Abstract
To evaluate the immunomodulating responses in intestine, spleen and liver, 50–200 mg/kg of DHP was orally administrated to mice without or with methotrexate. The proliferation of marrow cells, which was performed with the addition of the supernatant of small intestinal lymphocytes isolated from the mice administrated orally with DHP, showed that the intestinal immune response was significantly enhanced in all DHP-treated groups. For the immune response in spleen, all tested doses of DHP remarkably promoted the proliferation of splenic cells and increased the secretion of interferon-γ (IFN-γ). For the immune responses in liver, DHP not only significantly stimulated the proliferation of hepatic cells and the secretion of IFN-γ at all tested doses of DHP, but also significantly elevated the secretion interleukin-4 (IL-4) at the doses of 100 and 200 mg/kg. Moreover, DHP could recover methotrexate-injured small intestinal immune function (100 and 200 mg/kg) and promoted cell proliferation and IFN-γ production (200 mg/kg) in spleen and liver of methotrexate-treated mice. These results suggested that DHP after oral administration possessed immunomodulating effects both in small intestine immune system and in systemic immune system, which were further proved by the mRNA expression of IFN-γ and IL-4.
Keywords: Dendrobium huoshanense, Polysaccharides, Immune response
1. Introduction
The stems of Dendrobium huoshanense C.Z. Tang et S.J. Cheng (Orchidaceae) have been used as a food material to make tea, soup and porridge in many Southeast Asian countries for centuries [1]. In recent years, this plant has been reported to have good potential for improving chronic superficial gastritis, throat inflammation, immune deficiencies, yin-yang disharmony problems and weak eyesight [1,2]. To understand these health functions, different compounds including alkaloids, trace elements, free amino acids and polysaccharides have been isolated from D. huoshanense and their biological activities have been determined [3]. Among these compounds, the polysaccharides have been reported to possess a variety of pharmacological activities. Using Sprague-Dawley rats, the water-soluble polysaccharides from D. huoshanense were demonstrated to attenuate the streptozotocin-induced cataract [2] and protect liver from sodium selenite-induced damage [4]. With in vitro experiments, the polysaccharides from D. huoshanense were reported to stimulate murine macrophages and splenocytes to secrete the various cytokines, including interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6) and interleukin-1α (IL-1α) [5,6], suggesting that D. huoshanense polysaccharides have the capability of modulating immune functions. However, in vivo immune responses caused by oral administration of D. huoshanense polysaccharides still remain to be investigated. Since the stems of D. huoshanense are traditionally taken orally and its beneficial effects on body including the activities of anti-cataract and liver injury protection are suggested to be related to the regulation of immune functions [1,2,4], it was necessary to investigate the immune responses following the oral administration of polysaccharides from D. huoshanense for its use in functional foods.
In our previous work, the water-soluble polysaccharide (DHP) with immunostimulating activity has been isolated from the stems of D. huoshanense and proved to be composed of galactoglucomannan by the identification of chemical structures using GC–MS and NMR techniques [7]. In the present study, the immune responses occurring in mouse small intestine, spleen and liver to the oral administration of DHP would be reported. First, the different doses of DHP were orally administrated to mice without or with methotrexate, a reagent of inducing intestinal injury. Then, the small intestinal immune activity as well as the cellular proliferation and cytokine expression of spleen and liver were measured to evaluate the immunomodulating effects of orally administrated DHP.
2. Materials and methods
2.1. Plant materials and reagents
D. huoshanense was collected from Dabieshan mountain region in China and propagated under controlled conditions [7]. Voucher specimen (No. DHP0001) was identified by Professor Zhong-Ze Zhou who worked in Anhui University of China.
Methotrexate was from Pfizer (Perth) Pty Ltd. (Bentley, West Australia, Australia). IFN-γ and IL-4 ELISA kits were from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Mouse lipopolysaccharides (LPS) ELISA kit was purchased from R&D System China Co. Ltd. (Shanghai, China). RevertAid first strand cDNA synthesis kit and RNAiso plus kit were from Thermo Scientific (Waltham, Massachusetts, USA) and TaKaRa Biotechnology Co. Ltd. (Dalian, China), respectively. Primers were obtained from Sangon Biotech Co. Ltd (Shanghai, China). Dextran T20 with molecular weight 2.0 × 104 Da was purchased from Duly Biotechnology Co. Ltd. (Nanjing, China). All other reagents were analytical grade.
2.2. Preparation of D. huoshanense polysaccharides (DHP) and its hydrolyzates
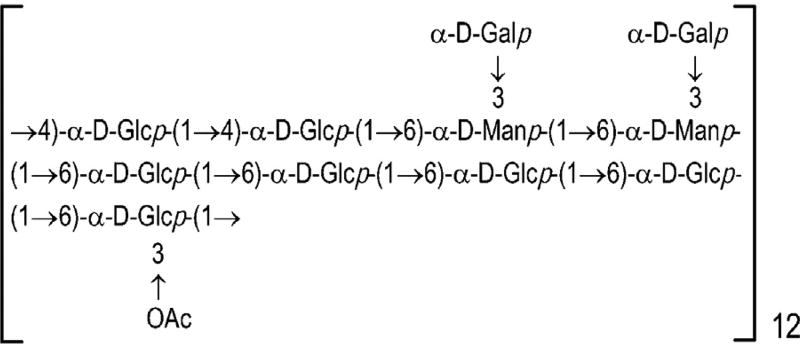
The fresh materials of D. huoshanense were used to prepare DHP by the method of hot water extraction and ethanol precipitation as described previously [7]. In short, the powdered materials were extracted with distilled water at 70 °C for 2 h (the ratio of materials to water was 1:3 g/mL). Extracts from two repeats after combination, filtration, concentration and centrifugation (1000 rpm for 10 min) were precipitated by the addition of ethanol to a final concentration of 80% (v/v). The precipitates were then dissolved in distilled water and deproteinized by Sevag method [8] to give polysaccharide extract, which was further fractionated by ultra-filtration membrane (molecular weight cut off: 2000 Da) and lyophilized. The resulting preparation was DHP, which mainly consisted of immunoregulatory galactoglucomannan (Fig. 1) with molecular weight 2.2 × 104 Da [7]. No toxicity and mutagenicity were observed with DHP [9]. For the preparation of DHP hydrolyzates, DHP was hydrolyzed with 2 M trifluoroacetic acid (TFA) at 110 °C for 3 h, evaporated to remove TFA and lyophilized. Both DHP and its hydrolysates were dissolved in normal saline solution for animal experiments. Endotoxin contamination was neglected because endotoxin levels were determined to be below the detection limit of 5 ng/mL with LPS ELISA kit.
Fig. 1.
The primary structure of in DHP.
2.3. Animal experiment
Male Kunming mice (25 ± 2 g) were purchased from the Experimental Animal Center in Anhui Medical University (Hefei, China). All mice were fed with standard chows and distilled water in an air-conditioned animal room (25 ± 2 °C, 50 ± 5% relative humidity and 12 h light–dark cycle). All animal handling procedures (used in this experiment) were performed strictly in accordance with the PR China Legislation on the Use and Care of Laboratory Animals. After being allowed to acclimatize for 7 days, the mice were randomly divided into seven groups (ten mice each): group 1 was orally administrated with solvent (normal saline solution), group 2 with 200 mg/kg of body weight (b.wt.) dextran, group 3 with 200 mg/kg b.wt. starch, group 4 with 200 mg/kg b.wt. hydrolyzates of DHP, group 5 with 200 mg/kg b.wt. DHP, group 6 with 100 mg/kg b.wt. DHP, and group 7 with 50 mg/kg b.wt. DHP. All groups were treated for 15 days. Then, the mice were euthanized and their small intestine, liver and spleen were collected for the following experiments.
To determine the effects of methotrexate, 50 male Kunming mice were divided into 5 groups including normal control, methotrexate treatment, methotrexate treatment in combination with 200, 100 and 50 mg/kg b.wt. of DHP. Methotrexate treatment was performed with a dose of 20 mg/kg b.wt. every three days starting from the first day. Other administration was the same as described above.
2.4. Determination of small intestinal immune activity
The effect of DHP on small intestinal immune activity was measured following the procedure described in the Refs. [10,11] with a minor modification. Briefly, the intestinal lymphocytes were isolated from the small intestine containing Peyer’s patches of mice and suspended into ice cold RPMI 1640 medium (containing 10% fetal bovine serum, 100 U/mL penicillin and 100 µg/mL streptomycin) at a density of 1 × 107 cells/mL. The cell suspension was cultured in the presence of 5 µg/mL Con A at 37 °C under an atmosphere of 5% CO2 for 3 days. Bone marrow cells were prepared from Kunming mice by excising femora and washing bone marrow cells using a 23-gauge needle in complete RPMI 1640 medium. The bone marrow cells were then washed and adjusted to 2.5 × 105 cells/mL with complete RPMI 1640 medium. Twenty microlitres of culture supernatant of small intestinal lymphocytes were added to 180 µL of bone marrow cell suspension to co-culture for 6 days under the same conditions as described above. The proliferation of bone marrow cells was measured with methylthiazoletetrazolium (MTT) method to evaluate small intestine immune activity [12]. To study the in vitro effect of DHP on small intestine immune activity, the small intestinal lymphocytes were isolated from the small intestine of untreated mice and were cultured at a density of 1 × 107 cells/mL either with or without DHP at different concentrations for 3 days. The resulting supernatant was used to stimulate the proliferation of bone marrow cells at initial cell density of 2.5 × 106 cells/mL and 1.25 × 106 cells/mL as described above.
2.5. Proliferation assay of splenic and hepatic cells
After the mice were sacrificed, the growth indices of spleen and liver were calculated, respectively. Subsequently, the spleen or the liver was exposed on a culture dish containing 5–10 mL ice cold Hank’s Balanced Salt Mixture (HBSS) and dissected into small pieces. Then, the splenic or hepatic cells were obtained by tapping gently tissue pieces with a rod on a 200-gauge sterile stainless sieve. The collected cells were centrifugated (4 °C, 1000 rpm) for 5 min and treated with 1.0% NH4Cl solution to lyse red blood cells. After centrifugated and washed with the cold HBSS twice, the cells were suspended in ice cold RPMI1640 medium at a density of 1 × 105 cells/mL. The cellular number and viability were determined by trypan blue assay. Cell suspension (195 µL) and 5 µL of Con A (200 µg/mL) were mixed and cultured in the CO2 incubator (the atmosphere of 5% CO2) at 37 °C for 48 h. Finally, MTT method was used to measure the proliferation of splenic or hepatic cells [12].
2.6. Measurements of cytokines in liver and spleen
Cultured splenic or hepatic cells as described above were centrifugated at 2000 rpm for 10 min and the resulting supernatant was used to measure cytokines IFN-γ and IL-4 by the ELISA kits according to the manufacturer’s instructions.
2.7. RT-PCR assays
Total RNA was isolated from aforementioned splenic or hepatic cells with RNAiso plus kit. Reverse transcription reaction was performed with RevertAid first strand cDNA synthesis kit according to the manufacturer’s protocol. PCR was performed on a GeneAmp PCR system (Applied Biosystems, Foster, California, USA). The temperature of initial denaturation remained at 95 °C for 5 min, and the cycles (denaturation at 95 °C for 30 s, annealing at 54 °C for 30 s, and extension at 72 °C for 60 s) were performed 30 times. The primers used in the PCR were as follows: 5′-AAAGAGATAATCTGGCTCTGC-3′ (upper primer) and 5′-GCTCTGAGACAATGAACGTC-3′ (lower primer) for IFN-γ, 5′-TGATGGGTC TCAGCCCCCACCT-3′ (upper primer) and 5′-CTTTCAGTGTTGTGAGCGTG GACTC-3′ (low primer) for IL-4, and 5′-AGGGAAATCGTGGGTGACATCAAA-3′ (upper primer) and 5′-ACTCATCGTACTCCTGCTTGCTGA-3′ (lower primer) for β-actin. The resulting PCR products were analyzed by agarose gel electrophoresis (1%, g/mL) and stained by ethidium bromide (EB).
2.8. Statistical analysis
The data were analyzed by the SPSS software (version 16.0, Chicago, USA). The values were expressed as mean ± standard deviation (SD). The differences between the experimental groups and the control group were analyzed using one-way analysis of variance (ANOVA) and Dunnett’s t-test.
3. Results
3.1. Small intestinal immune activity regulated by DHP
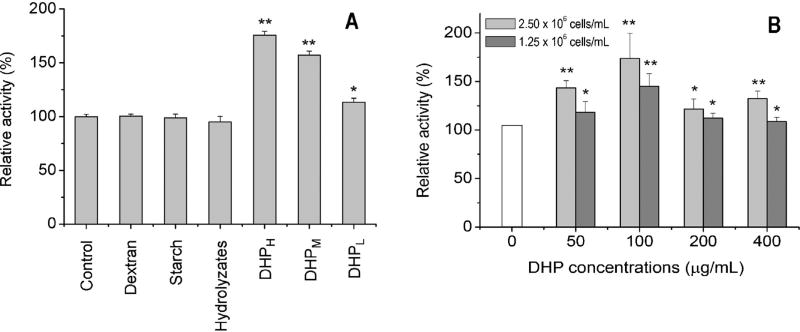
The oral administration of DHP exhibited the potential to stimulate small intestinal immune activity as evidenced by the proliferation of bone marrow cells (Fig. 2A). In response to the different doses (50, 100 and 200 mg/kg), the proliferation of bone marrow cells increased to 113%, 160% and 176% of the control group, respectively. On the contrary, other polysaccharides including dextran and starch did not show any stimulating effect on intestinal immune activity as compared to the control group. Furthermore, when DHP was completely hydrolyzed, the hydrolyzates lost the immunostimulating activity. DHP was also employed to directly stimulate small intestinal lymphocytes isolated from mice without oral administration of DHP. The resulting supernatant in different doses of DHP significantly enhanced the proliferation of bone marrow cells in this case too (Fig. 2B).
Fig. 2.
Effect of DHP on intestine immune activity based on the proliferation of bone marrow cells mediated by the supernatant of small intestinal lymphocytes from mice administered DHP orally (A) or by the supernatant of small intestinal lymphocytes from untreated mice cultured in vitro with DHP (B). ‘*’ and ‘**’ indicate the significant difference of P < 0.05 and P < 0.01 as compared with the control, respectively.
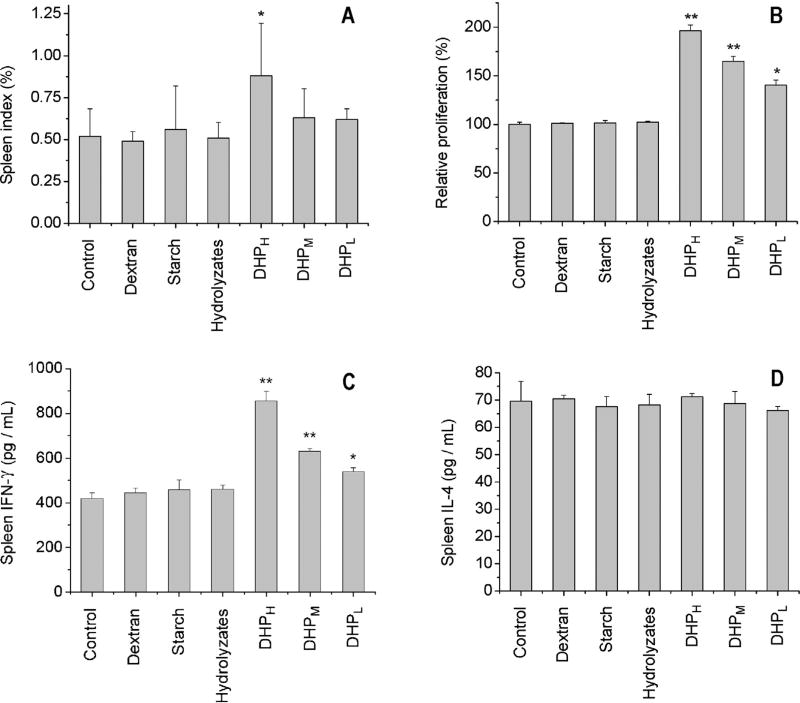
3.2. Immune responses to DHP in the spleen
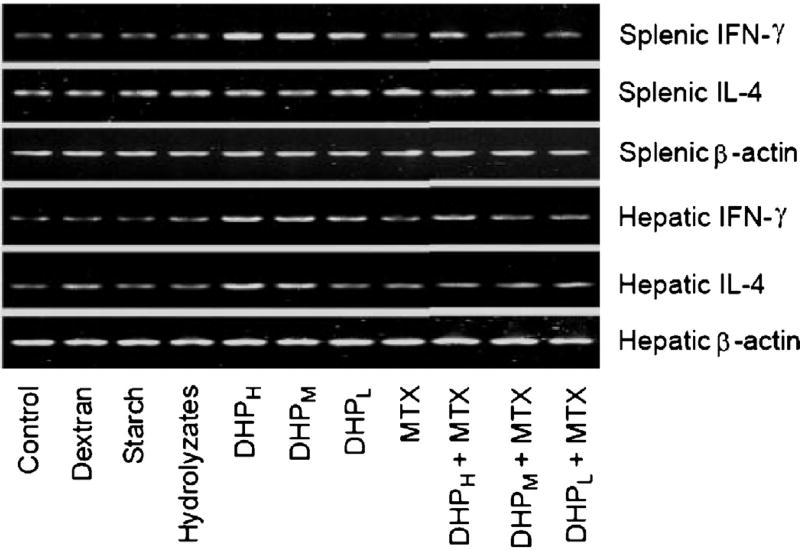
As compared to the control group, DHP at 200 mg/kg obviously increased the spleen growth index of the mice (Fig. 3A). In the presence of Con A, splenocyte proliferation promoted by DHP at 50, 100 and 200 mg/kg increased to 140%, 165% and 196% of the control group, respectively (Fig. 3B). Two cytokines IFN-γ and IL-4 were also measured to evaluate immune responses in the spleen. The results showed that the oral administration of DHP significantly stimulated the secretion of IFN-γ in a dose-dependent manner and the peak level of IFN-γ occurring in 200 mg/kg treatment group was 195% of the control group (Fig. 3C). However, the level of IL-4 in the spleen was not affected by the oral administration of DHP (Fig. 3D). Correspondingly, the mRNA expression of IFN-γ not IL-4 was enhanced by DHP at different doses (Fig. 5). Other polysaccharides (dextran and starch) and DHP hydrolyzates did not affect spleen index, splenocyte proliferation as well as cytokine expression and secretion.
Fig. 3.
Effect of DHP on spleen immune activity based the spleen growth index (A), splenocyte proliferation (B), secretion of IFN-γ (C) and IL-4 (D) in culture medium of splenocytes. ‘*’ and ‘**’ indicate the significant difference of P < 0.05 and P < 0.01 as compared with the control, respectively.
Fig. 5.
Effect of orally administration DHP with or without methotrexate on mRNA expression of IFN-γ and IL-4 in splenocytes and hepatocytes. β-Actin transcript was used as the external standard to eliminate the effects of the amount of mRNA present in tested samples.
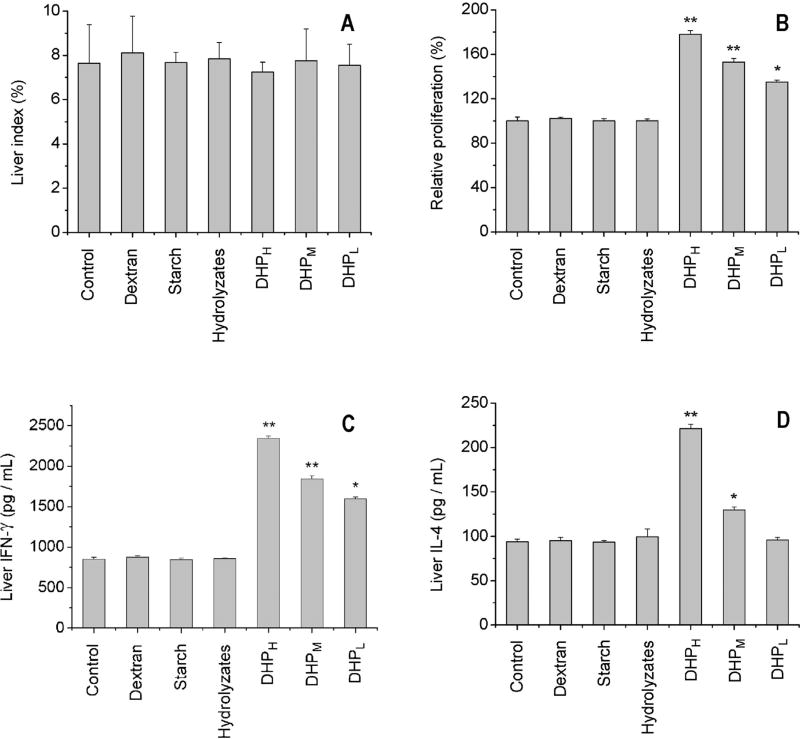
3.3. Immune responses to DHP in the liver
Experimental results indicated that although the oral administration of DHP did not change liver growth index (Fig. 4A), hepatocyte proliferation was significantly increased by DHP in a dose-dependent manner (Fig. 4B). In contrast to responses in the spleen, the oral administration of DHP stimulated the secretion of both IFN-γ and IL-4 in the liver. The maximum levels of IFN-γ and IL-4 observed in 200 mg/kg treatment group were 274% and 237% of the control group, respectively (Fig. 4C and D). Furthermore, the oral administration of DHP was found to promote the mRNA expression of IFN-γ and IL-4 (Fig. 5). As observed in the spleen, other polysaccharides (dextran and starch) and DHP hydrolyzates did not affect liver index, hepatocyte proliferation as well as cytokine expression and secretion.
Fig. 4.
Effect of DHP on liver immune activity based on liver growth index (A), hepatocyte proliferation (B), secretion of IFN-γ (C) and IL-4 (D) in culture medium of hepatocytes. ‘*’ and ‘**’ indicate the significant difference of P < 0.05 and P < 0.01 as compared with the control, respectively.
3.4. Effects of methotrexate on the immune responses to DHP
To elucidate the roles of small intestine in immune response to oral administration of DHP, methotrexate, a well-known anti-cancer drug which has been commonly used for induction of acute small intestinal injury and intestinal immune system impairment [13,14], was administered orally to the mice alone or in combination with DHP. The results showed that methotrexate treatment effectively suppressed small intestinal immune activity but did not change growth index, cell proliferation or cytokine production in spleen and liver as compared to the control (Table 1). However, DHP not only recovered small intestinal immune function (100 and 200 mg/kg), but also promoted cell proliferation and IFN-γ production in spleen and liver (200 mg/kg) although IL-4 level did not change and the enhancement in cell proliferation and IFN-γ production was lower than in the corresponding groups without methotrexate treatment (Table 1). Moreover, the enhanced mRNA expression of IFN-γ also confirmed the recovery effects of high dose of DHP on immune responses suppressed by methotrexate treatment (Fig. 5).
Table 1.
Effects of methotrexate (MTX) on immune responses induced by orally administrated polysaccharides from Dendrobium huoshanense in intestine, spleen and liver of mice.
| Treatment | Intestinal immune modulating activity (%) |
Spleen | Liver | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| Growth index | Cell proliferation (%) | IFN-γ (pg/mL) | IL-4 (pg/mL) | Growth index | Cell proliferation (%) | IFN-γ (pg/mL) | IL-4 (pg/mL) | ||
| Control | 100.0 ± 2.2b | 0.52 ± 0.16 | 100.0 ± 2.2b | 419.7 ± 25.1b | 65.6 ± 7.2 | 7.65 ± 1.74 | 100.0 ± 3.6b | 852.1 ± 23.4b | 93.9 ± 3.0 |
| MTX | 69.7 ± 3.8c | 0.47 ± 0.03 | 101.2 ± 2.0b | 455.9 ± 17.2b | 67.9 ± 1.4 | 6.91 ± 0.55 | 101.0 ± 1.1b | 851.1 ± 16.2b | 94.6 ± 4.1 |
| DHPH + MTX | 111.1 ± 1.9a | 0.64 ± 0.07 | 163.7 ± 3.4a | 647.2 ± 20.7a | 67.3 ± 2.7 | 7.68 ± 0.76 | 159.1 ± 4.8a | 1805.9 ± 22.5a | 96.1 ± 2.3 |
| DHPM + MTX | 99.9 ± 2.1b | 0.41 ± 0.09 | 102.2 ± 2.3b | 454.0 ± 24.4b | 67.1 ± 3.4 | 7.61 ± 0.82 | 103.7 ± 0.8b | 865.7 ± 17.9b | 95.6 ± 3.4 |
| DHPL + MTX | 70.9 ± 3.0c | 0.45 ± 0.07 | 101.4 ± 0.7b | 456.9 ± 11.1b | 65.3 ± 4.1 | 7.61 ± 0.82 | 101.3 ± 1.6b | 859.9 ± 32.2b | 95.0 ± 4.0 |
Data from 10 mice in each treatment were expressed as mean ± standard deviation.
Different superscripts letters within a column indicate a significant difference (P< 0.05).
4. Discussion
It has been proved that polysaccharides, after oral administration, not only promote small intestinal immune functions but also regulate systemic immune functions. Yamada’s group reported that the oral administration of polysaccharides or lignin-polysaccharide complexes from traditional herbal medicines led to enhanced intestinal immune responses including small intestinal lymphocyte-mediated proliferation of bone marrow cells [10,15,16], cytokine secretion [17] and T-cell activation [10]. Mean-while, these polysaccharides after oral administration were found to stimulate antigen-specific antibody response [13,18] and change hepatic cytokine secretion and natural killer T cell (NKT) population [19] in systemic immune function. The oral administration of other polysaccharides including β-glucan and chitosan has also been reported to induce phenotypic and functional changes of immune cells in intestinal Peyer’s patches, mesenteric lymph nodes and spleen, activate the secretion of cytokines in Peyer’s patches and intestinal epithelial cells, recover splenocyte proliferation after irradiation damage and increase number of B lymphocytes forming IgM and IgG antibodies [20–23]. Similarly, the results presented in this study proved that the polysaccharides from D. huoshanense (DHP) after oral administration could exert immunomodulating functions both in small intestine as indicated by the proliferation of bone marrow cells and in spleen and liver as indicated by the production of cytokines IFN-γ and IL-4. Moreover, the immunomodulating effects of DHP depended on the existence of specific polysaccharide structure because dextran, starch or DHP hydrolyzates did not display any effects on bone marrow cell proliferation, spleen and liver indices, splenic and hepatic cell proliferation, or cytokine production. The present results obtained with in vitro experiments for evaluating the effect of DHP on intestine immune function also suggested that DHP could activate intestine immunostimulating response directly, which was similar to the result reported by Hong et al. [10]. Because IFN-γ is Th1-associated cytokine and IL-4 is Th2-associated cytokine [24], the different enhancements in the levels of IFN-γ and IL-4 secretion caused by DHP in spleen and liver suggested that DHP exerted immunomodulating responses possibly by changing the balance of Th1/Th2.
The mucosal surface of intestine tract is the site both for digestion and absorption of nutrients and for sensing and promoting the local intestine immune response to foreign antigens, which may regulate systemic immune response by homing of lymphocytes and cytokines [25]. It has been suggested that orally administered polysaccharides express their intestine immunomodulating effects first through interaction between polysaccharides and immunocompetent cells in intestinal mucosal immune system, and then express their systemic immunomodulating effects through the migration of those functionally modulated lymphocytes from intestinal mucosal immune system to spleen and peripheral lymph nodes because of the guidance of homing-related molecules [18]. However, the possibility that polysaccharides directly regulate systemic immune functions should not be ruled out because different polysaccharides after oral administration have been detected in intestinal epithelial cells, T cell area of follicles of Peyer’s patches, and macrophages in spleen, lymph nodes and bone marrow [26–28]. In the present study, the decrease in normal intestinal immune activity but not systemic immune responses in the spleen or liver caused by the oral administration of methotrexate alone was recovered to a great extent by high doses of DHP, indicating that DHP could ameliorate methotrexate-induced intestinal injury and the stimulating activity of DHP on systemic immune system depended on intestinal immune function because methotrexate at the used concentration did not influence growth index, cell proliferation or cytokine production in spleen and liver. Similarly, methotrexate-induced impairment of intestinal immune function not systemic immune function has also been reported to be recovered by polysaccharides from other plants [13,29].
5. Conclusions
As the food materials, the stem of D. huoshanense has long been claimed to have the ability to enhance body’s immune function. The present study confirmed that the polysaccharides from D. huoshanense (DHP), after oral administration, possessed immunomodulating effects both in small intestine immune system and in systemic immune function. Thus, we can conclude that the polysaccharides may be one of main active ingredients that mediate the immunomodulating function of D. huoshanense.
Acknowledgments
This study was financially supported by the National Natural Science Foundation of China (Grant Nos. 21006019, 20872024 and 31271814) and the Project for Science and Technology Research Plan from Anhui Province of China (No. 12010402088).
References
- 1.Bao XS, Shun QS, Chen LZ. Medicinal Dendrobii in China. Fudan University Press; Shanghai: 2001. pp. 1–75. [Google Scholar]
- 2.Luo JP, Deng YY, Zha XQ. Pharm. Biol. 2008;46:243–249. [Google Scholar]
- 3.Wu HQ, Luo JP. Lishizhen Med. Mat. Med. Res. 2010;21:208–211. (in Chinese, with English abstract) [Google Scholar]
- 4.Pan LH, Lu J, Luo JP, Zha XQ, Wang JH. Exp. Toxicol. Pathol. 2012;64:899–904. doi: 10.1016/j.etp.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 5.Zha XQ, Luo JP, Jiang ST. Pharm. Biol. 2007;45:71–76. [Google Scholar]
- 6.Hsieh SY, Chien C, Liao SK, Liao SF, Hung WT, Yang WB, Lin CC, Cheng TJ, Chang CC, Fang JM, Wong CH. Bioorg. Med. Chem. 2008;16:6054–6068. doi: 10.1016/j.bmc.2008.04.042. [DOI] [PubMed] [Google Scholar]
- 7.Zha XQ, Luo JP, Luo SZ, Jiang ST. Carbohydr. Polym. 2007;69:86–93. [Google Scholar]
- 8.Staub AM. Methods Carbohydr. Chem. 1965;5:5–6. [Google Scholar]
- 9.Li SL, Chen C, Yang SL, Pan LH, Zhang HL, Zhang LL, Luo JP. Drug. Eva. Res. 2012;35:321–327. (in Chinese) [Google Scholar]
- 10.Hong T, Matsumoto T, Kiyohara H, Yamada H. Phytomedicine. 1998;5:353–360. doi: 10.1016/S0944-7113(98)80017-2. [DOI] [PubMed] [Google Scholar]
- 11.Kanaya T, Miyazawa K, Takakura I, Itani W, Watanabe K, Ohwada S, Kitazawa H, Rose MT, McConochite HR, Okano H, Yamaguchi T, Aso H. Am. J. Physiol. Gastrointest. Liver Physiol. 2008;295:273–284. doi: 10.1152/ajpgi.00378.2007. [DOI] [PubMed] [Google Scholar]
- 12.Fan Y, Liu J, Wang D, Song X, Hu Y, Zhang C, Zhao X, Nguyen TL. Carbohydr. Polym. 2013;94:24–30. doi: 10.1016/j.carbpol.2012.12.071. [DOI] [PubMed] [Google Scholar]
- 13.Kiyohara H, Nagai T, Munakata K, Nonaka K, Hanawa T, Kim SJ, Yamada H. Evid. Based Complement. Alternat. Med. 2006;3:459–467. doi: 10.1093/ecam/nel030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gulguna M, Erdemb O, Oztasc E, Kesik V, Balamtekin N, Vurucu S, Kul M, Kismet E, Koseoglu V. Exp. Toxicol. Pathol. 2010;62:109–115. doi: 10.1016/j.etp.2009.02.120. [DOI] [PubMed] [Google Scholar]
- 15.Kiyohara H, Matsumoto T, Yamada H. Planta Med. 2000;66:20–24. doi: 10.1055/s-2000-11116. [DOI] [PubMed] [Google Scholar]
- 16.Kiyohara H, Matsunoto T, Yamada H. Phytomedicine. 2002;9:614–624. doi: 10.1078/094471102321616427. [DOI] [PubMed] [Google Scholar]
- 17.Kiyohara H, Uchida T, Takakiwa M, Matsuzaki T, Hada N, Takeda T, Shibata T, Yamada H. Phytochemistry. 2010;71:280–293. doi: 10.1016/j.phytochem.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 18.Kiyohara H, Nonaka K, Sekiya M, Matsumoto T, Nagai T, Tabuchi Y, Yamada H. Evid. Based Complement. Alternat. Med. 2011;2011:1–13. doi: 10.1093/ecam/nep193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsumoto T, Sakurai MH, Kiyohara H, Yamada H. Immunopharmacology. 2000;46:149–161. doi: 10.1016/s0162-3109(99)00166-6. [DOI] [PubMed] [Google Scholar]
- 20.Vetvicka V, Dvorak B, Vetvickova J, Richter J, Krizan J, Sima P, Yvin JC. Int. J. Biol. Macromol. 2007;40:291–298. doi: 10.1016/j.ijbiomac.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 21.Porporatto C, Bianco ID, Correa SG, Leukoc J. Biol. 2005;78:62–69. doi: 10.1189/jlb.0904541. [DOI] [PubMed] [Google Scholar]
- 22.Canali MM, Porporatto C, Aoki MP, Bianco ID, Correa SG. Vaccine. 2010;28:5718–5724. doi: 10.1016/j.vaccine.2010.06.027. [DOI] [PubMed] [Google Scholar]
- 23.Xia L, Liu X, Guo H, Zhang H, Zhu J, Ren F, Funct J. Foods. 2012;4:294–301. [Google Scholar]
- 24.Borchers AT, Krishnamurthy A, Keen CL, Meyers FJ, Gershwin ME. Exp. Biol. Med. 2008;233:259–276. doi: 10.3181/0708-MR-227. [DOI] [PubMed] [Google Scholar]
- 25.Maldonado-Contreras AL, McCormick BA. Cell Tissue Res. 2011;343:5–12. doi: 10.1007/s00441-010-1082-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rice PJ, Adams EL, Ozment-Skelton T, Gonzalez AJ, Goldman MP, Lock-hart BE, Barker LA, Breuel KF, Deponti WK, Kalbfleisch JH, Ensley HE, Brown GD, Gordon S, Williams DL, Pharmacol J. Exp. Ther. 2005;314:1079–1086. doi: 10.1124/jpet.105.085415. [DOI] [PubMed] [Google Scholar]
- 27.Hong F, Yan J, Baran JT, Allendorf DJ, Hansen RD, Ostroff GR, Xing PX, Cheung NK, Ross GD. J. Immunol. 2004;173:797–806. doi: 10.4049/jimmunol.173.2.797. [DOI] [PubMed] [Google Scholar]
- 28.Sakurai MH, Matsumoto T, Kiyohara H, Yamada H. Planta Med. 1996;62:341–346. doi: 10.1055/s-2006-957898. [DOI] [PubMed] [Google Scholar]
- 29.Chen LH, Lin ZB, Li WD. Acta Pharmacol. Sin. 2011;32:1505–1512. doi: 10.1038/aps.2011.126. [DOI] [PMC free article] [PubMed] [Google Scholar]