Abstract
Pulmonary fibrosis is the leading cause of death in systemic sclerosis (SSc). Sirtuin1 (SIRT1) is a deacetylase with known antiinflammatory and antifibrotic activity in the liver, kidney, and skin. The role of SIRT1 in SSc-related pulmonary fibrosis is unknown. In the present work, we determined that the expression of SIRT1 in peripheral blood mononuclear cells of patients with SSc with pulmonary fibrosis is lower than that in patients with SSc without pulmonary fibrosis. In in vivo studies of bleomycin-induced lung fibrosis in mice, SIRT1 activation with resveratrol reduced collagen production when it was administered either prophylactically during the inflammatory stage or after the development of fibrosis. Furthermore, SIRT1 activation or overexpression inhibited tumor necrosis factor-α–induced inflammatory responses in vitro in human fetal lung fibroblasts, depletion of SIRT1 in fibroblasts enhanced inflammation, and these effects were related to changes in the acetylation of NF-κB. In addition, SIRT1 activation or exogenous overexpression inhibited collagen production in vitro, and these manipulations also inhibited fibrosis via inactivation of transforming growth factor-β/mothers against decapentaplegic homolog and mammalian target of rapamycin signaling. Taken together, our results show that a loss of SIRT1 may participate in the pathogenesis of SSc-related pulmonary fibrosis, and that SIRT1 activation is an effective treatment for both the early (inflammatory) and late (fibrotic) stages of pulmonary fibrosis. Thus, SIRT1 may be a promising therapeutic target in the management of SSc-related pulmonary fibrosis.
Keywords: systemic sclerosis, pulmonary fibrosis, inflammation, NF-κB pathway, transforming growth factor-β/mammalian target of rapamycin pathway
Clinical Relevance
Pulmonary fibrosis is the leading cause of death in patients with systemic sclerosis (SSc), with more than 70% of patients with SSc having some degree of pulmonary involvement. However, the treatment of SSc-related pulmonary fibrosis is limited, owing to insufficient understanding of its pathogenesis. In this work, we specified the role of sirtuin1 (SIRT1) in the pathogenesis of pulmonary fibrosis. It was demonstrated that SIRT1 was reduced in peripheral blood mononuclear cells of patients with SSc with pulmonary fibrosis as well as lung tissues of mice with bleomycin-induced lung fibrosis. In addition, SIRT1 activation with resveratrol reduced collagen production when it was administered either prophylactically during the inflammatory stage or after the development of fibrosis; therefore, SIRT1 activation may be a promising approach for both the prevention and treatment of SSc-related pulmonary fibrosis.
Scleroderma or systemic sclerosis (SSc) is a severe and devastating autoimmune disorder characterized by immune alterations, microvascular injury, and fibrosis of the skin and internal organs. SSc is classified into two subsets according to the extent of fibrosis of the skin: limited cutaneous SSc (lcSSc) and diffuse cutaneous SSc (dcSSc) (1). Pulmonary fibrosis is the leading cause of death in patients with SSc, with more than 70% of patients with SSc having some degree of pulmonary involvement (2–4). However, the treatment of SSc-related pulmonary fibrosis is limited due to insufficient understanding of its pathogenesis, and greater knowledge of the underlying processes is needed.
Pulmonary fibrosis is characterized by the accumulation of fibroblasts and extracellular matrix (ECM) in the lung (5–9). The disorder arises from a variety of etiologies that initiate inflammation (9–11) and excess production of inflammatory cytokines (e.g., IL-1β, IL-6), which promote the progression to lung fibrosis (10, 11). In later stages of lung injury, a variety of proinflammatory and profibrotic mediators, including transforming growth factor (TGF)-β and connective tissue growth factor (CTGF) interact to maintain fibrosis (12). A pivotal role for profibrotic inflammatory processes in the development of pulmonary fibrosis has been suggested in a large number of studies (6).
Sirtuin1 (SIRT1; a.k.a., silence information regulator 1) is an nicotinamide (NAM) adenine dinucleotide+–dependent class III histone deacetylase (13) that participates in many physiological processes, such as cell survival, antioxidative stress and antiinflammatory pathways, metabolic regulation, aging, and DNA repair (14–17). SIRT1 regulates inflammation in fibroblasts, chondrocytes (18, 19), and adipose tissue (20) via inactivation of the NF-κB pathway that is dependent on its deacetylase activity; SIRT1 abundance is decreased in the skin in SSc (21, 22), and it attenuates fibrotic processes in the skin (21), kidney (23), and heart (24) by targeting TGF-β/mothers against decapentaplegic homolog (Smad3) signaling. It has been previously determined that low expression of SIRT1 plays an important promoting role in the development of liver fibrosis, and its restoration contributes to the amelioration of liver fibrosis (25). In contrast, how SIRT1 function is potentially altered and how its manipulation may impact SSc-related pulmonary fibrosis are unknown.
In the present study, we investigated the function of SIRT1 and its links to proinflammatory and profibrotic pathways in SSc-related lung fibrosis in clinical samples, in vitro in cell culture, and in vivo in a model of bleomycin-induced pulmonary fibrosis. Our results demonstrate that SIRT1 mRNA abundance is decreased in peripheral blood mononuclear cells of patients with SSc with lung fibrosis compared with those without lung fibrosis. In addition, we found that Bleomycin-induced lung inflammation and fibrosis in mice were both alleviated by activating SIRT1 with resveratrol. Furthermore, we determined that SIRT1 exerts these effects by decreasing NF-κB acetylation and by inhibiting TGF-β– and mammalian target of rapamycin (mTOR)–related signaling.
Methods
Human Study
A total of 145 Chinese patients who met diagnostic criteria for SSc were enrolled from Shanghai Traditional Chinese Medicine–Integrated Hospital (Table E1 in the data supplement) (26). The study was approved by and performed in accordance with the guidelines of the School of Life Sciences, Fudan University (Shanghai, China). Full details about the patients are provided in the data supplement.
Lung Fibrosis Model
Pathogen-free, C57BL/6 female mice of 6–8 weeks of age were maintained with water and pelleted food at the Animal Centre of the State Key Laboratory of Genetic Engineering, School of Life Sciences, Fudan University. Bleomycin-induced lung fibrosis was generated using established procedures (27). BAL fluid (BALF) collection and analysis, lung histologic analysis and immunohistochemical staining of SIRT1, and total soluble collagen isolation and analysis were done using previously reported approaches (28). IL-1β and IL-6 concentrations in BALF and plasma were measured by ELISA (Abcam Systems) according to the manufacturer’s instructions. All the animal protocols were approved by the School of Life Sciences, Fudan University (28). The detailed methods are provided in the data supplement.
Cell Culture Models
Experiments in cell culture were performed in human fetal lung fibroblast cells (MRC-5 line) and NIH/3T3 cells. SIRT1 expression was silenced, SIRT1 was overexpressed, and luciferase reporter gene assays were done as described in the data supplement.
Quantitative RT-PCR Analysis
RNA was isolated from mouse lungs and cultured cells, and inflammation- and ECM-related gene expression was evaluated by quantitative RT-PCR, as described in detail in the data supplement.
Western Blot Analysis
Methods used to analyze SIRT1, mTOR, phospho (p)-mTOR, p-S6R, Smad3, pSmad3, and collagen (COL) type I polyclonal protein expression by immunoblotting are described in detail in the data supplement.
SIRT1 Activity Assay
SIRT1 activity was assessed using a SIRT1 deacetylase activity assay kit (CS1040; Sigma-Aldrich) according to the manufacturer’s instructions as described in the data supplement.
Statistical Analysis
Data are expressed as mean (±SEM). Student’s t tests or one-way ANOVA with least significant difference (LSD) multiple comparison test were used for the evaluation of significance between two groups or three or more groups, respectively. A P value less than 0.05 was considered statistically significant.
Results
Peripheral Blood Mononuclear Cell SIRT1 Levels Are Decreased in SSc-Related Pulmonary Fibrosis
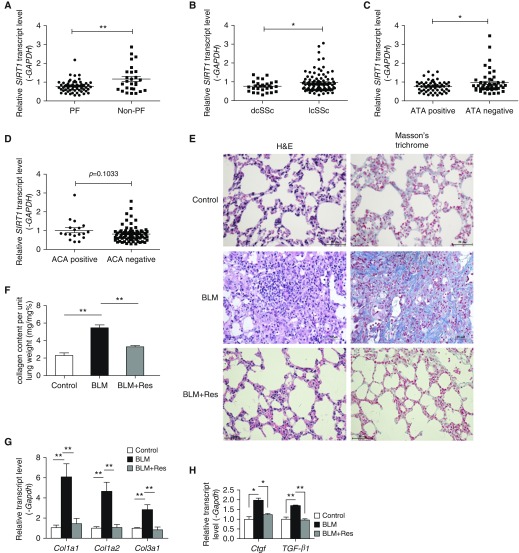
Although it is known that SIRT1 is involved in the development of SSc-related skin fibrosis (21, 22), how SIRT1 participates in the development of SSc-related pulmonary fibrosis is unknown. We examined the expression of SIRT1 in the peripheral blood mononuclear cells (PBMCs) of patients with SSc with pulmonary fibrosis versus those without pulmonary fibrosis. Transcript levels for SIRT1 in the PBMCs of patients with SSc with pulmonary fibrosis were decreased compared with patients with SSc without pulmonary fibrosis (P < 0.0006, Figure 1A). In addition, the level of SIRT1 mRNA in the PBMCs from patients with dcSSc was lower than that in PBMCs from patients with limited cutaneous (P = 0.0219, Figure 1B). One of the main characteristics of SSc is the presence of autoantibodies, such as anti-DNA topoisomerase I (ATA), anticentromeric proteins (ACA), and anti-RNA polymerase III, and the autoantibodies are associated with certain SSc features. For instance, the presence of ATA in patients with SSc is strongly associated with dcSSc and pulmonary fibrosis, whereas ACA is correlated with less pulmonary involvement (1). Therefore, SIRT1 expression was also analyzed in patients with different autoantibody subsets, and it was found to be decreased in patients with ATA compared with those without ATA (Figure 1C, P = 0.0320). In contrast, there was a modest directional change in the level of SIRT1 related to ACA, with SIRT1 mRNA greater in patients with ACA than in those without ACA (Figure 1D, P = 0.1033). These findings suggest that a deficiency in SIRT1 may play a role in SSc-related pulmonary fibrosis.
Figure 1.
Sirtuin1 (SIRT1) mRNA level is decreased in peripheral blood mononuclear cells (PBMCs) of patients with systemic sclerosis (SSc) with pulmonary fibrosis (PF), and SIRT1 activation attenuates experimental PF. Relative PBMC mRNA level of SIRT1 in (A) patients with SSc with PF versus those without PF, (B) patients with diffuse cutaneous SSc (dcSSc) versus patients with limited cutaneous SSc (lcSSc), (C) patients with SSc with anti-DNA topoisomerase I (ATA) versus those without ATA, and (D) patients with SSc with anticentromeric proteins (ACA) versus those without ACA. (E) Effect of resveratrol (Res) on bleomycin (BLM)-induced lung inflammation and fibrosis. Magnification, ×400. Scale bars: 50 μm. (F) Lung collagen content determined by Sircol assay. (G) Lung collagen mRNA levels. (H) Lung levels of connective tissue growth factor (Ctgf) and transforming growth factor (TGF)-β mRNA were measured by real-time PCR and normalized to Gapdh. *P < 0.05, **P < 0.001. (F–H) Mean (±SEM); n = 6 mice per group. H&E = hematoxylin and eosin.
SIRT1 Activation Attenuates Pulmonary Fibrosis in Bleomycin-Treated Mice
To directly determine how relative SIRT1 activity influences pulmonary fibrosis, we investigated the effect of SIRT1 activation on bleomycin-induced pulmonary fibrosis in mice. As an activator of SIRT1, resveratrol has been shown to be an effective treatment for experimental renal and liver fibrosis (23, 29–32). First, we evaluated the effect of resveratrol administered for a period of 24 days, from 3 days before bleomycin through Day 21 after bleomycin. Hematoxylin and eosin staining showed disruption of the alveolar units and infiltration of lymphocytes into the interstitial and peribronchial space after bleomycin treatment. In addition, Masson’s trichrome staining revealed increased collagen deposition in the interstitial space after bleomycin treatment. In contrast, resveratrol treatment ameliorated these changes (Figure 1E). Furthermore, compared with controls, bleomycin treatment increased collagen content 2.3-fold (P < 0.001), and resveratrol reduced collagen by 40% (Figure 1F, P < 0.001). Compared with controls, bleomycin administration also enhanced the transcript level for Col1a1, Col1a2, and Col3a1 (P < 0.001), whereas resveratrol inhibited collagen transcript up-regulation by bleomycin (Figure 1G, P < 0.001). Ctgf and Tgf-β are two potent stimulators of collagen production. Bleomycin enhanced Ctgf and Tgf-β mRNA levels (P < 0.05 and P < 0.001, respectively), and resveratrol prevented the increases in Ctgf and Tgf-β mRNA (Figure 1H, P < 0.05 and P < 0.001, respectively). Recognizing that a model of bleomycin-induced pulmonary fibrosis was employed to represent the clinical condition, these results suggest that SIRT1 activation may be effective in inhibiting the development of SSc-related pulmonary fibrosis.
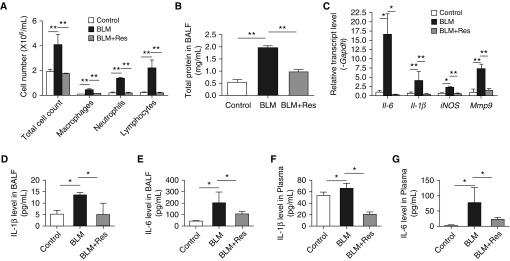
SIRT1 Activation Attenuates Pulmonary Inflammation in Bleomycin-Treated Mice
The first 7 days after bleomycin administration is the acute inflammatory stage. To more subtly investigate the effect of SIRT1 on pulmonary fibrosis–associated inflammation, we treated mice with resveratrol for 10 days, which began 3 days before bleomycin administration through Day 7 after bleomycin. Inflammatory cells in BALF were counted to determine whether resveratrol prevents their bleomycin-induced infiltration into the airways and parenchyma. The total number of inflammatory cells, macrophages, neutrophils, and lymphocytes in the BALF of bleomycin-injected mice was elevated compared with saline-injected controls, and the increases were blunted by resveratrol treatment (Figure 2A, P < 0.001). Total protein in BALF from bleomycin-treated mice was also reduced by resveratrol treatment (Figure 2B, P < 0.001). Next, we examined transcript levels for the inflammatory cytokines, Il-6, Il-1β, iNOS, and Mmp-9. The mRNA levels of these inflammatory cytokines were markedly increased in lungs from bleomycin-treated mice (Figure 2C, P < 0.05 or P < 0.001), and resveratrol decreased the abundance of the mRNAs to normal levels (Figure 2C, P < 0.05 or P < 0.001). We also measured the levels of IL-6 and IL-1β in BALF and plasma. Both cytokines were elevated in BALF and plasma after bleomycin treatment, and the increases were attenuated by resveratrol (Figures 2D–2G, P < 0.001). We further evaluated where SIRT1 is expressed. Immunofluorescence analysis of adjacent lung sections revealed that SIRT1 was abundantly expressed in CD11c+ dendritic cells and CD68+ macrophages, and was partially expressed in CD3+ T cells and CD11b+ monocytes (Figure E1A). These results indicate that SIRT1 is expressed by immune cells and has antiinflammatory activity in bleomycin-induced pulmonary fibrosis.
Figure 2.
SIRT1 attenuates inflammation induced by bleomycin in mouse lung. (A and B) Cells were counted and total protein was determined in BAL fluid (BALF). (C) RT-PCR was used to quantify Il-6, Il-1β, nitric oxide synthase (iNOS), and matrix metallopeptidase 9 (Mmp-9) mRNA abundance in lung tissues. mRNA levels were calculated relative to Gapdh. IL-1β (D) and IL-6 (E) BALF levels and IL-6 (F) and IL-1β (G) plasma levels were determined by ELISA. Mean (±SEM); *P < 0.05, **P < 0.001 versus mice treated with BLM plus vehicle. For each group, n = 6.
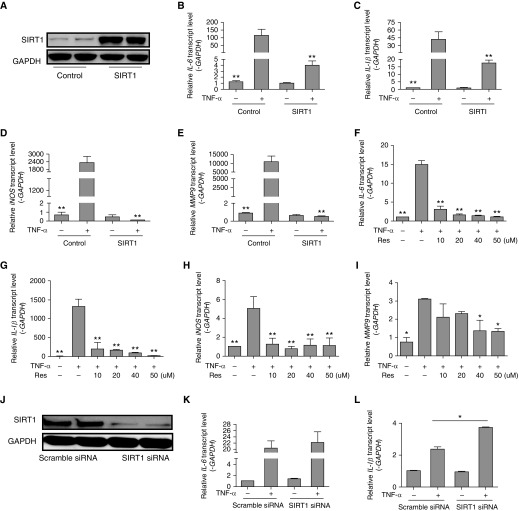
SIRT1 Represses TNF-α–induced Inflammation
To gain better insight into the antiinflammatory effects of SIRT1 in a lung cell type of relevance to pulmonary fibrosis, MRC-5 human fetal lung fibroblasts were transfected with control plasmid or SIRT1-expressing plasmid, and then exposed to TNF-α. SIRT1 expression was effectively increased after transfection (Figure 3A). Levels of inflammatory cytokine mRNAs (IL-6, IL-1β, iNOS, and MMP-9) increased in control cells in response to TNF-α (Figures 3B–3E, P < 0.001), and SIRT1 overexpression prevented the increases (Figures 3B–3E, P < 0.001), suggesting that SIRT1 inhibits the production of inflammatory cytokines. We then examined the effects of resveratrol on proinflammatory genes. Once again, TNF-α treatment increased IL-6, IL-1β, iNOS, and MMP-9 mRNAs (Figures 3F–3I, P < 0.001 or P < 0.05), and the increases were prevented by resveratrol in a dose-dependent manner (Figures 3F–3I, P < 0.001 or P < 0.05). Resveratrol also dose-dependently inhibited proinflammatory transcript induction by TNF-α in NIH/3T3 fibroblasts (data not shown), confirming an antiinflammatory role for SIRT1.
Figure 3.
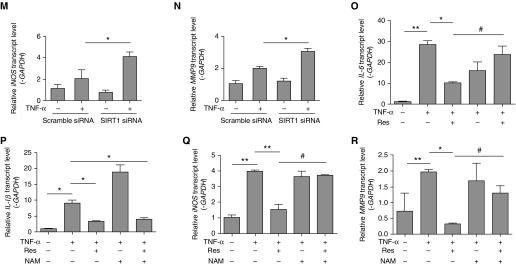
SIRT1 inhibits inflammation induced by TNF-α. (A) MRC-5 human fetal lung fibroblasts were transiently transfected with SIRT1 plasmid or control plasmid and whole-cell lysates were used for immunoblotting of SIRT1. (B–E) MRC-5 cells were transiently transfected with control or SIRT1 plasmid for 6 hours, then incubated with TNF-α (10 ng/ml) for another 6 hours, and transcript abundance was quantified. (F–I) Cells were stimulated with TNF-α in the absence or presence of Res (0, 10, 20, 40, or 50 μM) for 6 hours, and transcript abundance was quantified. (J) Cells were transfected with control silence RNA (siRNA) or SIRT1 siRNA, and whole-cell lysates were used for immunoblotting of SIRT1. (K–N) Cells were transiently transfected with siRNA for 6 hours, then incubated with exogenous TNF-α (10 ng/ml) for another 6 hours, and transcript levels were evaluated. (O–R) Cells were treated with vehicle or 20 mM nicotinamide (NAM) for 6 hours, and then exposed to 10 ng/ml TNF-α with or without added 50 μM Res for another 6 hours. RT-PCR was used to quantify the expression of IL-6, IL-1β, iNOS, and MMP9 mRNA. The mRNA levels were calculated using a relative ratio to GAPDH. Mean (±SEM), n = 3; *P < 0.05, **P < 0.001 versus cell exposure to TNF-α only. (O–R) #P < 0.05 versus cell exposure to NAM. Findings were confirmed in three separate experiments.
Further evidence for SIRT1 inhibition of inflammation in lung fibroblasts was then obtained by the finding that silence RNA (siRNA)-based depletion of SIRT1 in fibroblasts (Figure 3J) enhanced inflammation-related gene up-regulation by TNF-α (Figures 3K–3N). To further confirm the antiinflammatory effects of SIRT1, NAM, an inhibitor of SIRT1, was used. As shown in Figures 3O–3R, the inhibition of TNF-α–induced inflammatory cytokine expression of IL-6, iNOS, and MMP-9 by resveratrol was reversed by NAM. These data suggest a pivotal role for SIRT1 in mediating antiinflammatory responses.
SIRT1 Inhibits Inflammation through NF-κB Signaling
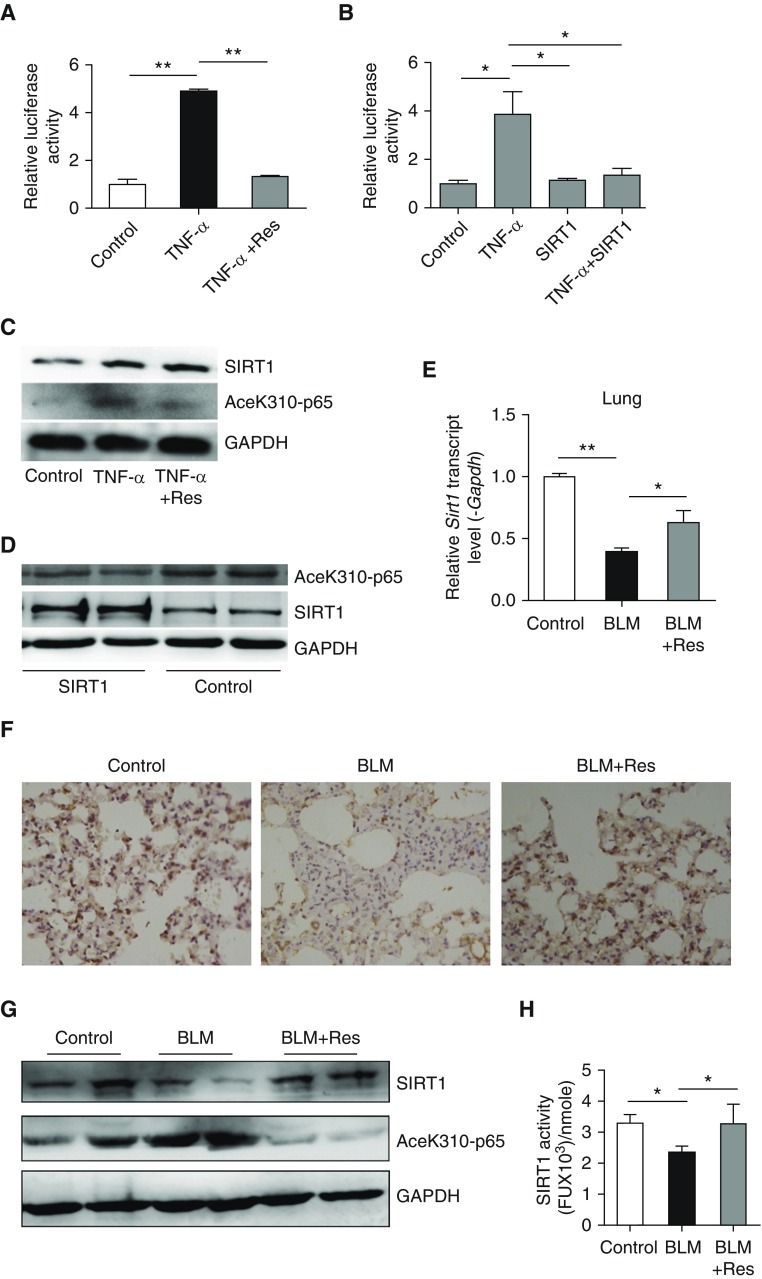
The NF-κB pathway is a canonical proinflammatory signaling pathway. We used luciferase activity as a reporter of NF-κB activation to investigate the effects of SIRT1 on NF-κB signaling in fibroblasts. Resveratrol or SIRT1 overexpression decreased NF-κB activation by TNF-α (Figures 4A and 4B). SIRT1 is a histone deacetylase, and the acetylation of NF-κB subunit, RelA/p65, at lysine 310 is critical for its proinflammatory activity (33). We found that p65 acetylation was increased after TNF-α treatment, whereas resveratrol inhibited the increase (Figure 4C). When MRC-5 cells were transiently transfected with a plasmid encoding SIRT1, the overexpression of SIRT1 also caused a decrease in RelA/p65 acetylation (Figure 4D). Then the expression and activation of SIRT1 and RelA/p65 acetylation in vivo were evaluated. The mRNA level of SIRT1 was decreased by bleomycin in mouse lung tissues, BALF cells, blood, and spleen; in contrast, resveratrol treatment restored SIRT1 expression in the mouse lung and BALF cells (Figure 4E, Figure E2A). In addition, the number of SIRT1-positive cells in the lungs of mice was reduced by bleomycin, and resveratrol treatment markedly increased the number of lung cells expressing SIRT1 (Figure 4F). Bleomycin caused a decrease in SIRT1 protein level and an increase in the acetylation level of NF-κB p65. In contrast, SIRT1 was increased by resveratrol treatment, whereas the acetylation level of NF-κB p65 was subsequently inhibited (Figure 4G). Furthermore, compared with control, bleomycin caused a decrease in SIRT1 activity (Figure 4H), and the activity was rescued by resveratrol treatment (Figure 4H). These results suggest that SIRT1 increase or activation prevents inflammation by inhibiting the NF-κB pathway. In addition, SIRT1 likely protects against pulmonary fibrosis by causing a decrease in NF-κB activation via a decline in its acetylation.
Figure 4.
SIRT1 inhibits inflammation by blunting NF-κB acetylation. (A) Effect of Res on TNF-α–induced activation of an NF-κB reporter (luciferase activity) in NIH/3T3 fibroblasts. (B) Effect of SIRT1 overexpression on TNF-α–induced activation of NF-κB reporter (luciferase activity) in NIH/3T3 fibroblasts. (C) MRC-5 fibroblasts were treated with TNF-α (10 ng/ml) in the absence or presence of Res (50 μM). Western blot analysis was performed to evaluate SIRT1 protein abundance and p65 acetylation (lysine 310). (D) MRC-5 cells were transiently transfected with SIRT1 plasmid or empty vector for 48 hours. SIRT1 protein abundance and the acetylation of p65 (lysine310) were determined by Western blot analysis. (E) SIRT1 mRNA levels were measured in lungs from control, bleomycin or bleomycin plus Resveratrol–treated mice. mRNA levels were normalized to Gapdh. (F) Immunohistochemistry for SIRT1 in mouse lung tissue. Magnification, ×400. (G) SIRT1 protein and p65 acetylation were measured by Western blotting. (H) SIRT1 deacetylase activity was determined in mouse lung tissues by SIRT1 assay kit. (A, B, E, and H) Mean (±SEM). **P < 0.001 versus cell exposure to TNF-α only (A) and *P < 0.05 versus cell exposure to TNF-α plus negative control (B). (C and D) Findings were confirmed in three separate experiments. (E and H) *P < 0.05, **P < 0.001 versus mice treated with bleomycin plus vehicle. For each group, n = 6.
SIRT1 Activation Attenuates Established Pulmonary Fibrosis in Bleomycin-treated Mice
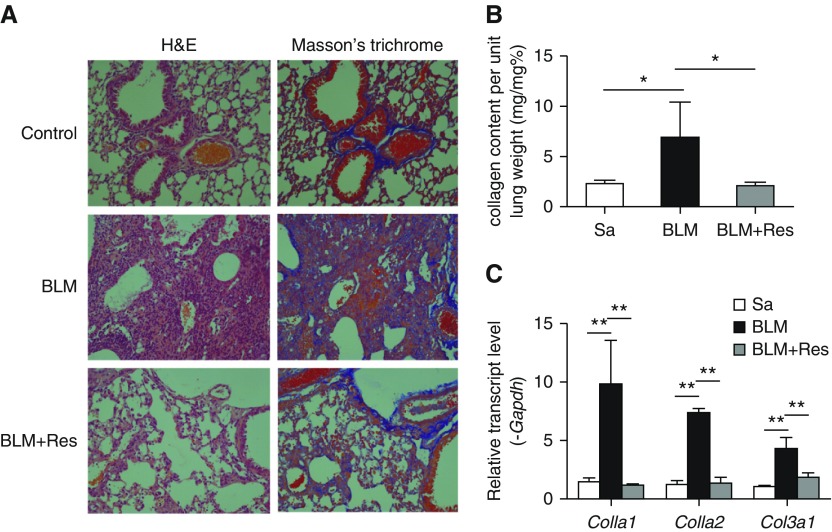
Fibrosis begins 7–9 days after bleomycin administration (34, 35). To determine the effects of SIRT1 on established fibrosis, resveratrol was administered daily beginning at Day 10 after bleomycin for 14 days. Resveratrol treatment alleviated pulmonary fibrosis progression (Figure 5), as assessed by histological analysis (Figure 5A), collagen content (Figure 5B), and ECM gene expression (Figure 5C). In addition, immunofluorescence analyses of lung tissues revealed a complete colocalization of SIRT1 and α-smooth muscle actin (α-SMA), indicating that the SIRT1 was abundantly expressed in myofibroblast to exert the antifibrotic ability (Figure E1B). Taken together, these results show that SIRT1 has both antiinflammatory and antifibrotic activity, and that activating SIRT1 with resveratrol at either the inflammatory or fibrogenic stage of disease development ameliorates bleomycin-induced pulmonary fibrosis.
Figure 5.
SIRT1 reverses bleomycin-induced pulmonary fibrosis. (A) Mice received BLM and then Res beginning 10 days after bleomycin treatment. Lung inflammation and fibrosis were evaluated by H&E and Masson’s trichrome staining. Magnification, ×400. (B) Lung collagen, measured by Sircol assay (n = 4 mice per group). (C) Lung collagen mRNA was quantified by real-time PCR. mRNA levels were normalized to Gapdh. (B and C) Mean (±SEM); *P < 0.05, **P < 0.001 versus mice treated with BLM plus vehicle. n = 4 mice per group.
SIRT1 Inhibits TGF-β–induced Fibrosis In Vitro
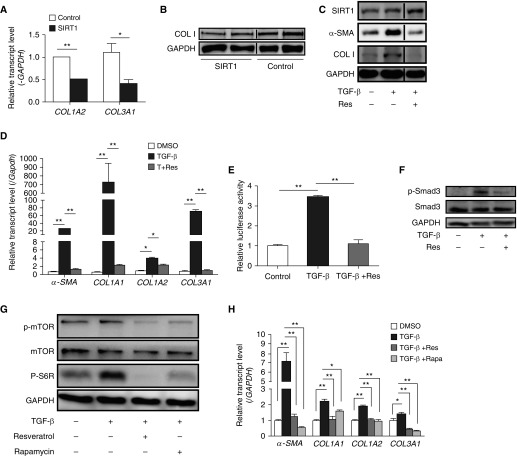
To evaluate the antifibrotic activity of SIRT1 in a lung cell of relevance to pulmonary fibrosis, MRC-5 cells were transfected with a control plasmid or a plasmid encoding SIRT1, and collagen mRNA and protein abundance were measured. Lower COL1A2 mRNA (P < 0.001), COL3A1 mRNA (P < 0.05), and COL1 protein expression was found in the cells overexpressing SIRT1 (Figures 6A and 6B). TGF-β was then used to assess a fibrotic response and resveratrol was used to activate SIRT1. Resveratrol inhibited increases in α-SMA (myofibroblasts marker) and COL1 protein abundance stimulated by TGF-β (Figure 6C). TGF-β also upregulated α-SMA (P < 0.001), COL1A1 (P < 0.001), COL1A2 (P < 0.001), and COL3A1 (P < 0.001) expression, and resveratrol prevented the increases (Figure 6D). A luciferase reporter gene assay was then employed to determine the effect of SIRT1 on the activity of the Smad-binding element (SBE) sequence in the collagen promoter in response to TGF-β. As shown in Figure 6E, SBE activity was increased 3.45-fold (P < 0.001) after stimulation with TGF-β, and resveratrol treatment attenuated the increase, returning SBE activity to nearly normal levels (P < 0.001). We next assessed the phosphorylation of Smad3, an important downstream transducer of TGF-β action. As shown in Figure 6F, the TGF-β–induced increase in p-Smad3 was blunted by resveratrol.
Figure 6.
SIRT1 inhibits fibrosis induced by TGF-β. (A) Levels of COL1A2 and COL3A1 mRNA in MRC-5 cells transiently transfected with control or SIRT1 plasmid for 24 hours. (B) Type I collagen (COL1) protein in MRC-5 cells transiently transfected with control or SIRT1 plasmid for 48 hours was measured by Western blot analysis. (C) Levels of α-smooth muscle actin (α-SMA) and COL1 protein were analyzed by Western blotting in TGF-β (10 ng/ml)–stimulated MRC-5 cells with or without Res treatment T+Res, TGF-β+Res. (D) Levels of α-SMA and collagen mRNA in TGF-β–stimulated MRC-5 cells with or without Res (50 μM) treatment. mRNA levels were calculated relative to GAPDH. (E) Relative activity of Smad-binding element in the COL1 promoter in MRC-5 fibroblasts treated with vehicle, TGF-β or TGF-β plus Res. (F) Western blot analysis of phospho (p)Smad3 and total Smad3 protein content in MRC-5 fibroblasts treated with vehicle, TGF-β, or TGF-β plus Res. (G) Western blot analysis of p-mammalian target of rapamycin (mTOR), total mTOR, and p-S6R protein in MRC-5 fibroblasts treated with vehicle, TGF-β, or TGF-β plus Res (50 μM) or rapamycin 10 μM. (H) Relative transcript levels of α-SMA, COL1A1, COL1A2, and COL3A1 in MRC-5 fibroblasts that were treated with vehicle, TGF-β, or TGF-β plus resveratrol or rapamycin. mRNA levels were calculated relative to GAPDH. (A, D, E, and H) Values are mean (±SEM); n = 3. *P < 0.05, **P < 0.001. (B, C, F, and G) Findings were confirmed in three independent experiments. Smad3 = mothers against decapentaplegic homolog.
The phosphatidylinositol 3-kinase/protein kinase B/mTOR pathway has recently been implicated in fibrosis via the actions of its downstream mediator, p70S6K1 (36), and activation of the mTOR pathway has been observed both in an animal model (37) and in human patients (38) with pulmonary fibrosis. The blockade of mTOR with rapamycin has already been used as a treatment for scleroderma-related fibrosis (39, 40). Because SIRT1 has been shown to inhibit mTOR signaling in TNF-α–induced inflammation and diet-induced obesity (18, 41), we postulated that SIRT1 impairs mTOR signaling in lung fibroblasts, and tested this possibility in MRC-5 cells. TGF-β increased mTOR activity, as indicated by enhanced mTOR and S6R phosphorylation (Figure 6G), and the increase was attenuated by both rapamycin and resveratrol (Figure 6G). The increases in expression of α-SMA (P < 0.001), COL1A1 (P < 0.001), COL1A2 (P < 0.001), and COL3A1 (P < 0.001) mRNAs induced by TGF-β were also inhibited by resveratrol and rapamycin, and the effect of resveratrol was comparable to that of rapamycin (Figure 6H). These data suggest that the antifibrotic effects of SIRT1 may involve mTOR pathway inhibition.
Discussion
The present work demonstrates that SIRT1 is downregulated in PBMCs of patients with SSc with pulmonary fibrosis, and that the down-regulation of SIRT1 is related to dcSSc and the presence of ATA in SSc. Further studies found that SIRT1 activation prevents pulmonary fibrosis by down-regulating proinflammatory and profibrotic signaling. In addition, we determined that these actions of SIRT1 require its deacetylase activity to block NF-κB signaling in inflammation, and the inhibition of mTOR pathway activity to attenuate fibrosis.
SIRT1 plays critical roles in aging, metabolism, and cancer. The function of SIRT1 in SSc is now beginning to be understood. Recent studies have delineated the role of SIRT1 in SSc skin fibrosis, finding that SIRT1 targets canonical TGF-β signaling (21, 22). However, the role of SIRT1 in SSc-related pulmonary fibrosis is unknown. In this study, we evaluated SIRT1 mRNA expression in the PBMCs of Chinese Han patients with SSc. We found that SIRT1 mRNA levels are downregulated in patients with SSc with pulmonary fibrosis compared with those without pulmonary fibrosis (Figure 1A). Pulmonary fibrosis is the leading cause of death in patients with SSc (42). Our previous study suggested that the presence of ATA in Chinese patients with SSc is strongly associated with dcSSc and pulmonary fibrosis, whereas ACA is associated with less pulmonary involvement (1). In addition, further analysis found that the decreased expression of SIRT1 was correlated with dcSSc (Figure 1B) and the presence of ATA (Figure 1C). Taken together, these results suggest involvement of SIRT1 loss in the development of SSc-related pulmonary fibrosis.
The bleomycin-induced pulmonary fibrosis model is a well characterized, commonly used model. Bleomycin induces lung injury by causing DNA strand damage, then endothelial injury, and then leakage of inflammatory cells into the alveolar space. This is followed by the development of fibrosis. In this study, it was found that the activation of SIRT1 by resveratrol reduced the lung inflammation and fibrosis invoked by bleomycin (Figures 1E–1H, Figure 2, and Figure 5). It was further determined that the basis for SIRT1 effect on fibrosis involves multiple mechanisms.
The overexpression or activation of SIRT1 reduced the inflammatory responses induced by bleomycin in vivo and by TNF-α in vitro (Figures 1–3). These findings support a role for SIRT1 as an antiinflammatory mediator in pulmonary fibrosis development. Further studies found that SIRT1 activation ameliorated established fibrosis (Figure 5). SIRT1 effectively inhibited collagen production and myofibroblast differentiation in TGF-β–induced fibrosis in lung fibroblast in the absence of inflammation (Figures 6A–6D), and, consistent with our previous observation that SIRT1 has antifibrotic effects by targeting TGF-β signaling (21), we observed that SIRT1 inhibits the TGF-β pathway in a cellular context (Figures 6E and 6F). Therefore, the enhancement of SIRT1 activity is a potential treatment for patients with established pulmonary fibrosis.
SIRT1 is a widely expressed NAM adenine dinucleotide–dependent deacetylase, and studies in vitro and in vivo have implicated SIRT1 in the regulation of inflammation due to its deacetylase activity. NF-κB, a key intracellular mediator of inflammatory responses, is a deacetylation target of SIRT1, responsible for the expression of many cytokines, such as TNF-α, IL-6, and IL-1β (18, 43, 44). NF-κB is sequestered in the cytoplasm through interactions with the inhibitor protein, IκB. Numerous extracellular stimuli can activate NF-κB through signal transduction pathways that activate an IκB kinase complex that phosphorylates IκBα on serines 32 and 36. The phosphorylation of IκBα leads to its ubiquitination and ultimate degradation by the proteasome, allowing NF-κB to translocate to the nucleus, where it activates the expression of genes. Recently, it was found that resveratrol decreases the protein level of NF-κB to modulate the activity of vascular endothelial growth factor and IL-8 (45). In vivo, resveratrol has been demonstrated to reduce NF-κB p65 protein expression and exert protection in an experimentally induced colitis model (46). Resveratrol may also inhibit the activation of the IκB kinase, thereby inhibiting the activation of NF-κB (47). As exhibited by this work and that of others, resveratrol may additionally cause a decrease in the acetylation of NF-κB p65 to inhibit the activation of NF-κB via SIRT1 (18, 33). Our results in vitro demonstrated that NF-κB signaling and p65 acetylation increased in response to TNF-α and decreased after SIRT1 activation or overexpression (Figures 4A–4D). These findings are consistent with the results of our experiments in vivo, in which resveratrol treatment increased SIRT1 expression levels and activity and reduced NF-κB signaling (Figures 4E–4H). As such, our studies revealed that actions on NF-κB signaling participate in the antiinflammatory actions of SIRT1 in pulmonary fibrosis.
Along with targeting NF-κB, resveratrol had been identified to have additional effects on other regulatory pathways. It has been shown that resveratrol activates mitogen-activated protein kinase in a SIRT1-dependent manner (48). In addition, it has been found that resveratrol prevents inflammation and fibrosis through inhibiting the activation of signal transducer and activator of transcription 3 (49). Furthermore, both phosphatidylinositol 3-kinase and vascular endothelial growth factor have been implicated in pulmonary fibrosis in the bleomycin mouse model (50), and resveratrol administration inhibited their functions (29, 51). Moreover, basic fibroblast growth factor, a hallmark of idiopathic pulmonary fibrosis (52), may exert fibrogenic effects, and it may also be regulated by resveratrol (53). Therefore, it is possible that these various processes may additionally contribute to the effects of resveratrol observed in the present study.
The present work is the first to investigate the role of SIRT1 in SSc-related pulmonary fibrosis. We reveal that SIRT1 expression is downregulated in patients with SSc with pulmonary fibrosis, and in vivo and in vitro studies demonstrated that SIRT1 prevents inflammation and fibrosis during pulmonary fibrosis development via the inactivation of NF-κB and TGF-β/mTOR signaling, respectively. We propose that SIRT1 activation may be a promising approach for both the prevention and treatment of SSc–related pulmonary fibrosis.
Acknowledgments
Acknowledgments
The authors appreciate the computational support provided by the High-End Computing Center located at Fudan University (Shanghai, China).
Footnotes
This work was partially supported by grants from the National Science Foundation of China grants 31521003 and 81470254, International S&T Cooperation Program of China grant 2013DFA30870, National Institutes of Health National Institute of Allergy and Infectious Diseases U01 grant 1U01AI090909, and the 111 Project grant B13016 from the Ministry of Education of China.
Author Contributions: J.W. and H.C. planned the project, designed experiments, analyzed the data, interpreted the results, and wrote the manuscript; H.C. performed the animal studies, the clinical study, most of the in vitro studies, and the corresponding molecular studies, as well as the histopathological studies; S.J. performed part of the in vitro study and its corresponding molecular studies and performed the evaluations of infiltrated leukocytes in the in vivo studies; Q.L., Y.M., and M.L. assisted with the in vivo studies; X. Zhu, X.S., and W.D. assisted with the in vitro studies; F.Q. helped design the project; X. Zhou, H.Z., P.W.S., and L.J. edited the manuscript; all authors read and approved the final manuscript.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2016-0192OC on August 11, 2017
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Wang J, Assassi S, Guo G, Tu W, Wu W, Yang L, et al. Clinical and serological features of systemic sclerosis in a Chinese cohort. 2013;32:617–621. doi: 10.1007/s10067-012-2145-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Homer RJ, Herzog EL. Recent advances in pulmonary fibrosis: implications for scleroderma. 2010;22:683–689. doi: 10.1097/BOR.0b013e32833ddcc9. [DOI] [PubMed] [Google Scholar]
- 3.Steen VD, Owens GR, Fino GJ, Rodnan GP, Medsger TA., Jr Pulmonary involvement in systemic sclerosis (scleroderma) 1985;28:759–767. doi: 10.1002/art.1780280706. [DOI] [PubMed] [Google Scholar]
- 4.Wu T, Chu H, Tu W, Song M, Chen D, Yuan J, et al. Dissection of the mechanism of traditional Chinese medical prescription–Yiqihuoxue formula as an effective anti-fibrotic treatment for systemic sclerosis. 2014;14:224. doi: 10.1186/1472-6882-14-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolters PJ, Collard HR, Jones KD. Pathogenesis of idiopathic pulmonary fibrosis. 2014;9:157–179. doi: 10.1146/annurev-pathol-012513-104706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herzog EL, Mathur A, Tager AM, Feghali-Bostwick C, Schneider F, Varga J. Review: interstitial lung disease associated with systemic sclerosis and idiopathic pulmonary fibrosis: how similar and distinct? 2014;66:1967–1978. doi: 10.1002/art.38702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Veraldi KL, Hsu E, Feghali-Bostwick CA. Pathogenesis of pulmonary fibrosis in systemic sclerosis: lessons from interstitial lung disease. 2010;12:19–25. doi: 10.1007/s11926-009-0071-8. [DOI] [PubMed] [Google Scholar]
- 8.Noble PW, Barkauskas CE, Jiang D. Pulmonary fibrosis: patterns and perpetrators. 2012;122:2756–2762. doi: 10.1172/JCI60323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Toonkel RL, Hare JM, Matthay MA, Glassberg MK. Mesenchymal stem cells and idiopathic pulmonary fibrosis: potential for clinical testing. 2013;188:133–140. doi: 10.1164/rccm.201207-1204PP. [DOI] [PubMed] [Google Scholar]
- 10.Vaillant P, Menard O, Vignaud JM, Martinet N, Martinet Y. The role of cytokines in human lung fibrosis. 1996;51:145–152. [PubMed] [Google Scholar]
- 11.Di Paola R, Talero E, Galuppo M, Mazzon E, Bramanti P, Motilva V, et al. Adrenomedullin in inflammatory process associated with experimental pulmonary fibrosis. 2011;12:41. doi: 10.1186/1465-9921-12-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wynn TA. Integrating mechanisms of pulmonary fibrosis. 2011;208:1339–1350. doi: 10.1084/jem.20110551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blander G, Guarente L. The Sir2 family of protein deacetylases. 2004;73:417–435. doi: 10.1146/annurev.biochem.73.011303.073651. [DOI] [PubMed] [Google Scholar]
- 14.Chen B, Zang W, Wang J, Huang Y, He Y, Yan L, et al. The chemical biology of sirtuins. 2015;44:5246–5264. doi: 10.1039/c4cs00373j. [DOI] [PubMed] [Google Scholar]
- 15.Matsushima S, Sadoshima J. The role of sirtuins in cardiac disease. 2015;309:H1375–H1389. doi: 10.1152/ajpheart.00053.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharples AP, Hughes DC, Deane CS, Saini A, Selman C, Stewart CE. Longevity and skeletal muscle mass: the role of IGF signalling, the sirtuins, dietary restriction and protein intake. 2015;14:511–523. doi: 10.1111/acel.12342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ming M, Zhao B, Shea CR, Shah P, Qiang L, White SR, et al. Loss of sirtuin 1 (SIRT1) disrupts skin barrier integrity and sensitizes mice to epicutaneous allergen challenge. 2015;135:936–945.e4. doi: 10.1016/j.jaci.2014.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu X, Liu Q, Wang M, Liang M, Yang X, Xu X, et al. Activation of Sirt1 by resveratrol inhibits TNF-α induced inflammation in fibroblasts. 2011;6:e27081. doi: 10.1371/journal.pone.0027081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moon MH, Jeong JK, Lee YJ, Seol JW, Jackson CJ, Park SY. SIRT1, a class III histone deacetylase, regulates TNF-α–induced inflammation in human chondrocytes. 2013;21:470–480. doi: 10.1016/j.joca.2012.11.017. [DOI] [PubMed] [Google Scholar]
- 20.Gillum MP, Kotas ME, Erion DM, Kursawe R, Chatterjee P, Nead KT, et al. SirT1 regulates adipose tissue inflammation. 2011;60:3235–3245. doi: 10.2337/db11-0616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wei J, Ghosh AK, Chu H, Fang F, Hinchcliff ME, Wang J, et al. The histone deacetylase sirtuin 1 is reduced in systemic sclerosis and abrogates fibrotic responses by targeting transforming growth factor β signaling. 2015;67:1323–1334. doi: 10.1002/art.39061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zerr P, Palumbo-Zerr K, Huang J, Tomcik M, Sumova B, Distler O, et al. Sirt1 regulates canonical TGF-β signalling to control fibroblast activation and tissue fibrosis. 2016;75:226–233. doi: 10.1136/annrheumdis-2014-205740. [DOI] [PubMed] [Google Scholar]
- 23.Huang XZ, Wen D, Zhang M, Xie Q, Ma L, Guan Y, et al. Sirt1 activation ameliorates renal fibrosis by inhibiting the TGF-β/Smad3 pathway. 2014;115:996–1005. doi: 10.1002/jcb.24748. [DOI] [PubMed] [Google Scholar]
- 24.Cappetta D, Esposito G, Piegari E, Russo R, Ciuffreda LP, Rivellino A, et al. SIRT1 activation attenuates diastolic dysfunction by reducing cardiac fibrosis in a model of anthracycline cardiomyopathy. 2016;205:99–110. doi: 10.1016/j.ijcard.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 25.Wu Y, Liu X, Zhou Q, Huang C, Meng X, Xu F, et al. Silent information regulator 1 (SIRT1) ameliorates liver fibrosis via promoting activated stellate cell apoptosis and reversion. 2015;289:163–176. doi: 10.1016/j.taap.2015.09.028. [DOI] [PubMed] [Google Scholar]
- 26.Preliminary criteria for the classification of systemic sclerosis (scleroderma). Subcommittee for Scleroderma Criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. 1980;23:581–590. doi: 10.1002/art.1780230510. [DOI] [PubMed] [Google Scholar]
- 27.Hattori N, Degen JL, Sisson TH, Liu H, Moore BB, Pandrangi RG, et al. Bleomycin-induced pulmonary fibrosis in fibrinogen-null mice. 2000;106:1341–1350. doi: 10.1172/JCI10531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Q, Chu H, Ma Y, Wu T, Qian F, Ren X, et al. Salvianolic acid B attenuates experimental pulmonary fibrosis through inhibition of the TGF-β signaling pathway. 2016;6:27610. doi: 10.1038/srep27610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang M, Li W, Yu L, Wu S. The suppressive effect of resveratrol on HIF-1α and VEGF expression after warm ischemia and reperfusion in rat liver. 2014;9:e109589. doi: 10.1371/journal.pone.0109589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ponnusamy M, Zhou X, Yan Y, Tang J, Tolbert E, Zhao TC, et al. Blocking sirtuin 1 and 2 inhibits renal interstitial fibroblast activation and attenuates renal interstitial fibrosis in obstructive nephropathy. 2014;350:243–256. doi: 10.1124/jpet.113.212076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liang J, Tian S, Han J, Xiong P. Resveratrol as a therapeutic agent for renal fibrosis induced by unilateral ureteral obstruction. 2014;36:285–291. doi: 10.3109/0886022X.2013.844644. [DOI] [PubMed] [Google Scholar]
- 32.Hong SW, Jung KH, Zheng HM, Lee HS, Suh JK, Park IS, et al. The protective effect of resveratrol on dimethylnitrosamine-induced liver fibrosis in rats. 2010;33:601–609. doi: 10.1007/s12272-010-0415-y. [DOI] [PubMed] [Google Scholar]
- 33.Ito K. Impact of post-translational modifications of proteins on the inflammatory process. 2007;35:281–283. doi: 10.1042/BST0350281. [DOI] [PubMed] [Google Scholar]
- 34.Chaudhary NI, Schnapp A, Park JE. Pharmacologic differentiation of inflammation and fibrosis in the rat bleomycin model. 2006;173:769–776. doi: 10.1164/rccm.200505-717OC. [DOI] [PubMed] [Google Scholar]
- 35.Moeller A, Ask K, Warburton D, Gauldie J, Kolb M. The bleomycin animal model: a useful tool to investigate treatment options for idiopathic pulmonary fibrosis? 2008;40:362–382. doi: 10.1016/j.biocel.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mitra A, Luna JI, Marusina AI, Merleev A, Kundu-Raychaudhuri S, Fiorentino D, et al. Dual mTOR inhibition is required to prevent TGF-β–mediated fibrosis: implications for scleroderma. 2015;135:2873–2876. doi: 10.1038/jid.2015.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gui YS, Wang L, Tian X, Li X, Ma A, Zhou W, et al. mTOR overactivation and compromised autophagy in the pathogenesis of pulmonary fibrosis. 2015;10:e0138625. doi: 10.1371/journal.pone.0138625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park JS, Park HJ, Park YS, Lee SM, Yim JJ, Yoo CG, et al. Clinical significance of mTOR, ZEB1, ROCK1 expression in lung tissues of pulmonary fibrosis patients. 2014;14:168. doi: 10.1186/1471-2466-14-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fried L, Kirsner RS, Bhandarkar S, Arbiser JL. Efficacy of rapamycin in scleroderma: a case study. 2008;6:217–219. doi: 10.1089/lrb.2008.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tamaki Z, Asano Y, Kubo M, Ihn H, Tada Y, Sugaya M, et al. Effects of the immunosuppressant rapamycin on the expression of human α2(I) collagen and matrix metalloproteinase 1 genes in scleroderma dermal fibroblasts. 2014;74:251–259. doi: 10.1016/j.jdermsci.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 41.Zhou XL, Xu JJ, Ni YH, Chen XC, Zhang HX, Zhang XM, et al. SIRT1 activator (SRT1720) improves the follicle reserve and prolongs the ovarian lifespan of diet-induced obesity in female mice via activating SIRT1 and suppressing mTOR signaling. 2014;7:97. doi: 10.1186/s13048-014-0097-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wells AU, Steen V, Valentini G. Pulmonary complications: one of the most challenging complications of systemic sclerosis. 2009;48:iii40–iii44. doi: 10.1093/rheumatology/kep109. [DOI] [PubMed] [Google Scholar]
- 43.Xie J, Zhang X, Zhang L. Negative regulation of inflammation by SIRT1. 2013;67:60–67. doi: 10.1016/j.phrs.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 44.Yang SR, Wright J, Bauter M, Seweryniak K, Kode A, Rahman I. Sirtuin regulates cigarette smoke–induced proinflammatory mediator release via RelA/p65 NF-κB in macrophages in vitro and in rat lungs in vivo: implications for chronic inflammation and aging. 2007;292:L567–L576. doi: 10.1152/ajplung.00308.2006. [DOI] [PubMed] [Google Scholar]
- 45.Tino AB, Chitcholtan K, Sykes PH, Garrill A. Resveratrol and acetyl-resveratrol modulate activity of VEGF and IL-8 in ovarian cancer cell aggregates via attenuation of the NF-κB protein. 2016;9:84. doi: 10.1186/s13048-016-0293-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martín AR, Villegas I, Sánchez-Hidalgo M, de la Lastra CA. The effects of resveratrol, a phytoalexin derived from red wines, on chronic inflammation induced in an experimentally induced colitis model. 2006;147:873–885. doi: 10.1038/sj.bjp.0706469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Holmes-McNary M, Baldwin AS., Jr Chemopreventive properties of trans-resveratrol are associated with inhibition of activation of the IκB kinase. 2000;60:3477–3483. [PubMed] [Google Scholar]
- 48.Price NL, Gomes AP, Ling AJ, Duarte FV, Martin-Montalvo A, North BJ, et al. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. 2012;15:675–690. doi: 10.1016/j.cmet.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kessoku T, Imajo K, Honda Y, Kato T, Ogawa Y, Tomeno W, et al. Resveratrol ameliorates fibrosis and inflammation in a mouse model of nonalcoholic steatohepatitis. 2016;6:22251. doi: 10.1038/srep22251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kulkarni YM, Dutta S, Iyer AK, Venkatadri R, Kaushik V, Ramesh V, et al. A proteomics approach to identifying key protein targets involved in VEGF inhibitor mediated attenuation of bleomycin-induced pulmonary fibrosis. 2016;16:33–46. doi: 10.1002/pmic.201500171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Poolman TM, Ng LL, Farmer PB, Manson MM. Inhibition of the respiratory burst by resveratrol in human monocytes: correlation with inhibition of PI3K signaling. 2005;39:118–132. doi: 10.1016/j.freeradbiomed.2005.02.036. [DOI] [PubMed] [Google Scholar]
- 52.Inoue Y, King TE, Jr, Barker E, Daniloff E, Newman LS.Basic fibroblast growth factor and its receptors in idiopathic pulmonary fibrosis and lymphangioleiomyomatosis 2002166765–773 [DOI] [PubMed] [Google Scholar]
- 53.Uchiyama T Toda K Takahashi S Resveratrol inhibits angiogenic response of cultured endothelial f-2 cells to vascular endothelial growth factor, but not to basic fibroblast growth factor 2010331095–1100 [DOI] [PubMed] [Google Scholar]