Abstract
The gastrointestinal (GI) barrier serves a critical role in survival and overall health of animals and humans. Several layers of barrier defense mechanisms are provided by the epithelial, immune and enteric nervous systems. Together they act in concert to control normal gut functions (e.g., digestion, absorption, secretion, immunity, etc.) whereas at the same time provide a barrier from the hostile conditions in the luminal environment. Breakdown of these critical GI functions is a central pathophysiological mechanism in the most serious GI disorders in pigs. This review will focus on the development and functional properties of the GI barrier in pigs and how common early life production stressors, such as weaning, can alter immediate and long-term barrier function and disease susceptibility. Specific stress-related pathophysiological mechanisms responsible for driving GI barrier dysfunction induced by weaning and the implications to animal health and performance will be discussed.
Keywords: Intestinal barrier, Pigs, Stress, Weaning, Gut health, Mast cells
1. Introduction
The gastrointestinal (GI) epithelium and underlying lamina propria are continually exposed to a harsh luminal environment, which includes massive amounts of toxins, antigens, pathogens, etc. In this environment, the gut must provide a barrier to pathogenic and antigenic components in the lumen to prevent an overwhelming immune activation and potentially sepsis, which is critical for host survival. However, simultaneously, the GI system must efficiently transport luminal nutrients, water, and electrolytes, which are vital for maintenance and growth, and selectively uptake dietary and microbial antigens to facilitate proper development and education of the mucosal immune system. To perform these divergent functions, the GI system is equipped with multiple layers of sophisticated barrier mechanisms. This review will focus on the postnatal development of specific barrier properties, which are provided by the GI epithelium and enteric immune and nervous systems. This review will also provide the supporting evidence from the literature on the impact of common early life production stressors, such as early weaning stress, on the developmental trajectory and long-term integrity of GI barrier properties and insight into the pathophysiologic mechanisms driving these changes. Whereas the microbiome has received much attention as an important defense barrier through several proposed mechanisms, reviewing the body of microbiome studies is beyond the scope of this review, but we direct readers to other excellent reviews on the topic (Backhed et al., 2005, Honda and Littman, 2016, Kelly et al., 2015).
2. Gastrointestinal barriers and their importance to GI health and disease resistance
The GI barrier is comprised of a multi-layered system of host defense mechanisms provided by the intestinal epithelial cells, and components of the immune and enteric nervous system. Given the importance of these barrier mechanisms to health and disease, a large amount of research has been conducted on these specific functions and a number of review papers have been published; therefore, an extensive review of these functions is beyond the scope of this review. However, to provide the framework for the relevance of the GI barrier mechanisms in animal production, nutrition and health, a brief overview of the major GI barrier mechanisms is provided below.
2.1. Barrier properties of the GI epithelium
The single layer of epithelial cells lining the GI tract represents the largest interface between the host and the outside world. The intestinal epithelium facilitates the breakdown and uptake of nutrients via brush border enzyme activity and an array of apical and basolateral nutrient transporters, while at the same time facilitating a massive amount of bidirectional water movement via electrolyte transporters, channels and pumps. At the same time, the epithelium must provide a barrier to the harsh luminal contents, which include pathogens, antigens, toxins, and serves as the first line of defense. Several extrinsic and intrinsic mechanisms of defense are provided by the GI epithelium. One of the most critical mechanisms is the establishment of a permeability barrier, which is regulated predominantly by the tight junctions (TJs), which consist of numerous intracellular and apical intercellular membrane proteins (e.g., zonula occludens, occludin, and claudins) (Edelblum and Turner, 2009, Groschwitz and Hogan, 2009, Marchiando et al., 2010, Turner, 2009). Tight junction proteins regulate the leakiness of the epithelium by modulating ion selectively and pore size of the epithelium known as the “gate function”. Tight junctions also play a critical role in establishing epithelial polarity important for the compartmentalization of apical and basolateral transporter and receptor function known as the “fence function”. The polarization provided by the fence function is important to maintain the apical Na+ gradients needed for efficient nutrient (glucose, amino acids) and water transport. Whereas a tight or resistant epithelium is critical for barrier function, the epithelium must exhibit a normal physiologic degree of paracellular and transcellular permeability required for solute-driven water absorption and transcellular antigen uptake (Turner et al., 1997). The critical importance of the appropriate regulation of epithelial permeability is highlighted by the number of important diseases linked with “leaky gut” including chronic inflammatory, allergic, and functional disorders and life threatening conditions such as sepsis and multiple organ dysfunction (Barbara, 2006, Camilleri and Gorman, 2007, Edelblum and Turner, 2009, Marchiando et al., 2010). The intestinal epithelial barrier is also supported by specialized epithelial cell types such as goblet cells, which provide protective mucous layer and Paneth cells, which secrete antimicrobial peptides. Intestinal epithelial cells provide buffering and pH regulation through the secretion of Cl− and HCO3− ions, which is performed in large part by the crypt epithelium. The ability to upregulate ion and fluid secretion is considered to be an important mechanism to flush out pathogens in response to stress and pathogenic challenges (Moeser and Blikslager, 2007). Enteroendocrine cells play important roles in pathogen sensing and can synthesize and release neuropeptides such as serotonin and Peptide YY (PYY), which have a diverse range of physiologic functions from pathogen defense to metabolic regulation of appetite (Argenzio et al., 1997, Duca et al., 2013, Little and Feinle-Bisset, 2011). The intestinal epithelial cells act as immune sentinel cells by recognizing pathogenic signal molecules and secreting interleukins (IL) and growth factors (e.g., IL-17A, IL-33, IL-23 and transforming growth factor-β), which have important immunomodulatory properties (Schiering et al., 2014).
2.2. Barrier properties of the GI immune system
The resident immune cells and related lymphoid structures in the gut constitute the largest immune organ in the body. Given the massive antigenic luminal environment and continual exposure to luminal products, the GI immune system is tightly regulated via a number of molecular mechanisms, to prevent excessive activation and inflammation in response to the continual exposure to highly antigenic and inflammatory substances. However, the GI immune system must also be able to rapidly and robustly respond to any breach in the epithelial barrier or in the event of a pathogenic/antigenic challenge to mobilize innate and adaptive immune responses, which is critical in preventing the systemic spread of infection and inflammation. In summary, a delicate balance between control and reactiveness of the GI immune system is critical for optimal GI health and disturbances in this balance is central to GI inflammatory disorders and disorders associated with immune suppression.
2.3. Enteric nervous system barrier mechanisms
The enteric nervous system contains >100 million neurons, which is as many as the spinal cord, and plays a central role in gut and overall systemic health. The enteric nervous system consists of 2 major neural ganglia located in the muscle (myenteric plexus) and submucosa (submucosal plexus), which control motility and peristalsis and mucosal and epithelial functions, respectively. The enteric nervous system, through the constant release of an array of neurochemicals, plays a central role in gut motility, secretion and absorption, and modulation of epithelial permeability. The nervous system is also a major regulator of systemic and local GI immune responses via neuro-immune synapses, and can modulate bacterial toxin sensing and adherence (Dhawan et al., 2012, Downing and Miyan, 2000, Fernandez-Cabezudo et al., 2010). Due to its critical role in normal gut functions, disturbances in enteric nervous system function can have deleterious impacts on GI health. This concept is highlighted by the growing evidence for enteric nervous system dysfunction in as a mechanism in important GI diseases states relevant to pig GI health, including secretory diarrhea, altered motility and inflammation. For example, many diarrheal pathogens including enterotoxigenic Escherichia coli enterotoxins, rotavirus mediate diarrhea activation of neural secretory reflexes involving the sensing of toxins by sensory fibers beneath the epithelium, which in turn convey signals and activation of secretomotor neuron activation, mediated via release of neurochemicals vasoactive intestinal peptide and acetylcholine, which drive electrolyte and fluid secretion (Field, 2003, Lundgren, 2002, Wood, 2010). Stress neuroendocrine mediators such as catecholamines and adrenocorticotropic hormone (ACTH) can influence binding and adherence of important swine enteric pathogens to the intestinal mucosa (Brown and Price, 2008, Chen et al., 2006, Schreiber and Brown, 2005). Significant alterations in the enteric nervous system phenotype and function can change in response to stressors such as weaning (Medland et al., 2016, Moeser et al., 2007a) and pathogenic challenges from Lawsonia intracellularis (Pidsudko et al., 2008, Wojtkiewicz et al., 2012). There is an increased interest in studying the neuron-immune communication as a way to explain mechanisms of GI diseases. An important example of such communication in GI disease is nerve-mast cell interactions, which are increased in IBS (Barbara et al., 2004, 2007). Similar to human IBS, Pohl et al. (2017) demonstrated that adult pigs that were previously early weaned (16 d wean age) exhibited enhanced co-localization of intestinal mast cells with enteric nerves, compared with late weaned pigs (weaned at 28 d of age) (Pohl et al., 2017).
3. The critical window of postnatal GI barrier development
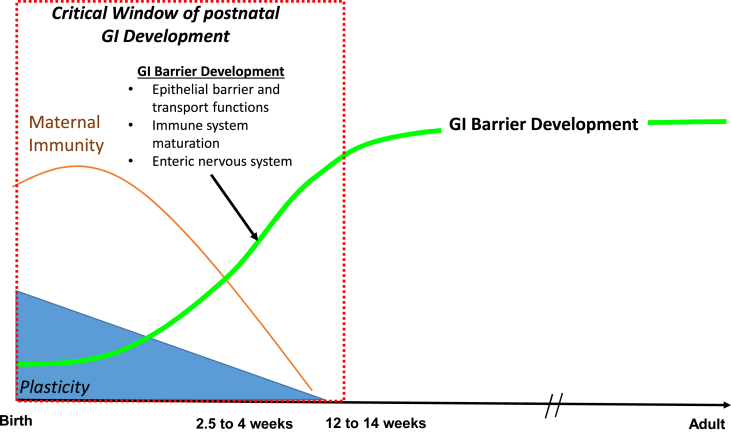
The first three months of postnatal life represent a major maturational period of GI development in the pig. During this time, intestinal epithelial, immune and enteric nervous system (ENS) phenotype and function change dramatically as the neonate adapts to life in the extra-uterine environment (Fig. 1). Whereas some developmental changes are a result of intrinsic genetic programming or biological clocks, many changes are influenced by changing environmental cues. Many developmental processes exhibit a high degree of plasticity during this time and thus perturbations occurring in this critical window can largely shape the long-term phenotype and GI function. The postnatal GI developmental aspects in the pig has been reviewed in detail previously (Pohl et al., 2015) but here we will cover some of the major concepts of postnatal GI barrier function development relevant to GI barrier components discussed above.
Fig. 1.
Ontogeny of postnatal gastrointestinal (GI) barrier function development in the pig. During the first 12 weeks of postnatal life, the GI system in the pigs undergoes significant development. Colostrum and sow's milk initially provides the piglet protective passive immunity as well as important growth and immune factors. The postnatal period is marked by maturation of the epithelial barrier and transport functions, and immune and enteric nervous systems (indicated by green line) that are almost complete by 12 to 14 weeks of age. Developmental processes occurring this time exhibit a high degree of plasticity and shape the adult phenotype and function of the GI barrier.
3.1. Postnatal intestinal epithelial barrier development
The establishment of the epithelial barrier develops rapidly in postnatal life and is characterized by a rapid decline in intestinal permeability. The process is critical to prevent exposure of the immune system to new environmental antigens from food and the colonizing microbiota, which would otherwise trigger massive inflammation. Although species differences exist, in most animals the development of intestinal epithelial barrier function occurs within the first 2 to 3 weeks of postnatal life characterized by a decline in intestinal permeability (Catassi et al., 1995, De Quelen et al., 2011, Mackey et al., 2016, Patel et al., 2012). The precise mechanisms driving early postnatal barrier development are not well-defined but are thought to be driven by several factors including inherent genetic programming, microbial colonization, and colostrum and milk factors (Fawzy et al., 2011, Rogier et al., 2014).
3.2. Postnatal intestinal immune barrier development
Birth and weaning represent a major challenge to the developing immune system as it must adapt to GI microbial colonization and milk and feed antigens. In addition to a rapid epithelial barrier establishment, additional exogenous and endogenous factors act to suppress immune activation. For example, milk-derived immunoglobulins (e.g., immunoglobulin A, IgA), maternal leukocytes, and milk glycans can act to modulate and neutralize intestinal microbes. Additionally, mothers milk provides anti-inflammatory cytokines and peptides, which suppress neonatal toll like receptor (TLR) and inflammatory cytokine expression (Newburg and Walker, 2007). An additional mechanism to prevent over-activation of the immune system during GI immune development, neonates are born with few lymphocytes and reduced co-stimulatory molecules expression (Nguyen et al., 2010, Upham et al., 2006). The neonates immune system is biased toward a T helper 2 (Th2: humoral) immune response as their ability to produce T helper1 (Th1) cytokines such as interferon gamma (IFNγ) and interleukin-12 (IL-12) is suppressed whereas Th2 cytokines such as IL-4, IL-10 and IL-13 are comparatively higher (Beverley, 1997). Additionally, neonatal T cells show hyper responsiveness to IL-4 and hypo-responsiveness to IL-12, which further skews the immune response toward Th2 (Beverley, 1997). Moreover, a shorter complementarity-determining region 3 (CDR3), where binding of molecules to their specific antigen on T cell receptors (TcR) occur, makes neonatal T cells less reactive (Garcia et al., 2000). Several weeks after birth, neonates overcome the neonatal immunosuppressive stage when lymphocytes start developing functionally as well as structurally (Butler et al., 2000, Butler et al., 2009; Butler and Wertz, 2012). Unweaned (nursing) piglets achieve a stable number of lymphocytes at about 6 weeks of age (Blikslager et al., 1997). Around the same time, secondary lymphoid organs such as jejunal and ileal Peyer's patches rapidly mature characterized by an infiltration of lymphocytes (Barman et al., 1997, Pabst et al., 1988). Interaction of lymphocytes to a wide variety of antigen including self-antigen and bacteria, help in differentiating between self and non-self-antigen and further facilitate to achieve homeostasis (Martin et al., 2010, Wu and Wu, 2012). The proper interactions of lymphocytes with antigen also lead to their proliferation, immunoglobulins and balanced Th1/Th2 cytokine production (Blikslager et al., 1997, Frenyo et al., 1981, Pomorska-Mol and Markowska-Daniel, 2010). In summary, several maternal and host mechanisms act to limit immune activation during early GI development indicating the importance of an immunosuppressive environment for optimal and long-term maturation of the immune system. Therefore, inappropriate or excessive immune stimulation during this critical period has potential to disrupt the development and long-term function of the gut immune system.
3.3. Postnatal development of the enteric nervous system
During postnatal life, major changes take place in the enteric nervous system including formation of functional neurocircuits, gangliogenesis, and changes in the neurochemical phenotype (Lake and Heuckeroth, 2013, Sasselli et al., 2012). Following neurogenesis and proliferation is a period of neuronal number decline, or “neuron pruning”, resulting in the adult neuronal phenotype (Aoki et al., 2007, Gabella, 1971, Medland et al., 2016, Schafer et al., 1999, Wester et al., 1999). Cholinergic neurons represent an import neuronal system that exhibits significant developmental changes in postnatal life. Cholinergic innervation of the gut is characterized by the proportion of neurons expressing choline acetyltransferase (ChAT), the rate limiting enzyme for the synthesis of acetylcholine, and the major excitatory neurotransmitter in the GI tract. The proportion of ChAT neurons in postnatal life increases dramatically and can account for approximately 44% of all neurons in the submucosal plexus, and 62% in the myenteric plexus by maturity (Furness, 2000, Hao et al., 2013). In addition to cholinergic neurons, serotonergic and adrenergic neurons undergo major changes throughout postnatal GI development (Medland et al., 2016).
In summary, major developmental changes in the GI intestinal barrier function take place postnatally. The GI systems undergoing development during this time exhibit a high degree of plasticity and are modified by environmental cues. Therefore stressful or inflammatory disturbances during this time can have long-last consequences. Exogenous and endogenous protective mechanisms discussed, such as rapid epithelial barrier function and an immunosuppressive immune phenotype, support the concept that this critical period of development remains protected or undisturbed. Unfortunately, this vulnerable developmental period for the GI system coincides with the most stressful production practices including early weaning, vaccination, transport, diet change, and more. Therefore current production practices can have a profound effect on shaping long-term GI development and health of the pig. Current knowledge on how certain early life stressors associated with weaning in the pig influence GI barrier development and function is reviewed below.
4. Early weaning stress and GI barrier development: Immediate and long-term impacts
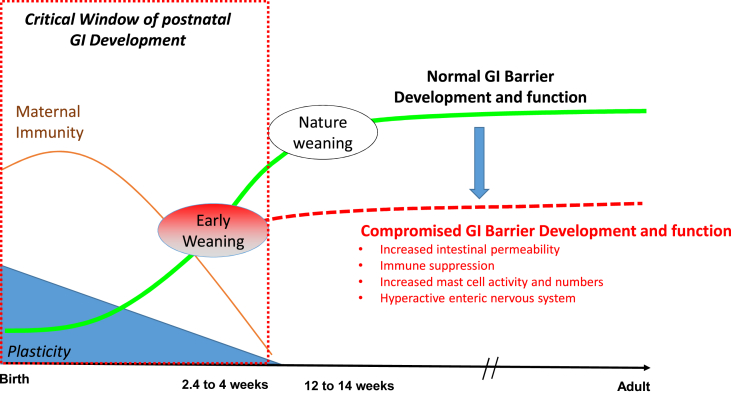
In nature, weaning in pigs is a gradual process that approaches completion at around 10 to 12 weeks of age, which coincides with the near complete maturation of the GI epithelial, immune and nervous systems described above. However, in commercial pig production, weaning is abrupt occurring between 14 and 30 days of age (Fig. 2). Whereas maternal separation is a major stressor to the weaned pig, additional psychosocial and immunological stressors, compound the stress load during this time, including transportation, mixing, fighting and establishment of a new social hierarchy, vaccination, etc. The timing of commercial weaning also coincides with a period of declining passive immunity from sow milk contributing an additional challenge to the pig. Weaned pigs are able to survive and overcome the stress of weaning; however, it is important to recognize early weaning stressors occurs during the critical window of GI barrier development. Therefore, stressors associated with early weaning have the potential to disrupt normal epithelial, immune, and enteric nervous system developmental processes.
Fig. 2.
Impact of early weaning on the developmental trajectory of gastrointestinal (GI) barrier function. In nature, weaning is a gradual process occurring at ∼12 to 14 weeks of age, at which time the GI barrier function is near complete. In commercial production systems, weaning is abrupt and compounded by multiple social and environmental stressors and occurs at the height of GI barrier development between 2 and 4 weeks of age. Early weaning pigs exhibit an altered GI barrier developmental trajectory (red dashed line) resulting in permanent, suboptimal barrier function and increased disease susceptibility.
4.1. Weaning and immediate alterations in GI barrier function
In response to weaning there are a number of morphological, enzymatic, and inflammatory changes, which take place in the GI tract of pigs and have been reviewed previously (Boudry et al., 2004, Montagne et al., 2007, Wijtten et al., 2011). Here, we will focus on the impacts of weaning and associated stressors on critical components of the intestinal barrier including intestinal epithelial permeability, immune system activation, and the enteric nervous system.
Results of several studies have demonstrated that weaning in pigs induces a breakdown in intestinal barrier function characterized by a significant decline in intestinal transepithelial electrical resistance and increased permeability to paracellular probes in Ussing chamber experiments (Hu et al., 2013, Moeser et al., 2007a 2007b). Moeser et al. (2007a) demonstrated that, compared with age-matched, non-weaned littermate pigs, weaned pigs exhibited increased intestinal permeability that was most pronounced at 24 h post weaning and then gradually declined over the first 2 weeks post-weaning (Moeser et al., 2007a, Peace et al., 2011). At the same time that epithelial barrier function is disrupted, an upregulation of proinflammatory cytokines has been reported indicating a robust activation of the GI immune system following weaning (Hu et al., 2013, McCracken et al., 1999). A specific immune reaction that plays a central role in the intestinal barrier pathophysiology observed in the weaned pigs is intestinal mast cell activation, which is discussed in more detail later in this review (Moeser et al., 2007b). The influence of weaning on enteric nervous system function has not been extensively studied in the pig. However, weaning-induced elevations in transepithelial short circuit current (Isc), a marker of intestinal secretion, were inhibited with the neural inhibitor toxin, tetrodotoxin, demonstrating that weaning induced Isc, and therefore secretory activity is mediated in large part by activation of enteric nerves (Moeser et al., 2007a).
4.2. The influence of wean age on short and long-term intestinal barrier function
In commercial pig production, weaning age can vary between 14 and 30 days of age depending on several management factors (e.g., lactation space, disease status, and weaning schedule). Our laboratory had demonstrated in several published investigations that weaning age is a major factor increasing the severity of intestinal barrier injury following weaning. Compared with pigs weaned at 28 d of age, 21 d weaned pigs exhibit increased intestinal permeability and Isc responses, when measured at 24 h post-weaning (Moeser et al., 2007b). Smith et al. (2010) demonstrated that increases in weaning age from 15 to 28 d of age results in graded reductions in intestinal permeability when measured 2 weeks post-weaning.
The majority of research on gut function in the weaned pigs has focused on the first 1 to 2 weeks post-weaning. The long-term impacts on pig health and gut function have only recently begun to be realized. Main et al. (2004) demonstrated in a commercial multisite swine production system that increases in weaning age from 12 to 21.5 d resulted in linear improvements in growth rate, feed efficiency and reductions in mortality to market (Main et al., 2004). In agreement with production data provided by Main et al., 2004, Smith et al., 2010 showed that increasing weaning age linearly reduced intestinal permeability when measured at 2 weeks post-weaning and intestinal permeability differences between early and late-weaned pigs persisted when measured at 9 weeks post-weaning (Smith et al., 2010). Recent evidence by demonstrates that functional GI disturbances in GI barrier, immune and nervous system function in early weaned pigs persists into adulthood (Medland et al., 2016, Pohl et al., 2017).
Along with disturbances in intestinal permeability, there is also accumulating evidence for lasting functional changes in the GI immune barrier function and disease susceptibility. In response to a post-weaning F18 enterotoxigenic E. coli challenge, early weaned pigs (15 to 16 d weaning age) had increased incidence of clinical disease (diarrhea and growth performance reductions) and increased intestinal permeability compared with later-weaned pigs (22 d weaning age) (McLamb et al., 2013). Evaluation of the mucosal innate immune responses in this study revealed that early weaned pigs exhibited suppressed IL-6, IL-8 and neutrophil responses to E. coli challenge, compared with late weaned pigs, thus suggesting a compromised or suppressed immune response in early weaned pigs. In a study comparing 14 d vs. 21 d weaned pigs, white blood cell concentrations, specifically lymphocytes, were found to be higher in 21-d weaned pigs following mixing and resorting at 37 d post-weaning, whereas no differences were observed after mixing and resorting in 14-d weaned pigs suggesting a lower immunological response to commingling stress in early weaned pigs (Davis et al., 2006). In the same study, lower blood eosinophils were observed in 14 d weaned pigs at 44 d post-weaning whereas at 65 d post-weaning, 14-d weaned pigs had higher neutrophil percent but lower lymphocyte percent compared with 21-d weaned pigs (Davis et al., 2006). Together, these studies provide supporting evidence for lasting changes in peripheral and mucosal immune cells as a result of wean age. Whereas evidence supports a compromised immune response in early weaned pigs, our laboratory has demonstrated that early weaned pigs have marked and persistent mast cell hyperplasia in the small and large intestine when measured at 2 and 9 weeks post-weaning (McLamb et al., 2013, Smith et al., 2010). Recent data form our laboratory confirmed that mast cell hyperplasia persists into adulthood in early weaned pigs (Pohl et al., 2017).
As described above, the enteric nervous system plays central role in the regulation of the intestinal epithelial and immune barriers. In a study by Medland et al. (2016), early weaned pigs exhibited lasting phenotypic and functional changes in the enteric nervous system, compared with late weaned pigs. This study showed that late weaned pigs exhibited a normal decline or pruning of enteric neurons numbers between 60 and 170 d post-weaning; however, enteric neuron numbers persisted in early weaned pigs. A large percentage of the persistent enteric neurons in early weaned pigs were shown to be positive for choline acetyltransferase (ChAT), the rate limiting enzyme in acetylcholine synthesis. An upregulation in acetylcholinesterase activity was also observe in the early weaned pig. Ussing chambers experiments revealed that ileum from early weaned pigs exhibited heightened neural- and CRF-evoked secretory responses that were blocked with the cholinergic muscarinic receptor blocker atropine. Together, these experiments demonstrated that early weaning induces a persistent upregulation of the enteric cholinergic system. In this study, early weaned female pigs exhibited greater neural-evoked secretory responses and more pronounced cholinergic activation, compared with early weaned male pigs provided new evidence for sex-specific changes in the enteric nervous system triggered by early weaning (Medland et al., 2016). Given the role of the cholinergic nervous system in modulating immune responses, secretory diarrhea and the epithelial barrier (Dhawan et al., 2012, Hirota and McKay, 2006, Matteoli and Boeckxstaens, 2013), there are potential implications for an upregulated cholinergic system in early weaned pigs as a pathogenic mechanism for increased disease susceptibility associated with early life stressors such as weaning (McLamb et al., 2013).
5. Mechanisms driving intestinal barrier dysfunction in early weaned pigs
Weaning encompasses several stressors (e.g., maternal separation, mixing stress, transportation, diet change, etc.), which collectively contribute to compromised gut health and well-being of the weaned pig. The impact of weaning on GI health and disease has long been known; however, the underlying mechanisms remain poorly understood. This knowledge gap is a key factor limiting targeted interventions to ameliorate the adverse effects of weaning on gut function.
5.1. Stress signaling and the intestinal corticotropin releasing factor (CRF) system
Weaning is a stressful event in pigs as evidenced by the activation of hypothalamic pituitary adrenal axis (HPA) and elevation of stress related mediators, including CRF and cortisol in the circulation. Activation of the HPA axis is a critical survival mechanism to respond to a stressor and return to homeostasis. The precise role of this system in modulating GI physiology remains poorly understood in the pig. Serum CRF levels were shown to be elevated following weaning for the first week post-weaning, which mirrored changes in intestinal transepithelial permeability and electrogeni ion transport properties (determine by measurement of transepithelial Isc) in pigs weaned at 19 d of age (Moeser et al., 2007a). In the same study, serum cortisol was also elevated in response to weaning; however, cortisol levels remained elevated throughout the post-weaning period and thus did not coincide with post-weaning changes in GI barrier function. During the first week post-weaning, early weaned pigs had greater serum CRF levels but lower cortisol levels compared with late-weaned pigs (Moeser et al., 2007b). At the intestinal level, the expression of CRF receptors in the jejunum, ileum, and colon were upregulated in early weaned pigs but not in late-weaned pigs (Moeser et al., 2007b). At 2 weeks post-weaning, early weaned pigs had higher jejunal CRF levels and decreased intestinal mucosal expression of CRF receptor subtypes, which might indicate a downregulation of CRF receptor expression in response to chronically elevated CRF ligand (Smith et al., 2010). In contrast, glucocorticoid receptor expression was found to be markedly higher in the jejunum of early weaned pigs compared with late weaned pigs (Smith et al., 2010). More importantly, administration of CRF receptor antagonists to early weaned pigs at weaning, or 9 weeks post-weaning, reduced the early (Moeser et al., 2007a) and chronic (Smith et al., 2010) elevations in intestinal permeability thus demonstrating a central role for the intestinal CRF receptor system in modulating intestinal permeability in the weaned pig. In addition weaning-associated changes in the expression of the CRF system, we have also demonstrated that the CRF system is upregulated in pigs undergoing other stressors including Salmonella typhimurium challenge (Boyer et al., 2015) and chronic mixing and crowding stress (Li et al., 2017), suggesting a potential common pathophysiologic mechanism underlying stress-induced gut dysfunction in pigs. Together, these studies demonstrate that weaning and other stressors induce early and long-term changes in the intestinal CRF system which are central to intestinal barrier disturbances in pigs.
5.2. The role of intestinal mast cells in gastrointestinal barrier dysfunction induced by early weaning in pigs
Mast cells are hematopoetically-derived innate immune cells that play important roles in host defense and disease pathogenesis. Mast cells are strategically located at host-environmental interfaces such as the skin and gut mucosa and serve as important immune sentinel cells and immune modulators (Abraham and St John, 2010). Mast cells are required for host defense and wound healing; however, excessive activation of mast cells is a central pathophysiologic mechanisms in inflammatory disease such as allergy and asthma and functional stress-related GI disorders including irritable bowel syndrome in humans (Boeckxstaens, 2015, Galli and Tsai, 2012, Hamilton et al., 2014, Wouters et al., 2016). Intestinal mast cells were shown to be activated within 24 h of weaning in pigs (Moeser et al., 2007b). Wean age has a major impact on intestinal mast cell activity in pigs. Compared with late-weaned pigs, early weaned pigs exhibit greater intestinal mast cell activation measured at 24 h post-weaning (Moeser et al., 2007b) and develop a persistent intestinal mast cell hyperplasia that was evident at 9 weeks post-weaning (Smith et al., 2010) and into adulthood (Pohl et al., 2017). The importance of increased intestinal mast cell activity in early weaned pigs was demonstrated in experiments where early weaned pigs were administered the mast cell stabilizer drug sodium cromolyn, which was shown to prevent weaning-induced increases in intestinal permeability and changes in short-circuit current (Isc) (Moeser et al., 2007b). In vivo and ex vivo studies demonstrated an interplay between the CRF receptor activation and mast cells as CRF applied to porcine intestine was shown to increase intestinal permeability via a mechanism involving release of mast cell proteases and TNFα (Overman et al., 2012, Smith et al., 2010). Together, these studies provide strong evidence for a central role for mast cells in driving intestinal epithelial permeability disturbances in the early weaned pig (Fig. 3). Given that mast cells are also potent positive and negative modulators of immune responses (Abraham and St John, 2010, Chan et al., 2013, Choi et al., 2013, Galli et al., 2008) and enteric neurons (Barbara et al., 2004, Kirsch and Riddell, 2006, Kobayashi et al., 1999, Wouters et al., 2016), mast cells might be major players regulating immune and ENS dysfunction observed in early weaned pigs, but the precise role of mast cells as immune modulators in pigs remains to be elucidated.
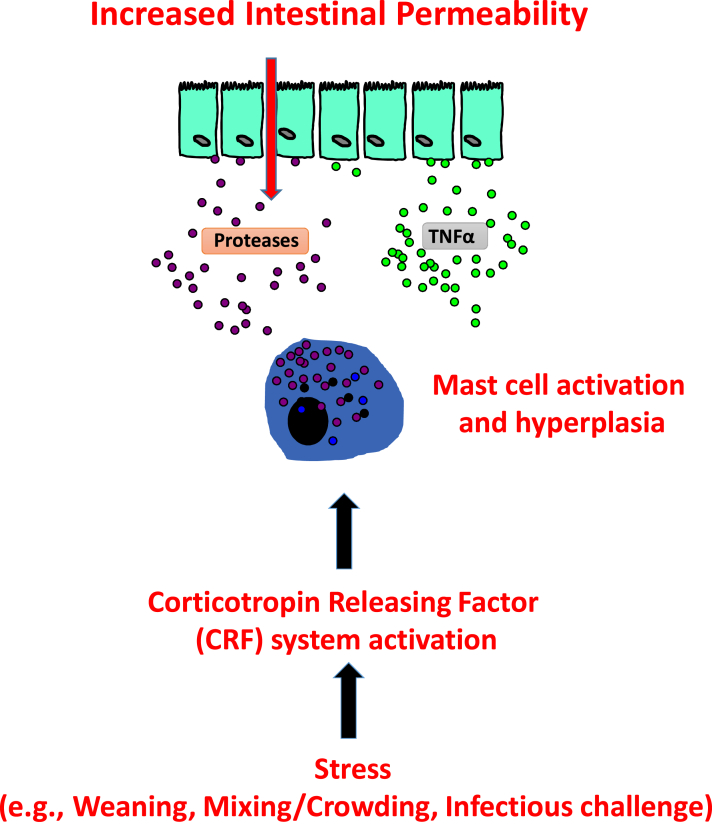
Fig. 3.
Mechanisms of intestinal barrier dysfunction induced by production stressors in pigs: corticotropin releasing factor (CRF)-mast cell axis. Production stressors such as weaning, mixing/crowding and gastrointestinal (GI) infections, trigger activation of intestinal CRF and receptors. The CRF receptor activation enhances intestinal mast cell activation and hyperplasia and the release of mast cell mediators including proteases (e.g., tryptase and chymase) and tumor necrosis factor-α (TNF-α). Mast cell proteases and TNF-α induce increases in intestinal epithelial permeability.
6. Conclusion
Under natural conditions, the early postnatal period is characterized by a critical period of immunological and neuroendocrine quiescence, which is required for normal development and function of the GI system. However, in commercial production, many stressors are imposed upon the pig during this critical period. Although piglets overcome the weaning period and can perform well, new research has demonstrated that early life stressors such as weaning alter the developmental trajectory of GI barrier functions leading to long-lasting deleterious consequences on gut health and disease susceptibility of the animal throughout the production lifespan (Fig. 3). This review presents a summary of that, which is currently known about the deleterious impacts of current early weaning practices on the development and long-term GI epithelial, immune, and ENS barrier functions in the pig. The concept of early life origins of GI disease susceptibility in the pig is supported by paradigms in humans where early life adverse events (e.g., psychological trauma, inflammation, infection) are risk factors for GI inflammatory and functional diseases later in life (Bradford et al., 2012, Chaloner and Greenwood-Van Meerveld, 2013, Maccari et al., 2014, Park et al., 2016, Sanchez et al., 2001). Increasing our understanding of how early life stressors such as weaning in the pig shape long-term epithelial, immune, and ENS barrier function is needed to discover new targets and (or) management interventions to promote optimal development and long-term gut health in pigs.
Acknowledgements
This work was supported by a grant from the National Institutes of Health HD072968 (to A.J.M.) and by Agriculture and Food Research Initiative Competitive Grant No. 2017-67015-26673 from the USDA National Institute of Food and Agriculture (to A.J.M.). Dr. Adam Moeser is the Matilda R. Wilson Endowed Chair of Large Animal Clinical Sciences Professor.
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- Abraham S.N., St John A.L. Mast cell-orchestrated immunity to pathogens. Nat Rev Immunol. 2010;10:440–452. doi: 10.1038/nri2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki T., Jusuf A.A., Iitsuka Y., Isono K., Tokuhisa T., Hatano M. Ncx (Enx, Hox11L.1) is required for neuronal cell death in enteric ganglia of mice. J Pediatr Surg. 2007;42:1081–1088. doi: 10.1016/j.jpedsurg.2007.01.064. [DOI] [PubMed] [Google Scholar]
- Argenzio R.A., Armstrong M., Blikslager A., Rhoads J.M. Peptide YY inhibits intestinal Cl- secretion in experimental porcine cryptosporidiosis through a prostaglandin-activated neural pathway. J Pharmacol Exp Ther. 1997;283:692–697. [PubMed] [Google Scholar]
- Backhed F., Ley R.E., Sonnenburg J.L., Peterson D.A., Gordon J.I. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- Barbara G. Mucosal barrier defects in irritable bowel syndrome. Who left the door open? Am J Gastroenterol. 2006;101:1295–1298. doi: 10.1111/j.1572-0241.2006.00667.x. [DOI] [PubMed] [Google Scholar]
- Barbara G., Stanghellini V., De Giorgio R., Cremon C., Cottrell G.S., Santini D. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology. 2004;126:693–702. doi: 10.1053/j.gastro.2003.11.055. [DOI] [PubMed] [Google Scholar]
- Barbara G., Wang B., Stanghellini V., de Giorgio R., Cremon C., Di Nardo G. Mast cell-dependent excitation of visceral-nociceptive sensory neurons in irritable bowel syndrome. Gastroenterology. 2007;132:26–37. doi: 10.1053/j.gastro.2006.11.039. [DOI] [PubMed] [Google Scholar]
- Barman N.N., Bianchi A.T., Zwart R.J., Pabst R., Rothkotter H.J. Jejunal and ileal Peyer's patches in pigs differ in their postnatal development. Anat Embryol Berl. 1997;195:41–50. doi: 10.1007/s004290050023. [DOI] [PubMed] [Google Scholar]
- Beverley P.C. Vaccine immunity. Immunol Today. 1997;18:413–415. doi: 10.1016/s0167-5699(97)01120-1. [DOI] [PubMed] [Google Scholar]
- Blikslager A.T., Roberts M.C., Rhoads J.M., Argenzio R.A. Is reperfusion injury an important cause of mucosal damage after porcine intestinal ischemia? Surgery. 1997;121:526–534. doi: 10.1016/s0039-6060(97)90107-0. [DOI] [PubMed] [Google Scholar]
- Boeckxstaens G. Mast cells and inflammatory bowel disease. Curr Opin Pharmacol. 2015;25:45–49. doi: 10.1016/j.coph.2015.11.005. [DOI] [PubMed] [Google Scholar]
- Boudry G., Peron V., Le Huerou-Luron I., Lalles J.P., Seve B. Weaning induces both transient and long-lasting modifications of absorptive, secretory, and barrier properties of piglet intestine. J Nutr. 2004;134:2256–2262. doi: 10.1093/jn/134.9.2256. [DOI] [PubMed] [Google Scholar]
- Boyer P.E., D'Costa S., Edwards L.L., Milloway M., Susick E., Borst L.B. Early-life dietary spray-dried plasma influences immunological and intestinal injury responses to later-life Salmonella typhimurium challenge. Br J Nutr. 2015;113:783–793. doi: 10.1017/S000711451400422X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford K., Shih W., Videlock E.J., Presson A.P., Naliboff B.D., Mayer E.A. Association between early adverse life events and irritable bowel syndrome. Clin Gastroenterol Hepatol. 2012;10(385–390):e381–e383. doi: 10.1016/j.cgh.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D.R., Price L.D. Catecholamines and sympathomimetic drugs decrease early Salmonella Typhimurium uptake into porcine Peyer's patches. FEMS Immunol Med Microbiol. 2008;52:29–35. doi: 10.1111/j.1574-695X.2007.00348.x. [DOI] [PubMed] [Google Scholar]
- Butler J.E., Lager K.M., Splichal I., Francis D., Kacskovics I., Sinkora M. The piglet as a model for B cell and immune system development. Veterinary Immunol Immunopathol. 2009;128:147–170. doi: 10.1016/j.vetimm.2008.10.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler J.E., Weber P., Sinkora M., Sun J., Ford S.J., Christenson R.K. Antibody repertoire development in fetal and neonatal piglets. II. Characterization of heavy chain complementarity-determining region 3 diversity in the developing fetus. J Immunol Baltim Md 1950. 2000;165:6999–7010. doi: 10.4049/jimmunol.165.12.6999. [DOI] [PubMed] [Google Scholar]
- Butler J.E., Wertz N. The porcine antibody repertoire: variations on the textbook theme. Front Immunol. 2012;3:153. doi: 10.3389/fimmu.2012.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camilleri M., Gorman H. Intestinal permeability and irritable bowel syndrome. Neurogastroenterol Motil. 2007;19:545–552. doi: 10.1111/j.1365-2982.2007.00925.x. [DOI] [PubMed] [Google Scholar]
- Catassi C., Bonucci A., Coppa G.V., Carlucci A., Giorgi P.L. Intestinal permeability changes during the first month: effect of natural versus artificial feeding. J Pediatr Gastroenterol Nutr. 1995;21:383–386. doi: 10.1097/00005176-199511000-00003. [DOI] [PubMed] [Google Scholar]
- Chaloner A., Greenwood-Van Meerveld B. Sexually dimorphic effects of unpredictable early life adversity on visceral pain behavior in a rodent model. J Pain. 2013;14:270–280. doi: 10.1016/j.jpain.2012.11.008. [DOI] [PubMed] [Google Scholar]
- Chan C.Y., St John A.L., Abraham S.N. Mast cell interleukin-10 drives localized tolerance in chronic bladder infection. Immunity. 2013;38:349–359. doi: 10.1016/j.immuni.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Lyte M., Stevens M.P., Vulchanova L., Brown D.R. Mucosally-directed adrenergic nerves and sympathomimetic drugs enhance non-intimate adherence of Escherichia coli O157:H7 to porcine cecum and colon. Eur J Pharmacol. 2006;539:116–124. doi: 10.1016/j.ejphar.2006.03.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H.W., Brooking-Dixon R., Neupane S., Lee C.J., Miao E.A., Staats H.F. Salmonella typhimurium impedes innate immunity with a mast-cell-suppressing protein tyrosine phosphatase, SptP. Immunity. 2013;39:1108–1120. doi: 10.1016/j.immuni.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M.E., Sears S.C., Apple J.K., Maxwell C.V., Johnson Z.B. Effect of weaning age and commingling after the nursery phase of pigs in a wean-to-finish facility on growth, and humoral and behavioral indicators of well-being. J Anim Sci. 2006;84:743–756. doi: 10.2527/2006.843743x. [DOI] [PubMed] [Google Scholar]
- De Quelen F., Chevalier J., Rolli-Derkinderen M., Mourot J., Neunlist M., Boudry G. n-3 polyunsaturated fatty acids in the maternal diet modify the postnatal development of nervous regulation of intestinal permeability in piglets. J Physiol. 2011;589:4341–4352. doi: 10.1113/jphysiol.2011.214056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhawan S., Cailotto C., Harthoorn L.F., de Jonge W.J. Cholinergic signalling in gut immunity. Life Sci. 2012;91:1038–1042. doi: 10.1016/j.lfs.2012.04.042. [DOI] [PubMed] [Google Scholar]
- Downing J.E., Miyan J.A. Neural immunoregulation: emerging roles for nerves in immune homeostasis and disease. Immunol Today. 2000;21:281–289. doi: 10.1016/s0167-5699(00)01635-2. [DOI] [PubMed] [Google Scholar]
- Duca F.A., Sakar Y., Covasa M. The modulatory role of high fat feeding on gastrointestinal signals in obesity. J Nutr Biochem. 2013;24:1663–1677. doi: 10.1016/j.jnutbio.2013.05.005. [DOI] [PubMed] [Google Scholar]
- Edelblum K.L., Turner J.R. The tight junction in inflammatory disease: communication breakdown. Curr Opin Pharmacol. 2009;9:715–720. doi: 10.1016/j.coph.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawzy A., Arpadi S., Kankasa C., Sinkala M., Mwiya M., Thea D.M. Early weaning increases diarrhea morbidity and mortality among uninfected children born to HIV-infected mothers in Zambia. J Infect Dis. 2011;203:1222–1230. doi: 10.1093/infdis/jir019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Cabezudo M.J., Lorke D.E., Azimullah S., Mechkarska M., Hasan M.Y., Petroianu G.A. Cholinergic stimulation of the immune system protects against lethal infection by Salmonella enterica serovar typhimurium. Immunology. 2010;130:388–398. doi: 10.1111/j.1365-2567.2009.03238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M. Intestinal ion transport and the pathophysiology of diarrhea. J Clin Invest. 2003;111:931–943. doi: 10.1172/JCI18326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenyo V.L., Pethes G., Antal T., Szabo I. Changes in colostral and serum IgG content in swine in relation to time. Veterinary Res Commun. 1981;4:275–282. doi: 10.1007/BF02278503. [DOI] [PubMed] [Google Scholar]
- Furness J.B. Types of neurons in the enteric nervous system. J Aut Nerv Syst. 2000;81:87–96. doi: 10.1016/s0165-1838(00)00127-2. [DOI] [PubMed] [Google Scholar]
- Gabella G. Neuron size and number in the myenteric plexus of the newborn and adult rat. J Anat. 1971;109:81–95. [PMC free article] [PubMed] [Google Scholar]
- Galli S.J., Grimbaldeston M., Tsai M. Immunomodulatory mast cells: negative, as well as positive, regulators of immunity. Nat Rev Immunol. 2008;8:478–486. doi: 10.1038/nri2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli S.J., Tsai M. IgE and mast cells in allergic disease. Nat Med. 2012;18:693–704. doi: 10.1038/nm.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia A.M., Fadel S.A., Cao S., Sarzotti M. T cell immunity in neonates. Immunol Res. 2000;22:177–190. doi: 10.1385/IR:22:2-3:177. [DOI] [PubMed] [Google Scholar]
- Groschwitz K.R., Hogan S.P. Intestinal barrier function: molecular regulation and disease pathogenesis. J Allergy Clin Immunol. 2009;124:3–20. doi: 10.1016/j.jaci.2009.05.038. quiz 21–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M.J., Frei S.M., Stevens R.L. The multifaceted mast cell in inflammatory bowel disease. Inflamm Bowel Dis. 2014;20:2364–2378. doi: 10.1097/MIB.0000000000000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao M.M., Bornstein J.C., Young H.M. Development of myenteric cholinergic neurons in ChAT-Cre;R26R-YFP mice. J Comp Neurol. 2013;521:3358–3370. doi: 10.1002/cne.23354. [DOI] [PubMed] [Google Scholar]
- Hirota C.L., McKay D.M. Cholinergic regulation of epithelial ion transport in the mammalian intestine. Br J Pharmacol. 2006;149:463–479. doi: 10.1038/sj.bjp.0706889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda K., Littman D.R. The microbiota in adaptive immune homeostasis and disease. Nature. 2016;535:75–84. doi: 10.1038/nature18848. [DOI] [PubMed] [Google Scholar]
- Hu C.H., Xiao K., Luan Z.S., Song J. Early weaning increases intestinal permeability, alters expression of cytokine and tight junction proteins, and activates mitogen-activated protein kinases in pigs. J Anim Sci. 2013;91:1094–1101. doi: 10.2527/jas.2012-5796. [DOI] [PubMed] [Google Scholar]
- Kelly J.R., Kennedy P.J., Cryan J.F., Dinan T.G., Clarke G., Hyland N.P. Breaking down the barriers: the gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front Cell Neurosci. 2015;9:392. doi: 10.3389/fncel.2015.00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch R., Riddell R.H. Histopathological alterations in irritable bowel syndrome. Mod Pathol. 2006;19:1638–1645. doi: 10.1038/modpathol.3800704. [DOI] [PubMed] [Google Scholar]
- Kobayashi H., Yamataka A., Fujimoto T., Lane G.J., Miyano T. Mast cells and gut nerve development: implications for Hirschsprung's disease and intestinal neuronal dysplasia. J Pediatr Surg. 1999;34:543–548. doi: 10.1016/s0022-3468(99)90069-6. [DOI] [PubMed] [Google Scholar]
- Lake J.I., Heuckeroth R.O. Enteric nervous system development: migration, differentiation, and disease. Am J Physiol Gastrointest Liver Physiol. 2013;305:G1–G24. doi: 10.1152/ajpgi.00452.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Song Z., Kerr K.A., Moeser A.J. Chronic social stress in pigs impairs intestinal barrier and nutrient transporter function, and alters neuro-immune mediator and receptor expression. PLoS One. 2017;12:e0171617. doi: 10.1371/journal.pone.0171617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little T.J., Feinle-Bisset C. Effects of dietary fat on appetite and energy intake in health and obesity–oral and gastrointestinal sensory contributions. Physiol Behav. 2011;104:613–620. doi: 10.1016/j.physbeh.2011.04.038. [DOI] [PubMed] [Google Scholar]
- Lundgren O. Enteric nerves and diarrhoea. Pharmacol Toxicol. 2002;90:109–120. doi: 10.1034/j.1600-0773.2002.900301.x. [DOI] [PubMed] [Google Scholar]
- Maccari S., Krugers H.J., Morley-Fletcher S., Szyf M., Brunton P.J. The consequences of early-life adversity: neurobiological, behavioural and epigenetic adaptations. J Neuroendocrinol. 2014;26:707–723. doi: 10.1111/jne.12175. [DOI] [PubMed] [Google Scholar]
- Mackey E.M., Ayyadurai S., Pohl C.S., D'Costa S.D., Li Y., Moeser A.J. Sexual dimorphism in the mast cell transcriptome and the pathophysiological responses to immunological and psychological stress. Biol Sex Differ. 2016;7:60. doi: 10.1186/s13293-016-0113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Main R.G., Dritz S.S., Tokach M.D., Goodband R.D., Nelssen J.L. Increasing weaning age improves pig performance in a multisite production system. J Anim Sci. 2004;82:1499–1507. doi: 10.2527/2004.8251499x. [DOI] [PubMed] [Google Scholar]
- Marchiando A.M., Graham W.V., Turner J.R. Epithelial barriers in homeostasis and disease. Annu Rev pathology. 2010;5:119–144. doi: 10.1146/annurev.pathol.4.110807.092135. [DOI] [PubMed] [Google Scholar]
- Martin R., Nauta A.J., Ben Amor K., Knippels L.M., Knol J., Garssen J. Early life: gut microbiota and immune development in infancy. Benef Microbes. 2010;1:367–382. doi: 10.3920/BM2010.0027. [DOI] [PubMed] [Google Scholar]
- Matteoli G., Boeckxstaens G.E. The vagal innervation of the gut and immune homeostasis. Gut. 2013;62:1214–1222. doi: 10.1136/gutjnl-2012-302550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken B.A., Spurlock M.E., Roos M.A., Zuckermann F.A., Gaskins H.R. Weaning anorexia may contribute to local inflammation in the piglet small intestine. J Nutr. 1999;129:613–619. doi: 10.1093/jn/129.3.613. [DOI] [PubMed] [Google Scholar]
- McLamb B.L., Gibson A.J., Overman E.L., Stahl C., Moeser A.J. Early weaning stress in pigs impairs innate mucosal immune responses to enterotoxigenic E. coli challenge and exacerbates intestinal injury and clinical disease. PLoS One. 2013;8:e59838. doi: 10.1371/journal.pone.0059838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medland J.E., Pohl C.S., Edwards L.L., Frandsen S., Bagley K., Li Y. Early life adversity in piglets induces long-term upregulation of the enteric cholinergic nervous system and heightened, sex-specific secretomotor neuron responses. Neurogastroenterol Motil. 2016;9:1317–1329. doi: 10.1111/nmo.12828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeser A.J., Blikslager A.T. Mechanisms of porcine diarrheal disease. J Am Vet Med Assoc. 2007;231:56–67. doi: 10.2460/javma.231.1.56. [DOI] [PubMed] [Google Scholar]
- Moeser A.J., Klok C.V., Ryan K.A., Wooten J.G., Little D., Cook V.L. Stress signaling pathways activated by weaning mediate intestinal dysfunction in the pig. Am J Physiol Gastrointest Liver Physiol. 2007;292:G173–G181. doi: 10.1152/ajpgi.00197.2006. [DOI] [PubMed] [Google Scholar]
- Moeser A.J., Ryan K.A., Nighot P.K., Blikslager A.T. Gastrointestinal dysfunction induced by early weaning is attenuated by delayed weaning and mast cell blockade in pigs. Am J Physiol Gastrointest Liver Physiol. 2007;293:G413–G421. doi: 10.1152/ajpgi.00304.2006. [DOI] [PubMed] [Google Scholar]
- Montagne L., Boudry G., Favier C., Le Huerou-Luron I., Lalles J.P., Seve B. Main intestinal markers associated with the changes in gut architecture and function in piglets after weaning. Br J Nutr. 2007;97:45–57. doi: 10.1017/S000711450720580X. [DOI] [PubMed] [Google Scholar]
- Newburg D.S., Walker W.A. Protection of the neonate by the innate immune system of developing gut and of human milk. Pediatr Res. 2007;61:2–8. doi: 10.1203/01.pdr.0000250274.68571.18. [DOI] [PubMed] [Google Scholar]
- Nguyen M., Leuridan E., Zhang T., De Wit D., Willems F., Van Damme P. Acquisition of adult-like TLR4 and TLR9 responses during the first year of life. PLoS One. 2010;5:e10407. doi: 10.1371/journal.pone.0010407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overman E.L., Rivier J.E., Moeser A.J. CRF induces intestinal epithelial barrier injury via the release of mast cell proteases and TNF-alpha. PLoS One. 2012;7:e39935. doi: 10.1371/journal.pone.0039935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabst R., Geist M., Rothkotter H.J., Fritz F.J. Postnatal development and lymphocyte production of jejunal and ileal Peyer's patches in normal and gnotobiotic pigs. Immunology. 1988;64:539–544. [PMC free article] [PubMed] [Google Scholar]
- Park S.H., Videlock E.J., Shih W., Presson A.P., Mayer E.A., Chang L. Adverse childhood experiences are associated with irritable bowel syndrome and gastrointestinal symptom severity. Neurogastroenterol Motil. 2016;8:1252–1260. doi: 10.1111/nmo.12826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R.M., Myers L.S., Kurundkar A.R., Maheshwari A., Nusrat A., Lin P.W. Probiotic bacteria induce maturation of intestinal claudin 3 expression and barrier function. Am J Pathol. 2012;180:626–635. doi: 10.1016/j.ajpath.2011.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peace R.M., Campbell J., Polo J., Crenshaw J., Russell L., Moeser A. Spray-dried porcine plasma influences intestinal barrier function, inflammation, and diarrhea in weaned pigs. J Nutr. 2011;141:1312–1317. doi: 10.3945/jn.110.136796. [DOI] [PubMed] [Google Scholar]
- Pidsudko Z., Kaleczyc J., Wasowicz K., Sienkiewicz W., Majewski M., Zajac W. Distribution and chemical coding of intramural neurons in the porcine ileum during proliferative enteropathy. J Comp Pathology. 2008;138:23–31. doi: 10.1016/j.jcpa.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Pohl C.S., Medland J.E., Mackey E., Edwards L.L., Bagley K.D., DeWilde M.P. Early weaning stress induces chronic functional diarrhea, intestinal barrier defects, and increased mast cell activity in a porcine model of early life adversity. Neurogastroenterol Motil. 2017 doi: 10.1111/nmo.13118. http://dx.doi.org/10.1111/nmo.13118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl C.S., Medland J.E., Moeser A.J. Early life stress origins of gastrointestinal disease: animal models, intestinal pathophysiology, and translational implications. Am J Physiol Gastrointest Liver Physiol. 2015;309:G927–G941. doi: 10.1152/ajpgi.00206.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomorska-Mol M., Markowska-Daniel I. Interferon-gamma secretion and proliferative responses of peripheral blood mononuclear cells after vaccination of pigs against Aujeszky's disease in the presence of maternal immunity. FEMS Immunol Med Microbiol. 2010;58:405–411. doi: 10.1111/j.1574-695X.2010.00651.x. [DOI] [PubMed] [Google Scholar]
- Rogier E.W., Frantz A.L., Bruno M.E., Wedlund L., Cohen D.A., Stromberg A.J. Secretory antibodies in breast milk promote long-term intestinal homeostasis by regulating the gut microbiota and host gene expression. Proc Natl Acad Sci U S A. 2014;111:3074–3079. doi: 10.1073/pnas.1315792111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez M.M., Ladd C.O., Plotsky P.M. Early adverse experience as a developmental risk factor for later psychopathology: evidence from rodent and primate models. Dev Psychopathol. 2001;13:419–449. doi: 10.1017/s0954579401003029. [DOI] [PubMed] [Google Scholar]
- Sasselli V., Pachnis V., Burns A.J. The enteric nervous system. Dev Biol. 2012;366:64–73. doi: 10.1016/j.ydbio.2012.01.012. [DOI] [PubMed] [Google Scholar]
- Schafer K.H., Hansgen A., Mestres P. Morphological changes of the myenteric plexus during early postnatal development of the rat. Anat Rec. 1999;256:20–28. doi: 10.1002/(SICI)1097-0185(19990901)256:1<20::AID-AR4>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Schiering C., Krausgruber T., Chomka A., Frohlich A., Adelmann K., Wohlfert E.A. The alarmin IL-33 promotes regulatory T-cell function in the intestine. Nature. 2014;513:564–568. doi: 10.1038/nature13577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber K.L., Brown D.R. Adrenocorticotrophic hormone modulates Escherichia coli O157:H7 adherence to porcine colonic mucosa. Stress. 2005;8:185–190. doi: 10.1080/10253890500188732. [DOI] [PubMed] [Google Scholar]
- Smith F., Clark J.E., Overman B.L., Tozel C.C., Huang J.H., Rivier J.E. Early weaning stress impairs development of mucosal barrier function in the porcine intestine. Am J Physiol Gastrointest Liver Physiol. 2010;298:G352–G363. doi: 10.1152/ajpgi.00081.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner J.R. Intestinal mucosal barrier function in health and disease. Nature reviews. Immunology. 2009;9:799–809. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- Turner J.R., Rill B.K., Carlson S.L., Carnes D., Kerner R., Mrsny R.J. Physiological regulation of epithelial tight junctions is associated with myosin light-chain phosphorylation. Am J Physiol. 1997;273:C1378–C1385. doi: 10.1152/ajpcell.1997.273.4.C1378. [DOI] [PubMed] [Google Scholar]
- Upham J.W., Rate A., Rowe J., Kusel M., Sly P.D., Holt P.G. Dendritic cell immaturity during infancy restricts the capacity to express vaccine-specific T-cell memory. Infect Immun. 2006;74:1106–1112. doi: 10.1128/IAI.74.2.1106-1112.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wester T., O'Briain D.S., Puri P. Notable postnatal alterations in the myenteric plexus of normal human bowel. Gut. 1999;44:666–674. doi: 10.1136/gut.44.5.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijtten P.J., van der Meulen J., Verstegen M.W. Intestinal barrier function and absorption in pigs after weaning: a review. Br J Nutr. 2011;105:967–981. doi: 10.1017/S0007114510005660. [DOI] [PubMed] [Google Scholar]
- Wojtkiewicz J., Rowniak M., Gonkowski S., Crayton R., Majewski M., Robak A. Proliferative enteropathy (PE)-induced changes in the calbindin-immunoreactive (CB-IR) neurons of inferior mesenteric ganglion supplying the descending colon in the pig. J Mol Neurosci MN. 2012;48:757–765. doi: 10.1007/s12031-011-9691-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood J.D. Enteric nervous system: sensory physiology, diarrhea and constipation. Curr Opin Gastroenterol. 2010;26:102–108. doi: 10.1097/MOG.0b013e328334df4f. [DOI] [PubMed] [Google Scholar]
- Wouters M.M., Vicario M., Santos J. The role of mast cells in functional GI disorders. Gut. 2016;65:155–168. doi: 10.1136/gutjnl-2015-309151. [DOI] [PubMed] [Google Scholar]
- Wu H.J., Wu E. The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes. 2012;3:4–14. doi: 10.4161/gmic.19320. [DOI] [PMC free article] [PubMed] [Google Scholar]