Abstract
Chlorine is a highly reactive gas that can cause significant injury when inhaled. Unfortunately, its use as a chemical weapon has increased in recent years. Massive chlorine inhalation can cause death within 4 hours of exposure. Survivors usually require hospitalization after massive exposure. No countermeasures are available for massive chlorine exposure and supportive-care measures lack controlled trials. In this work, adult rats were exposed to chlorine gas (LD58–67) in a whole-body exposure chamber, and given oxygen (0.8 FiO2) or air (0.21 FiO2) for 6 hours after baseline measurements were obtained. Oxygen saturation, vital signs, respiratory distress and neuromuscular scores, arterial blood gases, and hemodynamic measurements were obtained hourly. Massive chlorine inhalation caused severe acute respiratory failure, hypoxemia, decreased cardiac output, neuromuscular abnormalities (ataxia and hypotonia), and seizures resulting in early death. Oxygen improved survival to 6 hours (87% versus 42%) and prevented observed seizure-related deaths. However, oxygen administration worsened the severity of acute respiratory failure in chlorine-exposed rats compared with controls, with increased respiratory acidosis (pH 6.91 ± 0.04 versus 7.06 ± 0.01 at 2 h) and increased hypercapnia (180.0 ± 19.8 versus 103.2 ± 3.9 mm Hg at 2 h). In addition, oxygen did not improve neuromuscular abnormalities, cardiac output, or respiratory distress associated with chlorine exposure. Massive chlorine inhalation causes severe acute respiratory failure and multiorgan damage. Oxygen administration can improve short-term survival but appears to worsen respiratory failure, with no improvement in cardiac output or neuromuscular dysfunction. Oxygen should be used with caution after massive chlorine inhalation, and the need for early assisted ventilation should be assessed in victims.
Keywords: chlorine gas, hypoxemia, oxygen, respiratory distress, seizure
Clinical Relevance
Intentional chlorine gas attacks are on the rise globally, without any known efficacious treatments to improve outcomes after exposure. In our studies, we found that oxygen administration after chlorine gas inhalation exposure in rats improved survival but worsened other morbidity measures that correlate with deleterious outcomes in the clinical setting. Although oxygen administration will be helpful in the treatment of acute hypoxemia after chlorine inhalation, caution must be exercised if there is evidence of respiratory acidosis, as oxygen treatment alone can worsen respiratory failure.
Chlorine (Cl2) is a yellow-green gas that is slightly water soluble and about two times heavier than air. It is a highly reactive oxidant gas that is commonly used in the bleaching of paper, production of hydrocarbon solvents, and disinfection of swimming pools, and as a chemical weapon (1). Over 14 million tons of chlorine are produced and transported on freight trains and highways annually in the United States (2, 3), increasing the potential for accidents or misuse.
Chlorine was first used as a chemical weapon in Ypres, France, during the First World War (4). Since then, due to its readiness and availability, it has been used on numerous occasions. In the ongoing Syrian civil war, the intentional use of chlorine to cause widespread casualties was seen in the towns of Kafr Zita, Harasta, and Damascus in April 2014. Such events have raised concerns that chlorine will be used more frequently in the near future as a weapon of opportunity by terrorists, globally as well as within the United States.
Chlorine is a known pulmonary irritant gas that causes acute damage in the respiratory system after inhalation (5). Chlorine inhalation can be acutely fatal and this effect appears to be dose dependent, with the greatest number of fatalities occurring in the immediate vicinity of a spill. The lethal effects are dependent not only on the inhaled concentration but also on the duration of exposure, and death is due to cardiopulmonary failure (6). After inhalation, chlorine reacts with water in the mucous membranes and airways to form hydrogen chloride and hypochlorous acid (7), which causes significant damage. The clinical symptoms after mild inhalation include eye, nose, and throat irritation, with progression to bronchoconstriction, cough, wheezing, and dyspnea after moderate exposure (8). Inhalation of massive doses of chlorine results in multiorgan injury, with abnormalities seen in the pulmonary, cardiovascular, and neuromuscular systems (9, 10). Acute pulmonary injury after massive chlorine inhalation involves bronchoconstriction, drastic hypoxemia, with a potential for interstitial pneumonia, pulmonary edema, and acute lung injury (ALI). Acute cardiovascular changes that occur after massive chlorine inhalation include decreased cardiac performance, acute pulmonary arterial hypertension (PAH), and myocardial infarction (3, 11, 12). Neuromuscular changes include ataxia and syncope with a potential for seizures.
Therapies for acute chlorine exposure include supportive-care measures such as oxygen administration for hypoxemia, bronchodilators for wheezing, and positive-pressure ventilation and intubation for severe symptoms (1). Several other treatments are also used on occasion, including the use of steroids and nebulized bicarbonate, but they are controversial (13). None of these supportive measures have been well studied after chlorine inhalation, and there are only anecdotal reports of their use in exposed individuals.
Oxygen is routinely administered to individuals with hypoxemia after chlorine inhalation, both in transport and in tertiary-care centers. In this work, we report on the effects of supplemental oxygen after acute high-dose chlorine inhalation exposure in rats. We assessed the ability of oxygen to alter not only acute mortality but also serious morbidity, including neuromuscular and cardiopulmonary parameters.
Methods
Complete details regarding the materials and methods used in this work can be found in the data supplement.
Animals
Sprague-Dawley rats (male, 300–340 g; Charles River Laboratories) were housed in an animal care facility at the University of Colorado Denver that was fully accredited by the Association for Assessment and Accreditation of Laboratory and Animal Care International before experiments were performed. The animals were allowed to acclimate to the altitude in Denver (5,280 ft) for 8–14 days before the start of experiments.
Chlorine Exposure
Whole-body exposure to chlorine gas was performed in two glass chambers (Specialty Glass Inc.) connected in parallel (two rats per chamber) by chlorine gas tanks (Airgas Specialty Gases). The lethal dose (LD) is an indication of the lethal toxicity of a substance within a set time-frame (6 h in this study), at which a given percentage of subjects will die. We achieved an LD17 by giving 500 ppm (30 min), an LD58 by giving 600 ppm (27 min), and an LD75 by giving 600 ppm (30 min) of chlorine. Rats with invasive intravascular catheters were more susceptible to chlorine injury, and an LD67 was achieved by administering 250 ppm of chlorine for 30 minutes. For the times of death for each group shown in the figures, see Table E1 in the data supplement.
Oxygen Administration
Rats were placed in plexiglass chambers (four rats per chamber) and given room air (0.21 fraction of inspired oxygen [FiO2]) or oxygen (0.8 FiO2), to normalize oxygen saturation to 90–92% (normal at Denver altitude) at 5.0 liters/min.
Pulse Oximetry and Respiratory Rate Assessments
A pulse oximeter (Starr Life Sciences) was used to collect peripheral oxygen saturation (SpO2) data from the rats before and hourly after chlorine exposure. The respiratory rate (RR) was obtained manually over 1 minute (bpm).
Respiratory Distress and Neuromuscular Scoring
Respiratory quality was scored on a scale of 0–6 using a previously described method (14, 15). Neuromuscular changes were scored on a scale of 0–10, using a scoring system developed by our group to encompass evaluations of four criteria: posture, ataxia, seizure activity, and tone (Table 1). Seizure was defined as a sudden paroxysmal episode of involuntary muscle contractions, which included muscle stiffening, uncontrolled jerking, flailing violently, and falling over.
Table 1.
Neuromuscular Scoring Criteria
| Posture | Ataxia | Seizure Activity | Tone |
|---|---|---|---|
| 0: normal | 0: normal | 0: normal | 0: normal |
| 1: low posture | 1: ataxia, no falling | 1: twitches/shuddering | 1: mild hypotonia |
| 2: lying on ground, straight posture | 2: ataxia with falling | 2: active myoclonic seizure | 2: moderate hypotonia |
| 3: lying on ground, leaning posture | 3: severe hypotonia |
Arterial Blood Gas Analysis and Po2/FiO2 Ratio Calculation
Blood was collected from the descending aorta after the rats were given ketamine (75 mg/kg), xylazine (7.5 mg/kg), and acepromazine (1.5 mg/kg) anesthesia. Blood samples were analyzed using the EPOC-Vet blood analysis system (Epocal Inc.). The Po2/FiO2 (P/F) ratio was back-calculated from So2 values, corrected to 620 mm Hg altitude in Denver.
Cardiac Catheterization
Two days before chlorine exposure, jugular venous and carotid artery access ports were placed in a group of rats under general anesthesia. Cardiac output (CO) was measured using a hemodilution method, heart rate (HR) was directly measured via a sensor within the indwelling arterial line, and stroke volume (SV) was calculated using the formula CO = SV × HR.
Animal Deaths
Rats were killed as planned at either 2 hours or 6 hours, or died spontaneously before the end of study. The rats were killed using a combination of intraperitoneal ketamine (75 mg/kg), xylazine (7.5 mg/kg), and acepromazine (1.5 mg/kg), with subsequent diaphragmatic rupture and exsanguination.
Statistical Analysis
For statistical analysis, Prism 6 software (GraphPad) was used, with one-way ANOVA followed by Tukey’s post hoc analysis for studies with more than two groups. When only two groups were compared, a t test (unpaired, two-tailed) was used for analysis. All mean values are reported with SEM. A P value < 0.05 was considered significant. Survival data were analyzed via the Mantel–Cox log-rank test.
Results
Survival
We observed differences in the rats’ survival after varying the levels of chlorine gas inhalation, with and without oxygen administration (Figures 1A and 1B). We found that higher doses of chlorine caused lower survival in the acute period (to 6 h), with most mortalities occurring in the earliest hours after exposure (Figure 1A). Previous studies by our group showed no acute deaths after the initial 6-hour period (data not shown) in any group, monitored for up to 7 days, and therefore in this study we focused on a 6-hour endpoint. Next, we evaluated the effect of oxygen administration on survival after chlorine exposure (Figure 1B). Using the LD58 chlorine inhalation model, we administered oxygen (0.8 FiO2) after an initial assessment by placing the rats into an oxygen chamber. We found that administration of oxygen after chlorine inhalation significantly improved survival compared with no treatment (87% survival with oxygen versus 42% without; P < 0.05). Of note, all animals in the untreated group died during an active myoclonic seizure, whereas the one animal that died in the oxygen-treated group had apnea instead of seizure just before death. Thus, oxygen administration improved survival to 6 hours, and prevented seizure-associated deaths caused by chlorine gas inhalation within this time period.
Figure 1.
Effect of oxygen administration on survival (to 6 h) after chlorine (LD58) inhalation exposure in rats. (A) Lethal-dose evaluation of escalating doses of chlorine gas in rats (LD17, n = 6; LD58, n = 12; LD75, n = 8). (B) Effects of oxygen treatment on survival after high-level chlorine (LD58) inhalation exposure. Oxygen treatment (0.8 fraction of inspired oxygen [FiO2], dashed line; n = 8) improved survival after chlorine inhalation exposure compared with control (solid line; n = 12). P = 0.0467, log-rank, Mantel–Cox test.
Acidosis, Hypercapnia, and Lactic Acidosis
To evaluate the effects of oxygen administration on blood acidosis and ventilation parameters, we analyzed arterial blood gas values after chlorine inhalation exposure (LD58) with and without oxygen treatment (Figures 2A–2C and E1). We found that chlorine inhalation caused severe blood acidosis by 2 hours after exposure to chlorine gas, compared with naive, and oxygen administration significantly worsened the acidosis to an extremely severe level (pH of 7.06 ± 0.01 in chlorine only versus 6.87 ± 0.06 in chlorine plus oxygen, and 7.43 ± 0.01 in the naive group; P < 0.0001, ANOVA; Figure 2A). By 6 hours, the blood pH improved only slightly in all survivors, with moderate acidosis in the chlorine-alone group, and severe acidosis with oxygen administration (pH of 7.23 ± 0.05 in chlorine only versus 7.10 ± 0.05 in chlorine plus oxygen). Similarly, we noted a significant 3-fold elevation in the arterial partial pressure of carbon dioxide (PaCO2) after chlorine exposure PaCO2 after chlorine exposure compared with naive values, which further worsened after oxygen administration at 2 hours (PaCO2 of 103.2 ± 3.9 mm Hg with chlorine only, versus 194.4 ± 20.1 mm Hg with chlorine plus oxygen, and 35.7 ± 0.9 in the naive group; P < 0.0001, ANOVA; Figure 2B). By 6 hours, PaCO2 improved greatly in survivors, with near-normal levels in the chlorine-only group, but with continued severely elevated levels in the oxygen-treatment group (PaCO2 of 69.9 ± 4.3 mm Hg with chlorine only, versus 104.9 ± 11.5 mm Hg with chlorine plus oxygen). Similarly, the levels of bicarbonate, a marker of chronic CO2 retention, were elevated at 2 hours after chlorine inhalation, and were significantly worse after oxygen administration (bicarbonate levels of 29.1 ± 0.9 mmol/liter with chlorine only, versus 35.9 ± 0.6 mmol/liter in chlorine plus oxygen, and 23.6 ± 0.2 mmol/liter in the naive group; P < 0.001, ANOVA; Figure E1). At 6 hours, bicarbonate levels continued to be elevated in both groups but were no longer significantly different (bicarbonate levels of 29.3 ± 1.9 mmol/liter with chlorine only, versus 31.0 ± 0.7 mmol/liter in chlorine plus oxygen). The hypercapnia observed after chlorine inhalation indicated a severe ventilation defect leading to acute respiratory failure, which significantly improved by 6 hours in survivors. However, oxygen administration caused a worsening in CO2 retention at all time points compared with no treatment, potentially due to a decreased hypoxia-induced ventilator drive.
Figure 2.
Effects of oxygen administration (0.8 FiO2) on arterial blood pH, arterial partial pressure of carbon dioxide (PaCO2), and lactate levels after LD58 inhalation exposure in rats, obtained at 2 hours. (A) Arterial pH in oxygen-treated versus untreated groups after chlorine exposure, compared with naive values over time. Note the severe blood acidosis after chlorine exposure, which significantly worsened with oxygen therapy at both 2 hours and 6 hours (P < 0.01). Horizontal dotted lines show clinical grades of acidosis severity. (B) PaCO2 in oxygen-treated versus untreated groups after chlorine exposure compared with naive values over time. Note the severely increased PaCO2 at 2 hours after chlorine exposure, which significantly worsened with oxygen therapy (P < 0.001) and sustained to above respiratory failure levels at 6 hours. Horizontal dotted lines show clinical grades of CO2 retention severity. (C) Lactate levels in oxygen-treated versus untreated groups after chlorine exposure compared with naive values over time. Note the increased lactate levels after chlorine exposure, which improved with oxygen therapy (P = 0.0001) at 2 hours but worsened with oxygen at 6 hours. Values represent means ± SEM; ANOVA for repeated measures, Tukey’s post hoc analysis; ****P < 0.0001; ***P < 0.001; **P < 0.01; *P < 0.05. n = 4 per group at 2 hours, n = 7 per group at 6 hours.
Although ventilation was worsened with oxygen administration after chlorine exposure, the levels of lactate, a biomarker of tissue hypoxia, improved shortly after the start of oxygen therapy at 2 hours (lactate of 4.4 ± 0.4 mmol/liter with chlorine only, versus 0.7 ± 0.1 mmol/liter with chlorine plus oxygen, and 2.1 ± 0.4 mmol/liter in the naive group; P < 0.001, ANOVA; Figure 2C). However, at 6 hours, lactate levels were increased with oxygen therapy but were trending down in the chlorine-only group (lactate of 2.7 ± 1.1 mmol/liter with chlorine only, versus 4.0 ± 0.7 mmol/liter with chlorine plus oxygen). Thus, although oxygen administration improved tissue oxygenation acutely, this beneficial effect of oxygen was not sustained over 6 hours of observation.
Hypoxemia and Decreased P/F Ratio
To evaluate whether oxygen administration after high-level chlorine inhalation could improve the observed hypoxemia due to exposure, we next assessed SpO2 over time, and calculated the P/F ratio hourly for 6 hours (Figures 3A and 3B). We found that immediately after chlorine inhalation exposure (LD58), SpO2 severely decreased from pre-exposure levels (mean SpO2 of 92.9 ± 0.4% versus 50.9 ± 4.8% immediately after exposure; P < 0.0001, t test; Figure 3A). Oxygen administration (0.8 FiO2) resulted in an improvement of SpO2 to near-normal levels compared with untreated controls (mean SpO2 over 6 hours of 85–90% with chlorine plus oxygen, versus 46–52% with chlorine only; P < 0.0001 at all time points, t test).
Figure 3.
Peripheral oxygen saturation (SpO2) and partial pressure of arterial oxygen/fraction of inspired oxygen (PaO2/FiO2) trends after LD58 inhalation exposure with and without oxygen administration (0.8 FiO2), obtained at Denver altitude. (A) SpO2 values obtained hourly after chlorine inhalation with and without oxygen therapy. Oxygen administration (squares with dashed line) significantly improved SpO2 to near-normal levels compared with untreated controls (triangles with solid line) throughout the entire study (P < 0.0001). (B) PaO2/FiO2 ratios calculated hourly after chlorine inhalation with (triangles with solid line) and without (squares with dashed line) oxygen therapy. Note the worsening of PaO2/FiO2 ratios immediately after chlorine exposure, which worsened with oxygen therapy. Values represent means ± SEM; statistical analysis via unpaired t test; ****P < 0.0001; ***P < 0.001; **P < 0.01; *P < 0.05. n = 8 per group.
To further assess acute hypoxic respiratory failure due to chlorine exposure, and the effects of oxygen administration on such failure, we next evaluated the P/F ratio over time. The P/F ratio is used as a marker of an impaired physiological relationship between inspired oxygen and the arterial partial pressure of oxygen, and it is a valuable clinical measure of respiratory status in individuals receiving oxygen. We found that in our model, the P/F ratio calculated for Denver altitude showed a severe decrease from normal after chlorine exposure (P/F ratio of 322 ± 8 versus 92 ± 5 immediately after exposure; P < 0.0001, t test; Figure 3B), consistent with severe hypoxic respiratory failure. After oxygen was administered, the P/F ratio did not improve, and instead it worsened the hypoxic respiratory failure to an extremely severe level, which persisted over the entire study period (P/F ratio over 6 hours of 65–72 with chlorine plus oxygen, versus 85–113 with chlorine alone; P < 0.001 at 0.5 h; P < 0.01 at 1 and 2 h; P < 0.05 at 3 and 6 h; t test). We conclude that although oxygen administration after acute chlorine inhalation will improve the apparent hypoxemia caused by chlorine exposure, oxygen therapy alone will lead to a worsening of acute respiratory failure due to chlorine.
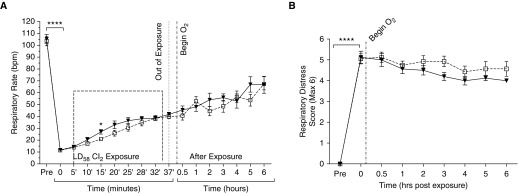
Bradypnea and Respiratory Distress
The RR and work of breathing were assessed after chlorine inhalation exposure (LD58), with and without oxygen administration (Figures 4A and 4B). Within minutes after the start of chlorine exposure, the RR of the rats in the inhalation chambers showed a 10-fold decrease from normal (RR of 105 ± 4 bpm versus 11 ± 1 bpm immediately at the start of exposure; P < 0.0001, t test; Figure 4A). During the entire exposure, the RR improved only slightly, but consistently, and was still only 30% of normal at the end of exposure (42 ± 2 bpm). When we compared oxygen-treated (0.8 FiO2) rats with untreated rats for 6 hours after exposure, we noticed a slow and steady improvement in RR in both groups, which was not significantly different between the groups at any time point assessed.
Figure 4.
Respiratory parameters after LD58 inhalation exposure with and without oxygen administration (0.8 FiO2). (A) Variations in the respiratory rate with oxygen before, during, and after chlorine inhalation. Chlorine exposure resulted in severe bradypnea (triangles with solid line), with slow improvement over 6 hours, and oxygen administration (squares with dashed line) did not improve respiratory rates throughout the study. (B) The respiratory distress score, a measure of the work of breathing, was obtained hourly after chlorine inhalation. Note the worsening of respiratory distress scores immediately after chlorine exposure, which did not improve with oxygen therapy (squares with dashed line) compared with untreated controls (triangles with solid line). Values represent means ± SEM; statistical analysis via unpaired t test; ****P < 0.0001; *P < 0.05. n = 8 per group.
Together with the low RR, or bradypnea, we noticed alterations in the rats’ quality of breathing after chlorine exposure, including retractions, open-mouth breathing, and gasping. Therefore, we employed a semiquantitative respiratory scoring system to help assess the degree of change in respiratory quality with and without oxygen therapy (Figure 4B). We found that chlorine inhalation caused a significant worsening of respiratory quality, or work of breathing, compared with normal immediately after the end of exposure (respiratory distress score of 0 versus 5.1 ± 0.3 immediately after exposure; P < 0.0001, t test). This corresponded with severe retractions and severely increased work of breathing. Oxygen administration did not significantly improve respiratory distress scores throughout the entire 6-hour study. In fact, oxygen-treated rats tended to have worse respiratory distress than untreated controls over the last 4 hours of the study. Thus, oxygen administration (0.8 FiO2) failed to improve chlorine inhalation–induced bradypnea and respiratory distress, despite the relative normoxia achieved with oxygen administration.
Neuromuscular Defects and Seizure Activity
During preliminary studies, we observed that high-level chlorine–exposed rats acutely developed neuromuscular abnormalities, including poor posture, ataxia, hypotonia, and seizures, and these abnormalities were predictive of mortality. To further monitor this phenomenon, we developed a semiquantitative neuromuscular scoring system to help evaluate treatment effects in our model (minimum of 0, maximum of 10; Figure 5). We noted that chlorine inhalation exposure caused an acute worsening of neuromuscular scores compared with normal (neuromuscular score of 6.0 ± 0.4 in the chlorine-only group immediately after exposure versus 0 in the naive group; P < 0.0001, t test). The oxygen-treated group appeared to have a mildly decreased neuromuscular score compared with the untreated control group. Most notably, we observed no seizure activity in any rats treated with oxygen, whereas all untreated rats that spontaneously died in the study did so after an observed myoclonic seizure. Thus, it is likely that the chlorine exposure–induced seizures were hypoxemia related, since we effectively eliminated seizures in chlorine-exposed rats with oxygen therapy.
Figure 5.
Neuromuscular scores after LD58 inhalation exposure with and without oxygen administration (0.8 FiO2). Neuromuscular scores (observation scoring of ataxia, posture, tone, and seizure activity) were persistently elevated after chlorine inhalation (triangles with solid line) and minimally improved with oxygen therapy (squares with dashed line). Values represent means ± SEM; statistical analysis via unpaired t test; ****P < 0.0001; **P < 0.01. n = 12 per group.
Poor CO and Pulmonary Hypertension
To examine acute hemodynamic changes that occurred after chlorine gas inhalation, we placed intravascular catheters into the jugular vein and carotid artery of rats before exposing them to chlorine. Surprisingly, we found that rats with intravascular catheters were more susceptible to chlorine inhalation injury, with higher mortality observed at lower levels of inhaled chlorine, compared with noncatheterized rats (Figure E2). Thus, we used a lower level of chlorine gas (250 ppm × 30 min) to obtain an LD67 model (at 6 h) for our studies.
We first evaluated CO, SV, and HR after chlorine inhalation (LD67) with and without oxygen administration (1.0 FiO2; Figures 6A–6C). We found that CO, a correlate of end-organ perfusion, was reduced 2-fold immediately after chlorine exposure compared with normal (CO of 159 ± 22 ml/min pre-exposure versus 73 ± 10 ml/min immediately after exposure; P < 0.05, t test; Figure 6A), and this reduced-CO state was sustained throughout the 6-hour study period. Oxygen administration did not improve the CO compared with untreated controls at any time point evaluated. Similarly, we found that HR was greatly reduced after chlorine inhalation (HR of 436 ± 17 bpm pre-exposure versus 268 ± 11 bpm immediately after exposure; P < 0.0001, t test; Figure 6B), and this relative bradycardia persisted throughout the entire study period. Oxygen administration did not improve the bradycardia. Next, we calculated SV, a correlate of cardiac function, after chlorine inhalation, with and without oxygen therapy. We found no significant change in SV after chlorine exposure from normal pre-exposure values (SV of 0.27 ± 0.02 ml pre-exposure versus 0.33 ± 0.05 ml immediately after exposure; P > 0.05; t test; Figure 6C). Oxygen administration caused no significant change in SV after chlorine inhalation exposure. Therefore, the observed poor-CO state, and thus poor end-organ perfusion, after chlorine inhalation exposure was due mainly to bradycardia and not to any defect in cardiac contractility, as this was not observed. Moreover, as oxygen administration to normoxia did not improve the CO, hypoxemia was not the cause of the decreased CO and HR after chlorine inhalation.
Figure 6.
Cardiovascular parameters obtained after LD67 inhalation exposure in invasively catheterized rats with oxygen administration (0.8 FiO2, squares with dashed line) compared with untreated controls (triangles with solid line). (A) Cardiac output (CO) measurements obtained via the hemodilution method after chlorine inhalation. Note the precipitous decline in CO in both groups after exposure, with no improvement noted after oxygen administration. (B) Heart rates (HRs) obtained hourly after chlorine inhalation. Note the immediate and sustained decline in HR in both groups. (C) Stroke volumes (SVs) obtained after chlorine inhalation. SVs were not significantly altered at any time point after chlorine exposure and only tended to mildly decrease after oxygen therapy. Values represent means ± SEM; statistical analysis via unpaired t test; ****P < 0.0001; *P < 0.05. n = 5 in control group, n = 6 in oxygen-treated group. ns = not significant.
We next evaluated arterial pressures within the pulmonary and systemic beds to assess the effects of chlorine gas and oxygen administration on these compartments (Figures 7A and 7B). We found a significant increase in mean pulmonary artery pressure (MPAP) immediately after chlorine inhalation (MPAP of 22.7 ± 1.5 mm Hg pre-exposure versus 32.2 ± 3.5 mm Hg immediately after exposure; P < 0.05, t test; Figure 7A), consistent with acute mild pulmonary hypertension. This pulmonary hypertension worsened over time, peaking at 1 hour after exposure (39.5 ± 3.6 mm Hg), consistent with acute moderate pulmonary hypertension, and then decreased slightly over the rest of the study period. Oxygen administration (1.0 FiO2), a potent vasodilator, decreased MPAP at the 1-, 2-, and 3-hour time points compared with untreated controls, but this decrease was not statistically significant. Conversely, we found no significant change in the mean systemic artery pressure (MAP) immediately after chlorine inhalation (MAP of 121 ± 1.9 mm Hg pre-exposure versus 114 ± 3.2 mm Hg immediately after exposure; P > 0.05, t test; Figure 7B), or at any other time throughout the study. Although oxygen administration appeared to slightly increase the MAP after chlorine, this increase was not statistically significant. In summary, lethal chlorine gas inhalation causes an acute pulmonary hypertension state without systemic hypertension, and oxygen administration does not appear to alter this condition significantly.
Figure 7.
Pulmonary and systemic arterial pressures after LD67 inhalation exposure in invasively catheterized rats with oxygen administration (0.8 FiO2, squares with dashed line) compared with untreated controls (triangles with solid line). (A) The mean pulmonary artery pressure (MPAP) was obtained hourly after chlorine inhalation exposure with and without oxygen administration. Note the abrupt increase in MPAP early after chlorine inhalation, with oxygen therapy tending to normalize MPAP after administration (not significant). (B) Mean systemic artery pressure (MAP) obtained hourly after chlorine inhalation exposure with and without oxygen. No significant change occurred in MAP after chlorine inhalation or with oxygen. Values represent means ± SEM; statistical analysis via unpaired t test; *P < 0.05. n = 14 in control group, n = 5 in oxygen-treated group.
Discussion
In our rat model of chlorine inhalation exposure, we found that oxygen administration (0.8 FiO2) was effective in improving survival to 6 hours, although it also increased disease morbidity. In practice, oxygen therapy is routinely given for acute chlorine toxicity, as was the case after a train derailment in Graniteville, South Carolina, which released tons of chlorine gas. Nearly all of the people hospitalized after exposure to this gas received supplemental oxygen (11). The goals of oxygen therapy are said to be to relieve hypoxemia by increasing alveolar tension, to reduce the work of breathing, and to decrease the work on the myocardium. To date, no clinical trials to study the efficacy of oxygen administration after chlorine exposure in humans have been performed, largely because such studies would be grossly unethical. Only anecdotal evidence exists regarding oxygen use in humans for this indication (16). Therefore, studies in animals are the only appropriate way to study treatment interventions after chlorine exposure. Herein, we show the outcomes after acute high-dose chlorine inhalation exposure in rats, with and without oxygen therapy as the primary intervention.
In the Graniteville incident, eight fatalities were reported within 4 hours of exposure (11), and one person died after admission to the hospital. To mirror human disease, we chose a dose of chlorine that also caused death within the first 4 hours of exposure. In our model, we noted several serious morbidities as well, the most important of which were acute hypercarbic respiratory failure and severe hypoxemia preceding seizure activity that resulted in death. We tested oxygen therapy in an attempt to improve morbidity and mortality after exposure to high-dose chlorine. We found that oxygen administration did not improve acute hypercarbic respiratory failure due to chlorine, and instead worsened it, but that oxygen did correct hypoxemia and diminish seizure activity, resulting in an improved overall survival to 6 hours.
High-dose chlorine exposure evoked signs of respiratory distress immediately after the start of exposure, as evidenced by decreased RR (bradypnea), gasping, and severe abdominal retractions. These signs of distress continued during and after exposure, with slow improvements over 6 hours. Oxygen administration starting 10 minutes after exposure did not improve chlorine inhalation–associated respiratory distress in our model, as shown by unchanged respiratory distress scores. Thus, the severe respiratory distress seen after high-level chlorine inhalation in the rats was not due to hypoxemia, as its correction by oxygen administration did not alter the level of distress.
In addition to significant respiratory distress, we observed severe acute respiratory failure after high-dose chlorine exposure, as measured by the severity of blood acidosis and hypercarbia. The presence of severe hypercarbia points to a type II acute respiratory failure classification rather than the type I (acute hypoxemic respiratory failure) classification that is most associated with ALI/acute respiratory distress syndrome (ARDS) (17, 18). Although there was also hypoxemia in our model, it was corrected with supplemental oxygen, a phenomenon that is commonly seen in type II respiratory failure, where the problem is usually inadequate alveolar ventilation. Type I respiratory failure is most often refractory to supplemental oxygen, as it is caused by intrapulmonary shunting of blood resulting from airspace filling or collapse. With ARDS, oxygen administration does not usually improve hypoxemia. However, in our model, oxygen administration improved hypoxemia but worsened CO2 retention. Worsening hypercarbia is a known danger of oxygen therapy, particularly in type II acute respiratory failure, due to loss of hypoxia-induced ventilatory drive, loss of hypoxic vasoconstriction, increased dead space, and thus a ventilation/perfusion mismatch (19). Indeed, that is what happened when oxygen was administered to high-dose chlorine–exposed rats. Therefore, our model suggests that ALI/ARDS is not present within the first 6 hours after exposure, when mortality is the highest. Indeed, we found no alveolar infiltrates in our model via histological assessment before the 6-hour observation period (Figure 3E). The etiology of the type II acute respiratory failure in our model may be multifactorial, and could include 1) direct stimulation by chlorine of irritant receptors within the respiratory tract, such as transient receptor potential channels, causing an altered breathing pattern (20); 2) direct or indirect effects of chlorine on the brain respiratory centers, resulting in a reduced ventilatory drive and hypoventilation; 3) increased airway resistance, causing longer breathing cycles and increased use of accessory muscles for breathing; 4) decreased pulmonary perfusion, causing a ventilation/perfusion mismatch; and/or 5) neuromuscular abnormalities resulting in ineffective muscles for respiration. Indeed, we found evidence of not only decreased CO but also decreased muscle tone in all animals exposed to high-level chlorine, neither of which was affected by oxygen treatment.
In our studies, mortality was high, with only 47% of untreated chlorine-exposed rats surviving to 6 hours. In those that survived, we did see a steady improvement in respiratory failure parameters, such as blood pH, PaCO2, and lactate levels. Interestingly, oxygen administration worsened respiratory parameters (and acute respiratory failure) in chlorine-exposed rats compared with untreated chlorine-exposed rats, yet survival was greatly improved, with 87% of the oxygen-treated animals alive at 6 hours. It is unclear whether this speaks to the resilient nature of the animal species used in this study. It is possible that rats can tolerate hypercapnic-only respiratory failure better than a combination of hypercapnic and hypoxemic respiratory failure. This may be different for humans, who would not be able to tolerate either one for very long. In addition, the fact that survival was improved with oxygen must mean that hypercarbia is not the single cause of death after chlorine in this rat model. Organ dysfunction may be a contributor to death in this model, as hypoxemia poses a major immediate threat to organ function (especially cardiac function and tissue oxygen delivery). With oxygen administration, organs are protected from hypoxemia-induced dysfunction, which should lead to improved survival. The lower lactate levels observed at 2 hours with oxygen compared with no treatment corroborated this hypothesis. However, this organ protection with oxygen administration was not sustained, as higher lactate levels were noted at 6 hours in oxygen-treated rats versus controls. It is likely that organs and tissues will eventually succumb to injury from acidosis and high CO2 levels. It is unclear how long animals would stay alive with oxygen therapy only, without additional interventions such as mechanical ventilation, to help prevent hypercarbic respiratory failure.
Oxygen administration after high-dose chlorine exposure normalized SpO2 but worsened the P/F ratio. This discrepancy is likely due not only to oxygen-associated hypoventilation, which worsens PaO2, but also to oxygen causing pulmonary vasodilation and thereby worsening the ventilation/perfusion mismatch. However, although we reported this value in our model, it is greatly misleading due to the high CO2 levels. The P/F ratio is calculated to reflect how well oxygen is absorbed from expired air. It is used to diagnose and grade the severity of ALI/ARDS, which is measured at a positive end-expiratory pressure of 5 according to the Berlin definition (2–4). At our altitude in Denver, a P/F ratio of >250 is considered normal. A severe decline in the P/F ratio is known to be associated with mortality in ARDS. However, the P/F ratio is used as a diagnostic tool only in type I acute hypoxemic respiratory failure, and not in type II hypercarbic respiratory failure. In standard practice, the P/F ratio should only be used to detect an alveolar diffusion abnormality when the PaCO2 is normal, and/or in patients who are mechanically ventilated (6). We had neither in our model. Moreover, a low P/F ratio can also occur due to poor hemodynamics (low CO), poor pulmonary perfusion, or a nonaerated lung (i.e., due to atelectasis or airway obstruction) (5). Indeed, we found that the CO was severely decreased immediately after high-dose chlorine exposure, consistent with a low perfusion state, and this was not improved with oxygen.
With severe acute respiratory failure, hemodynamic function is usually also affected (21). The resulting low organ perfusion, in particular the low pulmonary perfusion, likely caused decreased blood flow through the lungs, thereby diminishing oxygen/CO2 exchange at the alveolar level. Interestingly, the SV, a surrogate measure of cardiac pump function, was not altered with chlorine or oxygen. However, the HR was affected by chlorine, with a relative bradycardia observed after exposure, and this was not changed by oxygen treatment. As the HR is directly correlated with the CO along with the SV, bradycardia appears to be the sole cause of the decreased CO in our model. Not surprisingly, along with systemic hypoperfusion after acute chlorine inhalation, we also found a likely compensatory acute PAH within the pulmonary bed, with no significant change in systemic pressures. Acute PAH can occur physiologically to maintain adequate blood flow to the lungs (22). Oxygen, a potent pulmonary vasodilator, tended to decrease MPAPs to near-normal levels after chlorine exposure, albeit not significantly, in our studies. Therefore, there was a component of hypoxic pulmonary hypertension in our model, and likely an additional component due to acute pulmonary disease. The decrease in MPAP likely worsened blood flow to the lungs in the low-CO state, thereby worsening gas exchange and leading to our finding of worsening respiratory failure with oxygen. As the relatively normalized MPAP with oxygen did not improve respiratory failure, the acute PAH does not appear to be the cause of acute respiratory failure in this model.
In addition to respiratory and hemodynamic dysfunction, we observed a profound neuromuscular dysfunction immediately after high-level chlorine exposure, as indicated by findings of significant ataxia, hypotonia, and seizures leading to death in exposed rats. Although oxygen administration appeared to prevent seizure-related deaths, it did not improve ataxia or hypotonia. The cause of this neuromuscular dysfunction is uncertain, but direct injury to the neuromuscular system may be involved. Seizures have been documented sporadically in chlorine-exposed individuals (10, 23), but our finding of ataxia and hypotonia is novel. Together with bradycardia and bradypnea, both of which may have neurologic etiologies, we believe that the neuromuscular system is significantly involved in high-level acute chlorine exposure, similar to organophosphate exposures (24), albeit not to that severity. Future studies focusing on neuromuscular dysfunction after chlorine exposure are needed, as researchers investigating countermeasures against chlorine toxicity may need to include drugs that target the neuromuscular system.
In summary, oxygen administration in our model improved survival up to 6 hours, but worsened other morbidity measures that correlate with deleterious outcomes in the clinical setting, such as increased hypercarbia and blood acidosis. Although oxygen administration may be helpful in the treatment of acute hypoxemia after chlorine inhalation, caution must be exercised if there is evidence of respiratory acidosis, as oxygen treatment alone can decrease the ventilator drive without concurrent administration of ventilator support. In addition, the observation of multiorgan effects after high-level chlorine inhalation, particularly involving the cardiovascular and neuromuscular systems, highlights the need to develop effective countermeasures for rescue after massive chlorine exposure.
Acknowledgments
Acknowledgments
The authors thank Julie Harral (Hemodynamics CORE Laboratory, University of Colorado Denver) for her assistance in performing vascular access catheter placements and hemodynamic measurements.
Footnotes
This work was supported by the CounterACT Program, Office of the Director, National Institutes of Health, and grant R21 ES094111 from the National Institute of Environmental Health Sciences (L.A.V.).
Author Contributions: O.C.O. and L.A.V. were involved in the conception and design of the study, hypothesis delineation, interpretation of the data, and writing and revision of manuscript. O.C.O., M.D.M., M.M.D., R.B.G., J.S.R., A.L.M., G.B.R., C.W.W., and L.A.V. were involved in acquisition and analysis of the data.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2016-0223OC on August 28, 2017
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.McGovern T, Day BJ, White CW, Powell WS, Martin JG. AEOL10150: a novel therapeutic for rescue treatment after toxic gas lung injury. 2011;50:602–608. doi: 10.1016/j.freeradbiomed.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duncan MA, Drociuk D, Belflower-Thomas A, Van Sickle D, Gibson JJ, Youngblood C, et al. Follow-up assessment of health consequences after a chlorine release from a train derailment—Graniteville, SC, 2005. 2011;7:85–91. doi: 10.1007/s13181-010-0130-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wenck MA, Van Sickle D, Drociuk D, Belflower A, Youngblood C, Whisnant MD, et al. Rapid assessment of exposure to chlorine released from a train derailment and resulting health impact. 2007;122:784–792. doi: 10.1177/003335490712200610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Budko AA, Ivanovskii YV. [Use of chemical war gases at the Russian-German front during the First World War] 2016;337:75–81. [PubMed] [Google Scholar]
- 5.Chang W, Chen J, Schlueter CF, Rando RJ, Pathak YV, Hoyle GW. Inhibition of chlorine-induced lung injury by the type 4 phosphodiesterase inhibitor rolipram. 2012;263:251–258. doi: 10.1016/j.taap.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carlisle M, Lam A, Svendsen ER, Aggarwal S, Matalon S. Chlorine-induced cardiopulmonary injury. 2016;1374:159–167. doi: 10.1111/nyas.13091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evans RB. Chlorine: state of the art. 2005;183:151–167. doi: 10.1007/s00408-004-2530-3. [DOI] [PubMed] [Google Scholar]
- 8.Parimon T, Kanne JP, Pierson DJ. Acute inhalation injury with evidence of diffuse bronchiolitis following chlorine gas exposure at a swimming pool. 2004;49:291–294. [PubMed] [Google Scholar]
- 9.Zaky A, Bradley WE, Lazrak A, Zafar I, Doran S, Ahmad A, et al. Chlorine inhalation-induced myocardial depression and failure. 2015;3:e12439. doi: 10.14814/phy2.12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kilburn KH. Chlorine-induced damage documented by neurophysiological, neuropsychological, and pulmonary testing. 2000;55:31–37. doi: 10.1080/00039890009603382. [DOI] [PubMed] [Google Scholar]
- 11.Van Sickle D, Wenck MA, Belflower A, Drociuk D, Ferdinands J, Holguin F, et al. Acute health effects after exposure to chlorine gas released after a train derailment. 2009;27:1–7. doi: 10.1016/j.ajem.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kose A, Kose B, Açikalin A, Gunay N, Yildirim C. Myocardial infarction, acute ischemic stroke, and hyperglycemia triggered by acute chlorine gas inhalation. 2009;27:1022.e1–4. doi: 10.1016/j.ajem.2008.12.029. [DOI] [PubMed] [Google Scholar]
- 13.Aslan S, Kandiş H, Akgun M, Cakir Z, Inandi T, Görgüner M. The effect of nebulized NaHCO3 treatment on “RADS” due to chlorine gas inhalation. 2006;18:895–900. doi: 10.1080/08958370600822615. [DOI] [PubMed] [Google Scholar]
- 14.Veress LA, Hendry-Hofer TB, Loader JE, Rioux JS, Garlick RB, White CW. Tissue plasminogen activator prevents mortality from sulfur mustard analog-induced airway obstruction. 2013;48:439–447. doi: 10.1165/rcmb.2012-0177OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Veress LA, Anderson DR, Hendry-Hofer TB, Houin PR, Rioux JS, Garlick RB, et al. Airway tissue plasminogen activator prevents acute mortality due to lethal sulfur mustard inhalation. 2015;143:178–184. doi: 10.1093/toxsci/kfu225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Monto RW. Positive pressure oxygen therapy in treatment of pulmonary edema caused by chlorine gas. 1946;5:233–235. [PubMed] [Google Scholar]
- 17.Epstein SK, Singh N. Respiratory acidosis. 2001;46:366–383. [PubMed] [Google Scholar]
- 18.Ferguson ND, Fan E, Camporota L, Antonelli M, Anzueto A, Beale R, et al. The Berlin definition of ARDS: an expanded rationale, justification, and supplementary material. 2012;38:1573–1582. doi: 10.1007/s00134-012-2682-1. [DOI] [PubMed] [Google Scholar]
- 19.Broccard AF. Making sense of the PaO2/FiO2 in patients with acute respiratory distress syndrome. 2013;1:9. [Google Scholar]
- 20.Pilcher J, Perrin K, Beasley R. The effect of high concentration oxygen therapy on PaCO2 in acute and chronic respiratory disorders. 2013;1:8. doi: 10.1186/2213-0802-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahmad S, Ahmad A, Hendry-Hofer TB, Loader JE, Claycomb WC, Mozziconacci O, et al. Sarcoendoplasmic reticulum Ca(2+) ATPase. A critical target in chlorine inhalation-induced cardiotoxicity. 2015;52:492–502. doi: 10.1165/rcmb.2014-0005OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sommer N, Strielkov I, Pak O, Weissmann N. Oxygen sensing and signal transduction in hypoxic pulmonary vasoconstriction. 2016;47:288–303. doi: 10.1183/13993003.00945-2015. [DOI] [PubMed] [Google Scholar]
- 23.Kilburn KH. Brain but not lung functions impaired after a chlorine incident. 2003;41:299–305. doi: 10.2486/indhealth.41.299. [DOI] [PubMed] [Google Scholar]
- 24.Muro T, Shimada Y, Yano Y, Mozai T. [Neuropathy caused by organic phosphate and organic chlorine poisoning] 1971;28:509–519. [PubMed] [Google Scholar]