Abstract
Gluconeogenesis responses was assessed during a short starvation period and subsequent refeeding in Siberian sturgeon (Acipenser baerii) previously fed different dietary carbohydrates levels and experienced to a glucose stimuli during early life. The sturgeon larvae were previously fed either a high glucose diet (G) or a low glucose diet (F) from the first feeding to yolk absorption (8 to 12 d post-hatching [dph]). Each group of fish was sub-divided into 2 treatments at 13 dph and was fed either a high-carbohydrate diet (H) or a low carbohydrate diet (L) until 20 wk. In the current study, the fish in 4 groups (GL, FL, GH and FH) were experienced to starvation for 21 d following by re-feeding of their corresponding diets for 21 d. Fish were sampled at postprandial 6 and 24 h before starvation (P6h and P24h), starvation 7, 14 and 21 d (S7, S14 and S21) and 1, 7, 14 and 21 d during refeeding (R1, R7, R14 and R21). Plasma samples during refeeding were taken at P6h at each time point. Glycaemia levels, liver and muscle glycogen contents, activities and mRNA levels of hepatic gluconeogenic enzymes were examined. We found that both dietary carbohydrate levels and early glucose stimuli significantly affected the metabolic responses to starvation and refeeding in Siberian sturgeon (P < 0.05). During prolonged starvation, Siberian sturgeon firstly mobilized the liver glycogen and then improved gluconeogenesis when the dietary carbohydrates were abundant, whereas preserved the liver glycogen stores at a stable level and more effectively promoted gluconeogenesis when the dietary carbohydrates are absent to maintain glucose homoeostasis. During refeeding, as most teleostean, Siberian sturgeon failed controlling the activities and mRNA levels of phosphoenolpyruvate carboxykinase cytosolic forms (PEPCK-C), fructose-1,6-bisphosphatase (FBPase), but particularly controlled phosphoenolpyruvate carboxykinase mitochondrial forms (PEPCK-M) activities and mRNA expression of glucose-6-phosphatase (G6Pase, except in GL group). Siberian sturgeon has a full compensatory ability on growth, but this ability would be obstructed by early glucose stimuli when refeeding the low carbohydrate diet after S21.
Keywords: Early nutritional programming, Fasting, Refeeding, Gluconeogenesis, Acipenser baeri
1. Introduction
Both in aquaculture and in natural water, fish could experience periods of food deprivation or starvation, which are caused by seasonal fluctuations, reproductive process, or imposed by routine aquaculture procedures. Fish in northern latitudes experience low winter water temperatures often accompanied with limited food availability and low apatite controlled by endocrine system (Pottinger et al., 2003, Brodersen et al., 2011). Other factors may induce long-term fasting includes the abnormal increased ocean water temperature by changing climate, salinity changes and reproduction, etc. (Polakof et al., 2006, Brodersen et al., 2011). To face these nutritional stresses, fish mobilize their endogenous reserves to obtain the energy to maintain vital processes, which impose metabolic adjustments that are species dependent (Wang et al., 2006). Intraspecific adjustments to these conditions also depend on different factors such as fish age or nutritional status (Navarro and Gutiérrez, 1995).
In most species, liver glycogen is generally the first substrate used as an energy source, and mobilized to maintain the glucose homoeostasis or normoglycaemia during the first stages of starvation (Figueiredo-Garutti et al., 2002, Metón et al., 2003, Furné et al., 2012). Together with glycogen mobilization, reserved lipids are used to obtain energy, and the protein, mainly from skeletal muscle would be mobilized subsequently (Navarro and Gutiérrez, 1995, Metón et al., 2003). The muscle glycogen may be depleted during starvation or maintained stable through the continuous generation of glucose in the liver (Navarro and Gutiérrez, 1995). In contrast to this pre-established dogma, some species try to preserve liver glycogen stores by degrading protein for gluconeogenesis and use lipid and/or protein as energy substrates (Sheridan and Mommsen, 1991, Gillis and Ballantyne, 1996). The different responses of fish refed after a starvation period may depend on species, environmental conditions, starvation period, and the feeding history prior to starvation (Navarro and Gutiérrez, 1995). In most species, the metabolic profiles would return to pre-starvation levels after a short refeeding period with a fast weight recovery known as compensatory growth (Metón et al., 2003, Furné et al., 2012, Morshedi et al., 2013).
Sturgeon are the only members of a primitive group of fish, the chondrostean, surviving today. They occupy an intermediate position between elasmobranches and teleosts. Siberian sturgeon distributes in almost all river systems of northern latitudes. They have a very long-life span (up to 100 years), and become sexual maturity at 9 to 15 years for males or 16 to 20 years for females in natural environment. In water recirculation systems, sexual maturity can firstly occur at 5 years (http://www.fishbase.org/ Kottelat and Freyhof, 1972). Owing to its long-life span and the distribution condition, sturgeon encounters regular periods of low food availability, making it a suitable species for studies of gluconeogenesis strategies to face starvation and refeeding. It has been reported that the metabolic responses to some nutritional conditions, including starvation and refeeding on Adriatic sturgeon (Acipenser naccarii), are different from rainbow trout (Oncorhynchus mykiss) (Furné et al., 2009, Furné et al., 2012). Besides, some studies showed the dietary carbohydrates can be utilized effectively by Siberian sturgeon (A. baerii) (Kaushik et al., 1989, Yun et al., 2014, Gong et al., 2015) and other sturgeon species (Lin et al., 1997, Furné et al., 2005). A few studies have reported the responses of compensatory growth, plasma performances and/or body compositions in white sturgeon (A. transmontanus), Chinese sturgeon (A. sinensis), Persian sturgeon (A. persicus) and Siberian sturgeon facing starvation and refeeding respectively (Liu et al., 2011, Yarmohammadi et al., 2012, Morshedi et al., 2013), but there are no reports on the effects of nutritional history, including early programming and latter dietary carbohydrates levels on metabolic responses on any sturgeon species.
Early nutritional programming might be a way to modify metabolic responses during later life. The programming stimulus exerted in early ontogeny stages may have long-term consequences on physiological functions in later life stages (Lucas, 1998). Several studies demonstrated that fish also showed an obvious developmental plasticity by nutritional conditioning during the critical developmental stages early in life, which is just similar to the responses in mammals (Geurden et al., 2007, Vagner et al., 2007, Vagner et al., 2009, Fang et al., 2014, Gong et al., 2015). Gong et al. (2015) had found that high glucose intake during start feeding stage disturbed gluconeogenesis regulation in later life of Siberian sturgeon (Gong et al., 2015). However, no any reports on the adaptability to nutritional history of this primitive species in its long-life span. Therefore, the objectives of present study were to evaluate the possible influence of dietary carbohydrates on the metabolic strategy of Siberian sturgeon during a short starvation period and subsequent refeeding, and to determine whether an acute glucose stimulus during start feeding period could modify later gluconeogenesis response.
2. Materials and methods
The experimental protocols used for sturgeon in this study have been approved by Chinese Academy of Agricultural Sciences Animal Care and Use Committee following the principle of the State Council Regulation on Laboratory Animal Administration (July 18, 2013).
2.1. Experimental diets, fish husbandry and sampling
The present study was conducted on the leftover fish of Gong et al. (2015), in which 3 experimental diets were prepared and the formulation and compositions are shown in Table 1. The diet G contained 57% glucose and was used during the first feeding period of larvae as a hyperglucidic stimulus. Glucose was chosen as the carbohydrate source in this diet because glucose may allow the larvae to bypass the carbohydrate digestion step and induce pronounced stimulation (Geurden et al., 2007). Two other isoenergetic (19.6 kJ/g gross energy) diets were fed to fish after the stimulus until 20 wk. One contained a high level of digestible carbohydrates (35% dextrin, H diet), the other was with very low carbohydrates (3.6%, F/L diet). All ingredients were thoroughly mixed and formed into pellets (0.4, 0.6, 1, and 2.5 mm in diameter) with an extrusion-bending roller (Yanggong Machine, Beijing, China). All diets were air-dried and stored at −20 °C throughout the experimental period. Chemical compositions of experimental diets were determined using the methods of AOAC (2006) and the data are shown in Table 1.
Table 1.
Composition and proximate analysis of experimental diets (g/kg).
| Item1 | Low carbohydrate diet (F/L) | High carbohydrate diet (H) | High glucose diet (G) |
|---|---|---|---|
| Ingredients | |||
| LT-FM | 855 | 465 | 350 |
| Fish oil | 50 | 90 | 50 |
| Dextrin | 0 | 350 | 0 |
| Glucose | 0 | 0 | 570 |
| Premix2 | 10 | 10 | 10 |
| Sodium carboxymethyl cellulose | 20 | 20 | 20 |
| Fish soluble protein | 20 | 20 | 0 |
| Soy lecithin | 15 | 15 | 0 |
| Choline chloride | 5 | 5 | 0 |
| Calcium dihydrogen phosphate | 5 | 5 | 0 |
| Brewer's yeast | 20 | 20 | 0 |
| Analyzed composition, g/100 g DM | |||
| Dry matter | 93.2 | 90.4 | 92.3 |
| Crude protein | 67.7 | 39.3 | 21.5 |
| Crude fat | 12.7 | 14.3 | 12.0 |
| Gross energy, kJ/g DM | 19.9 | 19.3 | 17.1 |
| Ash | 16.0 | 8.8 | 6.7 |
| Carbohydrates3 | 3.6 | 37.6 | 59.8 |
All diets were the same as Gong et al. (2015). LT-FM: low temperature steam-dried fishmeal from anchovy, TripleNine Fish Protein. Esbjerg, Denmark. Anchovy fish oil and fish soluble protein were supplied by Coland Group, Fujian, China. The carbohydrate sources (glucose and dextrin) were both analytical reagents (Sinopharm Chemical Reagent Beijing Co., Ltd, Beijing, China).
Including vitamin premix (mg/kg diet): vitamin A 20; vitamin B1 12; vitamin B2 10; vitamin B6 15; Vitamin B12 8; niacinaminde 100; ascorbic acid 1,000; calcium pantothenate 40; biotin 2; folic acid 10; vitamin E 400; vitamin K3 20; vitamin D3 10; inositol 200; corn protein powder 150. Mineral premix (mg/kg diet): CuSO4·5H2O 10; FeSO4·H2O 300; ZnSO4·H2O 200; MnSO4·H2O 100; KIO3 (10%) 80; Na2SeO3 (10% Se) 67; CoCl2·6H2O (10% Co) 5; NaCl 100; Zeolite 638.
Content of carbohydrate is calculated as 100% − (% lipid + % protein + % ash).
The feeding and sampling protocol are shown in Fig. 1. The sturgeon larvae were fed diet G (high glucose stimulation) or diet F (free from stimulation) from the first feeding to yolk absorption (8 to 12 d post-hatching [dph]). At 13 dph, each group of fish was assigned to 2 treatments. One treatment was fed the high-carbohydrate diet (as groups GH and FH), and the other treatment was fed the low-carbohydrate diet (as groups GL and FL) until 20 wk with 6 replicates for each treatment and 30 fish in each tank (diameter: 80 cm; volume: 0.3 m3). All fish were fed 3 times per day at 08:00, 14:00 and 20:00 (published in Gong et al., 2015). After 20 weeks feeding, all fish were starved for 21 d and then refed for 21 d. During refeeding period, the fish were fed to apparent satiation 3 times daily with the corresponding diet (diet H or diet L) following the feeding protocol before starvation. The water temperature was maintained at 18 to 20 °C, with dissolved oxygen levels of 6.8 to 7.8 mg/L, pH = 8.5 and NH4–N < 0.5 mg/L. Aeration was supplied to each tank 24 h per day and fluorescent light was separately designed above the tanks and kept on from 08:00 to 21:00 for photoperiod of 13 D: 11 L.
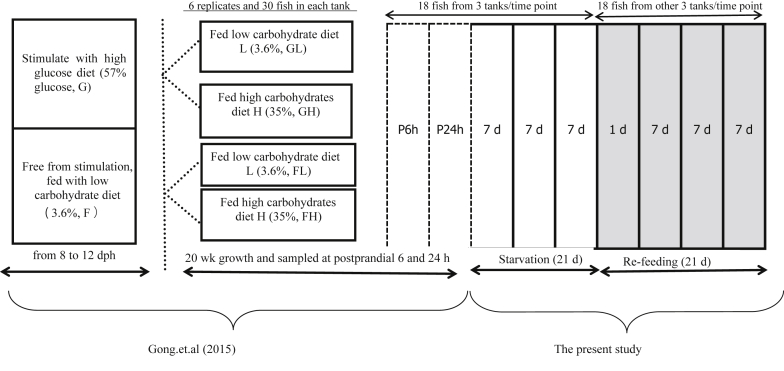
Fig. 1.
The protocols for the experiment design used in Gong et al. (2015) and the present study. After 20 weeks feeding, all fish were starved for 21 d and then re-fed for 21 d. Six fish in each tank from 3 of 6 replicates (n = 18) were individually weighed and sampled at postprandial 6 and 24 h before starvation (P6h and P24h), starvation 7, 14 and 21 d (S7, S14 and S21). Eighteen fish from other 3 tanks were sampled at P6h in re-feeding 1, 7, 14 and 21 d (R1, R7, R14 and R21). Partial data of P6h and P24h in the present study were published in Gong et al. (2015). dph = day post-hatching.
Six fish in each tank from 3 of 6 replicates (n = 18) were individually weighed and sampled at postprandial 6 (the glycaemia peak time point, Gong et al., 2015) and 24 h before starvation (P6h and P24h), starvation 7, 14 and 21 d (S7, S14 and S21). Eighteen fish from other 3 tanks were sampled at P6h at refeeding 1, 7, 14 and 21 d (R1, R7, R14 and R21). Body weight and liver weight were recorded and hepatosomatic index (HSI) was calculated by the ratio of liver weight and body weight. The P6h and P24h sampling points were used as pre-starvation control in the present study. The fish were anaesthetised with trichloro-tert-butyl alcohol (1 mg/mL) before sampling. Blood samples were taken from the caudal vein using heparinised syringes to obtain plasma samples after centrifugation (845 × g for 10 min) at 4 °C, which were maintained at −80 °C until analysis. The livers, white and red muscles were quickly removed, frozen in liquid nitrogen, and stored at −80 °C until the analyses.
2.2. Plasma glucose and tissue glycogen measurement
The plasma glucose contents were measured via the enzymatic colourimetric method using a commercial kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China, No. F006). Liver, white muscle (fast-twitch muscle) and red muscles (slow-twitch muscle) were homogenized and the glycogen concentrations were determined spectrophotometrically at 620 nm following the protocol of a commercial kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China, No. A043). The glycogen contents are expressed as mg glucose equivalent per 100 mg fresh liver or muscle tissues.
2.3. Liver gluconeogenic enzyme activities
The activities of phosphoenolpyruvate carboxykinase cytosolic forms (PEPCK-C), phosphoenolpyruvate carboxykinase mitochondrial forms (PEPCK-M), fructose-1,6-bisphosphatase (FBPase) and glucose-6-phosphatase (G6Pase) in Siberian sturgeon liver were measured as Gong et al. (2015) using a PowerWaveXS2 Microplate Spectrophotometer. In order to distinguish the activities between cytosolic (PEPCK-C) and mitochondrial (PEPCK-M) isoforms, the cytosolic (supernatant) and mitochondrial (pellet) fractions of homogenate were isolated by density gradient centrifugation. All enzyme activities were expressed as unit per mg of total protein (specific activity). One unit of enzyme activity was defined as the amount of enzyme capable of catalyzing the hydrolysis of 1 μmol of substrate per min at 37 °C.
2.4. Liver gluconeogenic gene mRNA levels
The mRNA level of gluconeogenic genes, including PEPCK-C (EC 4.1.1.32, GenBank accession No. JQ995142), PEPCK-M (EC 4.1.1.32, GenBank accession No. JQ995143), FBPase (EC 3.1.3.11, GenBank accession No. JF834907) and G6Pase (EC 3.1.3.9, GenBank accession No. JF834908) in Siberian sturgeon liver were measured by RT-qPCR analysis using anIQ 5 Multicolour Real-Time PCR Detection System (Bio-Rad, Hercules, U.S.). Elongation factor 1-alpha (EF1α, GenBank accession No. JQ995144) was used as a housekeeping gene whose expression was found to be unaffected by the treatment in the present experiment, and was used as an endogenous reference to normalise the template amount. The gene-specific primers used for mRNA quantification by RT-qPCR were designed by Primer Premier 5.0 and are shown in Table 2. The RNA extracting, cDNA synthesis and RT-qPCR process were carried out as Gong et al. (2015).
Table 2.
Primers used for mRNA quantification by RT-qPCR.
| Gene | Sense primer (5′ to 3′) | Antisense primer (5′ to 3′) | Target size, bp |
|---|---|---|---|
| PEPCK-C | GTCAAACACAACAACTACCAAACC | CAAAGCATACAATCAGTGCCTACA | 143 |
| PEPCK-M | GTTTTCTGACGCCTCTTTTG | CTCTGGATCGATTTGAATTTCC | 157 |
| FBPase | AAAGCCAAAGGGACGGGAGA | TAGAGCACAGGTGGTGAAGGAG | 216 |
| G6Pase | CTGCTTCTCCAATAGCCATCC | ATACCCCCTAACAACCTCACACT | 97 |
| EF1α | TACGAGGAAATCAGCAAGGAAG | AGCCAGAGATGGGCACAAAG | 88 |
PEPCK-C = phosphoenolpyruvate carboxykinase cytosolic forms; PEPCK-M = phosphoenolpyruvate carboxykinase mitochondrial forms; FBPase = fructose-1,6-bisphosphatase; G6Pase = glucose-6-phosphatase; EF1α = elongation factor 1-alpha.
2.5. Statistical analysis
Expression of mRNA of target genes is shown as n-fold difference relative to the calibrator (EF1α) following the Pfaffle (2001) methods. Statistical analyses were performed using Statistica8.0 (Statsoft, Tulsa, OK, U.S.). The parameters of carbohydrate metabolites always vary along “time”, so the “sampling time” factor is expected to be significantly different in all parameters. Therefore, the data of each sampling point were analyzed by repeated measures two-way ANOVA followed by Tukey's multiple-range test to inspect the differences affected by dietary carbohydrate level and high glucose stimuli during early life or the interception of both factors (Wang and Goonewardene, 2004). Then, one-way ANOVA was used for analyzing the difference among pre-starvation (P6h), S21 and R21. Homogeneity of variance was confirmed before ANOVA and differences were regarded as significant when P < 0.05, and data were reported as means ± standard error of mean (SEM).
3. Results
3.1. Body weight, liver weight and hepatosomatic index on various sampling point
The body weight and HSI of Siberian sturgeon are shown in Table 3. Fish received a high glucose stimuli during early stage showed significantly lower body weight at S14 than those fish without stimuli (P < 0.05) and high dietary carbohydrate improved the fish body weight at R7 (P < 0.01). Siberian sturgeon lost weight along with longer starvation period, and were down to the lowest level at S21. Except for the fish of GL, fish body and HSI gradually increased and regained to the level of pre-starvation (P24h) after refeeding. Fish from GL treatment failed recovering their body weight and HSI. Early glucose stimuli did not affect HSI and generally, fish fed high carbohydrate showed significantly higher HSI than those fed low carbohydrate (P < 0.05). No interaction between the factors of dietary carbohydrate level and early glucose stimuli was observed.
Table 3.
Body weight (g) and hepatosomatic index (HSI, %) in Siberian sturgeon at each sampling point during starvation and refeeding based on the main effects ANOVA (mean values with their standard errors, n = 3).
| Item1 | Sampling time points2 |
|||||||
|---|---|---|---|---|---|---|---|---|
| P24h | S7 | S14 | S21 | R1 | R7 | R14 | R21 | |
| Body weight | ||||||||
| FL | 231 ± 10.7B | 209 ± 8.13 | 244 ± 13.1Y | 175 ± 13.5A | 151 ± 10.9 | 185 ± 12.8a | 212 ± 11.2 | 214 ± 10.7AB |
| GL | 206 ± 12.8B | 214 ± 5.32 | 180 ± 5.16X | 195 ± 2.14A | 183 ± 12.0 | 174 ± 16.6a | 177 ± 4.81 | 188 ± 15.2A |
| FH | 234 ± 8.51B | 211 ± 6.12 | 225 ± 12.6Y, AB | 198 ± 15.2A | 190 ± 5.81 | 218 ± 12.0b | 209 ± 13.4 | 215 ± 8.16AB |
| GH | 227 ± 6.64C | 209 ± 13.1 | 191 ± 10.3X | 184 ± 9.45A | 186 ± 19.6 | 215 ± 10.0b | 197 ± 9.84 | 216 ± 14.2B |
| HSI3 | ||||||||
| FL | 2.35 ± 0.07a,B | 1.98 ± 0.04a | 1.52 ± 0.120a | 1.34 ± 00.4a,A | 1.52 ± 0.14a | 1.24 ± 0.09a | 1.81 ± 0.02a | 2.08 ± 0.07a,AB |
| GL | 2.20 ± 0.17a,B | 2.20 ± 0.15a | 1.26 ± 0.06a | 1.49 ± 0.12a,A | 1.57 ± 0.30a | 2.23 ± 0.11a | 2.17 ± 0.12a | 1.59 ± 0.08a,A |
| FH | 4.06 ± 0.13b,C | 2.62 ± 0.09b | 2.41 ± 0.08b | 1.95 ± 0.09b,A | 1.87 ± 0.11b | 2.85 ± 0.05b | 3.31 ± 0.21b | 3.51 ± 0.09b,B |
| GH | 4.06 ± 0.10b,C | 2.79 ± 0.08b | 2.31 ± 0.08b | 1.54 ± 0.06b,A | 1.82 ± 0.11b | 2.60 ± 0.09b | 3.78 ± 0.06b | 3.55 ± 0.14b,B |
| Carbohydrate level (C) | ns | ns | ns | ns | ns | * | ns | ns |
| High glucose stimuli (G) | ns | ns | * | ns | ns | ns | ns | ns |
| C × G | ns | ns | ns | ns | ns | ns | ns | ns |
| HSI | ||||||||
| Carbohydrate level (C) | ** | * | * | * | ** | * | ** | ** |
| High glucose stimuli (G) | ns | ns | ns | ns | ns | ns | ns | ns |
| C × G | ns | ns | ns | ns | ns | ns | ns | ns |
a,bDifferent superscript lowercase letters denote significant differences (P < 0.05) among experimental groups fed different dietary carbohydrate levels; X,Ydifferent capital letters denote significant differences (P < 0.05) between groups with or without high glucose stimuli during early life; A,Bdifferent capital letters denote significant difference (P < 0.05) among P6h, S21 and R21 within the same group by one-way ANOVA. ** means P < 0.01, * means P < 0.05, ns means not significant.
FL: fish fed low carbohydrate diet without high glucose stimulation during early programming stage; GL: fish fed low carbohydrate diet with high glucose stimulation during early programming stage; FH: fish fed high carbohydrate diet without high glucose stimulation during early programming stage; GH: fish fed high carbohydrate diet with high glucose stimulation during early programming stage.
Postprandial 6 and 24 h before starvation were abbreviated as P6h and P24h; Starvation 7, 14 and 21 days were abbreviated as S7, S14 and S21; 1, 7, 14 and 21 days during refeeding were abbreviated as R1, R7, R14 and R21.
Hepatosomatic index (HSI, %) = 100 × Liver weight/Whole body weight.
3.2. Plasma glucose
The plasma glucose concentrations at each sampling point are shown in Table 4. The plasma glucose concentrations were both affected by dietary carbohydrates level (P < 0.01) and early high glucose stimuli (P < 0.05). The plasma glucose concentrations were significantly higher in the H groups than in the L groups at most sampling point (P < 0.01). Glycaemia in H groups were significantly higher at P6h, but lower at P24h, and fish of GL group showed slightly higher glycaemia than other fish at P24h. However, the plasma glucose concentration of Siberian sturgeon at all duration for FL group were generally stable (average 4.93 at starvation stage to 5.62 μmol/mL at refeeding). In groups of GL and GH, both glycaemia at P6h and refeeding were significantly higher than that of at starvation, but no difference between pre-starvation (P6h) and refeeding. Only in FH group, glycaemia after R21 was significantly higher than that of P6h and S21.
Table 4.
Plasma glucose concentrations (μmol/mL) in Siberian sturgeon at each sampling point during starvation and refeeding (mean values with their standard errors, n = 3).
| Item1 | Sampling time points2 |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| P6h3 | P24h3 | S7 | S14 | S21 | R1 | R7 | R14 | R21 | |
| FL | 5.72 ± 0.16a,X | 4.80 ± 0.12b | 4.62 ± 0.17a | 4.51 ± 0.13 | 5.81 ± 0.28 | 4.93 ± 0.12a | 5.52 ± 0.14a | 6.16 ± 0.14a,Y | 5.90 ± 0.15a |
| GL | 5.87 ± 0.15a,Y,B | 5.42 ± 0.20b | 3.97 ± 0.11a | 4.20 ± 0.15 | 4.48 ± 0.22A | 4.65 ± 0.16a | 5.66 ± 0.11a | 5.99 ± 0.21a,X | 5.48 ± 0.20a,B |
| FH | 7.04 ± 0.12b,X, AB | 4.69 ± 0.05a | 5.24 ± 0.21b | 4.75 ± 0.21 | 5.66 ± 0.19A | 9.08 ± 0.45b | 7.92 ± 0.20b | 9.08 ± 0.18b,Y | 10.42 ± 0.30b,B |
| GH | 8.01 ± 0.18b,Y,B | 4.45 ± 0.07a | 5.34 ± 0.10b | 4.71 ± 0.09 | 5.87 ± 0.24A | 8.66 ± 0.39b | 8.25 ± 0.25b | 7.92 ± 0.22b,X | 9.80 ± 0.36b,B |
| Statistical analysis by repeated measures two-way ANOVA | |||||||||
| Carbohydrate level (C) | ** | * | ** | ns | ns | ** | ** | ** | ** |
| High glucose stimuli (G) | * | ns | ns | ns | ns | ns | ns | * | ns |
| C × G | ns | * | * | ns | ns | ns | ns | ns | ns |
a,bDifferent superscript lowercase letters denote significant differences (P < 0.05) among experimental groups fed different dietary carbohydrate levels; X,Ydifferent capital letters denote significant differences (P < 0.05) between groups with or without high glucose stimuli during early life; A,Bdifferent capital letters denote significant difference (P < 0.05) among P6h, S21 and R21 within the same group by one-way ANOVA. ** means P < 0.01, * means P < 0.05, ns means not significant.
FL: fish fed low carbohydrate diet without high glucose stimulation during early programming stage; GL: fish fed low carbohydrate diet with high glucose stimulation during early programming stage; FH: fish fed high carbohydrate diet without high glucose stimulation during early programming stage; GH: fish fed high carbohydrate diet with high glucose stimulation during early programming stage.
Postprandial 6 and 24 h before starvation were abbreviated as P6h and P24h; Starvation 7, 14 and 21 days were abbreviated as S7, S14 and S21; 1, 7, 14 and 21 days during refeeding were abbreviated as R1, R7, R14 and R21.
The data for P6h and P24h have been published in Gong et al. (2015).
3.3. Tissues glycogen
The liver, white and red muscle glycogen contents at each sampling point are shown in Table 5. The glycogen contents in liver, white and red muscle were affected significantly both by dietary carbohydrates level and early glucose stimuli (P < 0.05). Liver glycogen contents were significantly higher in the H groups than in the L groups at P6h, P24h, S7, R7, R14 and R21 (P < 0.01). Liver glycogen contents of L groups were generally kept at a stable low level, and in H groups the values were significantly decreased with the prolonged starvation (P < 0.05) and then gradually increased with refeeding to the pre-starvation levels (P6h) (P < 0.05).
Table 5.
Glycogen contents (g/100 g tissues) in liver, white muscle and red muscle of Siberian sturgeon at each sampling point during starvation and refeeding (mean values with their standard errors, n = 3).
| Item1 | Sampling time points2 |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| P6h3 | P24h3 | S7 | S14 | S21 | R1 | R7 | R14 | R21 | |
| Liver glycogen | |||||||||
| FL | 2.39 ± 0.22a | 2.35 ± 0.17a | 3.48 ± 0.21a,Y | 3.48 ± 0.32 | 1.97 ± 0.26 | 1.98 ± 0.14 | 2.29 ± 0.16a | 3.85 ± 0.11a | 2.5 ± 0.13a |
| GL | 2.06 ± 0.23a | 1.88 ± 0.26a | 2.38 ± 0.33a,X | 2.26 ± 0.41 | 1.29 ± 0.21 | 0.72 ± 0.09 | 1.88 ± 0.23a | 3.91 ± 0.58a | 2.32 ± 0.14a |
| FH | 9.25 ± 0.28b,B | 7.08 ± 0.46b | 6.23 ± 0.58b,Y | 1.61 ± 0.36 | 2.30 ± 0.47A | 1.70 ± 0.22 | 5.92 ± 0.11b | 9.33 ± 0.36b | 8.51 ± 0.85b,B |
| GH | 8.89 ± 0.59b,B | 6.66 ± 0.62b | 5.04 ± 0.26b,X | 3.87 ± 0.21 | 1.57 ± 0.14A | 2.18 ± 0.36 | 7.49 ± 0.52b | 8.64 ± 0.24b | 7.61 ± 0.33b,B |
| White muscle glycogen | |||||||||
| FL | 0.10 ± 0.01a | 0.12 ± 0.02a | 0.13 ± 0.02a | 0.17 ± 0.02 | 0.11 ± 0.01a,Y | 0.11 ± 0.02Y | 0.09 ± 0.02a | 0.17 ± 0.02a | 0.12 ± 0.01a |
| GL | 0.09 ± 0.02a | 0.13 ± 0.01a | 0.12 ± 0.02a | 0.17 ± 0.01 | 0.09 ± 0.01a,X | 0.08 ± 0.01X | 0.15 ± 0.01a | 0.15 ± 0.02a | 0.16 ± 0.02a |
| FH | 0.18 ± 0.02b | 0.18 ± 0.03b | 0.20 ± 0.02b | 0.20 ± 0.03 | 0.17 ± 0.01b,Y | 0.10 ± 0.02Y | 0.30 ± 0.03b | 0.27 ± 0.03b | 0.24 ± 0.03b |
| GH | 0.16 ± 0.01b | 0.20 ± 0.02b | 0.18 ± 0.02b | 0.15 ± 0.02 | 0.13 ± 0.02b,X | 0.08 ± 0.01X | 0.27 ± 0.02b | 0.34 ± 0.04b | 0.30 ± 0.04b |
| Red muscle glycogen | |||||||||
| FL | 0.12 ± 0.02a,Y | 0.07 ± 0.01a | 0.16 ± 0.02 | 0.09 ± 0.02a | 0.14 ± 0.01a,Y | 0.18 ± 0.02Y | 0.19 ± 0.04a | 0.22 ± 0.03a | 0.09 ± 0.01a,X |
| GL | 0.11 ± 0.02a,X | 0.11 ± 0.02a | 0.15 ± 0.01 | 0.09 ± 0.02a | 0.12 ± 0.01a,X | 0.14 ± 0.01X | 0.20 ± 0.02a | 0.13 ± 0.04a | 0.11 ± 0.03a,Y |
| FH | 0.20 ± 0.03b,Y | 0.17 ± 0.02b | 0.15 ± 0.03 | 0.14 ± 0.02b | 0.20 ± 0.02b,Y | 0.17 ± 0.03Y | 0.55 ± 0.04b | 0.43 ± 0.05b | 0.19 ± 0.03b,X |
| GH | 0.15 ± 0.01b,X,A | 0.18 ± 0.03b | 0.15 ± 0.02 | 0.10 ± 0.01b | 0.18 ± 0.01b,X,A | 0.14 ± 0.01X | 0.46 ± 0.03b | 0.45 ± 0.05b | 0.27 ± 0.03b,Y,B |
| Statistical analysis by repeated measures two-way ANOVA | |||||||||
| Liver glycogen | |||||||||
| Carbohydrate level (C) | ** | ** | ** | ns | ns | ns | ** | ** | ** |
| High glucose stimuli (G) | ns | ns | * | ns | ns | ns | ns | ns | ns |
| C × G | ns | ns | ns | ** | ns | ns | ns | ns | ns |
| White muscle glycogen | |||||||||
| Carbohydrate level (C) | ** | ** | ** | ns | ** | ns | ** | ** | ** |
| High glucose stimuli (G) | ns | ns | ns | ns | ** | * | ns | ns | ns |
| C × G | ns | ns | ns | ns | ns | ns | ** | * | ns |
| Red muscle glycogen | |||||||||
| Carbohydrate level (C) | ** | ** | ns | * | ** | ns | ** | ** | ** |
| High glucose stimuli (G) | * | ns | ns | ns | * | * | ns | ns | * |
| C × G | ns | ns | ns | ns | ns | ns | * | ns | ns |
a,bDifferent superscript lowercase letters denote significant differences (P < 0.05) among experimental groups fed different dietary carbohydrate levels; X,Ydifferent capital letters denote significant differences (P < 0.05) between groups with or without high glucose stimuli during early life; A,Bdifferent capital letters denote significant difference (P < 0.05) among P6h, S21 and R21 within the same group by one-way ANOVA. ** means P < 0.01, * means P < 0.05, ns means not significant.
FL: fish fed low carbohydrate diet without high glucose stimulation during early programming stage; GL: fish fed low carbohydrate diet with high glucose stimulation during early programming stage; FH: fish fed high carbohydrate diet without high glucose stimulation during early programming stage; GH: fish fed high carbohydrate diet with high glucose stimulation during early programming stage.
Postprandial 6 and 24 h before starvation were abbreviated as P6h and P24h; Starvation 7, 14 and 21 days were abbreviated as S7, S14 and S21; 1, 7, 14 and 21 days during refeeding were abbreviated as R1, R7, R14 and R21.
The data for P6h and P24h have been published in Gong et al. (2015).
In the whole duration, white muscle glycogen contents were averagely stable in each group. However, the white muscle glycogen was significantly higher in the H groups than in the L groups at most time points, except for the fish sampled at S14 and R1 (P < 0.05). Fish in glucose stimulation groups showed significantly lower glycogen content in white muscle than those of fish without glucose stimulation at S21 and R1, but no difference was observed at other sampling points. Red muscle glycogen contents were significantly higher in the fish fed high carbohydrate diets than those fed the low carbohydrate diet at most sampling points, except for S7 and R1 (P < 0.05). Red muscle glycogen of Siberian sturgeon were stable in L groups and FH group at P6h, S21 and R21, but significantly increased after refeeding in fish fed diet GH.
3.4. Liver gluconeogenic enzyme activities
The data for the activity of gluconeogenic enzymes including PEPCK-C PEPCK-M, FBPase and G6Pase in liver at each sampling point are shown in Table 6. Dietary carbohydrates level significantly affected the activities of PEPCK-C (at S7, S14 and R1) and PEPCK-M (at P6h, R7 and R21) with very different pattern. Fish fed H diets showed higher PEPCK-C activities at S7 and S14, but lower at R1 (P < 0.05), whereas kept lower PEPCK-M activities than those of L groups at P6h, R7 and R21 (satiation status). Fish with high glucose stimuli showed lower PEPCK-C (at S21, R21) and PEPCK-M (at P24h and S14) activities, but adversely higher at R7 for PEPCK-C and at S21, R7, R14 for PEPCK-M than PEPCKs activities of fish without experimented glucose stimuli. In general, PEPCK-C activities were increased at S21, but further increased during refeeding period. However, the activities of PEPCK-M were increased with prolonged starvation (S21) and then decreased, but not drop to the levels of before starvation (P6h) at R21 in all groups (P < 0.05).
Table 6.
Specific activities (mU/mg protein) of gluconeogenic enzyme in the liver of Siberian sturgeon at each sampling point during starvation and refeeding (mean values with their standard errors, n = 3).
| Item1 | Sampling time points2 |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| P6h3 | P24h3 | S7 | S14 | S21 | R1 | R7 | R14 | R21 | |
| PEPCK-C | |||||||||
| FL | 52.6 ± 2.30A | 35.5 ± 1.26 | 45.4 ± 2.14a | 65.2 ± 1.62a | 72.2 ± 1.44Y,B | 58.9 ± 1.23b | 99.2 ± 3.43X | 112 ± 4.15 | 112 ± 3.18Y,C |
| GL | 43.0 ± 4.13A | 43.8 ± 1.06 | 35.7 ± 1.61a | 61.8 ± 0.94a | 63.1 ± 5.20X,B | 89.8 ± 6.87b | 88.2 ± 1.37Y | 88.7 ± 3.36 | 105 ± 4.01X,C |
| FH | 62.0 ± 3.01A | 44.6 ± 1.94 | 55.2 ± 2.30b | 73.3 ± 1.78b | 80.0 ± 1.24Y,B | 60.4 ± 1.25a | 81.9 ± 2.05X | 102 ± 1.84 | 133 ± 2.81Y,C |
| GH | 48.1 ± 2.29A | 39.6 ± 1.68 | 70.4 ± 1.58b | 107 ± 19.2b | 69.9 ± 1.38X,B | 53.6 ± 3.25a | 113 ± 3.14Y | 113 ± 2.68 | 85.2 ± 1.64X,C |
| PEPCK-M | |||||||||
| FL | 4.28 ± 0.21b,A | 3.44 ± 0.16Y | 12.10 ± 0.89 | 16.5 ± 0.47Y | 23.2 ± 2.18Y,C | 22.8 ± 1.93 | 16.0 ± 0.89b,X | 12.9 ± 1.67X | 14.1 ± 0.82b,B |
| GL | 2.70 ± 0.10b,A | 2.63 ± 0.11X | 11.9 ± 1.18 | 13.7 ± 0.28X | 17.2 ± 1.92X,B | 16.3 ± 0.98 | 16.8 ± 0.91b,Y | 16.6 ± 0.98Y | 13.7 ± 1.68b,B |
| FH | 2.43 ± 0.23a,A | 3.14 ± 0.18Y | 9.84 ± 0.34 | 7.91 ± 0.79Y | 27.3 ± 2.30Y,C | 19.3 ± 0.29 | 9.82 ± 1.20a,X | 12.0 ± 0.87X | 9.30 ± 0.41a,B |
| GH | 2.84 ± 0.14a,A | 2.41 ± 0.09X | 10.9 ± 0.28 | 18.4 ± 1.90X | 19.8 ± 0.80X,C | 20.9 ± 1.59 | 13.0 ± 1.07a,Y | 14.6 ± 0.75Y | 11.3 ± 0.17a,B |
| FBPase | |||||||||
| FL | 131 ± 8.47b | 87.1 ± 3.7.2b | 132 ± 4.17b,Y | 184 ± 11.9Y | 140 ± 7.4.2b,Y | 84.2 ± 5.36b,Y | 107 ± 8.42b | 110 ± 5.24b | 153 ± 6.01b |
| GL | 117 ± 6.49b | 94.2 ± 3.12b | 93.5 ± 6.84b,X | 126 ± 8.56X | 85.0 ± 5.43b,X | 50.5 ± 2.64b,X | 58.8 ± 3.30ab | 108 ± 15.7b | 137 ± 14.3b |
| FH | 68.2 ± 3.38a | 53.5 ± 1.21a | 65.5 ± 5.70a,Y | 89.2 ± 4.83Y | 42.4 ± 3.67a,Y | 35.5 ± 2.72a,Y | 48.6 ± 2.59a | 47.1 ± 2.05a | 67.1 ± 7.16a |
| GH | 56.6 ± 2.90a | 40.3 ± 0.98a | 63.9 ± 1.68a,X | 84.8 ± 3.95X | 39.0 ± 2.69a,X | 31.6 ± 1.94a,X | 67.9 ± 6.12a | 45.9 ± 1.84a | 79.9 ± 4.90a |
| G6Pase | |||||||||
| FL | 10.0 ± 0.51Y,B | 6.88 ± 0.05b,Y | 4.74 ± 0.37 | 3.61 ± 0.47a | 3.78 ± 0.46a,A | 3.27 ± 0.26X | 4.24 ± 0.21b | 4.60 ± 0.44 | 4.14 ± 0.25A |
| GL | 4.35 ± 0.11X | 4.99 ± 0.18b,X | 6.63 ± 0.92 | 4.54 ± 0.22a | 4.21 ± 0.78a | 5.34 ± 0.43Y | 6.18 ± 0.48b | 4.75 ± 0.46 | 3.99 ± 0.16 |
| FH | 11.2 ± 0.88Y,B | 5.54 ± 0.19a,Y | 4.77 ± 0.47 | 7.20 ± 0.81b | 6.63 ± 1.02b,A | 3.68 ± 0.12X | 4.26 ± 0.39a | 5.61 ± 0.12 | 4.60 ± 0.14A |
| GH | 5.97 ± 0.29X | 4.85 ± 0.14a,X | 4.89 ± 0.48 | 4.84 ± 0.47b | 6.36 ± 0.70b | 3.73 ± 0.27Y | 3.73 ± 0.35a | 4.86 ± 0.38 | 4.10 ± 0.36 |
| Statistical analysis by repeated measures two-way ANOVA | |||||||||
| PEPCK-C | |||||||||
| Carbohydrate level (C) | ns | ns | ** | ** | ns | * | ns | ns | ns |
| High glucose stimuli (G) | ns | ns | ns | ns | * | ns | * | ns | ** |
| C × G | ns | * | * | * | ns | * | * | * | ** |
| PEPCK-M | |||||||||
| Carbohydrate level (C) | * | ns | ns | ns | ns | ns | ** | ns | ** |
| High glucose stimuli (G) | ns | ** | ns | * | * | ns | * | * | ns |
| C × G | * | ns | ns | ** | ns | ns | ns | ns | ns |
| FBPase | |||||||||
| Carbohydrate level (C) | ** | * | ** | ns | ** | ** | * | ** | ** |
| High glucose stimuli (G) | ns | ns | * | * | * | * | ns | ns | ns |
| C × G | ns | ns | * | * | * | * | ** | ns | ns |
| G6Pase | |||||||||
| Carbohydrate level (C) | ns | * | ns | ** | ** | ns | * | ns | ns |
| High glucose stimuli (G) | ** | ** | ns | ns | ns | ** | ns | ns | ns |
| C × G | ns | ns | ns | * | ns | ns | * | ns | ns |
PEPCK-C = phosphoenolpyruvate carboxykinase cytosolic forms; PEPCK-M = phosphoenolpyruvate carboxykinase mitochondrial forms; FBPase = fructose-1,6-bisphosphatase; G6Pase = glucose-6-phosphatase.
a,bDifferent superscript lowercase letters denote significant differences (P < 0.05) among experimental groups fed different dietary carbohydrate levels; X,Ydifferent capital letters denote significant differences (P < 0.05) between groups with or without high glucose stimuli during early life; A,Bdifferent capital letters denote significant difference (P < 0.05) among P6h, S21 and R21 within the same group by one-way ANOVA. **means P < 0.01, *means P < 0.05, ns means not significant.
FL: fish fed low carbohydrate diet without high glucose stimulation during early programming stage; GL: fish fed low carbohydrate diet with high glucose stimulation during early programming stage; FH: fish fed high carbohydrate diet without high glucose stimulation during early programming stage; GH: fish fed high carbohydrate diet with high glucose stimulation during early programming stage.
Postprandial 6 and 24 h before starvation were abbreviated as P6h and P24h; Starvation 7, 14 and 21 days were abbreviated as S7, S14 and S21; 1, 7, 14 and 21 days during refeeding were abbreviated as R1, R7, R14 and R21.
The data for P6h and P24h have been published in Gong et al. (2015).
The FBPase activities for H groups were significantly lower than those of L groups (P < 0.01) at all sampling points except for at S14. High glucose stimuli reduced the FBPase activities at S7, S14, S21 and R1 (P < 0.05).
The activities of G6Pase were significantly lower in H groups at P24h and R7, but adversely higher at S14 and S21 (P < 0.05). Early high glucose stimulus significantly inhibited G6Pase activities at P6h and P24h, but enhanced the activities at R1 (P < 0.05). The activities of G6Pase in F (FL and FH) groups were highest at P6h, then rapidly decreased with starvation and kept stable at low level after refeeding (R21). G6Pase in G (GL and GH) groups were kept stable during P6h, starvation (S21) and refeeding (R21) (P < 0.05).
3.5. Gluconeogenic gene mRNA levels
The data for the mRNA level of gluconeogenic genes including duplicated genes PEPCK-C PEPCK-M and theirs downstream genes, FBPase and G6Pase in liver at each sampling point are shown in Table 7. The mRNA levels of PEPCK-C and PEPCK-M were significantly lower in H groups than in L groups in most cases (P6h, P24h, S7, R7, R14 and R21 for PEPCK-C and P6h, P24h, R1 and R21 for PEPCK-M, P < 0.05). High glucose stimuli in early life only induced higher expression of PEPCK-C at P24h and PEPCK-M at S21, but at R21, the stimuli down-regulated the PEPCK-M mRNA levels (P < 0.05). Both of PEPCKs mRNA levels were significantly decreased with the feed deprivation (S21), but showed different patterns at R21 for L and H groups. Fish fed low carbohydrate diet showed increased PEPCKs mRNA levels at R21, but decreased expression of PEPCK-C and kept stable low levels of PEPCK-M mRNA expression in H groups (P < 0.05).
Table 7.
The mRNA levels of gluconeogenic gene in the liver of Siberian sturgeon at each sampling point during starvation and refeeding (mean values with their standard errors, n = 3).
| Item1 | Sampling time points2 |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| P6h3 | P24h3 | S7 | S14 | S21 | R1 | R7 | R14 | R21 | |
| PEPCK-C | |||||||||
| FL | 1.42 ± 0.27b,C | 0.10 ± 0.04b,X | 0.05 ± 0.03b | 0.02 ± 0.01 | 0.04 ± 0.02A | 0.17 ± 0.04 | 2.15 ± 0.47b | 0.91 ± 0.31b | 0.26 ± 0.06b,B |
| GL | 2.55 ± 0.69b,C | 1.02 ± 0.23b,Y | 0.07 ± 0.02b | 0.02 ± 0.01 | 0.07 ± 0.02A | 0.16 ± 0.06 | 1.28 ± 0.39b | 0.32 ± 0.14b | 0.26 ± 0.08b,B |
| FH | 0.12 ± 0.05a,C | 0.04 ± 0.01a,X | 0.03 ± 0.01a | 0.29 ± 0.12 | 0.05 ± 0.03B | 0.19 ± 0.08 | 0.28 ± 0.08a | 0.03 ± 0.01a | 0.01 ± 0.00a,A |
| GH | 0.08 ± 0.05a,B | 0.07 ± 0.04aY | 0.03 ± 0.00a | 0.01 ± 0.00 | 0.02 ± 0.01A | 0.36 ± 0.17 | 0.19 ± 0.06a | 0.01 ± 0.01a | 0.01 ± 0.00a,A |
| PEPCK-M | |||||||||
| FL | 1.18 ± 0.18b,B | 1.29 ± 0.12b | 0.41 ± 0.05 | 0.35 ± 0.07 | 0.33 ± 0.04X,A | 2.88 ± 0.35b | 3.40 ± 0.98 | 1.57 ± 0.66 | 1.77 ± 0.19b,Y,C |
| GL | 1.40 ± 0.13b,C | 1.20 ± 0.15b | 0.39 ± 0.09 | 0.31 ± 0.03 | 0.31 ± 0.05Y,A | 3.08 ± 0.88b | 2.20 ± 0.47 | 1.15 ± 0.11 | 0.88 ± 0.09b,X,B |
| FH | 0.74 ± 0.11a,B | 0.49 ± 0.10a | 0.30 ± 0.06 | 0.19 ± 0.06 | 0.21 ± 0.04X,A | 2.09 ± 0.21a | 1.79 ± 0.24 | 0.83 ± 0.27 | 0.34 ± 0.05a,Y,A |
| GH | 0.56 ± 0.06a | 0.62 ± 0.09a | 0.41 ± 0.04 | 0.21 ± 0.07 | 0.54 ± 0.09Y | 1.86 ± 0.18a | 1.88 ± 0.37 | 0.71 ± 0.14 | 0.53 ± 0.11a,X |
| FBPase | |||||||||
| FL | 1.08 ± 0.09b,B | 0.77 ± 0.08b | 0.38 ± 0.09 | 0.21 ± 0.04 | 0.47 ± 0.09A | 0.22 ± 0.01 | 1.54 ± 0.18 | 0.73 ± 0.04 | 0.57 ± 0.09b,A |
| GL | 1.15 ± 0.16b,B | 0.86 ± 0.09b | 0.34 ± 0.05 | 0.16 ± 0.03 | 0.32 ± 0.05A | 0.39 ± 0.08 | 1.29 ± 0.21 | 0.87 ± 0.15 | 0.67 ± 0.12b,A |
| FH | 0.47 ± 0.05a | 0.27 ± 0.04a | 0.25 ± 0.04 | 0.12 ± 0.04 | 0.29 ± 0.03 | 0.67 ± 0.16 | 1.11 ± 0.18 | 0.62 ± 0.25 | 0.28 ± 0.08a |
| GH | 0.4 ± 0.04a | 0.36 ± 0.08a | 0.29 ± 0.07 | 0.13 ± 0.08 | 0.58 ± 0.07 | 0.48 ± 0.13 | 1.49 ± 0.89 | 0.46 ± 0.17 | 0.26 ± 0.06a |
| G6Pase | |||||||||
| FL | 1.17 ± 0.11b,X | 2.17 ± 0.15X | 2.21 ± 0.17b,X | 0.8 ± 0.14 | 1.18 ± 0.07b | 1.42 ± 0.27 | 1.13 ± 0.11b | 0.6 ± 0.08 | 1.08 ± 0.07b |
| GL | 2.21 ± 0.24b,Y,B | 4.18 ± 0.34Y | 1.51 ± 0.09b,Y | 0.63 ± 0.07 | 1.34 ± 0.04b,A | 1.52 ± 0.11 | 1.08 ± 0.17b | 0.61 ± 0.14 | 1.11 ± 0.14b,A |
| FH | 0.73 ± 0.07a,X | 2.76 ± 0.48X | 1.48 ± 0.08a,X | 0.73 ± 0.09 | 0.77 ± 0.08a | 1.73 ± 0.14 | 0.59 ± 0.10a | 0.44 ± 0.09 | 0.64 ± 0.10a |
| GH | 0.74 ± 0.10a,Y | 2.72 ± 0.27Y | 1.27 ± 0.05a,Y | 0.73 ± 0.10 | 0.83 ± 0.07a | 1.31 ± 0.09 | 0.53 ± 0.07a | 0.40 ± 0.03 | 0.53 ± 0.05a |
| Statistical analysis by repeated measures two-way ANOVA | |||||||||
| PEPCK-C | |||||||||
| Carbohydrate level (C) | ** | * | ** | ns | ns | ns | ** | ** | ** |
| High glucose stimuli (G) | ns | * | ns | ns | ns | ns | ns | ns | ns |
| C × G | ns | * | ns | ns | ns | ns | ns | ns | ns |
| PEPCK-M | |||||||||
| Carbohydrate level (C) | * | ** | ns | ns | ns | * | ns | ns | ** |
| High glucose stimuli (G) | ns | ns | ns | ns | * | ns | ns | ns | * |
| C × G | ns | ns | ns | ns | * | ns | ns | ns | ** |
| FBPase | |||||||||
| Carbohydrate level (C) | ** | ** | ns | ns | ns | ns | ns | ns | ** |
| High glucose stimuli (G) | ns | ns | ns | ns | ns | ns | ns | ns | ns |
| C × G | ns | ns | ns | ns | ns | ns | ns | ns | ns |
| G6Pase | |||||||||
| Carbohydrate level (C) | ** | ns | ** | ns | ** | ns | ** | ns | ** |
| High glucose stimuli (G) | * | * | ** | ns | ns | ns | ns | ns | ns |
| C × G | * | * | ns | ns | ns | ns | ns | ns | ns |
PEPCK-C = phosphoenolpyruvate carboxykinase cytosolic forms; PEPCK-M = phosphoenolpyruvate carboxykinase mitochondrial forms; FBPase = fructose-1,6-bisphosphatase; G6Pase = glucose-6-phosphatase; EF1α = elongation factor 1-alpha.
a,bDifferent superscript lowercase letters denote significant differences (P < 0.05) among experimental groups fed different dietary carbohydrate levels; X,Ydifferent capital letters denote significant differences (P < 0.05) between groups with or without high glucose stimuli during early life; A,Bdifferent capital letters denote significant difference (P < 0.05) among P6h, S21 and R21 within the same group by one-way ANOVA. **means P < 0.01, *means P < 0.05, ns means not significant.
FL: fish fed low carbohydrate diet without high glucose stimulation during early programming stage; GL: fish fed low carbohydrate diet with high glucose stimulation during early programming stage; FH: fish fed high carbohydrate diet without high glucose stimulation during early programming stage; GH: fish fed high carbohydrate diet with high glucose stimulation during early programming stage.
Postprandial 6 and 24 h before starvation were abbreviated as P6h and P24h; Starvation 7, 14 and 21 days were abbreviated as S7, S14 and S21; 1, 7, 14 and 21 days during refeeding were abbreviated as R1, R7, R14 and R21.
The data for P6h and P24h have been published in Gong et al. (2015).
The mRNA levels of FBPase were significantly lower in fish fed high carbohydrate diet than in fish fed low carbohydrate diet at P6h, P24h and R21 (P < 0.05). High glucose stimuli did not affect the FBPase expression generally (P > 0.05). Fish of L groups got the highest FBPase mRNA levels at P6h, then decreased at S21 and kept stable low levels at R21. The FBPase mRNA levels were relatively stable at P6h, S21 and R21 for fish fed high carbohydrate diets (FH and GH) (P < 0.05).
The mRNA levels of G6Pase were significantly higher in L groups than those of H groups at P6h, S7, S21, R7 and R21 (P < 0.05). High glucose stimuli significantly up-regulated the G6Pase expression at P6h, P24h and S7, but no significant effect during refeeding periods. The G6Pase mRNA levels were stable at P6h, S21 and R21 for FL, FH and GH groups. Fish of GL group significantly down-regulated mRNA level of G6Pase during starvation and kept low level at R21 (P < 0.05).
4. Discussion
During the long evolutionary history, sturgeon faced various harsh climate changes on earth, which could induce short or long-term food deprivation for this ancient fish species. The maintenance of energy homoeostasis during food deprivation in fish is directly related to the capacity for mobilization of energy reserves such as hepatic glycogen during initial stages of fasting and depends on subsequent activation of hepatic gluconeogenesis (Polakof et al., 2006). The complete compensation growth observed in Siberian sturgeon after 2, 4 or 8 d fasting then refeeding until for 40 d, indicated a high ability of the species to grow to fully compensate for weight loss during starvation (Morshedi et al., 2013). Liu et al. (2011) found that Chinese sturgeon resumed body weight after 3 or 7 d fasting then refeeding to 70 d with similar final body weight and HSI, but only showed partial compensation for the longer starvation (14 to 28 d) groups, which with significantly higher mortality than those not be fasted. The complete compensation of fish could be a promising feeding management option for aquaculture. In the present study, we similarly observed resumed body weight after 21 d refeeding except in the group GL. All groups of fish lost weight gradually during starvation stage, but only fish of GL group failed recovering body and liver weight after R21. Fish experienced glucose stimuli during early stage might increase carbohydrate requirement for maintaining body energy retention or as Gong et al. (2015) supposed that the glucose stimuli during the early stage have long-term, even life-span interference on gluconeogenesis of Siberian sturgeon. Polakof et al., 2011, Polakof et al., 2012 reviewed the glucose metabolism in fish, and demonstrated that although fish do have an active glucose homoeostatic system, the phenotype of postprandial hyperglycemia and slow recovery to basal blood glucose levels in fish, including sturgeon are similar to the common features of human diabetes (Hung, 1991, Polakof et al., 2011, Polakof et al., 2012). Different from mammal and birds, fish could keep alive and adapt to a long-term (several month) food deprivation, which could be related to the lower glucose turnover rates (Garin et al., 1987, Hoenig et al., 2010). The natural diet of sturgeon is poor in carbohydrate (Sokolov, 1966), but glucose is an essential energy substrate for tissues and organs for all animals. Siberian sturgeon in FL group showed relatively stable glycaemia level even met 21 d starvation and thereafter refeeding. This showed that Siberian sturgeon, similar to the other carnivorous fish species, can use dietary source triglyceride-derived glycerol and gluconeogenic amino acid as substrates for glucose synthesis (Geurden et al., 2007, Gong et al., 2015). The plasma glucose levels of all groups were recovered to baseline (4 to 5 μmol/mL) into 24 h and kept stable over the S21. These results were consistent with the findings in lake sturgeon (A. fulvescens) and Persian sturgeon (A. persicus) (Gillis and Ballantyne, 1996, Yarmohammadi et al., 2012), but different from white sturgeon (A. transmontanus), which with diabetes-like characters as much slow glycaemia recovery (at least 48 h) and constant decreasing plasma glucose (from 3.6 to 2.6 μmol/mL) during 4-week starvation (Hung, 1991, Hung et al., 1997). Several studies in our lab had observed that the Siberian sturgeon, as an omnivorous species, has good abilities to utilize carbohydrate from starch or plant protein when fed a fishmeal free diet (Yun et al., 2014, Gong et al., 2015). The different feeding habits could be a reason to explain the different patterns of glucose metabolism between Siberian sturgeon and white sturgeon.
Liver glycogen is the first substrate for maintaining plasma glucose levels, which demonstrated as the rapidly reduced content at the initial stage of starvation in most fish (Pérez-Jiménez et al., 2007, Pérez-Jiménez et al., 2012, Barcellos et al., 2010, Furné et al., 2012). However, some studies showed that fish also try to preserve the liver glycogen stores when they experience some nutritional challenge (Sheridan and Mommsen, 1991, Navarro and Gutiérrez, 1995, Gillis and Ballantyne, 1996). In the present study, fish fed high carbohydrate diets preserved much higher liver glycogen, which accompanied with larger liver size than those of L groups. At the same time, we found that Siberian sturgeon rapidly mobilize the liver glycogen to maintain the plasma glucose levels when this substrate was abundant (mainly derived from dietary carbohydrates), and can preserve the stores to avoid depleted when they were insufficient (lack of dietary carbohydrates or starvation prolonged). These results were different from the findings in gilthead sea bream (Sparus aurata) and European sea bass (Dicentrarchus labrax), which showed that the reduced liver glycogen response to starvation in all groups fed with different protein and carbohydrates contents diets (Metón et al., 1999, Pérez-Jiménez et al., 2007). The different responses to starvation of liver glycogen induced by nutritional history before starvation in Siberian sturgeon suggested that this species, or maybe the lake sturgeon and the Persian sturgeon have stronger adaptability to glucose metabolism than the teleost carnivorous fish (Gillis and Ballantyne, 1996, Yarmohammadi et al., 2012). The lateral muscle of sturgeon mainly consisted of a large deep layer of fast-white muscle covered by a superficial layer of slow-red muscle (Radaelli et al., 1999). The significant decrease of white muscle glycogen during prolonged starvation was observed for several carnivorous fish species, such as Plaice (Pleuronectes platessa), Channa punctatus, and Northern pike (Esox lucius L.) (Johnston and Goldspink, 1973, Ince and Thorpe, 1976, Namrata et al., 2011). The fast running time and the glycogen content in white muscles of diabetes mice were significantly lower than that of wild type ones. Significantly, increased glycolytic flux was observed in diabetes mice fast muscle (Meng et al., 2013). In the present study, glycogen contents in red and white muscle were not obviously changed during starvation period in all groups, which suggested muscle carbohydrate storage are not the primary substrates mobilized as an energy source during the starvation in Siberian sturgeon. The similar results were observed in omnivorous carp, Cyprinus carpio L., (Blasco et al., 1992). Jundiá, Rhamdia quelen (Barcellos et al., 2010) and even a carnivorous species European sea bass (Gutiérrez et al., 1991, Chatzifotis et al., 2011).
During the refeeding period, fish fed high carbohydrates diet showed significantly higher levels of plasma glucose and glycogen than the fish fed low carbohydrates diet, which suggested the dietary carbohydrates has important contribution to the recovery of these indexes in Siberian sturgeon. In contrast, for European sea bass, the rapidly repletion of hepatic glycogen during refeeding was completely sustained by de novo gluconeogenesis, and synthesized from dietary carbohydrates was negligible (Viegas et al., 2012). These differences may also due to the different feeding habits between Siberian sturgeon and the carnivorous teleosts (Kaushik et al., 1989, Gong et al., 2015). Furthermore, the plasma glucose levels and red muscle glycogen contents exceed over the levels at pre-starvation when the fish fed with high carbohydrates diet in the present study. These glycogen overshoots after fasting period seem to be a tactic for rapid storage of food energy, and to be used later for the synthesis of body materials (Barcellos et al., 2010). Besides, early high glucose stimuli changed the metabolism pattern in fish both fed low or high carbohydrate diets. This confirmed the long-term effects of an early nutrition programming on the later life as reported by Gong et al. (2015).
The regulation of hepatic gluconeogenesis is mainly affected by natural feeding habits for a healthy animal. In the omnivorous carp, as in nondiabetic mammals, gluconeogenesis is activated during starvation and switched off when glucose is available from dietary sources (Panserat et al., 2002a, Pilkis and Granner, 1992, Van Schaftingen and Gerin, 2002). Some studies have focused on the gluconeogenesis response to starvation and thereafter-refeeding based activities and/or mRNA levels of key gluconeogenic enzymes, but the results were contradictory. For example, in European sea bass, fasting provoked a significant decrease in G6Pase activity and mRNA level (Viegas et al., 2013), but increased G6Pase activity for gilthead sea bream (Caseras et al., 2002, Metón et al., 2004, Sangiao-Alvarellos et al., 2005) and unchanged activity for rainbow trout (Panserat et al., 2001, Panserat et al., 2002a, Panserat et al., 2002b, Kirchner et al., 2008). The activities of FBPase were unchanged during starvation and subsequent refeeding in European sea bass, Adriatic sturgeon and rainbow trout (Pérez-Jiménez et al., 2007, Furné et al., 2012), or decreased with refeeding and recovered to the pre-starvation levels in rainbow trout (Soengas et al., 2006) but significantly enhanced during early refeeding period and higher than the continuingly feeding control in gilthead sea bream (Metón et al., 1999, Metón et al., 2003). Phosphoenolpyruvate carboxykinase cytosolic forms has been studied almost to the complete exclusion of PEPCK-M in mammals (Hanson, 2009, Stark and Kibbey, 2014). For a long term, PEPCK-M is generally considered irrelevant for glucose production in mammals until Stark and Kibbey (2014) found that PEPCK-M loss impaired gluconeogenesis from lactate, which is similar to the concept only supported for birds (they have PEPCK-M and no PEPCK-C activity in liver) (Watford et al., 1981). Panserat et al. (2001) cloned the PEPCKs cDNA sequence and demonstrate the PEPCK-M is the main source in rainbow trout. Different from mammals, PEPCK-M gene of rainbow trout is kept at an even level expression and not influenced by nutritional status (feeding, starvation and dietary carbohydrate level). The function of PEPCK-C could be ignored by its low expression. This could be the key reason of long-last postprandial hyperglycemia for carnivorous fish, at least for rainbow trout. However, in limited reports related to PEPCKs functions in fish, including common carp, grass carp and gilthead sea bream, the forms are not distinguished by sources from cytoplasm (PEPCK-C) or mitochondrion (PEPCK-M) (Panserat et al., 2002a, Tian et al., 2015). In the present study, PEPCK-M well responded the energy status of Siberian sturgeon, with significantly increased enzyme activities during starvation, then decreased after refeeding. Phosphoenolpyruvate carboxykinase cytosolic forms activities were significantly increased at S21. However, this relatively normal gluconeogeneic enzyme reaction is not controlled during the refeeding period. The constantly increased mRNA levels of PEPCK-C during refeeding period of Siberian sturgeon indicated the similarly incontrollable gluconeogenesis after meal as carnivorous fish, like rainbow trout. Both dietary carbohydrate levels and early glucose stimuli affected the specific gluconeogenesis enzyme activities and mRNA levels (Table 6, Table 7). Compare with the F groups, fish experienced early glucose stimuli showed less regulating ability in gluconeogenesis response (lower PEPCKs, FBPase activities during starvation), which could be an important reason for losing more body weight for G groups during starvation. In Siberian sturgeon, much higher activities of PEPCK-C were detected than those of PEPCK-M, we speculate PEPCK-C could be the main source for Siberian sturgeon as mammals, but PEPCK-M simultaneously works important role in regulating gluconeogenesis. The activities of FBPase were kept stable, and did not give corresponding response at mRNA levels for starvation or refeeding. Glucose-6-phosphatase is the final step for gluconeogenesis in animal. Different from other groups, G6Pase mRNA level in GL group significantly down-regulated at S21 and kept low expression at R21 even a very low carbohydrate diet was supplied. The uncontrolled gluconeogenesis response could be the reason for failing to recover the body weight after refeeding in this group. Siberian sturgeon could relatively control the plasma glucose level during starvation or refeeding fed with low or high carbohydrate diets. Conversely, carnivorous fish failed to inhibit the hepatic gluconeogenesis fed with high carbohydrates. Hyperglycemia or glucose intolerance phenotype in carnivorous species rainbow trout was associated with higher G6Pase gene expression and activity in the liver (Panserat et al., 2002b). Besides, the differences between the mRNA levels and activities of gluconeogenic enzymes in response to nutritional status were also found in our previous study in Siberian sturgeon (Gong et al., 2015) and European sea bass (Viegas et al., 2013). The genes expression could be subject to negative feedback by enzyme activities (Schunkert et al., 1993). These results suggested that Siberian sturgeon are able to effectively mobilize the gluconeogenesis pathway and enhancing endogenous glucose in response to food deprivation and refeeding with a particular way combined mammal and fish, but also different from mammal and most teleostean. The glucose metabolism in the ancient fish species needs to be further studied.
5. Conclusion
Both dietary carbohydrate levels and early glucose stimuli significantly affected the gluconeogenesis responses to starvation and refeeding in Siberian sturgeon. During prolonged starvation and thereafter refeeding, Siberian sturgeon combines the gluconeogenesis regulation characters of mammals (glucostasis, well controlled PEPCK-M activity and stable G6Pase mRNA expression) and carnivorous teleostean (uncontrolled PEPECK-C, FBPase mRNA levels and/or enzyme activities). Siberian sturgeon has a full compensatory ability on growth, but this ability would be obstructed by early glucose stimuli when refeeding the low carbohydrate diet after S21. The stable glycaemia under all conditions could be relied on the well-controlled regulation of glucose metabolism in this species, although the gluconeogenesis of the species is not perfectly responded to the nutrition status as mammals.
Acknowledgements
This study was supported by the National Basic Research Program of China (2014CB138601); The National Natural Science Foundation of China (No. 31572631, No. 31372539); Beijing Technology System for Sturgeon and Salmonids (SCGWZJ 20171103-1); the National Key Research and Development Program of China (2016YFF0201900); The special Fund for Agro-Scientific Research in the Public Interest (201203015).
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- Association of Official Analytical Chemist (AOAC) AOAC Inc.; Gaithersburg, MD, USA: 2006. Official methods of analysis of AOAC international. [Google Scholar]
- Barcellos L.J.G., Marqueze A., Trapp M., Quevedo R.M., Ferreira D. The effects of fasting on cortisol, blood glucose and liver and muscle glycogen in adult jundiá Rhamdia quelen. Aquaculture. 2010;300:231–236. [Google Scholar]
- Blasco J., Fernández J., Gutiérrez J. Fasting and refeeding in carp, Cyprinus carpio L.: the mobilization of reserves and plasma metabolite and hormone variations. J Comp Physiol B. 1992;162:539–546. [Google Scholar]
- Brodersen J., Luis Rodriguez-Gil J., J önsson M., Hansson L., Brönmark C., Nilsson P.A. Temperature and resource availability may interactively affect over-wintering success of juvenile fish in a changing climate. PLoS One. 2011;6:e24022. doi: 10.1371/journal.pone.0024022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caseras A., Metón I., Vives C., Egea M., Fernández F., Baanante I.V. Nutritional regulation of glucose-6-phosphatase gene expression in liver of the gilthead sea bream (Sparus aurata) Br J Nutr. 2002;88:607–614. doi: 10.1079/BJN2002701. [DOI] [PubMed] [Google Scholar]
- Chatzifotis S., Papadaki M., Despoti S., Roufidou C., Antonopoulou E. Effect of starvation and re-feeding on reproductive indices, body weight, plasma metabolites and oxidative enzymes of sea bass (Dicentrarchus labrax) Aquaculture. 2011;316:53–59. [Google Scholar]
- Fang L., Liang X.F., Zhou Y., Guo X.Z., He Y., Yi T.L. Programming effects of high-carbohydrate feeding of larvae on adult glucose metabolism in zebrafish, Danio rerio. Br J Nutr. 2014;111:808–818. doi: 10.1017/S0007114513003243. [DOI] [PubMed] [Google Scholar]
- Figueiredo-Garutti M.L., Navarro I., Capilla E., Souza R.H., Moraes G., Gutiérrez J. Metabolic changes in Brycon cephalus (Teleostei, Characidae) during post-feeding and fasting. Comp Biochem Physiol A Mol Integr Physiol. 2002;132:467–476. doi: 10.1016/s1095-6433(02)00094-6. [DOI] [PubMed] [Google Scholar]
- Furné M., Hidalgo M.C., López A., García-Gallego M., Morales A.E., Domezain A. Digestive enzyme activities in Adriatic sturgeon Acipenser naccarii and rainbow trout (Oncorhynchus mykiss). A comparative study. Aquaculture. 2005;250:391–398. [Google Scholar]
- Furné M., Sanz A., García-Gallego M., Carmen Hidalgo M., Domezain A., Domezain J. Metabolic organization of the sturgeon Acipenser naccarii. A comparative study with rainbow trout Oncorhynchus mykiss. Aquaculture. 2009;289:161–166. [Google Scholar]
- Furné M., Morales A.E., Trenzado C.E., García-Gallego M., Carmen Hidalgo M., Domezain A. The metabolic effects of prolonged starvation and refeeding in sturgeon and rainbow trout. J Comp Physiol B. 2012;182:63–76. doi: 10.1007/s00360-011-0596-9. [DOI] [PubMed] [Google Scholar]
- Garin D., Rombaut A., Freminet A. Determination of glucose turnover in sea bass Dicentrarchus labrax. Comparative aspects of glucose utilization. Comp Biochem Physiol B Biochem Mol Biol. 1987;87:981–988. doi: 10.1016/0305-0491(87)90422-6. [DOI] [PubMed] [Google Scholar]
- Geurden I., Aramendi M., Zambonino-Infante J., Panserat S. Early feeding of carnivorous rainbow trout (Oncorhynchus mykiss) with a hyperglucidic diet during a short period: effect on dietary glucose utilization in juveniles. Am J Physiol. 2007;292:R2275–R2283. doi: 10.1152/ajpregu.00444.2006. [DOI] [PubMed] [Google Scholar]
- Gillis T.E., Ballantyne J.S. The effects of starvation on plasma free amino acid and glucose concentrations in lake sturgeon Acipenser fulvescens. J Fish Biol. 1996;49:1306–1316. [Google Scholar]
- Gong G., Xue M., Wang J., Wu X.F., Zheng Y.H., Han F. The regulation of gluconeogenesis in the Siberian sturgeon (Acipenser baerii) affected later in life by a short-term high-glucose programming during early life. Aquaculture. 2015;436:127–136. [Google Scholar]
- Gutiérrez J., Pérez J., Navarro I., Zanuy S., Carrillo M. Changes in plasma glucagon and insulin associated with fasting in sea bass (Dicentrarchus labrax) Fish Physiol Biochem. 1991;9:107–112. doi: 10.1007/BF02265126. [DOI] [PubMed] [Google Scholar]
- Hanson R.W. Thematic minireview series: a perspective on the biology of phosphoenolpyruvate carboxykinase 55 years after its discovery. J Biol Chem. 2009;284:27021–27023. doi: 10.1074/jbc.R109.040519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoenig M., Jordan E.T., Ferguson D.C., de Vries F. Oral glucose leads to a differential response in glucose, insulin, and GLI-1 in lean versus obese cats. Domest Anim Endocrinol. 2010;38:95–102. doi: 10.1016/j.domaniend.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Hung S.S.O. Carbohydrate utilization by white sturgeon as assessed by oral administration tests. J Nutr. 1991;121:1600–1605. doi: 10.1093/jn/121.10.1600. [DOI] [PubMed] [Google Scholar]
- Hung S.S.O., Liu W., Li H., Storebakken T., Cui Y. Effect of starvation on some morphological and biochemical parameters in white sturgeon, Acipenser transmontanus. Aquaculture. 1997;151:357–363. [Google Scholar]
- Ince B.W., Thorpe A. The effects of starvation and force-feeding on the metabolism of the Northern pike, Esox lucius L. J Fish Biol. 1976;8:79–88. [Google Scholar]
- Johnston I.A., Goldspink G. Some effects of prolonged starvation on the metabolism of the red and white myotomal muscles of the plaice Pleuronectes platessa. Mar Biol. 1973;19:348–353. [Google Scholar]
- Kaushik S.J., Luquet P., Blanc D., Paba A. Studies on the nutrition of Siberian sturgeon, Acipenser baeri: I. Utilization of digestible carbohydrates by sturgeon. Aquaculture. 1989;76:97–107. [Google Scholar]
- Kirchner S., Panserat S., Lim P.L., Kaushik S., Ferraris R.P. The role of hepatic, renal and intestinal gluconeogenic enzymes in glucose homeostasis of juvenile rainbow trout. J Comp Physiol B. 2008;178:429–438. doi: 10.1007/s00360-007-0235-7. [DOI] [PubMed] [Google Scholar]
- Kottelat M., Freyhof J. 1972. Handbook of European freshwater fishes; p. 646. Berlin. [Google Scholar]
- Liu W., Wei Q.W., Wen H., Jiang M., Wu F., Shi Y. Compensatory growth in juvenile Chinese sturgeon (Acipenser sinesis) effects of starvation and subsequent feeding on growth and body composition. 2011;27:749–754. [Google Scholar]
- Lin J.H., Cui Y., Hung S.S.O., Shiau S.Y. Effect of feeding strategy and carbohydrate source on carbohydrate utilization by white sturgeon (Acipenser transmontanus) and hybrid tilapia (Oreochromis niloticus × O. aureus) Aquaculture. 1997;148:201–211. [Google Scholar]
- Lucas A. Programming by early nutrition: an experimental approach. J Nutr. 1998;458:S401–S406. doi: 10.1093/jn/128.2.401S. [DOI] [PubMed] [Google Scholar]
- Meng Z.X., Li S.M., Wang L., Ko H.J., Lee Y.J., Jung D.Y. Baf60C drives glycolytic metabolism in the muscle and improves systemic glucose homeostasis through Deptor-mediated Akt activation. Nat Med. 2013;19:640–645. doi: 10.1038/nm.3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metón I., Caseras A., Cantó E., Fernández F., Baanante I.V. Effect of diet composition and ration size on key enzyme activities of glycolysis-gluconeogenesis, the pentose phosphate pathway and amino acid metabolism in liver of gilthead sea bream (Sparus aurata) Br J Nutr. 1999;82:223–232. [PubMed] [Google Scholar]
- Metón I., Fernández F., Baanante I.V. Short- and long-term effects of refeeding on key enzyme activities in glycolysis–gluconeogenesis in the liver of gilthead seabream (Sparus aurata) Aquaculture. 2003;225:99–107. [Google Scholar]
- Metón I., Caseras A., Fernández F., Baanante I.V. Molecular cloning of hepatic glucose-6-phosphatase catalytic subunit from gilthead sea bream (Sparus aurata): response of its mRNA levels and glucokinase expression to refeeding and diet composition. Comp Biochem Physiol B. 2004;138:145–153. doi: 10.1016/j.cbpc.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Morshedi V., Kochanian P., Bahmani M., Yazdani-Sadati M.A., Pourali H.R., Ashouri G. Compensatory growth in sub-yearling Siberian sturgeon, Acipenser baerii Brandt, 1869: effects of starvation and refeeding on growth, feed utilization and body composition. J Appl Ichthyol. 2013;29:978–983. [Google Scholar]
- Namrata S., Sanjay N., Pallavi C. Effect of starvation on the biochemical composition of freshwater fish Channa punctatus. Rec Res Sci Tech. 2011;3:17–19. [Google Scholar]
- Navarro I., Gutiérrez J. Fasting and starvation. In: Hochachka P.W., Mommsen T., editors. Biochem mol biol fish. Elsevier Science BV; 1995. pp. 393–434. [Google Scholar]
- Panserat S., Plagnes-Juan E., Brèque J., Kaushik S. Hepatic phosphoenolpyruvate carboxykinase gene expression is not repressed by dietary carbohydrates in rainbow trout (Oncorhynchus mykiss) J Exp Biol. 2001;204:359–365. doi: 10.1242/jeb.204.2.359. [DOI] [PubMed] [Google Scholar]
- Panserat S., Plagnes-Juan E., Kaushik S. Gluconeogenic enzyme gene expression is decreased by dietary carbohydrates in common carp (Cyprinus carpio) and gilthead seabream (Sparus aurata) Biochim Biophys Acta. 2002;1579:35–42. doi: 10.1016/s0167-4781(02)00501-8. [DOI] [PubMed] [Google Scholar]
- Panserat S., Perrin A., Kaushik S. High dietary lipids induce liver glucose-6-phosphatase expression in rainbow trout (Oncorhynchus mykiss) J Nutr. 2002;132:137–141. doi: 10.1093/jn/132.2.137. [DOI] [PubMed] [Google Scholar]
- Pérez-Jiménez A., Guedes M.J., Morales A.E., Oliva-Teles A. Metabolic responses to short starvation and refeeding in Dicentrarchus labrax. Effect of dietary composition. Aquaculture. 2007;265:325–335. [Google Scholar]
- Pérez-Jiménez A., Cardenete G., Hidalgo M.C., García-Alcázar A., Abellán E., Morales A.E. Metabolic adjustments of Dentex dentex to prolonged starvation and refeeding. Fish Physiol Biochem. 2012;38:1145–1157. doi: 10.1007/s10695-011-9600-2. [DOI] [PubMed] [Google Scholar]
- Pilkis S.J., Granner D.K. Molecular physiology of the regulation of hepatic gluconeogenesis and glycolysis. Annu Rev Physiol. 1992;54:885–909. doi: 10.1146/annurev.ph.54.030192.004321. [DOI] [PubMed] [Google Scholar]
- Polakof S., Arjona F.J., Sangiao-Alvarellos S., Martín del Río M.P., Mancera J.M., Soengas J.L. Food deprivation alters osmoregulatory and metabolic responses to salinity acclimation in gilthead sea bream Sparus auratus. J Comp Physiol B. 2006;176:441–452. doi: 10.1007/s00360-006-0065-z. [DOI] [PubMed] [Google Scholar]
- Polakof S., Mommsen T.P., Soengas J.L. Glucosensing and glucose homeostasis: from fish to mammals. Comp Biochem Physiol A Mol Integr Physiol. 2011;160:123–149. doi: 10.1016/j.cbpb.2011.07.006. [DOI] [PubMed] [Google Scholar]
- Polakof S., Panserat S., Soengas J.J., Moon T.W. Glucose metabolism in fish: a review. J Comp Physiol B. 2012;182:1015–1045. doi: 10.1007/s00360-012-0658-7. [DOI] [PubMed] [Google Scholar]
- Pottinger T.G., Rand-Weaver M., Sumpter J.P. Overwinter fasting and re-feeding in rainbow trout: plasma growth hormone and cortisol levels in relation to energy mobilization. Comp Biochem Physiol B. 2003;136:403–417. doi: 10.1016/s1096-4959(03)00212-4. [DOI] [PubMed] [Google Scholar]
- Radaelli B.G., Domeneghini C., Arrighi S., Vaine F., Mascarello F. Histochemical and immunohistochemical investigation of muscle fibres in the sturgeon (Chondrostei; Acipenser) J Appl Ichthyol. 1999;15:87–91. [Google Scholar]
- Sangiao-Alvarellos S., Guzmán J.M., Láiz-Carrión R., Míguez J.M., Martín Del Río M.P., Mancera J.M. Interactive effects of high stocking density and food deprivation on carbohydrate metabolism in several tissues of gilthead sea bream Sparus auratus. J Exp Zool A Comp Exp Biol. 2005;303:761–775. doi: 10.1002/jez.a.203. [DOI] [PubMed] [Google Scholar]
- Schunkert H., Ingelfinger J.R., Hirsch A.T., Pinto Y., Remme W.J., Jacob H. Feedback regulation of angiotensin converting enzyme activity and mRNA levels by angiotensin II. Cir Res. 1993;72:312–318. doi: 10.1161/01.res.72.2.312. [DOI] [PubMed] [Google Scholar]
- Sheridan M.A., Mommsen T.P. Effects of nutritional state on in vivo lipid and carbohydrate metabolism of cohosalmon, Oncorhynchus kisutch. Gen Comp Endocr. 1991;81:473–483. doi: 10.1016/0016-6480(91)90175-6. [DOI] [PubMed] [Google Scholar]
- Soengas J.L., Polakof S., Chen X., Sangiao-Alvarellos S., Moon T.W. Glucokinase and hexokinase expression and activities in rainbow trout tissues: changes with food deprivation and refeeding. Am J Physiol Regul Integr Comp Physiol. 2006;291:810–821. doi: 10.1152/ajpregu.00115.2006. [DOI] [PubMed] [Google Scholar]
- Sokolov L.I. Feeding of Siberian sturgeon Acipenser baeri Brandt of the Lena River. Vorposy Ikhtiologii. 1966;6:550–560. [Google Scholar]
- Stark R., Kibbey R.G. The mitochondrial isoform of phophoenolpyruvate carboxykinase (PEPCK-M) and glucose homeostais: has it been overlooked? Biochim Biophys Acta. 2014;1840:1313–1330. doi: 10.1016/j.bbagen.2013.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian J.J., Lu R.H., Ji R., Sun J., Li C., Liu P. Comparative analysis of the hepatopancreas transcriptome of grass carp (Ctenopharyngodon idellus) fed with lard oil and fish oil diets. Gene. 2015;565:192–200. doi: 10.1016/j.gene.2015.04.010. [DOI] [PubMed] [Google Scholar]
- Vagner M., Zambonino Infante J.L., Robin J.H., Person-Le Ruyet J. Is it possible to influence European sea bass (Dicentrarchus labrax) juvenile metabolism by a nutritional conditioning during larval stage? Aquaculture. 2007;267:165–174. [Google Scholar]
- Vagner M., Robin J.H., Zambonino-Infante J.L., Tocher D.R., Person-Le Ruyet J. Ontogenic effects of early feeding of sea bass (Dicentrarchus labrax) larvae with a range of dietary n-3 highly unsaturated fatty acid levels on the functioning of polyunsaturated fatty acid desaturation pathways. Br J Nutr. 2009;101:1452–1462. doi: 10.1017/S0007114508088053. [DOI] [PubMed] [Google Scholar]
- Van Schaftingen E., Gerin I. The glucose-6-phosphatase system. Biochem J. 2002;362:513–532. doi: 10.1042/0264-6021:3620513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viegas I., Rito J., Jarak I., Leston S., Carvalho R.A., Metón I. Hepatic glycogen synthesis in farmed European seabass (Dicentrarchus labrax L.) is dominated by indirect pathway fluxes. Comp Biochem Physiol A. 2012;163:22–29. doi: 10.1016/j.cbpa.2012.04.023. [DOI] [PubMed] [Google Scholar]
- Viegas I., Rito J., González J.D., Jarak I., Carvalho R.A., Metón I. Effects of food-deprivation and refeeding on the regulation and sources of blood glucose appearance in European seabass (Dicentrarchus labrax L.) Comp Biochem Physiol A Mol Integr Physiol. 2013;166:399–405. doi: 10.1016/j.cbpa.2013.07.013. [DOI] [PubMed] [Google Scholar]
- Wang Z., Goonewardene L.A. The use of MIXED models in the analysis of animal experiments with repeated measures data. Can J Anim Sci. 2004;84:1–11. [Google Scholar]
- Wang T., Hung C.C.Y., Randall D.J. The comparative physiology of food deprivation: from feast to famine. Ann Rev Physiol. 2006;68:223–251. doi: 10.1146/annurev.physiol.68.040104.105739. [DOI] [PubMed] [Google Scholar]
- Watford M., Hod Y., Chiao Y.B., Utter M.F., Hanson R.W. The unique role of the kidney in gluconeogenesis in the chicken. The significance of a cytosolic form of phosphoenolpyruvate carboxykinase. J Biol Chem. 1981;256:100023–100027. [PubMed] [Google Scholar]
- Yarmohammadi M., Shabani A., Pourkazemi M., Soltanloo H., Imanpour M.R. Effect of starvation and re-feeding on growth performance and content of plasma lipids, glucose and insulin in cultured juvenile Persian sturgeon (Acipenser persicus Borodin, 1897) J Appl Ichthyol. 2012;28:692–696. [Google Scholar]
- Yun B., Xue M., Wang J., Sheng H., Zheng Y., Wu X. Fishmeal can be totally replaced by plant protein blend at two protein levels in diets of juvenile Siberian sturgeon, Acipenser baerii Brandt. Aquacult Nutr. 2014;20:69–78. [Google Scholar]