Abstract
The immediate post-weaning period is one of the most stressful phases in a pig's life, and during this period, piglets are usually exposed to environmental, social and psychological stressors which have direct or indirect effects on gut health and overall growth performance. In this review, the impact of husbandry practices on gut health outcomes and performance of piglets is discussed. Husbandry practices in the swine barn generally include nutrition and management practices, maintenance of hygienic standards and disease prevention protocols, and animal welfare considerations. Poor husbandry practices could result in reduced feed intake, stress and disease conditions, and consequently affect gut health and performance in weaned piglets. Reduced feed intake is a major risk factor for impaired gut structure and function and therefore a key goal is to maximize feed intake in newly weaned piglets. In weaned piglets, crowding stress could reduce pig performance, favor the proliferation of pathogenic bacteria resulting in diarrhea, stimulate immune responses and interfere with beneficial microbial activities in the gut. Sanitation conditions in the swine barn plays an important role for optimal piglet performance, because unclean conditions reduced growth performance, shifted nutrient requirements to support the immune system and negatively affected the gut morphology in weaned piglets. Appropriate biosecurity measures need to be designed to prevent disease entry and spread within a swine operation, which in turn helps to keep all pigs and piglets healthy. Collectively, husbandry practices relating to feeding and nutrition, animal welfare, biosecurity and disease prevention are important determinants of gut health and piglet performance. Thus, it is suggested that adopting high husbandry practices is a critical piece in strategies aimed at raising pigs without the use of in-feed antibiotics.
Keywords: Gut health, Husbandry practices, Weaned piglets
1. Introduction
Weaning is one of the most challenging phases in a pig's life, often accompanied by reduced growth performance and increased incidences of diarrhea (Leibbrandt et al., 1975, Vente-Spreeuwenberg et al., 2003). This phase is frequently characterized by reduced feed intake (Bruininx et al., 2002a, Vente-Spreeuwenberg et al., 2003) which, in concert with an immature digestive and immune systems, predisposes the piglet to gastrointestinal disturbances. The major effects of an immature digestive system in weaned piglets include reduced activity of digestive enzymes, changes in intestinal morphology (Hampson, 1986, Boudry et al., 2004) and reduced digestion in the small intestine. In relation to the immune system, nursery piglets are extremely immune deficient and extensively rely on sow's milk for protection of immunity, growth and survival (Stokes et al., 2001). Hence, piglet management during weaning is one of the most challenging tasks in swine production.
Husbandry practices in the swine barn generally include nutrition and management practices, maintenance of hygienic standards and disease prevention protocols, and animal welfare considerations (e.g., space allowance and ambient temperature for piglets) (Canadian Pork Council (CPC), 2014). Husbandry practices and gut health in weaned piglets are strongly inter-related as these factors have a direct effect on gut structure and function. The term gut health in animals is not well-defined, however, numerous indices, such as those relate to gut structure and function, and microbial population, incidences of diarrhea have been used to describe gut health outcomes (Lalle's et al., 2007). The factors that affect gut health and growth performance in piglet husbandry practices include feeding strategies (Dong and Pluske, 2007), exposure to crowding stress (Khafipour et al., 2014), sanitation (Jayaraman et al., 2016; Kahindi et al., 2014) and disease conditions (Opapeju et al., 2009). To minimize the adverse effects of weaning and their subsequent consequences, appropriate husbandry management strategies need to be taken to maximize post-weaning performance. The objective of this paper is to review the effects of husbandry practices on gut health and growth performance of weaned piglets. It is suggested that to effectively manage gut health of piglets raised under production systems that do not rely on in-feed antibiotics, intervention strategies must include a consideration of husbandry practices. Such knowledge will allow a more robust assessment of the effectiveness of any one intervention strategy.
2. Factors affecting feed intake in weaned piglets
The effects of feed intake on gut morphology and growth performance in weaned piglets has been well reviewed by Dong and Pluske (2007). For a detailed discussion on the determinants of voluntary feed intake in swine, please refer to the review by Nyachoti et al. (2004). For the newly-weaned piglets, various factors affecting feed intake have been identified and these include creep feeding (Bruininx et al., 2002a), weaning age (Davis et al., 2006), mixing of different litters after weaning (Bjork, 1989, McGlone and Curtis, 1985), dietary nutrient level and balance (D'Mello, 2003), diseases or the immune activation status (Edmonds et al., 1997, Jayaraman et al., 2015, Williams et al., 1997), environmental factors (Bruininx et al., 2002b), palatability of feed stuffs (Bell, 1984, van Heugten, 2001), physical form of diets (Hancock and Behnke, 2001, Patridge, 1989), feeding practices (Han et al., 2006), and water supply and quality (Dybkjaer et al., 2006, Thacker, 2001).
2.1. Feed intake affects gut health and function
Reduced feed intake immediately after weaning could lead to adverse morphological and functional changes in the intestine (Dong and Pluske, 2007). The major adverse changes in intestinal morphology include shortening of the villi, hyperplasia of crypt cells, and increased epithelial cell mitosis (Nabuurs et al., 1993, Van Beers-Schreurs et al., 1995). Due to these changes in intestinal morphology, gut functions can be incomplete, resulting in decreased brush-border enzyme activity and absorptive capacity (Hampson and Kidder, 1986, Nabuurs et al., 1993, Vente-Spreeuwenberg et al., 2004). Furthermore, low feed intake and stress in piglets could lead to reduced gut mucosal integrity confirmed by an increase in paracellular transport, and a decrease in villous height (Spreeuwenberg et al., 2001). Because of increased paracellular permeability, luminal antigens rather than bacteria may enter the lamina propria, resulting in inflammation (Spreeuwenberg et al., 2001). Available evidences indicate that reduced feed intake is a major contributing factor to the abruptly-reduced intestinal villus height (VH) (Cera et al., 1988, Spreeuwenberg et al., 2001).
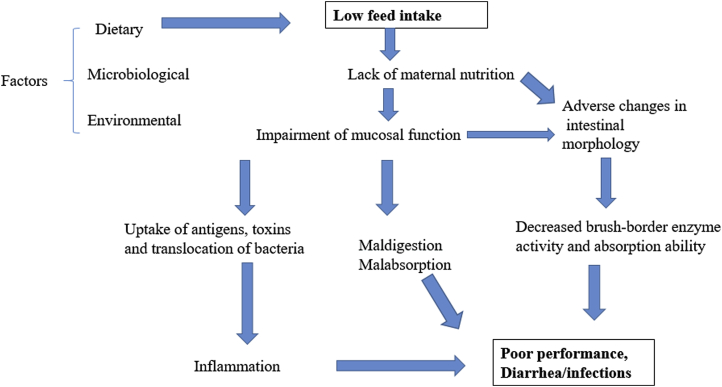
The brush border enzyme activities have been used as indicators of maturation and digestive capacity of the small intestine (Hampson and Kidder, 1986). Low feed intake associated with weaning negatively affects brush-border enzyme activity and absorption ability of the gut (Fig. 1; Dong and Pluske, 2007, Vente Spreeuwenberg and Beynen, 2003). For optimal digestive and absorptive function of the small intestine, longer VH is desirable (Pluske et al., 1997). Pluske et al. (1997) indicated that feed intake linearly and positively correlates with VH in piglets. Moreover, restricted feed intake negatively affects intestinal morphology in piglets (Verdonk et al., 2007). Furthermore, adequate feed intake prevents the loss of the barrier function of the tight junctions in piglets after weaning which indicates the significance of a sufficient luminal nutrient supply to maintain the barrier function. Hence, sufficient feed intake could be particularly essential for the proximal small intestine because this part depends more on luminal nutrient supply than the distal small intestine (Stoll et al., 2000). Verdonk et al. (2007) studied the effects of feed intake level on gut structure and permeability as determined using the Ussing chambers. The authors indicated that restricted feed intake negatively affected gut morphology compared to high level of feed intake in weaned piglets but did not affect trans-epithelial transport.
Fig. 1.
Effects of low feed intake on brush-border enzyme activity and absorption ability after weaning in piglets. Adapted from Dong and Pluske, 2007, Vente Spreeuwenberg and Beynen, 2003.
Collectively, low feed intake is a major risk factor for impaired structure and function, which negatively affect gut morphology and barrier function. Hence, the key goal in swine husbandry practice is to maximize feed intake in newly weaned piglets. Efforts to achieve this goal must start in farrowing room with managing feed intake in the lactating sow. Sows nursing newborns require essentially full feeding during lactation, therefore, supplementing the sow's diet during late gestation and lactation periods may improve performance of the sow and her piglets (Kirkden et al., 2013).
2.2. Creep feeding
In commercial swine production, creep feeding during the suckling period prior to weaning has been a common husbandry practice because it increases weaning weight of piglets and leads to a smooth transition period for the piglets from sow's milk to the dry feed (Cabrera et al., 2013, Dong and Pluske, 2007). Previous studies demonstrated that creep feed intake has a positive effect on post-weaning feed intake (Bruininx et al., 2002a, Cabrera et al., 2013, Kuller et al., 2007), and it is assumed that nursery piglets offered creep feed prompts them to get adapted to solid feed (Dong and Pluske, 2007). In addition, Bruininx et al. (2002a) investigated whether consumption of creep feed prior to weaning stimulated the increase in post-weaning feed intake and performance. In this study, 149 piglets were provided creep feed containing chromic oxide (1%) and the remaining 49 piglets were not offered creep feed. Fecal samples were collected at 3 different time points (18, 22 and 27 d of age) and the visual appearance of green color of feces indicated the consumption of creep feed. The piglets were designated as eaters, which showed green colored feces at all-time points, and the piglets that never showed green colored feces as non-eaters. In addition, the piglets were grouped as non-eaters which were not provided creep feed. The authors showed that feed intake and daily gain during the first 8 days after weaning was higher for eaters than for non-eaters of creep feed in lactation or for those piglets not provided with creep feed during this period. These studies indicate that piglets consuming more creep feed during lactation get adapted to solid diets, which promotes gut development and therefore helps them more easily manage with a dietary change after weaning.
With respect to gut health, creep feeding helps to maintain nutrient supply after weaning, and consequently prevents villous atrophy, thereby reducing the chances of post-weaning diarrhea in piglets (Pluske et al., 1996). Furthermore, creep feed intake during lactation improved net absorption in the small intestine after weaning which could decrease the risk of post-weaning diarrhea (Kuller et al., 2007).
The management strategies for creep feeding in piglets include offering fresh and palatable creep feed at less than 2 weeks of age (Appleby et al., 1991), frequent feeding, feed should be accessible (Wattanakul et al., 2005), increasing feeder space (Appleby et al., 1992), and supplementation of feed additives in creep feed (Cabrera et al., 2013, Shim et al., 2005). For example, supplementation of the feed additive oligo-fructose in antibiotic-free creep feed favored the growth of beneficial bacteria (Bifidobacterium species) and reduced the harmful bacteria (coliforms) in the colon (Shim et al., 2005). Similarly, supplementation of creep feed with L-glutamine has been shown to improve feed conversion possibly due to improved intestinal health (Cabrera et al., 2013). Collectively, creep feeding is advantageous in improving gut health in weaned piglets, and thus promotes growth performance in piglets.
2.3. Feeder space
Pigs are highly social animals and their feeding behavior is to eat in a group (Figueroa et al., 2013), and therefore enough feeder space need to be provided to allow group feeding. Restricted feeder space could increase the competition among the pigs for the feed, which would result in reduced growth performance. Hence, providing adequate feeder space is critical to weaned pig performance (CPC, 2014) because limited feeder space can increase competition at the feeder, which is likely to compromise feed intake thus leading to reduced growth rates (Averos et al., 2012, Georgsson and Svendsen, 2001). In a growth study, Lindemann et al. (1987) demonstrated that weaned piglets offered 9 cm per pig feeder space allowance had significantly higher growth performance compared to those provided 3 cm per pig feeder space allowance. Recently, He et al. (2016) evaluated the effect of restricted feeder space (2 spaces per pen versus 5 spaces per pen; area of each feeding space was 15 cm × 15 cm) on growth performance of weaned piglets. The authors indicated that limited feeder space (5 spaces per pen) is associated with increased risk of mortality or slow growth in weaned piglets. Overall, restricted feeder space can compromise growth performance and welfare of pigs, which may contribute to depressed performance in piglets. Because feed intake is an important factor in gut development, any factor such as space allowance, which influences feed intake is therefore potentially capable of impacting gut health outcomes in weaned piglets.
3. Effects of stress in pigs
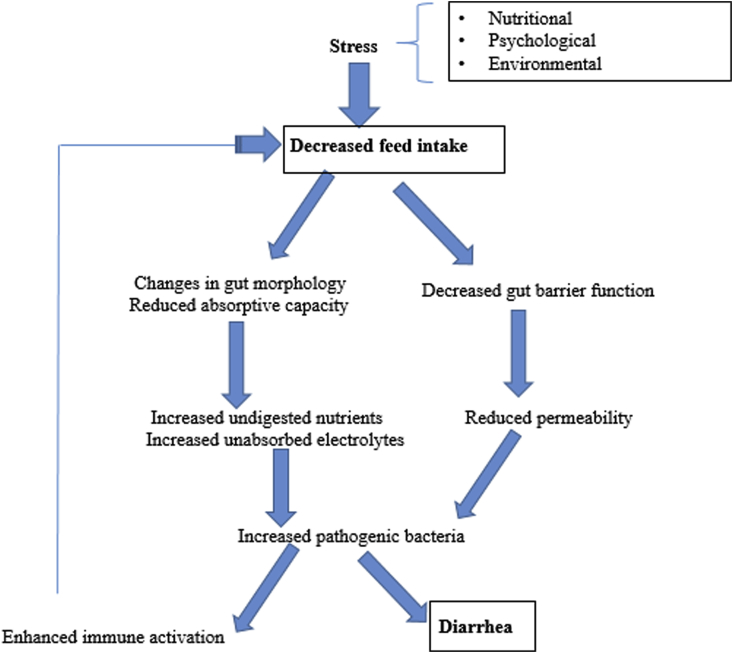
Stress is generally defined as any threat to an animal's homeostasis (Mawdsley and Rampton, 2005). During stress, physiological mechanisms get activated to maintain homeostasis, which might also compromise productivity. The major stressors during the post-weaning phase include nutritional (e.g., removal of sow's milk), psychological (e.g., mixing with other littermates) and environmental (e.g., change in ambient temperature) (Campbell et al., 2013), which result in drastic reduction in feed intake (Fig. 2). Weaning anorexia is associated with significant gastrointestinal tract (GIT) disturbances characterized by decreased digestive and absorptive capacity and consequently results in an increase in pathogenic bacteria (Pluske et al., 1997, Lalle’s et al., 2004). Moreover, crowding stress also decreases immune competence by decreasing immune cell numbers or increasing immunosuppressive mechanisms, and reduced growth rate (Khafipour et al., 2014). Therefore, appropriate husbandry practices and nutritional management need to be taken to minimize stressful conditions and optimize growth performance in piglets.
Fig. 2.
Schematic diagram illustrating the effects of stress in weaned piglets.
At weaning, the piglet is exposed to various stressors and pathogenic infections due to restricted space allowance, mingling with unfamiliar animals, unfavorable ambient temperature, contaminated air, and low bio-security. The combined and additive effects of these stressors and pathogenic threats accelerate the detrimental effects on the growth performance of pigs (Hyun et al., 1998). The health of the pig will, therefore, be improved if the number of stressors in the pigs' environment are reduced.
3.1. Effects of crowding stress on gut health
In weaned piglets, crowding escalates social stress and adversely affect feed consumption and growth performance (Kil and Stein, 2010). Khafipour et al. (2014) investigated the effects of crowding stress on performance and immunological parameters in weaned piglets challenged with enterotoxigenic Escherichia coli (ETEC). The authors demonstrated that crowding stress increased plasma cortisol in stressed piglets compared to non-stressed piglets. In animals, plasma cortisol concentration has been considered as a stress indicator. Moreover, crowding stress had a direct impact on immune response in pigs as indicated by an elevated serum cytokines and plasma cortisol (Khafipour et al., 2014, Oha et al., 2010). In a recent study, Li et al. (2017) demonstrated that weaned piglets under chronic social stress had reduced growth performance and altered gut barrier and nutrient transport function.
Restricted space allowance is one of the stressors for piglets at the time of weaning (Kil and Stein, 2010) which results in crowding stress and as a result negatively affects feed intake and growth performance (Khafipour et al., 2014). Crowding stress also results increase in abnormal behavior and levels of aggression, which increases non-growth energy expenditure (Turner et al., 2003, Nyachoti et al., 2004, Khafipour et al., 2014). The current recommended space allowance for weaned pig is at least 0.34 m2 per pig in slatted pens (AAFC, 1993), however, in antibiotic-free feeding regimen, more space allowance might be required (Varley, 2004).
In relation to gut health, crowding stress reduced resistance to bacterial infection as well as increasing fecal shedding of weaned piglets (Jones et al., 2001). Similarly, Khafipour et al. (2014) demonstrated that weaned piglets exposed to crowding stress and challenged with ETEC had increased proliferation of ETEC in the ileal and colon digesta compared to those provided with adequate space. Crowding stress leads to an increase in intestinal pH, which may create favorable conditions for ETEC colonization resulting in diarrhea. In general, low intestinal pH favor establishment of beneficial bacteria and inhibit harmful bacteria (Ohland and MacNaughton, 2010). Collectively, crowding stress can directly or indirectly deteriorate gut health and growth performance of weaned piglets.
4. Effects of sanitation on performance and gut health
In commercial swine production, clean and hygienic sanitation play an important role in growth performance of healthy pigs (CPC, 2014). Le Floc'h et al. (2006) proposed that degradation of sanitary conditions could provoke moderate inflammation in weaned piglets. In swine studies, poor sanitary conditions could be created by keeping the animal room uncleaned and non-disinfected prior housing and during the growth period (Kahindi et al., 2014; Lee et al., 2005, Le Floc’h et al., 2009). Under poor sanitary conditions, piglets had depressed growth performance, stimulated immune system (Williams et al., 1997) and a provoked inflammatory response (Le Floc'h et al., 2006) which interferes with growth because of competition for nutrients between structural tissues and immune function (Le Floc'h et al., 2009). Due to immune system stimulation, amino acids are the most commonly affected nutrients and the requirements of amino acids could be increased in weaned piglets (Kahindi, 2015, Jayaraman et al., 2015). For instance, the optimal standardized ileal digestible threonine:lysine for weaned pigs raised under unclean sanitary conditions was higher (66.5% vs. 65%) than for those raised under clean sanitary conditions (Jayaraman et al., 2015). In a growth study, Jayaraman et al. (2016) demonstrated that a room with unclean sanitary conditions had higher NH3 (26.65 vs. 18.17 ppm) and H2S (0.099 vs. 0.010 ppm) compared to the room with clean sanitary conditions. In this study, unclean sanitary conditions increased aerial NH3 and H2S concentrations in the room. The deterioration of air quality could have caused distress and a drop in feed intake, thus leading to the observed reduced growth performance of piglets reared under unclean sanitary conditions. The underlying mechanism could be that, under unclean conditions, piglets had reduced feed intake which could have caused alterations in gut morphology as reported by Pluske et al. (1996). After weaning, negative alterations in intestinal morphology of pigs occur which includes reduced villous height and, to a lesser extent, reduced crypt depth. Zhao et al. (2007) indicated that piglets raised under unclean conditions had shorter villous height and less crypt depth compared to those raised under clean conditions, which could be the influence of sanitary conditions. In addition, Pastorelli et al. (2012) demonstrated that weaned piglets raised under unclean sanitary conditions had higher incidences of soft feces and diarrhea compared to those raised under clean sanitary conditions. Results of these studies collectively demonstrate that, maintaining high standards of cleanliness in the nursery is critical for optimal performance of piglets partly because of its direct or indirect effects on gut health and function.
5. Biosecurity measures to promote gut health in pigs
Biosecurity is defined as the implementation of measures that reduce the risk of disease agents being introduced and spread in an animal and its environment. It requires that people adopt a set of attitudes and behaviors to reduce risk in all activities involving domestic, exotic and wild animals and their products (Food and Agriculture Organization (FAO, 2010)). Biosecurity and security procedures are entwined to improve the health status and productivity of pigs (Levis and Baker, 2011). In general, swine herd maintenance depends on 1) development of herd immunity, 2) biosecurity, 3) pig flow, 4) medicine management, 5) stock health management, 6) pig environment, and 7) disease management (Kyriazakis and Whittemore, 2006). However, it is important to note that biosecurity of a pig farm and security risk factors are unique to that farm and, therefore, each biosecurity plan should be farm specific (Levis and Baker, 2011).
Biosecurity includes bio-exclusion, bio-containment, and bio-management (Levis and Baker, 2011). Bio-exclusion (also known as external biosecurity) is preventing the entry of undesirable pathogens into the farm. For example, preventing the entry of common enteric pathogens such as ETEC, Salmonella and porcine proliferative ileitis into the pig farm. Most pig producers concentrate on bio-exclusion as these types of practices are vital indicators for the chances of pathogen introduction, particularly in regards to the porcine reproductive and respiratory syndrome virus (Bottoms et al., 2013). Bio-management is the combined effort to control economically infectious diseases that are already present in the farm population. Proper cleanliness and disinfection of the pig rooms, vaccinations, all-in/all-out pig movement are some of the important procedures to minimize the pathogen build-up or enhance immunity levels in the pigs are the key components of bio-management. In pigs, enteric disease is an intricate interaction between the number of pathogens present in the environment, immune competence of the pigs, and concomitant stresses occurring within the pigs' environment (Pluske et al., 2002). Therefore, control of enteric disease is one of the most challenging areas for pig producers, regardless of whether the animals are housed indoors or outdoors. In the pig farm environment, though it is impossible to accomplish without any disease risk, biosecurity practices aid in reducing the disease risk (Bottoms et al., 2013).
5.1. The cost of enteric disease in the pig industry
Kyriazakis and Whittemore (2006) indicated that for unhealthy pigs, the price paid by a pig industry is the sum of the following: the costs of veterinarian visits, veterinary medicine, vaccines, the cost of in-feed medicines, cost of lost efficiency pre-farm gate due to decrease in sow productivity, reduced growth rate and fee efficiency, loss of product quality, and loss of saleable product through meat processing condemnation.
5.2. Pig flow – managing an all-in/all-out system
The practice of all-in/all-out management system breaks the disease cycle by preventing the sharing of air-space of pigs carrying clinical disease with pigs susceptible to the infection by that disease. The principle behind all-in/all-out system is that between batches of pigs, the location is completely cleared, disinfected and rested to ensure the cycle of infection is broken and premises do not themselves serve as a reservoir for infective material (Kyriazakis and Whittemore, 2006).
The key reasons for using all-in/all-out system are to minimize exposure levels to pathogenic organisms in the pig farm, to avoid the spread of diseases from adult pigs to younger pigs, and to increase feed efficiency and rate of gain by maintaining a high health status (Levis and Baker, 2011). Furthermore, it can be argued that by minimizing exposure to pathogenic organisms, and especially those that cause enteric diseases, this management strategy will be invaluable to production systems raising pigs without the use of in-feed antimicrobial agents.
6. Conclusions
Husbandry practices are one of the most important determinants of piglet performance. Poor husbandry practices could result in low feed intake, stress and disease conditions, and consequently affect gut health and performance of weaned piglets. Therefore, appropriate feeding and nutrition strategies, management practices and animal welfare activities, biosecurity and disease prevention measures are critical determinants of gut health and piglet performance. Thus, it is suggested that husbandry practices must be considered as a critical piece in the overall strategy of raising weaned piglets without in-feed antibiotics.
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- AAFC . Agriculture and Agri-Feed Canada Publication; 1993. Recommended code of practice for the care and handling of farm animals: pigs; p. 55. [Google Scholar]
- Appleby M.C., Pajor E.A., Fraser D. Effects of management options on creep feeding by piglets. Anim Prod. 1991;53:361–366. [Google Scholar]
- Appleby M.C., Pajor E.A., Fraser D. Individual variation in feeding and growth of piglets: effects of increased access to creep food. Anim Prod. 1992;55:147–152. [Google Scholar]
- Averos X., Brossard L., Dourmad J.Y., de Greef K.H., Edwards S.A., Meunier-Salaun M.C. Meta-analysis on the effects of the physical environment, animal traits, feeder and feed characteristics on the feeding behaviour and performance of growing-finishing pigs. Animal. 2012;6:1275–1289. doi: 10.1017/S1751731112000328. [DOI] [PubMed] [Google Scholar]
- Bell J.M. Nutrients and toxicants in rapeseed meal: a review. J Anim Sci. 1984;58:996–1010. doi: 10.2527/jas1984.584996x. [DOI] [PubMed] [Google Scholar]
- Bjork A.K.K. Is social stress in pigs a detrimental factor to health and growth that can be avoided by amperzoide treatment. Appl Anim Behav Sci. 1989;23:39–47. [Google Scholar]
- Bottoms K., Poljak Z., Dewey C., Deardon R., Holtkamp D., Friendship R. Evaluation of external biosecurity practices on Southern Ontario sow farms. Prev Vet Med. 2013;109:58–68. doi: 10.1016/j.prevetmed.2012.08.013. [DOI] [PubMed] [Google Scholar]
- Boudry G., Péron V., Le Huérou-Luron I., Lallès J.P., Sève B. Weaning induces both transient and long-lasting modifications of absorptive, secretory, and barrier properties of piglet intestine. J Nutr. 2004;134:2256–2262. doi: 10.1093/jn/134.9.2256. [DOI] [PubMed] [Google Scholar]
- Bruininx E.M.A.M., Binnendijk G.P., van der PeetSchwering C.M.C., Schrama J.W., den Hartog L.A., Everts H. Effect of creep feed consumption on individual feed intake characteristics and performance of group-housed weanling pigs. J Anim Sci. 2002;80:1413–1418. doi: 10.2527/2002.8061413x. [DOI] [PubMed] [Google Scholar]
- Bruininx E.M.A.M., Heetkamp M.J.W., van den Bogaart D., van der Peet- Schwering C.M.C., Beynen A.C., Everts H. A prolonged photoperiod improves feed intake and energy metabolism of weanling pigs. J Anim Sci. 2002;80:1736–1745. doi: 10.2527/2002.8071736x. [DOI] [PubMed] [Google Scholar]
- Cabrera R.A., Usry J.L., Arrellano C., Nogueira E.T., Kutschenko M., Moeser A.J. Effect of creep feeding and supplemental glutamine or glutamine plus glutamate (Amino gut) on pre- and post-weaning growth performance and intestinal health of piglets. J Anim Sci Biotechnol. 2013;4:29. doi: 10.1186/2049-1891-4-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell J.M., Crenshaw J.D., Polo J. The biological stress of early weaned piglets. J Anim Sci Biotechnol. 2013;4:19. doi: 10.1186/2049-1891-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canadian Pork Council (CPC) Canada Pork Council and National Farm Animal Care Council; 2014. Code of practice: for the care and handling of pigs. [Google Scholar]
- Cera K.R., Mahan D.C., Cross R.F., Reinhart G.A., Whitmoyer R.E. Effect of age, weaning and postweaning diet on small intestinal growth and jejunal morphology in young swine. J Anim Sci. 1988;66:574–584. doi: 10.2527/jas1988.662574x. [DOI] [PubMed] [Google Scholar]
- Davis M.E., Sears S.C., Apple J.K., Maxwell C.V., Johnson Z.B. Effect of weaning age and co-mingling after the nursery phase of pigs in a wean-to-finish facility on growth, humoral and behavioral indicators of well-being. J Anim Sci. 2006;84:743–756. doi: 10.2527/2006.843743x. [DOI] [PubMed] [Google Scholar]
- D'Mello J.P.F. Adverse effects of amino acids. In: D'Mello J.P.F., editor. Amino acids in animal nutrition. 2nd ed. CAB International; Wallingford: 2003. pp. 125–142. [Google Scholar]
- Dong G.Z., Pluske J.R. The low feed intake in newly-weaned pigs: problems and possible solutions. Asian-Aust J Anim Sci. 2007;20:440–452. [Google Scholar]
- Dybkjaer L., Jacobsen A.P., Togersen F.A., Poulsen H.D. Eating and drinking activity of newly weaned piglets: effects of individual characteristics, social mixing, and addition of extra zinc to the feed. J Anim Sci. 2006;84:702–711. doi: 10.2527/2006.843702x. [DOI] [PubMed] [Google Scholar]
- Edmonds M.S., Arentson B.E., Nipper W.A., Froe D.L. Segregated early weaning: effects of raising pigs on-site and off-site with and without vaccination on performance and economics. J Anim Sci. 1997;75:195. [Google Scholar]
- Food and Agriculture Organization (FAO) Animal Production and Health . 2010. Good biosecurity practices for the pig sector: issues and options in developing and transition countries.http://www.fao.org/docrep/012/i1435e/i1435e00.pdf [Google Scholar]
- Figueroa J., Solà-Oriol D., Manteca X., Pérez J.F. Social learning of feeding behaviour in pigs: effects of neophobia and familiarity with the demonstrator conspecific. Appl Anim Behav Sci. 2013;148(1–2):120–127. [Google Scholar]
- Georgsson L., Svendsen J. One or two feeders for groups of 16 growing-finishing pigs: effects on health and production. Acta Agric Scand Sect A Anim Sci. 2001;51:257–264. [Google Scholar]
- Hampson D.J. Alterations in piglet small intestinal structure at weaning. Res Vet Sci. 1986;40:32–40. [PubMed] [Google Scholar]
- Hampson D.J., Kidder D.E. Influence of creep feeding and weaning on brush border enzyme activities in the piglet small intestine. Res Vet Sci. 1986;40:24–31. [PubMed] [Google Scholar]
- Han Y.K., Thacker P.A., Yang J.S. Effects of the duration of liquid feeding on performance and nutrient digestibility in weaned pigs. Asian-Aust J Anim Sci. 2006;19:396–401. [Google Scholar]
- Hancock J.D., Behnke K.C. Use of ingredient and diet processing technologies (grinding, mixing, pelleting, and extruding) to produce quality feeds for pigs. In: Lewis A.J., Southern L.L., editors. Swine nutrition. 2nd. CRC Press LLC; Boca Raton, Florida: 2001. pp. 469–497. [Google Scholar]
- He Y., Deen J., Shurson G.C., Wang L., Chen C., Keisler D.H. Identifying factors contributing to slow growth in pigs. J Anim Sci. 2016;94:2103–2116. doi: 10.2527/jas.2015-0005. [DOI] [PubMed] [Google Scholar]
- Hyun Y., Ellis M., Riskowski G., Johnson R.W. Growth performance of pigs subjected to multiple concurrent environmental stressors. J Anim Sci. 1998;76:721–727. doi: 10.2527/1998.763721x. [DOI] [PubMed] [Google Scholar]
- Jayaraman B., Htoo J.K., Nyachoti C.M. Effects of dietary threonine:lysine ratios and sanitary conditions on performance, plasma urea nitrogen, plasma-free threonine and lysine of weaned pigs. Anim Nutr. 2015;1:1–6. doi: 10.1016/j.aninu.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaraman B., Htoo J.K., Nyachoti C.M. Effects of different dietary tryptophan: lysine ratios and sanitary conditions on growth performance, plasma urea nitrogen, serum haptoglobin and ileal histomorphology of weaned pigs. Anim Sci J. 2016;88:763–771. doi: 10.1111/asj.12695. [DOI] [PubMed] [Google Scholar]
- Jones P.H., Roe J.M., Miller B.G. Effects of stressors on immune parameters and on the faecal shedding of enterotoxigenic Escherichia coli in piglets following experimental inoculation. Res Vet Sci. 2001;70:9–17. doi: 10.1053/rvsc.2000.0436. [DOI] [PubMed] [Google Scholar]
- Kahindi R. University of Manitoba; 2015. Assessment of standardized ileal digestible lysine and sulphur amino acids to lysine ratio for weaned piglets fed antibiotic-free diets. [Doctoral thesis dissertation] [Google Scholar]
- Kahindi R.K., Htoo J.K., Nyachoti C.M. Effect of dietary lysine content and sanitation conditions on performance of weaned pigs fed antibiotic-free diets. Can J Anim Sci. 2014;94:115–118. [Google Scholar]
- Khafipour E., Munyaka P.M., Nyachoti C.M., Krause D.O., Rodriguez-Lecompte J.C. Effect of crowding stress and Escherichia coli K88+ challenge in nursery pigs supplemented with anti-Escherichia coli K88+ probiotics. J Anim Sci. 2014;92:2017–2029. doi: 10.2527/jas.2013-7043. [DOI] [PubMed] [Google Scholar]
- Kil D.Y., Stein H.H. Invited review: management and feeding strategies to ameliorate the impact of removing antibiotic growth promoters from diets fed to weanling pigs. Can J Anim Sci. 2010;90:447–460. [Google Scholar]
- Kirkden R.D., Broom D.M., Andersen I.L. Invited review: piglet mortality: management solutions. J Anim Sci. 2013;91:3361–3389. doi: 10.2527/jas.2012-5637. [DOI] [PubMed] [Google Scholar]
- Kuller W.I., Soede N.M., van Beers-Schreurs H.M.G., Langendijk P., Taverne M.A.M., Kemp B. Effects of intermittent suckling and creep feed in creep feeding duration for piglets take on pig performance from birth to slaughter. J Anim Sci. 2007;85:1295–1301. doi: 10.2527/jas.2006-177. [DOI] [PubMed] [Google Scholar]
- Kyriazakis I., Whittemore C.T. 3rd ed. 2006. Chapter 7 the maintenance of health. Whittenmore's science and practice of pig production; pp. 263–315. [Google Scholar]
- Lalle's J.P., Boudry G., Favier C., Le Floc'h N., Lurona I., Montagne L. Gut function and dysfunction in young pigs: physiology. Anim Res. 2004;53:301–316. [Google Scholar]
- Lalle's J.P., Bosi P., Smidt H., Stokes C.R. Weaning – a challenge to gut physiologists. Livest Sci. 2007;108:82–93. [Google Scholar]
- Lee C.L., Giles R., Bryden W.L., Downing J.L., Owens P.C., Kirby A.C. Performance and endocrine responses of group housed weaner pigs exposed to the air quality of a commercial environment. Livest Prod Sci. 2005;93:255–262. [Google Scholar]
- Le Floc'h N., Jondreville C., Matte J.J., Seve B. Importance of sanitary environment for growth performance and plasma nutrient homeostasis during the post-weaning period in piglets. Arch Anim Nutr. 2006;60:23–34. doi: 10.1080/17450390500467810. [DOI] [PubMed] [Google Scholar]
- Le Floc'h N., Lebellago L., Matte J.J., Melchior D., Seve B. The effect of sanitary status degradation and dietary tryptophan content on growth rate and tryptophan metabolism in weaning pigs. J Anim Sci. 2009;87:1686–1694. doi: 10.2527/jas.2008-1348. [DOI] [PubMed] [Google Scholar]
- Leibbrandt V.D., Ewan R.C., Speer V.C., Zimmerman D.R. Effect of weaning and age at weaning on baby pig performance. J Anim Sci. 1975;40:1077–1080. [Google Scholar]
- Levis D.G., Baker R.B. University of Nebraska Lincoln Extension publications; 2011. Biosecurity of pigs and farm security.http://extesnion.unl.edu/publications [Google Scholar]
- Li Y., Song Z., Kerr K.A., Moeser A.J. Chronic social stress in pigs impairs intestinal barrier and nutrient transporter function, and alters neuro-immune mediator and receptor expression. PLoS ONE. 2017;12(2):e0171617. doi: 10.1371/journal.pone.0171617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindemann M.D., Kornegay E.T., Meldrum J.B., Shurig G., Gwazdauskas F.C. The effect of feeder space allowance on weaned pig performance. J Anim Sci. 1987;64:8–14. [Google Scholar]
- Mawdsley J.E., Rampton D.S. Psychological stress in IBD: new insights into pathogenic and therapeutic implications. Gut. 2005;54:1481–1491. doi: 10.1136/gut.2005.064261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlone J.J., Curtis S.E. Behaviour and performance of weanling pigs in pens equipped with hide area. J Anim Sci. 1985;60:20–24. doi: 10.2527/jas1985.60120x. [DOI] [PubMed] [Google Scholar]
- Nabuurs M.J.A., Hoogendoorn A., van der Molden E.J., Van Osta A.L.M. Villous height and crypt depth in weaned and unweaned pigs, reared under various circumstances in The Netherlands. Res Vet Sci. 1993;55:78–84. doi: 10.1016/0034-5288(93)90038-h. [DOI] [PubMed] [Google Scholar]
- Nyachoti C.M., Zijlstra R.T., de Lange C.F.M., Patience J.F. Voluntary feed intake in growing-finishing pigs: a review of the main determining factors and potential approaches for accurate predictions. Can J Anim Sci. 2004;84:549–566. [Google Scholar]
- Oha H.K., Choia H.B., Jua W.S., Chungb C.S., Ki Y.Y. Effects of space allocation on growth performance and immune system in weaning pigs. Livest Sci. 2010;132:113–118. [Google Scholar]
- Ohland C.L., MacNaughton W.K. Probiotic bacteria and intestinal epithelial barrier function. Am J Physiol Gastrointest Liver Physiol. 2010;298:G807–G819. doi: 10.1152/ajpgi.00243.2009. [DOI] [PubMed] [Google Scholar]
- Opapeju F.O., Krause D.O., Payne R.L., Rademacher M., Nyachoti C.M. Effect of dietary protein level on growth performance, indicators of enteric health and gastrointestinal microbial ecology of weaned pigs induced with post-weaning colibacillosis. J Anim Sci. 2009;87:2635–2643. doi: 10.2527/jas.2008-1310. [DOI] [PubMed] [Google Scholar]
- Pastorelli H., Le Floc'h N., Merlot E., Meunier-Salau M.C., van Milgen J., Montagne L. Sanitary housing conditions modify the performance and behavioural response of weaned pigs to feed- and housing-related stressors. Animal. 2012;6:1811–1820. doi: 10.1017/S1751731112001231. [DOI] [PubMed] [Google Scholar]
- Patridge I.G. Alternative feeding strategies for weaner pigs. Manipulation of pig production. 1989:160–169. [Google Scholar]
- Pluske J.R., Williams I.H., Aherne F.X. Maintenance of villous height and crypt depth in piglets by providing continuous nutrition after weaning. Anim Sci. 1996;62:131–144. [Google Scholar]
- Pluske J.R., Hampson D.J., Williams I.H. Factors influencing the structure and function of the small intestine in the weaned pig: a review. Livest Prod Sci. 1997;51:215–236. [Google Scholar]
- Pluske J.R., Pethick D.W., Hopwood D.E., Hampson D.J. Nutritional influences on some major enteric bacterial diseases of pigs. Nutr Res Rev. 2002;15:333–371. doi: 10.1079/NRR200242. [DOI] [PubMed] [Google Scholar]
- Shim S.B., Verstegen M.W.A., Kim I.H., Kwon O.S., Verdonk J.M.A. Effects of feeding antibiotic-free creep feed supplemented with oligofructose, probiotics or synbiotics to suckling piglets increases the preweaning weight gain and composition of intestinal microbiota. Arch Anim Nutr. 2005;59:419–427. doi: 10.1080/17450390500353234. [DOI] [PubMed] [Google Scholar]
- Spreeuwenberg M.A.M., Verdonk J.M.A.J., Gaskins H.J., Verstegen M.W.A. Small intestine epithelial barrier function is compromised in pigs with low feed intake at weaning. J Anim Nutr. 2001;131:1520–1527. doi: 10.1093/jn/131.5.1520. [DOI] [PubMed] [Google Scholar]
- Stokes C.R., Bailey M., Haverson K. Development and function of the pig gastrointestinal immune system. In: Lindberg J.E., Ogle B., editors. Digestive physiology of pigs. CAB International; Wallingford, UK: 2001. pp. 59–65. [Google Scholar]
- Stoll B., Chang X., Fan M.Z. Enteral nutrient intake level determines intestinal protein synthesis and accretion rates in neonatal pigs. Am J Physiol Gastrointest Liver Physiol. 2000;279:G288–G294. doi: 10.1152/ajpgi.2000.279.2.G288. [DOI] [PubMed] [Google Scholar]
- Thacker P.A. Water in swine nutrition. In: Lewis A.J., Southern L.L., editors. Swine nutrition. 2nd. CRC Press LLC; Boca Raton, Florida: 2001. pp. 381–398. [Google Scholar]
- Turner S.P., Allcroft D.J., Edwards S.A. Housing pigs in large social groups: a review of implications for performance and other economic traits. Livest Prod Sci. 2003;82:39–51. [Google Scholar]
- van Heugten E. Mycotoxins and other anti-nutritional factors in swine feeds. In: Lewis A.J., Southern L.L., editors. Swine nutrition. 2nd. CRC Press LLC; Boca Raton, Florida: 2001. pp. 563–583. [Google Scholar]
- Van Beers-Schreurs H.M.G., Nabuurrs M.J.A., Vellenga L., Breukink H.J. Proc of IXth Int'l Conf Prod Dise Farm Anim Berlin, Germany. 1995. The effect of weaning and diets on villous height and crypt depth in the small intestines of piglets; p. 103. [Google Scholar]
- Varley M.A. Alternatives to antibiotics growth promoters for post-weaned piglets. Pig J. 2004;54:161–170. [Google Scholar]
- Vente Spreeuwenberg M.A.M., Beynen A.C. Diet mediated modulation of small intestine integrity in weaned pigs. In: Pluske J.R., Le Dividich J., Verstegen M.W.A., editors. Weaning the pig: concepts and consequences. Wageningen Academic Publishers; Wageningen, Netherlands: 2003. pp. 145–198. [Google Scholar]
- Vente-Spreeuwenberg M.A.M., Verdonk J.M.A.J., Beynen A.C., Verstegen M.W.A. Interrelationships between gut morphology and feces consistency in newly weaned piglets. Anim Sci. 2003;77:85–93. [Google Scholar]
- Vente-Spreeuwenberg M.A.M., Verdonk J., Bakker G.C.M., Beynen A.C., Verstegen M.W.A. Effect of dietary protein source on feed intake and small intestinal morphology in newly weaned piglets. Livest Prod Sci. 2004;86:169–177. [Google Scholar]
- Verdonk J.M.A.J., Bruininx E.M.A.M., van der Meulen J. Post-weaning feed intake level modulates gut morphology but not gut permeability in weaned piglets. Livest Sci. 2007;108:146–149. [Google Scholar]
- Wattanakul W., Bulman C.A., Edge H.L., Edwards S.A. The effect of creep feed presentation method on feeding behaviour, intake and performance of suckling piglets. Appl Anim Behav Sci. 2005;92:27–36. [Google Scholar]
- Williams N.H., Stahly T.S., Zimmerman D.R. Effect of chronic immune system activation on body nitrogen retention, partial efficiency of lysine utilization, and lysine needs of pigs. J Anim Sci. 1997;75:2472–2480. doi: 10.2527/1997.7592472x. [DOI] [PubMed] [Google Scholar]
- Zhao J., Harper A.F., Estienne M.J., Webb K.E., McElroy A.P., Denbow D.M. Growth performance and intestinal morphology responses in early weaned pigs to supplementation of antibiotic-free diets with an organic copper complex and spray-dried plasma protein in sanitary and nonsanitary environments. J Anim Sci. 2007;85:1302–1310. doi: 10.2527/jas.2006-434. [DOI] [PubMed] [Google Scholar]