission for observation only |
| Major complications |
| Require therapy, minor hospitalization (<48 h) |
| Require major therapy, unplanned increase in lever of care, prolonged hospitalization (>48 h) |
| Permanent adverse sequelae |
| Death |
SIR, Society of Interventional Radiology.
Statistical analysis
Descriptive statistics and parameters, such as frequencies and percentages, were used and provided in order to accurately describe our experience regarding the embolization of peripheral lesions using Onyx®. Due to the retrospective nature of this study, our Ethics Committee waived the requirement for informed patient consent.
Results
Population study
No missing data was noted. From May 2014 to November 2016, 50 consecutive patients underwent peripheral embolization using Onyx® at Dijon University Hospital (Table 2). The mean age was 56±18 years (range, 15–89 years). Among the studied cases, we identified 34 (68%) males and 16 (32%) females. Twenty-nine (58%) patients underwent an emergency hemostatic embolization whereas 21 (42%) of them underwent a scheduled non-hemostatic embolization. Onyx®-18 (6% EVOH) was the concentration used in 37 cases (74%) whereas Onyx®-34 (8% EVOH) was used in 9 of them (18%). Besides, a combination of different concentrations (Onyx®-18 and Onyx®-34) was used in 4 cases (8%). It is worth noting that Onyx® was the sole agent used in 25 patients (50%) and that concomitant embolic agents included coils (n=15, 30%), NBCA-glue (n=4, 8%), stents (n=1, 2%), gelatin sponge (n=1, 2%) or a combination of embolic agents (n=4, 8%).
Table 2. Patient demographics and embolization data.
| Variables | Data |
|---|---|
| Age at presentation, years | |
| Mean ± SD | 56±18 |
| Median [range] | 54 [15–89] |
| Gender, n [%] | |
| Female | 16 [32] |
| Male | 34 [68] |
| Procedure, n [%] | |
| Hemostatic embolization | 29 [58] |
| Non-hemostatic embolization | 21 [42] |
| Embolized vessel, n [%] | |
| Artery | 35 [70] |
| Vein | 15 [30] |
| Onyx concentration, n [%] | |
| Onyx-18 | 37 [74] |
| Onyx-34 | 9 [18] |
| Both | 4 [8] |
| Onyx alone, n [%] | 25 [50] |
| Onyx with other embolic agents, n [%] | 25 [50] |
| Coils | 15 [30] |
| NBCA glue | 4 [8] |
| Stent | 1 [2] |
| Gelatin sponge | 1 [2] |
| Combination of embolic agents | 4 [8] |
NBCA, N-butyl-cyanoacrylate; SD, standard deviation.
The embolization data, complications and recurrence according to the indications are presented in Table 3. Indications were classified into four groups: hemorrhagic lesions (n=29, 58%), arterial aneurysms (n=8, 16%) (Figure 1), preoperative portal vein embolization (n=6, 12%) (Figure 2) and other indications (n=7, 14%). The main indications for Onyx® embolization were arterial gastrointestinal hemorrhages (n=14, 28%) (Figure 3). The most common etiologies of arterial gastrointestinal hemorrhage included gastrointestinal ulcers (n=4), false post-pancreatitis aneurysms (n=4) and diverticulosis (n=2). Etiologies of non-gastrointestinal arterial hemorrhages (n=8, 16%) included hemoptysis due to lung cancer (n=2), hematuria from post-traumatic kidney fracture (n=4) and muscular hematoma (n=2). Etiologies of venous gastrointestinal hemorrhages included rupture of esophageal varices in cirrhotic patients (n=6) and rupture of perigastric varices favored by superior mesenteric vein thrombosis in a pancreatic cancer patient (n=1). The other indications included embolization of type II endoleaks (n=2), angiomyolipoma (n=2), varicocele (n=2) and cutaneous AVM (n=1).
Table 3. Procedural data, complications and recurrence according to the indications.
| Indications | n [%] | Anesthesia, n [%] | Onyx concentration, n [%] | Onyx vial, mean [range] | Other embolic agent, n [%] | Pain, n [%] | Complications, n [%] | Recurrence, n [%] | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LA | GA | Onyx 18 | Onyx 34 | Both | Coils only | Other | |||||||
| Hemorrhagic lesions | 29 [58] | 22 [44] | 7 [14] | 24 [48] | 4 [8] | 1 [2] | 2.2 [1–6] | 7 [14] | 7 [14] | 2 [4] | 3 [6] | 2 [4] | |
| Arterial | |||||||||||||
| Gastrointestinal hemorrhages | 14 [28] | 14 [28] | 0 | 11 [22] | 3 [6] | 0 | 1.4 [1–3] | 5 [10] | 3 [6] | 1 [2] | 2 [4] | 1 [2] | |
| Non-gastrointestinal hemorrhages* | 8 [16] | 8 [16] | 0 | 7 [14] | 1 [2] | 0 | 2 [1–4] | 1 [2] | 3 [6] | 1 [2] | 0 | 1 [2] | |
| Venous | 7 [14] | 0 | 7 [14] | 6 [12] | 0 | 1 (2) | 4 [2–6] | 1 [2] | 1 [2] | NA | 1 [2] | 0 | |
| Aneurysms | 8 [16] | 8 [16] | 0 | 4 [8] | 4 [8] | 0 | 3 [1–6] | 7 [14] | 1 [2] | 0 | 2 [4] | 0 | |
| Preoperative portal vein embolization | 6 [12] | 0 | 6 [12] | 4 [8] | 0 | 2 [4] | 12.3 [5–17] | 0 | 1 [2] | NA | 0 | 0 | |
| Other indications** | 7 [14] | 7 [14] | 0 | 5 [10] | 1 [2] | 1 [2] | 3 [1–6] | 1 [2] | 1 [2] | 2 [4] | 0 | 0 | |
| Total | 50 [100] | 37 [74] | 13 [26] | 37 [74] | 9 [18] | 4 [8] | 3.7 [1–17] | 15 [30] | 10 [20] | 4 [8] | 5 [10] | 2 [4] | |
*, hemoptysis, hematuria, muscular hematoma; **, type II endoleaks, arteriovenous malformations, varicoceles, angiomyolipoma. n, number of patient(s); LA, local anesthesia; GA, general anesthesia; NA, not assessable due to general anesthesia.
Figure 1.
Embolization of a splenic artery aneurysm with Onyx®. (A) Unexpected detection of a large asymptomatic aneurysm of the splenic artery on axial CT view in a 39-year-old male; (B) selective angiography of the splenic artery showing a 3.5 cm aneurysm of the distal part of the splenic artery; (C) coils were first positioned into the aneurysmal sac to achieve packing and reduce the intra-aneurysmal flow; (D) complete embolization of the aneurysm was achieved using Onyx-34 to fill in the sac; arrowhead indicates the presence of Onyx; (E) control angiography confirmed satisfactory embolization with complete occlusion of the aneurysmal sac; (F,G,H) at 1-month follow-up, axial T2-weighted MR view and axial T1-weighted contrast-enhanced MR images showed complete exclusion of the aneurysm with preservation of the splenic artery patency and normal spleen parenchyma; arrow indicates complete thrombosis of the aneurysmal sac. CT, computed tomography; MR, magnetic resonance.
Figure 2.
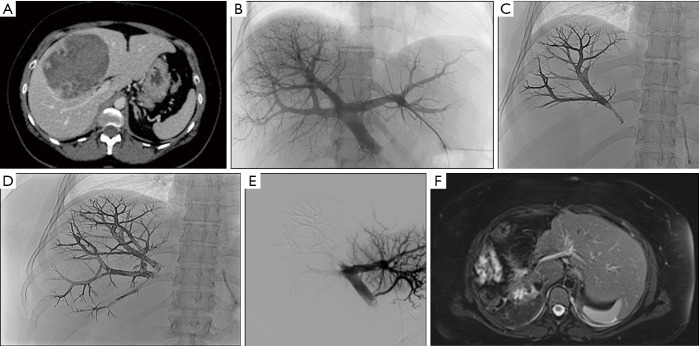
Preoperative portal vein embolization with Onyx®. (A) 35-year-old female patient with voluminous hepatocellular carcinoma of the right liver on axial CT view. After consulting with the multidisciplinary board, a right portal vein embolization was carried out to allow left liver hypertrophy followed by a right hepatectomy; (B) 3D portography following percutaneous left portal branch puncture showing a portal trifurcation; (C,D) Onyx embolization of anterior and posterior sectoral portal branches with Onyx-18;. (E) control portography showing good exclusion of anterior and posterior sectoral portal branches; (F) axial fat-sat T2-weighted MRI view showing a good hypertrophy of the left liver and the sequelae of a right hepatectomy. CT, computed tomography; MRI, magnetic resonance imaging.
Figure 3.
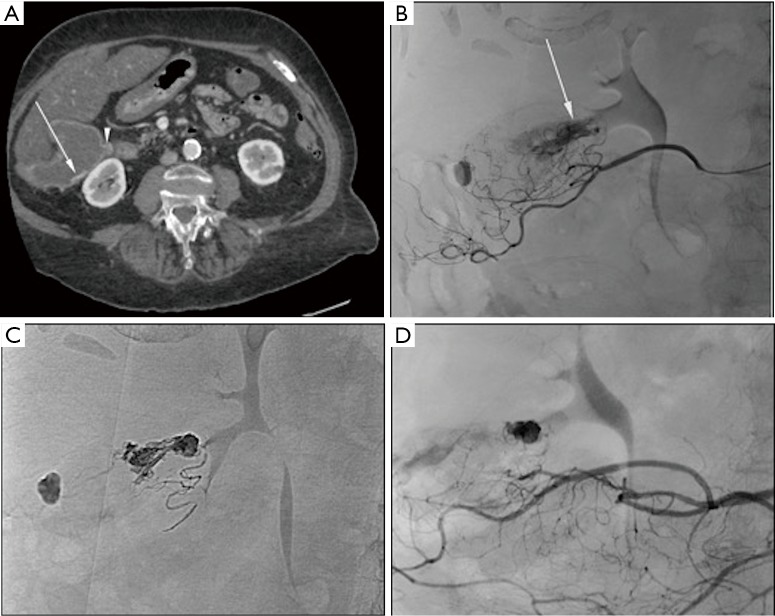
Arterial embolization of acute lower gastrointestinal bleeding with Onyx®. (A) Axial CT view of an 89-year-old female patient with gastrointestinal hemorrhage showing extravasation of iodinated contrast (arrow) in the right diverticular colon (triangle); (B) super-selective angiography of the superior mesenteric artery showing extravasation of iodinated contrast (arrow) from the middle colic artery branch; (C) Onyx embolization of the middle colic artery branch using Onyx-18; (D) control angiography after Onyx embolization showing complete occlusion of the branch responsible for the bleeding. CT, computed tomography.
Unlike preoperative portal vein embolization and gastrointestinal venous hemorrhage (treated via transjugular intrahepatic portosystemic shunt or portal vein transhepatic puncture) which were carried out under general anesthesia, all remaining angiographies were performed under local anesthesia. Due to its low viscosity and its ability to travel more distally and penetrate deeper, Onyx®-18 (6% EVOH) was the most widely used concentration, especially in the treatment of hemorrhagic lesions (n=24, 48%). As for Onyx®-34 (8% EVOH), it was mostly used with vascular coils in treating aneurysms. Vascular coils were first positioned in the aneurysmal sac to achieve packing and reduce the aneurysmal flow. Embolization was then completed with Onyx®-34 in order to completely exclude the aneurysm. A combination of different concentrations (Onyx®-18 and Onyx®-34) was mainly used for preoperative portal vein embolization procedures. After using Onyx®-18 to fill in the distal portal branches, Onyx®-34 was used to safely supplement embolization by avoiding reflux. The number of Onyx® vials used ranged from 1 to 17, and in 27 (54%) cases ≤2 Onyx® vials were necessary. It is worth noting that a large amount of Onyx® vials was used for preoperative portal vein embolization [mean, 12.3 vials (range, 5–17 vials)] compared to a low amount for the other indications [mean, 2.5 vials (range, 1–6 vials)].
Outcomes
Angiographic success was achieved in all patients. Rebleeding occurred in 2 patients (4%), namely, one case of hematochezia in a cirrhotic patient suffering from hemorrhoids secondary to portal hypertension and another case of hematoma of the rectus sheath muscle. Regarding the hematoma case, it should be noted that said rebleeding did not occur at the initial embolization site and that the patient underwent an additional embolization the following day, which enabled to effectively treat the bleeding. Unlike the rectus abdominis muscle hematoma, in the case of hemorrhoids due to post-embolic rectal ischemia, the rebleeding occurred at the same initial embolization site. This recurrence was later successfully managed by endoscopic treatment. In this case, the patient had already undergone several unsuccessful endoscopic hemostatic treatments prior to Onyx® embolization. No aneurysmal recanalization occurred. Surgery was possible in all patients after portal vein embolization within 4 weeks.
Complications
Based on the Society of Interventional Radiology classification system, two and three minor complications were identified. Two patients passed away within 1 month following peripheral embolization with Onyx® but only one patient died from the embolization procedure. Said patient suffered from venous gastrointestinal hemorrhage secondary to perigastric varices favored by superior mesenteric vein thrombosis in a context of metastatic pancreatic cancer. Prior to his death, said patient had undergone a recanalization of the superior mesenteric vein along with an Onyx® embolization of the perigastric varices under favorable circumstances. Unfortunately, the percutaneous portal vein puncture site could not be controlled upon removal of the sheath, which resulted in the patient’s death from excessive bleeding at the transhepatic puncture site in the resuscitation room immediately following the procedure. As for the other patient, he died from multisystem organ failure in the medical intensive care unit few days after undergoing the embolization of a gastrointestinal arterial hemorrhage secondary to postoperative pseudoaneurysm following sleeve gastrectomy. It is worth noting that death was due to postoperative complications and not to peripheral embolization. Besides, another patient presented cholangitis with hepatic abscesses due to the migration of his biliary prosthesis, following a preoperative portal embolization. Here again, no correlation could be made between the cholangitis and the embolization procedure. The last major complication was abscess formation in the infarcted splenic parenchyma following the preventive embolization of an asymptomatic splenic artery aneurysm. This splenic abscess was drained radiologically with good clinical outcome.
Finally, minor embolization-related complications were identified in three patients. These complications included one case of small subcapsular splenic hematoma following a false aneurysm embolization of the splenic artery due to an acute pancreatitis and two cases of embolization material reflux, which were inconsequential to the patient’s health. In addition, 4 (11%) patients out of the 37 who underwent peripheral embolization under local anesthesia experienced pain during Onyx® injection caused by the diffusion of DMSO in the vessels. In all cases, the pain was easily controlled using usual analgesic drugs.
Discussion
Onyx® is a well-known non-adhesive, non-absorbable and permanent liquid embolic agent that has gained popularity outside of neurovascular applications (3). It is available in two product formulations, Onyx®-18 and Onyx®-34, Onyx®-34 offering a better control while injecting the embolic agent. This essential feature ensures an optimal control of the Onyx® injection with minimal risk of non-target embolization in case the microcatheter tip is close to the target lesion or inside the latter. Besides, Onyx®-34 may also be used in case of risks of migration due to high vascular flow in order to maintain better control over the embolization. As for Onyx®-18 and its lower viscosity formulation, it is preferred when a lesion is distant from the microcatheter tip as it allows the liquid embolic agent to travel more distally and to penetrate deeper, thus ensuring a more appropriate lesion embolization. In addition, Onyx® is equally effective in the treatment of peripheral lesions whether it is used alone or combined with other embolic agents. Finally, the use of Onyx® in peripheral applications offers numerous benefits due to its physical properties such as its viscous nature, its non-adherent features, its slow polymerization, its ability to conform to any vessel shape and its high fluoroscopic visibility (14,15).
With a total of 50 patients, our series is one of the largest on peripheral embolization using Onyx®. In accordance with the literature, this series confirms that Onyx® is both effective and safe in treating peripheral lesions, which strengthens the possibility to use it in different anatomical sites. As already highlighted by various authors, Onyx® can be used for the embolization of all peripheral hemorrhages (3,16), aneurysms, type II endoleaks (17), hemoptysis (18), cutaneous AVMs (6), angiomyolipoma (19). Besides, in our study, 6 patients (12%) underwent preoperative portal embolization using Onyx® without any complication, a fact that has yet never been described in the literature. Portal embolization allowed hypertrophy of the remaining liver and improved liver function, which enabled the patients to benefit from a curative surgery in a second phase.
The main advantages of this liquid embolic agent compared to other similar agents such as NBCA-glue are its non-adherence, its progressive solidification, its cohesiveness, its high vascular penetration and its very weak inflammatory effect on the endothelium (4). Said non-adherent property avoids the main problems usually associated with the use of glue, especially the one regarding adherence to the internal and external walls of the microcatheters. As a result, the use of Onyx® prevents any theoretical risk of obstructing the microcatheters during procedure, any adhesion of Onyx® fragments to the distal end of the microcatheters as well as any risk of adhesive trapping of the microcatheters. Not only do all of these features make the procedure extremely safe, but they also enable the operator to pause the injection at any time leaving the distal end of the catheter in place before resuming the procedure a few minutes later.
Unlike glue polymerization, which takes place immediately upon contact with ionic solutions such as blood, Onyx® has a liquid form at the time of injection that will progressively turn into a solid form upon contact with blood through precipitation upon DMSO dissipation. Therefore, this property allows slower and longer injection rates that are easier to control. Most importantly, Onyx® has a lava-like flow pattern within blood vessels without any fragmentation during injection. When using Onyx®, one method used consists of waiting 2 to 3 minutes for the reflux to solidify before resuming the injection. That way, the product released from the distal end of the catheter can no longer reflux as it anterogradely reaches the territory to be embolized. Finally, Onyx® does not need to be injected directly at its target site. Indeed, in case tortuous vessels prevent access via the microcatheters, the slow polymerization of Onyx® will keep the latter in a semisolid state for approximately 5 minutes after deployment, hence allowing the product to be pushed forward by the operator or carried away by the bloodstream, adapting itself acutely to the vessels as it progresses (20).
Onyx® embolization has proven to cause a smaller area of extravascular inflammatory tissue compared to glue, which results in a more tolerable experience for patients. Due to a possible vasospasm reaction related to vasculitic phenomena, a slow and careful injection speed (0.16–0.3 mL/min) is essential to prevent it. Vasospasm reaction can be very dangerous considering it increases the risks of both reflux and non-target embolization. In the same way, a painful reaction during injection related to vasculitic phenomena caused by DMSO on the vascular endothelium may be observed. However, these painful reactions amounting to 11% in our study are usually rare and can be controlled using usual analgesic drugs. As for the embolized vessels, they are completely filled with embolic material and are less fragile due to the lower inflammatory reaction.
Onyx® radio-opacity using tantalum powder was optimal and enabled us to closely monitor the entire procedure without any complication. Both this good radio-opacity and the Onyx® injection under permanent subtracted radioscopy allowed preventing non-target embolizations. It should be noted that tantalum powder is likely to cause significant streak artifacts on follow-up CT imaging. Indeed, more artifacts are caused by tantalum powder than by lipiodol used with glue but less than those caused by coils. Hence, follow-up by ultrasound and MRI imaging should be preferred over CT. Onyx® appears as a hypointensity on all MRI images, with no artifact (21).
While performing damaged vessels embolization, coil or vascular plug deployment within a fragile artery may rupture said vessels and result in massive hemorrhage. In this case, a liquid embolic agent such as Onyx® was preferred as it deploys without applying any radial force to vessel walls.
In high-flow lesions, where particles and glue are difficult to control, Onyx® was chosen for its high viscosity and lava-like flow pattern as it decreases the risk of non-target embolization and organ infarction. Slow, careful, and repeated injections of Onyx® may be performed without risk of catheter entrapment. It should also be noted that the effectiveness of both microcoils and particles depends on a normal coagulation status and that the level of post-embolization clinical failure is higher when the patient suffers from coagulopathy (22). The homeostatic power of Onyx® is not influenced by the patient’s coagulation status, which represents a major advantage in compromised patients. Onyx® was injected through the thinnest most flexible catheters that could be placed most distally and close to the lesion, providing full control over the bleeding. It is interesting to highlight that coils and catheters compatible with particulate agents may not go as distally far. Finally, deposition of the embolic material close to the lesion was essential in order to cure the lesion in one treatment session.
Onyx® vials need to be shaken for 15–20 minutes to obtain a consistent mixture of EVOH and tantalum powder in order to achieve homogeneous radio-opacity of said mixture (18), a necessary precaution prior to use that potentially limits its use in case of emergency. To address that issue, in some cases, vials are shaken before the arrival of the patient in the angiography room. In our study, 39 patients (78%) underwent emergency embolizations using Onyx®. Although very expensive, it is important to use DMSO compatible microcatheters since the use of another type of device could cause Onyx® percolation that may result in a fractured microcatheter or a in a breach through which Onyx® could accidentally leak.
If subcutaneous vessels such as cutaneous vascular malformations are embolized, the tantalum powder present in Onyx® may leave deposits in the tissues and cause permanent tattooing of the skin. As the DMSO dissipates, patients may experience a peculiar garlic-like smell and taste in their sweat and breath. This inconvenience usually disappears within 2 days following treatment.
The use of Onyx® requires an essential specific training. In our opinion, however, a complete and proper formal training in catheter-based endovascular techniques seems even more important. We are convinced that the existing training on glue could be applied to the use of Onyx® and that any interventionists should become familiar with such promising embolic material. Finally, it seems relevant to mention that the high cost of Onyx® could be a potential factor limiting its use in peripheral applications (23) and that the catheters recommended for use with DMSO are also more expensive than their more common counterparts.
As a monocentric, retrospective and non-randomized descriptive analysis study, the latter may present some limitations. Some indications of peripheral embolization such as hemorrhages are potentially deadly emergency situations, which make a prospective randomized study very difficult to conduct. In addition, the presented case series is non-homogenous since all peripheral embolizations with Onyx® are included, regardless of whether or not they were emergency procedures, not taking into account the fact that Onyx® is used alone or with other embolic agents. However, in our study, hemorrhages, aneurysms and preoperative portal embolizations were the three most frequent indications for Onyx® embolization. Besides, since preoperative portal embolization with Onyx® has not yet been described in the literature, it might be interesting to further study this subgroup in the future.
Conclusions
Embolization with Onyx® is feasible, safe and effective in treating peripheral lesions besides the usual neurovascular applications. Currently, in France, the only peripheral indications eligible for Onyx® reimbursement are treatment of AVMs, type II endoleaks and hemoptysis. It is thus important to balance cost versus benefits when selecting any embolic agents. Due to its physical properties, Onyx® may be useful in many other peripheral applications, especially for treatment of hemorrhages, aneurysms or for preoperative portal vein embolization. More liberal use of Onyx® will allow interventional radiologist to be trained in all the existing embolic agents.
Acknowledgements
None.
Ethical Statement: The study was approved by the Dijon University Ethics Committee. Written informed consent was obtained from all patients for publication of this original series and any accompanying images.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Molyneux AJ, Cekirge S, Saatci I, Gál G. Cerebral Aneurysm Multicenter European Onyx (CAMEO) trial: results of a prospective observational study in 20 European centers. AJNR Am J Neuroradiol 2004;25:39-51. [PMC free article] [PubMed] [Google Scholar]
- 2.Vaidya S, Tozer KR, Chen J. An overview of embolic agents. Semin Intervent Radiol 2008;25:204-15. 10.1055/s-0028-1085930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kolber MK, Shukla PA, Kumar A, Silberzweig JE. Ethylene vinyl alcohol copolymer (onyx) embolization for acute hemorrhage: a systematic review of peripheral applications. J Vasc Interv Radiol 2015;26:809-15. 10.1016/j.jvir.2015.02.025 [DOI] [PubMed] [Google Scholar]
- 4.Guimaraes M, Wooster M. Onyx (Ethylene-vinyl Alcohol Copolymer) in peripheral applications. Semin Intervent Radiol 2011;28:350-6. 10.1055/s-0031-1284462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Urbano J, Manuel Cabrera J, Franco A, Alonso-Burgos A. Selective arterial embolization with ethylene-vinyl alcohol copolymer for control of massive lower gastrointestinal bleeding: feasibility and initial experience. J Vasc Interv Radiol JVIR 2014;25:839-46. 10.1016/j.jvir.2014.02.024 [DOI] [PubMed] [Google Scholar]
- 6.Numan F, Ömeroğlu A, Kara B, Cantaşdemir M, Adaletli İ, Kantarcı F. Embolization of peripheral vascular malformations with ethylene vinyl alcohol copolymer (Onyx). J Vasc Interv Radiol 2004;15:939-46. 10.1097/01.RVI.0000130862.23109.52 [DOI] [PubMed] [Google Scholar]
- 7.Abularrage CJ, Patel VI, Conrad MF, Schneider EB, Cambria RP, Kwolek CJ. Improved results using Onyx glue for the treatment of persistent type 2 endoleak after endovascular aneurysm repair. J Vasc Surg 2012;56:630-6. 10.1016/j.jvs.2012.02.038 [DOI] [PubMed] [Google Scholar]
- 8.Bommart S, Bourdin A, Giroux MF, Klein F, Micheau A, Bares VM, Kovacsik H. Transarterial ethylene vinyl alcohol copolymer visualization and penetration after embolization of life-threatening hemoptysis: technical and clinical outcomes. Cardiovasc Intervent Radiol 2012;35:668-75. 10.1007/s00270-011-0270-3 [DOI] [PubMed] [Google Scholar]
- 9.Pan JW, Zhou HJ, Zhan RY, Wan S, Yan M, Fan WJ, Wu ZX, Zheng SS. Supratentorial brain AVM embolization with Onyx-18 and post-embolization management. A single-center experience. Interv Neuroradiol 2009;15:275-82. 10.1177/159101990901500304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hauck EF, Welch BG, White JA, Purdy PD, Pride LG, Samson D. Preoperative embolization of cerebral arteriovenous malformations with Onyx. AJNR Am J Neuroradiol 2009;30:492-5. 10.3174/ajnr.A1376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Izaaryene J, Vidal V, Bartoli JM, Gaubert JY. Multiple bronchial artery aneurysms: Successful treatment with ethylene-vinyl alcohol copolymer (Onyx®). Diagn Interv Imaging 2016;97:125-7. 10.1016/j.diii.2015.05.009 [DOI] [PubMed] [Google Scholar]
- 12.Chevallier O, Gehin S, Foahom-Kamwa A, Pottecher P, Favelier S, Loffroy R. Ethylene-vinyl alcohol copolymer (Onyx®) transarterial embolization for post-traumatic high-flow priapism. Quant Imaging Med Surg 2016;6:323-7. 10.21037/qims.2016.06.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Regine R, Palmieri F, De Siero M, Rescigno A, Sica V, Cantarela R, Villari V. Embolization of traumatic and non-traumatic peripheral vascular lesions with Onyx. Interv Med Appl Sci 2015;7:22-9. 10.1556/IMAS.6.2014.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duffner F, Ritz R, Bornemann A, Freudenstein D, Wiendl H, Siekmann R. Combined therapy of cerebral arteriovenous malformations: histological differences between a non-adhesive liquid embolic agent and n-butyl 2-cyanoacrylate (NBCA). Clin Neuropathol 2002;21:13-7. [PubMed] [Google Scholar]
- 15.Rennert J, Herold T, Schreyer AG, Banas B, Jung EM, Feuerbach S, Lenhart M, Mueller-Wille R, Zorger N. Evaluation of a liquid embolization agent (Onyx) for transcatheter embolization for renal vascular lesions. Rofo 2009;181:996-1001. 10.1055/s-0028-1109741 [DOI] [PubMed] [Google Scholar]
- 16.Müller-Wille R, Heiss P, Herold T, Jung EM, Schreyer AG, Hamer OW, Rennert J, Hoffstetter P, Stroszczynski C, Zorger N. Endovascular treatment of acute arterial hemorrhage in trauma patients using ethylene vinyl alcohol copolymer (Onyx). Cardiovasc Intervent Radiol 2012;35:65-75. 10.1007/s00270-011-0134-x [DOI] [PubMed] [Google Scholar]
- 17.Nevala T, Biancari F, Manninen H, Aho PS, Matsi P, Mäkinen K, Roth WD, Ylönen K, Lepäntalo M, Perälä J., Type II. Endoleak after endovascular repair of abdominal aortic aneurysm: effectiveness of embolization. Cardiovasc Intervent Radiol 2010;33:278-84. 10.1007/s00270-009-9685-5 [DOI] [PubMed] [Google Scholar]
- 18.Khalil A, Parrot A, Fartoukh M, Djibre M, Tassart M, Carette MF. Pulmonary artery occlusion with ethylene vinyl alcohol copolymer in patients with hemoptysis: initial experience in 12 cases. AJR Am J Roentgenol 2012;198:207-12. 10.2214/AJR.10.5370 [DOI] [PubMed] [Google Scholar]
- 19.Katsanos K, Sabharwal T, Ahmad F, Dourado R, Adam A. Onyx embolization of sporadic angiomyolipoma. Cardiovasc Intervent Radiol 2009;32:1291-5. 10.1007/s00270-008-9481-7 [DOI] [PubMed] [Google Scholar]
- 20.Lenhart M, Paetzel C, Sackmann M, Schneider H, Jung EM, Schreyer AG, Feuerbach S, Zorger N. Superselective arterial embolisation with a liquid polyvinyl alcohol copolymer in patients with acute gastrointestinal haemorrhage. Eur Radiol 2010;20:1994-9. 10.1007/s00330-010-1762-2 [DOI] [PubMed] [Google Scholar]
- 21.Saeed Kilani M, Izaaryene J, Cohen F, Varoquaux A, Gaubert JY, Louis G, Jacquier A, Bartoli JM, Moulin G, Vidal V. Ethylene vinyl alcohol copolymer (Onyx®) in peripheral interventional radiology: indications, advantages and limitations. Diagn Interv Imaging 2015;96:319-26. 10.1016/j.diii.2014.11.030 [DOI] [PubMed] [Google Scholar]
- 22.Carberry G, Dalvie P, Ozkan O. Onyx as a second-line embolic agent in peripheral applications. J Vasc Interv Radiol 2013;24:abstr S103.
- 23.Loffroy R, Favelier S, Genson PY, Guiu B. Onyx for embolization of life-threatening hemoptysis: a promising but luxury embolic agent! Cardiovasc Intervent Radiol 2012;35:221; author reply 222. 10.1007/s00270-011-0331-7 [DOI] [PubMed] [Google Scholar]