Abstract
Although supplementing the diet with zinc oxide and arginine is known to improve growth in weanling piglets, the mechanism of action is not well understood. We measured the antioxidant status and inflammatory response in 48 weanling castrated male piglets fed diets supplemented with or without zinc oxide (2,500 mg Zn oxide per kg) and arginine (1%) starting at the age of 20 days. The animals were injected with lipopolysaccharide (100 μg/kg) on day 5. Half of them received another injection on day 12. Blood samples were taken just before and 6, 24 and 48 h after injection and the mucosa lining the ileum was recovered following euthanizing on days 7 and 14. Zinc supplementation increased reduced and total glutathione (GSH) (reduced and total) during days 5 to 7 and arginine decreased oxidized GSH measured on days 5 and 12 and the ratio of total antioxidant capacity to total oxidative status during days 12 to 14. Zinc decreased plasma malondialdehyde measured on days 5 and 12 and serum haptoglobin measured on day 12 and increased both metallothionein-1 expression and total antioxidant capacity measured in the ileal mucosa on day 14. Tumour necrosis factor α concentration decreased from days 5 to 12 (all effects were significant at P < 0.05). This study shows that the zinc supplement reduced lipid oxidation and lipopolysaccharide-induced inflammation during the post-weaning period, while the arginine supplementation had only a limited effect.
Keywords: Antioxidant, Inflammation, Zinc, Arginine, Piglet, Weanling
1. Introduction
In weanling piglets, the abrupt change in diet from milk to cereal-based solid feed leads to specific systemic and intestinal perturbations, including over-expression of pro-inflammatory cytokines (Pié et al., 2004) and increased levels of haptoglobin, an acute-phase protein in blood (Petersen et al., 2004, Sauerwein et al., 2005). An increase in oxidative stress is also noted (Sauerwein et al., 2005, Zhu et al., 2012, Yin et al., 2014). Oxidative stress results from production of reactive oxygen species (ROS) exceeding the capacity of the antioxidant system. Its indicators include increases in oxidation products such as malondialdehyde and oxidized glutathione (GSH) (Jaeschke, 2011).
Numerous experiments have shown that feeding pharmacological doses of inorganic zinc (2,000 to 3,000 mg Zn oxide per kg of feed) to weanling piglets improves growth performance, reduces diarrhoea, has a positive impact on the immune response and reduces intestinal malformations (Hu et al., 2012a, Hu et al., 2012b, Hu et al., 2013, Sales, 2013). In our recent study of piglets, we found that zinc supplementation at 2,500 mg/kg after weaning decreased lipid oxidation measured as plasma malondialdehyde concentration (Bergeron et al., 2014). The supplementation improves the total antioxidant capacity measured in the mucosae of the jejunum and ileum 3 h after an acute inflammatory challenge with lipopolysaccharide (Bergeron et al., 2014). It remains to be determined whether or not similar effects are observed for a lipopolysaccharide-induced chronic inflammatory condition. Being major components of the outer membrane of Gram-negative bacteria, lipopolysaccharide injected into the bloodstream can cause a wide variety of pathological effects, including tissue injury, release of various pro-inflammatory cytokines and increased production of ROS, nitric oxide (NO) and lipid peroxides (Wyns et al., 2015). The intensity of these effects depends on the dose and time after injection (Wyns et al., 2015).
Studies have also shown that arginine supplements (0.5% to 1% of the diet) improve growth and feed efficiency in weaned piglets (Wu et al., 2010, Yao et al., 2011). We have observed that this supplement improves systemic antioxidant status 3 h after acute challenge with lipopolysaccharide (Bergeron et al., 2014). Arginine is an essential precursor for the synthesis of NO, a key mediator in several physiological functions. Increased production and concentration of NO is known to cause Zn2+ release in endothelial cells and to increase metallothionein-1 expression (Wiseman et al., 2006, Li et al., 2010). Metallothionein-1 (MT-1) is a zinc-storing sulpho-protein involved in zinc homoeostasis and has significant antioxidant properties (Formigari et al., 2007). Arginine supplementation could therefore activate zinc release from MT-1 via NO production.
The goal of this study was to determine the impact of zinc and arginine supplementation on antioxidant and inflammatory status in weaning piglets using a model of chronic inflammation and oxidative stress obtained by injecting coliform lipopolysaccharide. Our hypothesis is that this supplementation should improve antioxidant status and reduce inflammation.
2. Materials and methods
2.1. Animals and housing
Forty-eight 20-day-old (±1 day) male-castrated and weaned piglets (Yorkshire × Landrace) were moved from the farrowing room of a commercial farm (La Coopérative Fédérée, QC, Canada) to an experimental farm (Centre de recherche en sciences animales de Deschambault, Québec, Canada) and distributed in pairs to pens (1 m × 2 m) each equipped with a feeder and watering nipple and maintained at 32 °C. The ambient temperature was decreased gradually to 26 °C over the next 7 days, at which point one pig per pen was euthanized (48 h after injection of lipopolysaccharide as described below). The photoperiod was 12 h light and 12 h dark for the duration of the experiment. All animal procedures were conducted according to the guidelines set by the Canadian Council on Animal Care (2009), and the experimental protocol received approval from the Université Laval animal use and care committee.
2.2. Experimental design and diets
Four diets (Table 1) were formulated to meet or exceed recommendations suggested by the NRC (2012) for piglets. The diets were designated as follows: ZN0ARG0 or control, ZN2500ARG0 (containing 2,500 mg Zn/kg), ZN0ARG1 (containing 1% arginine) and ZN2500ARG1 (containing 2,500 mg Zn/kg and 1% arginine). Animals were distributed according to their initial body weight among the 4 treatments in a randomized complete block design (initially 12 per treatment) and were paired according to weight (difference minimized). Feed was provided daily in 3 equal portions throughout the 14-day period for a total of 60 g per piglet per day on day 0 and increased daily by 45% of the intake on the previous day. During the lipopolysaccharide challenge periods, feeding was maintained at the same level as before the injection and refusal was noted daily. In spite of this limiting of feed, refusals were observed each day in all pens, indicating that feed intake was not a limiting factor. The piglets had ad libitum access to water during the experiment. They were weighed on days 0, 7 and 14.
Table 1.
Composition of experimental diets (as fed basis).
| Item | ZN0ARG0 | ZN2500ARG0 | ZN0ARG1 | ZN2500ARG1 |
|---|---|---|---|---|
| Ingredient, % of total | ||||
| Ground corn | 31.5 | 31.5 | 31.5 | 31.5 |
| Soybean meal | 22.5 | 22.5 | 22.5 | 22.5 |
| Whey powder | 20.0 | 20.0 | 20.0 | 20.0 |
| Hamlet protein 300 | 9.5 | 9.5 | 9.5 | 9.5 |
| Choice fat | 5.0 | 5.0 | 5.0 | 5.0 |
| Spray-dried animal plasma | 3.5 | 3.5 | 3.5 | 3.5 |
| Blood meal | 2.5 | 2.5 | 2.5 | 2.5 |
| Limestone | 1.1 | 1.1 | 1.1 | 1.1 |
| Di-calcium phosphate | 1.0 | 1.0 | 1.0 | 1.0 |
| NaCl (salt) | 0.3 | 0.3 | 0.3 | 0.3 |
| Vitamins1 | 0.25 | 0.25 | 0.25 | 0.25 |
| Minerals2 | 0.25 | 0.25 | 0.25 | 0.25 |
| Lysine HCl | 0.25 | 0.25 | 0.25 | 0.25 |
| dl-methionine | 0.15 | 0.15 | 0.15 | 0.15 |
| l-threonine | 0.10 | 0.10 | 0.10 | 0.10 |
| Corn starch | 0.40 | – | 1.1 | 0.70 |
| Zinc oxide | – | 0.40 | – | 0.40 |
| l-arginine-HCl | – | – | 1.00 | 1.00 |
| l-alanine | 1.70 | 1.70 | – | – |
| Analysed nutrient composition, % | ||||
| Gross energy, MJ/kg | 18.67 ± 0.14 | 18.72 ± 0.10 | 18.68 ± 0.15 | 18.69 ± 0.12 |
| Crude protein | 25.18 ± 0.42 | 24.49 ± 0.38 | 24.77 ± 0.40 | 24.81 ± 0.35 |
| Calcium | 0.78 ± 0.05 | 0.78 ± 0.05 | 0.73 ± 0.04 | 0.77 ± 0.04 |
| Phosphorus | 0.58 ± 0.02 | 0.61 ± 0.02 | 0.53 ± 0.01 | 0.57 ± 0.02 |
| Zinc, mg/kg | 134 ± 10 | 2,224 ± 20 | 123 ± 9 | 2,342 ± 25 |
| Total lysine | 1.69 ± 0.05 | 1.65 ± 0.04 | 1.67 ± 0.04 | 1.67 ± 0.04 |
| Total arginine | 1.52 ± 0.04 | 1.49 ± 0.04 | 2.30 ± 0.05 | 2.31 ± 0.04 |
| Calculated nutrient composition3 | ||||
| Net energy, MJ/kg | 10.96 | 10.96 | 10.98 | 10.98 |
| Digestible lysine, % | 1.52 | 1.52 | 1.52 | 1.52 |
| Digestible arginine, % | 1.37 | 1.37 | 2.17 | 2.17 |
ZN0ARG0 = control diet (n = 6); ZN2500ARG0 = control diet + 2,500 mg of zinc (zinc oxide) (n = 6); ZN0ARG1 = control diet + 1% arginine (n = 6); ZN2500ARG1 = control diet + 2,500 mg of zinc (zinc oxide) + 1% arginine (n = 6).
Provided per kilogram of diet: vitamin A palmitate 5,000 IU; vitamin D3 1,000 IU; vitamin E acetate 22.5 IU; menadione sodium bisulphite 3.75 mg; thiamine HCl 1.0 mg; riboflavin 4.5 mg; niacin 20.0 mg; calcium pantothenate 25.0 mg; pyridoxine HCl 1.5 mg; biotin 0.2 mg; choline bitartrate 375 mg; vitamin B12 25.0 μg.
Provided per kilogram of diet: Zn (as zinc carbonate) 100 mg; Fe (as ferric citrate) 100 mg; Cu (as cupric carbonate) 25 mg; I (as potassium iodate) 0.28 mg; Mn (as manganous carbonate) 46 mg; Se (as sodium selenite) 0.30 mg.
Values for nutritional composition were calculated according to Sauvant et al. (2004).
2.3. Challenge with lipopolysaccharide
On day 5, lipopolysaccharide (Escherichia coli LPS, K-235 Sigma Aldrich, St-Louis, MO, USA) was administered to all piglets by intramuscular injection (100 μg per kg of body weight). Blood samples were taken just before the injection and then 6, 24 and 48 h after from one piglet per pen. One piglet was sacrificed on day 7. On day 12, the remaining piglet was injected again with the same dose and blood samples were collected according to the same sampling schedule. All samples were placed on ice and then centrifuged at 2,000 × g for 15 min at 4 °C. Plasma was stored at −80 °C for further analysis.
2.4. Tissue collection
Shortly after obtaining the 48 h post-injection blood sample on day 7 or 14, animals were sedated with an intramuscular injection of azaperone (Stresnil, Vetoquinol Canada Inc. QC, Canada) at 2 mg/kg. The sedated animals were euthanized by CO2 inhalation. The entire intestine was removed and freed from the mesentery. The segment ending 50 cm cranial from the caecum was considered the ileum (Yen, 2001). The middle 20 cm this segment was used for biochemical analyses and determination of mRNA expression levels of MT-1, tumour necrosis factor-α (TNF-α) and inductive nitric oxide synthase (iNOS). The segment was rinsed with ice-cold saline solution (0.9% NaCl), opened lengthwise and blotted dry. The mucosa was scraped from the underlying tissue using a glass slide, snap-frozen immediately in liquid nitrogen and then stored at −80 °C until analysis.
2.5. Biochemical analysis
Malondialdehyde generation in samples of plasma and ileum mucosa was measured according to the method of Jain et al. (1989) as an index of lipid peroxidation and oxidative status (Michel et al., 2008). Although the spectrometric determination may have given higher values than an HPLC reference method, the concentrations measured in the present study were close to values published in other studies of weanling piglets. Intra-assay and inter-assay coefficients of variability (CV) values were 6.0% and 5.5%, respectively. Samples of ileum tissue (0.5 g) were homogenized directly (Ultra-Turrax T18, IKA-Labortechnick, Stenfer, Germany) with 5 mL of ice-cold PBS pH 7.4 and then centrifuged at 2,000 × g for 15 min. The supernatant (100 μL) was used for the assay as described previously for plasma. The intra-assay and inter-assay CV values were 7.0% and 6.0%, respectively.
Plasma TNF-α concentrations were determined using an ELISA kit (# KSC3012/KSC3011, Invitrogen Corporation, Carlsbad, USA). The intra-assay and inter-assay CV values were 6.0% and 7.2%, respectively.
Total antioxidant capacities (TAC) of plasma and intestinal mucosa (supernatant obtained as described above) were assayed (80 μL sample volume) according to the method of Erel (2004) and Maurice et al. (2007). Total antioxidant capacities are a measurement of the concentration of antioxidants including vitamin C, vitamin E, reduced GSH, polyphenol compounds and protein thiol groups (Erel, 2004). The intra-assay and inter-assay CV values were 2.5% and 3.0%, respectively. Serum total oxidant status (TOS) was assayed according to the method of Erel (2005). Total oxidant status is a linear function of the molar concentration of oxidant substances (hydrogen peroxide, cumene hydroperoxide, tert-butyl hydroperoxide). The intra-assay and inter-assay CV values were 2.0% and 3.5%, respectively.
Reduced and total GSH in plasma were determined using fluorescent detection kit K006-F5 (Arbor Assays, Ann Arbor, USA) according to the manufacturer's instructions. The intra-assay and inter-assay CV values were respectively 3.7% and 9.1% for reduced GSH and 3.6% and 10.0% for total GSH. The difference between total and reduced GSH was presumed to be oxidized (disulphide-linked) GSH (GSSG).
Serum haptoglobin was determined using pig haptoglobin ELISA kit KT-349 (Kamiya Biomedical Company, Seattle, USA) according to the manufacturer's instructions. The intra-assay and inter-assay CV values were 5.0% and 6.2%, respectively.
Plasma nitrite + nitrate (NOx) concentration was assayed using fluorometric kit 780051 (Cayman Chemical Company, Ann Arbor, Michigan, USA). The intra-assay and inter-assay CV values were 2.5% and 3.5%, respectively.
2.6. Analysis of MT1, TNF-α and NOS2 mRNA expression
Total RNA was extracted from 170 mg of intestinal mucosa homogenized (Ultra-Turrax T18, IKA-Labortechnick) in 1 mL of TRIzol reagent (Invitrogen, Ontario, Canada). RNA extracts were purified on RNeasy Mini Spin Columns including the RNase-free DNase step (Qiagen, Ontario, Canada) and quantified with a Nanodrop ND-1000 (NanoDrop Technologies). The quality of each sample was analysed on the Agilent 2100 Bioanalyzer using the Agilent RNA 6000 Nano Assay Protocol (Agilent Technologies, Waldbronn, Germany). Samples with an RNA integrity number (RIN) over 7.5 were kept for further analysis. Samples with a RIN under 7.5 were re-extracted.
The Sensiscript reverse transcription kit (Qiagen) was used according to the manufacturer's instructions with a fixed RNA input. The sample was heated to 72 °C for 2 min to disrupt RNA secondary structure and then placed immediately on ice to increase primer annealing and thereby increase transcript yield. Two micrograms of denatured RNA were then mixed with random decamer solution (10 μmol/L, Ambion), held for 5 min at 65 °C and then placed on ice. Four units of Sensiscript reverse transcriptase (Qiagen) in 20 μL of 1× buffer with 0.5 mmol/L dNTP and 10 U RNase inhibitor (Promega) were added and the reaction mixture was held for 10 min at 25 °C to allow priming of the random decamers, followed by 1 h at 37 °C.
The resulting cDNA (2 μL) was then amplified on the Roche LightCycler by real-time PCR in buffer containing 0.25 mmol/L of each primer, 3 mmol/L MgCl2 in 1× SYBR green, dNTPs and FastStart Taq DNA polymerase enzyme (Roche) in total volume of 20 μL. Primer sequences are shown in Table 2. Primers were designed using the Primer3 Web interface (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi). The PCR conditions used for all genes were as follows: denaturing cycle of 10 min at 95 °C, 45 PCR cycles (denaturing at 95 °C for 5 s; 5 s at the annealing temperature in Table 2; extension at 72 °C for 20 s), followed by a melting cycle. DNA was quantified using LightCycler Software Version 3.5 and comparison with the standard curve.
Table 2.
Primers used for measurement of gene expression level by quantitative PCR.
| Gene description | Gene symbol | Primer sequences | qPCR efficiency, % | Amplicon size, bp | Annealing temperature, °C | Fluorescence acquisition temperature, °C | GeneCards identifiers |
|---|---|---|---|---|---|---|---|
| Actin, beta | ACTB | Up 5′ -CACGCCATCCTGCGTCTGGA- 3′ Low 5′ -AGCACCGTGTTGGCGTAGAG- 3′ |
93 | 452 | 58.7 | 72 | GC07M005566 |
| Glyceraldehyde-3-phosphate dehydrogenase | GAPDH | Up 5′ -ACACTCACTCTTCTACCTTTG- 3′ Low 5′ -CAAATTCATTGTCGTACCAG- 3′ |
95 | 90 | 49.3 | 81 | GC12P006643 |
| Ribosomal protein L4 | RPL4 | Up 5′ -CAAGAGTAACTACAACCTTC- 3′ Low 5′ -GAACTCTACGATGAATCTTC- 3′ |
94 | 635 | 60.0 | 72 | GC15M066790 |
| Metallothionein | MT1 | Up 5′ -TTGCTCTCTGCTTGGTCTCACCT -3′ Low 5′- GGGATGTAGCATGAAGTCAGTGCATGTG -3′ |
98 | 378 | 60.3 | 72 | NM 001001266 |
| Tumour necrosis factor-α | TNF-α | Up 5′- GCCCACGTTGTAGCCAATGTCAAA -3′ Low 5′- TTGTCTTTCAGCTTCACGCCGTTG -3′ |
99 | 98 | 58.0 | 72 | GC06P031543 |
| Nitric oxide synthase 2, inducible | NOS2 | Up 5′- TCCAGAAGCAGAACGTGACCATCA -3′ Low 5′- GAGCACGGCTTTGACCAAGACTTT -3′ |
93 | 293 | 58.0 | 72 | GC17M026083 |
The standard curve consisted of 5 dilutions of purified amplicon (diluted from 0.1 pg to 0.1 fg). Quantitative PCR was performed using a LightCycler apparatus with SYBR green incorporation (Roche Diagnostics, Laval, QC, Canada). Amplicon specificity was confirmed by analysis of the melting curve given by the Lightcycler software. The presence of a single amplicon was checked using the melting curve and amplified products were then run on a 2% agarose gel and visualized with ethidium bromide under UV light with a Bio-DocIt imaging system (UVP, Upland CA, USA) to confirm the expected amplicon size. Sanger sequencing using one primer to initiate the sequencing reaction was performed to confirm amplicon identity. The geometric means of the three housekeeping genes (ACTB, GAPDH and RPL4) were analysed statistically as unaffected by dietary treatments and were used as a normalization factor using GeNorm version 3.5 (Chapman and Waldenström, 2015). These housekeeping genes are usually used for gene expression studies in pig tissues using SYBR green qPCR (Nygard et al., 2007).
2.7. Feed analysis
Feed samples were finely ground using a sample mill (Cyclotec 1093, Foss Tecator, Sweden). Energy content was determined using a bomb calorimeter (Parr Instruments Co., Moline, IL, USA). Nitrogen content was obtained by the combustion method using the Leco Nitrogen Determinator (model TruSpec v1.10, Leco, MI, USA). The mineral contents (P, Ca, and Zn) were analysed according to AOAC procedure 985.01 (2005) using an ICP-OES device (Optima 430DV, Perkin Elmer. MA, USA). Lysine and arginine were determined by HPLC (Water HPLC system, Water Corporation, MA, USA) as described previously (Guay et al., 2006).
2.8. Statistical analysis
The effect of post-weaning period (before LPS injection) was analysed using the SAS MIXED procedure (SAS Inst. Inc. Cary, NC) according to a 2 × 2 × 2 factorial arrangement in a randomized complete block design (initial body mass) with zinc and arginine supplementation as the 2 main independent variables and post-weaning (5 and 12 days) added as a third factor. The experimental unit was the individual pen containing 1 or 2 piglets (n = 6 for each treatment/challenge condition). Treatment means and interactions were calculated for blood parameters (i.e., malondialdehyde, TAC, TOS, GSH [reduced, total, oxidized], TNF-α, haptoglobin and NOx concentrations), mucosal mRNA expression (TNF-α, MT-1 and iNOS) and mucosal antioxidant and oxidative status (malondialdehyde and TAC). The model was: Yijk = μ + Bi + Fj + (Bi × Fj) + Pk + (Bi × Pk) + (Fj × Pk) + (Bi × Fj × Pk) + eijk, where Yij = dependent variable, Bi = zinc, Fj = arginine, Pk = post weaning day and eij = residual error. To study the effect of time after a lipopolysaccharide injection, blood parameters were analysed using the SAS MIXED procedure according to a 2 × 2 × 4 factorial arrangement with zinc and arginine supplementation as the 2 main independent variables with the time post injection added as a third factor and analysed using the repeated option of SAS. The post-weaning periods between days 5 and 7 and between days 12 and 14 were analysed separately. The model was: Yijk = μ + Bi + Fj + (Bi × Fj) + Tk + (Bi × Tk) + (Fj × Tk) + (Bi × Fj × Tk) + eijk, where Yij = dependent variable, Bi = zinc factor, Fj = arginine, Tk = time after the LPS injection and eij = residual error. For growth parameters (i.e. average daily weight gain, average daily feed intake, and growth-to-feed ratio), only the supplementations were including in the model as the 2 main independent variables. For each analysis, the pen was considered as an experimental unit. Differences between means were considered significant at P < 0.05. For parameters measured in plasma, the baseline value (at day 1) was added as a co-variable in statistical models.
3. Results
3.1. Growth performance
The feed supplements had no effect on growth performance (Table 3). There was an interaction between zinc and arginine for average daily feed intake but only for the 48 h after the first injection of lipopolysaccharide (days 5 to 7, P < 0.05).
Table 3.
Average daily gain (ADG), average daily feed intake (ADFI), feed efficiency (gain:feed), body weight (BW) of weanling piglets fed diets supplemented with or without zinc and arginine.
| Item1 | Initial BW, kg | 7 days BW, kg | 14 days BW, kg | ADG, g/d |
Gain:feed ratio |
ADFI, g/d |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 to 7 d | 8 to 14 d | 0 to 7 d | 8 to 14 d | 0 to 7 d | 5 to 7 d | 12 to 14 d | 7 to 14 d | ||||
| ZN0ARG0 | 6.93 | 7.78 | 10.60 | 137 | 431 | 0.389 | 0.836 | 355 | 540a | 514 | 468 |
| ZN0ARG1 | 7.39 | 7.90 | 10.72 | 107 | 433 | 0.283 | 0.835 | 358 | 306b | 519 | 499 |
| ZN2500ARG0 | 7.39 | 7.85 | 10.95 | 80 | 435 | 0.237 | 0.863 | 302 | 313b | 507 | 490 |
| ZN2500ARG1 | 6.81 | 7.52 | 10.42 | 125 | 437 | 0.358 | 0.865 | 347 | 490ab | 505 | 515 |
| SEM | 0.31 | 0.33 | 0.49 | 30 | 23 | 0.078 | 0.046 | 26 | 93 | 10 | 28 |
| Zinc | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| Arginine | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| Zinc × Arginine | NS | NS | NS | NS | NS | NS | NS | NS | 0.019 | NS | NS |
a,b Within a column, means without a common superscript differ (P < 0.05).
ZN0ARG0: control diet (n = 6); ZN0ARG1: control diet + 1% arginine (n = 6); ZN2500ARG0: control diet + 2,500 mg of zinc (zinc oxide) (n = 6); ZN2500ARG1: control diet + 2,500 mg of zinc (zinc oxide) + 1% arginine (n = 6).
3.2. Effect of post-weaning time before lipopolysaccharide injection
Lower plasma malondialdehyde was associated with the zinc-supplemented diet during the post-weaning period prior to lipopolysaccharide injection (P < 0.05, Table 4) but no other effect on oxidative status was noted. Arginine alone tended to decrease GSH but increase it when combined with the zinc supplement (Zn × Arg, P = 0.075). Arginine decreased the GSSG concentration significantly (P < 0.05) and the GSSG:total GSH ratio during the post-weaning period and tended to reduce TAC (P = 0.059) and the TAC:TOS ratio (P = 0.086). As shown in Table 4, reduced and total GSH concentrations but not GSSG were significantly higher on day 12 than on day 5 (P < 0.001).
Table 4.
Malondialdehyde, reduced and total glutathione (GSH) and oxidized GSH (GSSG) concentrations in plasma before lipopolysaccharide injection on days 5 and 12 post-weaning from piglets fed diets supplemented with or without zinc and arginine.
| Item1 | Malondialdehyde, μmol/L |
Reduced GSH, μmol/L |
GSSG, μmol/L |
Total GSH, μmol/L |
GSSG:total GSH |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Day 5 | Day 12 | Day 5 | Day 12 | Day 5 | Day 12 | Day 5 | Day 12 | Day 5 | Day 12 | |
| ZN0ARG0 | 2.73 | 3.05 | 1.62 | 3.04 | 0.42 | 0.59 | 2.37 | 4.46 | 0.183 | 0.142 |
| ZN0ARG1 | 3.04 | 3.01 | 0.77 | 2.61 | 0.15 | 0.43 | 1.14 | 3.54 | 0.126 | 0.132 |
| ZN2500ARG0 | 2.73 | 2.56 | 0.87 | 2.15 | 0.55 | 0.65 | 1.85 | 3.46 | 0.277 | 0.205 |
| ZN2500ARG1 | 2.83 | 2.35 | 1.91 | 2.30 | 0.49 | 0.39 | 2.76 | 3.05 | 0.149 | 0.134 |
| SEM | 0.25 | 0.50 | 0.13 | 0.64 | 0.040 | |||||
| Arginine | NS | NS | 0.039 | NS | 0.021 | |||||
| Zinc | 0.041 | NS | NS | NS | NS | |||||
| Day | NS | 0.001 | NS | 0.001 | NS | |||||
| Zinc × Arginine | NS | 0.075 | NS | NS | NS | |||||
| Day × Zinc | NS | NS | NS | NS | NS | |||||
| Day × Arginine | NS | NS | NS | NS | NS | |||||
| Day × Zn × Arginine | NS | NS | NS | NS | NS | |||||
ZN0ARG0: control diet (n = 6); ZN0ARG1: control diet + 1% arginine (n = 6); ZN2500ARG0: control diet + 2,500 mg of zinc (zinc oxide) (n = 6); ZN2500ARG1: control diet + 2,500 mg of zinc (zinc oxide) + 1% arginine (n = 6).
Tumour necrosis factor-α concentration was higher on day 5 than on day 12 (Table 5, P < 0.01) but was not affected by the dietary treatments. Meanwhile, zinc decreased the haptoglobin concentration measured on day 12 (377 vs. 1,074 mg/L) but had not effect on it on day 5 (1,046 vs. 897 mg/L, Table 5, Zn × day, P < 0.05). Finally, zinc alone tended to reduce NOx concentration (P = 0.099).
Table 5.
Plasma total antioxidant capacity (TAC), total oxidant status (TOS), TAC:TOS ratio, tumour necrosis factor-α (TNF-α), haptoglobin (HAPT) and nitrite/nitrate (NOx) concentrations in piglets fed diets supplemented with or without zinc and arginine (measured before immunological challenge with lipopolysaccharide on days 5 and 12 post-weaning).
| Treatments1 | TAC, μmol/L |
TOS, μmol/L |
TAC:TOS ratio |
TNF-α, ng/L |
HAPT, mg/L |
NOx, nmol/L |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 5 | Day 12 | Day 5 | Day 12 | Day 5 | Day 12 | Day 5 | Day 12 | Day 5 | Day 12 | Day 5 | Day 12 | |
| ZN0ARG0 | 169 | 158 | 10.39 | 6.93 | 38.46 | 97.04 | 178 | 107 | 1,083 | 1,173 | 635 | 949 |
| ZN0ARG1 | 156 | 122 | 7.94 | 9.40 | 23.76 | 12.70 | 185 | 88 | 710 | 975 | 1,354 | 752 |
| ZN2500ARG0 | 151 | 151 | 8.22 | 9.24 | 49.45 | 51.03 | 166 | 139 | 1,279 | 322 | 652 | 598 |
| ZN2500ARG1 | 131 | 95 | 5.70 | 8.18 | 54.35 | 13.45 | 177 | 142 | 812 | 432 | 698 | 650 |
| SEM | 28 | 4.75 | 25.70 | 30 | 307 | 246 | ||||||
| Arginine | 0.059 | NS | 0.086 | NS | NS | NS | ||||||
| Zinc | NS | NS | NS | NS | NS | 0.099 | ||||||
| Day | NS | NS | NS | 0.010 | NS | NS | ||||||
| Zinc × Arginine | NS | NS | NS | NS | NS | NS | ||||||
| Day × Zinc | NS | NS | NS | NS | 0.047 | NS | ||||||
| Day × Arginine | NS | NS | NS | NS | NS | NS | ||||||
| Day × Zinc × Arginine | NS | NS | NS | NS | NS | NS | ||||||
ZN0ARG0: control diet (n = 6); ZN0ARG1: control diet + 1% arginine (n = 6); ZN2500ARG0: control diet + 2,500 mg of zinc (zinc oxide) (n = 6); ZN2500ARG1: control diet + 2,500 mg of zinc (zinc oxide) + 1% arginine (n = 6).
3.3. Effect of diet under conditions of induced inflammation
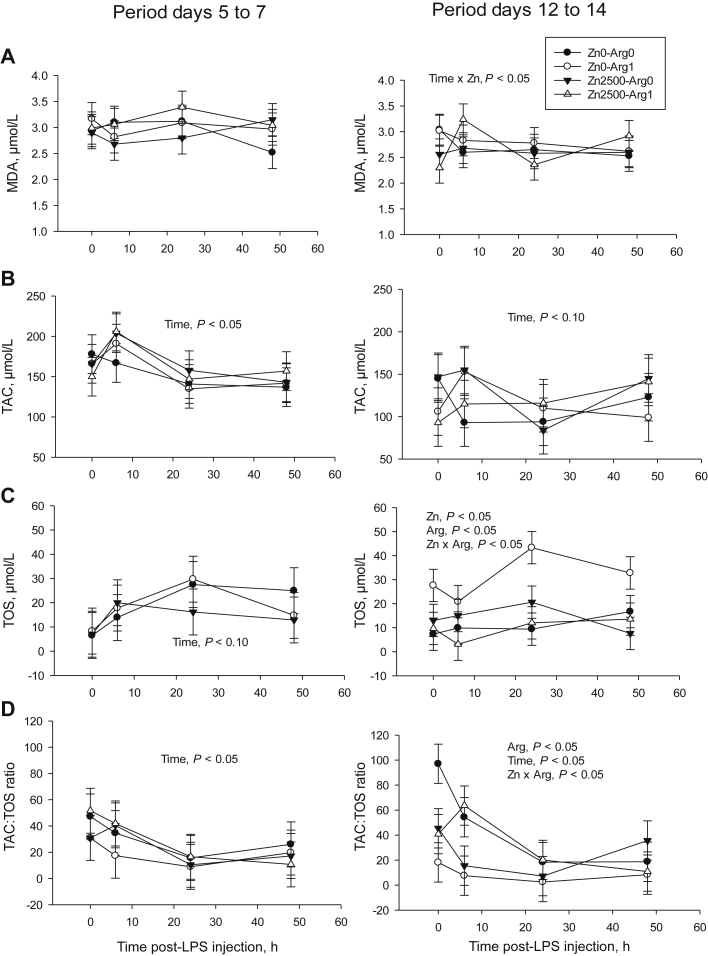
During the first challenge with lipopolysaccharide, the supplements had no effect on malondialdehyde, TAC or TOS (Fig. 1). However, TAC reached its maximum 6 h after injection (192 ± 24 μmol/L) then dropped below its 0 h value (165 μmol/L) and remained there (145 μmol/L at 24 h, 144 μmol/L at 48 h, time effect, P < 0.05). The TAC:TOS ratio also decreased during this period (time effect, P < 0.05). In piglets fed the zinc supplement, plasma malondialdehyde was reduced before but not after the second challenge (Fig. 1, Zn × Time, P < 0.05). However, arginine increased TOS during this challenge, but only in comparison with the diet containing no supplement (Zn × Arg, P < 0.05). The TAC:TOS ratio was also reduced in ZN2500ARG0 and ZN0ARG1 compared to ZN2500ARG1 and ZN0ARG0 (Zn × Arg, P < 0.05).
Fig. 1.
(A) Plasma malondialdehyde (MDA) concentration, (B) total antioxidant capacity (TAC), (C) total oxidant status (TOS) and (D) TAC:TOS ratio measured before (0 h) and after (6, 24 and 48 h) inducing inflammation (by lipopolysaccharide [LPS] injection) in weaned piglets fed corn/soy/whey-based diets supplemented with or without zinc (2,500 mg/kg) and arginine (1%). For both post-injection periods, n = 6.
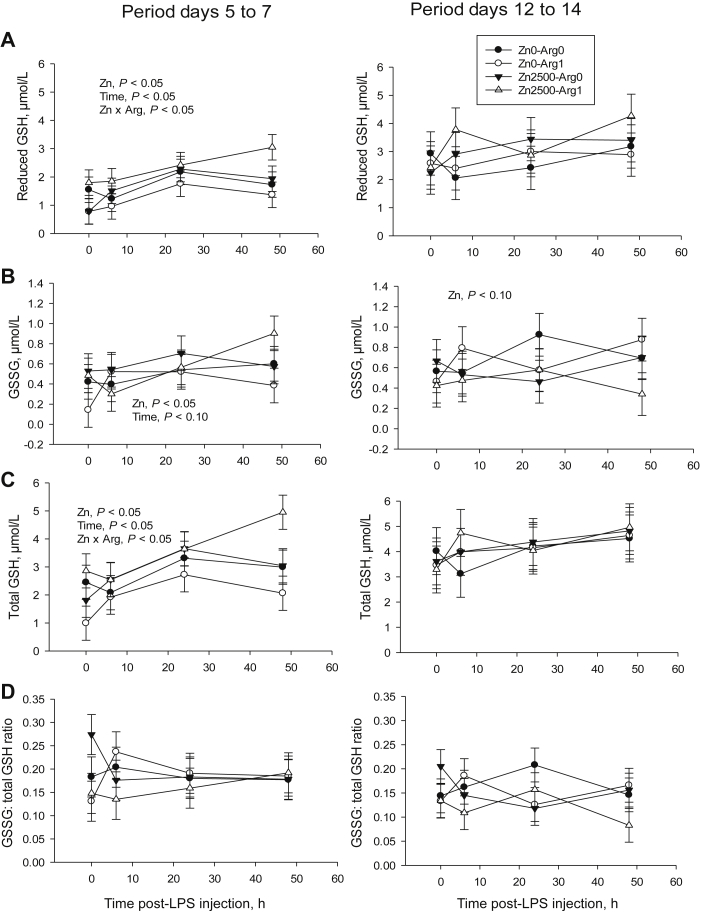
From days 5 to 7, reduced and total GSH increased significantly in response to zinc supplementation (Fig. 2, P < 0.05), especially when diet was also supplemented with arginine (Zn × Arg, P < 0.05). Oxidized GSH concentration also increased (P < 0.05). Reduced and total GSH concentrations were higher at 24 and 48 h than at 0 and 6 h after lipopolysaccharide injection for all dietary treatments (time effect, P < 0.05). During days 12 to 14, GSSG tended to decrease in response to the zinc supplement (P = 0.062).
Fig. 2.
Plasma glutathione (GSH) concentrations measured before (0 h) and after (6, 24 and 48 h) inducing inflammation (by lipopolysaccharide [LPS] injection) in weaned piglets fed corn/soy/whey-based diets supplemented with or without zinc (2,500 mg/kg) and arginine (1%). (A) Reduced GSH, (B) oxidized GSH (GSSG), (C) total GSH, (D) GSSH:total GSH ratio. For both post injection periods, n = 6.
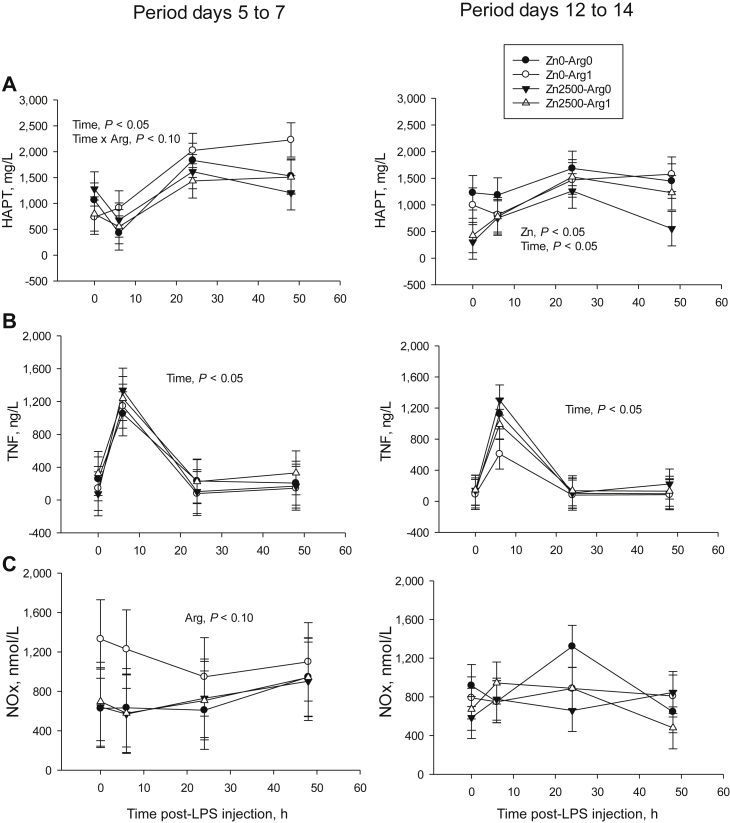
Haptoglobin increased to a maximum at 24 and 48 h after both challenges while TNF-α did likewise at 6 h (Fig. 3, time effect, P < 0.01). However, from days 5 to 7, haptoglobin tended to increase more in piglets on the arginine-supplemented diet (Time × Arg, P = 0.096) and decreased between days 12 and 14 in those on the zinc-supplemented diet (854 ± 284 vs. 1,300 ± 324 mg/L, P < 0.05). The only response of NOx concentration was a tendency to increase during days 5 to 7 in piglets on the arginine-supplemented diet (P = 0.098).
Fig. 3.
Plasma concentrations measured before (0 h) and after (6, 24 and 48 h) inducing inflammation (by lipopolysaccharide [LPS] injection) in weaned piglets fed corn/soy/whey-based diets supplemented or not with zinc (2,500 mg/kg) and arginine (1%). (A) Haptoglobin (HAPT), (B) tumour necrosis factor-α (TNF-α), (C) nitrite/nitrate (NOx). For both post-injection periods, n = 6.
3.4. Antioxidant and oxidative status and Metallothionein-1 expression in the ileum
Malondialdehyde in the intestinal mucosa was lowest in the ZN0ARG1 group while the highest and intermediary values were observed in the ZN0ARG0 and ZN2500 groups respectively, but only on day 14 (Zn × Arg × Day, Table 6, P < 0.05). Zinc increased TAC mainly on day 14 (Zn × Day effect, P < 0.05) and MT-1 expression (mRNA) also on day 14 (Zn × Day, P < 0.05). It tended to increase TNF-α mRNA expression from days 7 to 14 (Zn × Day, P = 0.098). Expression of iNOS mRNA tended to decrease from days 7 to 14 (day, P = 0.093) but not in response to either supplement.
Table 6.
Malondialdehyde, total antioxidant capacity (TAC), expression of metallothionein-1 (MT-1), tumour necrosis factor-α (TNF-α) and inductive nitric oxide synthase (iNOS) in ileal mucosa of weanling piglets fed diets supplemented with or without zinc and arginine (48 h after lipopolysaccharide injection).
| Item1 | Malondialdehyde, μmol/g |
TAC, mmol/g |
MT-12 |
TNF-α2 |
iNOS2 |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Day 7 | Day 14 | Day 7 | Day 14 | Day 7 | Day 14 | Day 7 | Day 14 | Day 7 | Day 14 | |
| ZN0ARG0 | 136 | 156b | 3.99 | 3.86a | 0.008a | 0.074a | 1.042 | 0.810 | 3.320 | 2.200 |
| ZN0ARG1 | 147 | 89a | 4.19 | 3.39a | 0.012a | 0.039a | 1.460 | 1.000 | 5.154 | 2.234 |
| ZN2500ARG0 | 200 | 122ab | 4.71 | 4.73b | 2.200b | 3.300b | 0.905 | 2.720 | 3.342 | 2.845 |
| ZN2500ARG1 | 139 | 146ab | 4.60 | 4.88b | 0.630ab | 3.930b | 0.720 | 1.290 | 7.984 | 2.647 |
| SEM | 33 | 0.24 | 0.690 | 0.100 | 1.228 | |||||
| Arginine | NS | NS | NS | NS | NS | |||||
| Zinc | NS | 0.001 | 0.001 | NS | NS | |||||
| Day | NS | 0.048 | 0.028 | NS | 0.093 | |||||
| Zinc × Arginine | NS | NS | NS | NS | NS | |||||
| Day × Zinc | NS | 0.050 | 0.036 | 0.098 | NS | |||||
| Day × Arginine | NS | NS | NS | NS | NS | |||||
| Day × Zinc × Arginine | 0.050 | NS | NS | NS | NS | |||||
a,b Within a column, means without a common superscript differ (P < 0.05).
ZN2500ARG1: control diet + 2,500 mg of zinc (zinc oxide) + 1% arginine (n = 6); ZN2500ARG0: control diet + 2,500 mg of zinc (zinc oxide) (n = 6); ZN0ARG1: control diet + 1% arginine (n = 6); ZN0ARG0: control diet (n = 6).
Gene expression (mRNA) in arbitrary units.
4. Discussion
The goal of this study was to investigate the effect of diets containing zinc and arginine supplements on systemic and intestinal antioxidant and inflammatory status in weanling piglets challenged with an injection of lipopolysaccharide to induce a condition similar to chronic inflammation.
4.1. Growth performance
According to published studies, a weaning diet containing a high zinc supplement (2,000 to 3,000 mg Zn oxide per kg) improves body weight gain in piglets (Wang et al., 2009, Hu et al., 2012a, Hu et al., 2012b, Hu et al., 2013, Sales, 2013). However, our findings corroborate other studies in which zinc had no effect on growth (Broom et al., 2006, Bergeron et al., 2014). These results suggest that the ZN0 diets met the Zn requirement (123 to 134 mg/kg) of piglets growing under experimental conditions (disease-free, no crowding). Some estimate this requirement to be as low as 100 mg/kg (NRC, 2012). Others have shown that a high-dose supplement (2,500 mg/kg) may have no effect on growth performance but does improve average daily gain after oral challenge with enterotoxigenic E. coli (Kim et al., 2015). Although the lipopolysaccharide challenge used in this study did alter inflammatory and oxidative status, it appears not to have affected growth performance to an extent that could be countered with a zinc supplement. This challenge is known to decrease feed intake by 30% to 70% over the 48 h following the injection (Frank et al., 2005). In the present study, feed intake was reduced slightly during the first challenge and only in the ZN0ARG1 and ZN2500ARG0 groups. This is rather surprising since increases in markers of inflammation were observed (TNF-α and haptoglobin on days 5 to 7), regardless of supplementation. Reduced feed intake 48 h after injection has been correlated with increased blood acute-phase protein (Frank et al., 2005), further suggesting that the piglets in the present study had only a weak reaction to the lipopolysaccharide injection. Furthermore, the second injection seemed to have no significant effect on feed intake, even though this challenge also increased the concentrations of TNF-α and haptoglobin.
Published studies are divided also on the effectiveness of arginine supplementation, some finding that it does improve the growth performance of weanling piglets (Wu et al., 2010, Yao et al., 2011) and others finding no effect (Liu et al., 2008; Zhan et al., 2008, Zheng et al., 2013). It has been observed that after (not before) an injection of diquat (which increases oxidative stress), supplementing the diet with 0.8% or 1.6% arginine improves (or counteracts the drop in) growth performance (Zheng et al., 2013). Similar results have been obtained with lipopolysaccharide (Liu et al., 2008). However, the levels of total arginine were lower in these studies (0.95% and 1.28%) than in the present study (1.49% to 1.52%) suggesting that the ARG0 diets met the requirement for piglet growth under our experimental conditions.
4.2. Antioxidants and inflammatory response to challenge with lipopolysaccharide
Weaning is well known to decrease antioxidant status and increase oxidative stress as measured in plasma and the intestinal mucosa (Zhu et al., 2012, Yin et al., 2014). Haptoglobin, a serum protein marker of the acute phase of inflammation, is increased in weaned piglets (Petersen et al., 2004, Sauerwein et al., 2005). We found increases in reduced and total GSH and decreased TNF-α from days 5 to 12 of the post-weaning period, suggesting a lowering of oxidative stress and systemic inflammation.
Although the zinc supplement had no effect on growth performance, it decreased lipid oxidation by maintaining lower malondialdehyde concentrations, as reported previously (Bergeron et al., 2014). This effect was associated with increased concentrations of GSSG, reduced and total GSH, but only during the first challenge. Zinc thus appears to promote synthesis or release of GSH under conditions of lipopolysaccharide challenge, but mainly when arginine is also added to the diet. Large amounts of zinc (3,000 mg/kg) can decrease GSSG in the jejunal mucosa of weanling piglets, suggesting reduced oxidative stress (Wang et al., 2009). Zinc injections have been shown to increase the concentration of GSH in the liver of rats (Iszard et al., 1995) and this effect could be protective. Increased hepatic concentrations of GSSG in piglets fed diets supplemented with 0.5% or 1% arginine have been noted after injection of lipopolysaccharide (Li et al., 2012). However, there is no obvious explanation of how arginine increases the zinc-driven increase in the release of GSH. Although arginine is an essential precursor for the synthesis of NO, which is known to stimulate Zn2+ release in endothelial cells (Wiseman et al., 2006, Li et al., 2010), it did not clearly increase the NOx concentration measured during the first lipopolysaccharide post-injection period. It is possible that plasma NOx concentration and NO synthesis in tissues are not correlated (Poeze et al., 2011). Reduced and total GSH were already elevated (2.5 to 3.5 μmol/L) before the second challenge with lipopolysaccharide, suggesting that synthesis or release of GSH was responsible and the effect of diet was small and non-significant.
In addition to the positive effect on oxidative status, zinc supplementation reduced serum haptoglobin concentration, but only after 12 days into the experiment. It therefore might have reduced the inflammation caused by the second lipopolysaccharide injection. However, zinc did not affect TNF-α concentration before or after either injection. This has been observed previously in piglets (Namkung et al., 2006), but so has increased TNF-α under similar conditions (Yu et al., 2000, Bergeron et al., 2014), specifically 3 h after injection. It is possible that the effect of zinc is lessened after 6 h or more.
Although arginine supplementation had a limited effect on oxidative and inflammatory status during the first challenge with lipopolysaccharide, piglets on this diet had a lower GSSG concentration and lower GSSG:total GSH ratio than those fed ARG0 diets. However, the supplement tended to decrease TAC value. Moreover, during the second challenge, the supplement increased TOS concentration and reduced the TAC:TOS ratio, suggesting a pro-oxidative effect of arginine at this point. However, this effect was lessened when the diet also included the zinc supplement, suggesting that the pro-oxidative effect depends partially on Zn status. Arginine in excess could increase oxidative stress by contributing to over-production of NO, which is a reactive nitrogen species (Valko et al., 2007). Injected arginine decreases plasma total-radical-trapping antioxidant capacity in rats (de Lima et al., 2012), and a total intake exceeding 2% of the diet (it was 2.3% in our study) can impair vascular development in intestinal tissue of weaning piglets (Zhan et al., 2008). However, as mentioned above, the arginine supplement in the present study did not increase plasma NOx. It has also been observed that supplementation at 1.6% (equivalent to 2.48% of the diet) arginine reduced malondialdehyde and increased GSH peroxidase activity in the plasma of weaning piglets (Zheng et al., 2013), suggesting that its overall effect on antioxidant status is positive.
In the intestinal mucosa of piglets receiving the arginine supplement, the malondialdehyde concentration was decreased 48 h after the second inflammatory challenge, but only in the absence of the zinc supplement. This suggests that arginine could improve oxidative status in the intestine. Arginine can provide protection against reactive oxygen species by direct chemical interaction with O2− (Lass et al., 2002). However, the effect observed in this study was not associated with a higher intestinal TAC and would depend on the level of zinc in the diet.
Although the zinc supplement increased both MT-1 expression and TAC, as observed previously (Bergeron et al., 2014), this appears not to have affected lipid oxidation in the intestinal mucosa, as measured in terms of malondialdehyde. Metallothioneins are rich in thiol groups (cysteine residues) and are known to sequester reactive oxygen and nitrogen species, suggesting an antioxidant role (Formigari et al., 2007).
5. Conclusion
The results of this study show that antioxidant and inflammatory status in weanling piglets improved in terms of increased plasma GSH and decreased TNF-α concentrations. Supplementing the post-weaning diet with zinc reduced plasma lipid oxidation and the haptoglobin concentration, and arginine supplementation did not modify these effects. Both supplements had inconsistent effects on inflammatory and oxidative parameters in response to inflammation by lipopolysaccharide. It nevertheless appears that zinc modifies GSH metabolism and arginine could affect negatively the antioxidant status of piglets with a sensitized immune system. The level of arginine in a piglet feed should be chosen carefully in order to avoid possible negative effects on antioxidant status during the post-weaning period.
Acknowledgement
Financial support for this work was provided by a grant from the Natural Science and Engineering Research Council (NSERC) awarded to F. Guay and by a studentship from La Fédération des producteurs de porcs du Québec (FPPQ) awarded to N. Bergeron.
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- Bergeron N., Robert C., Guay F. Antioxidant status and inflammatory response in weanling piglets fed diets supplemented with arginine and zinc. Can J Anim Sci. 2014;94(1):87–97. [Google Scholar]
- Broom L.J., Miller H.M., Kerr K.G., Knapp J.S. Effects of zinc oxide and Enterococcus faecium SF68 dietary supplementation on the performance, intestinal microbiota and immune status of weaned piglets. Res Vet Sci. 2006;80(1):45–54. doi: 10.1016/j.rvsc.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Care C.C.O.A. CCAC; Ottawa, ON: 2009. The care and use of farm animals in research, teaching and testing; pp. 12–15. [Google Scholar]
- Chapman J.R., Waldenström J. Whit reference to reference genes: a systematic review of endogenous controls in gene expression studies. PLoS One. 2015;10(11):e0141853. doi: 10.1371/journal.pone.0141853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lima D.D., Delwing F., da Cruz J.G., Wyse A.T., Delwing-Dal Magro D. Protective effect of antioxidants on blood oxidative stress caused by arginine. Fundam Clin Pharmacol. 2012;26(2):250–258. doi: 10.1111/j.1472-8206.2010.00909.x. [DOI] [PubMed] [Google Scholar]
- Erel O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin Biochem. 2004;37(4):277–285. doi: 10.1016/j.clinbiochem.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem. 2005;38(12):1103–1111. doi: 10.1016/j.clinbiochem.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Formigari A., Irato P., Santon A. Zinc, antioxidant systems and metallothionein in metal mediated-apoptosis: biochemical and cytochemical aspects. Comp Biochem Physiol C. 2007;146(4):443–459. doi: 10.1016/j.cbpc.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Frank J.W., Mellencamp M.A., Carroll J.A., Boyd R.D., Allee G.L. Acute feed intake and acute-phase protein responses following a lipopolysaccharide challenge in pigs from two dam lines. Vet Immunol Immunopatol. 2005;107(3):179–187. doi: 10.1016/j.vetimm.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Guay F., Donovan S.M., Trottier N.L. Biochemical and morphological developments are partially impaired in intestinal mucosa from growing pigs fed reduced-protein diets supplemented with crystalline amino acids. J Anim Sci. 2006;84(7):1749–1760. doi: 10.2527/jas.2005-558. [DOI] [PubMed] [Google Scholar]
- Hu C., Song J., Li Y., Luan Z., Zhu K. Diosmectite-zinc oxide composite improves intestinal barrier function, modulates expression of pro-inflammatory cytokines and tight junction protein in early weaned pigs. Br J Nutr. 2012;110(4):681–688. doi: 10.1017/S0007114512005508. [DOI] [PubMed] [Google Scholar]
- Hu C., Song J., You Z., Luan Z., Li W. Zinc oxide-montmorillonite hybrid influences diarrhea, intestinal mucosal integrity, and digestive enzyme activity in weaned pigs. Biol Trace Elem Res. 2012;149(2):190–196. doi: 10.1007/s12011-012-9422-9. [DOI] [PubMed] [Google Scholar]
- Hu C.H., Xiao K., Song J., Luan Z.S. Effects of zinc oxide supported on zeolite on growth performance, intestinal microflora and permeability, and cytokines expression of weaned pigs. Anim Feed Sci Technol. 2013;181(1–4):65–71. [Google Scholar]
- Iszard M.B., Liu J., Klaassen C.D. Effect of several metallothionein inducers on oxidative stress defense mechanisms in rats. Toxicology. 1995;104(1):25–33. doi: 10.1016/0300-483x(95)03118-y. [DOI] [PubMed] [Google Scholar]
- Jaeschke H. Reactive oxygen and mechanisms of inflammatory liver injury: present concepts. J Gastroenterol Hepatol. 2011;26:173–179. doi: 10.1111/j.1440-1746.2010.06592.x. [DOI] [PubMed] [Google Scholar]
- Jain S.K., McVie R., Duett J., Herbst J.J. Erythrocyte membrane lipid peroxidation and glycosylated hemoglobin in diabetes. Diabetes. 1989;38(12):1539–1543. doi: 10.2337/diab.38.12.1539. [DOI] [PubMed] [Google Scholar]
- Kim S., Kwon C.H., Park B.C., Lee C.Y., Han J.H. Effects of a lipid-encapsulated zinc oxide dietary supplement, on growth parameters and intestinal morphology in weanling pigs artificially infected with enterotoxigenic Escherichia coli. J Anim Sci Technol. 2015;57(1):4–8. doi: 10.1186/s40781-014-0038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lass A., Suessenbacher A., Wölkart G., Mayer B., Brunner F. Functional and analytical evidence for scavenging of oxygen radicals by L-arginine. Mol Pharmacol. 2002;61(5):1081–1088. doi: 10.1124/mol.61.5.1081. [DOI] [PubMed] [Google Scholar]
- Latimer J.W., Horwitz W., editors. Association of Official Analytical Chemists (AOAC) Official methods of analysis, Arlington, VA, USA. 2005. [Google Scholar]
- Li H., Cao R., Wasserloos K.J., Bernal P., Liu Z.-Q., Pitt B.R. Nitric oxide and zinc homoeostasis in pulmonary endothelium. Ann N Y Acad Sci. 2010;1203:73–78. doi: 10.1111/j.1749-6632.2010.05558.x. [DOI] [PubMed] [Google Scholar]
- Li Q., Liu Y., Che Z., Zhu H., Meng G., Hou Y. Dietary L-arginine supplementation alleviates liver injury caused by Escherichia coli LPS in weaned pigs. Innate Immun. 2012;18(6):804–814. doi: 10.1177/1753425912441955. [DOI] [PubMed] [Google Scholar]
- Liu Y., Huang J., Hou Y., Zhu H., Zhao S., Ding B. Dietary arginine supplementation alleviates intestinal mucosal disruption induced by Escherichia coli lipopolysaccharide in weaned pigs. Br J Nutr. 2008;100(3):552–560. doi: 10.1017/S0007114508911612. [DOI] [PubMed] [Google Scholar]
- Maurice D., Lightsey S.F., Toler J.E., Canty S. Effect of chronic oxidative/corticosterone-induced stress on ascorbic acid metabolism and total antioxidant capacity in chickens (Gallus gallus domesticus) J Anim Physiol Anim Nutr. 2007;91(2):355–360. doi: 10.1111/j.1439-0396.2006.00662.x. [DOI] [PubMed] [Google Scholar]
- Michel F., Bonnefont-Rousselot D., Mas E., Drai J., Thérond P. Biomarqueurs de la peroxydation lipidique : aspects analytiques. Ann Biol Clin. 2008;66(6):605–620. doi: 10.1684/abc.2008.0283. [DOI] [PubMed] [Google Scholar]
- Namkung H., Gong J., Yu H., De Lange C.F.M. Effect of pharmacological intakes of zinc and copper on growth performance, circulating cytokines and gut microbiota of newly weaned piglets challenged with coliform lipopolysaccharides. Can J Anim Sci. 2006;86(4):511–522. [Google Scholar]
- NRC . twelfth revised ed. The National Academies Press; Washington, DC: 2012. Nutrient requirements of swine. [Google Scholar]
- Nygard A.B., Jørgensen C.B., Cirera S., Fredholm M. Selection of reference genes for gene expression studies in pig tissues using SYBR green qPCR. BMC Mol Biol. 2007;8(1):67. doi: 10.1186/1471-2199-8-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen H.H., Nielsen J.P., Heegard P.M.H. Application of acute phase protein measurements in veterinary clinical chemistry. Vet Res. 2004;35(2):163–187. doi: 10.1051/vetres:2004002. [DOI] [PubMed] [Google Scholar]
- Pié S., Lallès J.P., Blazy F., Laffitte J., Sève B., Oswald I.P. Weaning is associated with an upregulation of expression of inflammatory cytokines in the intestine of piglets. J Nutr. 2004;134(3):641–647. doi: 10.1093/jn/134.3.641. [DOI] [PubMed] [Google Scholar]
- Poeze M., Bruins M.J., Kessels F., Luiking Y.C., Lamers W.H., Deutz N.E.P. Effects of L-arginine pretreatment on nitric oxide metabolism and hepatosplanchnic perfusion during porcine endotoxemia. Am J Clin Nutr. 2011;93(6):1237–1247. doi: 10.3945/ajcn.110.007237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sales J. Effects of pharmacological concentrations of dietary zinc oxide on growth of post-weaning pigs: a metaanalysis. Biol Trace Elem Res. 2013;152(3):343–349. doi: 10.1007/s12011-013-9638-3. [DOI] [PubMed] [Google Scholar]
- Sauerwein H., Schmitz S., Hiss S. The acute phase protein haptoglobin and its relation to oxidative status in piglets undergoing weaning-induced stress. Redox Rep. 2005;10(6):295–302. doi: 10.1179/135100005X83725. [DOI] [PubMed] [Google Scholar]
- Sauvant D., Perez J.M., Tran G. INRA Éditions; Versailles, France: 2004. Tables de composition et de valeur nutritive des matières premières destinées aux animaux d’élevage: porcs, volailles, bovins, ovins, lapins, chevaux, poisson. [Google Scholar]
- Valko M., Leibfritz D., Moncol J., Cronin M.T., Mazur M., Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39(1):44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Wang X., Ou D., Yin J., Wu G., Wang J. Proteomic analysis reveals altered expression of proteins related to glutathione metabolism and apoptosis in the small intestine of zinc oxide-supplemented piglets. Amino Acids. 2009;37(1):209–218. doi: 10.1007/s00726-009-0242-y. [DOI] [PubMed] [Google Scholar]
- Wiseman D.A., Wells S.M., Wilham J., Hubbard M., Welker J.E., Black S.M. Endothelial response to stress from exogenous Zn2+ resembles that of NO-mediated nitrosative stress, and is protected by MT-1 overexpression. Am J Physiol Cell Physiol. 2006;291(3):555–568. doi: 10.1152/ajpcell.00509.2005. [DOI] [PubMed] [Google Scholar]
- Wu X., Ruan Z., Gao Y., Yin Y., Zhou X., Wang L. Dietary supplementation with L-arginine or N-carbamylglutamate enhances intestinal growth and heat shock protein-70 expression in weanling pigs fed a corn- and soybean meal-based diet. Amino Acids. 2010;39:831–839. doi: 10.1007/s00726-010-0538-y. [DOI] [PubMed] [Google Scholar]
- Wyns H., Plessers E., De Backer P., Meyer E., Croubels S. In vivo porcine lipopolysaccharide inflammation models to study immunomodulation of drugs. Vet Immunol Immunopathol. 2015;166(3–4):58–69. doi: 10.1016/j.vetimm.2015.06.001. [DOI] [PubMed] [Google Scholar]
- Yao K., Guan S., Li T., Huang R., Wu G., Ruan Z. Dietary L-arginine supplementation enhances intestinal development and expression of vascular endothelial growth factor in weanling piglets. Br J Nutr. 2011;105(5):703–709. doi: 10.1017/S000711451000365X. [DOI] [PubMed] [Google Scholar]
- Yen J. Anatomy of the digestive system and nutritional physiology. In: Lewis A.J., Southern L.L., editors. Swine nutrition. CRC Press; Florida, USA: 2001. p. 32. [Google Scholar]
- Yin J., Wu M.M., Xiao H., Ren W.K., Duan J.L., Yang G. Development of an antioxidant system after early weaning in piglets. J Anim Sci. 2014;92(2):612–619. doi: 10.2527/jas.2013-6986. [DOI] [PubMed] [Google Scholar]
- Yu I.T., Lin J., Lee D.N. Effect of various levels of zinc and copper in diet on growth and immune responses of weaning pigs. Asian Australas J Anim Sci. 2000;13 A(Suppl.):81. [Google Scholar]
- Zhan Z., Ou D., Piao X., Kim S.W., Liu Y., Wang J. Dietary arginine supplementation affects microvascular development in the small intestine of early-weaned pigs. J Nutr. 2008;138(7):1304–1309. doi: 10.1093/jn/138.7.1304. [DOI] [PubMed] [Google Scholar]
- Zheng P., Yu B., He J., Tian G., Luo Y., Mao X. Protective effects of dietary arginine supplementation against oxidative stress in weaned piglets. Br J Nutr. 2013;109(12):2253–2260. doi: 10.1017/S0007114512004321. [DOI] [PubMed] [Google Scholar]
- Zhu L.H., Zhao K.L., Chen X.L., Xu J.X. Impact of weaning and an antioxidant blend on intestinal barrier function and antioxidant status in pigs. J Anim Sci. 2012;90(8):2581–2589. doi: 10.2527/jas.2012-4444. [DOI] [PubMed] [Google Scholar]