Abstract
Epigenetic modulation is critical for regulating the development and function of T cells. In this issue of Immunity, DuPage et al. (2015) show that the chromatin-modifying enzyme Ezh2 induced by CD28 costimulation is essential for regulatory T (Treg) cell maintenance during activation and differentiation.
Regulatory T (Treg) cells are a specialized T cell subset critical for immune maintenance and homeostasis and considered to be the guardian of life. It is well established that the transcription factor Foxp3 defines the functions of Treg cells. T cell receptor (TCR), costimulatory, and cytokine signals, however, are also critical for their development. Here DuPage et al. suggest that CD28 signals induce the chromatin-remodeling enzyme Ezh2 during the Treg cell development, which regulates Treg cell activation and function (DuPage et al., 2015).
Ezh2, the histone methyltransferase of multi-subunit polycomb repressive complex 2 (PRC2), is an essential epigenetic regulator of development of multiple cell types in mice and functions as a key factor for regulating histone H3 methylation of lysine 27 (H3K27me3) in both B cells and T cells (O’Carroll et al., 2001; Su et al., 2003). During normal development of Tregs cells, the position and frequency of H3K27me3 across the span of genes within Treg cells is different compared with other T cell subsets. Ezh2 is recruited by Foxp3 during Treg cell development and not only controls Foxp3 expression (Arvey et al., 2014) but also genomic landscape as reported in the study highlighted here (DuPage et al., 2015).
By performing cDNA transcript analysis on human naive CD4+ T cells co-stimulated in the presence of CD28, the authors showed that Ezh2 was the most highly expressed gene among different chromatin modifiers. The induction of Ezh2 by CD28 co-stimulation was further confirmed in murine Treg cells, which correlated with Ezh2 enzymatic activity and abundance of H3K27me3 histone marks. Furthermore, higher amounts of Ezh2 expression and H3K27me3 were observed in the activated Treg cell compartment defined by expression of CD44 and CD62L (CD44+CD62L−), versus the resting CD44−CD62L+ Treg cells, suggesting that the expression of Ezh2 coordinates Treg cell activation and potentially regulates Treg cell functions.
By generating Ezh2 Treg cell-specific deficient (Treg.Ezh2Δ/Δ) mice, the authors observed enhanced frequency of Treg cells in lymph nodes and thymus, along with higher expression of surface proteins such as CTLA-4, PD-1, CD103, and GITR on the Tregs of the aged Treg.Ezh2Δ/Δ mice. However, the in vitro analysis of Treg cells from the Treg.Ezh2Δ/Δ mice did not show enhanced Treg cell suppressive capacity in CD62Lhi cells.
In contrast to the in vitro data, further in vivo investigation on the Treg.Ezh2Δ/Δ mice showed that there was a higher frequency of activated CD4+ and CD8+ T cells, together with enhanced lymph node cellularity. More importantly, the Treg.Ezh2Δ/Δ mice developed systemic autoimmune and inflammatory phenotype, which was caused by massive lymphocytic infiltration. To further address whether it is a cell intrinsic effect of Ezh2, the authors introduced Foxp3Yfp-cre knockin allele to the female Ezh2fl/fl mice. Due to random X-inactivation, the same amount of normal and Ezh2- deficient Treg cells could be found in the Foxp3Yfp-cre/WT Ezh2fl/fl mice. Under such settings, these mice exhibited normal immune status as the wild-type (WT) mice, suggesting the WT Treg (Ezh2fl/fl) cells overcame the deficiency of losing Ezh2 (Treg.Ezh2Δ/Δ) in the Treg cells. Then the authors refined the experiments by introducing the Foxp3DTR allele together with the Foxp3Yfp-cre allele into the Ezh2fl/fl mice (Foxp3Yfp-cre/DTR Ezh2fl/fl). In this way, the Treg cells carrying diphtheria toxin receptor (DTR) with WT phenotype could be deleted by diphtheria toxin (DT) treatment so that the immediate effect of immune regulation in the mice could be controlled by the Ezh2-deficient Treg cells that remained in the repertoire. Indeed, after WT Treg cell depletion, these mice developed similar pathological symptoms as Treg.Ezh2Δ/Δ mice.
The authors then analyzed Treg cells in non-lymphoid tissues including skin, lung, liver, and pancreas. The reduction of Foxp3+ Ezh2-deficient Treg cells was seen in all of these tissues. The authors then utilized the Foxp3 fate-mapping mouse as previously reported (Komatsu et al., 2014). Using this strategy, the Treg.Ezh2Δ/Δ mice were first observed to have more newly generated Treg (CD4+GFP+RFP−) cells originated from the thymus compared to the controls. More importantly, these mice exhibited an increased frequency of “Ex-Treg” (CD4+GFP−RFP+) cells within all tissues, particularly in non-lymphoid tissues. Interestingly, all the Ex-Treg cells were found to display the activated phenotype (CD44+CD62L−), indicating the critical role of Ezh2 for both activation and stability of Treg cells.
In order to understand the molecular mechanism by which Ezh2 regulates Treg cell functions, DuPage et al. performed RNA-seq by employing a sophisticated sorting strategy. They isolated different cell populations including CD62L+ or CD62L− Ezh2Δ/Δ and control Treg cells from Foxp3Yfp-cre/WT; Ezh2fl/+ or Foxp3Yfp-cre/WT; Ezh2fl/fl mice. Between the WT and Ezh2-deficient Treg cells, the most gene-expression differences were found within the activated (CD62L−) Treg cell populations. The genes that were overexpressed in Ezh2-deficient Treg cells were found to be repressed in CD62L− T cells. These genes were associated with a decrease in H3K27me3 level by cross-referencing the RNA-seq data with the previous dataset by Arvey et al. (2014). Furthermore, the comparison between the Ezh2-deficient and Foxp3- deficient Treg cells revealed an overlap of differentially expressed genes (DEG) within the CD62L− cell compartment. These data suggest that the Ezh2-mediated H3K27me3 is not only important for Foxp3-dependent Treg cell program but also for maintaining Treg cell activation program.
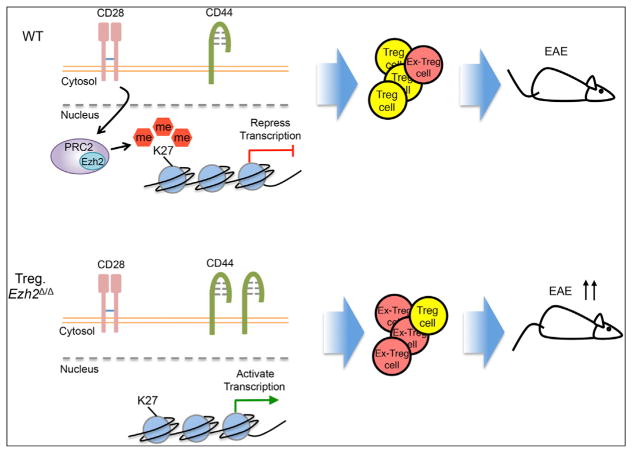
Finally, the authors tested the impact of loss of Ezh2 in Treg cells in vivo using a murine model of experimental autoimmune encephalomyelitis (EAE). Treg. Ezh2Δ/Δ mice developed more severe disease than the control mice with reduction in the frequency of Treg cells in the central nervous system (CNS). The Ezh2- deficient Treg cells could not control the disease because of decreased stability of Foxp3, consistent with the data in the naive mice. Employing the antigen tetramers further showed less antigen-specific Treg cells in the absence of Ezh2 within the CNS at the peak of the disease (Figure 1).
Figure 1. Ezh2 Regulates Function and Stability of Treg Cells.
(Top) Within activated (CD44+) WT Treg cells, CD28 signal enhances the expression of Ezh2, leading to the deposition of genomic H3K27me3 and the repression of transcription of genes that inhibit Treg cell development. Induction of Ezh2 maintains the expression of Foxp3 and stabilizes the identity of Treg cells (shown in yellow). Therefore, the WT Treg cells exert strong suppressive function even in the face of tissue inflammation and during autoimmune reaction in EAE.
(Bottom) In contrast to WT Treg cells, the Ezh2-deficient activated Treg cells fail to promote the repressive gene program for Treg cell development and display a more activated phenotype (CD44+). Therefore, the Ezh2-deficient mice develop more systemic autoimmune reactions with more “Ex-Treg” cells (shown in red), which lose Foxp3 expression and have no suppressive function. The mice show a defect in Treg cell function and massive infiltration of effector and Ex-Tregs in multiple organs. Finally, the absence of Ezh2 within Treg cells leads to the development of more severe EAE compared to WT mice, and the Treg cells lose their identity and function.
This study provides detailed characterization and comprehensive analysis of the effect on loss of an enzyme Ezh2 in Treg cell maintenance and function, using both in vitro and in vivo functional assays, genomic computational analysis, and autoimmune disease models to analyze the impact of the Ezh2 enzyme on the development of Treg cells. The authors demonstrated that Ezh2, as an epigenetic modifier, is a key factor required in Treg cell activation and stabilization. The paper links Treg cell induction by CD28 costimulation with chromatin modification, which reconciles the old data showing a critical role of CD28 costimulation in the generation and function of Foxp3+ Treg cells (Tang et al., 2003).
Activated Treg cells, which are induced in the peripheral or secondary lymphoid organs, are found to have enhanced ability to migrate and mediate suppression. In comparison to the resting Treg cells, activated Treg cells show distinct surface markers, chemokine receptors, gene expression, and epigenetic modifications, although Foxp3 expression is essential in the maintenance of both resting and activated Treg cells. As reported by DuPage et al., the deficiency of Ezh2 destabilized Treg cells only in the activated pool, suggesting the dynamic epigenetic regulation in Treg cells, during activation and in various microenvironments. Moreover, Treg cells change their phenotype and function in different inflammatory conditions and tissue environments and become specialized to mediate their specific effector in defined tissues (Burzyn et al., 2013; Cipolletta et al., 2012). The requirement of Ezh2 in Treg cell populations in non-lymphoid tissues suggests that Ezh2 might also play an important role in inducing genomic and transcriptional changes in tissue Tregs. In addition, Treg cells were also found to be inactivated during acute inflammation (Fontenot et al., 2005). This study raises an interesting issue of whether Treg cell destabilization observed at the sites of acute inflammation is due to downregulation or inactivation of Ezh2 by certain tissue cues and inflammatory stimuli.
The function of Ezh2 has been well characterized in cancer. Either hyperactivation or mutations are found in a variety of malignancies (Simon and Lange, 2008). Ezh2 was now shown to participate in the regulation of Treg cell activation and T helper 1 (Th1) and Th2 cell differentiation. Thus, it is worthwhile to study the application of several potent Ezh2 inhibitors developed in the research of cancer for the treatment of autoimmune diseases. The predominant role of Ezh2 in many cell types implicates a common mechanism of regulation of H3K27me3 status, due to activation or costimulation, in coordination of gene expression and cell function.
References
- Arvey A, van der Veeken J, Samstein RM, Feng Y, Stamatoyannopoulos JA, Rudensky AY. Nat Immunol. 2014;15:580–587. doi: 10.1038/ni.2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burzyn D, Kuswanto W, Kolodin D, Shadrach JL, Cerletti M, Jang Y, Sefik E, Tan TG, Wagers AJ, Benoist C, Mathis D. Cell. 2013;155:1282–1295. doi: 10.1016/j.cell.2013.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipolletta D, Feuerer M, Li A, Kamei N, Lee J, Shoelson SE, Benoist C, Mathis D. Nature. 2012;486:549–553. doi: 10.1038/nature11132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuPage M, Chopra G, Quiros J, Rosenthal WL, Morar MM, Holohan D, Zhang R, Turka L, Marson A, Bluestone JA. Immunity. 2015;42(this issue):227–238. doi: 10.1016/j.immuni.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Komatsu N, Okamoto K, Sawa S, Nakashima T, Oh-hora M, Kodama T, Tanaka S, Bluestone JA, Takayanagi H. Nat Med. 2014;20:62–68. doi: 10.1038/nm.3432. [DOI] [PubMed] [Google Scholar]
- O’Carroll D, Erhardt S, Pagani M, Barton SC, Surani MA, Jenuwein T. Mol Cell Biol. 2001;21:4330–4336. doi: 10.1128/MCB.21.13.4330-4336.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon JA, Lange CA. Mutat Res. 2008;647:21–29. doi: 10.1016/j.mrfmmm.2008.07.010. [DOI] [PubMed] [Google Scholar]
- Su IH, Basavaraj A, Krutchinsky AN, Hobert O, Ullrich A, Chait BT, Tarakhovsky A. Nat Immunol. 2003;4:124–131. doi: 10.1038/ni876. [DOI] [PubMed] [Google Scholar]
- Tang Q, Henriksen KJ, Boden EK, Tooley AJ, Ye J, Subudhi SK, Zheng XX, Strom TB, Bluestone JA. J Immunol. 2003;171:3348–3352. doi: 10.4049/jimmunol.171.7.3348. [DOI] [PubMed] [Google Scholar]